Abstract
Glutathione (GSH) is the most abundant antioxidant and is believed to maintain redox potential in tissues, cells, and individual compartments. However, GSH concentrations in some tumor cells and tissues have been reported to be as high as 1–10 mM, a concentration that is up to 10,000-fold higher than that of reactive oxygen species. Critical quantitative evaluation of glutathione’s proposed functions suggests that glutathione is an amino acid checkpoint. In this role, glutathione contributes to regulating cell proliferation and apoptosis, pending amino acid availability.
Graphical Abstract
INTRODUCTION
Glutathione (GSH) is the most abundant antioxidant and is believed to maintain redox potential in tissues, cells, and individual compartments. Glutathione concentrations in healthy tissues have been reported in the μM range and up to 10 mM in tumor tissues.1–3 We recently reported that lung tumor cells excrete glutathione actively at a rate of at least ∼33 nmol/min/100,000 cells, demonstrating the huge effort that tumor cells devote to the synthesis and excretion of glutathione.4 This led us to a simple question: How much glutathione does a biological system need to fulfill the antioxidant function? Some research indicates that concentrations of reactive oxygen species (ROS) under normal circumstances are in the low nM range and may reach as high as 1 μM during inflammation response.5–7 If true, this means that cells, especially tumor cells, produce up to 10,000-fold more glutathione than they need to scavenge all ROS.
This stoichiometric disconnect suggests that there is another important function requiring significantly larger amounts of glutathione. To begin addressing the question of glutathione overproduction, we first must consider glutathione’s function as an antioxidant and defender against immune response-derived or stress-induced ROS. The antioxidant capacity of glutathione is usually expressed as a ratio of reduced-to-oxidized glutathione (GSH/GSSG), a measure that does not indicate actual glutathione concentrations. In general, a decrease in the GSH/GSSG ratio is correlated with an increase in ROS, a condition that is thought to induce apoptosis.6 Although this notion is plausible, it is confounded by the fact that only a small fraction of glutathione is oxidized. Further, cells undergoing apoptosis are known to switch from biosynthetic to catalytic metabolism, including reduced or ceased glutathione synthesis. Therefore, apoptotic cells are expected to show a decrease in the GSH/GSSG ratio because GSH is not being replenished and the remaining GSH spontaneously oxidizes to GSSG.8 As a result, an observed change in the GSH/GSSG ratio may be indicative of the apoptotic state of a cell, but its causality in triggering apoptosis cannot be assumed.
Moreover, the stoichiometric disconnect becomes clear when one envisions ROS reaching 10 mM, a concentration that is very unlikely to be reached under physiological conditions. Indeed, macrophages are reported to produce the highest level of extracellular peroxide (3.1 ± 0.09 nmol/min/100,000 cells), followed by Type II alveolar cells (0.035 ± 0.07 nmol/min/100,000 cells), and then by vascular endothelial cells (0.013 nmol/min/100,000 cells).6 H2O2 formation in tumor cells has been reported to range from 0.03 to 0.08 nmol/min/100,000 cells.7 4-Hydroxynonenal, a lipid peroxidation product and biomarker for oxidative stress, is usually in the range of 1 to 180 nmol/100,000 cells.9 Although comparing these formation rates with the GSH excretion rates of the lung tumor cells is not optimal, it still demonstrates the significant quantitative difference in the formation of GSH and ROS. Finally, if glutathione’s function is simply to improve cellular defense against ROS, cells could use other less valuable antioxidants, such as ascorbic acid, α-tocopherol, or α-lipoic acid.10 Together, it becomes apparent that glutathione’s antioxidant function does not explain why tumor cells devote so much energy to its synthesis and excretion.
Harsh treatments such as chemotherapy or acetaminophen overdose deplete glutathione and cause adverse side effects and toxicities, suggesting that glutathione is essential for normal viability. Therefore, it is not surprising that an increase in glutathione is correlated with increased resistance to common chemotherapeutic agents in tumor cells.11,12 This resistance is a result of glutathione’s conjugation with electrophilic drugs through reactions catalyzed by glutathione S-transferase. The resulting conjugates are exported from the cell by the GS-X pump and eliminated from the body.13 However, because of the fast turnover and rapid synthesis of glutathione under normal conditions, only small amounts of glutathione are needed to facilitate the elimination of xenobiotic and exogenous toxins to maintain viability in healthy systems.
Glutathione has also been implicated in maintaining intracellular cysteine levels. Cells grown in a cysteine-free medium require expression of γ-glutamyl transpeptidase to utilize extracellular glutathione as a cysteine source.14,15 This suggests that extracellular glutathione can serve as a valuable cysteine source, particularly when the cysteine is needed later to synthesize intracellular glutathione. This notion is challenged by the fact that cysteine concentrations in the plasma of healthy adults are approximately 3-fold higher than concentrations of glutathione (9.7 ± 3.2 μM cysteine compared to 2.80 ± 0.91 μM glutathione), disqualifying glutathione as a suitable buffer for maintaining cysteine homeostasis.1 Instead, amino acid transport systems, such as the xC-system, have been shown to be cysteine-specific and essential for maintaining intracellular cysteine.16
In general, the biological functions described above require only nM concentrations of glutathione. So why do tumor cells and some others cells make so much glutathione? Over 50 years ago, Meister and colleagues proposed the γ-glutamyl cycle and suggested that it participates in amino acid transport.17–20 In brief, the γ-glutamyl cycle starts with the synthesis of glutathione, which occurs in two ligation reactions. After synthesis, excess glutathione is exported from the cells, and extracellular γ-glutamyl transpeptidase transfers the γ-glutamyl group of glutathione to various acceptor amino acids or dipeptides.21 The resulting γ-glutamyl amino acids are supposed to be readily imported into the cytosol, where the γ-glutamyl bonds are broken by γ-glutamyl cyclotransferase, releasing the imported amino acids or dipeptides and 5-oxoproline.22 The latter is decyclized by 5-oxoprolinase, releasing glutamate that feeds back into glutathione synthesis, thereby completing the γ-glutamyl cycle. This cycle has been the subject of heated discussions,8,23 and it is considered to have been disproven mainly because proteins transporting γ-glutamyl amino acids have not been identified and most amino acids are transported in their free form. Furthermore, Km values for the involved enzymes are in the mM range, suggesting that this pathway is physiologically irrelevant. However, upregulation of several of its enzymes in lung tumor cells and tissues suggests that part of this cycle is critical in cancer promotion and progression.
GLUTATHIONE AS AN AMINO ACID CHECKPOINT
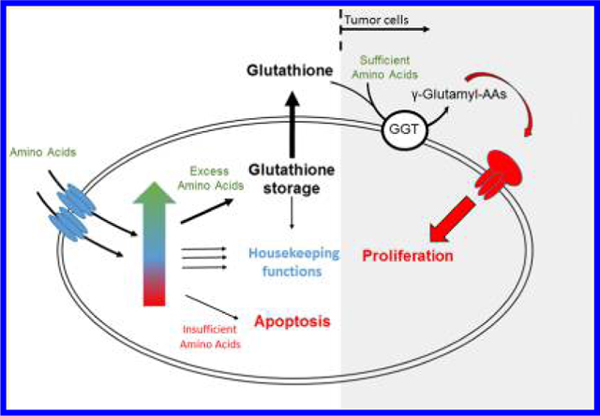
One can postulate that glutathione functions as a checkpoint to indicate sufficient amino acids are available, both intercellularly to share with neighboring cells and extracellularly to trigger cell and tissue grow. As the intracellular amino acid pool increases, most amino acids are degraded to TCA cycle intermediates. If these accumulate, they are converted to glutamate. Others are converted to glycine or serine, and the latter promotes cysteine synthesis to retain sulfur. As these three accumulate, they are stored as glutathione. The relatively high Km values for the enzymes involved in glutathione synthesis support this theory because cells need to maintain free amino acids for normal cellular functions and only convert surplus amino acids to glutathione. Glutathione subsequently accumulates and functions as a checkpoint to indicate sufficient amounts of intracellular amino acids for growth. At this stage, glutathione is exported to supply neighboring cells and tissues with building blocks for biosynthesis. As the extracellular glutathione concentration increases, γ-glutamyl transpeptidase forms γ-glutamyl amino acid conjugates, which function as a checkpoint to indicate sufficient amounts of extracellular amino acids are available for cell proliferation. This notion is supported by the fact that glutathione supplementation increases cell proliferation and can, at least partially, overcome the effects of compounds that inhibit the uptake of key amino acids.24–26 In fact, γ-glutamyl transpeptidase-positive tumors have been shown to have a 2-fold greater growth rate than γ-glutamyl transpeptidase-negative tumors.27 In contrast, in cases of insufficient nutrients, cells undergo growth arrest, and glutathione is utilized for housekeeping functions. Under conditions of severe nutrient deficiency, glutathione synthesis halts and glutathione is depleted, which may also function as checkpoint involved in initiating apoptosis. The signal from the glutathione checkpoint is then processed and integrated with other biochemical signals that control cell growth or apoptosis.
This idea opposes the common belief that increased oxidative stress leads to glutathione oxidation and subsequent apoptosis. However, as discussed above, the actual amount of glutathione being oxidized is small compared to the total amount of glutathione present. Therefore, it seems likely that the observed inverse relationship between glutathione and apoptosis might be a result of reduced nutrient uptake and lack of glutathione de novo synthesis rather than small increases in the oxidative status of glutathione. If this is true, it will replace the “unstable radical chemistry” commonly believed to regulate cell proliferation and apoptosis with a “stable molecule chemistry”. Hence, when glutathione is high (approximately 1 mM), it promotes proliferation; when it is normal, it maintains cell senescence, and when it is low (< ∼1 nM), it induces apoptosis.
Why would glutathione be used for this purpose rather than another polypeptide?
Glutathione is a valuable tripeptide and acts as a carbon, nitrogen, and sulfur source. The glutamate, cysteine, and glycine groups of glutathione represent C2, C3, and C5 carbon backbones and are the basic building blocks for essential biosynthesis pathways. Hence, glutathione might function as a universal amino acid pool or precursor for various biosynthesis pathways. The γ-glutamyl bound between glutamate and cysteine prevents degradation by proteases, making glutathione the perfect storage molecule.
Why has this been ignored in the past decades?
When Meister et al. proposed the γ-glutamyl cycle in the 1950s and 1960s, most studies were conducted in tissues of normal and mainly senescent cells or tissues, and Km values above 1 mM were considered biologically irrelevant. In the 1970s, two discoveries opened the path of departure. First, glutathione S-transferase (GST) enzymes were shown to utilize glutathione for conjugation of drugs and carcinogens to facilitate their elimination. Subsequent research efforts in drug development flooded the literature. Km values for GST-catalyzed reactions were reported to be in the nM range, outcompeting Km values of γ-glutamyl cycle enzymes. Second, studying the importance of redox chemistry in biological systems led to the notion that oxidative stress is the ultimate culprit of diseases, and efforts were devoted to understanding radical chemistry and moved away from effects of nutrients.
In summary, critical quantitative evaluation of glutathione’s proposed role suggests that glutathione functions as a universal amino acid pool and checkpoint. In doing so, glutathione promotes cell proliferation or induces apoptosis depending on the amino acid availability. Further studies are needed to better understand how this signal is processed and integrated with biochemical signals needed to initiate proliferation, such as glucose availability, cell cycle checkpoints, and others. In healthy cells and tissues, secondary functions of GSH, such as serving as an antioxidant or a substrate for conjugation reactions, utilize only a small fraction of the overall glutathione and do not fully account for its high abundance observed in tumors. The huge efforts in drug development and cultural opinion devoted to elucidating the potential of antioxidants have led to a literature bias that, in the case of glutathione, is not sustainable. Although the proposed paradigm is partially derived from observations in a lung tumor cell model, the generality of these results to other normal and tumor cell lines remains to be determined.
Acknowledgments
Funding
Support has been provided in part by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, Grants UL1TR000039 and KL2TR000063, the Arkansas Bioscience Institute, and the Envoys, an advocacy group of the UAMS Cancer Institute Foundation.
ABBREVIATIONS
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- GST
glutathione S-transferase
- ROS
reactive oxygen species
Biography
Gunnar Boysen is an Associate Professor of Environmental and Occupational Health at the University of Arkansas for Medical Sciences. He received his Ph.D. from the University of Kaiserslautern, Germany for studies on tobacco-specific DNA and protein adducts conducted at the University Minnesota Cancer Center. He moved to the University of North Carolina at Chapel Hill for a postdoctoral fellowship and advanced to Assistant Professor before relocating to the University of Arkansas for Medical Sciences. He specializes in the application of mass spectrometry for biomarker quantitation to better understand toxicant exposure and metabolic alterations in disease progression.
Footnotes
The author declares no competing financial interest.
REFERENCES
- (1).Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, and Sternberg P. (2000) Redox state of glutathione in human plasma. Free Radical Biol. Med 28, 625–635. [DOI] [PubMed] [Google Scholar]
- (2).Blair SL, Heerdt P, Sachar S, Abolhoda A, Hochwald S, Cheng H, and Burt M. (1997) Glutathione metabolism in patients with non-small cell lung cancers. Cancer Res. 57, 152–155. [PubMed] [Google Scholar]
- (3).Liu Y, Hyde AS, Simpson MA, and Barycki JJ (2014) Emerging regulatory paradigms in glutathione metabolism. Adv. Cancer Res 122, 69–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Sappington DR, Siegel ER, Hiatt G, Desai A, Penney RB, Jamshidi-Parsian A, Griffin RJ, and Boysen G. (2016) Glutamine drives glutathione synthesis and contributes to radiation sensitivity of A549 and H460 lung cancer cell lines. Biochim. Biophys. Acta, Gen. Subj 1860, 836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Dedon PC, and Tannenbaum SR (2004) Reactive nitrogen species in the chemical biology of inflammation. Arch. Biochem. Biophys 423, 12–22. [DOI] [PubMed] [Google Scholar]
- (6).Day RM, and Suzuki YJ (2005) Cell proliferation, reactive oxygen and cellular glutathione. Dose-Response 3, 425–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Szatrowski TP, and Nathan CF (1991) Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 51, 794–798. [PubMed] [Google Scholar]
- (8).Flohe L. (2013) The fairytale of the GSSG/GSH redox potential. Biochim. Biophys. Acta, Gen. Subj 1830, 3139–3142. [DOI] [PubMed] [Google Scholar]
- (9).Schaur RJ, Siems W, Bresgen N, and Eckl PM (2015) 4-Hydroxy-nonenal-A Bioactive Lipid Peroxidation Product. Biomolecules 5, 2247–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Rahman K. (2007) Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2, 219–236. [PMC free article] [PubMed] [Google Scholar]
- (11).Lewis AD, Hayes JD, and Wolf CR (1988) Glutathione and glutathione-dependent enzymes in ovarian adenocarcinoma cell lines derived from a patient before and after the onset of drug resistance: intrinsic differences and cell cycle effects. Carcinogenesis 9, 1283–1287. [DOI] [PubMed] [Google Scholar]
- (12).Ahmad S, Okine L, Wood R, Aljian J, and Vistica DT (1987) gamma-Glutamyl transpeptidase (gamma-GT) and maintenance of thiol pools in tumor cells resistant to alkylating agents. J. Cell. Physiol 131, 240–246. [DOI] [PubMed] [Google Scholar]
- (13).Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, and Bannai S. (2000) Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-. Antioxid. Redox Signaling 2, 665–671. [DOI] [PubMed] [Google Scholar]
- (14).Hanigan MH (2014) Gamma-glutamyl transpeptidase: redox regulation and drug resistance. Adv. Cancer Res 122, 103–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Hanigan MH, and Ricketts WA (1993) Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry 32, 6302–6306. [DOI] [PubMed] [Google Scholar]
- (16).Okuno S, Sato H, Kuriyama-Matsumura K, Tamba M, Wang H, Sohda S, Hamada H, Yoshikawa H, Kondo T, and Bannai S. (2003) Role of cystine transport in intracellular glutathione level and cisplatin resistance in human ovarian cancer cell lines. Br. J. Cancer 88, 951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Orlowski M, and Meister A. (1963) Gamma-Glutamyl-p-nitoranilide: A new convenient substrate for determination and study of L- and D-gamma-glutamyltranspeptidase activities. Biochim. Biophys. Acta, Spec. Sect. Enzymol. Subj 73, 679–681. [DOI] [PubMed] [Google Scholar]
- (18).Orlowski M, and Meister A. (1970) The gamma-glutamyl cycle: a possible transport system for amino acids. Proc. Natl. Acad. Sci. U. S. A 67, 1248–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Meister A. (1973) On the enzymology of amino acid transport. Science 180, 33–39. [DOI] [PubMed] [Google Scholar]
- (20).Meister A. (1974) Glutathione, metabolism and function via the gamma-glutamyl cycle. Life Sci. 15, 177–190. [DOI] [PubMed] [Google Scholar]
- (21).Griffith OW, Bridges RJ, and Meister A. (1979) Transport of gamma-glutamyl amino acids: role of glutathione and gamma-glutamyl transpeptidase. Proc. Natl. Acad. Sci. U. S. A 76, 6319–6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Lu SC (2009) Regulation of glutathione synthesis. Mol. Aspects Med 30, 42–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Inoue M. (2016) Glutathionists in the battlefield of gamma-glutamyl cycle. Arch. Biochem. Biophys 595, 61–63. [DOI] [PubMed] [Google Scholar]
- (24).Kang YJ (1994) Exogenous glutathione attenuates the antiproliferative effect of buthionine sulfoximine. Toxicology 88, 177–189. [DOI] [PubMed] [Google Scholar]
- (25).Shaw JP, and Chou IN (1986) Elevation of intracellular glutathione content associated with mitogenic stimulation of quiescent fibroblasts. J. Cell. Physiol 129, 193–198. [DOI] [PubMed] [Google Scholar]
- (26).Kang YJ, Feng Y, and Hatcher EL (1994) Glutathione stimulates A549 cell proliferation in glutamine-deficient culture: the effect of glutamate supplementation. J. Cell. Physiol 161, 589–596. [DOI] [PubMed] [Google Scholar]
- (27).Hanigan MH, Gallagher BC, Townsend DM, and Gabarra V. (1999) Gamma-glutamyl transpeptidase accelerates tumor growth and increases the resistance of tumors to cisplatin in vivo. Carcinogenesis 20, 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]


