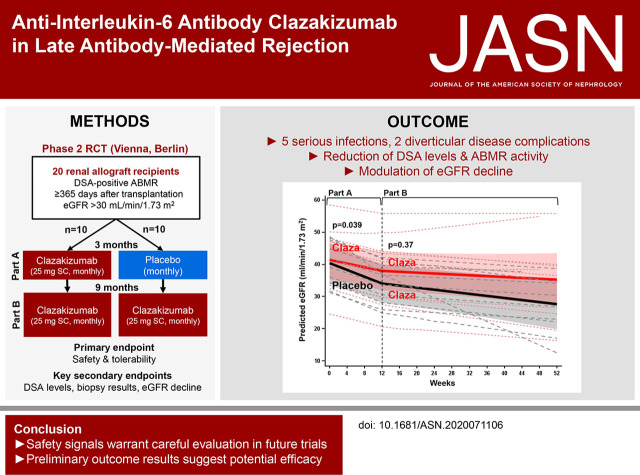
Significance Statement
There is no proven effective treatment for a major cause of graft failure, late antibody-mediated rejection, but IL-6, a cytokine known to promote B cell immunity, may be a promising therapeutic target. The authors describe the results of a phase 2 randomized clinical trial involving 20 patients, designed to evaluate the safety (primary endpoint) and efficacy (secondary endpoint analysis) of an anti–IL-6 antibody, clazakizumab, versus placebo in late antibody-mediated rejection. Although the occurrence of serious infections and diverticulitis presented important safety signals, clazakizumab was associated with an early decrease in donor-specific antibody levels, modulated antibody-mediated rejection activity, and slowed the decline of renal function. Preliminary efficacy results suggest a potentially beneficial effect of clazakizumab and may therefore support the design of larger trials with a longer duration of follow-up.
Keywords: antibody-mediated rejection, chronic rejection, interleukin-6, monoclonal antibody, renal transplantation, randomized controlled trial
Visual Abstract
Abstract
Background
Late antibody-mediated rejection (ABMR) is a leading cause of transplant failure. Blocking IL-6 has been proposed as a promising therapeutic strategy.
Methods
We performed a phase 2 randomized pilot trial to evaluate the safety (primary endpoint) and efficacy (secondary endpoint analysis) of the anti–IL-6 antibody clazakizumab in late ABMR. The trial included 20 kidney transplant recipients with donor-specific, antibody-positive ABMR ≥365 days post-transplantation. Patients were randomized 1:1 to receive 25 mg clazakizumab or placebo (4-weekly subcutaneous injections) for 12 weeks (part A), followed by a 40-week open-label extension (part B), during which time all participants received clazakizumab.
Results
Five (25%) patients under active treatment developed serious infectious events, and two (10%) developed diverticular disease complications, leading to trial withdrawal. Those receiving clazakizumab displayed significantly decreased donor-specific antibodies and, on prolonged treatment, modulated rejection-related gene-expression patterns. In 18 patients, allograft biopsies after 51 weeks revealed a negative molecular ABMR score in seven (38.9%), disappearance of capillary C4d deposits in five (27.8%), and resolution of morphologic ABMR activity in four (22.2%). Although proteinuria remained stable, the mean eGFR decline during part A was slower with clazakizumab compared with placebo (−0.96; 95% confidence interval [95% CI], −1.96 to 0.03 versus −2.43; 95% CI, −3.40 to −1.46 ml/min per 1.73 m2 per month, respectively, P=0.04). During part B, the slope of eGFR decline for patients who were switched from placebo to clazakizumab improved and no longer differed significantly from patients initially allocated to clazakizumab.
Conclusions
Although safety data indicate the need for careful patient selection and monitoring, our preliminary efficacy results suggest a potentially beneficial effect of clazakizumab on ABMR activity and progression.
Late antibody-mediated rejection (ABMR) is a common cause of kidney allograft failure.1 Its molecular mechanisms are increasingly understood, and diagnostic criteria have been refined continuously.2,3 Treating late ABMR, however, remains a significant challenge.4,5 Systematic trials have failed to demonstrate any benefit of therapies widely used for desensitization and acute ABMR, such as rituximab plus intravenous Ig6 or bortezomib.7 Consequently, there is a high unmet need for an effective treatment.4,5
One promising therapeutic target is IL-6, a cytokine that is critically involved in the regulation of inflammation and immune cell differentiation.8 Over the last decade, the concept of targeting IL-6 or its receptor (IL-6R) has entered clinical routine, and is well established in rheumatoid arthritis.9–11 More recently, IL-6/IL-6R interference has also become of interest in organ transplantation.8 An observational study evaluating anti–IL-6R antibody tocilizumab in chronic ABMR has suggested stabilization of allograft function, presumably resulting from reduced levels of donor-specific antibody (DSA).12,13 The occurrence of graft losses in four recipients in whom tocilizumab was prematurely stopped,12 however, suggested a rebound effect, triggered by IL-6 accumulated on treatment.14 Thus, the use of antibodies that directly neutralize IL-6 may be of particular interest.
Clazakizumab is a humanized monoclonal IgG1 antibody with high affinity for IL-6 and a long t1/2 of approximately 30 days. This antibody has been systematically evaluated in rheumatoid and psoriatic arthritis,10,15 but has not yet been approved for clinical use. We designed this phase 2 pilot trial, the first randomized controlled trial evaluating IL-6 signaling blockade in transplantation, to assess the safety and tolerability (primary endpoint) as well as efficacy (secondary endpoint analysis) of clazakizumab in late ABMR.
Methods
Trial Oversight and Design
This investigator-driven, randomized, double-blind, placebo-controlled, parallel-group phase 2 pilot trial was conducted at two sites (Medical University of Vienna, Austria; Charité Universitätsmedizin Berlin, Germany) from January 2018 to April 2020 (recruitment period: January 2018 to April 2019). Details of the protocol have been described previously.16 We hypothesized that clazakizumab is a safe treatment to counteract ABMR progression. The study consisted of two parts (Supplemental Figure 1); a 12-week randomized placebo-controlled phase to decipher the short-term effects of treatment (part A), followed by a 40-week open-label extension where all participants received clazakizumab (part B). The rationale behind this design was to offer all participants the option of a potentially effective treatment. Furthermore, two follow-up biopsies to analyze short- and intermediate-term treatment effects were considered unacceptable for patients on long-term placebo treatment. The study was approved by the institutional review board of the Medical University of Vienna (EK1428/2017), the Berlin State Ethics Committee (17/0485– EK 15), and the regulatory authorities in Austria (Federal Office for Safety in Health Care, Austrian Agency for Health and Food Safety) and Germany (Federal Institute for Vaccines and Biomedicines, Paul-Ehrlich Institute). All patients provided written informed consent before study inclusion, and the study was conducted in accordance with International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice requirements, Good Laboratory Practice, the principles of the Declaration of Helsinki 2008, and the Declaration of Istanbul. The trial was registered on the European Union Drug Regulating Authorities Clinical Trials Database (2017–001604–30) and ClinicalTrials.gov (NCT03444103).
Participants
The trial was designed to include 20 patients (Figure 1, Supplemental Figure 1). Eligible participants were adult kidney transplant recipients (>18 years) with late active or chronic active ABMR ≥365 days after transplantation (with or without C4d deposits along the peritubular capillaries), associated with a molecular pattern of ABMR in gene array analysis, preformed or de novo HLA class I and/or II DSA, and an eGFR (CKD Epidemiology Collaboration equation) >30 ml/min per 1.73 m2. Exclusion criteria were age ≤18 years, participation in another clinical trial, pregnancy or breastfeeding, T cell–mediated rejection classified Banff grade ≥I, de novo or recurrent severe thrombotic microangiopathy, polyoma virus nephropathy, de novo or recurrent GN, acute rejection treatment <3 months before screening, acute deterioration of graft function (eGFR decline within 1–3 months >25%), nephrotic range proteinuria >3500 mg/g protein/creatinine ratio, active viral, bacterial, or fungal infection precluding intensified immunosuppression, active malignant disease precluding intensified immunosuppressive therapy, abnormal liver-function tests (alanine aminotransferase, aspartate aminotransferase, bilirubin >1.5× upper limit of normal), other significant liver disease, latent or active tuberculosis (positive QuantiFERON-TB-Gold test, chest x-ray), administration of a live vaccine within 6 weeks of screening, neutropenia (<1 G/L) or thrombocytopenia (<100 G/L), history of gastrointestinal perforation, diverticulitis, or inflammatory bowel disease, history of alcohol or illicit substance abuse, or a serious medical or psychiatric illness likely to interfere with participation in the study.
Figure 1.
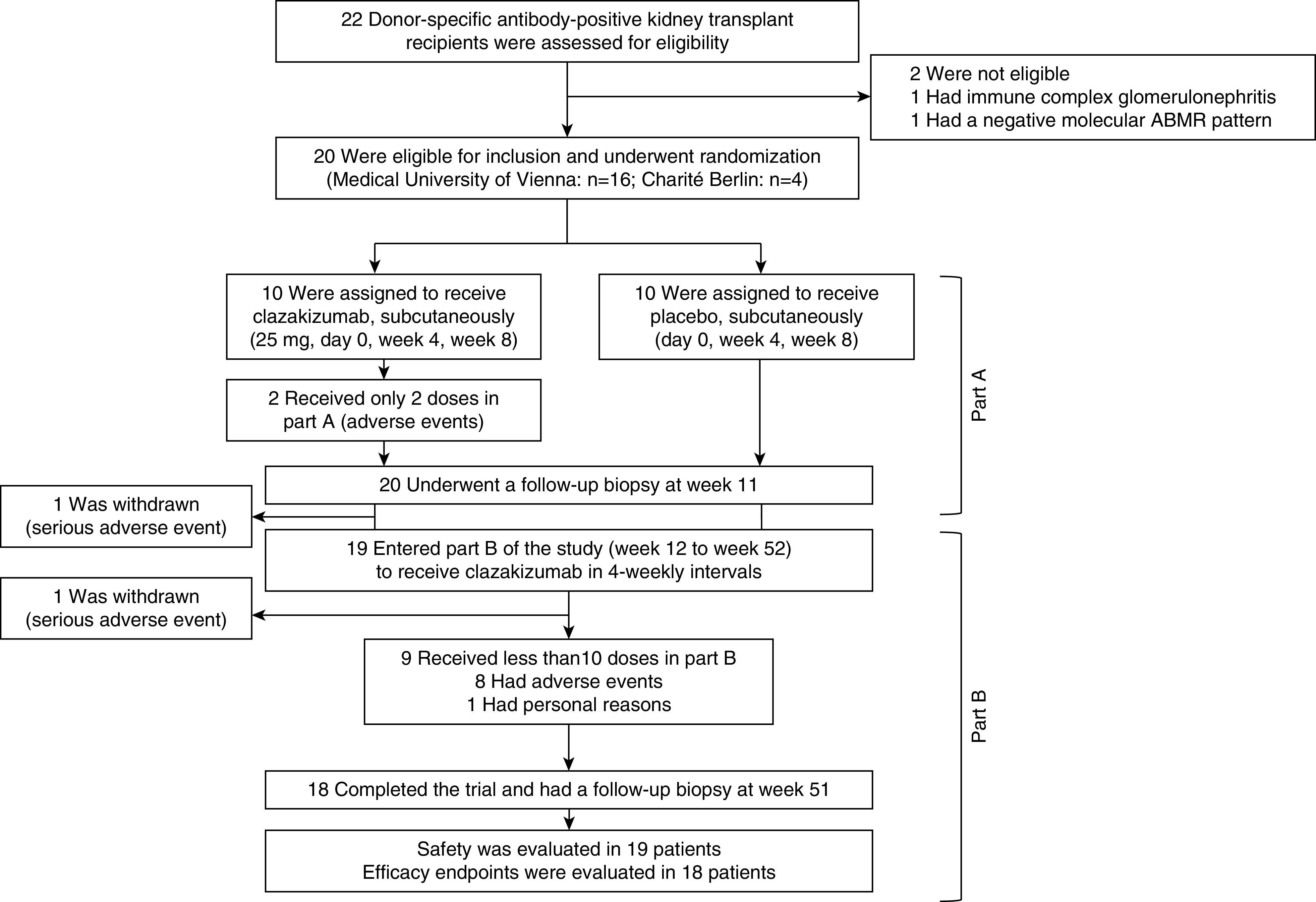
Trial flowchart.
Randomization
Patients were randomized 1:1 to one of the two study arms (clazakizumab versus placebo) in part A using a web-based randomization platform (www.meduniwien.ac.at/randomizer). Permuted block randomization with block sizes of two was stratified by study site (Vienna versus Berlin) and ABMR category (active ABMR versus chronic/active ABMR). Study participants, care providers, and those assessing outcomes were unaware of the randomization sequence. The allocation sequence was generated, and medication or placebo was prepared by independent nonblinded study pharmacists. Study physicians and nurses were provided with blinded subcutaneous medication. Subjects were enrolled and assigned to interventions by study physicians. The participating investigators, staff with medical interaction, and the study participants were blinded to group allocation until the last patient had completed part A. Treatment code envelopes were securely stored to be opened in case of emergency.
Study Medication
Clazakizumab (25 mg in 1 ml single-dose vials; Vitaeris Inc., Vancouver, Canada) and placebo (0.9% saline) were administered via subcutaneous injection in 4-weekly intervals. Due to adverse events (n=10) or personal reasons (n=1), 11 (55%) subjects did not receive all 13 scheduled clazakizumab injections (12 doses: n=3; 11 doses: n=2; ten doses: n=2; nine doses: n=1; eight doses: n=1; four doses: n=1; two doses: n=1). In accordance with a July 2019 trial amendment after two patients had serious gastrointestinal complications (diverticulitis; one patient requiring open surgery because of colon perforation), the dose of clazakizumab was reduced to 12.5 mg per injection in two other active patients with documented diverticulosis (last injection before completion of the study).
Immunosuppression
Tacrolimus, cyclosporin A, and everolimus doses were adjusted to trough levels of 5–10 ng/ml, 80–120 ng/ml, and 3–8 ng/ml, respectively. In total, 18 recipients were on calcineurin- or mTOR inhibitor–based triple immunosuppressive therapy, and two on dual therapy without steroids. The single patient on everolimus achieved levels within the target range throughout the study period (not shown). In one patient off steroids, prednisolone was initiated on trial inclusion. Dosages of steroids were not adjusted during the trial (prednisolone, 16 patients: 5 mg/d, n=15; 2.5 mg/day, n=1; methylprednisolone, four patients: 4 mg/d). The following medications were prohibited and not applied during the study: rituximab, eculizumab, proteasome inhibitors, intravenous Ig, plasma exchange, immunoadsorption, and other investigational drugs or treatments including commercially available anti–IL-6/IL-6R mAbs.
Endpoints
As effect sizes were unknown, the design of this pilot study—the first randomized trial evaluating clazakizumab in patients who had undergone a transplant—did not include sample size estimations. Trial visits were conducted at day 0 and weeks 1, 2, 3, 4, 8, 11, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48, 51, and 52. The primary endpoint was the assessment of safety and tolerability. Key secondary endpoints were the evolution of morphologic and molecular rejection patterns (index biopsy, week 11, and week 51 follow-up biopsies); DSA and non-DSA levels, IgG, IgM, and IgG subclass concentrations and Torque Teno virus (TTV) load (day 0, week 12, and week 52); the pharmacodynamics of clazakizumab (reduction of C-reactive protein, CRP); and the course of eGFR (CKD Epidemiology Collaboration), and protein/creatinine ratio (all visits).
Safety
Adverse events were closely monitored throughout the study and classified using the Medical Dictionary for Regulatory Activities Version 23.0. The study was monitored by an independent data and safety monitoring board (DSMB), and included two interim analyses, the first after 10 and the second after 20 subjects had completed part A of the study. The DSMB was instructed to consider stopping the trial if the pattern of related serious adverse events or safety laboratory results strongly supported a major safety signal. Exact statistical definitions of criteria for premature study termination were not defined. Stopping rules were defined as follows. Part A: if six subjects experience related (definitely and possibly) serious adverse events (common toxicity criteria > Grade 3 or severe/medically significant), and/or substantially elevated levels of liver parameters (alanine aminotransferase, aspartate aminotransferase, and/or bilirubin >3× upper limit of normal) or neutropenia (below 0.5 G/L), the DSMB would unblind the safety results. If five or all subjects were to be in the clazakizumab group, then the study would be stopped. Similarly, if six related serious adverse events and/or substantial abnormalities in liver enzymes or neutrophil counts occurred in the same system organ class, the DSMB would unblind these serious adverse events, and if five or all serious adverse events were in the clazakizumab group, then the study would be stopped. Part B: if ten or more subjects experienced drug-related serious adverse events or ten or more related serious adverse events occurred in the same system organ class, then the trial would be stopped (the total number of related serious adverse events should also include the related events occurring in the clazakizumab-treated subjects during part A).
Antibody Detection
For HLA antibody detection, LABscreen single-antigen flow-bead assays (One Lambda, Canoga Park, CA) were applied. Serum samples were incubated with EDTA (10 mM) to prevent complement interference. Data acquisition was performed via a LABScan 200 flow analyzer (Luminex Corporation, Austin, TX). For longitudinal analysis of DSA levels, bead assays were performed retrospectively (centralized analysis) to avoid influences of day-by-day variations in test results (test batches including samples from four to six patients each). Donor specificity was defined according to serological and/or low- or high-resolution donor/recipient HLA typing (HLA-A, -B, -Cw, -DR, -DQ, -DP on availability) provided either by the local HLA lab or the Eurotransplant database. Test results were documented as mean fluorescence intensity (MFI) of the immunodominant DSA. An MFI threshold >1000 was considered as positive. To estimate the effect of clazakizumab treatment on DSA levels, we documented the percent change in MFI. In an effort to quantify changes in DSA levels more accurately, we additionally performed dilution experiments after an earlier described protocol.17 In brief, nonlinear standard curves on the basis of raw DSA MFI levels (immunodominant DSA) were obtained by serial dilution of individual patient sera collected before the start of treatment (all samples were incubated with EDTA). According to computed standard curves, the fold change of antibody levels was then calculated from DSA MFI levels detected in the same experiment for undiluted week 12 and week 52 samples.
Total IgG, IgM, and IgG subclasses were assessed in serum applying immunonephelometry on a BN II analyzer (Siemens Healthineers, Erlangen, Germany).
Transplant Biopsies
The study included 20 index, 20 11-week follow-up, and 18 51-week follow-up biopsies. None of the patients underwent additional indication biopsies. Biopsies were performed using ultrasound-guided percutaneous techniques (1–2 cores per biopsy, 16-gauge needle). Histomorphology and C4d staining was evaluated on formalin-fixed paraffin-embedded sections. C4d in peritubular capillaries was scored as 0 (negative), 1 (minimal), 2 (focal), and 3 (diffuse), and a score ≥1 was considered positive. In total, 35 of the 58 study biopsies were additionally evaluated using electron microscopy to detect microcirculation injury. Morphologic results were read locally (Medical University of Vienna, Charité Universitätsmedizin Berlin) in a blinded fashion, following the rules of the Banff 2017 scheme. In addition, all biopsies were analyzed using a thoroughly validated molecular method (MMDx).18,19 For each biopsy, a 3 mm portion of one core was immediately placed in RNAlater, stored at −20°C, and shipped either at ambient temperature or on dry ice to the Alberta Transplant Applied Genomics Centre (University of Alberta, Edmonton, AB, Canada) for gene array analysis. Molecular scores on the basis of lesion-based classifiers related to rejection (ABMR, T cell–mediated rejection, all rejection), inflammation (global disturbance score), or chronic injury (atrophy/fibrosis score) were generated using a reference set of 1529 biopsies. After the 2017 update of the Banff classification,20 ABMR was defined and categorized on the basis of morphologic, immunohistochemical (C4d), ultrastructural (transplant glomerulopathy, multilayering of the peritubular capillary basement membranes), serological (DSA detection), and thoroughly validated molecular criteria (molecular ABMR score ≥0.2), respectively.
TTV Quantification
For TTV analysis, DNA was extracted from plasma (200 μl) using the NucliSENS easyMAG platform (bioMeriéux, France), and eluted in 50 μl of elution buffer. TTV DNA was quantitated by TaqMan real time PCR, according to earlier described protocols.21,22 The quantitative PCR reactions were performed in a volume of 25 µl using 2× TaqMan Universal PCR Master Mix, containing 5 µl of extracted DNA, 400 nM of each primer, and 80 nM of the probe. Thermal cycling was started for 3 minutes at 50°C, followed by 10 minutes at 95°C, and then by 45 cycles at 95°C for 15 seconds, at 55°C for 30 seconds, and at 72°C for 30 seconds, using the CFX96 Real-time System (Bio-Rad, Hercules, CA). Results were recorded as copies per ml.
Statistical Methods
For group comparisons we used Fisher’s exact, Mann–Whitney U, or Wilcoxon tests. GFR trajectories were analyzed using a linear mixed model with eGFR values from 0 to 12 weeks (day 0, week 1, 2, 3, 4, 8, 12) and from 12 to 52 weeks (week 12, 16, 20, 24, 28, 32, 36, 40, 44, 48, 52) as the dependent variable. Time, treatment, and their interaction were used as fixed effects. Furthermore, patient-specific random effects for intercept and slope were specified. Each patient’s trajectories were modeled as a hockey-stick spline with a fixed knot at 12 weeks, which is change of therapy for the placebo group. Due to the unevenly spaced time points, a spatial covariance matrix (power structure) was used. Intergroup differences were tested at a two-sided significance level of 5%. For statistical analysis, IBM SPSS Statistics version 24 (IBM Corporation, Armonk, NY) and SAS version 9.4. (The SAS Institute Inc., Cary, North Carolina) were applied.
Results
Patient Disposition and Characteristics
In total, 20 kidney transplant recipients (Vienna, n=16; Berlin, n=4) with DSA-positive ABMR after a median of 10.6 years post-transplantation were randomly assigned to clazakizumab or placebo (12-week randomized, placebo-controlled part A). In a subsequent 40-week open-label extension (part B), all participants received clazakizumab (Figure 1). Baseline characteristics are provided in Supplemental Tables 1 and 2, and Table 1. Ten (50%) patients were female, seven (35%) were retransplant recipients, and five (25%) had been subjected to desensitization because of preformed DSA and/or broad HLA reactivity.23 At study inclusion, 18 (90%) recipients had anti-HLA class II DSA (with or without HLA class I DSA), 15 (75%) against HLA-DQ. The immunodominant DSA MFI was 11,708 (median; interquartile range [IQR]: 1947–17,709). Two (10%) recipients had active and 18 (90%) chronic/active ABMR. Median eGFR and protein/creatinine ratios were 39.3 (IQR 33.6–49.7) ml/min per 1.73 m2 and 962 (IQR, 310–1863) mg/g, respectively. Important baseline variables relating to the severity of rejection, such as eGFR or morphologic results obtained in index biopsies were well balanced between patient groups. Despite stratified randomization, however, recipients allocated to placebo showed numerically higher median levels of DSA MFI, CRP levels, and proteinuria, whereas molecular rejection-related scores were higher in the clazakizumab arm (Supplemental Tables 1 and 2, Table 1).
Table 1.
Demographics and baseline characteristics
| Parameter | Total (n=20) | Clazakizumab (n=10) | Placebo (n=10) |
|---|---|---|---|
| Variables recorded at transplantation | |||
| Female sex, n (%) | 10 (50) | 3 (30) | 7 (70) |
| Recipient age (yr), median (IQR) | 34.2 (24.6–47.6) | 37.4 (27.1–57.9) | 31.4 (22.3–42.3) |
| Living donor, n (%) | 6 (30) | 3 (30) | 3 (30) |
| ABO-compatible transplant, n (%) | 20 (100) | 10 (100) | 10 (100) |
| Prior kidney transplant, n (%) | 7 (35) | 4 (40) | 3 (30) |
| Current CDC panel reactivity ≥10%, n (%)a | 6 (33.3) | 3 (33.3) | 3 (33.3) |
| Preformed anti-HLA DSA, n (%)b | 5 (45.5) | 3 (42.9) | 2 (50) |
| Donor age (yr), median (IQR)c | 49.0 (21.8–57.3) | 51.0 (21.8–57.3) | 44.0 (23.3–66.0) |
| HLA mismatch (A, B, DR), median (IQR)d | 3 (2–3) | 3 (3–3) | 3 (2–3) |
| Cold ischemia time (h), median (IQR)e | 13.0 (4.8–17.3) | 10.4 (3.4–14.6) | 15.5 (6.4–20.0) |
| Variables recorded at trial inclusion | |||
| Age of study patients (yr), median (IQR) | 41.5 (36.4–60.1) | 47.2 (38.7–62.1) | 39.6 (30.2–59.6) |
| Yr to inclusion in the trial | 10.6 (4.4–16.2) | 9.7 (4.1–16.7) | 11.4 (5.9–16.1) |
| eGFR (ml/min per 1.73 m2), median (IQR) | 39.3 (33.6–49.7) | 40.5 (33.3–49.8) | 39.2 (32.9–51.7) |
| Protein/creatinine ratio (mg/g), median (IQR) | 962 (310–1863) | 727 (197–1311) | 1387 (532–3575) |
| DSA characteristics | |||
| HLA class I DSA only, n (%) | 2 (10) | 1 (10) | 1 (10) |
| HLA class II DSA only, n (%) | 14 (70) | 7 (70) | 7 (70) |
| HLA class I and II DSA, n (%) | 4 (20) | 2 (20) | 2 (20) |
| Anti-DQ DSA, n (%) | 15 (75) | 7 (70) | 8 (80) |
| No. of DSA, median (IQR) | 1 (1–2) | 1 (1–2) | 1 (1–2) |
| MFI of the peak DSA, median (IQR) | 11,708 (1947–17,709) | 10,789 (3092–15,437) | 14,207 (1252–19,144) |
| MFI sum of detected DSA, median (IQR) | 13,130 (2137–18,962) | 10,789 (4244–18,102) | 16,126 (1252–19,302) |
| Banff 2017 rejection categories | |||
| ABMR, n (%) | 20 (100) | 10 (100) | 10 (100) |
| Active ABMR, n (%) | 2 (10) | 2 (20) | 0 |
| Chronic/active ABMR, n (%) | 18 (90) | 8 (80) | 10 (100) |
| C4d-positive ABMR, n (%) | 7 (35) | 4 (40) | 3 (30) |
| Banff borderline lesion, n (%) | 1 (5) | 0 | 1 (10) |
| Markers of inflammation and immunosuppressive load | |||
| CRP (mg/dl), median (IQR) | 0.20 (0.05–0.44) | 0.13 (0.04–0.26) | 0.42 (0.08–0.48) |
| TTV load (copies/ml), median (IQR)f | 1.9×105 (3.5×104–9.1×105) | 7.2×104 (3.5×104–2.1×105) | 6.0×105 (7.6×104–1.7×108) |
CDC, complement-dependent cytotoxicity.
CDC panel reactivity was not recorded for one recipient in the clazakizumab arm and one in the placebo arm.
Pretransplant DSA data were available for seven recipients in the clazakizumab arm and four in the placebo arm (solid-phase HLA antibody screening on the waitlist was implemented at the Vienna transplant unit in July 2009).23
Donor age was not recorded for two recipients in the placebo arm.
HLA mismatch was not recorded for one recipient in the placebo arm.
Cold ischemia time was not recorded for one recipient in the clazakizumab arm and two recipients in the placebo arm.
One patient had TTV levels below the detection threshold.
At study inclusion, 18 (90%) recipients were on triple and two (10%) were on dual immunosuppressive therapy (Supplemental Table 2). Immunosuppressant trough levels and doses are shown in Supplemental Figure 2. In total, 11 (55%) patients did not receive all scheduled clazakizumab injections, mostly because of adverse events. These patients were included in all endpoint analyses. Two (10%) patients were withdrawn from the study due to diverticular disease complications, one after completion of part A, and one shortly after initiation of part B (Figure 1).
Safety
In part A, overall incidences of adverse events were 50 in the clazakizumab and 44 in the placebo arm (Supplemental Table 3, Table 2). The most frequent events were infections and gastrointestinal disorders (Supplemental Table 3). Serious adverse events occurred in four (20%) patients, three of whom received clazakizumab (Table 2). One patient on clazakizumab developed diverticulitis, leading to withdrawal from the trial (stable graft function until the end of the trial). Diverticulitis resolved after percutaneous abscess drainage and antibiotic therapy.
Table 2.
Serious treatment emergent adverse events by system organ class
| Serious Adverse Events, n (%) | Part Aa | Part B | |
|---|---|---|---|
| Clazakizumab (n=10) | Placebo (n=10) | Clazakizumab (n=19)b | |
| Infections and infestations | 0 | 0 | 5 (26.3) |
| Pneumonia | 0 | 0 | 2 (10.5) |
| Pyelonephritis | 0 | 0 | 1 (5.3) |
| Ovarian abscess | 0 | 0 | 1 (5.3) |
| Aseptic meningitis | 0 | 0 | 1 (5.3) |
| Gastrointestinal disorders | 1 (10) | 0 | 1 (5.3) |
| Diverticulitis | 1 (10)c | 0 | 1 (5.3)d |
| General disease and administration site conditions | 0 | 1 (10) | 0 |
| Pyrexia | 0 | 1 (10) | 0 |
| Respiratory, thoracic, and mediastinal disorders | 1 (10) | 0 | 1 (5.3) |
| Pleural effusion | 1 (10) | 0 | 1 (5.3) |
| Surgical and medical procedures | 0 | 0 | 1 (5.3) |
| Pleurodesis | 0 | 0 | 1 (5.3) |
| Permanent thorax cavity drainage | 0 | 0 | 1 (5.3) |
| Renal and urinary disorders | 1 (10) | 0 | 0 |
| Acute renal injury | 1 (10) | 0 | 0 |
Bold text indicates the number (%) of adverse events for individual system organ classes.
Differences between groups in part A were NS.
One patient was withdrawn from the trial in part A and was not included in the safety analysis of part B.
Diverticulitis resolved after percutaneous abscess drainage and antibiotic therapy.
Diverticulitis was complicated by colon perforation requiring open surgery (Hartmann’s procedure).
Part B had 129 adverse events overall, of which nine were serious. Three (15.8%) patients reported mild injection site reactions. Serious adverse events included a case of complicated diverticulitis with colon perforation requiring surgery. This patient (clazakizumab in part A) was withdrawn from the study before the second visit in part B (return to dialysis 9 months after trial withdrawal). Other serious events included pneumonia (n=2), pyelonephritis (n=1), ovarian abscess (n=1), Coxsackie virus–associated meningitis (n=1), and recurrent pleural effusion requiring pleurodesis, and, subsequently, permanent thorax cavity drainage.
After the second case of diverticulitis, the DSMB requested a careful re-evaluation of all included participants and a reduced dose of clazakizumab in two active patients diagnosed with diverticulosis (12.5 mg, last injection in part B). Overall, nine (45%) patients had colon diverticulosis, among them the two patients who developed diverticulitis. Additional predisposing risk factors were polycystic kidney disease and a long history of immunosuppression and steroid exposure (Supplemental Table 4).
Laboratory findings are provided in Supplemental Figure 3 and Table 3. Mild liver parameter elevations were noted in a few patients; none met Hy’s law. Anemia, mostly graded I or II, was already prevalent in both arms before study inclusion, with no relevant differences in part A and stable hemoglobin levels in part B. There were also no relevant changes in leukocyte and platelet counts. Lipid abnormalities were frequent in both study arms, with peak (unfasted) triglyceride levels higher under clazakizumab (Table 3).
Table 3.
Laboratory variables
| Variables | Part A | Part B | ||
|---|---|---|---|---|
| Clazakizumab (n=10) | Placebo (n=10) | P Value | Clazakizumab (n=18) | |
| Liver parameters | ||||
| ALT levels (U/L) | ||||
| Highest level, median (IQR) | 29 (23–39) | 24 (18–36) | 0.39 | 30 (23–43) |
| >1–3 ULN, n (%) | 1 (10) | 1 (10) | 1 (5.6) | |
| >3–5 ULN, n (%) | 0 | 0 | 2 (11.1) | |
| >5–8 ULN, n (%) | 0 | 0 | 0 | |
| AST levels (U/L) | ||||
| Highest level, median (IQR) | 26 (22–34) | 28 (25–34) | 0.68 | 28 (24–35) |
| >1–3 ULN, n (%) | 1 (10) | 0 | 2 (11.1) | |
| >3–5 ULN, n (%) | 0 | 0 | 0 | |
| >5–8 ULN, n (%) | 0 | 0 | 0 | |
| Total bilirubin levels (mg/dl) | ||||
| Highest level, median (IQR) | 0.69 (0.41–0.94) | 0.38 (0.31–0.55) | 0.052 | 0.56 (0.43–1.0) |
| >1–3 ULN, n (%) | 0 | 0 | 3 (16.7) | |
| >3–5 ULN, n (%) | 0 | 0 | 0 | |
| >5–8 ULN, n (%) | 0 | 0 | 0 | |
| Blood count | ||||
| Hemoglobin levels (g/dl) | ||||
| Level at nadir, median (IQR) | 11.4 (9.8–12.2) | 9.4 (8.4–10.7) | 0.023 | 9.8 (8.5–11.0) |
| Anemia grade at nadir, n (%)a | ||||
| 0 | 1 (10) | 0 | 1 (5.6) | |
| I | 7 (70) | 3 (30) | 8 (44.4) | |
| II | 1 (10) | 6 (60) | 6 (33.3) | |
| IIII | 1 (10) | 1 (10) | 3 (16.7) | |
| IV | 0 | 0 | 0 | |
| Leukocyte count (x109/L) | ||||
| Level at nadir, median (IQR) | 5.3 (3.8–6.7) | 4.7 (3.3–8.9) | 0.80 | 4.6 (3.3–6.1) |
| Leukopenia grade at nadir, n (%)a | ||||
| 0 | 8 (80) | 5 (50) | 10 (55.6) | |
| I | 2 (20) | 5 (50) | 8 (44.4) | |
| II | 0 | 0 | 0 | |
| IIII | 0 | 0 | 0 | |
| IV | 0 | 0 | 0 | |
| Platelet count (x109/L) | ||||
| Level at nadir, median (IQR) | 172 (131–207) | 226 (130–284) | 0.14 | 162 (136–213) |
| Thrombocytopenia grade at nadir, na (%) | ||||
| 0 | 6 (60) | 7 (70) | 10 (55.6) | |
| I | 4 (40) | 3 (30) | 7 (38.9) | |
| II | 0 | 0 | 1 (5.6) | |
| IIII | 0 | 0 | 0 | |
| IV | 0 | 0 | 0 | |
| Lipids (unfasted) | ||||
| Cholesterol (mg/dl) | ||||
| LDL | ||||
| Level above threshold, n (%) | 4 (40) | 4 (40) | >0.99 | 12 (66.7) |
| Highest level, median (IQR) | 149 (132–166) | 142 (122–209) | 0.97 | 205 (142–253) |
| HDL | ||||
| Level below threshold, n (%) | 8 (80) | 8 (80) | >0.99 | 17 (94.4) |
| Level at nadir, median (IQR) | 45 (30–59) | 49 (39–61) | 0.48 | 47 (41–52) |
| Triglycerides (mg/dl) | ||||
| Level above threshold, n (%) | 9 (90) | 6 (60) | 0.30 | 18 (100) |
| Highest level, median (IQR) | 313 (213–655) | 205 (118–254) | 0.029 | 246 (201–352) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal.
For grading of hematologic toxicities we used the National Cancer Institute Common Terminology Criteria for Adverse Events.
Efficacy
Treatment with clazakizumab effectively suppressed CRP levels and slightly increased TTV viral load (Supplemental Figure 4).
HLA Antibody and Ig Levels
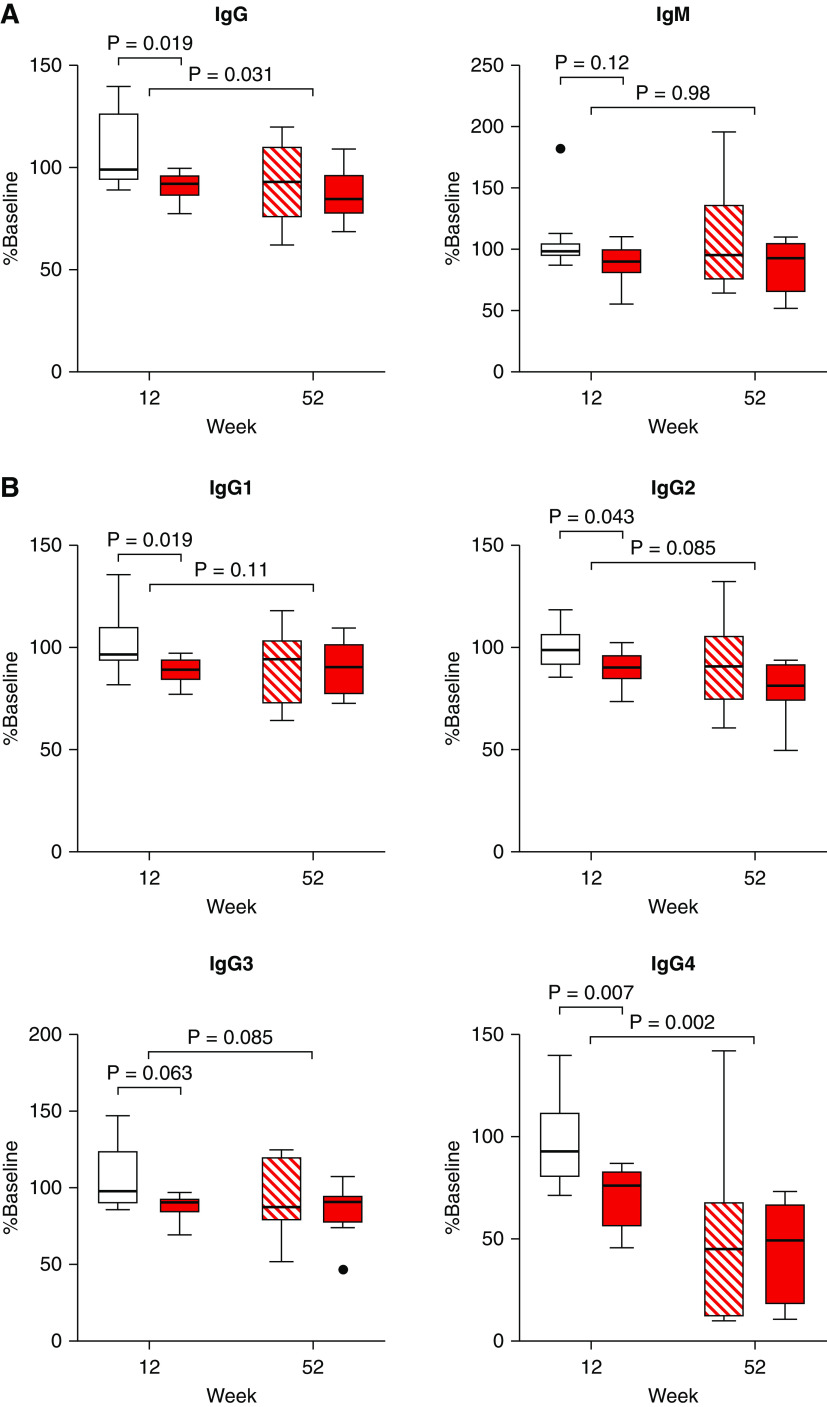
Within 12 weeks (part A), clazakizumab decreased DSA MFI to a median of 77% (IQR, 63%–101%) from baseline (placebo: 103% [IQR, 94%–104%]; P=0.035). Extension of treatment in part B led to a further decrease of DSA levels (P<0.001) (Figure 2A). Similar results were obtained for levels of DSA interpolated from dilution experiments, nondonor-specific HLA reactivity (Figure 2A), total IgG (but not IgM), and, among IgG subclasses, most prominently IgG4 (Figure 3).
Figure 2.
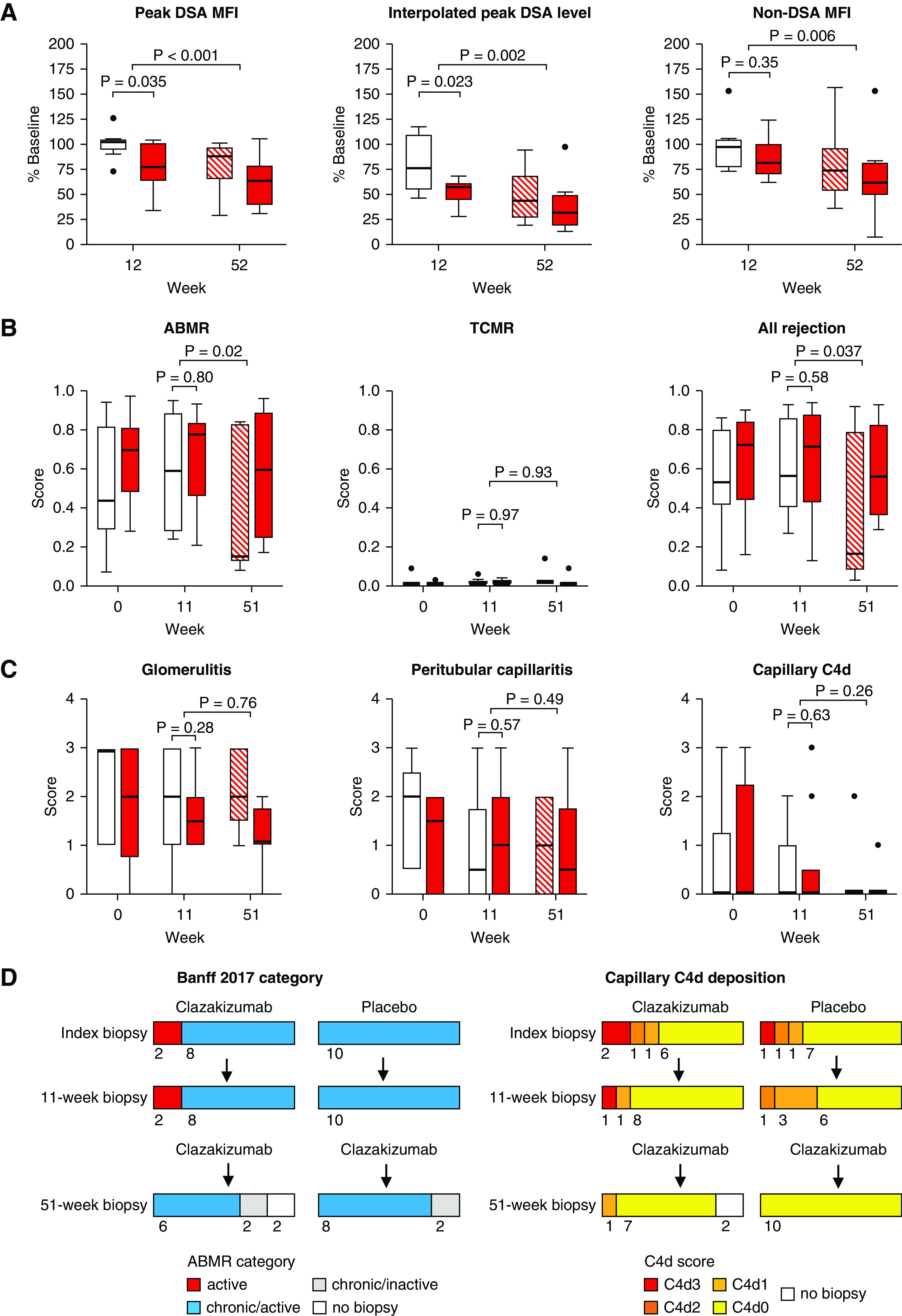
Antibody and biopsy results. Percentages (in relation to baseline values) of (A) peak DSA MFI, DSA levels interpolated from dilution experiments, and the median MFI detected for non-DSA, (B) rejection-associated molecular classifiers (ABMR, T cell–mediated rejection [TCMR], all rejection), and (C) morphologic single lesion scores (g, ptc, C4d) are shown in relation to treatment allocation (clazakizumab, part A/B: red closed boxplots; placebo, part A: open boxplots; placebo, part B [switch to clazakizumab]: red hatched boxplots). We applied unpaired Mann–Whitney U tests for group comparisons (clazakizumab versus placebo) at the end of part A, and paired Wilcoxon test to evaluate changes under clazakizumab (overall cohort) in the open-label extension (part B). (D) shows proportions of ABMR categories and C4d scoring results obtained in index, 11-week and 51-week biopsies.
Figure 3.
Serum Ig concentrations. Shown are the percent levels (in relation to values at baseline) of (A) IgG, IgM, and (B) IgG subclasses for patients randomized to clazakizumab (red closed boxplots) or placebo (part A: open boxplots; part B: red hatched boxplots). We used unpaired Mann–Whitney U test for group comparisons (clazakizumab versus placebo) at the end of part A and paired Wilcoxon test to evaluate changes under clazakizumab (overall cohort) in the open-label extension (part B).
Evolution of Rejection
The 11-week biopsies failed to demonstrate significant intergroup differences in rejection-related molecular and morphologic scores (Figure 2, B and C), and there were no meaningful changes in ABMR phenotypes (Figure 2D). In contrast, the 51-week biopsies performed after prolonged clazakizumab treatment in part B demonstrated a significant decrease in molecular ABMR (P=0.020) and “all rejection” scores (P=0.037), whereas T cell–mediated rejection scores remained negative at all time points (Figure 2B). After 51 weeks, seven of 18 patients (38.9%) had a negative ABMR score (<0.2) (not shown). Although microcirculation inflammation did not change significantly (Figure 2C), we found a resolution of ABMR activity in four (22.2%) and disappearance of capillary C4d deposits in five (27.8%) patients (Figure 2D). Supplemental Table 5 details serial biopsy results obtained in the four patients who showed transition of active ABMR to an inactive phenotype (cg in the absence of evidence of current/recent antibody interaction with the endothelium). At baseline, three of these recipients were C4d negative, with rather low levels of microcirculation inflammation (g+ptc sum score ≤3). On treatment, g+ptc scores decreased to <2, paralleled by a marked reduction in molecular ABMR scores (Supplemental Table 5).
Levels of interstitial fibrosis and tubular atrophy increased significantly from week 11 to week 51, but transplant glomerulopathy or a molecular classifier reflecting atrophy/fibrosis remained unchanged (Supplemental Figure 5).
Clinical Outcomes
In part A, the mean slope of eGFR differed significantly between clazakizumab and placebo (−0.96; 95% confidence interval [95% CI], −1.96 to 0.03 versus −2.43; 95% CI, −3.40 to −1.46 ml/min per 1.73 m2 per month, P=0.04) (Figure 4). In part B, patients switched from placebo to clazakizumab showed significant improvement in the eGFR slope compared with the slope calculated for part A (P<0.001), and differences to patients initially allocated to clazakizumab (no significant change on extension of treatment in part B) became NS (−0.29, 95% CI, −0.85 to 0.26 versus −0.64, 95% CI, −1.13 to −0.14 ml/min per 1.73 m2 per month, P=0.37) (Figure 3). Similar results were obtained in a separate analysis, including the two patients who were prematurely withdrawn from the trial (Supplemental Figure 6). Levels of proteinuria did not change over time (Supplemental Figure 7).
Figure 4.
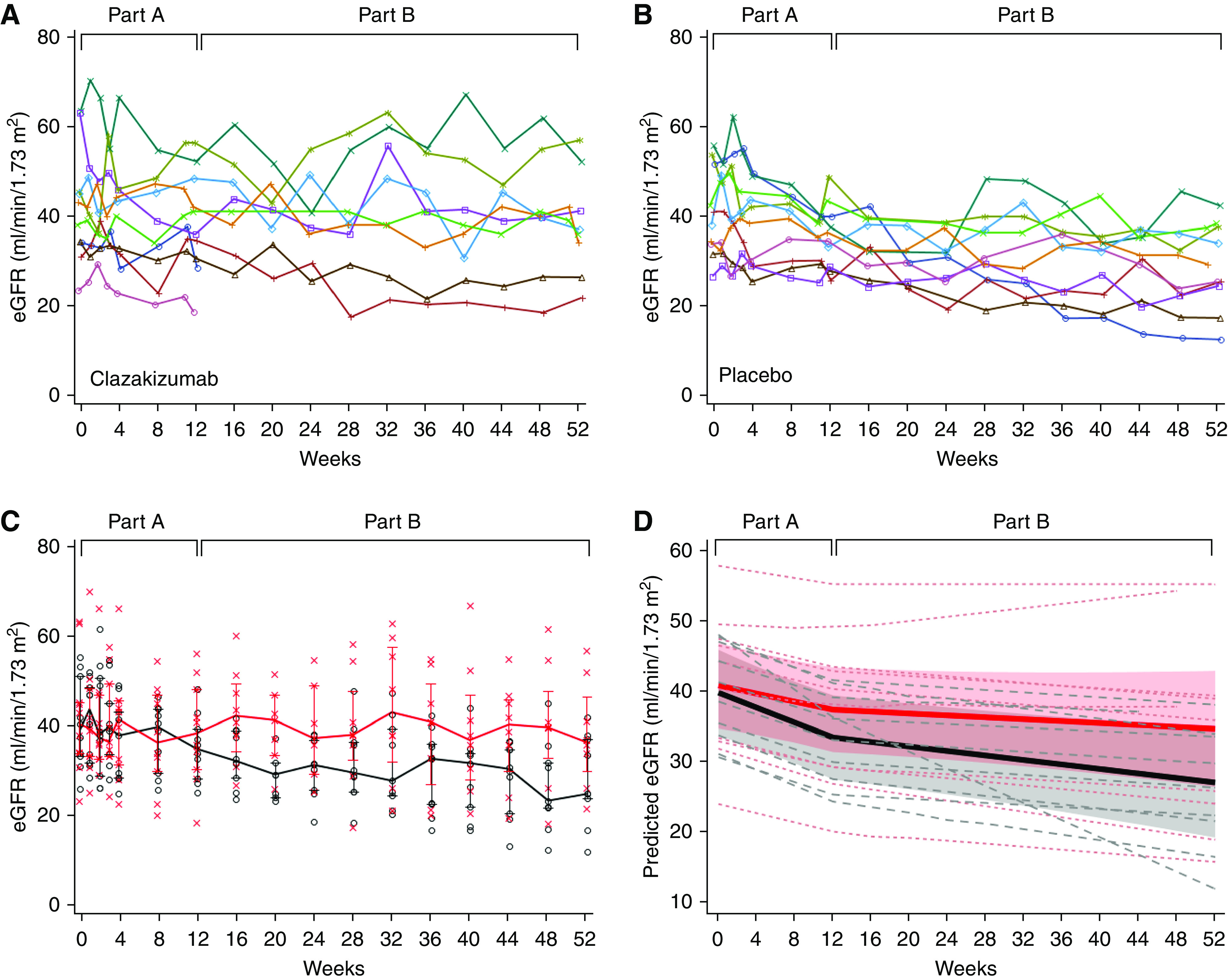
Kidney allograft function. The individual course of eGFR is shown for patients allocated to receive clazakizumab (A) versus placebo (B). (C) shows median, IQR, and individual levels of eGFR in relation to treatment allocation in part A (clazakizumab: red line, bars and asterisks; placebo: black lines, bars and circles). (D) shows individual (dashed lines) and mean eGFR slopes (solid lines; shaded areas represent 95% CIs) in relation to treatment in part A (clazakizumab: red lines; placebo: black lines).
As shown in Supplemental Figure 8, on 6 months follow-up after the end of the trial, we observed a slight increase in CRP levels to ranges detected before trial initiation. None of the patients developed acute graft dysfunction (or underwent indication biopsies), and as illustrated in Supplemental Figure 8, there was no major change in the mean slope of eGFR. One patient returned to dialysis 3 months after the last visit.
Discussion
This phase 2 trial evaluated the safety (primary endpoint) and efficacy (secondary endpoint analysis) of anti–IL-6 antibody clazakizumab in late ABMR. Major safety signals were the occurrence of serious infectious events and diverticular disease complications. Key results of secondary endpoint analysis were an early decrease in DSA levels, a slowed eGFR decline, and, after extended treatment, modulation of rejection-associated gene expression patterns, reduction of C4d scores, and, in some patients, resolution of ABMR activity.
The role of IL-6 in transplantation is supported by its marked upregulation on rejection,24,25 with HLA class II DSA being a potent trigger of endothelial IL-6 secretion.26 IL-6 is known to contribute to plasma cell formation, which may promote DSA formation.8 In line with an observational study evaluating tocilizumab,12,13 clazakizumab led to an early decrease in DSA, non-DSA, and total IgG levels. We found a pronounced reduction of IgG4, an IgG subclass previously postulated to play a critical role in chronic rejection.27 Interpreting our DSA results, we want to point out potential caveats associated with using MFI as a measure of antibody levels.28 It is well established that MFI levels detected in bead arrays do not simply reflect antibody concentrations in serum, but also depend on antibody avidity/affinity and the density or conformation of HLA antigens coated to individual microbeads. Reporting numerical changes in MFI may be problematic and not accurately reflect quantitative changes in antibody levels, especially in the case of bead saturation. In this respect, titer studies may be more informative. In an attempt to indirectly quantify changes in antibody levels, we applied dilution experiments creating standard curves for baseline sera. Another point is the earlier reported spontaneous fluctuation of DSA levels (independent of specific therapeutic interventions),29 which has to be taken into account when interpreting antibody changes in the open-label part of our trial.
Early follow-up biopsies did not show any effect of clazakizumab on morphologic and molecular results, despite an early decline in CRP and DSA levels. After prolonged treatment in part B (biopsies after 51 weeks), however, biopsy results suggested amelioration of rejection, at least in some of the included patients. These findings may relate to the observed decrease in DSA levels, but one may also argue that other factors have potentially influenced ABMR activity, such as the load of maintenance immunosuppression. There were, however, no meaningful adjustments in the composition and dose of immunosuppression, which, together with the outcome differences observed in placebo-controlled part A, supports a specific therapeutic effect of IL-6 signaling blockade. Our failure to detect any improvement in atrophy/fibrosis and transplant glomerulopathy (and proteinuria) was expected, as advanced chronic injury may not be reversible. Interpreting morphologic biopsy results, however, we are aware of the inherent limitation of poor reproducibility of Banff histology scores, which may support the use of more objective analysis of gene expression patterns, especially in patients with ambiguous histology.30
The slope of eGFR is a useful surrogate predicting graft survival, and in late ABMR, a 30% deterioration in eGFR slope was reported to be associated with a 10% increase in graft loss rates.31 We found a significant intergroup slope difference already in part A of the trial, and a slowed progression of renal dysfunction in part B. Our results are promising and in contrast to previous trials conducted in late ABMR, where other treatments, such as bortezomib7 or rituximab plus intravenous Ig,6 failed to slow the progression of renal dysfunction. Nevertheless, the outcome data in our trial, which was not primarily powered to detect differences in graft function, have to be interpreted with caution. Calculated eGFR slopes, both in the placebo and clazakizumab arms, were within a range earlier reported for a cohort of 91 recipients with late ABMR (most of them treated with intravenous Ig or steroids),31 and it remains unclear whether the slope differences in our trial would result in differences in graft survival. In this context, we want to mention a recently initiated large multicenter, randomized, placebo-controlled phase 3 trial evaluating clazakizumab in chronic ABMR (IL 6 Blockade Modifying Antibody-Mediated Graft Injury and eGFR Decline trial; ClinicalTrials.gov identifier: NCT03744910). The design of this trial, which aims to recruit 350 patients to detect differences in 5-year graft survival, will include an early evaluation of eGFR slope differences as an interim surrogate endpoint (after approximately 200 subjects have received at least 1 year of treatment).
Our trial did not include follow-up biopsies to investigate a potential role of rebound phenomena after stopping clazakizumab treatment. Nevertheless, a 6-month follow-up after the last visit revealed only a slight increase in CRP levels to values detected before trial initiation, and none of the patients developed acute graft dysfunction. Together with our finding of no major change in eGFR slope after the last visit, this may argue against major clinically relevant rebound phenomena.
In line with earlier studies,10,15 clazakizumab was associated with mild injection site reactions, increases in lipid levels, and mild abnormalities of liver enzymes or blood cell count. When combining clazakizumab with baseline immunosuppression, aggravated infectious complications may become a significant concern. Under prolonged treatment in part B, five serious infectious events were recorded. In parallel, we observed a significant reduction in serum IgG and a slight increase in TTV load as a surrogate marker of intensified immunosuppression.32 In trials performed in rheumatoid arthritis, IL-6 interference was reported to be associated with increases in serious infection incidence,9,10,33,34 although in a comprehensive analysis of available trials and their extension phases, intergroup differences were NS.35 The most important safety signal was the occurrence of diverticulitis in two patients with pre-existing diverticulosis, one event resulting in colon perforation. The risk of gastrointestinal perforation is known to be significant under IL-6 blockade.11,36 In a large US real-world dataset of patients with rheumatoid arthritis, tocilizumab was associated with a lower gastrointestinal perforation rate of 1.26 per 1000 patient-years.37 A study by Choi et al.12 showed zero of 36 patients receiving tocilizumab for ABMR experiencing diverticular disease complications; one case of colon perforation, however, was recorded in a small trial of tocilizumab for recipient desensitization.38 Given that none of the trials evaluating clazakizumab in rheumatologic disease (>500 treated patients) reported any patients with gastrointestinal perforation (as in our study, patients with a history of diverticulitis or gastrointestinal perforation were not included),10,15 one may assume that additional immunosuppression, in particular long-term steroid use, and the background of certain predisposing conditions, including diverticulosis and polycystic kidney disease, have increased the risk significantly.39 Our data suggest the need for careful evaluation of transplant patients considered for IL-6 signaling blockade, for example, in the context of future trials. This should include a thorough evaluation of the patient’s history, including documented colon diverticulosis and, at least for patients deemed to be at particular risk, such as those with polycystic kidney disease, use of diagnostic tests (e.g., colonoscopy or computer tomography) to rule out significant colon diverticulosis. Of note, the results of our safety analysis have significantly influenced the design of an ongoing pivotal phase 3 trial (IL 6 Blockade Modifying Antibody-Mediated Graft Injury and eGFR Decline; ClinicalTrials.gov identifier: NCT03744910), including a reduction in monthly clazakizumab doses (from 25 to 12.5 mg per month) and a strict definition of exclusion criteria (history of gastrointestinal perforation; diverticular disease or diverticulitis, except if disease has been fully excised; or inflammatory bowel disease).
A major limitation of our trial is its small sample size. A strength, however, may be the granularity of analyzed endpoints, including a detailed work-up of three sequential biopsies. Although the trial was not primarily powered to detect efficacy outcome differences, the double-blind phase of the trial revealed significant differences in DSA levels and progression of graft dysfunction, and observed changes in secondary outcomes were pronounced upon treatment in the open-label extension. One may argue that intergroup differences in the course of renal function in part A could partly relate to a bias by chance due to imbalances in baseline variables associated with the severity of rejection or immunosuppressive therapy (despite stratified randomization), in particular, higher numerical levels of DSA MFI, CRP, and proteinuria and a lower dose of enteric-coated mycophenolic acid in the placebo arm. In contrast, however, baseline eGFR and morphologic features of rejection and/or chronic injury were similar between groups, and molecular rejection-related scores were even higher in the clazakizumab arm.
In conclusion, this phase 2 trial suggests the efficacy of clazakizumab in late ABMR after kidney transplantation. Recorded safety outcomes—the primary trial endpoint—warrant careful patient selection and monitoring to minimize the risk of diverticular disease complications and serious infections. The results of secondary endpoint analysis, including first systematic data on the size of expected outcome effects, may provide a valuable basis for larger phase 3 trials with longer duration of follow-up to systematically evaluate the concept of IL-6/IL-6R blockade in transplantation (in comparison to placebo and/or other treatment concepts).
Disclosures
B. Jilma reports having consultancy agreements with Guardian Therapeutics; and Speakers Bureau from Sanofi. E. Chong is employed by and has ownership interest in Vitaeris Inc., Vancouver, Canada. G. Böhmig and K. Budde are members of the steering committee for an ongoing pivotal phase 3 trial evaluating clazakizumab in chronic active ABMR (ClinicalTrials.gov number, NCT03744910; sponsored by Vitaeris Inc.). G. Böhmig reports consultancy agreements from Vitaeris Inc., Vancouver, Canada; research funding from Vitaeris Inc., Vancouver, Canada; honoraria from Astellas, Fresenius Medical Care, OneLambda, Sandoz; and being a scientific advisor or membership of Vitaeris Inc, Vancouver, Canada. G. Bond reports receiving research funding from Chiesi. H. Regele reports receiving honoraria from Roche, Menarini, and other interests/relationships with Gerson Lehrman Group. K. Budde reports consultancy agreements with Abbvie, Alexion, Astellas, Bristol-Myers Squibb, Chiesi, CSL-Behring, Fresenius, Hansa, Hexal, Novartis, MSD, Otsuka, Pfizer, Quark, Roche, Sandoz, Shire, Veloxis, Vifor, and Vitaeris; reports receiving research funding from Abbvie, Alexion, Astellas, Bristol-Myers Squibb, Chiesi, CSL-Behring, Fresenius, Hansa, Hexal, Novartis, MSD, Otsuka, Pfizer, Quark, Roche, Sandoz, Shire, Veloxis, Vifor, and Vitaeris; receiving honoraria from Abbvie, Alexion, Astellas, Bristol-Myers Squibb, Chiesi, Fresenius, Hexal, Novartis, Otsuka, Pfizer, Quark, Roche, Sandoz, Shire, and Veloxis Pharma; being a scientific advisor or membership of Astellas, Bristol-Myers Squibb, Chiesi, Hansa, Hexal, MSD, Novartis, Pfizer, Roche, and Veloxis. M. Duerr reports receiving research funding from Bristol-Myers Squibb; honoraria from Shire, Novartis; being a scientific advisor or member of Novartis. N. Kozakowski reports receiving research funding from the Austrian Science Fund—Erwin Schrödinger Fellowship J-4377; being a scientific advisor or member as Associate Editor—BMC Nephrology; other interests/relationships with 2012–2020 Austrian, German, French, and European Societies for Pathology, 2018–2020 French Speaking Club of Renal Pathology, 2012–2020 Austrian, French, and European Societies for Transplantation, 2018–2020 Member then Co-chair of the Banff Working group for Thrombotic Microangiopathy, and 2020 Co-chair of the Banff Working group for Peritubular Capillaritis. N. Lachmann reports consultancy agreements with BmT GmbH. P. Halloran reports consultancy agreements with Astellas, CSL Behring; ownership interest in Transcriptome Sciences Inc., a University of Alberta research company with an interest in molecular diagnostics, has given lectures for Thermo Fisher, and is a consultant for CSL Behring; reports receiving research funding from Transcriptome Sciences Inc.; reports receiving honoraria from Astellas, One Lambda; is a scientific advisor or member of CEO, Transcriptome Sciences Inc., Editor-in-Chief emeritus, American Journal of Transplantation; Speakers Bureau for Astellas and One Lambda. All remaining authors have nothing to disclose.
Funding
The trial was funded by an investigator-initiated unrestricted grant from Vitaeris Inc., Vancouver, Canada (to G. Böhmig and B. Jilma).
Supplementary Material
Acknowledgments
The authors wish to thank Prof. Josef Smolen for his scientific advice, and Susanne Haindl, Sarah Ely, Dr. Christa Drucker, Rene Nadolny, and Silke Kasbohm for excellent assistance.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020071106/-/DCSupplemental.
Supplemental Table 1. Single lesions and molecular classifiers at baseline.
Supplemental Table 2. Baseline immunosuppression.
Supplemental Table 3. Adverse events by system organ class.
Supplemental Table 4. Diverticular disease and complications.
Supplemental Table 5. Resolution of ABMR activity in four recipients: serial biopsy results.
Supplemental Figure 1. Summary of trial protocol.
Supplemental Figure 2. Immunosuppression levels and dosage.
Supplemental Figure 3. Safety lab.
Supplemental Figure 4. CRP and Torque Teno virus (TTV) levels.
Supplemental Figure 5. Morphologic and molecular features of chronic injury.
Supplemental Figure 6. Analysis of renal function including two patients withdrawn from the trial.
Supplemental Figure 7. Spot urine protein/creatinine ratio.
Supplemental Figure 8. CRP and renal function after the end of the trial.
References
- 1.Loupy A, Lefaucheur C: Antibody-mediated rejection of solid-organ allografts. N Engl J Med 379: 1150–1160, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Halloran PF, Venner JM, Madill-Thomsen KS, Einecke G, Parkes MD, Hidalgo LG, et al.: Review: The transcripts associated with organ allograft rejection. Am J Transplant 18: 785–795, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, et al.: The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 18: 293–307, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Böhmig GA, Eskandary F, Doberer K, Halloran PF: The therapeutic challenge of late antibody-mediated kidney allograft rejection. Transpl Int 32: 775–788, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schinstock CA, Mannon RB, Budde K, Chong AS, Haas M, Knechtle S, et al.: Recommended treatment for antibody-mediated rejection after kidney transplantation: The 2019 expert consensus from the transplantion society working group. Transplantation 104: 911–922, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreso F, Crespo M, Ruiz JC, Torres A, Gutierrez-Dalmau A, Osuna A, et al.: Treatment of chronic antibody mediated rejection with intravenous immunoglobulins and rituximab: A multicenter, prospective, randomized, double-blind clinical trial. Am J Transplant 18: 927–935, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Eskandary F, Regele H, Baumann L, Bong G, Kozakowski N, Wahrmann M, et al.: A randomized trial of bortezomib in late antibody-mediated kidney transplant rejection. J Am Soc Nephrol 29: 591–605, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan SC, Ammerman N, Choi J, Kumar S, Huang E, Toyoda M, et al.: Interleukin-6: An important mediator of allograft injury. Transplantation 104: 2497–2506, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al.; OPTION Investigators: Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): A double-blind, placebo-controlled, randomised trial. Lancet 371: 987–997, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Weinblatt ME, Mease P, Mysler E, Takeuchi T, Drescher E, Berman A, et al.: The efficacy and safety of subcutaneous clazakizumab in patients with moderate-to-severe rheumatoid arthritis and an inadequate response to methotrexate: Results from a multinational, phase IIb, randomized, double-blind, placebo/active-controlled, dose-ranging study. Arthritis Rheumatol 67: 2591–2600, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Aletaha D, Bingham CO 3rd, Tanaka Y, Agarwal P, Kurrasch R, Tak PP, et al.: Efficacy and safety of sirukumab in patients with active rheumatoid arthritis refractory to anti-TNF therapy (SIRROUND-T): A randomised, double-blind, placebo-controlled, parallel-group, multinational, phase 3 study. Lancet 389: 1206–1217, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Choi J, Aubert O, Vo A, Loupy A, Haas M, Puliyanda D, et al.: Assessment of tocilizumab (anti-interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am J Transplant 17: 2381–2389, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Shin BH, Everly MJ, Zhang H, Choi J, Vo A, Zhang X, et al.: Impact of tocilizumab (Anti-IL-6R) treatment on immunoglobulins and anti-HLA antibodies in kidney transplant patients with chronic antibody-mediated rejection. Transplantation 104: 856–863, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T: Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 112: 3959–3964, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Mease PJ, Gottlieb AB, Berman A, Drescher E, Xing J, Wong R, et al.: The efficacy and safety of clazakizumab, an anti-interleukin-6 monoclonal antibody, in a phase IIb study of adults with active psoriatic arthritis. Arthritis Rheumatol 68: 2163–2173, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Eskandary F, Dürr M, Budde K, Doberer K, Reindl-Schwaighofer R, Waiser J, et al.: Clazakizumab in late antibody-mediated rejection: Study protocol of a randomized controlled pilot trial. Trials 20: 37, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doberer K, Kläger J, Gualdoni GA, Mayer KA, Eskandary F, Farkash EA, et al.: CD38 antibody daratumumab for the treatment of chronic active antibody-mediated kidney allograft rejection [published online ahead of print March 30, 2020]. Transplantation 10.1097/TP.0000000000003247 [DOI] [PubMed] [Google Scholar]
- 18.Halloran PF, Reeve J, Akalin E, Aubert O, Böhmig GA, Brennan D, et al.: Real time central assessment of kidney transplant indication biopsies by microarrays: The INTERCOMEX study. Am J Transplant 17: 2851–2862, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Reeve J, Böhmig GA, Eskandary F, Einecke G, Lefaucheur C, Loupy A, et al.; MMDx-Kidney study group: Assessing rejection-related disease in kidney transplant biopsies based on archetypal analysis of molecular phenotypes. JCI Insight 2: e94197, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al.; Banff meeting report writing committee: Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions [published correction appears in Am J Transplant 15: 2784, 2015 10.1111/ajt.13517]. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Maggi F, Pifferi M, Fornai C, Andreoli E, Tempestini E, Vatteroni M, et al.: TT virus in the nasal secretions of children with acute respiratory diseases: Relations to viremia and disease severity. J Virol 77: 2418–2425, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiemann M, Puchhammer-Stöckl E, Eskandary F, Kohlbeck P, Rasoul-Rockenschaub S, Heilos A, et al.: Torque Teno virus load-inverse association with antibody-mediated rejection after kidney transplantation. Transplantation 101: 360–367, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwaiger E, Eskandary F, Kozakowski N, Bond G, Kikić Ž, Yoo D, et al.: Deceased donor kidney transplantation across donor-specific antibody barriers: Predictors of antibody-mediated rejection. Nephrol Dial Transplant 31: 1342–1351, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Vandenbroecke C, Caillat-Zucman S, Legendre C, Noel LH, Kreis H, Woodrow D, et al.: Differential in situ expression of cytokines in renal allograft rejection. Transplantation 51: 602–609, 1991 [DOI] [PubMed] [Google Scholar]
- 25.Waiser J, Budde K, Katalinic A, Kuerzdörfer M, Riess R, Neumayer HH: Interleukin-6 expression after renal transplantation. Nephrol Dial Transplant 12: 753–759, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Cross AR, Lion J, Poussin K, Assayag M, Taupin JL, Glotz D, et al.: HLA-DQ alloantibodies directly activate the endothelium and compromise differentiation of FoxP3high regulatory T lymphocytes. Kidney Int 96: 689–698, 2019 [DOI] [PubMed] [Google Scholar]
- 27.Lefaucheur C, Viglietti D, Bentlejewski C, Duong van Huyen JP, Vernerey D, Aubert O, et al.: IgG donor-specific anti-human HLA antibody subclasses and kidney allograft antibody-mediated injury. J Am Soc Nephrol 27: 293–304, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan HC, Liwski RS, Bray RA, Gebel HM: The road to HLA antibody evaluation: Do not rely on MFI. Am J Transplant 17: 1455–1461, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schinstock CA, Bentall AJ, Smith BH, Cornell LD, Everly M, Gandhi MJ, et al.: Long-term outcomes of eculizumab-treated positive crossmatch recipients: Allograft survival, histologic findings, and natural history of the donor-specific antibodies. Am J Transplant 19: 1671–1683, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madill-Thomsen K, Perkowska-Ptasińska A, Böhmig GA, Eskandary F, Einecke G, Gupta G, et al.; MMDx-Kidney Study Group: Discrepancy analysis comparing molecular and histology diagnoses in kidney transplant biopsies. Am J Transplant 20: 1341–1350, 2020 [DOI] [PubMed] [Google Scholar]
- 31.Irish W, Nickerson P, Astor BC, Chong E, Wiebe C, Moreso F, et al.: Change in estimated GFR and risk of allograft failure in patients diagnosed with late active antibody-mediated rejection following kidney transplantation [published online ahead of print April 16, 2020]. Transplantation, 2020 [DOI] [PubMed] [Google Scholar]
- 32.Doberer K, Schiemann M, Strassl R, Haupenthal F, Dermuth F, Görzer I, et al.: Torque teno virus for risk stratification of graft rejection and infection in kidney transplant recipients-A prospective observational trial. Am J Transplant 20: 2081–2090, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, et al.: Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: The tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 58: 2968–2980, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Kremer JM, Blanco R, Brzosko M, Burgos-Vargas R, Halland AM, Vernon E, et al.: Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: Results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum 63: 609–621, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Genovese MC, Rubbert-Roth A, Smolen JS, Kremer J, Khraishi M, Gómez-Reino J, et al.: Longterm safety and efficacy of tocilizumab in patients with rheumatoid arthritis: A cumulative analysis of up to 4.6 years of exposure. J Rheumatol 40: 768–780, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Schiff MH, Kremer JM, Jahreis A, Vernon E, Isaacs JD, van Vollenhoven RF: Integrated safety in tocilizumab clinical trials. Arthritis Res Ther 13: R141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie F, Yun H, Bernatsky S, Curtis JR: Brief report: Risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol 68: 2612–2617, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vo AA, Choi J, Kim I, Louie S, Cisneros K, Kahwaji J, et al.: A phase I/II trial of the interleukin-6 receptor-specific humanized monoclonal (tocilizumab) + intravenous immunoglobulin in difficult to desensitize patients. Transplantation 99: 2356–2363, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Hwang SS, Cannom RR, Abbas MA, Etzioni D: Diverticulitis in transplant patients and patients on chronic corticosteroid therapy: A systematic review. Dis Colon Rectum 53: 1699–1707, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.