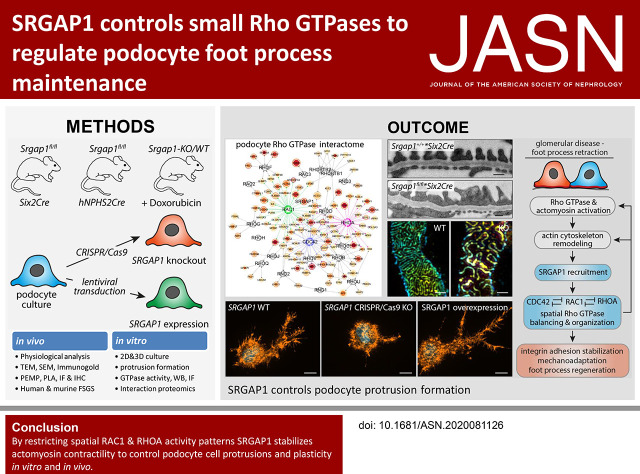
Significance Statement
Although the role of the podocyte cytoskeleton in the integrity of glomerular filtration is well established, the specific contribution of an upstream regulatory network comprising the Rho family of small GTPases—modulators of the actin cytoskeleton—is poorly characterized. The authors provide a comprehensive map of the podocyte Rho GTPase affinity interactome and identify a small GTPase-activating protein, SRGAP1, as a podocyte-specific RhoGAP. Through in vivo models of experimental FSGS, they demonstrate that SRGAP1 prevents podocyte foot-process effacement. They also show that SRGAP1 mediates spatial restriction of the activity of the Rho GTPase RAC1, thereby maintaining morphologic plasticity in disease conditions. These findings indicate that a comprehensive understanding of the regulatory networks of small Rho GTPases is needed for precisely targeted therapeutic interventions in glomerular diseases.
Keywords: cell adhesion, cell signaling, focal segmental glomerulosclerosis, glomerular epithelial cells, nephrotic syndrome, podocyte, Rho GTPase, RhoGAP, Srgap1
Visual Abstract
Abstract
Background
Previous research demonstrated that small Rho GTPases, modulators of the actin cytoskeleton, are drivers of podocyte foot-process effacement in glomerular diseases, such as FSGS. However, a comprehensive understanding of the regulatory networks of small Rho GTPases in podocytes is lacking.
Methods
We conducted an analysis of podocyte transcriptome and proteome datasets for Rho GTPases; mapped in vivo, podocyte-specific Rho GTPase affinity networks; and examined conditional knockout mice and murine disease models targeting Srgap1. To evaluate podocyte foot-process morphology, we used super-resolution microscopy and electron microscopy; in situ proximity ligation assays were used to determine the subcellular localization of the small GTPase-activating protein SRGAP1. We performed functional analysis of CRISPR/Cas9-generated SRGAP1 knockout podocytes in two-dimensional and three-dimensional cultures and quantitative interaction proteomics.
Results
We demonstrated SRGAP1 localization to podocyte foot processes in vivo and to cellular protrusions in vitro. Srgap1fl/fl*Six2Cre but not Srgap1fl/fl*hNPHS2Cre knockout mice developed an FSGS-like phenotype at adulthood. Podocyte-specific deletion of Srgap1 by hNPHS2Cre resulted in increased susceptibility to doxorubicin-induced nephropathy. Detailed analysis demonstrated significant effacement of podocyte foot processes. Furthermore, SRGAP1-knockout podocytes showed excessive protrusion formation and disinhibition of the small Rho GTPase machinery in vitro. Evaluation of a SRGAP1-dependent interactome revealed the involvement of SRGAP1 with protrusive and contractile actin networks. Analysis of glomerular biopsy specimens translated these findings toward human disease by displaying a pronounced redistribution of SRGAP1 in FSGS.
Conclusions
SRGAP1, a podocyte-specific RhoGAP, controls podocyte foot-process architecture by limiting the activity of protrusive, branched actin networks. Therefore, elucidating the complex regulatory small Rho GTPase affinity network points to novel targets for potentially precise intervention in glomerular diseases.
Podocytes are essential for the maintenance of the glomerular filtration barrier. These specialized epithelial cells form the outer part of the filtration barrier by covering glomerular capillaries with complex cellular protrusions (i.e., foot processes [FPs]).1,2 Podocytes rely on a tightly balanced and specialized cytoskeleton machinery to control FP morphology and adhesion via integrin adhesion complexes (IACs). Retraction and simplification of podocyte FPs (FP effacement) is the earliest common hallmark of glomerular disease,2–4 and one essential factor restricting the permeability of the filtration barrier.5 Mechanistically, FP retraction is linked to a fundamental rearrangement of the actin cytoskeleton and IACs, which finally translates into the disassembly of slit diaphragms and progressive detachment of podocytes from the glomerular basement membrane.2,4 The essential role of the podocyte cytoskeleton is evidenced by the identification of several disease-causing mutations affecting core components of the actin cytoskeleton (e.g., ACTN4, INF2).2,6
Rho guanosine 5ʹ-triphophatases (GTPases) are essential and versatile modulators of the actin cytoskeleton. In fact, there is ample evidence for the profound role of the prototype Rho GTPases RAC1, CDC42, and RHOA in podocyte development and disease.2,6 Increased activation of these Rho GTPases is frequently observed in glomerular disease.2,6 Moreover, in vivo models substantiated the causative relationship between RAC1, CDC42, and RHOA activity and podocyte disease phenotypes.7–12 On the contrary, inactivation of the very same proteins has also been shown in conditions of podocyte disease.9,12 These observations led to the speculation that context-dependent balancing and titration of Rho GTPase activity is a major determinant for the maintenance of the integrity of the kidney filtration barrier.6 Mechanistically, Rho GTPases form a molecular switch for cytoskeleton signaling by cycling between a GTP-bound (active) and GDP-bound (inactive) state.13 Activity of Rho GTPases is tightly regulated by Rho guanine nucleotide exchange factors (RhoGEFs) and Rho GTPase-activating proteins (RhoGAPs). In fact, at least 20 Rho GTPases and >140 RhoGEFs and RhoGAPs are described in humans, highlighting the complexity of this regulatory machinery.14,15 However, there is only limited knowledge concerning the regulation and cell type–specific composition of the Rho GTPase network in podocytes.16
Recently, advances in omics technologies led to the comprehensive description of the podocyte proteome and transcriptome in vivo. In addition, very recently, the Rho GTPase affinity and proximity interaction network and the activities of RhoGAP and RhoGEF were comprehensively described.15,17–19 Here, we provide a podocyte-specific expression atlas for Rho GTPases, RhoGEFs, and RhoGAPs, and defined the podocyte-specific Rho GTPase affinity interaction network. On the basis of this in silico analysis, we identify SRGAP1 as a podocyte-specific RhoGAP. By modulating Rho GTPase activity and actomyosin contractility, SRGAP1 controls podocyte cell protrusion and plasticity in vitro and in vivo.
Methods
Animals
Mice were housed, according to the German law for the welfare of animals and to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, in a specific pathogen–free facility and kept in a 12-hour day/night cycle with free access to chow and water. Genotyping and breeding of animals was performed according to standard procedures. Mice carrying a conditional allele for Srgap1 (Srgap1tm1a[KOMP]Wtsi) were purchased from the Knockout Mouse Project, University of California, Davis. To generate deletion of Srgap1 in the metanephric mesenchyme, Srgap1flox/flox mice were crossed to Six2-Cre animals.20,21 For generation of a podocyte-specific knockout (KO) model, Srgap1flox/flox mice were crossed to hNPHS2-Cre animals (generously provided by L. Holzmann, School of Medicine, University of Pennsylvania, Philadelphia, PA).22 Respective offspring were backcrossed on a C57BL6 (SV129/C57BL6-mixed) genetic background for six generations. In the nephropathy experiments, 6-week-old mice were challenged using doxorubicin according to standard protocols.23 The age of animals used for the respective experiments is stated in the figures and/or figure legends (male and female animals showed similar phenotypes and were combined for the analysis). All animal experiments were approved by the local authorities (Regierungspräsidium Freiburg, G14-43).
Measurement of Urinary Albumin and Creatinine
Albumin and creatinine levels were quantified by measuring spot urine from wild-type and KO mice at defined time points. Proteinuria was expressed as albumin-creatinine ratio. Assessment of urinary albumin was performed using a mouse-specific, albumin fluorescent–based kit (Progen Biotechnik GmbH, Heidelberg, Germany). Measurement of creatinine was performed using an enzymatic creatinine kit (Creatinine PAP LT-SYS; Labor & Technik Eberhard Lehmann GmbH, Berlin, Germany).
Antibodies
The antibodies used in this study were described in detail in Supplemental Table 1. Fluorescence-labeled phalloidin (Thermo Fisher Scientific) was used to visualize fibrillary actin (F-actin).
In Situ Hybridization and LacZ Staining
In situ hybridization and LacZ staining of kidney sections were previously described in detail.24 The primers used for the generation of in situ probes are described in Supplemental Table 2. Overviews images of in situ hybridizations are stitched from multiple pictures.
Scanning Electron Microscopy, Transmission Electron Microscopy, and Immunogold Transmission Electron Microscopy
Sample preparation, processing, and analysis for transmission electron microscopy (TEM), scanning electron microscopy, and Immunogold TEM were performed as previously described.24,25 In brief, for TEM, small pieces of the renal cortex were dissected and cut into cubes of about 2×2×2 mm, using razor blades. Samples were transferred into glass vials and immersion fixed using 4% paraformaldehyde (PFA) and 1% glutaraldehyde in PBS overnight at 4°C. The tissue was postfixed in 1% osmium tetroxide in 6.86% saccharose in 0.1 M phosphate buffer for 30 minutes, and washed six times in 0.1 M phosphate buffer. Dehydration was performed by 15-minute incubations in 30% ethyl alcohol (EtOH) and 50% EtOH. The tissue was incubated in 1% uranyl acetate in 70% EtOH overnight at 4°C, and then further dehydrated in increasing concentrations of EtOH and finally in propylene oxide. After embedding in Durcupan resin, ultrathin sections were sliced using a UC7 Ultramicrotome (Leica), collected on Formvar‐coated copper grids. Poststaining was done for 1 minute with 3% lead citrate, followed by imaging using a Zeiss Leo 912 transmission electron microscope. The Immunogold TEM procedures were as follows: kidney samples were fixed using 4% PFA in phosphate buffer by perfusion via renal arteries using small silicone catheters. After perfusion fixation, the kidneys were removed and sliced into 50-µm vibratome sections. Anti-SRGAP1 antibody was incubated overnight at 4°C, and sections were washed in phosphate buffer and incubated overnight in secondary antibody at 4°C (1.4 nm Nanogold; Nanoprobes Inc., Yaphank, NY). Gold labeling was enhanced using the HQsilver kit (Nanoprobes Inc.). Finally, sections were embedded, sectioned, and imaged as described above.
Super-Resolution Microscopy
For evaluation of slit-diaphragm density as a direct marker for podocyte FP effacement, we applied a recently established, super-resolution microscopy (SIM)–based technique known as the podocyte effacement measurement procedure (PEMP), as previously described.25,26 Filtration-slit-density values of 20 glomeruli of three mice per group were quantified.
Histology and Immunofluorescence Staining of Kidney Sections
In short, kidneys were perfusion fixed with 4% PFA in PBS via the renal artery and subsequently immersion fixed in 4% PFA in PBS for 24 hours. Kidney sections (1–2 µm thick) of formalin-fixed, paraffin-embedded (FFPE) tissue were generated as previously described.25 FFPE sections were deparaffinized, rehydrated, and then underwent heat-induced antigen retrieval (Tris-EDTA buffer, pH 9, 5–15 minutes, pressure cooker). Tissue was blocked with 5% BSA in PBS, incubated with primary antibodies in blocking solution for 2 hours, and then incubated with secondary antibodies for 45 minutes. We used Periodic acid–Schiff staining to analyze the histology of FFPE tissue sections. Glomerulosclerosis was scored as previously described by el Nahas et al.27 We performed immunofluorescence staining of frozen kidney sections (4 µm) as previously described.28 For the proximity ligation assay (PLA), the Duolink In Situ Orange Starter Kit Mouse/Rabbit (Sigma-Aldrich) was used according to the manufacturer’s instructions. Sections of Srgap1fl/fl*hNPHS2Cre mice were used as negative control, and quantification was performed using Fiji ImageJ. In brief, NPHS1 staining was used to create a mask of the podocyte compartment and the number of PLA dots was automatically counted within this selection mask. Use of human kidney material (samples were derived from unaffected areas of tumor nephrectomies and disease specimens from kidney biopsy samples) was approved by the Scientific-Ethics Committee of the Medical Center – University of Freiburg (EK-Freiburg 512/18).
Microscopy
Immunofluorescence analysis and image acquisition was performed using an inverted Zeiss Axio Observer and Axio Imager microscope equipped with an ApoTome.2, an Axiocam 702 mono camera, and the Colibri 7 illumination system. In addition, an inverted Zeiss Axio Imager microscope equipped with an Axiocam color was used. We used 100×, 63×, 40×, 20×, and 10× objectives and Zeiss fluorescence filter sets (49 4′,6-diamidino-2-phenylindole [DAPI], 38 green fluorescent protein [GFP], 43 HE dsRed, and 50 Cy5).
Cell Culture
Human immortalized podocytes29 were cultured in low-FCS epithelial growth medium (RPMI 1640 with l-glutamine, 0.2% FCS, 10 µg/ml ITS, 10 ng/ml recombinant human EGF, 3 µg/ml ascorbic acid, 20 ng/ml hydrocortisone, 50 pM triiodo-l-thyronine, 5 mM HEPES, 1:1000 Gibco MEM nonessential amino acids [11140050], 0.1 mM sodium pyruvate) according to standard cell-culture procedures. The cell lines were tested for mycoplasma contamination on a regular basis by PCR (Mycoplasma PCR Detection kit; Hiss Diagnostics GmbH) and by DAPI staining.
CRISPR/Cas9 and Lentiviral Transduction
SRGAP1-KO cells were generated using CRISPR/Cas9 genome editing technology in immortalized human podocytes. Single guide RNAs (gRNAs) targeting the human SRGAP1 gene were designed using the website-based platform CHOPCHOP (https://chopchop.cbu.uib.no).30 gRNA-1 (5′-ATTTGCTCCTGAACCAAGTA-3′) and gRNA-2 (5′-GGAGTCGTGCCGGCTGATGT-3′) were subcloned into the lentiCRISPRv2 plasmid, which was a gift from Feng Zhang (Addgene plasmid 52961; http://n2t.net/addgene:52961; RRID:Addgene_52961).31 Cloning was performed according to standard protocols. lentiCRISPRv2 plasmids (without gRNA) were used to generate negative (wild-type) control cells. Mouse full-length, amino-terminal GFP–tagged Srgap1, SRGAP1 F-BAR domain, or untagged Srgap1 were cloned in lentiviral pWPXLd plasmid, which was a gift from Didier Trono (Addgene plasmid 12258; http://n2t.net/addgene:12258; RRID:Addgene_12258). Template cDNA plasmids for SRGAP1 were a gift from Franck Polleux.32 Empty pWPXLd plasmid was used as control. Lentiviral particles were produced in HEK 293T cells according to standard protocols. Immortalized human podocytes were transduced by lentiviral particles and single cell clones of CRISPR/Cas9 genome-edited podocytes were generated. SRGAP1-KO clones and SRGAP1-overexpression podocytes were validated by Western blot analysis.
Analysis of Active Rho GTPase Levels
Cells were serum starved in RPMI 1640 medium containing 0% FCS for 24 hours. After serum starvation, cells were harvested and lysates were equalized due to protein content. GTP-CDC42, GTP-RAC1, and GTP-RHOA was measured using an ELISA-based activation assay kit according to manufacturer’s instructions (G-LISA, BK135; Cytoskeleton Inc.).
Cell-Spreading Assay
Cell-spreading assays were performed on collagen IV–coated, eight-well polymer coverslips (ibidi GmbH, Gräfelfing, Germany). Podocytes were detached by trypsinization, washed three times in culture medium, counted, diluted, and preincubated in culture medium (floating) in a 37°C cell-culture incubator for 20 minutes. Subsequently, podocytes were plated and spread for 30 minutes at 37°C. Cells were carefully washed with PBS containing 1 mM calcium chloride and magnesium chloride ions and fixed in 4% PFA in PBS for 20 minutes. Spreading experiments of GFP- and GFP-SRGAP1–expressing podocytes were performed in culture medium containing 10% FCS; the remaining spreading analysis was done in 0.2% FCS medium as outlined above. Cells were stained by phalloidin (F-actin) and analyzed by fluorescence microscopy. Image analysis of cell morphology was performed using Fiji ImageJ.
IAC, Podocyte Morphology, and Lamellipodia Analysis
Analysis of IACs, podocyte morphology, and lamellipodia was performed on human collagen IV–coated glass coverslips as previously described.28 In brief, cells were cultured for 24 hours on coverslips, carefully washed with PBS containing 1 mM calcium chloride and magnesium chloride ions, and fixed in 4% PFA in PBS for 15 minutes. Subsequently, podocytes were permeabilized using Triton X-100, blocked with 5% BSA in PBS for 1 hour, incubated with primary antibodies in blocking solution for 2 hours, and incubated with secondary antibodies including phalloidin (F-actin) and DAPI for 45 minutes. IACs were visualized by paxillin (PXN) staining. Cells were analyzed by fluorescence microscopy. Morphometric analysis of cells, IACs, and measurement of line scans were performed using Fiji ImageJ as previously described.28 Line scans, 3 µm long (starting at the cell edge), were used for the area-under-the-curve calculation of the lamellipodia region using GraphPad Prism 8. IACs >1.5 µm2 were defined as mature. For the respective treatment experiments, 1 µg/ml doxorubicin or 10 µM Y27632 was added to the culture medium for 24 hours.
Three-Dimensional Protrusion Formation of Human Podocytes
Three-dimensional (3D) protrusion formation of podocytes was performed similarly as previously described.25 In brief, 3D growth matrices were generated using Matrigel (Corning) according to the manufacturer’s instructions. We used a final Matrigel concentration of 6 mg/ml. We diluted 10,000 podocytes in a final amount of 60 μl of Matrigel solution and plated them in Matrigel-precoated, eight-well Labtek growth chamber slides. After 30 minutes, polymerized gels were covered by cell-culture medium containing 10 µM of the ROCK inhibitor Y27632 and cultivated for 24 hours. For the treatment experiments, 1 µg/ml doxorubicin was added to the culture medium for 24 hours. Samples were fixed in 4% PFA in PBS for 2 hours, washed, permeabilized using Triton X-100, stained for phalloidin (F-actin) and DAPI over 24 hours, and mounted with ProLong Gold Antifade Mountant. Maximum intensity projections of z-stack images were generated using the Zeiss ZEN2 software. Measurements of protrusions were done using Fiji ImageJ analysis software. The percentage of 3D “spread cells” was calculated by counting podocytes with visible protrusions as spread cells, using maximum intensity projections of z-stack from overview images. Overview images were acquired using a 10× objective.
Western Blot Analysis
Western blot analysis was performed using standard protocols. Cell lysates were generated by scraping podocytes in ice-cold RIPA lysis buffer containing protease and phosphatase inhibitors (Roche). After lysis for 15 minutes, we cleared cell detritus by centrifugation, and denatured cells in 2× Laemmli sample buffer with dithiothreitol at 95°C for 5 minutes.
Immunoprecipitation and Interactome Analysis
Immunoprecipitation (IP) and interactome analysis (Dataset 1) was performed as previously described.33 In brief, cells were labelled via stable isotope labeling by amino acids (SILAC) for 2 weeks (SILAC RPMI Lysine[6] Arginine[6] Kit; Silantes GmbH, Munich, Germany). Cells were lysed in Triton X-100 lysis buffer (1% Triton X-100, 20 mM Tris-hydrochloride, 50 mM sodium chloride, 50 mM sodium fluoride, 15 mM tetrasodium pyrophosphate, 1 mM EDTA in double-distilled water, pH 7.4) supplemented with proteinase and phosphatase inhibitors, and then cleared by centrifugation. Supernatants were adjusted to 2600 µg protein per sample and incubated for 1 hour at 4°C with 2 µg anti-SRGAP1 antibody (13252-1-AP) and subsequently with Protein A Affinity agarose gel for 30 minutes. Subsequently, four wash steps with IP lysis buffer were performed. Bound proteins were resolved in 2× Laemmli buffer with dithiothreitol and denatured at 95°C for 5 minutes. Lysates were separated by standard SDS-PAGE for Western blotting or processed for mass-spectrometry analysis. Mass spectrometry–based interactome analysis was performed as described.34 For data analysis, MaxQuant version 1.6.0.16 was used, requiring at least two unique peptides per protein, no requantifying option, requiring identification in all three replicates. For calculation of light/heavy ratios, zero-type signals were replaced with a minimum signal of 10,000 a.u. The proteome database (human reviewed entries) was downloaded from Uniprot on August 6, 2019. Annotation of Gene Ontology terms was performed using the Database for Annotation, Visualization and Integrated Discovery version 6.8.35 The Cytoscape version 3.8 software and the EnrichmentMap application were used for visualization of Gene Ontology terms and network generation.
Podocyte Rho GTPase Affinity Interactome
Datasets for analysis of podocyte-specific expression patterns of Rho GTPases, RhoGEFs, RhoGAPs, and for generation of a podocyte Rho GTPase affinity interactome were stated in Dataset 2 with all relevant information (for Rho GTPase, RhoGEF, and RhoGAP mapping also see Supplemental Tables 3 and 4). In brief, in vivo podocyte transcriptome and proteome datasets were merged on the basis of their UniProt accession number and Ensemble gene identifiers. Transcriptomics data were reanalyzed using GREIN (GEO RNA-seq Experiments Interactive Navigator) software36 to calculate transcripts per million. For differential-expression analysis, datasets were analyzed using the Galaxy platform (https://usegalaxy.eu/).37 Data were downloaded from the National Center for Biotechnology Information Sequence Read Archive and processed using the built in Mus musculus genome (mouse, December 2011, genome assembly GRCm38/mm10 [mm10]), fastp,38 HISAT2,39 featureCount,40 and DESeq2.41 RNA-sequencing data were combined and analyzed on the basis of this data. Literature-curated, Rho GTPase affinity interaction datasets were merged on the basis of their human UniProt/Ensembl identifiers, and cutoffs for Rho GTPase interactions were defined as stated in Dataset 2. Homologous genes for human and mouse were annotated using the Mouse Genome Informatics and UniProt database.42,43 A total of 20 reported members of the Rho GTPase family in mammals, RhoGEF, and RhoGAP proteins were analyzed for podocyte-specific expression.44 RhoGEFs (IPR035899) and RhoGAPs (IPR000198) were annotated using the InterPro database.45 Threshold values and generation of analyzed gene/protein lists are stated in respective figures, figure legends, and Dataset 2. The Cytoscape version 3.8 software was used for generation of a podocyte-specific Rho GTPase affinity interaction network (threshold values for podocyte-specific Rho GTPase interactors: transcripts per million >50, log2 fold-change transcriptome >0.5, and mean log2 fold-change proteome >0.5).46
Quantification and Statistical Analyses
If not stated otherwise, data are expressed as mean±SEM. Scatter dots indicating individual data points were used for statistical analysis. Unpaired t test, Welch t test, Mann–Whitney test, or one-way ANOVA test (Tukey multiple comparison test) were used on the basis of data distribution. GraphPad Prism 8 software was used for analysis. Statistical significance was defined as *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001. The number of independent experiments and total amount of analyzed cells/samples are stated in the figure legends. The Nephroseq database (www.nephroseq.org) was used for analysis of SRGAP1 expression in human glomerular disease.
Results
Generation of a Podocyte Rho GTPase Affinity Interactome Identifies SRGAP1 as a Podocyte-Specific RhoGAP
Rho GTPases form the main regulatory layer for actin cytoskeleton–associated cellular processes in podocytes (Figure 1A). Here, we generated a podocyte-specific expression atlas by in silico analysis of transcriptome and proteome data from isolated murine podocytes that was recently published by us and others.28,47–49 We used this multiomics dataset to analyze expression levels and specificity of 20 known Rho GTPases in podocytes (Figure 1, Supplemental Figure 1, Supplemental Tables 1 and 2, Dataset 2). This analysis demonstrated high expressional values for three well-established Rho GTPases—Rhoa, Rac1, and Cdc42—in podocytes (Figure 1, B and C). Although specificity for Rho GTPase expression was not detected, analyzing expression levels of RhoGEFs and RhoGAPs demonstrated a cell type–specific expression pattern (Figure 1, D and E). Thus far, this in silico analysis resulted in the identification of only less-recognized, podocyte-specific RhoGAP and RhoGEF proteins, such as SRGAP1. Furthermore, the hereditary nephrotic syndrome–associated RhoGAP ARHGAP24 and the recently described, podocyte-specific RhoGEF ARHGEF18 showed a very specific and strong enrichment, indirectly validating our mapping approach.28,50 To gain a more specific view of Rho GTPase signaling in podocytes on the level of individual Rho GTPases, we used recently published Rho GTPase affinity interaction screens for further in silico analysis (Figure 1F).15,17–19 Mapping of this podocyte-specific, enriched, Rho GTPase affinity interactome confirmed RAC1, CDC42, and RHOA as a central signaling hub of the podocyte cytoskeleton. Further validation of SRGAP family members by mRNA in situ hybridization confirmed specific expression for SRGAP1 and SRGAP2, but not SRGAP3, in podocytes (Figure 1G, Supplemental Figure 2).
Figure 1.
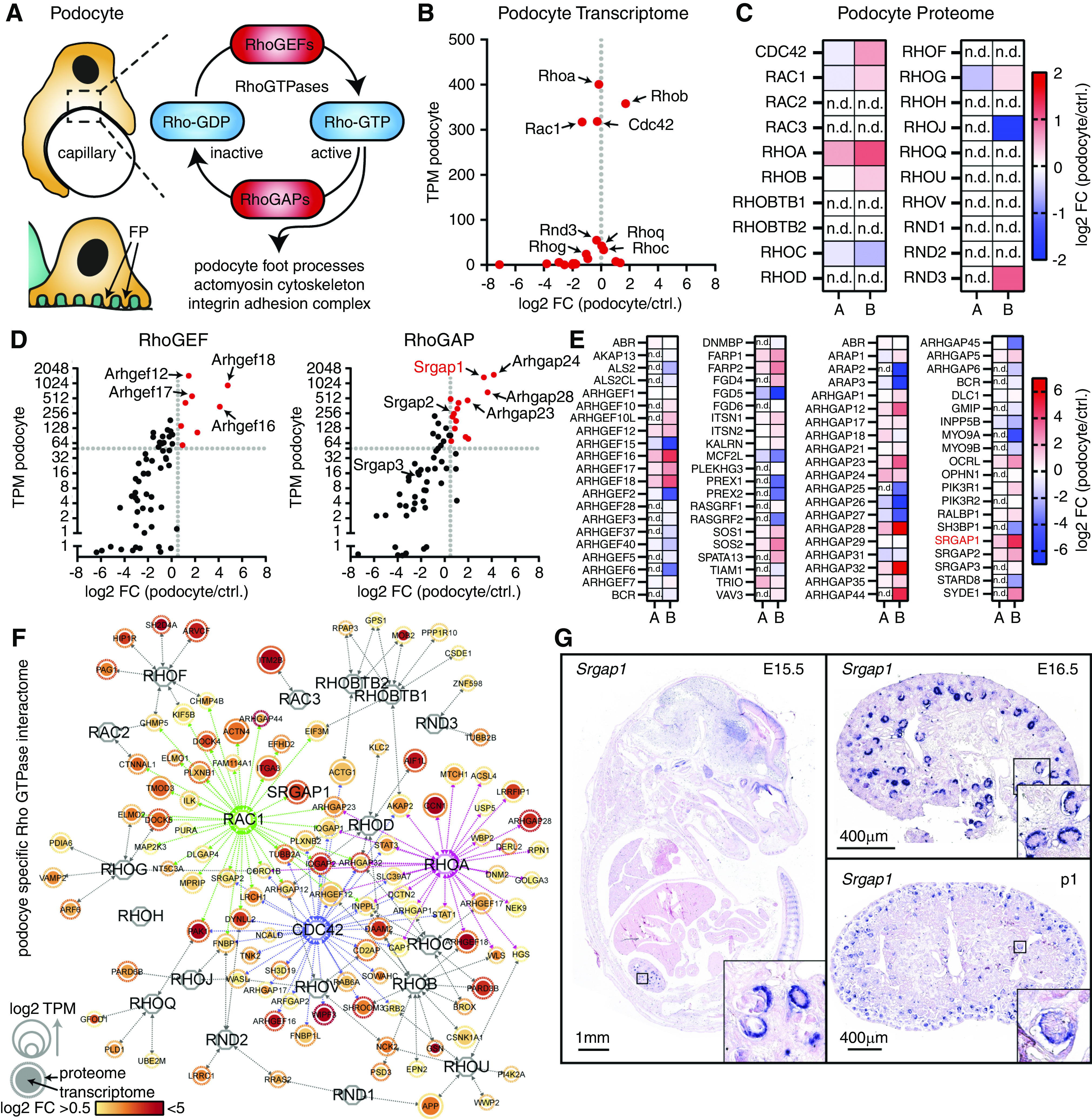
Generation of the podocyte Rho GTPase affinity interactome identifies SRGAP1 as a podocyte-specific RhoGAP for RAC1. (A) Schematic illustrating podocyte morphology. Rho GTPases and RhoGAP/RhoGEFs form a regulatory interface influencing central cytoskeletal functions in podocytes. (B and C) Analysis of podocyte transcriptome and proteome datasets for Rho GTPases demonstrate relative enrichment of Rac1, Cdc42, Rhoa, and Rhob. “A” indicates proteome by Schell et al.28 and “B” indicates proteome by Rinschen et al.49 (D and E) Analysis of podocyte transcriptome and proteome datasets allows for a selection of podocyte-enriched RhoGEF and RhoGAP proteins. (F) Rho GTPase affinity interactome mapping confirmed RAC1, CDC42, RHOA, and RHOB as central nodes for cytoskeletal signaling in podocytes. Nodes indicate proteins/genes and node size indicate TPMs; color gradients indicate log2FCs; arrows indicate GTPase proximity interactions for podocyte-specific proteins. (G) mRNA in situ hybridization confirmed podocyte-specific expression of Srgap1 starting in murine development at embryonic day 15.5 (E15.5) (boxes indicate magnified areas). Ctrl., control; FC, fold change; n.d., not detected; TPM, transcripts per million.
Loss of Srgap1 Causes Nephrotic Syndrome, FSGS, and Podocyte Depletion
These findings were further corroborated by analysis of a LacZ reporter mouse model combined with immunofluorescence studies on human and murine kidneys (Figure 2, A and B, Supplemental Figure 3). Hyperactivation of RAC1 is a common feature of glomerular disease.7,8 To evaluate the relevance of Srgap1 in limiting Rho GTPase activation, we generated a podocyte-specific KO mouse model. On the basis of the expression patterns of Srgap1 during early glomerular development (Figure 2B), we generated Srgap1fl/fl*Six2Cre mice (Figure 2, C and D).21 The KO mice did not show any developmental abnormalities and exhibited no increase in proteinuria levels at birth (Figure 2E). Follow-up analysis of Srgap1fl/fl*Six2Cre mice detected progressive loss of filtration-barrier function, starting at 6 weeks after birth. Moreover, albuminuria was accompanied by histopathologic alterations, including formation of proteinaceous casts, tubular dilation, and FSGS. These pathologic features resemble histologic criteria of human FSGS (Figure 2, F and G, Supplemental Figure 4). In addition, Srgap1fl/fl*Six2Cre-KO mice showed a pronounced rarefaction and loss of slit diaphragms, which was accompanied by progressive podocyte loss (Figure 2, H–L). Together, these findings indicate a critical and specific role of SRGAP1 in the maintenance of the function of podocytes and the kidney filtration barrier.
Figure 2.
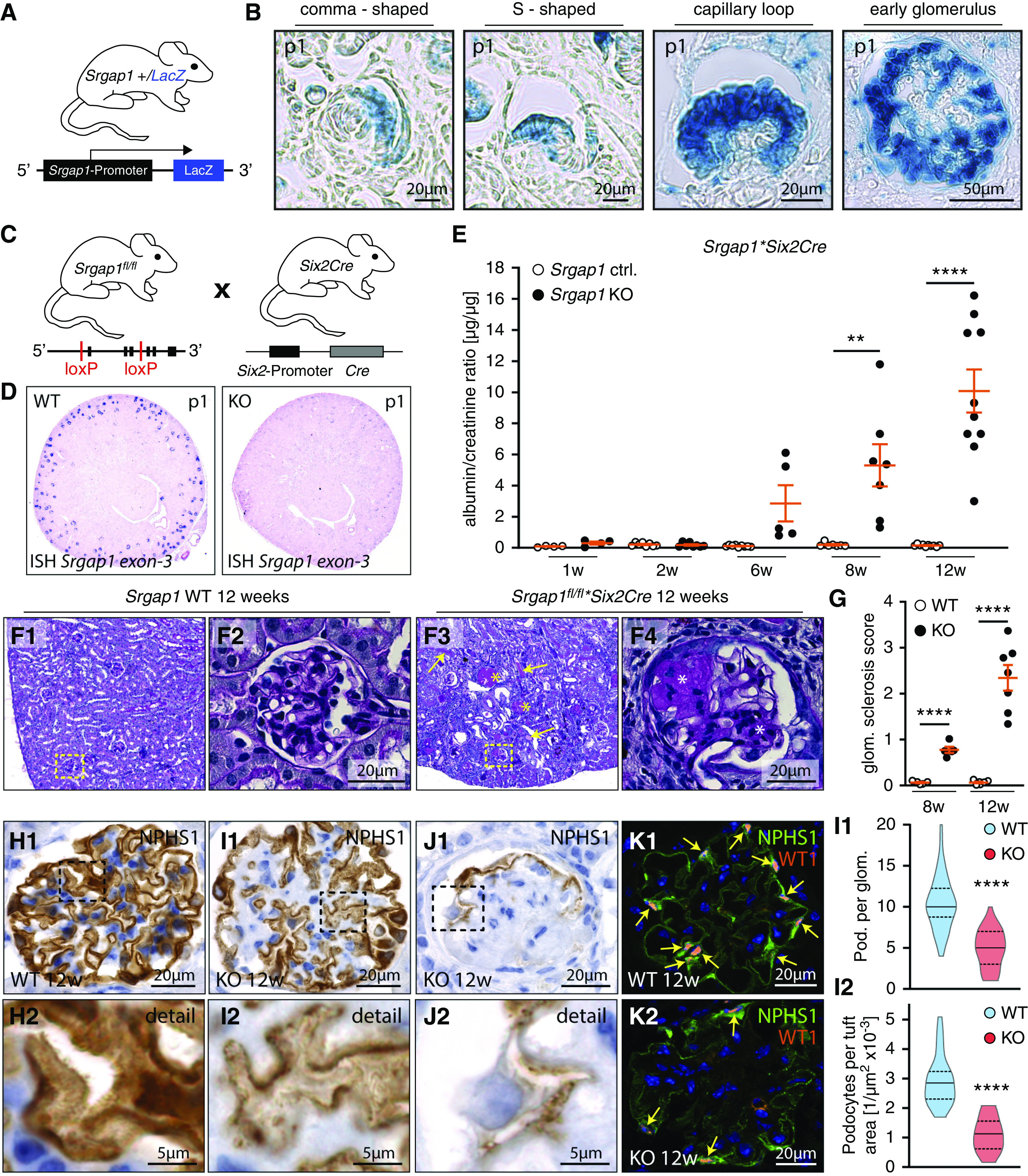
Loss of Srgap1 causes nephrotic syndrome, FSGS, and podocyte depletion. (A and B) Specific expression of Srgap1 in developing podocytes throughout all stages of glomerular maturation was demonstrated by the use of a LacZ reporter model. (C and D) Schematic illustration of the generation of Srgap1fl/fl*Six2Cre mice. Selective KO efficiency, restricted to the glomerular compartment, was confirmed by mRNA in situ hybridization (ISH) for Srgap1. (E) Progressive proteinuria was detected in adult Srgap1*SixCre-KO mice with an onset at 6 weeks after birth (dots indicate individual animals). (F) Loss of Srgap1 in podocytes led to FSGS accompanied by proteinaceus casts and tubular dilation. Yellow arrows indicate proteinaceous casts; yellow asterisks mark affected glomeruli; boxes show area of magnification; white asterisk indicate mesangial proliferation and sclerosis. (G) Quantification of glomerulosclerosis of 8- and 12-week-old Srgap1fl/fl*Six2Cre control and KO mice (dots indicate individual animals; >50 glomeruli per animal were analyzed). (H–L) Immunostaining demonstrated a rarefaction of the slit-diaphragm marker NPHS1 in Srgap1-KO mice accompanied by podocyte detachment. Nuclear staining of WT1 marks podocytes; yellow arrows indicate individual podocytes. Three animals for each genotype and at least 15 glomeruli per animal were analyzed. Violin plots indicate median and quantiles. **P<0.01, ****P<0.0001. Ctrl., control; glom., glomerulus; pod., podocyte; WT, wild type.
SRGAP1 Is Required for Podocyte FP Maintenance
Because Srgap1fl/fl*Six2Cre-KO mice presented with a late-onset phenotype, we aimed to evaluate the role of SRGAP1 in fully developed and differentiated podocytes by using the hNPHS2Cre deleter strain (Figure 3, A and B, Supplemental Figure 4). Interestingly, Srgap1fl/fl*hNPHS2Cre-KO mice did not develop any signs of proteinuria up to an age of 24 weeks (Figure 3, C and D). This discrepancy in phenotype presentation between both deletion approaches (Six2Cre versus hNPHS2Cre) might be attributed to a delayed deletion onset allowing for initiation of compensatory gene regulatory programs (e.g., expression of RhoGAPs). However, expression levels of SRGAP2 appeared only slightly increased in Srgap1fl/fl*hNPHS2Cre mice (Supplemental Figure 4). Therefore, developmental alterations due to a loss of Srgap1 can potentially explain the more severe phenotype observed in Srgap1fl/fl*Six2Cre-KO mice. Given the FSGS-like phenotype in Srgap1fl/fl*Six2Cre mice, we hypothesized that loss of SRGAP1 might exert protective functions in conditions of podocyte stress. To test this hypothesis, we analyzed localization patterns for SRGAP1 in well-established mouse models of glomerular disease and human biopsy specimens (Figure 3E, Supplemental Figure 5). Here, increased recruitment of SRGAP1 to regions devoid of slit diaphragms was observed in mildly diseased murine glomeruli and in human FSGS biopsy samples, implying a compensatory cytoskeletal response mediated by SRGAP1. The pathophysiologic relevance of SRGAP1 for the integrity of the glomerular filtration barrier was furthermore corroborated by mRNA expression analysis of various glomerular disease entities demonstrating a pronounced downregulation (Supplemental Figure 5). Finally, we evaluated these findings by applying the well-established doxorubicin nephropathy model to the Srgap1fl/fl*hNPHS2Cre strain (Figure 3F). Indeed, loss of SRGAP1 significantly increased the susceptibility of podocytes toward injury by doxorubicin, which was characterized by the detection of high levels of albuminuria (Figure 3G) and histologic lesions (Figure 3, H and I) in Srgap1fl/fl*hNPHS2Cre-KO mice. Together, these observations imply an adaptive and protective function for SRGAP1 in podocyte health and disease.
Figure 3.
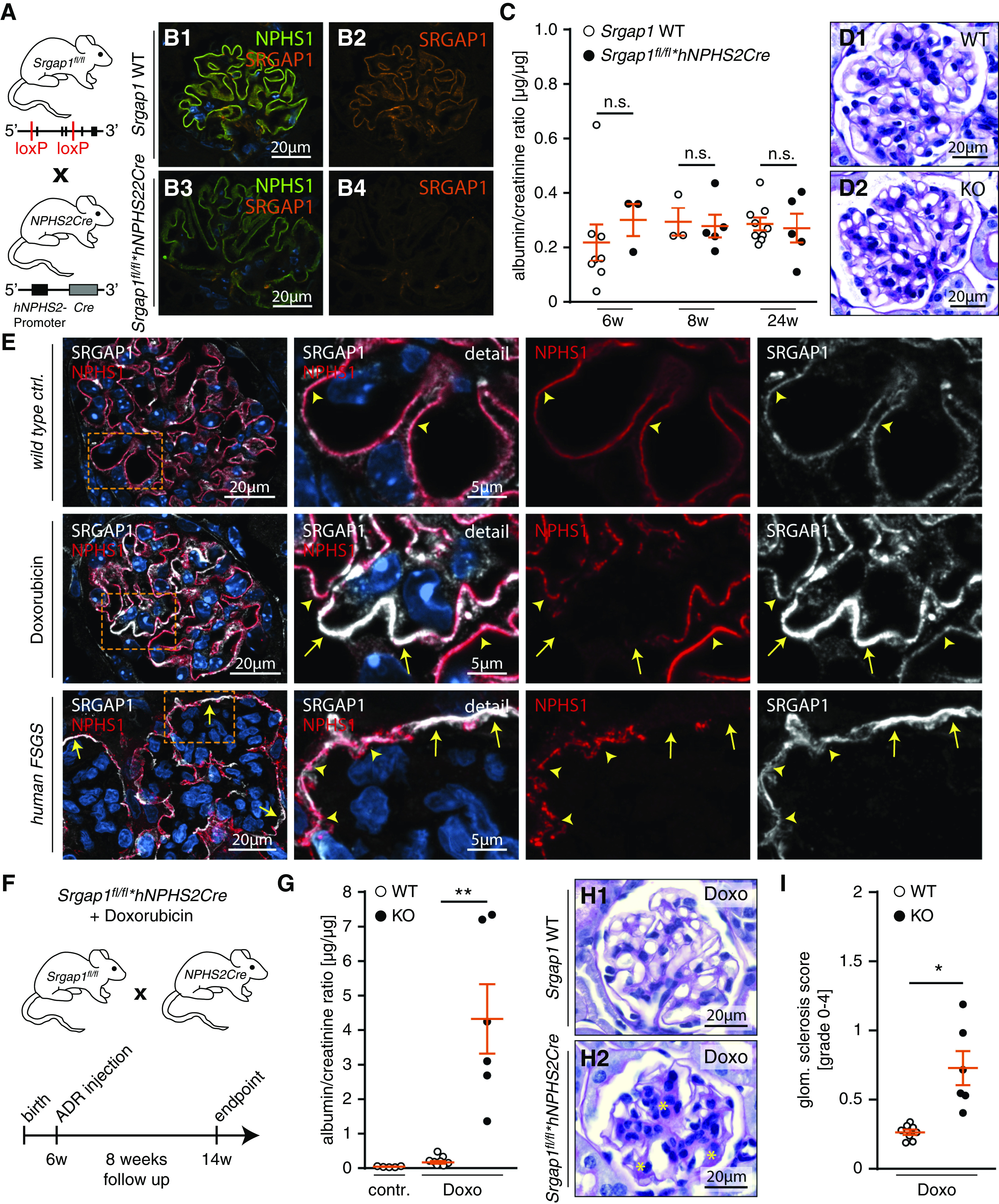
SRGAP1 is required for podocyte FP maintenance. (A) Schematic illustrating generation of podocyte-specific KO mice by crossing Srgap1fl/fl to hNPHS2Cre mice. (B) Immunofluorescence of SRGAP1 confirmed selective loss of SRGAP1 in podocytes of Srgap1fl/fl*hNPHS2Cre mice. (C and D) Loss of SRGAP1 did not result in an overt renal phenotype, as assessed by measurement of albuminuria and histologic analysis up to an age of 24 weeks (each dot indicates individual animals). (E) Immunofluorescence analysis of murine doxorubicin (Doxo) FSGS model and human FSGS demonstrated increased recruitment of SRGAP1 to glomerular regions with diminished levels of NPHS1 signal intensities (indicative of slit diaphragm degradation, yellow arrows). Yellow arrowheads mark regions with unaffected NPHS1 and SRGAP1 signals; boxes indicate magnified areas. (F) Schematic depicting the time course of the doxorubicin nephropathy model in Srgap1fl/fl*hNPHS2Cre mice. (G) Analysis of urinary albumin-creatinine ratio showed increased levels of proteinuria in Srgap1fl/fl*hNPHS2Cre-KO mice challenged with doxorubicin (dots indicate individual animals. (H and I) Histology and quantification of glomerulosclerosis confirmed increased susceptibility of Srgap1fl/fl*hNPHS2Cre mice to doxorubicin-induced podocyte injury. Yellow asterisks indicate segmental sclerosis and mesangial cell proliferation; dots indicate individual animals; >50 glomeruli per animal were analyzed. *P<0.01, **P<0.01. Ctrl., control; WT, wild type.
SRGAP1 Localizes to Podocyte Protrusions and Integrin Adhesions In Vivo and In Vitro
Given the profound translocalization of SRGAP1 in podocyte disease, we aimed to specify localization patterns of SRGAP1 under physiologic conditions. To determine the subcellular localization of SRGAP1, we performed PLAs and colocalization studies in vivo (Figure 4, A–C). This analysis demonstrated spatial recruitment patterns (<40 nm) of SRGAP1 to IACs, as indicated by integrin linked kinase and ITGB1, to the proximity of the slit-diaphragm complex, as indicated by NPHS2, and to the cortical actin cytoskeleton, as indicated by cortactin. These observations were further validated by Immunogold TEM for SRGAP1, showing SRGAP1 localization patterns to the basolateral FP compartment and to the plasma membrane of podocyte processes in vivo (Figure 4D). To gain further insights into the recruitment of SRGAP1 to these subcellular compartments, we used human immortalized podocytes stably expressing a GFP-SRGAP1 reporter protein (Figure 4, E and F, Supplemental Figure 6). In line with our in vivo observations, SRGAP1 localized to podocyte protrusions in two-dimensional (2D) and 3D culture environments, and to lamellipodia and matured IACs in 2D. Interestingly, plasma-membrane and filopodia recruitment of SRGAP1 was previously linked to its F-BAR domain function, allowing for direct binding to negatively charged phospholipids.32,51 As expected, sole expression of the SRGAP1 F-BAR domain was sufficient to localize an F-BAR reporter protein to cell membranes, lamellipodia, and filopodia in podocytes (Supplemental Figure 6). However, only very weak recruitment to IACs was detectable for the F-BAR domain reporter protein. Upon doxorubicin challenge, podocytes showed a pronounced translocation of SRGAP1 in vivo (Figure 3E). Similarly, in vitro treatment of podocytes with doxorubicin led to plaquelike IAC assembly and F-actin rearrangement showing colocalization with SRGAP1 (Figure 4G). Because IAC formation is a RHOA/ROCK/actomyosin signaling–dependent mechanism,52 we hypothesized treatment with the ROCK inhibitor Y27632 might influence SRGAP1 localization patterns. Indeed, we observed an enrichment of SRGAP1 to sites of cell-membrane protrusions as a consequence of the disassembly of IACs (Figure 4H). These observations imply an actomyosin-dependent mechanism overwriting F-BAR–mediated SRGAP1 localization patterns.
Figure 4.
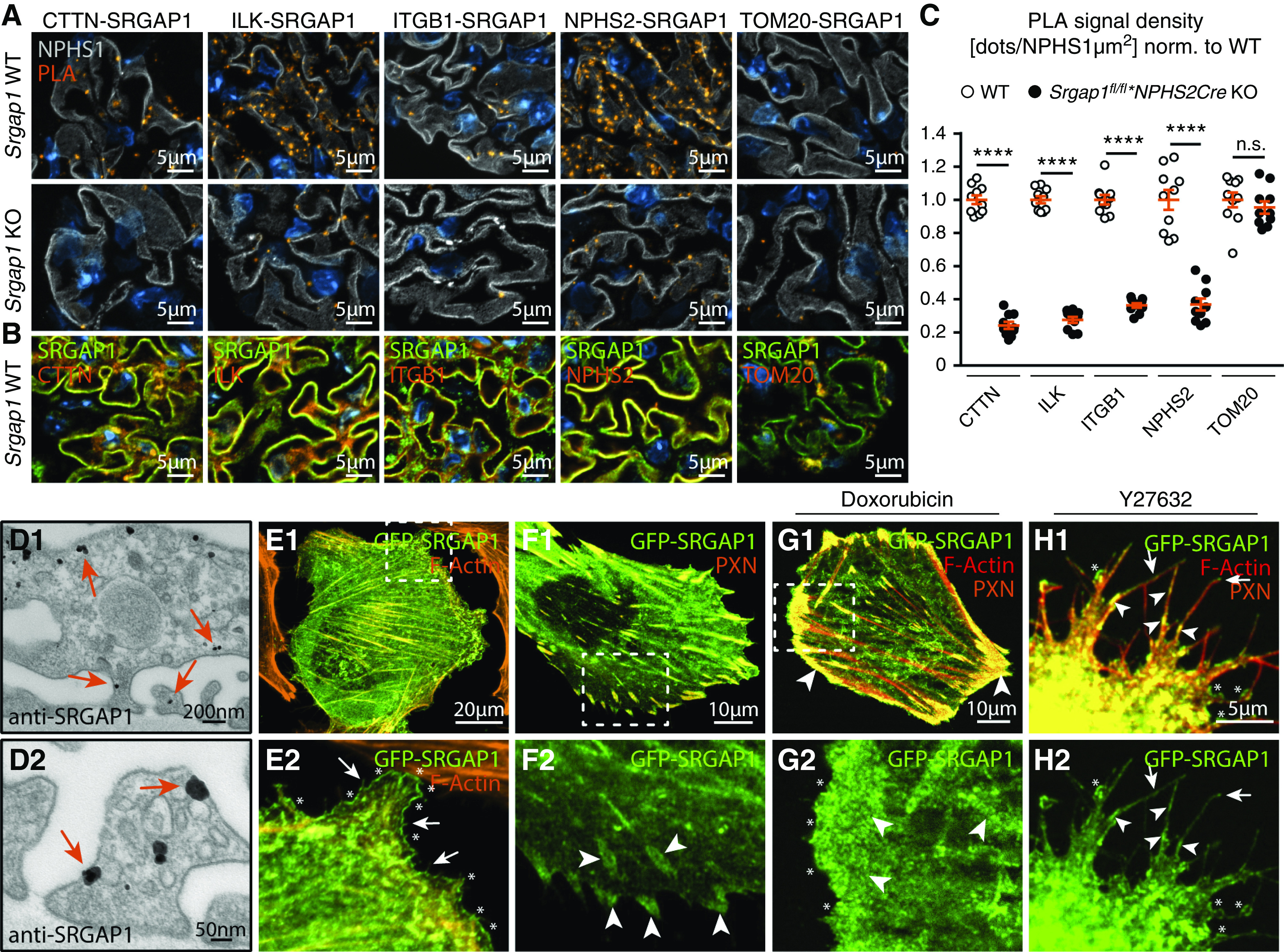
SRGAP1 localizes to podocyte protrusions and integrin adhesions in vivo and in vitro. (A) PLA for SRGAP1 and cortactin (CTTN), integrin linked kinase (ILK), integrin β-1 (ITGB1), podocin (NPHS2), and TOM20 indicated proximity recruitment of SRGAP1 to IACs, slit diaphragms, and to the cortical actin cytoskeleton in vivo (NPHS1 was used as podocyte marker). (B) Immunofluorescence staining confirmed colocalization of SRGAP1 with CTTN, ILK, ITGB1, and NPHS2. (C) Quantification of podocyte PLA signals (ten glomeruli per genotype were analyzed). (D) Immunogold TEM highlighted localization of SRGAP1 to the basolateral compartment of podocyte FPs and to the whole cortical cytoskeleton of podocytes in vivo (arrows indicate immunogold dots). (E and F) GFP-SRGAP1 localized to cell membranes, lamellipodia (asterisks), filopodia (arrows), and IACs (arrowheads) in 2D. Podocytes were costained for F-actin and the IAC marker paxillin (PXN) (boxes indicate magnified areas). (G) Challenging podocytes with doxorubicin induced formation of GFP-SRGAP1–positive IAC plaques. (H) Subcellular distribution of GFP-SRGAP1 depended on ROCK/actomyosin activity, as indicated by application of the ROCK inhibitor Y27632 (overexposed fluorescence signals to visualize cell protrusions). ****P<0.0001. Norm., normalized; WT, wild type.
SRGAP1 Controls Lamellipodial Extension and Spreading, and Stabilizes Actomyosin Contractility of Podocytes In Vitro
To further analyze SRGAP1-dependent mechanisms instructing the podocyte cytoskeleton, we used CRISPR/Cas9 genome editing technology to generate SRGAP1-deficient and -overexpressing immortalized human podocyte cell lines. We successfully generated monoclonal SRGAP1-KO clones from two independent single gRNAs and finally confirmed loss of SRGAP1 and overexpression by Western blot (Figure 5A, Supplemental Figure 7). In the SRGAP1-KO cells, we observed increased activity levels for RAC1 and RHOA, whereas a converse activity pattern was present in SRGAP1 overexpression lines (Supplemental Figure 7). Given the subcellular localization of SRGAP1 and the critical role of cell protrusions for podocyte function, we performed cell-spreading assays. SRGAP1-KO clones demonstrated an increased spreading ability on collagen IV, whereas SRGAP1 overexpression resulted in significantly impaired spreading capabilities (Figure 5, B–G). Detailed morphometry analysis showed that loss of SRGAP1 also resulted in an increased formation of lamellipodia under dynamic (Supplemental Figure 8) and stationary conditions (Figure 5, H and I, Supplemental Figure 8). In line with these observations, surface area/size was increased in KO podocytes and, conversely, decreased in SRGAP1-overexpression cells (Supplemental Figure 8). Together, these findings indicate a clear correlation between SRGAP1 expression levels and lamellipodia formation and cell protrusion. To further understand the underlying mechanisms of SRGAP1 signaling in regulating the protrusion machinery of podocytes, we performed SILAC-based, quantitative affinity interaction proteomics for SRGAP1 (Supplemental Figure 9 and Dataset 1). Interestingly, SRGAP1 associated to the myosin regulatory light chain 2 (MYL9), an essential regulator of the actomyosin machinery and protrusion formation.52–54 Cell protrusion and adhesion essentially relies on a fine-tuned coordination of RHOA-dependent contractility and RAC1-dependent protrusive signaling (e.g., by defining spatiotemporal activity zones for Rho GTPases55,56). To test whether SRGAP1 modulates localization and activity patterns of MYL9, we analyzed the spatial distribution and activation levels of phosphorylated MYL9 (indicative for contractile actomyosin activity). Here, SRGAP1-KO podocytes showed impaired actomyosin activation in the lamellipodia and lamella region and during cell spreading (Figure 5, J–L, Supplemental Figure 8). Together, these findings indicate an overall lower actomyosin contractility level in protrusive compartments of KO cells. The decoupling of globally increased, active RHOA levels and decreased phosphorylated-MYL9 signals in lamellipodia of KO cells can be attributed to the potential spatiotemporal separation of SRGAP1-dependent Rho GTPase regulation. However, other mechanisms, such as compensatory RHOA activation or altered cytoskeleton structure, might also contribute to this observation. In line with the first concept, a recent study suggested spatial stabilization of contractile signaling at mature IACs by tethering RAC1-specific RhoGEFs.15 On the basis of that hypothesis, we analyzed IACs of SRGAP1-KO podocytes and detected a reduced size of mature IACs, whereas SRGAP1 overexpression resulted in the opposite phenotype (Figure 5, M–O). Together, these significant phenotypic data support a RAC1-dependent concept, where SRGAP1 mediates spatial inhibition of RAC1-dependent signaling at IACs and lamellipodia to stabilize actomyosin-dependent contractility.15
Figure 5.
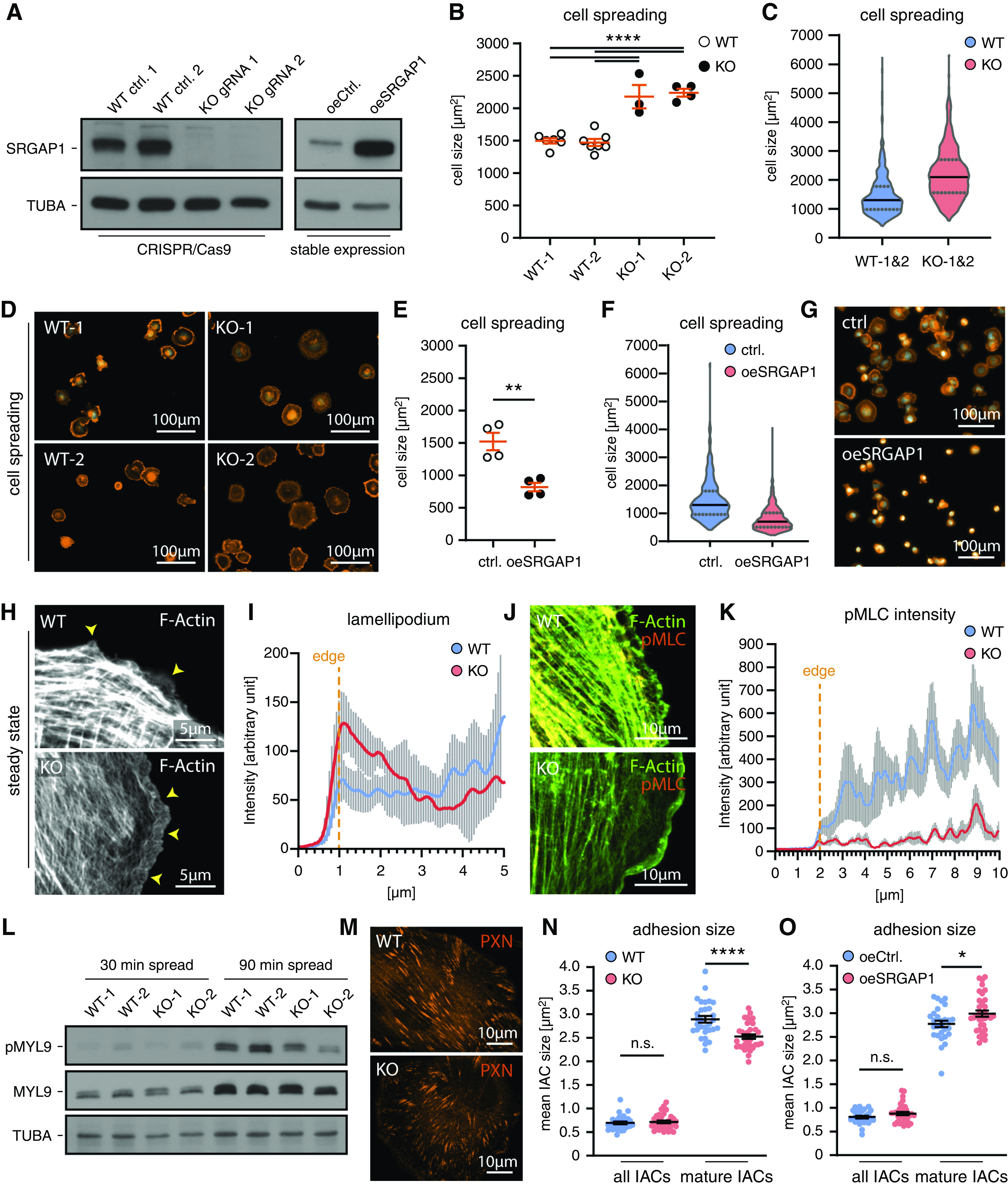
SRGAP1 controls lamellipodial extension and spreading and stabilizes actomyosin contractility of podocytes. (A) Immunoblot experiments confirmed efficient loss of protein in CRISPR/Cas9-engineered SRGAP1-KO podocytes and increased protein levels in stable SRGAP1-overexpressing cells. (B–D) Loss of SRGAP1 increased podocyte spreading on collagen IV (dots indicate independent experiments; >50 cells per experiment and genotype; violin plots show data distribution of 815 WT [WT1&2, pooled data from WT-1 & WT-2] and 578 SRGAP1 KO [KO1&2, pooled data from KO-1 & KO-2] podocytes). (E–G) Overexpression of SRGAP1 inhibited podocyte spreading (dots indicate independent experiments; >128 cells per experiment and genotype; violin plots of 638 control and 684 SRGAP1-overexpression podocytes). (H and I) SRGAP1-KO podocytes showed extended and widened lamellipodia (arrowheads) and lamella at steady state conditions. Line scan analysis indicated increased fluorescence intensity within lamellipodial F-actin networks (ten cells per condition were analyzed; lines show mean and error bars indicate SDs). (J and K) Immunofluorescence analysis of phosphorylated-Thr18/Ser19 myosin regulatory light chain 2 (pMYL9) demonstrated reduced signals at the cell periphery and concomitantly low pMYL9 activity at lamellipodia and lamella of SRGAP1 KO podocytes (line scans were analyzed for 11 cells per genotype; lines show mean and error bars indicate SEM). (L) Western blot analysis of pMYL9 demonstrated impaired pMYL9 activation in spreading SRGAP1-KO podocyte. (M–O) Quantification of IAC size show reduced size of matured IACs in SRGAP1-KO podocytes and increased size in SRGAP1-overexpressing podocytes (30 WT, 34 SRGAP1 KO, 28 control, and 35 SRGAP1-overexpression cells were analyzed; scatter dots indicate individual cells). *P<0.05, **P<0.01, ****P<0.0001. Ctrl., control; oe, overexpressed; WT, wild type.
SRGAP1 Instructs Podocyte 3D Protrusion Formation
Podocyte processes are specialized cellular protrusions with a complex morphology of primary and secondary processes. However, 2D cell-culture systems have certain limitations to model these 3D protrusion phenotypes. Using a recently established 3D podocyte culture system revealed impaired protrusion generation and length in SRGAP1-KO cells and strongly increased protrusion generation and length by overexpression of SRGAP1 (Figure 6, A–E).25 Moreover, protrusions of SRGAP1-KO podocytes appeared less branched and shorter, whereas SRGAP1-overexpression cells showed the opposite phenotype. Given the protective function and basal accumulation of SRGAP1 in podocyte nephropathy models in vivo (Figure 3), we tested if SRGAP1 mitigates podocyte injury in vitro. Here, doxorubicin treatment resulted in major impairment of protrusion formation of 3D cultured podocytes. On the contrary, high levels of SRGAP1 expression promoted protrusion formation (Figure 6, F and H). Multidimensional analysis, including SIM and TEM/scanning electron microscopy, of podocyte FP morphology in vivo further demonstrated that loss of SRGAP1 resulted in a global simplification of FPs (Figure 6, I–M, Supplemental Figure 10). In summary, these observations from in vitro and in vivo models highlight the morphofunctional role of SRGAP1 in podocytes, where SRGAP1 ensures podocyte FP plasticity under stress conditions and thereby maintains the integrity of the filtration barrier.
Figure 6.
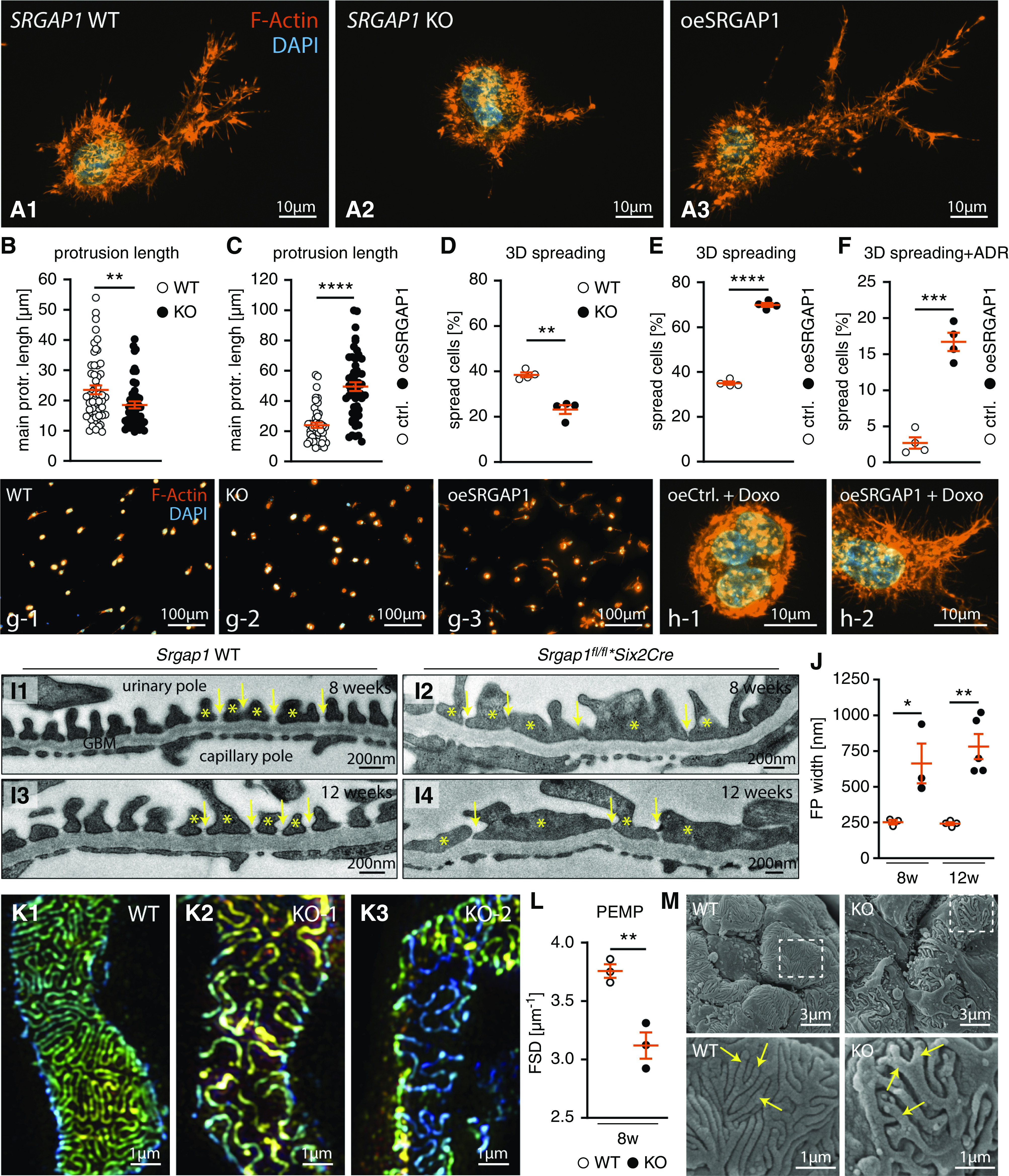
SRGAP1 instructs podocyte 3D protrusion formation. (A–H) Podocyte 3D culture demonstrated impaired protrusion generation and length in SRGAP1-KO cells, whereas overexpression of SRGAP1 resulted in the opposite phenotype. Individual protrusions of SRGAP1-KO podocytes were less branched and shorter. Application of doxorubicin (Doxo) diminished protrusion formation in wild-type (WT) podocytes, which was mitigated in SRGAP1-overexpressing cells. Under 3D culture conditions, SRGAP1 overexpression improved protrusion formation and filopodial length in the presence of doxorubicin. Representative images show maximum intensity projections of 3D-cultured podocytes stained for F-actin, main protrusion length per cell was quantified analyzing 53 WT, 48 SRGAP1 KO, 45 control, and 52 SRGAP1-overexpression podocytes as indicated by individual dots in (B and C); percentage of spread cells was quantified from four independent experiments per genotype as indicated by dots in (D–F). (I and J) TEM of KO and WT mice demonstrated pronounced widening and misconfiguration (FP effacement) of podocyte FPs (yellow asterisks) in KO mice. Slit diaphragm density (yellow arrows) was decreased and slit diaphragms were partially translocated to proximal parts of FPs. Glomerular basement membrane (GBM) and endothelial cells showed only modest changes (three to four animals per time point and genotype were analyzed for mean FP width; dots indicate individual animals). (K and L) 3D SIM microscopy in respective WT and KO animals identified reduced filtration slit density (FSD), indicative of aberrant FP architecture (SIM data of n=3 individual animals per genotype were quantified by PEMP; see Supplemental Figure 10 for overviews and z-scales). (M) Scanning electron microscopy showed simplification, reduced branching, and misconfiguration of podocyte FPs (yellow arrows) in Srgap1fl/fl*Six2Cre-KO mice. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Ctrl., control; oe, overexpression; protr., protrusion.
Discussion
Although it is well known that glomerular and podocyte disease is accompanied by a profound rearrangement of the actin cytoskeleton, including formation of contractile actomyosin filaments and imbalanced activity levels of Rho GTPases, a comprehensive understanding of these mechanisms is still lacking.
We mapped the podocyte-specific expression pattern of Rho GTPases and their regulatory RhoGEFs and RhoGAPs on the transcriptome and proteome level (Figure 1). On the basis of these datasets, we defined a podocyte-specific Rho GTPase affinity interaction network.15,17–19 This novel in silico analysis confirmed the central role of RAC1, RHOA, and CDC42 in the regulation of the podocyte actin cytoskeleton, and identified several new podocyte-specific RhoGEFs, RhoGAPs, and Rho GTPases. Generation and maintenance of the podocyte FP network critically relies on instructive CDC42/N-WASP/ARP2/3 signaling, as depicted by our mapping approach (Figure 1) and KO models in vivo.10,26,57,58 In contrast, increased RAC1 activity and consecutive RAC1/WAVE2/ARP2/3-dependent actin polymerization is associated with podocyte disease conditions.7,8 In this context, our mapping approach highlighted SRGAP1 as a podocyte-specific RhoGAP.
On the basis of previous work, SRGAP1 appears to physically interact with RAC1, RHOA, and CDC42, and functions as a cell type– and context-dependent RhoGAP that preferentially, but not exclusively, modulates RAC1 activity.15,59–63 More recently, it was proposed that SRGAP1 might act as a downstream effector of SLIT2/ROBO2 signaling in the nephrogenic mesenchyme (causing congenital anomalies of the kidney and urinary tract) and in podocytes.64,65 However, the in vivo relevance of SRGAP1 in podocytes has remained uncharacterized thus far. Here, two complementary podocyte-specific, Srgap1-KO mouse models have been generated by conditional deletion approaches (Six2Cre for deletion in the metanephric mesenchyme; hNPHS2Cre deleter strain for deletion in differentiated podocytes) (Figures 2 and 3). Srgap1fl/fl*Six2Cre mice develop a spontaneous insufficiency of the glomerular filtration barrier that resembles histologic features of FSGS in adult mice (Figure 2). In strong contrast to severe developmental phenotypes caused by conditional deletion of the CDC42/N-WASP/ARP2/3 machinery, SRGAP1 appeared to be dispensable for podocyte FP development.10,25,57 Remarkably, only Srgap1fl/fl*Six2Cre, but not Srgap1fl/fl*hNPHS2Cre, mice developed a spontaneous phenotype (Figures 2 and 3). Because Srgap1 exhibits a strict podocyte-specific expression pattern (Figures 1 and 2), we speculate that differences in the deletion onset (Six2Cre versus hNPHS2Cre) during podocyte development might translate into differential adaptive responses (e.g., via SRGAP2 or other RhoGAPs). However, developmental alterations due to loss of Srgap1 can also potentially explain the more severe phenotype observed in Srgap1fl/fl*Six2Cre-KO mice. Moreover, the Six2Cre deleter strain harbors a haploinsufficiency for the transcription factor Six2 gene, which might also sensitize these mice to additional co-occurring gene deletions (e.g., co-occurring mutation).66,67 This notion is supported by the observation that Srgap1fl/fl*hNPHS2Cre showed high levels of susceptibility when challenged in a toxic glomerulopathy model (Figure 3).
Detailed analysis of SRGAP1 localization patterns in human FSGS and podocyte disease models revealed a translocalization of SRGAP1 toward the basolateral compartment of podocyte processes (Figure 3). Within this compartment, accumulation of actin cytoskeleton in a matlike configuration and association with contractile actomyosin fibers has been observed in human and murine podocyte disease conditions.4,68,69 Due to its F-BAR domain, SRGAP1 shows a high affinity for localization toward the plasma membrane and cell protrusions (Supplemental Figure 6), in line with previous observations.32,51 Modeling podocyte stress response in vitro demonstrated a similar RHOA/ROCK-dependent translocalization of SRGAP1 to actin fibers and IACs (Figure 4).
To dissect the role and function of SRGAP1 for podocyte FP formation, we used CRISPR/Cas9 KO cells which showed increased lamellipodial extension and cell spreading (most likely due to RAC1 activation; Figure 5, Supplemental Figure 7). A similar role of SRGAP1/RAC1 in lamellipodia-based cell migration has already been reported in human fibrosarcoma cells.60 The process of cellular protrusion formation and adhesion underlies an intricate interplay involving reciprocal signaling between RHOA-mediated contractility and RAC1-promoted protrusive behavior (e.g., by defining spatiotemporal activity zones for Rho GTPases15,55,56). We observed decreased actomyosin activity in lamellipodia of SRGAP1-KO podocytes and association of SRGAP1 to protein complexes containing myosin II (Figure 5, Supplemental Figure 9). The highly context-dependent role of SRGAP1 is furthermore underlined by differential phenotypes observed in 2D and 3D environments. Whereas SRGAP1 loss in 2D translates into increased lamellipodia formation, loss of SRGAP1 in 3D environments is characterized by decreased protrusion formation, as also observed in Srgap1fl/fl*Six2Cre-KO mice in vivo (Figures 5 and 6). This 3D phenotype might be attributable to the SRGAP1-dependent suppression of RHOA.63,70 The relevance of SRGAP1 for podocyte FP plasticity is furthermore substantiated by its protective role in in vitro and in vivo podocyte stress models (Figures 3 and 6). Altogether, we describe here the Rho GTPase affinity network of podocytes and identify SRGAP1 as a podocyte-specific RhoGAP controlling podocyte FP plasticity in glomerular health and disease.
Disclosures
N. Endlich declares that PEMP, which has been used for this manuscript, is registered for a patent. N. Endlich is among the founders of the start-up NIKOKA, which will commercialize PEMP. T. Huber reports having consultancy agreements with AstraZeneca, Bayer, Boehringer-Ingelheim, DaVita, Deerfield, Fresenius Medical Care, GoldfinchBio, Mantrabio, Novartis, and Retrophin; receiving research funding from Amicus Therapeutics, Fresenius Medical Care; and being on the editorial board of Kidney International and the advisory board of Nature Review Nephrology. O. Schilling reports receiving research funding from Bayer/Monsanto and Roche. G. Walz reports being a scientific advisor for or member of the Meona Group. All remaining authors have nothing to disclose.
Funding
M. Rogg was supported by Else Kröner-Fresenius-Stiftung grant 2016_Kolleg.03. D. Sellung was supported by Else Kröner-Fresenius-Stiftung grant 2014_MOTI-VATE. T. Huber was supported by Deutsche Forschungsgemeinschaft (DFG) grants CRC1192, HU 1016/8-2, HU 1016/11-1, and HU 1016/12-1; Bundesministerium für Bildung und Forschung (BMBF) grants STOP-FSGS-01GM1518C and NephrESA-031L0191E; Else Kröner-Fresenius-Stiftung (Else Kröner-Promotionskolleg – iPRIME); and the H2020 European Research Council grants 616891 and BEAt-DKD (115974) (H2020-IMI2 consortium); this joint undertaking receives support from the European Union’s Horizon 2020 Framework Programme, European Federation of Pharmaceutical Industries and Associations, and JDRF. A. Abed was supported by the Alexander von Humboldt-Stiftung, European Renal Association, and European Dialysis and Transplant Association. N. Endlich was supported by BMBF grant STOP-FSGS-01GM1518B. C. Schell was supported by DFG grant DFG-SCHE 2092-1/1, Else Kröner-Fresenius-Stiftung grant EKFS A_09 Matriglom, and the Albert-Ludwigs-Universität Freiburg Berta-Ottenstein Programme.
Supplementary Material
Acknowledgments
The authors would like to thank Ms. Charlotte Meyer for expert technical assistance. In addition, the authors would like to express their gratitude to all members of our laboratories; the Core Facility Electron Microscopy, Department of Nephrology, Faculty of Medicine, University of Freiburg, Freiburg, Germany; and the Core Facility Proteomics, Institute of Surgical Pathology, Faculty of Medicine, University of Freiburg, Freiburg, Germany for helpful discussions and support.
This work contains parts of the doctoral thesis of Dr. Robert Dotzauer.
Dr. Manuel Rogg, Dr. Jasmin I. Maier, Dr. Gerd Walz, Dr. Nicole Endlich, Dr. Oliver Schilling, Dr. Tobias B. Huber, and Dr. Christoph Schell conceived of and analyzed experiments; Dr. Manuel Rogg, Dr. Gerd Walz, Dr. Martin Werner, Dr. Tobias B. Huber, and Dr. Christoph Schell supervised the study; Dr. Manuel Rogg, Dr. Jasmin I. Maier, Dr. Robert Dotzauer, Dr. Nadine Artelt, Dr. Oliver Kretz, Dr. Martin Helmstäder, Ms. Alena Sammarco, Dr. August Sigle, Mr. Dominik Sellung, Mr. Patrick Dinse, Ms. Karoline Reiche, Dr. Mako Yasuda-Yamahara, Dr. Martin L. Biniossek, Dr. Nicole Endlich, and Dr. Christoph Schell performed experiments; Dr. Manuel Rogg, Dr. Tobias B. Huber, and Dr. Christoph Schell wrote the manuscript with input and discussion from all authors.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Data Sharing Statement
All data associated with this study are available in the main text or in the Supplemental Material. The tandem mass spectrometry data of the SRGAP1 interactome are accessible at the MassIVE repository as ProteomeXchange dataset PXD020649.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020081126/-/DCSupplemental.
Supplemental Figure 1. Podocyte Rho GTPase network analysis, corresponding to Figure 1.
Supplemental Figure 2. ISH analysis of Srgap1/2/3 expression, corresponding to Figure 1.
Supplemental Figure 3. Analysis of SRGAP1 expression, corresponding to Figure 2.
Supplemental Figure 4. Characteristics of experimental mice, corresponding to Figures 2 and 3.
Supplemental Figure 5. Characteristics of experimental mice, corresponding to Figure 3.
Supplemental Figure 6. Analysis of subcellular SRGAP1 localization, corresponding to Figure 4.
Supplemental Figure 7. Analysis of SRGAP1 KO podocytes, corresponding to Figure 5.
Supplemental Figure 8. Analysis of SRGAP1 KO podocytes, corresponding to Figure 5.
Supplemental Figure 9. SRGAP1 interactome, corresponding to Figure 5.
Supplemental Figure 10. PEMP of Srgap1 mice, corresponding to Figure 6.
Supplemental Table 1. Antibodies.
Supplemental Table 2. In situ hybridization primer.
Supplemental Table 3. Rho GTPase expression in murine podocytes.
Supplemental Table 4. RhoGEF and RhoGAP expression in murine podocytes.
References
- 1.Grahammer F, Schell C, Huber TB: The podocyte slit diaphragm--from a thin grey line to a complex signalling hub. Nat Rev Nephrol 9: 587–598, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Perico L, Conti S, Benigni A, Remuzzi G: Podocyte-actin dynamics in health and disease. Nat Rev Nephrol 12: 692–710, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Maas RJ, Deegens JK, Smeets B, Moeller MJ, Wetzels JF: Minimal change disease and idiopathic FSGS: Manifestations of the same disease. Nat Rev Nephrol 12: 768–776, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV: The podocyte’s response to stress: The enigma of foot process effacement. Am J Physiol Renal Physiol 304: F333–F347, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Butt L, Unnersjö-Jess D, Höhne M, Edwards A, Binz-Lotter J, Reilly D, et al.: A molecular mechanism explaining albuminuria in kidney disease. Nat Metab 2: 461–474, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Schell C, Huber TB: The evolving complexity of the podocyte cytoskeleton. J Am Soc Nephrol 28: 3166–3174, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu H, Suleiman H, Kim AH, Miner JH, Dani A, Shaw AS, et al.: Rac1 activation in podocytes induces rapid foot process effacement and proteinuria. Mol Cell Biol 33: 4755–4764, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robins R, Baldwin C, Aoudjit L, Côté JF, Gupta IR, Takano T: Rac1 activation in podocytes induces the spectrum of nephrotic syndrome. Kidney Int 92: 349–364, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Blattner SM, Hodgin JB, Nishio M, Wylie SA, Saha J, Soofi AA, et al.: Divergent functions of the Rho GTPases Rac1 and Cdc42 in podocyte injury. Kidney Int 84: 920–930, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott RP, Hawley SP, Ruston J, Du J, Brakebusch C, Jones N, et al.: Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J Am Soc Nephrol 23: 1149–1154, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu L, Jiang R, Aoudjit L, Jones N, Takano T: Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1621–1630, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Ellis MJ, Gomez JA, Eisner W, Fennell W, Howell DN, et al.: Mechanisms of the proteinuria induced by Rho GTPases. Kidney Int 81: 1075–1085, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etienne-Manneville S, Hall A: Rho GTPases in cell biology. Nature 420: 629–635, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Aspenström P: Fast-cycling Rho GTPases. Small GTPases 11: 248–255, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller PM, Rademacher J, Bagshaw RD, Wortmann C, Barth C, van Unen J, et al.: Systems analysis of RhoGEF and RhoGAP regulatory proteins reveals spatially organized RAC1 signalling from integrin adhesions. Nat Cell Biol 22: 498–511, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Matsuda J, Maier M, Aoudjit L, Baldwin C, Takano T: ARHGEF7 (β-PIX) is required for the maintenance of podocyte architecture and glomerular function. J Am Soc Nephrol 31: 996–1008, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagci H, Sriskandarajah N, Robert A, Boulais J, Elkholi IE, Tran V, et al.: Mapping the proximity interaction network of the Rho-family GTPases reveals signalling pathways and regulatory mechanisms. Nat Cell Biol 22: 120–134, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Gillingham AK, Bertram J, Begum F, Munro S: In vivo identification of GTPase interactors by mitochondrial relocalization and proximity biotinylation. eLife 8: e45916, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul F, Zauber H, von Berg L, Rocks O, Daumke O, Selbach M: Quantitative GTPase affinity purification identifies rho family protein interaction partners. Mol Cell Proteomics 16: 73–85, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JS, Ma W, O’Brien LL, Chung E, Guo JJ, Cheng JG, et al.: Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev Cell 23: 637–651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, et al.: Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Two gene fragments that direct podocyte-specific expression in transgenic mice. J Am Soc Nephrol 13: 1561–1567, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Lee VW, Harris DC: Adriamycin nephropathy: A model of focal segmental glomerulosclerosis. Nephrology (Carlton) 16: 30–38, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Rogg M, Yasuda-Yamahara M, Abed A, Dinse P, Helmstädter M, Conzelmann AC, et al.: The WD40-domain containing protein CORO2B is specifically enriched in glomerular podocytes and regulates the ventral actin cytoskeleton. Sci Rep 7: 15910, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schell C, Sabass B, Helmstaedter M, Geist F, Abed A, Yasuda-Yamahara M, et al. ARP3 controls the podocyte architecture at the kidney filtration barrier. Dev cell 47: 741–757.e8, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegerist F, Ribback S, Dombrowski F, Amann K, Zimmermann U, Endlich K, et al.: Structured illumination microscopy and automatized image processing as a rapid diagnostic tool for podocyte effacement. Sci Rep 7: 11473, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.el Nahas AM, Bassett AH, Cope GH, Le Carpentier JE: Role of growth hormone in the development of experimental renal scarring. Kidney Int 40: 29–34, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Schell C, Rogg M, Suhm M, Helmstädter M, Sellung D, Yasuda-Yamahara M, et al.: The FERM protein EPB41L5 regulates actomyosin contractility and focal adhesion formation to maintain the kidney filtration barrier. Proc Natl Acad Sci U S A 114: E4621–E4630, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, et al.: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Labun K, Montague TG, Krause M, Torres Cleuren YN, Tjeldnes H, Valen E: CHOPCHOP v3: Expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res 47: W171–W174, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanjana NE, Shalem O, Zhang F: Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods 11: 783–784, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coutinho-Budd J, Ghukasyan V, Zylka MJ, Polleux F: The F-BAR domains from srGAP1, srGAP2 and srGAP3 regulate membrane deformation differently. J Cell Sci 125: 3390–3401, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasuda-Yamahara M, Rogg M, Yamahara K, Maier JI, Huber TB, Schell C: AIF1L regulates actomyosin contractility and filopodial extensions in human podocytes. PLoS One 13: e0200487, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knopf JD, Tholen S, Koczorowska MM, De Wever O, Biniossek ML, Schilling O: The stromal cell-surface protease fibroblast activation protein-α localizes to lipid rafts and is recruited to invadopodia. Biochim Biophys Acta 1853: 2515–2525, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Huang W, Sherman BT, Lempicki RA: Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Mahi NA, Najafabadi MF, Pilarczyk M, Kouril M, Medvedovic M: GREIN: An interactive web platform for re-analyzing GEO RNA-seq data. Sci Rep 9: 7580, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Afgan E, Baker D, van den Beek M, Blankenberg D, Bouvier D, Čech M, et al.: The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res 44: W3–W10, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen S, Zhou Y, Chen Y, Gu J: fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34: i884–i890, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim D, Langmead B, Salzberg SL: HISAT: A fast spliced aligner with low memory requirements. Nat Methods 12: 357–360, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao Y, Smyth GK, Shi W: featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Love MI, Huber W, Anders S: Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bult CJ, Blake JA, Smith CL, Kadin JA, Richardson JE; Mouse Genome Database Group: Mouse Genome Database (MGD) 2019. Nucleic Acids Res 47: D801–D806, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.UniProt Consortium: UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res 47: D506–D515, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heasman SJ, Ridley AJ: Mammalian Rho GTPases: New insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 9: 690–701, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Mitchell AL, Attwood TK, Babbitt PC, Blum M, Bork P, Bridge A, et al.: InterPro in 2019: Improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res 47: D351–D360, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al.: Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brunskill EW, Park JS, Chung E, Chen F, Magella B, Potter SS: Single cell dissection of early kidney development: Multilineage priming. Development 141: 3093–3101, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kann M, Ettou S, Jung YL, Lenz MO, Taglienti ME, Park PJ, et al.: Genome-wide analysis of Wilms’ tumor 1-controlled gene expression in podocytes reveals key regulatory mechanisms. J Am Soc Nephrol 26: 2097–2104, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rinschen MM, Gödel M, Grahammer F, Zschiedrich S, Helmstädter M, Kretz O, et al.: A multi-layered quantitative in vivo expression atlas of the podocyte unravels kidney disease candidate genes. Cell Rep 23: 2495–2508, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akilesh S, Suleiman H, Yu H, Stander MC, Lavin P, Gbadegesin R, et al.: Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J Clin Invest 121: 4127–4137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Itoh T, Erdmann KS, Roux A, Habermann B, Werner H, De Camilli P: Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev Cell 9: 791–804, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR: Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10: 778–790, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiggan O, Shaw AE, DeLuca JG, Bamburg JR: ADF/cofilin regulates actomyosin assembly through competitive inhibition of myosin II binding to F-actin. Dev Cell 22: 530–543, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delorme V, Machacek M, DerMardirossian C, Anderson KL, Wittmann T, Hanein D, et al.: Cofilin activity downstream of Pak1 regulates cell protrusion efficiency by organizing lamellipodium and lamella actin networks. Dev Cell 13: 646–662, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guilluy C, Garcia-Mata R, Burridge K: Rho protein crosstalk: Another social network? Trends Cell Biol 21: 718–726, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin K, Reimann A, Fritz RD, Ryu H, Jeon NL, Pertz O: Spatio-temporal co-ordination of RhoA, Rac1 and Cdc42 activation during prototypical edge protrusion and retraction dynamics. Sci Rep 6: 21901, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schell C, Baumhakl L, Salou S, Conzelmann AC, Meyer C, Helmstädter M, et al.: N-wasp is required for stabilization of podocyte foot processes. J Am Soc Nephrol 24: 713–721, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schell C, Wanner N, Huber TB: Glomerular development--shaping the multi-cellular filtration unit. Semin Cell Dev Biol 36: 39–49, 2014 [DOI] [PubMed] [Google Scholar]
- 59.Wong K, Ren XR, Huang YZ, Xie Y, Liu G, Saito H, et al.: Signal transduction in neuronal migration: Roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell 107: 209–221, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Yamazaki D, Itoh T, Miki H, Takenawa T: srGAP1 regulates lamellipodial dynamics and cell migratory behavior by modulating Rac1 activity. Mol Biol Cell 24: 3393–3405, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liang X, Budnar S, Gupta S, Verma S, Han SP, Hill MM, et al.: Tyrosine dephosphorylated cortactin downregulates contractility at the epithelial zonula adherens through SRGAP1. Nat Commun 8: 790, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang T, Zhou Y, Zhang J, Wong CC, Li W, Kwan JSH, et al.: SRGAP1, a crucial target of miR-340 and miR-124, functions as a potential oncogene in gastric tumorigenesis. Oncogene 37: 1159–1174, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kutys ML, Yamada KM: An extracellular-matrix-specific GEF-GAP interaction regulates Rho GTPase crosstalk for 3D collagen migration. Nat Cell Biol 16: 909–917, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hwang DY, Kohl S, Fan X, Vivante A, Chan S, Dworschak GC, et al.: Mutations of the SLIT2-ROBO2 pathway genes SLIT2 and SRGAP1 confer risk for congenital anomalies of the kidney and urinary tract. Hum Genet 134: 905–916, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fan X, Yang H, Kumar S, Tumelty KE, Pisarek-Horowitz A, Rasouly HM, et al.: SLIT2/ROBO2 signaling pathway inhibits nonmuscle myosin IIA activity and destabilizes kidney podocyte adhesion. JCI Insight 1: e86934, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Combes AN, Wilson S, Phipson B, Binnie BB, Ju A, Lawlor KT, et al.: Haploinsufficiency for the Six2 gene increases nephron progenitor proliferation promoting branching and nephron number. Kidney Int 93: 589–598, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guan J, Wang D, Cao W, Zhao Y, Du R, Yuan H, et al.: SIX2 haploinsufficiency causes conductive hearing loss with ptosis in humans. J Hum Genet 61: 917–922, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shirato I: Podocyte process effacement in vivo. Microsc Res Tech 57: 241–246, 2002 [DOI] [PubMed] [Google Scholar]
- 69.Suleiman HY, Roth R, Jain S, Heuser JE, Shaw AS, Miner JH: Injury-induced actin cytoskeleton reorganization in podocytes revealed by super-resolution microscopy. JCI Insight 2: e94137, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vega FM, Fruhwirth G, Ng T, Ridley AJ: RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. J Cell Biol 193: 655–665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.