Abstract
Background
Adequate reconstruction of the soft tissue defect following resection of bone tumors is challenging. Prolene mesh, despite being a useful tool, is not widely used due to the fear of deep infection. The aim of this study was to evaluate the functional outcome and complications of using a Prolene mesh in oncological reconstructions.
Methods
A retrospective study was conducted in bone tumor patients with soft tissue reconstruction using Prolene mesh between January 2017 and June 2019. Functional evaluation was done using MSTS 93 score. Complications were recorded and were classified as mechanical (dislocation and extension lag) or biological failure (wound problems and deep infection). Comparison was performed between groups with and without biological failure to identify predictive variables.
Results
Of 116 patients, 68 were males and 48 were females, with median age of 22.5 years. Thirty nine patients had tumors of proximal tibia, 23 of proximal femur, 25 of proximal humerus, 24 of pelvis, and five tumors at other sites. Approximately two-thirds (62.9%) of our patients underwent endoprosthetic reconstruction while the rest underwent either biological or cement spacer reconstructions. Excellent or good functional outcomes were reported in 98.3% patients as per MSTS 93 scoring. Complications were noted in 22 patients (18.9%), of which 16 had biological failure, with four patients requiring debridement and mesh removal. Dislocation of prosthesis occurred in 2 patients of proximal femur replacement. Overall re-surgery rate was 5.1% (6 patients). There was no statistically significant difference between the groups with or without biological failure with respect to demographics, site of tumor, type of procedure, blood loss, duration of surgery and history of chemotherapy.
Conclusion
Prolene mesh is a useful tool to reconstruct the soft tissue defects following bone tumor resections. It is readily available, reliable and provides reproducible results, with no added risk of wound complications.
Keywords: Prolene mesh, Soft tissue reconstruction, Bone tumor
1. Introduction
The peri-articular location of the bone tumors often necessitates removal of the part of the bone involved along with the surrounding soft tissue restraints of the joints (ligaments, tendons, muscles, joint capsule) which contribute to its stability. Loss of soft tissues stabilizers and constraints around the joints often leads to subluxation or dislocation of the prosthesis, as well as functional deficits.1,2 While there are multiple options to reconstruct the bone defect such as allograft, endoprosthesis or vascularised fibular grafting, soft tissue reconstruction has always been a challenge to the orthopaedic surgeon. For example, in case of a proximal tibial tumor, endoprosthetic reconstruction can provide an adequate mechanical support and functional joint following resection but reattaching the extensor mechanism to the prosthesis is not so simple.
Various modifications in surgical techniques and use of new biomaterials have been described in literature for soft tissue reconstruction following bone tumor resection. These include use of expensive tubes, Dacron sleeves, Merselene tapes, hydroxy apetite coating of the endoprosthesis, synthetic mesh etc.2,3 However, the ideal method for soft tissue reconstruction is still under debate, and at present, there is no perfect solution to this problem.
Few studies in literature have reported the use of synthetic mesh after resection of bone tumors for reconstruction of residual soft tissues with variable results.4, 5, 6, 7 However, most include a very small patient population and focus on a single anatomical site. To the best of our knowledge, no study till date has reported the use of mesh for soft tissue reconstruction involving various anatomical locations and evaluated the functional outcome, in a sufficiently large patient population. Our present study aims to find out whether use of Prolene mesh for soft tissue reconstruction following resection of bone tumors (1) enhances the stability of the reconstruct or (2) increases the risk of deep infection.
2. Materials & methods
We conducted a retrospective study to evaluate the functional outcome after use of Prolene mesh for soft tissue reconstruction following resection of bone tumors. The study was conducted at the Department of Orthopaedics, All India Institute of Medical Sciences, New Delhi, India, after institutional ethics committee approval.
Inclusion criteria:
-
1.
Patients of any age, who had biopsy proven primary malignant or benign aggressive bone tumors or secondary metastatic skeletal lesions involving various anatomical locations, and had undergone wide resection of the tumor with some form of soft tissue reconstruction using Prolene mesh between January 2017 and June 2019.
-
2.
Completed a minimum 12 months follow up.
Exclusion criteria:
-
1.
Patients not completing minimum 12 months follow up.
-
2.
Patients/Guardians not giving informed consent for inclusion in the study.
Demographic and clinical data on these patients were retrieved from a prospectively maintained bone tumor database. Functional evaluation was done using Musculoskeletal Tumor Society 93 (MSTS 93) score.8 Complications were recorded and classified following the Henderson classification system of failure of limb salvage after reconstructive surgery for bone tumors.9 We categorized complications as 1) Mechanical failure (type 1A Henderson failure including instability and extension lag) and 2) Biological failure (type 1B Henderson failure of soft tissue coverage including aseptic wound dehiscence and type 4 Henderson failures including deep infection). The incidence of re-surgeries to address these complications was also noted.
The Prolene mesh that we use at our centre is PROLENE™ Mesh (ETHICON™), manufactured by Johnson-Johnson Inc. (Langhorne, PA, USA) and distributed by Johnson-Johnson Inc. (Neuss, Germany). It is a construction of knitted non-absorbable filaments of polypropylene, identical in composition to that used in Prolene suture, that resists degradation by tissue enzymes and retains strength indefinitely in clinical use.10 The knitting process interlinks each fiber junction and provides for extensibility in both directions. The bi-directional extensible property allows adaptation to various stresses encountered in the body. According to manufacturer’s data, the mesh has a burst strength of approximately 14 kg/cm2, providing high burst strength and tensile strength for a strong repair.11,12 It is customizable and may be trimmed without unraveling.10 The mesh used in the study was free of cost for the patient.
All the collected data were entered in a Microsoft Excel Worksheet and tabulated.
Statistical analysis was done using the statistical computing software R version 2.15.0. Categorical variables were analyzed using Chi-square test. For continuous variables, normalcy of data distribution was assessed using Shapiro-Wilk test and Student t-test was used to assess significance. All the observations were considered to be significant when p-value was <0.05.
3. Results
A total of 116 patients met the inclusion criteria and were included in the study. Mean follow up period was 23.91 months (range 12–42).
The complications noted including mechanical failure (type 1A failure-instability and extension lag) and biological failure (type 1B failure of soft tissue coverage and type 4 failure with deep infection) are as shown in Table 1.
Table 1.
Complications noted including mechanical and biological failure.
| A. Mechanical failure | |
| A.1. Type 1A failure- instability after limb salvage surgery | |
|
Procedure |
No. of Patients |
| a. Proximal femur endoprosthesis | 2 |
| b. Proximal humerus endoprosthesis/nail cement spacer | 0 |
| c. Pelvis reconstruction | 0 |
| d. Proximal tibia endoprosthesis | 0 |
| e. Others | 0 |
|
Total |
2/116 (1.72%) |
|
A.2. Type 1A failure- extension lag after proximal tibia endoprosthesis | |
| a. No. of patients/total (%) without any lag (or with full active extension) | 31/39 (79.5%) |
| b. No. of patients/total (%) with lag | 8/39 (20.51%) |
| c. Mean extension lag (range) | 2.31° (0–20°) |
| d. Mean flexion (range) |
94.1° (70–110°) |
| B. Biological failure | |
| B.1. Type 1B-failure of coverage after limb salvage surgery | |
|
Procedure |
No. of Patients |
| a. Proximal femur endoprosthesis | 3 |
| b. Proximal tibia endoprosthesis | 8 |
| c. Proximal humerus endoprosthesis/nail cement spacer | 0 |
| d. Pelvis reconstruction | 1 |
| e. Others | 0 |
|
Total |
12/116 (10.3%) |
| B.2. Type 4 failure- deep infection after limb salvage surgery | |
|
Procedure |
No. of Patients |
| a. Proximal femur endoprosthesis | 1 |
| b. Proximal tibia endoprosthesis | 0 |
| c. Proximal humerus endoprosthesis/nail cement spacer | 0 |
| d. Pelvis reconstruction | 2 |
| e. Others | 1 |
| Total | 4/116 (3.45%) |
Patients without complications (group 1) and patients with complications (group 2) were compared for the demographic and outcome parameters (Table 2).
Table 2.
Comparison of demographics and outcome parameters between the Group 1 (without complications) and Group 2 (with complications).
| Demographics |
Group 1 (n=94) |
Group 2 (n=22) |
p value |
|---|---|---|---|
| 1. Median Age (years) | 23 | 19 | 0.54 |
| 2. Sex | |||
| Male (n=68) | 55 | 13 | 0.960 |
| Female (n=48) | 39 | 9 | |
| 3. Diagnosis | |||
| a. Osteosarcoma (n=32) | 27 | 5 | 0.187 |
| b. Ewing’s sarcoma (n=31) | 23 | 8 | |
| c. Chondrosarcoma (n=19) | 18 | 1 | |
| d. GCTB (n=23) | 16 | 7 | |
| e. Others (n=11) | 10 | 1 | |
| 5. Site of tumor | |||
| a. Proximal femur (n=23) | 18 | 5 | 0.032 |
| b. Proximal tibia (n=39) | 26 | 13 | |
| c. Proximal humerus (n=25) | 25 | 0 | |
| d. Pelvis (n=24) | 21 | 3 | |
| e. Others (n=5) | 4 | 1 | |
| 1. Proximal fibula | 1 | 1 | |
| 2. Proximal radius | 1 | 0 | |
| 3. Distal fibula | 2 | 0 | |
| 6. Reconstruction type) | |||
| 1. Endoprosthesis (n=73) | 55 | 18 | 0.070 |
| 2. Spacer (n=14) | 14 | 0 | |
| 3. Biological reconstruction (n=29) | 25 | 4 | |
| 7. Chemotherapy | |||
| a. Yes (n=63) | 50 | 13 | 0.617 |
| b. No (n=53) | 44 | 9 |
Other sites in Table 1, Table 2 include 2 cases of proximal fibula resection with reconstruction of posterolateral corner, 2 cases of distal fibula resection with reconstruction of tibio-fibular ligament and 1 case of proximal radius resection with reconstruction of lateral collateral ligament using Prolene mesh.
The requirement for a re-surgery procedure for the above mentioned complications was noted. A total of 6 re-surgery procedures were required with a re-surgery rate of 5.17%. Of the two patients with dislocation of proximal femur endoprosthesis, one was managed with closed reduction under anaesthesia followed by traction for 6 weeks, while the other also developed deep infection and required aggressive debridement, prosthesis and mesh removal and cement spacer. One patient with proximal tibia endoprosthesis reconstruction developed skin necrosis which required debridement and split skin grafting. Two patients with pelvic resections and one patient with proximal fibula resection with posterolateral corner reconstruction developed deep infection and required debridement and mesh removal.
Comparison of outcome parameters and intra-operative variables between patients with and without biological failure is as shown in Table 3.
Table 3.
Comparison of outcome parameters and intra-operative variables between patients with and without biological failure.
| Variables | Without Biological failure | With Biological failure | p-value |
|---|---|---|---|
| 1. Sex | |||
| a. Male | 60 | 8 | 0.451 |
| b. Female | 40 | 8 | |
| 2. Site | |||
| a. Proximal femur | 19 | 4 | 0.205 |
| b. Proximal tibia | 31 | 8 | |
| c. Proximal humerus | 25 | 0 | |
| d. Pelvis | 21 | 3 | |
| e. Others | 4 | 1 | |
| 3. Diagnosis | |||
| a. Osteosarcoma | 29 | 3 | 0.113 |
| b. Ewing’s sarcoma | 23 | 8 | |
| c. Chondrosarcoma | 18 | 1 | |
| d. GCTB | 19 | 4 | |
| e. Others | 11 | 0 | |
| 4.Reconstruction type | |||
| a. Endoprosthesis | 61 | 12 | 0.263 |
| b. Spacer | 14 | 0 | |
| c. Biological reconstruction | 25 | 4 | |
| 5. Chemotherapy | |||
| a. Yes | 52 | 11 | 0.212 |
| b. No | 48 | 5 | |
| 6. Median Blood loss (IQR) | 1150 ml (712.5 -1800) |
1100 ml (575-2750) |
0.985 |
| 7. Mean Duration of Surgery (min) | 240.47+/-50.31 | 281.58+/-83.80 | 0.143 |
| 8. ASA grade | Grade 1- 68 patients Grade 2- 6 patients |
Grade 1-10 patients Grade 2- 2 patients |
0.344 |
Functional outcome was evaluated using the MSTS 93 score (Table 4).
Table 4.
Functional outcome using MSTS 93 score.
|
No. Of Patients (%) | |||||
|---|---|---|---|---|---|
| Site of tumor | Mean MSTS 93 score (range) |
Excellent MSTS>23 |
Good MSTS 15-22 |
Fair MSTS 8-14 |
Poor MSTS<8 |
| 1. Proximal femur | 24.91 (12-29) | 19/23 (82.6%) | 3/23 (13%) |
1/23 (4.3%) |
0 |
| 2. Proximal tibia | 27.07 (23-30) | 39/39 (100%) | 0 | 0 | 0 |
| 3. Proximal humerus | 22.16 (19-25) | 12/25 (48%) |
13/25 (52%) |
0 | 0 |
| 4. Pelvis | 21.67 (14-25) | 8/24 (33.3%) |
15/24 (62.5%) |
1/24 (4.16%) |
0 |
| 5. Others | 24.6 (23-26) | 5/5 (100%) |
0 | 0 | 0 |
| Overall | 24.36 (12-30) | 83/116 (71.5%) |
31/116 (26.7%) |
2/116 (1.7%) |
0 |
4. Discussion
While obtaining adequate oncological margins during surgical resection is critical for optimum oncological outcome, reconstruction of the skeletal and soft tissue defect created following resection is the key to achieving good functional outcome. Soft tissue reconstruction should therefore be planned with the aim of achieving a reconstruction that is as close as possible to the original anatomy, enhancing stability of the skeletal reconstruction and facilitating early rehabilitation.
The ideal material for the reconstruction of the soft tissue deficiencies after resection of bone tumors should be able to provide long term stability to the skeletal reconstruct without itself fragmenting, and should not increase the likelihood of foreign body reaction and infections. Besides being non-absorbable, it should provide a degree of flexibility and should have high tensile strength to prevent early failure, especially around a joint that is capable of multidirectional movements. Prolene mesh is by far closest to an ideal material.
Synthetic mesh has been widely used in reconstructions of the abdominal and chest walls after major resections and closure of complex hernias.13, 14, 15, 16 We have used Prolene mesh for enhancing soft tissue repair and reconstruction following wide resection of bone tumors at various anatomical sites.
In oncological surgeries around the hip, obtaining a wide margin often necessitates sacrifice of important hip joint stabilizers including joint capsule and abductors. Dislocation is the most common complication of proximal femur endoprosthetic reconstruction.17 In literature, the reported dislocation rate ranges from 10 to 40%. These figures are much higher than the 2–8% dislocation rates reported for THR unrelated to bone tumors around the hip.18,19 While some studies advocate surgical repair of hip capsule and repair of abductors to the prosthesis as an effective method of preventing instability, others have found no such association.20, 21, 22, 23, 24, 25
Bickels et al. used Dacron tape capsulorrhaphy over the prosthetic neck in patients undergoing proximal or total femur resection with endoprosthetic reconstruction, and reported dislocation of the prosthesis in only one patient, with deep wound infection necessitating surgical intervention in two patients.1 Gosheger et al. used Trevira tube (polyethylene terephthalate knitted tube) for reconstruction of the capsule and noted dislocation of the prosthesis in two of 38 patients with late infections in three patients.3
The present study included 23 patients who underwent wide resection and proximal femur endoprosthetic reconstruction. We sutured a sheet of Prolene mesh to the capsular remnant and the acetabular labrum using a series of interrupted nonabsorbable sutures (Ethibond No. 5) in all cases. The mesh was then wrapped tightly around the femoral prosthesis and then sutured to itself over the prosthesis with a further series of nonabsorbable sutures to create a tight restraint. The abductors were then attached to the mesh. We found dislocation of the prosthesis in only two patients, both occurring within one month of index surgery, and deep infection in only one patient. Three patients developed wound complications which were managed conservatively. The mean MSTS score was 24.91, with 95.6% reporting an excellent or good functional outcome (Table 4). The results show that the application of a Prolene mesh to stabilize the proximal femur endoprosthesis helps achieve a stable hip with low dislocation and infection rates.
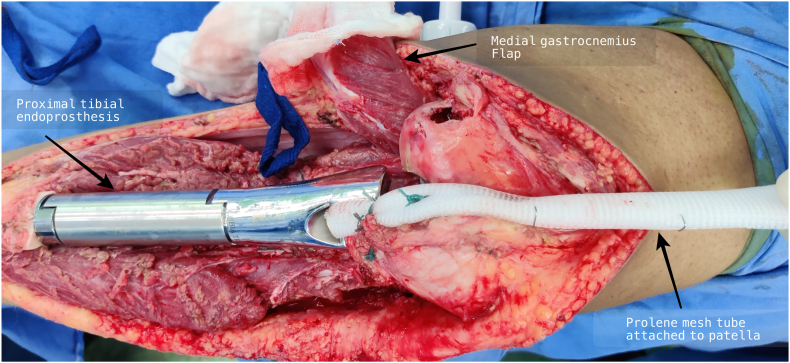
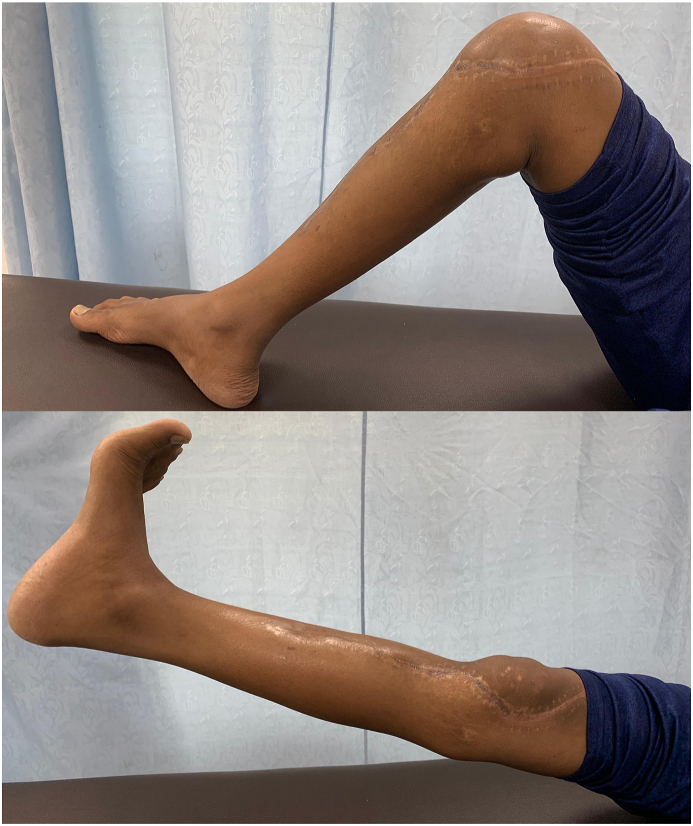
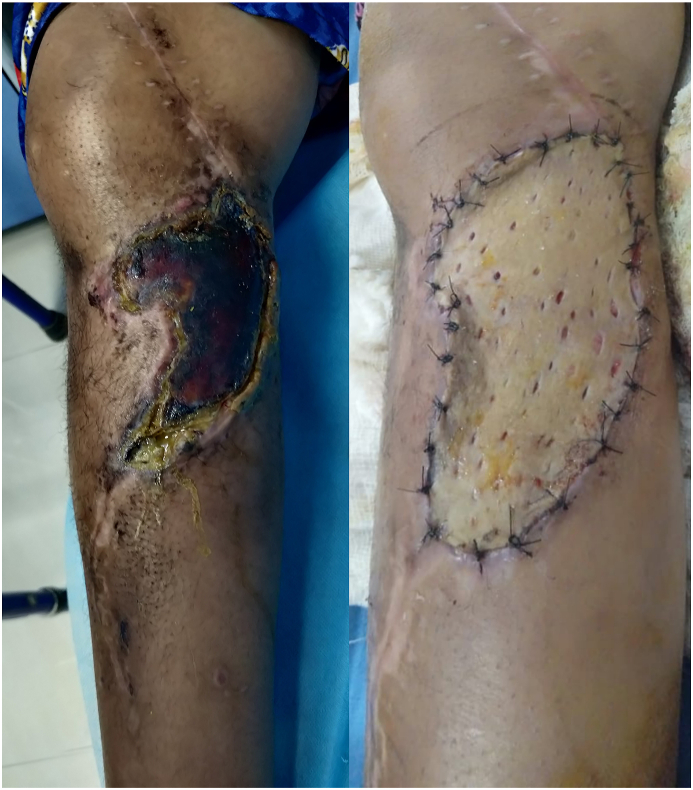
Wide resection of the proximal tibia involves disruption of the extensor mechanism of the knee. Various methods of reattachment of the patellar tendon to the prosthesis and gastrocnemius flap have been described in literature including direct fixation to the prosthesis using screws, sutures, loops and mechanical clamping, and augmentation with synthetic materials including synthetic ligaments (LARS, Leeds-Keio ligament, Gore-Tex1), Trevira tube, Aorta Graft, Dacron tape and synthetic mesh with variable results.3,26, 27, 28 We devised a new surgical technique of extensor mechanism reconstruction after proximal tibia resection using a cylindrical tube of Prolene mesh attached to the patellar tendon and medial patellar retinaculum. This tube is then passed through a slot in the prosthesis and reattached to itself under adequate tension with the knee in 15–20° of flexion (Fig. 1). Finally, medial gastrocnemius flap is rotated anteriorly for coverage of the mesh and prosthesis. Ichikawa et al. used a complex surgical technique for extensor mechanism reconstruction which included the attachment of mesh to the tibial component using a plate and screws, in a small series of 9 patients undergoing proximal tibia endoprosthetic reconstruction and reported a mean extension lag of 5°.6 Our technique is quite simple compared to other techniques reported in the literature and the mean extension lag reported in our study was 2.3° and mean flexion at the knee was 94.1° (range 70–110°). Most of the patients had good range of motion at the knee (Fig. 2). A reattachment tube (Implantcast) fixed around the prosthesis with non-absorbable sutures was used for extensor mechanism reconstruction by Hardes et al.29 with extension lag in 25% of patients as opposed to 20.5% patients in our study. Our method Prolene mesh reconstruction of extensor mechanism has comparable results with other reconstruction methods with the additional advantage of being cheap and widely available. Use of artificial ligaments and synthetic materials has been associated with synovitis, and loosening or stretching of the patellar tendon.26, 27, 28 Although we did not find any of these complications in our study cohort, eight patients developed aseptic wound necrosis, of which only one required debridement and Split Thickness Skin Grafting (STSG) (Fig. 3), while others were managed conservatively. Excellent functional outcome was reported in all the patients undergoing proximal tibia endoprosthesis, with mean MSTS score of 27.07 (Table 4).
Fig. 1.
Knee extensor mechanism reconstruction using Prolene mesh tube following proximal tibia resection and endoprosthesis reconstruction.
Fig. 2.
Clinical photograph showing range of motion at the knee after extensor mechanism reconstruction using Prolene mesh tube.
Fig. 3.
Wound necrosis following proximal tibial endoprosthetic reconstruction, which was managed with Split Thickness Skin Grafting.
Resection of tumors of proximal humerus often includes resection of major part of the rotator cuff muscles, the deltoid muscle and the axillary nerve, resulting in severe functional deficits. In such situations, a stable shoulder joint is critical for the preservation of the elbow and hand function. Marulanda et al. described the use of an aortic graft mesh (Gore graft) and bioabsorbable anchor screw for the attachment of endoprosthesis to glenoid and reported no subluxations or disocations using this technique, with infection rate of 6.25%.30 Kitagawa et al. reported subluxation or dislocation in 20% of cases in their method of attachment of prosthesis to the rotator cuff or acromion with synthetic sutures and to the bone projections by Dacron tape.31
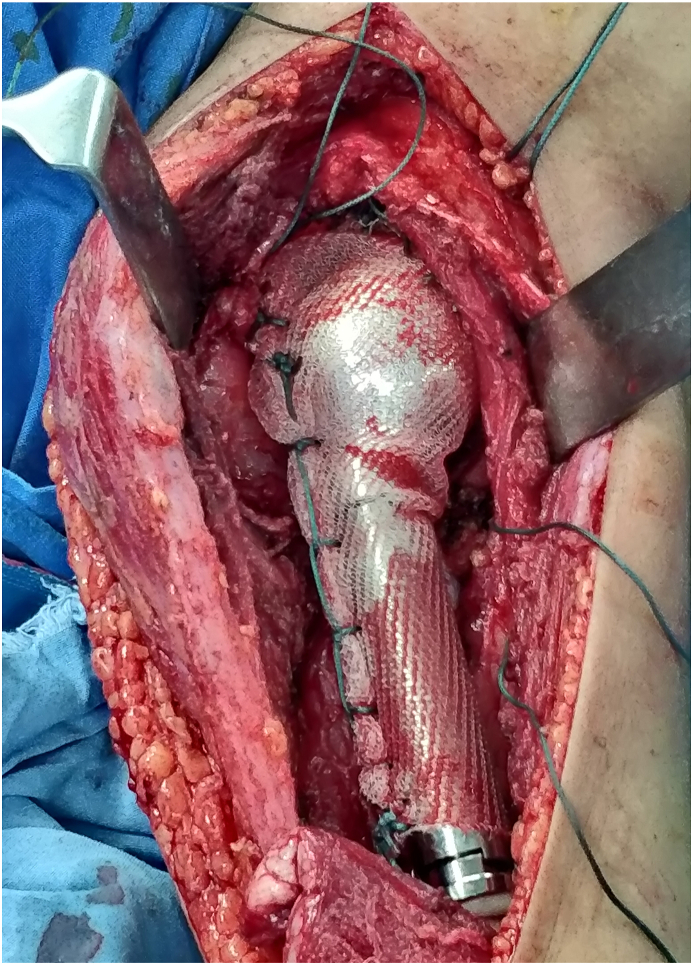
Our study included 25 patients with tumors involving proximal humerus who underwent wide resection followed by reconstruction with endoprosthesis or nail-cement spacer construct and stabilization of the reconstruct with Prolene mesh (Fig. 4). We found no dislocation of the prosthesis or nail-cement spacer construct. None of the patients developed any infection or wound complications. Wang et al. also reported similar results with no dislocations or infections while using polypropylene mesh for reconstruction following proximal humerus resection in 18 patients.32 Mean MSTS score recorded for proximal humerus resections in our study was 22.16, with excellent and good functional outcome reported in 48% and 52% respectively (Table 4). The results suggest that the use of Prolene mesh for stabilization of the reconstruct following proximal humerus resection is superior to any other reconstruction method reported till date.
Fig. 4.
Use of Prolene mesh for anchoring proximal humeral endoprosthesis to the glenoid following wide resection of tumor of proximal humerus.
Meshplasty is routinely performed at our centre following peri-acetabular resections where the femoral head is anchored to the nearest available healthy bone with the help of a Prolene mesh. 24 patients who underwent pelvic resections and reconstruction with Prolene mesh for pelvic tumors were included in the present study. Wound complications were noted in three patients in our study, of which two required debridement and mesh removal. One patient developed wound dehiscence which was managed conservatively with regular dressings. However, we did not report any dislocation with our reconstruction method. The mean MSTS 93 score at final follow up was 21.67. Although meshplasty may not restore normal hip joint kinematics, majority of these patients remained functionally active and were able to walk unassisted and returned to their occupation, with approximately 95% of the patients reporting excellent to good functional outcome (Table 4). Considering these favourable results of mesh reconstruction, we believe that future studies comparing the low cost meshplasty with the expensive prosthetic acetabular reconstruction techniques using objective parameters may help to establish meshplasty as a useful alternative to prosthesis reconstruction for pelvic resections.
The ill-founded fear of increased risk of deep prosthetic infection with the use of mesh has restricted the use of mesh in orthopaedic oncology. Whether or not there is an increased risk of infection due to the use of mesh has still not been validated in literature, as infections can also be attributed to inadequate soft tissue coverage, dead space after resection, and the immunosuppressive chemotherapy. Comparison of patients developing wound problems (biological failure) with those who did not develop wound problems in our study showed that age, sex, tumor type, site of involvement, type of reconstruction and chemotherapy did not influence the risk of wound related complications. Also, no association was found between duration of surgery, amount of intra-operative blood loss and ASA grade with wound related complications (Table 3).
The strength of our study lies in the inclusion of a large patient population, long follow up duration and application of mesh at various anatomical locations. There are few limitations in this study. Foremost, the data presented are retrospective. We have evaluated the outcomes following soft tissue reconstruction using only Prolene mesh because of which comparison could not be done with other forms of biomaterials in order to conclude whether prolene mesh is superior to other available biomaterials. While the study population includes different anatomical sites to discuss the various utilities of mesh in reconstructing soft tissue defects, it can also be considered as a limiting factor in analysis as the results may not be homogenous. However, the difference in anatomical location does not contribute to statistically significant difference in our outcomes (Table 2).
5. Conclusion
Our study results show that the use of Prolene mesh for soft tissue reconstruction following resection of bone tumors enhances the stability of the skeletal reconstruct without any increase in the risk of wound dehiscence or deep infection. Prolene mesh is a boon for orthopaedic onco-surgeons and an extremely useful tool to reconstruct the soft tissues especially in a developing country like ours. It is readily available, reliable and provides reproducible results. Prospective comparative studies are warranted to establish its superiority over other costlier alternatives.
Contribution
Love Kapoor: review of literature concerning the topic, data collection, statistical analysis, and assisted in surgeries, Roshan Banjara: data collection and assisted in surgeries, Dr. Ashish Ragase: data collection and assisted in surgeries, Abdul Majeed: data collection and assisted in surgeries, Venkatesan Sampath Kumar: designing the study, editing of the manuscript and statistical analysis, Shah Alam Khan: designing the study, final editing of the manuscript, supervised the study and was the senior surgeon in all the cases.
Declaration of competing interest
None.
There is no potential conflict of interest and there is nothing to disclose for all the authors.
Contributor Information
Love Kapoor, Email: lovekapoor871988@rediffmail.com.
Roshan Banjara, Email: roshan20440722@gmail.com.
Abdul Majeed, Email: amajeed2710@gmail.com.
Venkatesan Sampath Kumar, Email: venkatortho4@gmail.com.
Shah Alam Khan, Email: shahalamkhan70@gmail.com.
References
- 1.Bickels J., Meller I., Henshaw R.M., Malawer M.M. Reconstruction of hip stability after proximal and total femur resections. Clin Orthop Relat Res. 2000;375:218–230. doi: 10.1097/00003086-200006000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Ross A.C., Wilson J.N., Scales J.T. Endoprosthetic replacement of the proximal humerus. J Bone Joint Surg Br. 1987;69(4):656–661. doi: 10.1302/0301-620X.69B4.3611177. [DOI] [PubMed] [Google Scholar]
- 3.Gosheger G., Hillmann A., Lindner N. Soft tissue reconstruction of megaprostheses using a trevira tube. Clin Orthop Relat Res. 2001;393:264–271. doi: 10.1097/00003086-200112000-00030. [DOI] [PubMed] [Google Scholar]
- 4.Masterson E.L., Ferracini R., Griffin A.M., Wunder J.S., Bell R.S. Capsular replacement with synthetic mesh: effectiveness in preventing postoperative dislocation after wide resection of proximal femoral tumors and prosthetic reconstruction. J Arthroplasty. 1998;13(8):860–866. doi: 10.1016/s0883-5403(98)90190-5. [DOI] [PubMed] [Google Scholar]
- 5.Fujibuchi T., Matsumoto S., Shimoji T. New endoprosthesis suspension method with polypropylene monofilament knitted mesh after resection of bone tumors in proximal humerus. J Shoulder Elbow Surg. 2015;24(6):882–888. doi: 10.1016/j.jse.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Ichikawa J., Matsumoto S., Shimoji T., Ae K., Tanizawa T., Gokita T. A new technique using mesh for extensor reconstruction after proximal tibial resection. Knee. 2015;22(6):659–663. doi: 10.1016/j.knee.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Liu B., Tan J.C., Wang H.L., Wu Z., Yuan Z.C., Wei C.Y. The role of mesh technology with tumor prosthesis reconstruction to reconstruct the extensor mechanism of knee joint after resection of proximal tibial tumors. J Orthop Surg Res. 2019;14(1):64. doi: 10.1186/s13018-019-1105-1. Published 2019 Feb 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enneking W.F., Dunham W., Gebhardt M.C., Malawar M., Pritchard D.J. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 9.Henderson E.R., O’Connor M.I., Ruggieri P. Classification of failure of limb salvage after reconstructive surgery for bone tumours : a modified system Including biological and expandable reconstructions. Bone Joint Lett J. 2014;96-B(11):1436–1440. doi: 10.1302/0301-620X.96B11.34747. [DOI] [PubMed] [Google Scholar]
- 10.PROLENE® Polypropylene Mesh. Instructions for Use. Ethicon Inc. Somerville, NJ.
- 11.Cobb W.S., Warren J.A., Ewing J.A., Burnikel A., Merchant M., Carbonell A.M. Open retromuscular mesh repair of complex incisional hernia: predictors of wound events and recurrence. J Am Coll Surg. 2015;220(4):606–613. doi: 10.1016/j.jamcollsurg.2014.12.055. [DOI] [PubMed] [Google Scholar]
- 12.Holste J.L. Are meshes with lightweight construction strong enough? Int Surg. 2005;90(3 Suppl):S10–S12. [PubMed] [Google Scholar]
- 13.Molloy R.G., Moran K.T., Waldron R.P., Brady M.P., Kirwan W.O. Massive incisional hernia: abdominal wall replacement with Marlex mesh. Br J Surg. 1991;78(2):242–244. doi: 10.1002/bjs.1800780237. [DOI] [PubMed] [Google Scholar]
- 14.Liakakos T., Karanikas I., Panagiotidis H., Dendrinos S. Use of Marlex mesh in the repair of recurrent incisional hernia. Br J Surg. 1994;81(2):248–249. doi: 10.1002/bjs.1800810230. [DOI] [PubMed] [Google Scholar]
- 15.Kroll S.S., Walsh G., Ryan B., King R.C. Risks and benefits of using Marlex mesh in chest wall reconstruction. Ann Plast Surg. 1993;31(4):303–306. doi: 10.1097/00000637-199310000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Chapelier A., Macchiarini P., Rietjens M. Chest wall reconstruction following resection of large primary malignant tumors. Eur J Cardio Thorac Surg. 1994;8(7):351–357. doi: 10.1016/1010-7940(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 17.Anderson M.E., Hyodo A., Zehr R.J., Marks K.E., Muschler G.F. Abductor reattachment with a custom proximal femoral replacement prosthesis. Orthopedics. 2002;25(7):722–726. doi: 10.3928/0147-7447-20020701-11. [DOI] [PubMed] [Google Scholar]
- 18.Jacofsky D.J., Haidukewych G.J., Zhang H., Sim F.H. Complications and results of arthroplasty for salvage of failed treatment of malignant pathologic fractures of the hip. Clin Orthop Relat Res. 2004;427:52–56. doi: 10.1097/01.blo.0000143572.96021.93. [DOI] [PubMed] [Google Scholar]
- 19.Kunisada T., Choong P.F. Major reconstruction for periacetabular metastasis: early complications and outcome following surgical treatment in 40 hips. Acta Orthop Scand. 2000;71(6):585–590. doi: 10.1080/000164700317362217. [DOI] [PubMed] [Google Scholar]
- 20.Kawai A., Backus S.I., Otis J.C., Inoue H., Healey J.H. Gait characteristics of patients after proximal femoral replacement for malignant bone tumour. J Bone Joint Surg Br. 2000;82(5):666–669. doi: 10.1302/0301-620x.82b5.10264. [DOI] [PubMed] [Google Scholar]
- 21.Chandrasekar C.R., Grimer R.J., Carter S.R., Tillman R.M., Abudu A.T. Modular endoprosthetic replacement for metastatic tumours of the proximal femur. J Orthop Surg Res. 2008;3:50. doi: 10.1186/1749-799X-3-50. . Published 2008 Nov 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson E.R., Keeney B.J., Pala E. The stability of the hip after the use of a proximal femoral endoprosthesis for oncological indications: analysis of variables relating to the patient and the surgical technique. Bone Joint Lett J. 2017;99-B(4):531–537. doi: 10.1302/0301-620X.99B4.BJJ-2016-0960.R1. [DOI] [PubMed] [Google Scholar]
- 23.Tan A., Akagi M., Fukuda K., Hamanishi C. Modular prostheses for metastatic bone tumor of the proximal femur. Acta Med Kinki Univ. 2010;35:103–107. [Google Scholar]
- 24.Ogilvie C.M., Wunder J.S., Ferguson P.C., Griffin A.M., Bell R.S. Functional outcome of endoprosthetic proximal femoral replacement. Clin Orthop Relat Res. 2004;426:44–48. doi: 10.1097/01.blo.0000136840.67864.78. [DOI] [PubMed] [Google Scholar]
- 25.Morris H.G., Capanna R., Del Ben M., Campanacci D. Prosthetic reconstruction of the proximal femur after resection for bone tumors. J Arthroplasty. 1995;10(3):293–299. doi: 10.1016/s0883-5403(05)80177-9. [DOI] [PubMed] [Google Scholar]
- 26.Jentzsch T., Erschbamer M., Seeli F., Fuchs B. Extensor function after medial gastrocnemius flap reconstruction of the proximal tibia. Clin Orthop Relat Res. 2013;471(7):2333–2339. doi: 10.1007/s11999-013-2851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimose S., Sugita T., Kubo T., Matsuo T., Ochi M. Reconstructed patellar tendon length after proximal tibia prosthetic replacement. Clin Orthop Relat Res. 2005;439:176–180. doi: 10.1097/01.blo.0000176150.16509.33. [DOI] [PubMed] [Google Scholar]
- 28.Bickels J., Wittig J.C., Kollender Y. Reconstruction of the extensor mechanism after proximal tibia endoprosthetic replacement. J Arthroplasty. 2001;16(7):856–862. doi: 10.1054/arth.2001.25502. [DOI] [PubMed] [Google Scholar]
- 29.Hardes J., Henrichs M.P., Gosheger G. Tumour endoprosthesis replacement in the proximal tibia after intra-articular knee resection in patients with sarcoma and recurrent giant cell tumour. Int Orthop. 2018;42(10):2475–2481. doi: 10.1007/s00264-018-3893-z. [DOI] [PubMed] [Google Scholar]
- 30.Marulanda G.A., Henderson E., Cheong D., Letson G.D. Proximal and total humerus reconstruction with the use of an aortograft mesh. Clin Orthop Relat Res. 2010;468(11):2896–2903. doi: 10.1007/s11999-010-1418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitagawa Y., Thai D.M., Choong P.F. Reconstructions of the shoulder following tumour resection. J Orthop Surg. 2007;15(2):201–206. doi: 10.1177/230949900701500216. [DOI] [PubMed] [Google Scholar]
- 32.Wang B., Wu Q., Liu J., Yang S., Shao Z. Endoprosthetic reconstruction of the proximal humerus after tumour resection with polypropylene mesh. Int Orthop. 2015 Mar;39(3):501–506. doi: 10.1007/s00264-014-2597-2. [DOI] [PubMed] [Google Scholar]