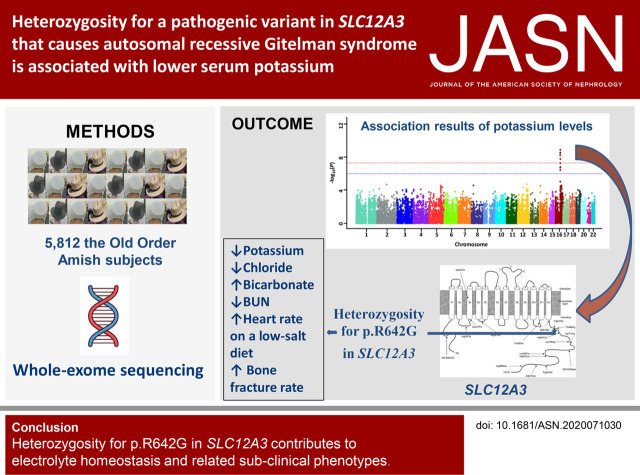
Significance Statement
Potassium regulates multiple pathophysiologic processes. Renal potassium handling is important for potassium homeostasis. The heritability of serum potassium level suggests genetic influences, however the genetic determinants are not known. Heterozygosity for a pathogenic variant (p.R642G) of SLC12A3, causing autosomal recessive Gitelman syndrome, is significantly associated with lower potassium and chloride levels, but not with sodium levels. Notably, p.R642G shows a novel role in modulation of serum BUN levels. This work provides new insights into SLC12A3 biology and the effects of heterozygosity on electrolyte homeostasis and related subclinical phenotypes that may have implications for personalized medicine.
Keywords: potassium, Gitelman syndrome, heterozygosity, whole-exome sequencing, genetic renal disease
Visual Abstract
Abstract
Background
Potassium levels regulate multiple physiologic processes. The heritability of serum potassium level is moderate, with published estimates varying from 17% to 60%, suggesting genetic influences. However, the genetic determinants of potassium levels are not generally known.
Methods
A whole-exome sequencing association study of serum potassium levels in 5812 subjects of the Old Order Amish was performed. A dietary salt intervention in 533 Amish subjects estimated interaction between p.R642G and sodium intake.
Results
A cluster of variants, spanning approximately 537 kb on chromosome 16q13, was significantly associated with serum potassium levels. Among the associated variants, a known pathogenic variant of autosomal recessive Gitelman syndrome (p.R642G SLC12A3) was most likely causal; there were no homozygotes in our sample. Heterozygosity for p.R642G was also associated with lower chloride levels, but not with sodium levels. Notably, p.R642G showed a novel association with lower serum BUN levels. Heterozygotes for p.R642G had a two-fold higher rate of self-reported bone fractures and had higher resting heart rates on a low-salt diet compared with noncarriers.
Conclusions
This study provides evidence that heterozygosity for a pathogenic variant in SLC12A3 causing Gitelman syndrome, a canonically recessive disorder, contributes to serum potassium concentration. The findings provide insights into SLC12A3 biology and the effects of heterozygosity on electrolyte homeostasis and related subclinical phenotypes that may have implications for personalized medicine and nutrition.
Potassium plays a key role in multiple pathophysiologic processes, including hypertension, cardiac arrhythmias, coronary heart disease, stroke, kidney disease, osteoporosis, impaired insulin secretion, and glucose intolerance.1–6 Moreover, recent studies have revealed that extracellular potassium levels modulate T-cell stemness and antitumor capacity.7 Serum potassium levels outside the normal range (3.5–5.0 mmol/L) are associated with increased mortality. Recent studies have shown that even mild reductions in serum potassium (3.5–4.0 mmol/L) are related to excess morbidity.8 Altered serum potassium concentration during treatment for hypertension can be clinically serious because it is associated with adverse cardiovascular effects.9 Thus, the maintenance of serum potassium within a narrow range is crucial for cell function and human health.
Potassium homeostasis is tightly maintained by multiple mechanisms.10 Approximately 90% of the potassium consumed is excreted in the urine, with the remaining 10% excreted by the gastrointestinal tract, and a very small amount lost in sweat. Interindividual variation in serum potassium is influenced by genetic and environmental factors, and their interactions. Serum potassium concentrations have been shown to have a moderate heritable component, with published heritability estimates varying from 17% to 60%, suggesting genetic influences on serum potassium levels.11–15 Several rare, monogenic disorders have been identified that cause abnormalities in potassium homeostasis, including Gitelman syndrome (Online Mendelian Inheritance in Man [OMIM] #263800), Bartter syndrome (OMIM #601678, #241200, #607364, and #602522), EAST syndrome (OMIM #612780), and pseudohypoaldosteronism (OMIM #177735 and #264350). To evaluate the contribution of common genetic variation to normal physiologic variation of serum potassium, Meyer et al.16 conducted a genome-wide association study of serum potassium concentrations in 15,366 participants of European descent from the Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium, in which they did not observe any common variants significantly associated with serum potassium concentrations. Uncovering the causal genetic components associated with serum potassium levels may provide insights into the mechanisms involved in potassium homeostasis, which may identify more effective pharmacologic targets or other interventions for disorders related to potassium homeostasis.
To further explore the contribution of genetics to variation in serum potassium levels, we performed whole-exome sequencing (WES) in Old Order Amish (OOA) subjects. The OOA are a culturally and genetically homogeneous population originating from a small number of founders who immigrated to the United States from Europe approximately 14 generations ago. We hypothesized that, through genetic drift, the OOA may be enriched for rare variants with large effects on serum potassium levels. Furthermore, increased genetic and lifestyle homogeneity in this population may increase power to identify causal variants.
Methods
Subjects
The OOA subjects (n=5812) from Lancaster, Pennsylvania who were included in this study were adults, aged ≥18 years, who participated in the Heredity and Phenotype Intervention (HAPI) Heart Study, the Amish Family Osteoporosis Study (AFOS), Pharmacogenomics of Anti-Platelet Intervention (PAPI) Study, or the Amish Wellness Study (AWS). Details of the design and recruitment of these studies have been described previously.17–21 Briefly, the HAPI Heart Study measured the cardiovascular response to four short-term interventions affecting cardiovascular risk factors to identify the genetic and environmental determinants of these responses. The AFOS aimed to identify genes that are important for bone health. The goal of the PAPI Study was to understand variability in response to commonly used antiplatelet medications to prevent myocardial infarction, including aspirin and clopidogrel. The goal of AWS was to provide wellness screening for cardiometabolic health to members of the OOA community in Lancaster County and to perform genetic research related to cardiometabolic health. The protocols and procedures for those studies were approved by the University of Maryland Institutional Review Board, and all subjects gave written informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Trait Measurements
Details of methods for the phenotyping of subjects have been described previously.17–21 Briefly, all blood samples used in this study were drawn between 8 am and 10 am after at least an 8-hour overnight fast, except for the noted traits. Serum potassium, sodium (Na), calcium, chloride (Cl), urea nitrogen (BUN), creatinine, bicarbonate, and glucose levels were measured using standard protocols (Quest Diagnostics, Horsham, PA). Resting systolic and diastolic BP measures were obtained by trained staff, and the average of multiple readings was used. Height, weight, and waist and hip circumference were measured by trained study personnel. Fracture cases were defined as those individuals who had fractures at any skeletal site by self-report. High-trauma fractures were excluded whenever possible.
In the HAPI Heart Study, 533 subjects participated in a dietary salt intervention.17 They consumed a standardized high‐Na diet (280 mEq/d=6440 mg/d) for 6 days, and then, after a 6‐ to 14‐day washout period, consumed a standardized low‐Na diet (40 mEq/d=920 mg/d) for 6 days. The dietary potassium intake was held constant at 140 mEq/d during both diets. BP (24 hour) and heart rate measurements were recorded at 30‐minute intervals by an ambulatory BP monitor worn by study patients on the last day of each diet intervention.
WES
WES was performed at the Regeneron Genetics Center using established protocols as described.22 In brief, genomic DNA samples were sheared to an average fragment length of 150 bp using focused acoustic energy (Covaris LE220). The sheared genomic DNA was prepared for exome capture with xGen Exome Research Panel version 1.0 (Integrated DNA Technologies). Captured DNA was amplified, quantified, and subsequently sequenced on an Illumina version 4 HiSeq 2500 instrument (to approximately 80 times the mean haploid read depth of targeted bases). Sequence reads were aligned to the human reference build GRCh38, and single-nucleotide variants and insertion/deletion sequence variants were identified using the Genome Analysis Toolkit 3.0, and then annotated using SnpEff31.
Statistical Analyses
Summary statistics of baseline clinical characteristics were expressed as unadjusted means±SDs using SPSS statistics software version 23 (IBM Corporation, New York, NY). Estimation of the additive genetic heritability followed basic quantitative genetic theory, which models the phenotypic covariance (conditional upon covariate effects) between two individuals in a pedigree as a function of their degree of biologic relatedness. Maximum-likelihood methods were used to estimate the values of the parameters, such as heritability, that resulted in the highest likelihood obtained across all of the pedigrees. The association analyses were carried out using Mixed Model Analysis of Pedigrees and Populations (https://mmap.github.io/). The polygenic component was modeled using the relationship matrix derived from the complete 14-generation Amish pedigree structure. We included family structure, study, age, and sex as covariates in the association models. P values <5×10−8 were considered significant for WES. P values <0.05 were considered significant for trait tests with the p.R642G variant. Correction for multiple testing was performed using the Bonferroni method for the number of traits tested (P=0.05/17=2.94×10−3).
Results
Clinical Characteristics
The clinical characteristics of 5812 OOA subjects are summarized in Table 1. The study subjects included in this analysis consisted of 2524 males and 3288 females. The average age and body mass index were 42.5 years and 26.7 kg/m2, respectively. The mean serum potassium was 4.19±0.29 mmol/L. Serum potassium levels were significantly lower in females than in males (4.14±0.28 versus 4.26±0.27 mmol/L, respectively; P=2.27×10−60), as were serum Na, BUN, creatinine, bicarbonate, glucose, systolic BP, and diastolic BP (P<0.001). Serum Cl and calcium were not significantly different between females and males. The mean heart rate was significantly higher in females than in males (P<0.001).
Table 1.
Clinical characteristics of the study population
| Characteristics | Total | Male | Female | P Valuea |
|---|---|---|---|---|
| Number (n) | 5812 | 2524 | 3288 | <0.001 |
| Age (yr) | 42.5±15.9 | 43.4±15.5 | 41.8±16.2 | <0.001 |
| BMI (kg/m2) | 26.7±5.0 | 26.1±4.1 | 27.1±5.6 | <0.001 |
| Potassium (mmol/L) | 4.19±0.29 | 4.26±0.27 | 4.14±0.28 | <0.001 |
| Na (mmol/L) | 138.5±2.0 | 138.8±1.8 | 138.3±2.1 | <0.001 |
| Cl (mmol/L) | 103.5±2.1 | 103.5±2.1 | 103.5±2.2 | 0.44 |
| Calcium (mg/dl) | 9.32±0.35 | 9.33±0.33 | 9.31±0.37 | 0.09 |
| BUN (mg/dl) | 15.1±4.4 | 17.1±4.1 | 13.5±4.0 | <0.001 |
| Creatinine (mg/dl) | 0.77±0.17 | 0.86±0.14 | 0.70±0.15 | <0.001 |
| Bicarbonate (mmol/L) | 24.4±2.4 | 24.7±2.2 | 24.1±2.4 | <0.001 |
| Glucose (mg/dl) | 86.1±15.5 | 87.4±14.5 | 85.0±16.1 | <0.001 |
| Heart rate (beats/min) | 65.0±9.9 | 61.6±8.9 | 67.5±9.8 | <0.001 |
| SBP (mm Hg) | 115.4±16.0 | 116.3±13.8 | 114.6±17.4 | <0.001 |
| DBP (mm Hg) | 71.2±9.7 | 72.8±9.8 | 69.9±9.5 | <0.001 |
Data are shown as mean±SD. BMI, body mass index; SBP, systolic BP; DBP, diastolic BP.
Comparison between male and female.
Heritability of Serum Potassium
We calculated the heritability of serum potassium in the Amish on the basis of the most parsimonious model of variance-component analysis. The heritability of potassium in the Amish was 18.25% (P=3.62×10−36). Because potassium levels were significantly different between males and females, we further evaluated heterogeneity in genetic variance between males and females. In the sex-stratified model, we found that heritability of potassium was generally higher in males than females (25.2% [P=2.13×10−17] and 17.2% [P=7.28×10−14], respectively), providing suggestive evidence for heterogeneity of genetic-variance components by sex.
Exome-Wide Association Study of Serum Potassium Levels
We performed an exome-wide association study (ExWAS) for serum potassium levels in 5812 OOA subjects, and identified a cluster of variants, spanning approximately 537 kb on chromosome 16q13, showing exome-wide significant association with potassium levels (lead single-nucleotide polymorphism [SNP], rs149279319; P=1.14×10−9) (Figure 1, Supplemental Table 1). Multiple highly associated variants were in linkage disequilibrium and were within, or flanking, 22 genes (Figure 2); among these was a missense variant in SLC12A3 (rs200697179, c.1924C>G, p.R642G). SLC12A3 encodes solute carrier family 12 member 3, a renal, thiazide-sensitive, Na-Cl cotransporter (NCC) that is important for electrolyte homeostasis. Mutations in this gene cause autosomal recessive Gitelman syndrome, which is characterized by hypokalemic alkalosis combined with hypomagnesemia, low urinary calcium, and increased renin activity, which is associated with low or normal BP. The mutation, p.R642G, in its recessive form is a known pathogenic variant; however, there were no homozygotes in our cohort.23 Consistent with a founder effect and genetic drift, the minor allele frequency (MAF) of p.R642G was 65 times higher in the Amish (MAF, 0.013; 147 heterozygotes, 5663 noncarriers) than in other populations (global MAF, 0.0002) (ClinVar, https://www.ncbi.nlm.nih.gov/clinvar/variation/VCV 000397523.1). The heterozygous carriers of rs200697179 (p.R642G) had significantly lower serum potassium levels than noncarriers (4.05 versus 4.20 mmol/L; P=2.39×10−9) (Figure 3).
Figure 1.
Manhattan plot showing genome-wide signficant association of a locus on chromosome 16 with serum potassium levels. SNPs are plotted on the x axis, according to their position on each chromosome, against association with serum potassium levels on the y axis (shown as −log10 P values).
Figure 2.
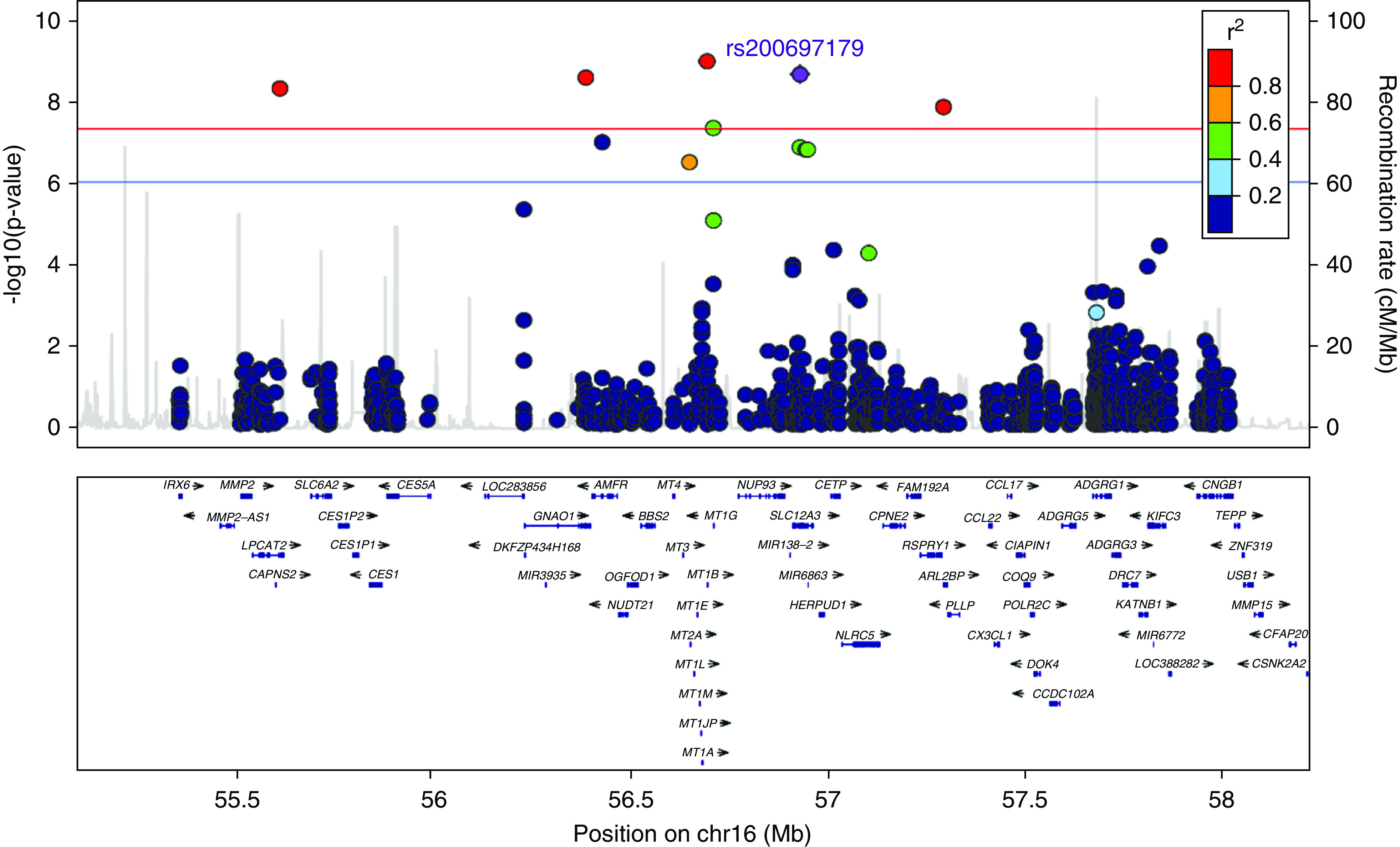
Regional association plot for SNPs and serum potassium levels shows that ExWAS significant variants are in high linkage disequilibrium between each other. The SNPs surrounding rs200697179 (p.R642G) are color coded to reflect their linkage disequilibrium with this SNP. Regional plots were generated using Locus Zoom (http://csg.sph.umich.edu/locuszoom). Chr16, chromosome 16.
Figure 3.
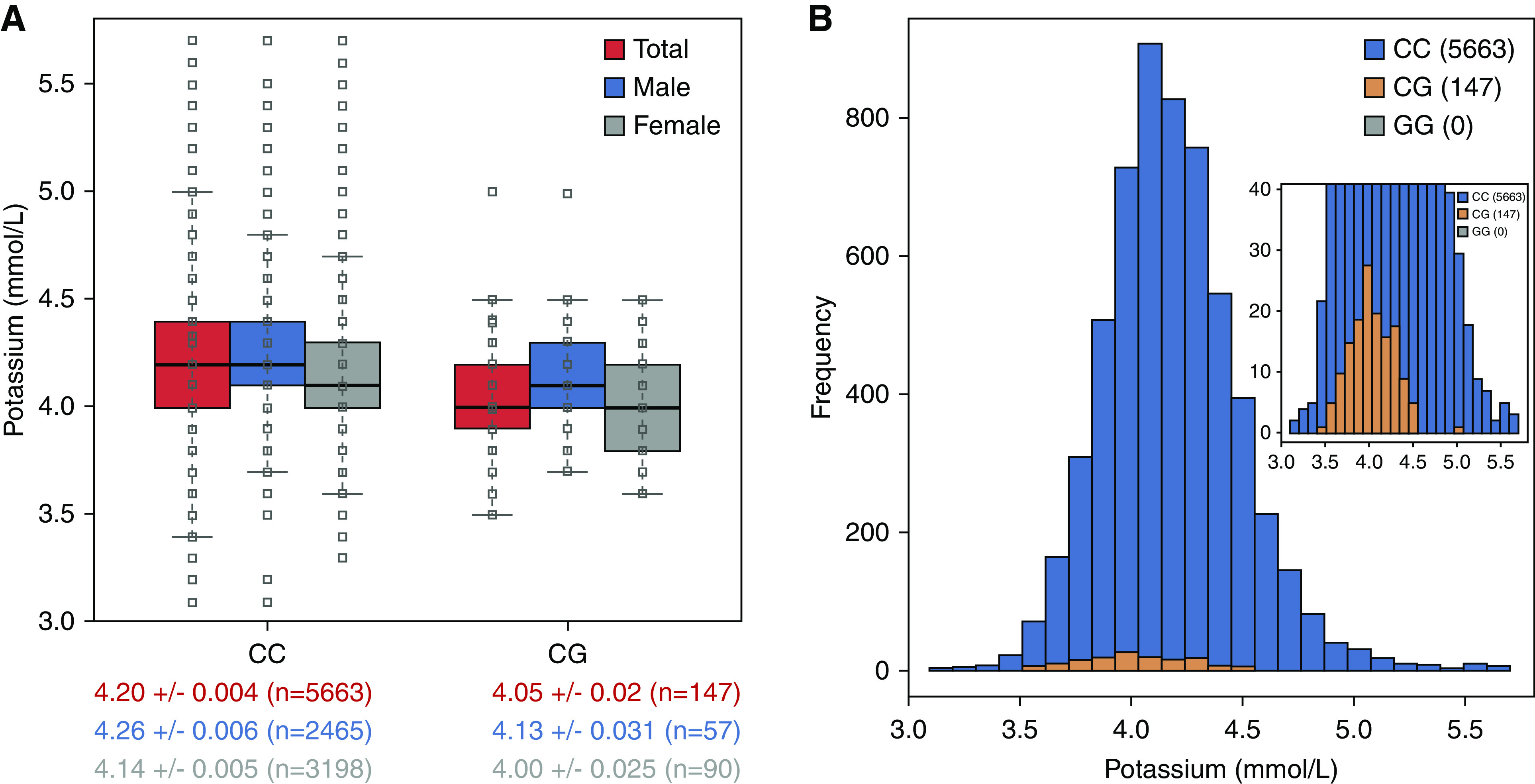
Heterozygous carriers of p.R642G SLC12A3 (CG) have lower serum potassium levels than noncarriers (CC). (A) Box plots are shown for individuals of each genotype, with the median potassium level represented by the black line. (B) Serum potassium distribution graph is shown for individuals of each genotype.
We observed that other variants (e.g., rs149279319) in high linkage disequilibrium with rs200697179 (p.R642G) had a marginally more significant P value for association with serum potassium levels than rs200697179. We performed further analysis, removing subjects who had missing genotypes, limiting analyses to the 5808 subjects with nonmissing genotypes for both SNPs. Indeed, rs200697179 had a slightly more significant P value for association with serum potassium levels than rs149279319 (β=−0.152 [P=1.02×10−9] versus β=−0.149 [P=1.61×10−9], respectively). Further, sequential conditional analysis revealed there was the only one signal associated with serum potassium in this region. After conditioning on rs200697179 (p.R642G), no other SNP in the region was associated with serum potassium levels (P>0.05) (Supplemental Table 2). We further evaluated the association of p.R642G with potassium levels in males and females separately, using a sex-stratified model. The reduction (from noncarriers) in potassium levels of p.R642G heterozygous carriers was observed in both males and females (P=2.28×10−5 and P=8.22×10−6, respectively) (Figure 3).
Biochemical Features of p.R642G SLC12A3 Heterozygotes
In addition to the effect on serum potassium, individuals inheriting one copy of p.R642G had significantly lower serum Cl levels (103.02±0.18 versus 103.48±0.028 mmol/L; P=7.93×10−4), lower BUN levels (13.84±0.34 versus 15.11±0.058 mmol/L; P=9.99×10−4), and nominally higher serum bicarbonate levels (24.88±0.20 versus 24.35±0.032 mmol/L; P=1.09×10−2) than noncarriers (Table 2). There were no statistically significant differences between heterozygous carriers and noncarriers in serum Na, creatinine, eGFR, calcium, or glucose (Table 2).
Table 2.
Association results of p.R642G SLC12A3 with biochemical and clinical features
| Trait | CC Genotype (Noncarriers) | CG Genotype (pR642G Heterozygotes) | Effect size (β) | P-value | ||
|---|---|---|---|---|---|---|
| Number | Mean±SEM | Number | Mean±SEM | |||
| Potassium (mmol/L) | 5663 | 4.20±0.004 | 147 | 4.05±0.02 | −0.15 | 2.39×10−9 |
| Cl (mmol/L) | 5663 | 103.48±0.03 | 147 | 103.02±0.18 | −0.66 | 7.93×10−4 |
| BUN (mg/dl) | 5663 | 15.11±0.06 | 147 | 13.84±0.34 | −1.16 | 9.99×10−4 |
| Bicarbonate (mmol/L) | 5473 | 24.35±0.03 | 137 | 24.88±0.20 | 0.56 | 1.09×10−2 |
| Na (mmol/L) | 5662 | 138.46±0.03 | 147 | 138.76±0.17 | 0.15 | 0.39 |
| Creatinine | 5681 | 0.77±0.002 | 147 | 0.76±0.012 | −0.0046 | 0.73 |
| eGFR (ml/min per 1.73 m2) | 5473 | 106.35±0.32 | 137 | 104.61±1.98 | −1.05 | 0.60 |
| Calcium (mg/dl) | 5676 | 9.32±0.005 | 147 | 9.34±0.029 | 0.030 | 0.36 |
| Glucose (mg/dl) | 5495 | 86.09±0.21 | 146 | 84.55±0.74 | −2.18 | 0.11 |
| SBP (mm Hg) | 5904 | 115.31±0.21 | 153 | 116.14±1.29 | −0.54 | 0.67 |
| DBP (mm Hg) | 5904 | 71.11±0.13 | 153 | 71.98±0.77 | −0.27 | 0.74 |
| Heart rate (beats/min) | 5407 | 64.94±0.13 | 137 | 65.45±0.97 | 0.78 | 0.39 |
| Height (m) | 5916 | 166.54±0.12 | 153 | 166.46±0.74 | 0.80 | 0.17 |
| Weight (kg) | 5913 | 73.87±0.19 | 153 | 73.96±1.14 | 1.38 | 0.28 |
| Waist circumference (cm) | 5644 | 88.68±0.16 | 143 | 88.13±0.95 | −0.24 | 0.81 |
| Hip circumference (cm) | 5639 | 102.27±0.14 | 143 | 103.55±0.90 | 1.19 | 0.20 |
| Bone fracture history (%) | 4856 | 6.75±0.36 | 124 | 12.9±3.02 | 0.062 | 9.89×10−3 |
SBP, systolic BP; DBP, diastolic BP.
Clinical Features of p.R642G SLC12A3 Heterozygotes
There were no statistically significant differences in diastolic or systolic BP, heart rate, height, weight, waist circumference, or hip circumference (Table 2). However, self-reported bone fracture rate was nominally higher in heterozygous carriers than noncarriers (12.9% [16/108] versus 6.75% [328/4528]; odds ratio, 2.05; P=9.89×10−3) (Table 2).
Response of p.R642G SLC12A3 to Low-Salt Intake
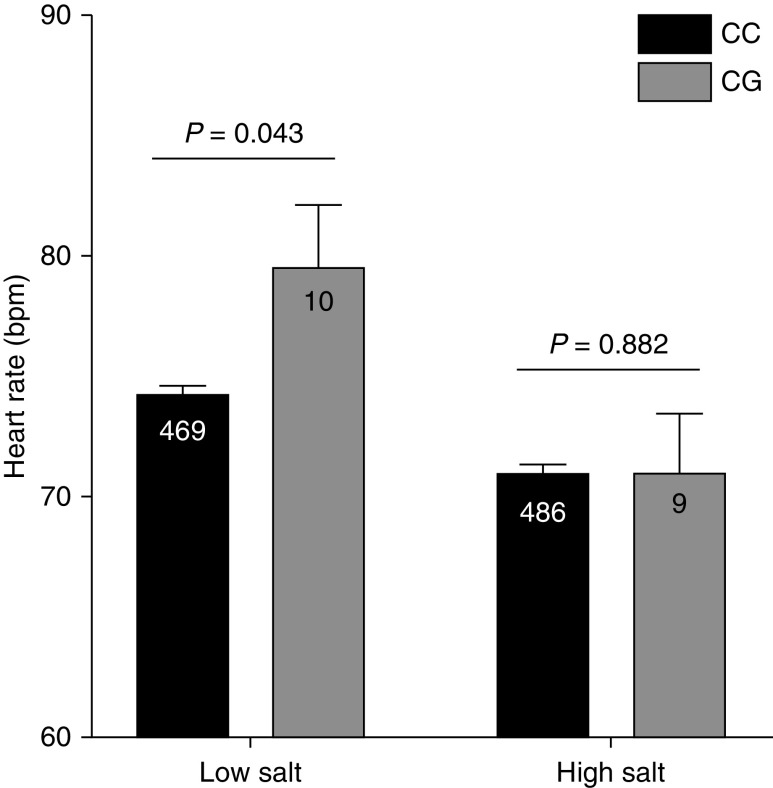
We investigated the hemodynamic response to 6 days of high-salt (300 mmol Na per day) and low-salt (40 mmol Na per day) diets. There were no significant differences in BP between pR642G heterozygotes and noncarriers on either diet (Supplemental Figure 1). However, on a low-salt diet, p.R642C heterozygotes had nominally higher resting heart rates than noncarriers (79.7±0.37 versus 74.2±2.41 bpm, respectively; P=0.043), whereas no significant difference in heart rate was observed in the same subjects in response to the high-salt diet, suggesting the increased heart rate on the low-salt diet may be a compensatory change for mild hypovolemia (Figure 4).
Figure 4.
Heterozygous carriers of p.R642G SLC12A3 (CG) have a nominally higher resting heart rate than noncarriers (CC) on a low-salt diet, but there is no significant difference in heart rate in the same subjects on a high-salt diet.
Discussion
In this study, we used an OOA cohort to investigate the effects of genetic components on serum potassium levels. We first estimated genetic influences on serum potassium in 5812 subjects and found that the heritability of serum potassium in the OOA was 18.25%. In agreement with our results, Pilia et al.13 found a heritability of 18.5% in 6148 Sardinians. Previous studies have estimated heritability of serum potassium to be 17%–60%.11–15 The variation in heritability estimates may be due to differences inherent to the ethnic group investigated, and in study design. For example, heritability estimates from twin studies are often higher than those observed in population-based studies. We also observed that the heritability of serum potassium was higher in males than in females, which is consistent with previous findings.11,13 The lower heritability in females may be due to sex-specific physiologic differences, such as the effects of hormone fluctuations in females.
Our ExWAS identified rs200697179, c.1924C>G, p.R642G in SLC12A3 to be highly enriched, through genetic drift, in the OOA and to be significantly associated with lower serum potassium levels genome wide. This finding supports the notion that pathogenic variants, known to cause many canonically recessive disorders, also contribute to complex traits. This understanding will help to generate a holistic biologic understanding of the genetic architecture of human diseases. To our knowledge, this is the largest study of heterozygous carriers for a pathogenic variant in SLC12A3, which causes autosomal recessive Gitelman syndrome. It provides the opportunity for a comprehensive assessment of the effects of heterozygosity of SLC12A3 on electrolyte homeostasis and related phenotypes.
The SLC12A3 gene encodes a thiazide-sensitive NCC protein expressed in the distal convoluted tubule, where it is responsible for the reabsorption of 5%–10% of filtered Na and Cl.1 NCC is the site of action of the thiazide diuretics (which are one of the most effective and widely used antihypertensive drugs) that act by inhibiting NCC transport activity, thus promoting the excretion of Na and Cl.24 The SLC12A3 mutation, p.R642G, is a known pathogenic variant that causes Gitelman syndrome in homozygotes or compound heterozygotes.23 However, whether heterozygotes of pathogenic variants in SLC12A3 have an intermediate phenotype has not been conclusively demonstrated. Indeed, previous studies have not shown differences in serum potassium in heterozygous carriers of pathogenic SLC12A3 variants, most likely due to an inadequate number of heterozygous carriers and low power.25,26 Ellison et al.27 reviewed heterozygous data from human and animal studies and pointed out that intermediate phenotypes may be present in SLC12A3 heterozygotes, although the reported differences were not statistically significant. Through a founder effect and genetic drift, the p.R642G variant is highly enriched in the OOA, thus providing among the largest cohorts of subjects heterozygous for the same pathogenic mutation in SLC12A3. With this large sample, we robustly observed that heterozygous carriers of p.R642G had significantly lower serum potassium levels than noncarriers, but these lower levels were less severe than that seen in Gitelman syndrome.23,24,28
Furthermore, we observed that mean serum Cl levels were significantly lower in p.R642G carriers than noncarriers, whereas the mean serum Na levels were not different (Supplemental Table 3). The underlying mechanism may be that heterozygosity for NCC function causes decreased Na and Cl reabsorption in the early distal convoluted tubule, which activates the renin-angiotensin system, increasing Na reabsorption via the epithelial Na channel in the distal nephron. Previous studies have shown that decreased Na and Cl reabsorption in the early distal convoluted tubule may lead to increased fractional excretion of Cl.28 We also observed a mild elevation in serum bicarbonate levels in heterozygous carriers, suggestive of mild metabolic alkalosis, as is often observed in patients with Gitelman syndrome.
Ji et al.29 investigated the association of rare, recessive mutations in renal salt-handling genes with hypertension and found heterozygous mutations in genes that alter renal salt handling are associated with BP variation in the general population. Despite finding heterozygosity for p.R642G SLC12A3 was associated with serum potassium, we did not observe statistically significant differences in diastolic or systolic BP or heart rate between heterozygous carriers and noncarriers. Because BP in this population is generally low, counter-regulatory mechanisms to defend against hypotension may mask any effect of p.R642G on BP. Indeed, Cruz et al.23 previously reported that Amish individuals heterozygous for SLC12A3 mutations self-selected higher salt intake to compensate, presumably, for a “mild salt-wasting defect.” Moreover, we observed that, on a low-salt diet, heterozygous p.R642G carriers had nominally higher resting heart rates compared with noncarriers, without a change in BP, suggesting increased heart rate as a compensatory change for mild hypovolemia. Previous studies have reported that a low-salt diet increases renal NCC expression and its phosphorylation to maintain Na and Cl homeostasis.30,31 These findings support an important role of genetic and environmental regulation of NCC on Na balance and hemodynamics.
To our knowledge, our data show, for the first time, that mean serum BUN levels are significantly lower in heterozygous carriers of pathogenic variants in SLC12A3 than in noncarriers. The mean serum creatinine and eGFR were not significantly different between p.R642G heterozygous carriers and noncarriers. The mechanism for lower serum BUN levels is not clear. The main causes of a decrease in BUN are decreased protein catabolic rate (as seen in severe liver disease and malnutrition), hypervolemia, and increased urine flow rate. The heterozygous carriers in our cohort did not suffer from severe liver disease or malnutrition (data not shown). Our results did provide evidence that, on a low-salt diet, heterozygous p.R642G carriers may have mild hypovolemia. As mentioned previously, on an unrestricted diet Amish individuals heterozygous for SLC12A3 mutations self-select higher salt and water intake, presumably to compensate for mild hypovolemia.23 How this observation may affect BUN is unclear. Morris et al.32 demonstrated a dramatic increase in urine flow when mice lacking NCC were given a potassium-restricted diet. Increased urine flow rate may contribute to BUN secretion. In addition, decreased NCC function leads to increased excretion of potassium ions (K+) and hydrogen ions into the distal tubule, which could facilitate ammonia secretion.33 Ammonium ions have the same hydrated ionic radius as K+ and can substitute for K+ in the Na+/K+-ATPase transporter and the Na+/2Cl/K+ cotransporter. Ammonium ions also interact with the hydrogen ion/Na+ exchanger, substituting for Na+.33 In these ways, increased ammonia secretion may contribute to lower serum BUN levels. Further investigation is required to identify the precise molecular mechanisms.
Previous studies have reported that patients with Gitelman syndrome have chondrocalcinosis, sclerochoroidal calcifications, increased renal calcium reabsorption, decreased rate of bone remodeling, and normal/low serum calcium levels.34–36 In our study, serum calcium levels were not significantly different between p.R642G heterozygous carriers and noncarriers. However, we found that p.R642G heterozygous carriers had a two-fold higher rate of self-reported bone fractures compared with noncarriers. The underlying mechanisms of this observation are not clear. Although the prevalence of p.R642G was higher in the OOA cohort, heterozygous individuals still made up a small group for evaluation of fracture rate. Therefore, further investigation of this phenotype is warranted.
Our results implicate rare pathogenic mutations in SLC12A3, causing autosomal recessive Gitelman syndrome, contribute in their heterozygous state to potassium variation. The prevalence of autosomal recessive Gitelman syndrome in the general European White population is 1:40,000. According to the Hardy–Weinberg equilibrium, this frequency would predict that approximately one in 100 individuals are heterozygous for pathogenic mutations in SLC12A3. Recently, Blanchard et al.26 analyzed the latest release of the gnomAD database version 2.1 and estimated a general population frequency of 3.6% for SLC12A3 heterozygosity (1.14% for well-established pathogenic mutations and 2.46% for low-frequency, nonclassified variants). The measured frequency in a Chinese population was estimated to be 3%.37 Although no heterozygous individuals had clinically defined hypokalemia, the modest reduction in serum potassium may have a protective health benefit from hypertension; however, they may be at increased risk for hypokalemia and its consequences in response to low-potassium dietary or therapeutic interventions. Additional studies will be required to better understand the implications of our findings toward personalized medicine and nutrition. Furthermore, intermediate phenotypic effects have been reported in heterozygotes for mutations in genes for Mendelian recessive forms of BP, HDL-cholesterol, and autism spectrum.29,38,39 These findings support the growing realization that the pathogenic variants causing many canonically recessive disorders contribute to complex traits and help to generate a more holistic biologic understanding of the genetic architecture of human diseases.
Our study has limitations. In this study, 24-hour urine samples were not available for more quantitative assessment of urinary electrolyte secretion. Fracture data were self-reported and, thus, the observed increase in fractures in p.R642G carriers will require further validation. Another potential limitation is that we do not have longer follow-up data on health outcomes of p.R642G heterozygotes. Further long-term data on clinical consequences of p.R642G heterozygotes are needed to better inform prognosis and management. Finally, while functional analyses have been published regarding similar mutations, including p.R642H and p.R642C, showing aberrant mRNA splicing,40,41 further investigation of the functional consequences of p.R642G is warranted.
In summary, we identified a locus on chromosome 16q13 that showed significant association with serum potassium levels in OOA. We identified a highly drifted, known autosomal recessive pathogenic variant (p.R642G) in SLC12A3 as the most likely causative variant, despite there being no homozygotes in our sample. Access to the largest cohort, to date, of subjects heterozygous for the same autosomal recessive pathogenic variant in SLC12A3 provided the opportunity to carry out a comprehensive assessment of the effects of heterozygosity of SLC12A3 on electrolytic homeostasis and related phenotypes. We estimate that 1% of the general population may carry pathogenic variants in SLC12A3 and, thus, these findings may be of relevance to electrolyte homeostasis and dysregulation in the general population, with clinical implications. Furthermore, our findings support the notion that heterozygosity for pathogenic variants for autosomal recessive disorders contribute to the genetic architecture of complex traits.
Disclosures
A. Baras, A.R. Shuldiner, C. Van Hout, J. Reid, and J. Overton report having ownership interest in, and receiving research funding from, Regeneron Pharmaceuticals. A. Baras, J. Overton, J. Reid, A.R. Shuldiner, and C. Van Hout are employees of, and receive compensation from, Regeneron Pharmaceuticals. Y. Li and X. Wan report receiving honoraria from The First Affiliated Hospital of Sun Yat-sen University. B.D. Mitchell and J. Perry reports receiving research funding from Regeneron Pharmaceuticals. J. Reid reports being a scientific advisor for or member of UK Biobank. All remaining authors have nothing to disclose.
Funding
The cohort studies were supported by National Heart, Lung, and Blood Institute grants HL69313, HL088119, and HL72515; National Institute of Diabetes and Digestive and Kidney Diseases grants DK54261 and P30 DK072488; National Institute on Aging grant AG1872801; National Institute of Arthritis and Musculoskeletal and Skin Diseases grant AR046838; American Heart Association grant 17GRNT33440151; Regeneron Pharmaceuticals; and the University of Maryland School of Medicine Program for Personalized and Genomic Medicine.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the support of the families participating in these studies, and the Amish Research Clinic Staff for their great efforts in study subject recruitment and characterization.
Dr. Alan R. Shuldiner and Dr. Mao Fu contributed to the design of study; Dr. Xuesi Wan, Dr. James Perry, Ms. Haichen Zhang, Dr. Feng Jin, Ms. Kathleen A. Ryan, Dr. Cristopher Van Hout, Dr. Zhe Han, Dr. Yanbing Li, and Dr. Mao Fu performed data acquisition and analysis; Dr. Xuesi Wan, Dr. Elizabeth Streeten, Dr. Braxton D. Mitchell, Dr. Alan R. Shuldiner, and Dr. Mao Fu drafted the manuscript; Dr. Aris Baras, Dr. John Overton, Dr. Jeffrey Reid, and the Regeneron Genetics Center contributed expertise in exome sequencing and genome informatics. All authors contributed to the interpretation of the results and participated in revision of the manuscript. All authors read and approved the final manuscript. The corresponding authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: Regeneron Genetics Center, Goncalo Abecasis, Aris Baras, Michael Cantor, Giovanni Coppola, Aris Economides, John D. Overton, Jeffrey G. Reid, Alan Shuldiner, Christina Beechert, Caitlin Forsythe, Erin D. Fuller, Zhenhua Gu, Michael Lattari, Alexander Lopez, Thomas D. Schleicher, Maria Sotiropoulos Padilla, Karina Toledo, Louis Widom, Sarah E. Wolf, Manasi Pradhan, Kia Manoochehri, Ricardo H. Ulloa, Xiaodong Bai, Suganthi Balasubramanian, Leland Barnard, Andrew Blumenfeld, Yating Chai, Gisu Eom, Lukas Habegger, Young Hahn, Alicia Hawes, Shareef Khalid, Evan K. Maxwell, John Penn, Jeffrey C. Staples, Ashish Yadav, Paloma M. Guzzardo, Marcus B. Jones, and Lyndon J. Mitnaul
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020071030/-/DCSupplemental.
Supplemental Table 1. The top ExWAS associations with serum potassium levels (P<10–3).
Supplemental Table 2. Association results of top SNPs with potassium levels adjusted for rs200697179.
Supplemental Table 3. Main phenotypes in p.R642G heterozygotes and Gitelman syndrome patients.
Supplemental Figure 1. No significant difference in blood pressure between carriers and noncarriers of p.R642G in either low salt or high salt diet intervention.
References
- 1.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, et al.: Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015. 10.1016/j.cmet.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Donnell M, Mente A, Rangarajan S, McQueen MJ, O’Leary N, Yin L, et al.; PURE Investigators: Joint association of urinary sodium and potassium excretion with cardiovascular events and mortality: Prospective cohort study. BMJ 364: l772, 2019. 10.1136/bmj.l772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watanabe D, Yatabe M, Ichihara A: Evaluation of insulin sensitivity and secretion in primary aldosteronism. Clin Exp Hypertens 38: 613–617, 2016. 10.1080/10641963.2016.1182176 [DOI] [PubMed] [Google Scholar]
- 4.He FJ, MacGregor GA: Beneficial effects of potassium on human health. Physiol Plant 133: 725–735, 2008 [DOI] [PubMed] [Google Scholar]
- 5.He FJ, MacGregor GA: Fortnightly review: Beneficial effects of potassium. BMJ 323: 497–501, 2001. 10.1136/bmj.323.7311.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson-Hughes B, Harris SS, Palermo NJ, Gilhooly CH, Shea MK, Fielding RA, et al.: Potassium bicarbonate supplementation lowers bone turnover and calcium excretion in older men and women: A randomized dose-finding trial. J Bone Miner Res 30: 2103–2111, 2015. 10.1002/jbmr.2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vodnala SK, Eil R, Kishton RJ, Sukumar M, Yamamoto TN, Ha NH, et al.: T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 363: eaau0135, 2019. 10.1126/science.aau0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins AJ, Pitt B, Reaven N, Funk S, McGaughey K, Wilson D, et al.: Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol 46: 213–221, 2017. 10.1159/000479802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alderman MH, Piller LB, Ford CE, Probstfield JL, Oparil S, Cushman WC, et al.; Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group: Clinical significance of incident hypokalemia and hyperkalemia in treated hypertensive patients in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Hypertension 59: 926–933, 2012. 10.1161/HYPERTENSIONAHA.111.180554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gumz ML, Rabinowitz L, Wingo CS: An integrated view of potassium homeostasis. N Engl J Med 373: 60–72, 2015. 10.1056/NEJMra1313341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moulin F, Ponte B, Pruijm M, Ackermann D, Bouatou Y, Guessous I, et al.: A population-based approach to assess the heritability and distribution of renal handling of electrolytes. Kidney Int 92: 1536–1543, 2017. 10.1016/j.kint.2017.06.020 [DOI] [PubMed] [Google Scholar]
- 12.Nilsson SE, Read S, Berg S, Johansson B: Heritabilities for fifteen routine biochemical values: Findings in 215 Swedish twin pairs 82 years of age or older. Scand J Clin Lab Invest 69: 562–569, 2009. 10.1080/00365510902814646 [DOI] [PubMed] [Google Scholar]
- 13.Pilia G, Chen WM, Scuteri A, Orrú M, Albai G, Dei M, et al.: Heritability of cardiovascular and personality traits in 6,148 Sardinians. PLoS Genet 2: e132, 2006. 10.1371/journal.pgen.0020132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitfield JB, Martin NG: The effects of inheritance on constituents of plasma: A twin study on some biochemical variables. Ann Clin Biochem 21: 176–183, 1984. 10.1177/000456328402100303 [DOI] [PubMed] [Google Scholar]
- 15.Whitfield JB, Martin NG: Genetic and environmental causes of variation in renal tubular handling of sodium and potassium: A twin study. Genet Epidemiol 2: 17–27, 1985. 10.1002/gepi.1370020103 [DOI] [PubMed] [Google Scholar]
- 16.Meyer TE, Verwoert GC, Hwang SJ, Glazer NL, Smith AV, van Rooij FJ, et al.; Genetic Factors for Osteoporosis Consortium; Meta Analysis of Glucose and Insulin Related Traits Consortium: Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six Loci influencing serum magnesium levels. PLoS Genet 6: e1001045, 2010. 10.1371/journal.pgen.1001045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell BD, McArdle PF, Shen H, Rampersaud E, Pollin TI, Bielak LF, et al.: The genetic response to short-term interventions affecting cardiovascular function: Rationale and design of the Heredity and Phenotype Intervention (HAPI) Heart Study. Am Heart J 155: 823–828, 2008. 10.1016/j.ahj.2008.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Streeten EA, McBride DJ, Pollin TI, Ryan K, Shapiro J, Ott S, et al.: Quantitative trait loci for BMD identified by autosome-wide linkage scan to chromosomes 7q and 21q in men from the Amish Family Osteoporosis Study. J Bone Miner Res 21: 1433–1442, 2006. 10.1359/jbmr.060602 [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Salimi S, Deng Z, Perry J, Ryan KA, Li Z, et al.: Evaluation of WISP1 as a candidate gene for bone mineral density in the Old Order Amish. Sci Rep 8: 7141, 2018. 10.1038/s41598-018-25272-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis JP, Ryan K, O’Connell JR, Horenstein RB, Damcott CM, Gibson Q, et al.: Genetic variation in PEAR1 is associated with platelet aggregation and cardiovascular outcomes. Circ Cardiovasc Genet 6: 184–192, 2013. 10.1161/CIRCGENETICS.111.964627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raheja UK, Fuchs D, Lowry CA, Stephens SH, Pavlovich MA, Mohyuddin H, et al.: Heritability of plasma neopterin levels in the Old Order Amish. J Neuroimmunol 307: 37–41, 2017. 10.1016/j.jneuroim.2017.02.016 [DOI] [PubMed] [Google Scholar]
- 22.Dewey FE, Gusarova V, O’Dushlaine C, Gottesman O, Trejos J, Hunt C, et al.: Inactivating variants in ANGPTL4 and risk of coronary artery disease. N Engl J Med 374: 1123–1133, 2016. 10.1056/NEJMoa1510926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruz DN, Simon DB, Nelson-Williams C, Farhi A, Finberg K, Burleson L, et al.: Mutations in the Na-Cl cotransporter reduce blood pressure in humans. Hypertension 37: 1458–1464, 2001. 10.1161/01.hyp.37.6.1458 [DOI] [PubMed] [Google Scholar]
- 24.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, et al.: Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996. 10.1038/ng0196-24 [DOI] [PubMed] [Google Scholar]
- 25.Fava C, Montagnana M, Rosberg L, Burri P, Almgren P, Jönsson A, et al.: Subjects heterozygous for genetic loss of function of the thiazide-sensitive cotransporter have reduced blood pressure. Hum Mol Genet 17: 413–418, 2008. 10.1093/hmg/ddm318 [DOI] [PubMed] [Google Scholar]
- 26.Blanchard A, Vallet M, Dubourg L, Hureaux M, Allard J, Haymann JP, et al.: Resistance to insulin in patients with Gitelman syndrome and a subtle intermediate phenotype in heterozygous carriers: A cross-sectional study. J Am Soc Nephrol 30: 1534–1545, 2019. 10.1681/ASN.2019010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellison DH, Lin SH: Enemy action in the distal convoluted tubule. J Am Soc Nephrol 30: 1345–1348, 2019. 10.1681/ASN.2019050475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filippatos TD, Rizos CV, Tzavella E, Elisaf MS: Gitelman syndrome: An analysis of the underlying pathophysiologic mechanisms of acid-base and electrolyte abnormalities. Int Urol Nephrol 50: 91–96, 2018. 10.1007/s11255-017-1653-4 [DOI] [PubMed] [Google Scholar]
- 29.Ji W, Foo JN, O’Roak BJ, Zhao H, Larson MG, Simon DB, et al.: Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet 40: 592–599, 2008. 10.1038/ng.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S: Expression and phosphorylation of the Na+-Cl- cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol 297: F704–F712, 2009. 10.1152/ajprenal.00030.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moes AD, van der Lubbe N, Zietse R, Loffing J, Hoorn EJ: The sodium chloride cotransporter SLC12A3: New roles in sodium, potassium, and blood pressure regulation. Pflugers Arch 466: 107–118, 2014. 10.1007/s00424-013-1407-9 [DOI] [PubMed] [Google Scholar]
- 32.Morris RG, Hoorn EJ, Knepper MA: Hypokalemia in a mouse model of Gitelman’s syndrome. Am J Physiol Renal Physiol 290: F1416–F1420, 2006. 10.1152/ajprenal.00421.2005 [DOI] [PubMed] [Google Scholar]
- 33.Wright PA: Nitrogen excretion: Three end products, many physiological roles. J Exp Biol 198: 273–281, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Calò L, Punzi L, Semplicini A: Hypomagnesemia and chondrocalcinosis in Bartter’s and Gitelman’s syndrome: Review of the pathogenetic mechanisms. Am J Nephrol 20: 347–350, 2000. 10.1159/000013614 [DOI] [PubMed] [Google Scholar]
- 35.Hsu YJ, Yang SS, Cheng CJ, Liu ST, Huang SM, Chau T, et al.: Thiazide-sensitive Na+ -Cl- cotransporter (NCC) gene inactivation results in increased duodenal Ca2+ absorption, enhanced osteoblast differentiation and elevated bone mineral density. J Bone Miner Res 30: 116–127, 2015. 10.1002/jbmr.2306 [DOI] [PubMed] [Google Scholar]
- 36.Nicolet-Barousse L, Blanchard A, Roux C, Pietri L, Bloch-Faure M, Kolta S, et al.: Inactivation of the Na-Cl co-transporter (NCC) gene is associated with high BMD through both renal and bone mechanisms: Analysis of patients with Gitelman syndrome and Ncc null mice. J Bone Miner Res 20: 799–808, 2005. 10.1359/JBMR.041238 [DOI] [PubMed] [Google Scholar]
- 37.Hsu YJ, Yang SS, Chu NF, Sytwu HK, Cheng CJ, Lin SH: Heterozygous mutations of the sodium chloride cotransporter in Chinese children: Prevalence and association with blood pressure. Nephrol Dial Transplant 24: 1170–1175, 2009. 10.1093/ndt/gfn619 [DOI] [PubMed] [Google Scholar]
- 38.Cohen JC, Kiss RS, Pertsemlidis A, Marcel YL, McPherson R, Hobbs HH: Multiple rare alleles contribute to low plasma levels of HDL cholesterol. Science 305: 869–872, 2004. 10.1126/science.1099870 [DOI] [PubMed] [Google Scholar]
- 39.Doan RN, Lim ET, De Rubeis S, Betancur C, Cutler DJ, Chiocchetti AG, et al.; Autism Sequencing Consortium: Recessive gene disruptions in autism spectrum disorder. Nat Genet 51: 1092–1098, 2019. 10.1038/s41588-019-0433-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lo YF, Nozu K, Iijima K, Morishita T, Huang CC, Yang SS, et al.: Recurrent deep intronic mutations in the SLC12A3 gene responsible for Gitelman’s syndrome. Clin J Am Soc Nephrol 6: 630–639, 2011. 10.2215/CJN.06730810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riveira-Munoz E, Chang Q, Godefroid N, Hoenderop JG, Bindels RJ, Dahan K, et al.; Belgian Network for Study of Gitelman Syndrome: Transcriptional and functional analyses of SLC12A3 mutations: New clues for the pathogenesis of Gitelman syndrome. J Am Soc Nephrol 18: 1271–1283, 2007. 10.1681/ASN.2006101095 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.