Abstract
Study design
Systematic Review.
Objectives
We performed this systematic overview on the overlapping meta-analyses that analyzed autologous bone marrow-derived mesenchymal stem cell(BM-MSC) therapy along with core decompression(CD) for the management of osteonecrosis of the femoral head(ONFH) and identify which study provides the current best evidence on the topic and generate recommendations for the same.
Materials and methods
We conducted independent and duplicate electronic database searches in PubMed, Web of Science, Embase, Cochrane Database of Systematic Reviews, and the Database of Abstracts of Reviews of Effects till September 2020 for meta-analyses that analyzed the efficacy of BM-MSC therapy along with CD for ONFH. Methodological quality assessment was made using Oxford Levels of Evidence, AMSTAR scoring, and AMSTAR 2 grades. We then utilized the Jadad decision algorithm to identify the study with the highest quality to represent the current best evidence to generate the recommendation.
Results
6 meta-analyses fulfilling the eligibility criteria were included. The AMSTAR scores of the included studies varied from 4 to 9 (mean:7) and all the included studies had critically low reliability in their summary of results due to their methodological flaws according to AMSTAR 2 grades. The current best evidence showed that utilization of BM-MSC therapy along with CD for ONFH resulted in significant improvement in Harris hip scores at 12 and 24 months along with a significant reduction in the necrotic area of the femoral head and the rate of conversion to total hip arthroplasty(THA) without a significant rise in adverse events due to the procedure.
Conclusion
Based on this systematic overview, we give a Level II recommendation that BM-MSC therapy is more efficacious along with CD in the management of ONFH compared to CD alone. BM-MSC therapy provides better pain relief with significant functional improvement and delaying the collapse of the femoral head thereby preventing further treatment such as THA.
1. Introduction
Osteonecrosis of the femoral head (ONFH), also called avascular necrosis or aseptic necrosis or ischaemic necrosis of femoral head, is a chronic progressive, debilitating degenerative disease of hip joint characterized by the progressive necrosis of osteogenic cells and the components of the bone marrow.1 If left untreated, the disease may progress and lead to complete deterioration of the hip joint.2 The incidence of ONFH cases were 20,000 to 30,000 cases per year.3 A multifactorial web of causation has been proposed for the pathogenesis of ONFH but still, the cause for the disease pathology is uncertain.
ONFH is a combination of osteological and vascular disease of the hip joint.4 Various researchers have postulated three important hypotheses for ONFH namely a) thrombosis or embolism of functional capillaries which serve as the conduit for stem cells, b) potential decline in the number of osteogenic cells, and mesenchymal stem cells in the infarcted region and c) apoptosis of osteogenic stem cells.5, 6, 7 These three postulates provide an environment where insufficient bone repair occurs and the disease pathology progresses. The management of the ONFH necessitates early diagnosis and management to preserve the vascularity of the femoral head. The effective treatment for ONFH is a challenging task for the orthopedic surgeons as the treatment protocol depends on the age of the patient, the stage of the disease, and the size of the necrosed lesions. The management protocols range from conservative protocol to hip replacement surgeries.8
Due to the evolving trends in regenerative medicine, researchers traced a path to regenerate the necrosed femoral head with orthobiologic therapy.9,10 The concept of cellular or cell-based therapy deals with the usage of autologous or allogenic cells with a higher regenerative potential to heal the degenerative diseases of bone and joints. Researchers demonstrated the superior role of potent orthobiologics such as bone marrow mesenchymal stem cells (BM-MSCs) in conjunction with the core decompression (CD) technique for ONFH.11, 12, 13 Core decompression relieves the intra-osseous pressure whereas BM-MSCs regenerates the osteogenic cells and induce vasculogenesis and helps in preserving the femoral head. However, there are conflicting evidences on their utility for acceptance into the management regime of ONFH.14
Recently, multiple meta-analyses have been published in this regard comparing the effectiveness of CD with and without BM-MSC therapy for the management of ONFH.15, 16, 17 However, these overlapping meta-analyses have a wider variability in the included studies due to the restriction in language and databases they employed for identifying primary studies. Every meta-analysis suffered from a limited pooled sample size. Hence, the objective of this systematic overview of the overlapping meta-analyses on BM-MSC therapy along with CD for ONFH is to provide the best evidence possible on this topic to generate recommendations and identify the potential limitations in the existing literature that needs further research.
2. Materials & methods
We present herewith a systematic overview which was being performed by duly cohering the guidelines of the Back Review Group of Cochrane Collaboration18 and aim to report the same based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).19
2.1. Search strategy
Two reviewers conducted an independent literature search for systematic reviews with meta-analysis evaluating BM-MSC therapy along with CD for ONFH. Electronic database search was conducted in PubMed, Web of Science, Embase, Cochrane Database of Systematic Reviews (CDSR), and the Database of Abstracts of Reviews of Effects (DARE) till September 2020. Our search was neither restricted to any particular language nor confined to a specific period. We designed our electronic search strategy following the Peer Review of Electronic Search Strategy(PRESS) guidelines.20 The major keywords used for the search were as follows: “Bone Marrow”, “Stem Cell”, “Osteonecrosis”, “Femur”, “BM-MSC”, “Core Decompression”, “Randomized Controlled Trial”, “Systematic Review”, “Meta-analysis” together with Boolean operators such as “AND”, “OR” and “NOT”. We made a manual search of the key journals and also searched the reference list of the selected articles to identify studies not identified in the primary search. We also made a search in the International prospective register of systematic reviews (PROSPERO) for any ongoing potential review that is nearing completion on the subject. We included and analyzed all the studies meeting the inclusion criteria. Any discrepancy between the reviewers was resolved through discussion until a consensus was obtained. A PRISMA flow diagram for study selection into the systematic overview has been depicted in Fig. 1.
Fig. 1.
PRISMA flow diagram of the included studies.
2.2. Eligibility criteria
Reviews were included if they satisfied the following criteria.
-
1.
Systematic review with a meta-analysis that compared CD with and without BM-MSC for ONFH.
-
2.
Should have analyzed at least one of the outcomes such as the Visual Analog Scale (VAS) score, Harris Hip Score (HHS), the Western Ontario and McMaster Universities Arthritis Index (WOMAC) score, the volume of the postoperative necrotic zone, number of hips undergoing total hip arthroplasty (THA) and adverse events.
2.3. Exclusion criteria
We excluded narrative reviews, correspondence articles, systematic reviews without data pooling or meta-analysis, systematic reviews with mixed intervention groups being analyzed for ONFH. Besides, we excluded pre-clinical studies, studies on animal models and cadaveric studies on the subject.
2.4. Data extraction
Data was extracted from the meta-analyses included in the analysis by two reviewers independently. Notably, the data extracted from the studies were as follows: first author, date of last literature search, year and journal of publication, number, and nature of studies included, language restrictions, inclusion/exclusion criteria, databases involved in literature search, software used for analysis, whether subgroup or sensitivity analysis, Grading of Recommendations Assessment, Development, and Evaluation (GRADE) summary, publication bias analysis, conflict of interest, I2 statistic value of the variables in each meta-analysis. Disagreements were settled by consensus.
2.5. Quality assessment
The methodological quality of the included reviews was evaluated using the Oxford Levels of Evidence.21 Besides, we also used the Assessment of Multiple Systematic Reviews (AMSTAR)22 and its updated grading tool AMSTAR 223 to assess their methodological robustness with good validity and reliability.24 Two reviewers independently assessed the methodological quality of the included studies. Disagreements were settled by consensus.
2.6. Heterogeneity assessment
I2 test was used for heterogeneity assessment.25 When I2 > 50% and p < 0.1, heterogeneity is deemed to exist among the included trials and the reviewers evaluated whether the studies have utilized sensitivity or subgroup analysis to assess the causes of heterogeneity and strengthen the robustness of the pooled data.
2.7. Application of Jadad decision algorithm
The variability in the findings among the included meta-analyses was interpreted with the help of the Jadad decision algorithm. As per Jadad et al.,26 the possible reasons for discordance in the results among the included studies include differences in study question, their inclusion and exclusion criteria, assessment of quality, data pooling and extraction, and statistical analysis. Currently, this is the commonly used algorithm for generating recommendations among the meta-analyses with discordant results.27, 28, 29, 30 Two reviewers independently used this algorithm to arrive at a single meta-analysis which represents the current best evidence to generate recommendations.
3. Results
3.1. Search results
A comprehensive search of the electronic database generated 84 articles and they were subjected to an initial screen for removing duplicate articles which resulted in 62 articles. Upon title and abstract screening of the resultant 62 articles, we excluded 52 articles. Therefore, 10 articles qualified for reviewing the full-text. On full-text review by both the reviewers 4 of them were excluded. A list of excluded articles with reasons was given in Supplementary File 1. Finally, 6 meta-analyses were included in this systematic review.15, 16, 17,31, 32, 33 These overlapping meta-analyses were published in different journals between 2014 and 2020 and the number of included studies ranged from 4 to 16 as shown in Table 1. The publication years of the included studies in these meta-analyses ranged between 2004 and 2019 as shown in Table 2.
Table 1.
Characteristics of the included studies.
| Author | Publication Date | Publication Journal | Literature search date | No. of Studies Included |
|---|---|---|---|---|
| X Li et al.17 (2014) | August 1, 2014 | International Journal of Clinical and Experimental Pathology | March 2013 |
4 |
| C Papakostidis et al.33 (2016) | February 2016 | Acta Orthopaedica | NA | 7 |
| S Xu et al.31 (2017) | August 3, 2017 | BioMed Research International | September 2016 | 11 |
| Z Wang et al.15 (2019) | July 10, 2019 | International Journal of Surgery | October 2018 | 14 |
| C Zhang et al.32 (2020) | February 28, 2020 | Journal of American Academy of Orthopedic Surgeons | November 12, 2019 | 16 |
| S Wang et al.16 (2020) | June 19, 2020 | Medicine | December 26, 2019 | 15 |
Table 2.
Primary studies included in each meta-analysis.
| Primary studies | Nature of Study | X Li17 (2014) | C Papakostidis33 (2016) | S Xu31 (2017) | Z Wang15 (2019) | C Zhang32 (2020) | S Wang16 (2020) |
|---|---|---|---|---|---|---|---|
| Gangji (2004) | CCT | + | + | + | |||
| Gangji (2005) | RCT | + | |||||
| Guo (2008) | RCT | + | + | + | |||
| Sun (2008) | RCT | + | + | + | |||
| Chang (2010) | RCT | + | + | + | |||
| Yamasaki (2010) | CCT | + | + | ||||
| Gangji (2011) | CCT | + | + | + | + | ||
| Zhao (2012) | RCT | + | + | + | + | + | + |
| Liu (2012) | CCT | + | |||||
| Liu (2013) | CCT | + | + | ||||
| Sen (2012) | RCT | + | + | + | + | + | + |
| Rastogi (2013) | RCT | + | + | + | |||
| Lim (2013) | CCT | + | + | ||||
| Ma (2014) | RCT | + | + | + | + | + | |
| Tabatabaee (2015) | RCT | + | + | + | + | ||
| Yang (2015) | RCT | + | + | + | |||
| Yan (2015) | RCS | + | |||||
| Mao (2015) | RCT | + | |||||
| Pepke (2016) | RCT | + | + | + | + | ||
| Cruz-Pardos (2016) | RCS | + | |||||
| Pilge (2016) | CCT | + | |||||
| Zhao (2016) | RCT | + | + | + | |||
| Hauzeur (2018) | RCT | + | + | ||||
| Hernigou (2018) | RCT | + | |||||
| Kang (2018) | CCT | + | |||||
| Liu (2019) | RCT | + | |||||
| Li (2020) | RCT |
RCT – randomized controlled trial; CCT – controlled clinical trial; RCS – retrospective cohort study.
3.2. Search methodology
Although the included meta-analyses made a comprehensive literature search, the search databases were discordant among them. All studies searched PubMed/Medline and Embase databases. While five of them searched the Cochrane library, two of them searched Web of Science, Google Scholar, China National Knowledge Infrastructure (CNKI) database, and Wan fang database. Apart from these Scopus, Springer, Cumulative Index to Nursing and Allied Health Literature (CINAHL) database, Allied and Complementary Medicine (AMED) database, Elsevier Science Direct, Chinese Biomedical (CBM) literature database, and VIP database were also used. Of the 6 studies, one included studies only in English,32 while 3 others mentioned no linguistic restriction in their selection criteria.15,31,33 Only 1 study searched grey literature for eligible studies.16 Further details on the search methodology employed by the included studies were presented in Table 3.
Table 3.
Search methodology used by each study.
| Search Parameters | X Li17 (2014) | C Papakostidis33 (2016) | S Xu31 (2017) | Z Wang15 (2019) | C Zhang32 (2020) | S Wang16 (2020) |
|---|---|---|---|---|---|---|
| Publication language restriction | NA | No | No | No | Yes | NA |
| Publication status restriction | NA | NA | NA | NA | Yes | No |
| PubMed | + | + | + | + | + | + |
| Medline | + | + | + | |||
| Embase | + | + | + | + | + | + |
| Cochrane library | + | + | + | + | + | |
| Web of Science | + | + | ||||
| Scopus | + | |||||
| Google Scholar | + | + | ||||
| CINAHL | + | |||||
| AMED | + | |||||
| CNKI | + | + | ||||
| Wan Fang | + | + | ||||
| CBM literature | + | |||||
| VIP | + | |||||
| Springer | + | |||||
| Elsevier Science Direct | + |
AMED – Allied and Complementary Medicine; CBM – Chinese BioMedical; CINAHL – Cumulative Index to Nursing and Allied Health Literature; CNKI – Chinese National Knowledge Infrastructure; NA – not available.
4. Methodological quality
Using Oxford Levels of Evidence, we determined the quality of the included studies based on the nature of the primary studies included in their analysis. Of the 6 studies analyzed, 3 were of level II evidence15,16,31 while the rest of them were of Level III evidence17,32,33 as shown in Table 4. Of the 6 included studies, 4 used RevMan for data analyses17,31, 32, 33 while others used Stata software.15,16 Besides, one study utilized the GRADE system,31 three studies conducted a sensitivity analysis,15,16,33 while three made subgroup analysis16,31,33 to explore the heterogeneity in their results, and 4 studies assessed for possible publication bias.15,16,31,33 As shown in Table 5, the AMSTAR scores of the included studies ranged from 4 to 9 (mean 7). Based on the AMSTAR 2 grading, none of the included studies were without critical methodological flaws in the conduction of the meta-analyses. Of all the included studies, the meta-analysis by S Wang et al.16 was found to be of the highest quality with an AMSTAR score of 9/11 as shown in Table 5. However, it also suffered from critical methodological flaws such as non-making a priori design for the conduction of the study and not providing the list of excluded studies with their reason for exclusion.
Table 4.
Methodological information of each study.
| Methodology | X Li17 (2014) | C Papakostidis33 (2016) | S Xu31 (2017) | Z Wang15 (2019) | C Zhang32 (2020) | S Wang16 (2020) |
|---|---|---|---|---|---|---|
| Primary study design | RCT & CCT | RCT & CCT | RCT | RCT | RCT, CCT | RCT |
| Level of Evidence | Level III | Level III | Level II | Level II | Level III | Level II |
| Software Used | RevMan | RevMan | RevMan | Stata | RevMan | Stata |
| GRADE Used | No | No | Yes | No | No | No |
| Sensitivity Analysis | No | Yes | No | Yes | No | Yes |
| Subgroup Analysis | No | Yes | Yes | No | No | Yes |
| Publication Bias | No | Yes | Yes | Yes | No | Yes |
CCT – controlled clinical trial; GRADE – Grading of Recommendations Assessment, Development and Evaluation system; RCTs – randomized controlled trials.
Table 5.
AMSTAR scores and AMSTAR 2 grading for included studies.
| Items | X Li17 (2014) | C Papakostidis33 (2016) | S Xu31 (2017) | Z Wang15 (2019) | C Zhang32 (2020) | S Wang16 (2020) |
|---|---|---|---|---|---|---|
| 1. Was a priori design provided? | 0 | 0 | 0 | 0 | 0 | 0 |
| 2. Were there duplicate study selection and data extraction? | 1 | 1 | 1 | 1 | 1 | 1 |
| 3. Was a comprehensive literature search performed? | 1 | 1 | 1 | 1 | 1 | 1 |
| 4. Was the status of publication (i.e. grey literature) used as an inclusion criterion? | 0 | 0 | 0 | 0 | 0 | 1 |
| 5. Was a list of studies (included and excluded) provided? | 0 | 0 | 0 | 0 | 0 | 0 |
| 6. Were the characteristics of the included studies provided? | 1 | 1 | 1 | 1 | 0 | 1 |
| 7. Was the scientific quality of the included studies assessed and documented? | 0 | 1 | 1 | 1 | 1 | 1 |
| 8. Was the scientific quality of the included studies used appropriately in formulating conclusions? | 0 | 1 | 1 | 1 | 0 | 1 |
| 9. Were the methods used to combine the findings of studies appropriate? | 1 | 1 | 1 | 1 | 1 | 1 |
| 10. Was the likelihood of publication bias assessed? | 0 | 1 | 1 | 1 | 0 | 1 |
| 11. Was the conflict of interest stated? | 1 | 1 | 1 | 1 | 0 | 1 |
| Total AMSTAR scores | 5 | 8 | 8 | 8 | 4 | 9 |
| Critical Methodological Flaw | 3 | 2 | 2 | 2 | 3 | 2 |
| Non-Critical Flaw | 4 | 2 | 2 | 3 | 4 | 1 |
| AMSTAR 2 Grade | Critically Low | Critically Low | Critically Low | Critically Low | Critically Low | Critically Low |
4.1. Heterogeneity assessment
All the studies included used I2 statistic for heterogeneity assessment. Mild heterogeneity was noted in outcomes like VAS, HHS at 24 months, and adverse events as shown in Table 6. Nevertheless, the heterogeneity of VAS at 24 months, HHS at 12 months, WOMAC, Femoral head collapse rate, and the number of patients getting converted to THA was significant. To explore the sources of heterogeneity two studies conducted a sensitivity analysis,15,16,33 and one made subgroup analysis16,31,33 as shown in Table 4.
Table 6.
I2 statistic values of variables analyzed in each meta-analysis.
| Outcome Variables | X Li17 (2014) | C Papakostidis33 (2016) | S Xu31 (2017) | Z Wang15 (2019) | C Zhang32 (2020) | S Wang16 (2020) |
|---|---|---|---|---|---|---|
| VAS - 6 months | 0% | |||||
| VAS - 12 months | 0% | |||||
| VAS - 24 months | 0% | 99% | 86.2% | |||
| HHS | 52% | 42% | ||||
| HHS – 12 months | 56.6% | 26.4% | ||||
| HHS – 24 months | 0% | 0% | 0% | |||
| WOMAC | 90.9% | |||||
| Femoral head collapse | 68% | 0% | 65% | |||
| Necrotic area reduction | 0% | 71.2% | 84.8% | |||
| Conversion to VBG | 0% | |||||
| Conversion to THA | 32% | 0% | 57.9% | 62% | 22.5% | |
| Adverse Events | 0% | 0% |
HHS – Harris Hip Score; THA – Total Hip Arthroplasty; VAS – Visual Analog Scale; VBG – Vascularised Bone Grafting; WOMAC - Western Ontario and McMaster Universities Arthritis Index.
It is important to probe into the source of discordances among the included studies since the recommendations generated as used in clinical practise and in developing public health policy.34 The heterogeneity of the results among the included meta-analyses was mostly due to the variability in the nature of the primary studies other than RCTs.
4.2. Results of Jadad decision algorithm
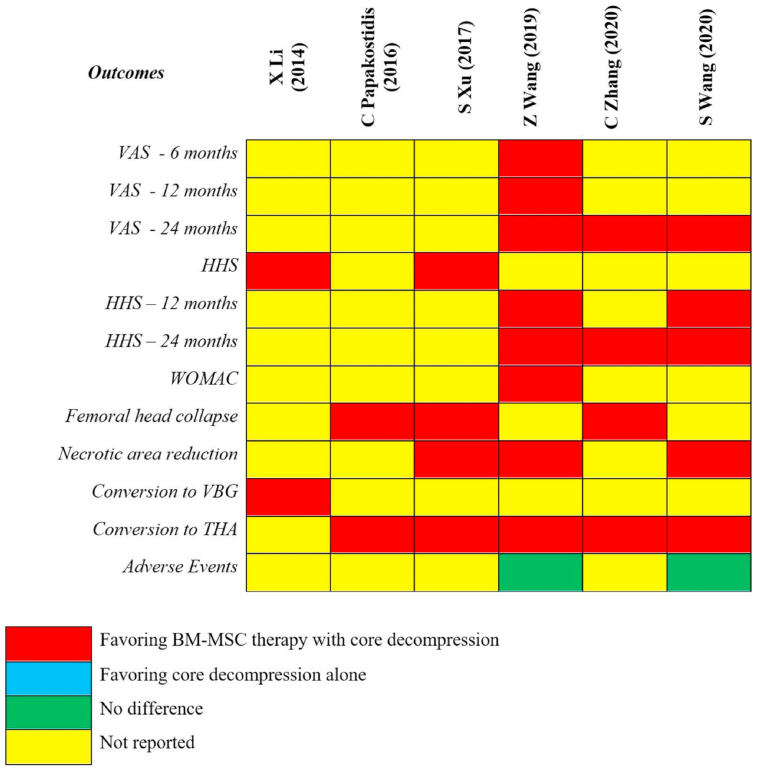
The pooled results from each of the included meta-analyses were given in Fig. 2. To identify the study that provides the best possible evidence to generate treatment recommendations, we adopted the Jadad decision algorithm. Considering that all the 6 included meta-analyses tried to answer the same clinical question despite having a varied spectrum of primary studies being analyzed, the study with the highest quality is selected based on its methodological quality, restrictions involved in study selection such as language, databases involved and data analysis protocols as shown in Fig. 3. Eventually, we identified the meta-analysis by S Wang et al.16 as the highest-quality study among the included meta-analyses based on the Jadad decision algorithm. This study has shown that BM-MSC with CD was more efficacious compared to CD alone, thereby delaying the progression of the ONFH, with a reduction in the necrotic area of the femoral head and decreasing the need for THA and improved HHS.
Fig. 2.
The pooled results of each included meta-analyses.
Fig. 3.
The flowchart of Jadad decision algorithm.
5. Discussion
To date, numerous RCTs have analyzed the utility of BM-MSC therapy in ONFH providing promising results in favor of the therapy.14,35, 36, 37, 38, 39 However, limited sample size and the paucity of long term results have been their major limitation. To further strengthen their results, multiple meta-analyses based on the RCTs have provided a higher level of evidence on the effectiveness of the intervention in ONFH.15, 16, 17,31, 32, 33 But the spectrum of primary studies included in the analysis and the databases analyzed were discordant among them. Hence, a systematic overview of the overlapping meta-analyses to identify the highest quality study among the meta-analyses to assist decision-makers in formulating recommendations is needed.
The main objective of this study was to make a systematic overview of the overlapping meta-analyses, analysing the effectiveness of BM-MSC therapy along with CD for ONFH, is to identify the study with the highest level of evidence to generate treatment recommendations. We did a comprehensive literature search and identified 6 potential meta-analyses to be included in this review.15, 16, 17,31, 32, 33 A decision tool was designed by Jadad et al.26 to select the highest-quality study from the meta-analyses under analysis which is widely used in varied fields of medicine.28,30,40 Finally, the meta-analysis by S Wang et al.16 with Level II evidence including only RCTs was chosen as the highest-quality study based on the decision algorithm by Jadad. This study identified as current best evidence has shown that BM-MSC with CD was more efficacious compared to CD alone, thereby delaying the progression of the ONFH, with a reduction in the necrotic area of the femoral head, decreasing the need for THA and improved HHS. In the prospective double-blinded RCTs by M Li et al.39 and P Hernigou et al.,41 the effectiveness of BM-MSC therapy along with CD in the management of ONFH is established with long term follow-up.
The pathogenesis of ONFH remains unclear to the global researchers despite a myriad of trials investigating its etiology. Cellular therapy has been a great boon in the area of bio-regeneration of bone and bone-related cells. Out of all the products of cell-based therapy, BM-MSCs are ubiquitous and proved the path of regenerating tissues in the area of interest. BM-MSCs possess multi-differential potential to differentiate into osteoblasts, endothelial progenitor cells, and hemangioblasts, which function to repair the necrotic region in the femoral head.
It is now established form various studies that implantation of MSCs in the area of femoral head necrosis resulted in a decreased conversion rate to total hip arthroplasty.12,13 Significant improvement in the femoral head survival rate and functional results were noted when the CD with BM-MSCs was done in the pre-collapse stage than the post-collapse stage of ONFH.36,42 The predictive factors for a successful outcome on treatment with CD along with BM-MSC therapy for ONFH are the size and number of necrotic areas, number of viable BM-MSCs delivered at the implantation site, and decreased intra-osseous pressure which prevents the apoptosis of the implanted BM-MSCs. The mechanism of repair in ONFH by BM-MSC implantation is by appositional bone growth on the old matrix with thickened trabeculae. The true volume of new bone formed by stem cell implantation can be assessed by histological examination when patients turn up for total hip arthroplasty.43 Kang et al.44 stated that BM-MSCs derived from older patients or patients with advanced lesions in the femoral head have lower differentiation potential and hence lead to failure of the procedure and an increased rate of conversion to THA.
Hernigou et al.45 evaluated the number of MSCs in 1 cm³ of a femoral head by femoral head fragmentation and found 700 ± 264 MSCs per cm³ in a normal femoral head. Given an average femoral head volume of 50 cm3, approximately 35,000 MSCs would be the critical number of MSCs to be loaded to re-establish the number of MSCs as in the normal femoral head. Hernigou et al.46 in their study identified that implantation of more than 2 million mononuclear cells per ml in the necrotic foci is necessary for a successful outcome from BM-MSC therapy. However, identification, quantification, and culture expansion of the MSCs from the mononuclear concentrate before injection remain a challenge due to economic constraints and stringent regulations in various countries laid down for cell-based therapies.
5.1. Directions for future
Current research on BM-MSC therapy is directed towards novel methods like implanting transfected targeted genes into the BM-MSC pool delivered to the osteonecrotic foci of the femoral head. Xiao et al.47 demonstrated the repairing of the experimental defect of ONFH with BM-MSC-seeded bio-derived bone material combined with rhBMP-2 to be even more beneficial. Wen et al.48 augmented the BM-MSC therapy with transplantation of hepatocyte growth factor to enhance blood vessel regeneration and bone reconstruction in an ONFH model.
Although our systematic overview establishes the efficacy of the BM-MSC therapy over CD alone for ONFH, there remains a lack of consensus on the quality and quantity of cell count with respect to the stage of the disease being treated, graft harvesting protocols, culture expansion, and cell delivery or implantation methods utilized for BM-MSC therapy for ONFH. To clarify these aspects, blinded RCTs investigating the above-mentioned lacunae are required in the future.
5.2. Limitations
This study has some limitations. This study identified meta-analyses of RCTs which were identified to be of Level II evidence. Hence, we cannot provide a Level I treatment recommendation with the current literature. This systematic overview may be influenced by the limitations and biases involved in the meta-analyses and their primary studies. Moreover, selecting the meta-analysis of highest quality based on Jadad algorithm generates recommendations based on the results of the selected meta-analysis at the cost of studies missed from their primary search as highlighted in Table 2.
6. Conclusion
Based on this systematic overview, we give a Level II recommendation that BM-MSC therapy is more efficacious along with CD in the management of ONFH compared to CD alone. BM-MSC therapy provides better pain relief with significant functional improvement and delaying the collapse of the femoral head thereby preventing further treatment such as THA.
Declaration of competing interest
Authors declare no potential conflicts of interest involved in the conduction of this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcot.2020.11.015.
CDSR – Cochrane Database of Systematic Reviews; DARE – Database of Abstracts of Reviews of Effects; # - List of excluded studies are given in Supplementary File 1.
VAS – Visual Analog Scale; HHS – Harris Hip Score; WOMAC - Western Ontario and McMaster Universities Arthritis Index; VBG – Vascularised Bone Grafting; THA – Total Hip Arthroplasty.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Baig S.A., Baig M.N. Osteonecrosis of the femoral head: etiology, investigations, and management. Cureus. 2018;10 doi: 10.7759/cureus.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Q.-Y., Li Z.-R., Gao F.-Q., Sun W. Pericollapse stage of osteonecrosis of the femoral head: a last chance for joint preservation. Chin Med J. 2018;131:2589–2598. doi: 10.4103/0366-6999.244111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X., Sun W., Tan M. Noncoding RNAs in steroid-induced osteonecrosis of the femoral head. BioMed Res Int. 2019;2019 doi: 10.1155/2019/8140595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah K.N., Racine J., Jones L.C., Aaron R.K. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med. 2015;8:201–209. doi: 10.1007/s12178-015-9277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi H.-R., Steinberg M.E., Cheng E.Y. Osteonecrosis of the femoral head: diagnosis and classification systems. Curr Rev Musculoskelet Med. 2015;8:210–220. doi: 10.1007/s12178-015-9278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moya-Angeler J., Gianakos A.L., Villa J.C., Ni A., Lane J.M. Current concepts on osteonecrosis of the femoral head. World J Orthoped. 2015;6:590–601. doi: 10.5312/wjo.v6.i8.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierce T.P., Jauregui J.J., Cherian J.J., Elmallah R.K., Mont M.A. Imaging evaluation of patients with osteonecrosis of the femoral head. Curr Rev Musculoskelet Med. 2015;8:221–227. doi: 10.1007/s12178-015-9279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.G E., Fl P., B J., B V., M A., U T. Conservative surgery for the treatment of osteonecrosis of the femoral head: current options. Clin Cases Miner Bone Metab Off J Ital Soc Osteoporos Miner Metab Skelet Dis. 2015;12:43–50. doi: 10.11138/ccmbm/2015.12.3s.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Im G.-I. Stem cell therapy in osteonecrosis of the femoral head. Hip Pelvis. 2018;30:135–137. doi: 10.5371/hp.2018.30.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mardones R., Camacho D., Monsalvo F. Treatment of osteonecrosis of the femoral head by core decompression and implantation of fully functional ex vivo-expanded bone marrow-derived mesenchymal stem cells: a proof-of-concept study. Stem Cell Clon Adv Appl. 2019;12:11–16. doi: 10.2147/SCCAA.S181883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z., Wei J., Xiao H. Bone-strengthening supplement (BSP) promotes bone and cartilage repair, for the treatment of Osteonecrosis of Femoral Head: an MRI-based study. Am J Transl Res. 2019;11:7449–7455. [PMC free article] [PubMed] [Google Scholar]
- 12.Sampson S., Botto-van Bemden A., Aufiero D. Stem cell therapies for treatment of cartilage and bone disorders: osteoarthritis, avascular necrosis, and non-union fractures. Pharm Manag PM R. 2015;7:S26–S32. doi: 10.1016/j.pmrj.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Piuzzi N.S., Chahla J., Schrock J.B. Evidence for the use of cell-based therapy for the treatment of osteonecrosis of the femoral head: a systematic review of the literature. J Arthroplasty. 2017;32:1698–1708. doi: 10.1016/j.arth.2016.12.049. [DOI] [PubMed] [Google Scholar]
- 14.Hauzeur J.-P., De Maertelaer V., Baudoux E., Malaise M., Beguin Y., Gangji V. Inefficacy of autologous bone marrow concentrate in stage three osteonecrosis: a randomized controlled double-blind trial. Int Orthop. 2018;42:1429–1435. doi: 10.1007/s00264-017-3650-8. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z., Sun Q.-M., Zhang F.-Q., Zhang Q.-L., Wang L.-G., Wang W.-J. Core decompression combined with autologous bone marrow stem cells versus core decompression alone for patients with osteonecrosis of the femoral head: a meta-analysis. Int J Surg Lond Engl. 2019;69:23–31. doi: 10.1016/j.ijsu.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Wang S., Hu Y., Chen H. Efficacy of bone marrow stem cells combined with core decompression in the treatment of osteonecrosis of the femoral head. Medicine (Baltim) 2020;99 doi: 10.1097/MD.0000000000020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X., Xu X., Wu W. Comparison of bone marrow mesenchymal stem cells and core decompression in treatment of osteonecrosis of the femoral head: a meta-analysis. Int J Clin Exp Pathol. 2014;7:5024–5030. [PMC free article] [PubMed] [Google Scholar]
- 18.Furlan A.D., Malmivaara A., Chou R. Updated Method guideline for systematic reviews in the Cochrane Back and neck group. Spine. 2015;40:1660–1673. doi: 10.1097/BRS.0000000000001061. 2015. [DOI] [PubMed] [Google Scholar]
- 19.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group T.P. Preferred reporting Items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGowan J., Sampson M., Salzwedel D.M., Cogo E., Foerster V., Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Slobogean G., Bhandari M. Introducing levels of evidence to the Journal of Orthopaedic Trauma: implementation and future directions. J Orthop Trauma. 2012;26:127–128. doi: 10.1097/BOT.0b013e318247c931. [DOI] [PubMed] [Google Scholar]
- 22.Shea B.J., Grimshaw J.M., Wells G.A. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shea B.J., Reeves B.C., Wells G. Amstar 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358 doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sathish M., Eswar R. Systematic reviews and meta-analysis in spine surgery—how good are they in methodological quality? A systematic review. Global Spine J. 2020 doi: 10.1177/2192568220906810. 2192568220906810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jadad A.R., Cook D.J., Browman G.P. A guide to interpreting discordant systematic reviews. CMAJ Can Med Assoc J J Assoc Medicale Can. 1997;156:1411–1416. [PMC free article] [PubMed] [Google Scholar]
- 27.Ding F., Jia Z., Zhao Z. Total disc replacement versus fusion for lumbar degenerative disc disease: a systematic review of overlapping meta-analyses. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2017;26:806–815. doi: 10.1007/s00586-016-4714-y. [DOI] [PubMed] [Google Scholar]
- 28.Fu B.-S., Jia H.-L., Zhou D.-S., Liu F.-X. Surgical and non-surgical treatment for 3-part and 4-Part Fractures of the proximal humerus: a systematic review of overlapping meta-analyses. Orthop Surg. 2019;11:356–365. doi: 10.1111/os.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mascarenhas R., Chalmers P.N., Sayegh E.T. Is double-row rotator cuff repair clinically superior to single-row rotator cuff repair: a systematic review of overlapping meta-analyses. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 2014;30:1156–1165. doi: 10.1016/j.arthro.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y., Yang S., Ding W. Unilateral versus bilateral pedicle screw fixation in lumbar fusion: a systematic review of overlapping meta-analyses. PloS One. 2019;14 doi: 10.1371/journal.pone.0226848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu S., Zhang L., Jin H. Autologous stem cells combined core decompression for treatment of avascular necrosis of the femoral head: a systematic meta-analysis. BioMed Res Int. 2017;2017 doi: 10.1155/2017/6136205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang C., Fang X., Huang Z., Li W., Zhang W., Lee G.-C. Addition of bone marrow stem cells therapy achieves better clinical outcomes and lower rates of disease progression compared with core decompression alone for early stage osteonecrosis of the femoral head: a systematic review and meta-analysis. J Am Acad Orthop Surg. 2020;28(23):973–979. doi: 10.5435/JAAOS-D-19-00816. [DOI] [PubMed] [Google Scholar]
- 33.Papakostidis C., Tosounidis T.H., Jones E., Giannoudis P.V. The role of “cell therapy” in osteonecrosis of the femoral head. Acta Orthop. 2016;87:72–78. doi: 10.3109/17453674.2015.1077418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moja L., Fernandez del Rio M.P., Banzi R. Multiple systematic reviews: methods for assessing discordances of results. Intern Emerg Med. 2012;7:563–568. doi: 10.1007/s11739-012-0846-1. [DOI] [PubMed] [Google Scholar]
- 35.Tabatabaee R., Saberi S., Parvizi J. Combining concentrated autologous bone marrow stem cells injection with core decompression improves outcome for patients with early-stage osteonecrosis of the femoral head: a comparative study. J Arthroplasty. 2015;30:11–15. doi: 10.1016/j.arth.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 36.Gangji V., De Maertelaer V., Hauzeur J., Gangji V., De Maertelaer V., Hauzeur J.-P. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled study. Bone. 2011;49:1005–1009. doi: 10.1016/j.bone.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 37.Pepke W., Kasten P., Beckmann N. Core decompression and autologous bone marrow concentrate for treatment of femoral head osteonecrosis: a randomized prospective study. Orthop Rev. 2016;8:5–9. doi: 10.4081/or.2016.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao D., Lu F., Wang W. Autologous bone marrow-derived and cultured mesenchymal stem cell therapy for avascular necrosis OF the femoral head. Vox Sang. 2012;103 263–263. [Google Scholar]
- 39.Li M., Ma Y., Fu G. 10-year follow-up results of the prospective, double-blinded, randomized, controlled study on autologous bone marrow buffy coat grafting combined with core decompression in patients with avascular necrosis of the femoral head. Stem Cell Res Ther. 2020:11. doi: 10.1186/s13287-020-01810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan G., Li F., Zhou D., Cai X., Huang Y., Liu F. Unilateral versus bilateral percutaneous balloon kyphoplasty for osteoporotic vertebral compression fractures: a systematic review of overlapping meta-analyses. Medicine (Baltim) 2018;97 doi: 10.1097/MD.0000000000011968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernigou P., Dubory A., Homma Y. Cell therapy versus simultaneous contralateral decompression in symptomatic corticosteroid osteonecrosis: a thirty year follow-up prospective randomized study of one hundred and twenty five adult patients. Int Orthop. 2018;42:1639–1649. doi: 10.1007/s00264-018-3941-8. [DOI] [PubMed] [Google Scholar]
- 42.Ma Y., Wang T., Liao J. Efficacy of autologous bone marrow buffy coat grafting combined with core decompression in patients with avascular necrosis of femoral head: a prospective, double-blinded, randomized, controlled study. Stem Cell Res Ther. 2014;5:115. doi: 10.1186/scrt505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gangji V., Hauzeur J.-P., Matos C., De Maertelaer V., Toungouz M., Lambermont M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am. 2004;86:1153–1160. doi: 10.2106/00004623-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Kang J., Suh Y., Moon K. Clinical efficiency of bone marrow mesenchymal stem cell implantation for osteonecrosis of the femoral head: a matched pair control study with simple core decompression. Stem Cell Res Ther. 2018;9 doi: 10.1186/s13287-018-1030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hernigou P., Trousselier M., Roubineau F. Stem cell therapy for the treatment of hip osteonecrosis: a 30-year review of progress. Clin Orthop Surg. 2016;8:1–8. doi: 10.4055/cios.2016.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernigou P., Poignard A., Manicom O., Mathieu G., Rouard H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg Br. 2005;87:896–902. doi: 10.1302/0301-620X.87B7.16289. [DOI] [PubMed] [Google Scholar]
- 47.Zm X., J H., Xl Z., Zg W., Xl Z. Treatment of osteonecrosis of femoral head with BMSCs-seeded bio-derived bone materials combined with rhBMP-2 in rabbits. Chin J Traumatol. 2008;11:165–170. doi: 10.1016/s1008-1275(08)60035-8. [DOI] [PubMed] [Google Scholar]
- 48.Wen Q., Ma L., Chen Y.-P., Yang L., Luo W., Wang X.-N. Treatment of avascular necrosis of the femoral head by hepatocyte growth factor-transgenic bone marrow stromal stem cells. Gene Ther. 2008;15:1523–1535. doi: 10.1038/gt.2008.110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.