Figure 1. Overall structure of mouse viperin.
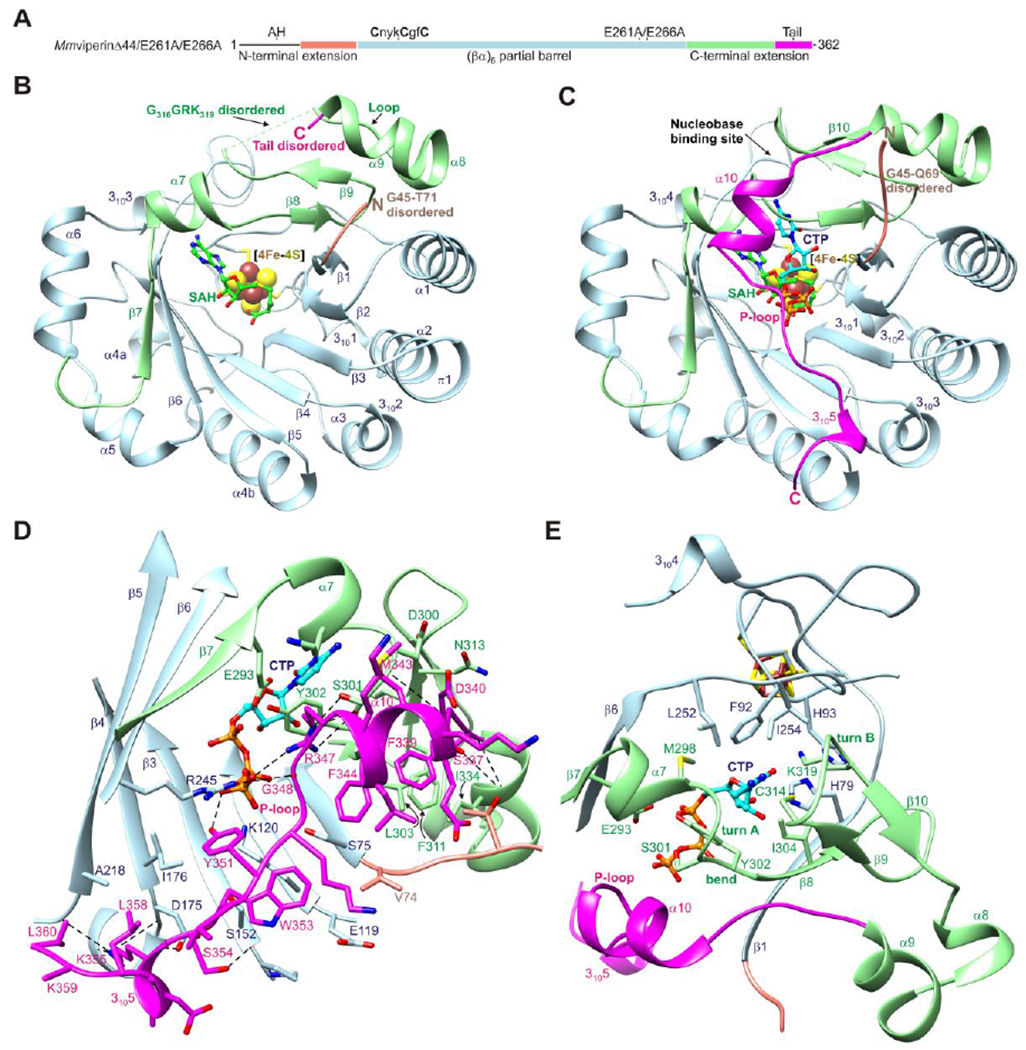
(A) One-dimensional schematic representation of viperin fragment cocrystallized with CTP or UTP showing structural organization and coloring scheme used in subsequent figures. AH denotes the amphipathic helix, which is not part of the viperin fragment. (B) Ribbon representation of MmviperinΔ44 bound to a [4Fe-4S] cluster and SAH (PDB code 5VSL).32 Secondary structure elements and disordered regions of the N- and C-terminal extensions are indicated. (C) Ribbon representation of MmviperinΔ44/E261A/E266A bound to a [4Fe-4S] cluster, SAH, and CTP. Changes in secondary structure assignments and new secondary structure elements observed in the CTP-bound complex are indicated. (D) Packing of C-terminal tail against the barrel opening and Pγ of CTP. (E) Nucleobase binding site. The residues surrounding the cytosine moiety from the core domain have a similar architecture in the nucleotide-free structure, whereas those from the C-terminal extension form turn A and a β-hairpin containing turn B. CTP and SAH are shown as balls and sticks and the [4Fe-4S] cluster as spheres. Potential hydrogen bonds and other electrostatic interactions are shown as dashed lines.
