Abstract
Background
Plantar fasciitis, which is a common cause of heel pain, often results in significant morbidity. In cases who are not responsive to initial conservative treatment, invasive procedures, often in the form of local infiltration of steroid are required. These procedures are associated with significant complications. Local Platelet Rich Plasma (PRP) infiltration is an emerging addition to these treatments. However, whether it is more effective in reducing pain and improving function than other treatments (such as steroid injections or whole blood) remains controversial.
Methods
Skeletally mature patients with plantar fasciitis who had failed conservative therapy were randomized using envelope method into 2 groups: PRP and Steroid group. The participants were assessed for pain using Visual Analog Scale on the day of presentation, and then after therapy at 2 weeks, 4 weeks, 3 months, and 6 months. They were additionally assessed on final follow-up using AOFAS hind-foot Score.
Results
118 patients were randomized into 2 groups: 58 patients to the PRP group and 60 to the Steroid group. PRP was associated with greater improvement in VAS score and resulted in superior AOFAS score at 6 months as compared to steroid injection. The authors did not find any local or systemic complications in any of the groups. The result and difference were more pronounced as the time from injection increased and maximal benefit was observed at 6 months follow-up. None of the patients needed a repeat injection at 6 months.
Conclusion
Our study expands on the previous studies to provide a better evidence for superiority of PRP over local injection of steroid in plantar fasciitis, and the authors conclude that PRP provides better pain relief and function as compared to steroid injection.
Level of evidence
Level 1 Prospective Randomized Control Trial (RCT);
Keywords: Platelet rich plasma (PRP), Plantar fasciitis, Heel pain: orthobiologics
1. Introduction
Plantar fasciitis, which is a common cause of heel pain, often results in significant morbidity. The plantar fascia is a band of connective tissue connecting the calcaneus to the tendons of the forefoot, and goes onto proximal phalanges of all toes. Its purpose is to support the arch of the foot and to act as a shock absorber for pressure placed on the foot.1,2 Plantar fasciitis is a degeneration of the plantar fascia as a result of repetitive microtears of the fascia leading to an inflammatory reaction and is not a primary inflammatory process as is customarily believed.2 The cause of plantar fasciitis is unknown, but is believed to be multifactorial, with abnormal biomechanics and delayed healing as likely contributors.3
Various conservative treatment options include non-weight bearing, eccentric stretching, night splints, orthotics, and non-steroidal anti-inflammatory drugs. These treatment measures can resolve nearly 80% of the cases.4 However, in cases who are not responsive to these treatments, invasive procedures in the form of local infiltration are required. Infiltration with intra-lesional steroids is commonly used in the treatment of chronic plantar fasciitis.5 This procedure is effective, but only produces short-term relief. Moreover, it is also accompanied by complications, such as local infections, heel fat pad atrophy, and in some cases even plantar fascia rupture in case of multiple injections.4,6
Local autologous Platelet Rich Plasma (PRP) infiltration is an emerging alternative for this condition. PRP is derived by centrifuging autologous whole blood and has a platelet concentration higher than that of blood.7 The platelets release a variety of growth factors and cytokines, which can stimulate and accelerate the nature physiological tissue healing process.7 Current evidence has shown promising results for PRP in the treatment of plantar fasciitis.8, 9, 10 However, whether it is more effective in reducing pain and improving function than other treatments (such as steroid injection, or whole blood) remains controversial.7 Aim of present study was thus to conduct a randomized control trial and to compare the effects of PRP and local depot preparation of methyl prednisone when injected locally in patients with plantar fasciitis who had failed conservative management.
2. Methods
The present study is a CTRI registered randomized control trial that took place in a tertiary level hospital over a period of one year from July 2018 to June 2019. The study was conducted on adults having heel pain for more than 4 weeks who were clinically diagnosed as plantar fasciitis, and were attending the outpatient department of the hospital. After taking due consent, participants were randomized using envelope method into 2 groups: Group A was given local injection of Platelet Rich Plasma and Group B was given local injection of Corticosteroid. All of the patients underwent conventional radiographs and magnetic resonance imaging (MRI) of the involved foot to rule out stress fractures, associated bone lesions or other causes of plantar heel pain. The inclusion criteria consisted of skeletally mature patients with heel pain at plantar fascia insertion, failure of conservative treatment for 4 weeks, and no previous injections. The exclusion criteria consisted of patients needing bilateral injections, patients with associated pathologies, inflammatory or degenerative osteoarthritis, uncontrolled diabetes, neurological conditions, skin infections, or a history of infection at the application site in the preceding 3 months. Approval was taken from the institute ethical and scientific committee. Informed written consent was taken from all of the included patients. Patients were assigned to one of the two groups in a randomized manner by selecting a sealed envelope (randomized using block randomization).
2.1. PRP preparation and injection
A bench-top centrifuge was used to concentrate platelets from autologous whole blood. 2 ml of PRP was obtained using a single step centrifugation procedure. 10 ml of blood was withdrawn from the Median Cubital Vein of the patient. The sample was collected in the EDTA Bulb and it was then centrifuged at 1800 rpm for 8 min in 2 centrifuge tubes. Bottom 1 ml of the plasma from each of the tubes, the platelet-rich plasma (PRP), was then harvested from each tube avoiding contamination by the buffy coat and red cell layers, for injection into the patient. The prepared PRP injection was given into the maximally tender point of the heel. Under aseptic precautions, patients in group A were given 1 ml of 2% Lignocaine at the medial side of the calcaneum which corresponded to the point of maximal tenderness which was infiltrated with an injection of 2 ml of autologous PRP.
Similarly, Group B patients were infiltrated with 2 ml of Depo-Medrol (40 mg methyl-prednisolone acetate) mixed with 1 ml of 2% Lignocaine into the point of maximal tenderness on the heel.
2.2. Post-procedure protocol
After the procedure, patients were instructed to apply ice, wear comfortable shoes. They were asked to avoid running, jumping and other high impact activities. Additional treatment permitted during the study included Analgesics like paracetamol only for one or two days to reduce the pain caused by the injection. NSAIDS were not advised after PRP injection.
2.3. Follow-up assessment
The participants were assessed for pain on the day of presentation and then after therapy at 2 weeks, 4 weeks, 3 months, and 6 months by Visual Analog Scale, and AOFAS hind-foot Score was taken at 6-month follow-up.
2.4. Sample size calculation
Power analysis for Unpaired t-test was conducted in G-POWER11 to determine a sufficient sample size using an alpha of 0.05, a power of 0.80, an effect size of 0.5 (Medium effect size using Cohen’s Convention), and two tails.12 There was an equal allocation of participants into each group. Based on the aforementioned assumptions, the desired sample size was as follows:
t tests - Means: Difference between two independent means (two groups)
Analysis:A priori: Compute required sample size.
Input:Tail(s) = Two.
Effect size d = 0.5
α err prob = 0.05.
Power (1-β err prob) = 0.8.
Allocation ratio N2/N1 = 1.
Output: Noncentrality parameter δ = 2.8284271.
Critical t = 1.9789706.
Df = 126.
Sample size group 1 = 64.
Sample size group 2 = 64.
Total sample size = 128.
Thus a sample size of at least 64 in each arm was taken. Allowing for a 15% loss to follow-up 75 patients were included in each group.
2.5. Statistical analysis
Data was coded and recorded in MS Excel spreadsheet program (Microsoft Redmond, WA). SPSS v23 (IBM Corp, Chicago, IL) was used for data analysis. Descriptive statistics were elaborated in the form of means/standard deviations and medians/IQRs for continuous variables, and frequencies and percentages for categorical variables. Data was presented in a graphical manner wherever appropriate for visualization. Group comparisons for continuously distributed data were made using independent sample ‘t’ test when comparing two groups. Chi-square test was used for group comparisons of categorical data. Continuously distributed paired variables were compared using Paired ‘t’ test when comparing two variables, and Repeated Measures ANOVA when comparing more than two variables. Statistical significance was kept at p < 0.05.
3. Results
3.1. Comparison of baseline parameters
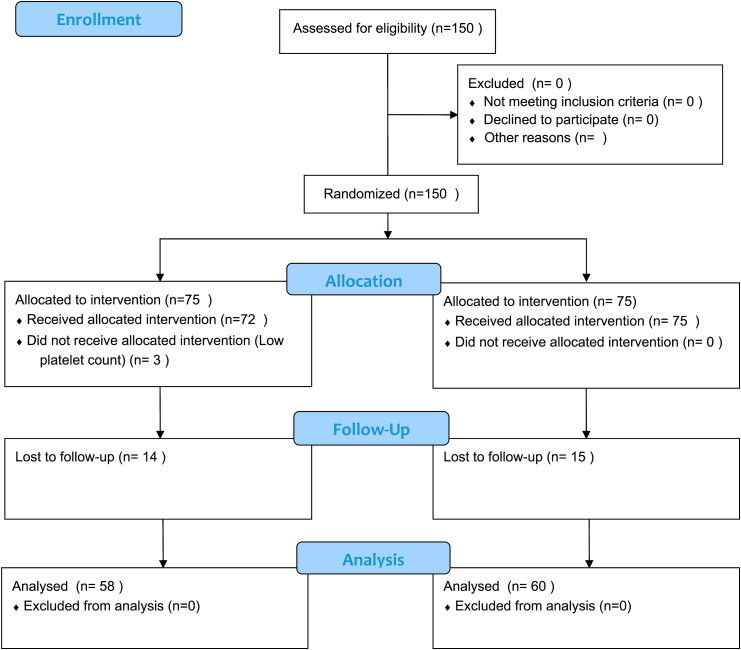
After obtaining institutional ethical and scientific committee clearance, 118 patients were recruited between July 2018 to June 2019 after necessary exclusions and loss to follow up. (CONSORT flow chart; Fig. 1). They were randomized into PRP group (n = 58) and Steroid group (n = 60). The two groups were comparable in terms of age, with the mean (SD) of age in PRP group being 32.57 (4.98) years while that in the steroid group being 34.70 (5.46) years (t = −2.215, p = 0.291). 86.2% of the participants in the PRP group had age less than 40 years, while 83.3% of the participants in the steroid group had age less than 40 years.
Fig. 1.
CONSORT flow diagram.
There was no significant difference between the various groups in terms of distribution of gender (χ2 = 0.576, p = 0.448). 58.6% of the participants in the PRP group were male while 51.7% of the participants in steroid group were male. There was no significant difference between the various groups in terms of distribution of side of disease (χ2 = 0.025, p = 0.875) (Table 1).
Table 1.
Association between group and parameters.
| Parameters | Group |
p value | |
|---|---|---|---|
| PRP (n = 58) | Steroid (n = 60) | ||
| Age (Years)∗∗∗ | 32.57 ± 4.98 | 34.70 ± 5.46 | 0.2911 |
| Age | 0.6642 | ||
| <40 Years | 50 (86.2%) | 50 (83.3%) | |
| ≥40 Years | 8 (13.8%) | 10 (16.7%) | |
| Gender | 0.4482 | ||
| Male | 34 (58.6%) | 31 (51.7%) | |
| Female | 24 (41.4%) | 29 (48.3%) | |
| Side | 0.8752 | ||
| Right | 33 (56.9%) | 35 (58.3%) | |
| Left | 25 (43.1%) | 25 (41.7%) | |
∗∗∗Significant at p < 0.05, 1: t-test, 2: Chi-Squared Test.
3.2. Comparison in terms of VAS score change
In PRP group, the mean VAS decreased from a maximum of 9.40 pre-injection to a minimum of 0.52 at 6 months (Repeated Measures ANOVA: F = 275.7, p = <0.001) (see Table 2). In steroid group, the mean VAS decreased from a maximum of 9.38 pre-injection to a minimum of 1.92 at 6 months (Repeated Measures ANOVA: F = 15.1, p = <0.001). The overall change in VAS over time was compared in the two groups using the generalized estimating equations (GEE) method. There was a significant difference in the trend of VAS over time in both the groups (p = <0.001) (Table 3). The maximum change from the pre-injection value was observed at the 6 months.
Table 2.
Comparison of the Two Groups in Terms of change in VAS over time (n = 118).
| VAS | Group |
P value for comparison of the two groups at each of the time points (t-test) | |||
|---|---|---|---|---|---|
| PRP |
Steroid |
||||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||
| Pre-Injection | 9.40 (0.72) | 10.00 (1.00) | 9.38 (0.74) | 10.00 (1.00) | 0.922 |
| 2 Weeks | 6.71 (0.99) | 6.50 (1.00) | 7.30 (1.09) | 7.00 (2.00) | 0.003 |
| 4 Weeks | 3.98 (1.03) | 4.00 (1.00) | 4.93 (1.07) | 5.00 (2.00) | <0.001 |
| 12 Weeks | 1.45 (0.75) | 1.00 (1.00) | 2.72 (0.98) | 3.00 (1.00) | <0.001 |
| 24 Weeks | 0.52 (0.60) | 0.00 (1.00) | 1.92 (1.03) | 2.00 (1.00) | <0.001 |
| P Value for change in VAS over time within each group (Repeated Measures ANOVA) | <0.001 | <0.001 | |||
| Overall P Value for comparison of change in VAS over time between the two groups (Generalized Estimating Equations Method) | <0.001 | ||||
Table 3.
Change in VAS from Pre-Injection to the various follow-up time points.
| Time point Comparison | Change in VAS from Pre-Injection to Follow-up Time points |
Comparison of the Two Groups in Terms of Difference of VAS from Pre-Injection to Follow-up Timepoints |
||||||
|---|---|---|---|---|---|---|---|---|
| Group: PRP |
Group: Steroid |
|||||||
| Mean (SD) of Absolute Change | Mean (SD) of % Change | P Value of Change Within Group | Mean (SD) of Absolute Change | Mean (SD) of % Change | P Value of Change Within Group | P Value of Absolute Change | P Value of % Change | |
| 2 Weeks - Pre-Injection | −2.69 (0.82) | −28.7% (8.3) | <0.001 | −2.08 (0.93) | −22.2% (9.7) | <0.001 | <0.001 | <0.001 |
| 4 Weeks - Pre-Injection | −5.41 (1.06) | −57.6% (10.2) | <0.001 | −4.45 (0.89) | −47.6% (9.8) | <0.001 | <0.001 | <0.001 |
| 12 Weeks - Pre-Injection | −7.95 (0.98) | −84.6% (8.0) | <0.001 | −6.67 (0.97) | −71.2% (9.6) | <0.001 | <0.001 | <0.001 |
| 24 Weeks - Pre-Injection | −8.88 (0.88) | −94.5% (6.3) | <0.001 | −7.47 (1.07) | −79.7% (10.5) | <0.001 | <0.001 | <0.001 |
Post-Hoc pairwise tests for Repeated Measures ANOVA performed using Tukey method were used to explore the statistical significance of the change in VAS from the Pre-Injection time point to the various follow-up time points. Group comparisons for change in VAS performed using Student’s t-test. Green background denotes statistically significant difference at p < 0.05.
3.3. AOFAS score at 6 months
The mean (SD) of AOFAS Score (6 Months) in the PRP group was 95.09 (4.60) while in the steroid group was 85.96 (5.34).There was a significant difference between the 2 groups in terms of AOFAS Score (6 Months) (t = 9.527, p = <0.001), with the mean AOFAS Score (6 Months) being higher in the PRP group (Table 4).
Table 4.
Comparison of the 2 subgroups of the variable group in terms of AOFAS score (6 Months) (n = 108).
| AOFAS Score (6 Months) | Group |
t-test |
||
|---|---|---|---|---|
| PRP | Steroid | t | p value | |
| Mean (SD) | 95.09 (4.60) | 85.96 (5.34) | 9.527 | <0.001 |
| Median (IQR) | 95 (90–100) | 86 (84–90) | ||
| Range | 85–100 | 73–97 | ||
3.4. Complications
No patient in any of the two groups suffered any complication (local or systemic) throughout their follow-up. There was no cross-over allowed in our study. None of the patients required a repeat injection till 6 months follow-up.
4. Discussion
Plantar fasciitis is common among general population and can have serious implications on a patient’s life and work. Successful and absolute treatment for plantar fasciitis remains an enigma till date. There have been several treatment options that have been used for plantar fasciitis, including orthoses, physical therapy, and steroid injections.13, 14, 15, 16, 17 Injectable therapy has been considered second line treatment, after conservative methods fail to provide relief.18 They are thought to reduce inflammation and pain, thereby improving functioning. The mainstay of such treatment, steroid injections, have been associated with infection, fat pad atrophy and in some cases even plantar fascia rupture.19, 20, 21
Plantar fasciitis is considered a degenerative condition of the plantar fascia with current evidence indicating the role of small tears of the plantar fascia. Normal plantar fascia has been observed to be replaced by angiofibroblastic hyperplastic tissue, with the lesion ironically not having any inflammatory cell invasion.22,23 Cytokines and growth factors play a significant role in the treatment of plantar fasciitis. PRP is rich in such factors, including TGF-B (Transforming Growth factor), VEGF (Vascular Endothelial Growth Factor), PDGF (Platelet Derived Growth Factor), and several other anti-inflammatory cytokines and interleukins. The combination of these growth factors and anti-inflammatory cytokines are postulated to heal and reverse the degenerative process at the insertion of plantar fascia.8,24 Recent evidence indicates that PRP increases collagen gene expression and production of vascular endothelial growth factor to promote healing.25 Local PRP injection also enables delivery of growth factors because the hypo-vascular and hypo-cellular nature of the plantar fascia and high local concentration of PRP allows the regenerative process to begin shortly following infiltration.26,27
Our findings suggest that PRP was associated with greater improvement in VAS score and resulted in superior AOFAS score at 6 months as compared to steroid injection. We did not find any local or systemic complications in any of the groups. The result and difference were more pronounced as the time from injection increased, and maximal benefit was observed at 6 months follow-up. None of the patients needed a repeat injection at 6 months.
There have been a few published reviews and meta-analysis to compare the results of PRP and steroids. Ling et al. in their meta-analysis have found that PRP was associated with greater changes in VAS and AOFAS scores than other treatments.28 The authors also found that the advantage of PRP over other treatments was only observed only at the 12 months, and not earlier. In contrast to these findings, our results show that the difference was significant at all time durations between 2 weeks and 6 months, but the difference was more marked at 6 months than at initial follow-up. Moreover, Ling et al. also found PRP to be more effective than steroid and placebo in the change of AOFAS score, which is similar to our results of AOFAS score at 6 months. Hsiao et al.29 in their meta-analysis of autologous blood derived products (ABP) for plantar fasciitis have included studies using PRP as the ABP and 3 such studies have found that PRP showed a significantly greater reduction in VAS score as compared to corticosteroids at 3 months, but the reduction at 6 months was comparable between the two treatments, which is in contrast to our findings. We have found more significant improvement with PRP with the passage of time. Thus, whether PRP is superior to corticosteroids in long term remains uncertain based on inconstant results in available literature.
Our results were inconsistent with previous findings of Aksahin and Jain et al.30,31 Aksahin et al. compared the effects of local injection of PRP with corticosteroids among sixty patients with plantar fasciitis who had failed conservative treatment.31 At 3 weeks and 6 months after the treatment, the VAS score and RMS were significantly improved in both groups, however, the differences between them were not significant. Similarly, Jain et al.30 compared the efficacy of PRP with steroid at 3, 6, and 12 months after injection and they found that, at 3 months, the VAS, AOFAS and RM scores were marginally better in steroid group than in the PRP group. At 6 months, these outcome scores were better in PRP group than in the steroid group. These differences in outcome scores, however, did not reach statistical significance at either 3 or 6 months.24
Contrary to the negative findings of Aksahin and Jain et al., some other studies observed entirely different results. In the study of Shetty et al., the authors compared the efficacy of corticosteroid injection (30 patients) with PRP injection (30 patients). At the 3-month of follow-up, the postoperative measure outcomes were significantly improved in both groups.32 And these results were much better in the PRP group than that in the steroid group.32 Similarly, Say et al. compared the effects of PRP and steroid in patients with plantar fasciitis.33 The authors assessed 50 patients divided among each group.22 PRP had a larger change in AOFAS and VAS scores than that in the steroid group, both at 6 weeks and 6 months.22 With regards to the long-term effect of PRP, our results suggested that PRP was associated with greater changes in VAS and AOFAS scores compared to local steroid injections at 6 months.30 Likewise, in the study of Monto et al., difference in AOFAS score between the PRP and steroid groups was clinically significant at the 12- and 24-month follow-up evaluations (P = 0.001).34 Thus, available evidence indicates that PRP is more effective than steroid in the long term for the management of plantar fasciitis, a finding which is consistent with our own (Table 5).
Table 5.
Randomized control trials comparing PRP to local steroid injection for plantar fasciitis.
| Study | Sample size |
Steroid preparation | PRP volume (ml) | Maximal follow-up (months) | Outcome measure | Superior outcomes | Complications |
||
|---|---|---|---|---|---|---|---|---|---|
| PRP | steroid | PRP | Steroid | ||||||
| Omar et al., 2012 | 15 | 15 | NS | NS | 1 | VAS, DASH, FHSQ | PRP | 0 | 0 |
| Tiwari and Bhargava 2013 | 30 | 30 | 40 methylprednisolone | 5 | 6 | VAS | PRP | NS | NS |
| Monto 2014 | 20 | 20 | 40 methylprednisolone | 3 | 24 | AOFAS | PRP | NS | NS |
| Sherpy et al., 2016 | 25 | 25 | 80 triamcinolone | 3 | 3 | VAS | Comparable | 0 | 0 |
| Jain et al., 2015 | 30 | 30 | 40 triamcinolone | 2.5 | 12 | VAS, AOFAS | PRP | NS | NS |
| Acosta-Olivo et al., 2017 | 14 | 14 | 8 mg Dexamethasone | 3 | 4 | VAS, AOFAS, FADI | Comparable | 0 | 0 |
| Mahindra et al., 2016 | 25 | 25 | 80 methylprednisolone | 3 | 3 | VAS, AOFAS | Comparable | NS | NS |
| Vahdatpour et al., 2016 | 16 | 16 | 40 methylprednisolone | 3 | 6 | VAS, RMS, Sonography | PRP | NS | NS |
| Ugurlar et al., 2018 | 39 | 40 | 40 betamethasone | 5 | 36 | VAS | Comparable | NS | NS |
| Jain et al., 2018 | 40 | 30 | 80 methylprednisolone | 3 | 6 | VAS, AOFAS, Sonography | Comparable | NS | NS |
| Shetty et al., 2019 | 30 | 30 | 80 methylprednisolone | 2 | 18 | VAS, RMS | PRP | NS | NS |
| PRESENT STUDY | 58 | 60 | 80 methylprednisolone | 2 | 6 | VAS, AOFAS | PRP | 0 | 0 |
NS=Not Specified.
In present study PRP was administered at the point of maximum tenderness of the heel. Previous studies have advocated the use of ultrasound guidance for the injection in plantar fasciitis,35,36 since this could allow for more accurate placement of the injection. However, results from the trials conducted by Tsai and Kane suggested that ultrasound-guided injection did not appear to more effective than palpation-guided injection in the treatment of idiopathic plantar fasciitis.37,38
VAS score is the more commonly used outcome score for evaluating effect of various treatment modalities for plantar fasciitis.9,33, 39, 40 Primary concerns in treating plantar fasciitis is not just pain relief but also functional improvement and early return to day to day activities. So, to give a better idea of improvement with any therapy used for plantar fasciitis, methodologically superior recent literature uses functional scores like AOFAS score to report improvement with any therapy used.22,24,25 Our study uses VAS as well as AOFAS to report outcomes of steroid and PRP injection for plantar fasciitis.
Large sample size in this study as compared to previous studies (Table 4) has enhanced the statistical power of our results, and the high quality in terms of level one evidence has ensured the reliability and credibility of the findings of present study. The credibility of our outcome is however limited by a follow-up of 6 months and that we were unable to determine if any patient had recurrence of symptoms with requirement of repeat injection later than that. Another limitation of our study was lack of blinding among treating physician and patients. Present study may additionally be underpowered as attrition was greater than the initial allowance of 15%. Despite some shortcomings, the current RCT suggests that Plantar fasciitis patients benefit from both PRP and steroid therapy. However, the benefit is more with PRP in terms of mid-term control of pain as well as functional improvement.
5. Conclusion
As shown in our RCT, significant differences were found in short-term and mid-term pain relief as well as mid-term functional benefit by use of PRP over steroid injection for plantar fasciitis. Considering the effectiveness of PRP, we recommend the use of PRP as the preferred treatment for Plantar fasciitis. Our study expands on the previous studies to provide a better characterization of evidence base for PRP over local steroid injection in plantar fasciitis.
References
- 1.Lee W.C.C., Wong W.Y., Kung E., Leung A.K.L. Effectiveness of adjustable dorsiflexion night splint in combination with accommodative foot orthosis on plantar fasciitis. J Rehabil Res Dev. 2012;49(10):1557. doi: 10.1682/jrrd.2011.09.0181. [DOI] [PubMed] [Google Scholar]
- 2.Tong K.B., Furia J. Economic burden of plantar fasciitis treatment in the United States. Am J Orthop Belle Mead NJ. 2010 May;39(5):227–231. [PubMed] [Google Scholar]
- 3.Monteagudo M., Maceira E., Garcia-Virto V., Canosa R. Chronic plantar fasciitis: plantar fasciotomy versus gastrocnemius recession. Int Orthop. 2013 Sep;37(9):1845–1850. doi: 10.1007/s00264-013-2022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toomey E.P. Plantar heel pain. Foot Ankle Clin. 2009 Jun;14(2):229–245. doi: 10.1016/j.fcl.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Cheung J.T.-M., An K.-N., Zhang M. Consequences of partial and total plantar fascia release: a finite element study. Foot Ankle Int. 2006 Feb;27(2):125–132. doi: 10.1177/107110070602700210. [DOI] [PubMed] [Google Scholar]
- 6.Tatli Y.Z., Kapasi S. The real risks of steroid injection for plantar fasciitis, with a review of conservative therapies. Curr Rev Musculoskelet Med. 2009 Mar;2(1):3–9. doi: 10.1007/s12178-008-9036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sánchez M., Anitua E., Orive G., Mujika I., Andia I. Platelet-rich therapies in the treatment of orthopaedic sport injuries. Sports Med. 2009;39(5):345–354. doi: 10.2165/00007256-200939050-00002. [DOI] [PubMed] [Google Scholar]
- 8.Martinelli N., Marinozzi A., Carnì S., Trovato U., Bianchi A., Denaro V. Platelet-rich plasma injections for chronic plantar fasciitis. Int Orthop. 2013 May;37(5):839–842. doi: 10.1007/s00264-012-1741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar V., Millar T., Murphy P.N., Clough T. The treatment of intractable plantar fasciitis with platelet-rich plasma injection. Foot. 2013 Jun;23(2–3):74–77. doi: 10.1016/j.foot.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 10.O’Malley M.J., Vosseller J.T., Gu Y. Successful use of platelet-rich plasma for chronic plantar fasciitis. HSS J ®. 2013 Jul;9(2):129–133. doi: 10.1007/s11420-012-9321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faul F., Erdfelder E., Buchner A., Lang A.-G. Statistical power analyses using G∗Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009 Nov;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 12.Chen H., Cohen P., Chen S. Biased odds ratios from dichotomization of age. Stat Med. 2007 Aug 15;26(18):3487–3497. doi: 10.1002/sim.2737. [DOI] [PubMed] [Google Scholar]
- 13.Wrobel J.S., Fleischer A.E., Crews R.T., Jarrett B., Najafi B. A randomized controlled trial of custom foot orthoses for the treatment of plantar heel pain. J Am Podiatr Med Assoc. 2015 Jul 1;105(4):281–294. doi: 10.7547/13-122.1. [DOI] [PubMed] [Google Scholar]
- 14.Piper S., Shearer H.M., Côté P. The effectiveness of soft-tissue therapy for the management of musculoskeletal disorders and injuries of the upper and lower extremities: a systematic review by the Ontario Protocol for Traffic Injury management (OPTIMa) collaboration. Man Ther. 2016 Feb;21:18–34. doi: 10.1016/j.math.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim M.I., Donatelli R.A., Hellman M., Hussein A.Z., Furia J.P., Schmitz C. Long-term results of radial extracorporeal shock wave treatment for chronic plantar fasciopathy: a prospective, randomized, placebo-controlled trial with two years follow-up. J Orthop Res Off Publ Orthop Res Soc. 2017;35(7):1532–1538. doi: 10.1002/jor.23403. [DOI] [PubMed] [Google Scholar]
- 16.Lynch D., Goforth W., Martin J., Odom R., Preece C., Kotter M. Conservative treatment of plantar fasciitis. A prospective study. J Am Podiatr Med Assoc. 1998 Aug 1;88(8):375–380. doi: 10.7547/87507315-88-8-375. [DOI] [PubMed] [Google Scholar]
- 17.Sun J., Gao F., Wang Y., Sun W., Jiang B., Li Z. Extracorporeal shock wave therapy is effective in treating chronic plantar fasciitis: a meta-analysis of RCTs. Medicine (Baltim) 2017 Apr;96(15) doi: 10.1097/MD.0000000000006621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beeson P. Plantar fasciopathy: revisiting the risk factors. Foot Ankle Surg. 2014 Sep;20(3):160–165. doi: 10.1016/j.fas.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Kirkland P., Beeson P. Use of primary corticosteroid injection in the management of plantar fasciopathy. J Am Podiatr Med Assoc. 2013 Sep 1;103(5):418–429. doi: 10.7547/1030418. [DOI] [PubMed] [Google Scholar]
- 20.Buccilli T.A., Hall H.R., Solmen J.D. Sterile abscess formation following a corticosteroid injection for the treatment of plantar fasciitis. J Foot Ankle Surg Off Publ Am Coll Foot Ankle Surg. 2005 Dec;44(6):466–468. doi: 10.1053/j.jfas.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Acevedo J.I., Beskin J.L. Complications of plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int. 1998 Feb;19(2):91–97. doi: 10.1177/107110079801900207. [DOI] [PubMed] [Google Scholar]
- 22.Lemont H., Ammirati K.M., Usen N. Plantar fasciitis. J Am Podiatr Med Assoc. 2003 May 1;93(3):234–237. doi: 10.7547/87507315-93-3-234. [DOI] [PubMed] [Google Scholar]
- 23.Barrett S.J., O’Malley R. Plantar fasciitis and other causes of heel pain. Am Fam Physician. 1999 Apr 15;59(8):2200–2206. [PubMed] [Google Scholar]
- 24.Molloy T., Wang Y., Murrell G.A.C. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33(5):381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 25.Anitua E., Pino A., Orive G. Plasma rich in growth factors promotes dermal fibroblast proliferation, migration and biosynthetic activity. J Wound Care. 2016 Nov 2;25(11):680–687. doi: 10.12968/jowc.2016.25.11.680. [DOI] [PubMed] [Google Scholar]
- 26.Fenwick S.A., Hazleman B.L., Riley G.P. The vasculature and its role in the damaged and healing tendon. Arthritis Res. 2002;4(4):252. doi: 10.1186/ar416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J.H.-C., Nirmala X. Application of tendon stem/progenitor cells and platelet-rich plasma to treat tendon injuries. Operat Tech Orthop. 2016 Jun;26(2):68–72. doi: 10.1053/j.oto.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ling Y., Wang S. Effects of platelet-rich plasma in the treatment of plantar fasciitis: a meta-analysis of randomized controlled trials. Medicine (Baltim) 2018 Sep;97(37) doi: 10.1097/MD.0000000000012110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsiao M.-Y., Hung C.-Y., Chang K.-V., Chien K.-L., Tu Y.-K., Wang T.-G. Comparative effectiveness of autologous blood-derived products, shock-wave therapy and corticosteroids for treatment of plantar fasciitis: a network meta-analysis. Rheumatol Oxf Engl. 2015 Sep;54(9):1735–1743. doi: 10.1093/rheumatology/kev010. [DOI] [PubMed] [Google Scholar]
- 30.Jain K., Murphy P.N., Clough T.M. Platelet rich plasma versus corticosteroid injection for plantar fasciitis: a comparative study. Foot. 2015 Dec;25(4):235–237. doi: 10.1016/j.foot.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Akşahin E., Doğruyol D., Yüksel H.Y. The comparison of the effect of corticosteroids and platelet-rich plasma (PRP) for the treatment of plantar fasciitis. Arch Orthop Trauma Surg. 2012 Jun;132(6):781–785. doi: 10.1007/s00402-012-1488-5. [DOI] [PubMed] [Google Scholar]
- 32.Shetty V.D., Dhillon M., Hegde C., Jagtap P., Shetty S. A study to compare the efficacy of corticosteroid therapy with platelet-rich plasma therapy in recalcitrant plantar fasciitis: a preliminary report. Foot Ankle Surg Off J Eur Soc Foot Ankle Surg. 2014 Mar;20(1):10–13. doi: 10.1016/j.fas.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Say F. Comparison of platelet-rich plasma and steroid injection in the treatment of plantar fasciitis. Acta Orthop Traumatol Turcica. 2014;48(6):667–672. doi: 10.3944/AOTT.2014.13.0142. [DOI] [PubMed] [Google Scholar]
- 34.Monto R.R. Platelet-rich plasma efficacy versus corticosteroid injection treatment for chronic severe plantar fasciitis. Foot Ankle Int. 2014 Apr;35(4):313–318. doi: 10.1177/1071100713519778. [DOI] [PubMed] [Google Scholar]
- 35.Tsai W.C., Wang C.L., Tang F.T., Hsu T.C., Hsu K.H., Wong M.K. Treatment of proximal plantar fasciitis with ultrasound-guided steroid injection. Arch Phys Med Rehabil. 2000 Oct;81(10):1416–1421. doi: 10.1053/apmr.2000.9175. [DOI] [PubMed] [Google Scholar]
- 36.Cunndne G., Brophy D.P., Gibney R.G., FitzGerald O. Diagnosis and treatment of heel pain in chronicinflammatory arthritis using ultrasound. Semin Arthritis Rheum. 1996 Jun;25(6):383–389. doi: 10.1016/s0049-0172(96)80003-5. [DOI] [PubMed] [Google Scholar]
- 37.Tsai W.-C., Hsu C.-C., Chen C.P.C., Chen M.J.L., Yu T.-Y., Chen Y.-J. Plantar fasciitis treated with local steroid injection: comparison between sonographic and palpation guidance. J Clin Ultrasound JCU. 2006 Jan;34(1):12–16. doi: 10.1002/jcu.20177. [DOI] [PubMed] [Google Scholar]
- 38.Kane D., Greaney T., Shanahan M. The role of ultrasonography in the diagnosis and management of idiopathic plantar fasciitis. Rheumatol Oxf Engl. 2001 Sep;40(9):1002–1008. doi: 10.1093/rheumatology/40.9.1002. [DOI] [PubMed] [Google Scholar]
- 39.Mahindra P., Yamin M., Selhi H.S., Singla S., Soni A. Chronic plantar fasciitis: effect of platelet-rich plasma, corticosteroid, and placebo. Orthopedics. 2016 Mar 1;39(2):e285–e289. doi: 10.3928/01477447-20160222-01. [DOI] [PubMed] [Google Scholar]
- 40.Tiwari M., Bhargava R. Platelet rich plasma therapy: a comparative effective therapy with promising results in plantar fasciitis. J Clin Orthop Trauma. 2013 Mar;4(1):31–35. doi: 10.1016/j.jcot.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]