1. Introduction
Portable methods for measurement and quantification of biological analytes have tremendous promise to advance non-invasive personal health monitoring devices. Exhaled breath metabolomics shares this promise and has been gaining popularity as a non-invasive technique amenable to a vast range of medical uses. Novel sampling technologies, rapid portable breath chemical analysis platforms, and miniaturized manufacturing methods have the potential to be scaled up for wide use in basic medical practice.
Exhaled breath is a complex mixture containing basic respiratory gases, hundreds of volatile organic compounds (VOCs) of exogenous and endogenous origin, and aerosolized droplets of non-volatile compounds from the liquid lining of the lung. Depending on the sampling method, there are two fractions of breath: exhaled breath vapor (EBV) and exhaled breath condensate (EBC). EBV mainly contains the gaseous fraction of the breath with VOCs. EBC is obtained when the exhaled breath is cooled and converted into a liquid phase comprising soluble exhaled gases and non-volatile metabolites of the extracellular lining fluid [1]. EBC is considered a simplified metabolite signature that only contains water soluble volatiles and non-volatile compounds [2]. EBC can be a valuable matrix for biomarker discovery, providing vital information about lung health.
EBC has been used to diagnose diseases, to monitor status of medical conditions using physiologic markers, and to identify presence of exogenous compounds that could be etiologic or exacerbating factors in specific diseases [3, 4]. Several publications have reported the application of EBC sampling as a quick screening tool for respiratory conditions focused on inflammatory markers. EBC is considered a filtrate of blood, reflects the lung epithelia [1], and has been used for diseases such as asthma [5], chronic obstructive pulmonary disease (COPD) [6], pneumonia [7] or cystic fibrosis [8]. EBC collection devices can be used in several locations, including intensive care units (ICUs), outpatient clinics, workplaces, and at home.
This current study conducts a simultaneous targeted and untargeted analysis of inflammatory and oxidative stress markers reported in studies of asthma [9–12]. Common inflammatory markers are formed by the lipid oxidation (oxylipins), mainly involving eicosanoids that are metabolites of arachidonic acid (C20:4n6). We analyze a balance between pro- and anti-inflammatory oxylipins commonly examined in EBC. A pro-inflammatory profile has been shown in patients with a pathophysiology of bronchial obstruction [13], that presented higher concentrations in EBC of leukotrienes (LTB4) and cysteinyl-leukotrienes (LTC4, LTD4, LTE4) [14–17], and thromboxanes (TXA2, TXB2 and 11-dehydro-TXB2) [18, 19]. Anti-inflammatory activity can be promoted in numerous diseases like asthma [20] by the presence in the airways of hydroxyeicosatetraenoic acid (5-, 12- and 15-HETE), its derivatives such as lipoxin A4 (LXA4) [19, 21]; and resolvin E1 (RvE1) [22], an EPA metabolite.
The oxidative stress markers can be linked to DNA damage, lipid peroxidation, redox enzyme activity and decreased antioxidant defense levels [23]. Some studies have determined in EBC that 8-hydroxy-2’-deoxyguanosine (8-OHdG) is generated by DNA degradation [24], prostaglandins (PGD2, PGE2, PGF2 and 8-iso-PGF2) are induced by lipid peroxidation [16, 25], and o-tyrosine modifies proteins [26]. These metabolites will serve as our targeted measure of inflammatory responses and oxidative stress markers. However, there are two main challenges in detecting these specific compounds using a single methodology. One problem is the low concentrations presented in EBC, which makes the detection of some of the compounds very difficult, and another issue is the difference in chemical structures of these compounds, which usually require specific sample treatments.
In order to overcome these limitations, parallel-untargeted metabolite analysis can offer a more general measure of the biological status in health, and has the potential to discover novel biomarkers, which can also be up- or down-regulated in diseased cells. Untargeted analysis allows a better comprehension of the specific breath compounds related to certain conditions, offering additional information that cannot be considered in an initial hypothesis. Additionally, untargeted analysis allows putative identification of potential metabolites related to both exogenous factors of potential etiologic interest and endogenous chemicals of possible mechanistic significance.
In this proof-of-principle pilot study, we describe the altered metabolites in a small cohort of healthy control subjects and mild asthmatic subjects. For this, breath samples were collected as condensates with a novel miniaturized sampler. The EBC obtained was analyzed with liquid chromatography-mass spectrometry (LC-MS) to discriminate asthma phenotypes or even define specific metabolite differences between subjects.
2. Materials and methods
2.1. Human subject pilot study
This research adhered to clinical practices and protocols as approved by the University of California, Davis Institutional Review Board (IRB Protocol #1055441). Informed consent was obtained from all subjects or their parents when subjects were under 18 years of age. Twelve adolescents were recruited in total, including subjects who had physician diagnosed and treated asthma and healthy control subjects with no history of lung disease. The age range from all subjects was between 14 and 18 years of age, and all EBC samples were collected longitudinally by the subjects in their homes. All subjects followed the same protocol and instructions for breath collection.
Briefly, all subjects underwent a training visit, in which they were instructed on the use of the handheld EBC collector. All subjects completed an intake questionnaire about their overall health, symptoms including fatigue, shortness of breath, weekly physical activities, medication use, as well as healthcare utilization including clinics and emergency department (ED) visits in the last 6 months. They were instructed to complete a daily diary about respiratory symptoms, medication use, time of collection, humidity, temperature and any comments about ease of use.
2.2. Exhaled breath condensate collection
Breath sample collection is achieved using a condenser surface in a miniature breath collector that has been previously published [27]. Briefly, the condenser surface is installed in a flow chamber and cooled with a thermoelectric element. The vapor in exhaled breath is condensed on the cooled surface as it passes. The conversion of exhaled breath to liquid phase facilitates sample manipulation and chemical analysis when metabolite concentrations are very low. The EBC sampler has a disposable mouthpiece, a set of inhale and exhale one-way flap valves to allow condensation of exhaled breath only, and a saliva filter [28]. The sampling time is 15–20 minutes, and the miniature EBC sampler collects between 200–500 μL of EBC during normal tidal breathing. EBC samples were stored at −15 °C in personal household freezers, until they were returned to the lab for analysis. Subjects collected breath samples twice per day, (morning and night, approximately 8 AM / 8 PM) for 2 weeks. No nose clip was worn during collection. Subjects performed normal tidal breathing. In order to reduce the effect of food related confounders, the subjects restrained from food consumption or brushing their teeth one hour before the EBC collection procedure and rinsed their mouth with water prior to sampling.
Prior to subject enrollment, the surfaces were immersed in a ‘Piranha’ bath (4:1, H2O2 (30%):H2SO4 (98%)) for one hour to clean the surface. The surfaces were thoroughly rinsed with deionized (DI) water, dried with nitrogen gas, and stored in a clean environment. The miniature EBC sampling device was reused by each subject. All parts of the device, including the glass pipettes, were thoroughly cleaned once per day by subjects. The cleaning protocol included: 70% ethanol disinfectant spray followed by a deionized (DI) water spray and air-dried. After breathing into the device, subjects collected the EBC sample into a clean borosilicate glass vial with a reusable glass pipette. The 28 EBC samples obtained for each subject were kept frozen during transport and then stored in a laboratory freezer at −80 °C until analysis. The analytical procedure workflow followed in this study is shown (Figure 1).
Figure 1.
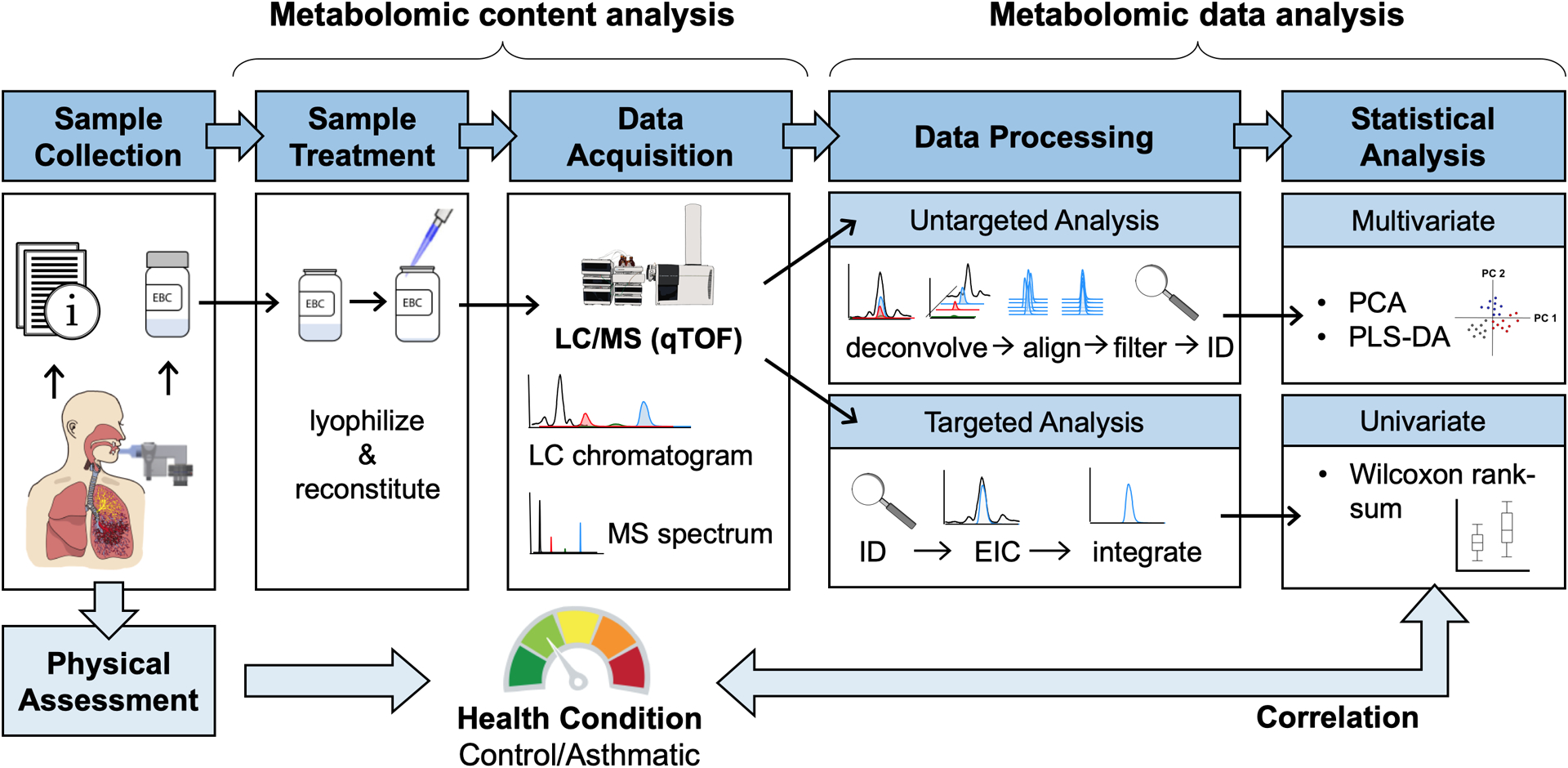
Metabolomics workflow for the human breath study.
Although the EBC sample volume is small, the sampling rate of this miniature EBC sampler is higher than commercial samplers, when compared by surface area of the condenser element [28]. We also note that EBC sampling for a shorter period would be more practical for portable self-diagnostic platforms. The time duration was required due to the size-scale mismatch between a miniature collection element and the large bench-top instruments used for chemical analysis of the collected EBC sample. The minimum sample volume for bench-top mass spectrometers exceeds that of microfluidic platforms. The amount of sample collection could be reduced to a few microliters and sampling time to a few minutes when collection and analysis are coupled with near-real-time detection micro-scaled systems [29].
2.3. Sample preparation
Frozen EBC (250 μL) was directly lyophilized inside the glass vials. The obtained dried extract was reconstituted using 25 μL of mobile phase (95% water in acetonitrile). Afterwards, samples were shortly vortexed, sonicated for 10 min at 4°C and centrifuged at 13,000 rpm for 10 min at 4 °C. The supernatant was transferred to a glass vial and stored at −80 °C until analysis. A pooled quality control (QC) sample was also prepared with each batch of samples by mixing healthy EBC and spiking it with a known concentration of eicosanoid standard mix. QCs, and non-spiked EBC samples (QC blank) were prepared following the sample preparation process for all samples. Blanks (mobile phase) were injected repeatedly during the analysis.
2.4. Instrumental analysis
A simultaneous untargeted and targeted metabolomics analysis was performed on an Agilent 1290 series HPLC system coupled with an Agilent 6530 quadrupole-time of flight (qTOF) mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). 20 μL of each sample was injected through an InfinityLab Poroshell 120 EC-C18 column (2.7 μm, 3.0 mm × 50 mm; Agilent Technologies, Palo Alto, CA, USA). The mobile phases consisted of water (A) and acetonitrile (B), both with 0.1% formic acid. The solvent flow rate was set to 0.6 ml min−1, the column temperature to 35 °C and the autosampler to 5 °C to increase sample stability. An electrospray ionization (ESI) source with an Agilent Jet Stream nebulizer was used in negative mode with the following operating parameters: capillary voltage, 4000 V; nebulizer pressure, 25 psi; drying gas, 10 L min−1; gas temperature, 250 °C; fragment voltage, 130 V. The qTOF calibration was performed daily with the manufacturer’s solution. Mass measurements were recalibrated using the reference mass m/z 112.9856 (deprotonated trifluoroacetic acid (TFA)) in negative ion mode. Mass spectra were acquired at MS resolution level at a scan rate of 2 spectra/s in the over a range of m/z 100–950, and for MS/MS over at a scan rate of 5 spectra/s in the over a range of m/z 50–750. Collision energies for precursor ions were set within the range 5–20 eV, after a preliminary optimization by injecting eicosanoid standard mix. The targeted compounds detected by LC-MS/MS are reported in Supplemental Table S1. Molecular formula and exact masses are provided together with LC-MS retention times and preferred precursor ions used for AutoMS/MS analysis. Confirmation qualifier masses and optimal collision energies used are also listed.
2.5. Data processing and statistical analysis
The metabolomic data analysis procedure workflow is shown in Figure 1. Untargeted and targeted information were acquired simultaneously in a single chemical analysis, but the treatment of the obtained data was performed separately. However, in both cases, LC-MS data were initially checked for qualitative purposes with Agilent’s Mass Hunter Qualitative Analysis B.06.00 software. For untargeted analysis, data mining was performed using an automated algorithm for peak finding, alignment and integration in Agilent’s Mass Hunter Profinder B.08.00 software. Because of the huge amount of data, raw data was treated by batches using Bach Recursive Feature Extraction with the following parameters: mass tolerance and window of 20 ppm and 0.025 Da, retention time (RT) range 0.2–16.5 min and window of 0.3 min, with minimum absolute abundance of 1000 counts. The obtained dataset was exported into a .cef format and imported to Agilent’s Mass Profiler Professional (MPP, V13.0) software for re-alignment, identification and initial statistical analysis. Same mass and retention time windows were set to compile all data into a single dataset. Afterwards, the identification of the obtained molecular features (markers), described as mass@retention time, was performed using ID browser, an integrated software in MPP. ID browser used the accurate mass information of the aligned spectra to calculate the proposed molecular formula and tentative compound name of each compound or marker. Based on matching experimental and theoretical isotope pattern of the markers, the software proposed formulas and names with scores above 70%. Compounds were identified by using the METLIN database. The dataset was filtered by removing compounds that appear in blank samples with signals higher than 10 (peak sample/blank ratio). Final data was normalized using probabilistic quotient normalization with median values per sample to correct the bias between sample collection and preparation [30].
Due to the low amount of signal detected for the targeted analysis, quantification was not possible. For that, we worked with the intensity of the peak detected corresponding to the listed compounds in Supplemental Table S1. Data was treated with Agilent’s Mass Hunter Quantitative (qTOF) Analysis B.07.00 software, where the peaks were compared and constrained into a MassHunter quantification method on the accurate mass precursor ion level, using the MS/MS information to confirm the compounds identification manually with adducts and spectral scoring accuracy. The targeted dataset containing integrated peak area values was corrected by batch analysis using prepared QCs.
Datasets were analyzed using univariate and chemometric data compression techniques. Both analyses were performed with MATLAB R2017a and PLS Toolbox V8.6.2 software. Tables were constructed using Excel software (Microsoft, Redmond, WA, USA). These techniques identified similarities and differences between analyzed samples. Univariate statistical analysis was performed using a two-sided Wilcoxon rank sum test (MATLAB command ranksum), which is a non-parametric statistical method testing the null hypothesis that data from two sets are samples from continuous distributions with equal medians, against the alternative that they are not. The test assumes that two samples are independent. Having a p value less than 0.05 indicates the rejection of the null hypothesis of equal medians at the 5% significance level. For untargeted analysis, Principal Components Analysis (PCA) was applied as an unsupervised method that explores the intrinsic variation of the data detecting potential patterns. PCA simplifies the complexity of high-dimensional data by transforming the data into fewer dimensions with a “best-fitting” straight line or plane [31]. Partial Least-Squares Discriminant Analysis (PLS-DA), however, is a supervised classification technique that uses a response category and allows the filtering of the discriminant compounds or markers [32]. All techniques provided a list of potential markers related to the health condition and other relevant biological information of the study.
3. Results and discussion
3.1. Demographic and clinical characteristics data from participants at baseline
From February 2018 to December 2018, six asthmatic patients and six healthy participants from the northern Central Valley California region were recruited. A total of 293 breath samples were collected and analyzed longitudinally in the study, with about 28 samples for each of the 11 subjects. One hundred sixty samples were collected from the six control subjects and 133 from five asthmatic subjects. Of these, samples from one asthmatic participant were excluded as contaminated. All samples from the subject who was excluded from the study contained a white solid powder that was only observed when the sample was lyophilized. This precipitate consisted of fibrin or mucous substances soluble with mobile phase, which clogged the LC system making the instrumental analysis not feasible. All control subjects were in good health and had no history of smoking. Health symptoms and medicinal use as reported by participants while collecting breath samples are represented in Supplemental Figure S1. Asthmatic subjects were 16.7 ± 1.3 years of age and healthy control subjects were 17.2 ± 1.5 years of age. For the control group there were three male and three female subjects, and for the asthmatic group three male and two female subjects. All asthmatic participants were prescribed daily inhaled corticosteroid. Mean prescribed inhaled corticosteroid dose range was 440 μg/day. Three of the five asthmatic participants were also on a leukotriene antagonist.
Participants were surveyed regarding overall health, quality of life, shortness of breath symptoms, physical activities capacity as well as healthcare utilization (Supplemental Table S2). Overall health score indicates better overall perceived health and less fatigue in healthy participants. No healthy participants reported shortness of breath in comparison to asthmatic subjects who reports average score of 4.2 while a score of 10 indicates severe shortness of breath within the last month. Healthy participants also did more demanding activities including walking, swimming, bicycling whereas asthmatic subjects who did more stretching and strengthening. Unsurprisingly, asthmatic subjects visited physician offices three times more frequently than healthy subjects did. One asthmatic participant went to the ED for health concerns within the last 6 months.
3.2. Untargeted metabolomics analysis
A total of 3,583 metabolite features were obtained from the LC-MS chromatograms in negative mode. Data was previously aligned and filtered, as described in section 2.5. The resulting data were autoscaled before building the multivariate models.
PCA was first established based on these features to discover the presence of inherent similarities in mass spectral profiles between groups of samples (Figure 2). Individual differences between subject batch of samples were studied (Figure 2a and 2b), as well as main differences between health conditions (Figure 2c), defined by control/healthy versus asthmatic. Although PCA shows low explained variance, the highest variability (13% in first principal component, PC1) is due to one of the subjects (S-01) front the rest of participants (Figure 2a). These results corresponded to the signal obtained by the Total Ion Chromatogram (TIC) from the samples (Supplemental Figure S2) and S-01 already presented higher LC-MS intensities on the TIC profile through all the samples. No parameters (ambient humidity, temperature, dryness of mouth, shortness of breath) from S-01 were significantly different from other subjects during time of sampling, except reports of high fatigue. S-01 was part of the control group and these differences among other controls could be explained by either an unknown health condition, an incorrect sample collection procedure, or sample contamination during the experimental process.
Figure 2.

Scores plot from the Principal Components Analysis (PCA) obtained with untargeted LC-MS data. All samples were distributed in two-dimensional score plots represented by PC1 and PC2 (a), as well as PC2 and PC4 (b and c). Samples were colored by subject batches (a and c) and by health condition (b).
When PC 2 and PC 4 were studied, even explaining only around 10% of the total variance, differences could be distinguished between most of the subjects (Figure 2b). That information can be used to define specific metabolites per subject by reaching the loadings or variables that describe those sample distributions in the PCA score plot. When the same data was presented by health conditions (Figure 2c); then, group differences were enhanced with a clear separation between subjects belonging to the control group and the ones belonging to asthmatic group.
Based on the preliminary PCA results, a PLS-DA model was constructed to discriminate the difference under the already established separation between health conditions. As shown in Figure 3, the subjects from the asthmatic and control groups were appreciably separated from each other using two latent variables (LV). The model was validated by applying cross-validation with random subsets, and a test validation using 66% of the samples randomly to build the PLS-DA model and the rest as external test samples. In both cases, all the samples were correctly classified by the health condition.
Figure 3.
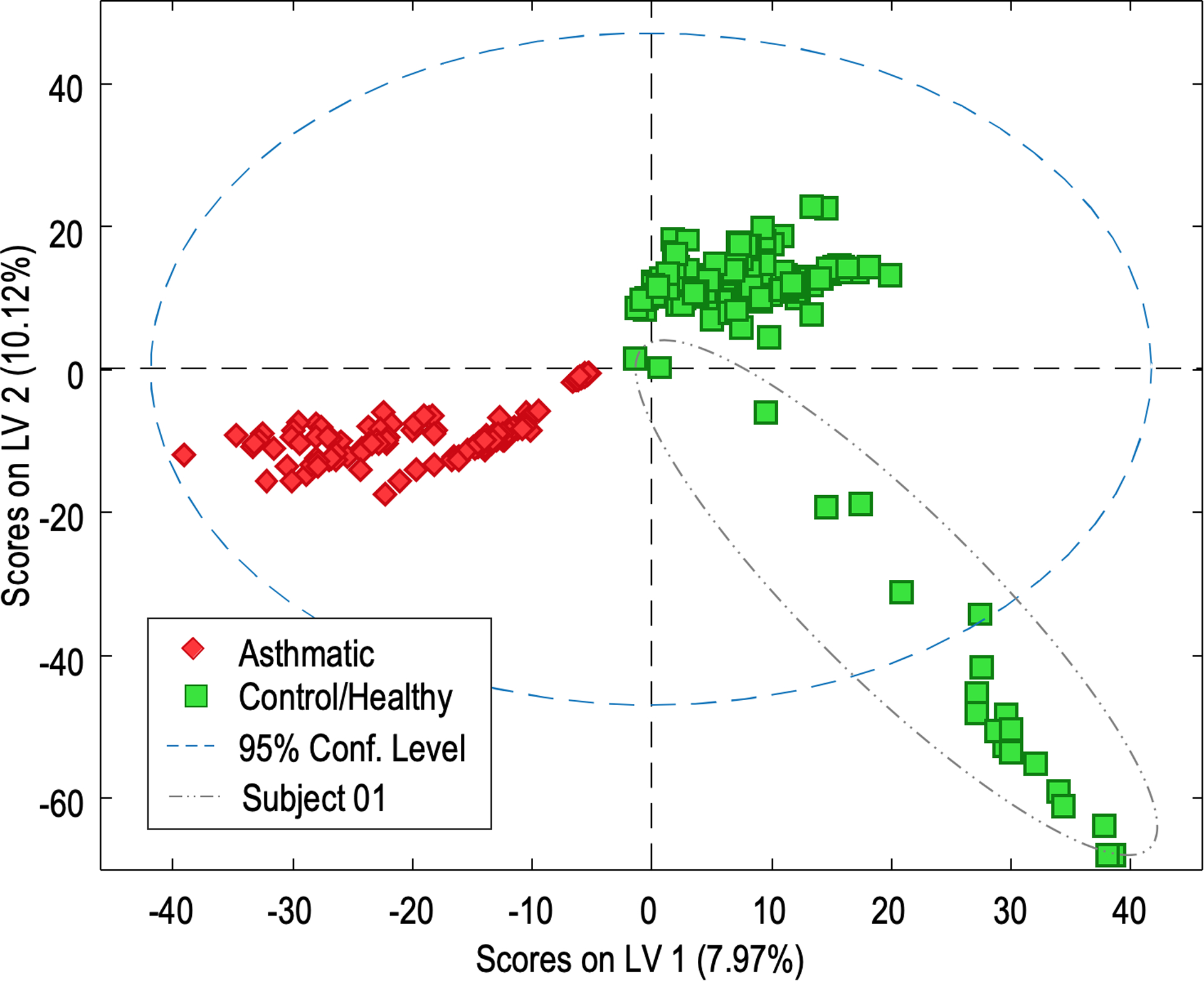
PLS-DA scores plot obtained with untargeted LC-MS data. Sample discrimination by health condition using 2 components (LV1 versus LV2). Control/healthy samples are colored in green and asthmatic samples are colored in red.
The clear sample separation by PLS-DA score plots indicates which loadings define the model components. Loadings are described by the data variables or features, which include the potential asthmatic marker metabolites that distinguish the sample groups. For example (Figure 3), positive values for LV1 allow the identification of characteristic features for control/healthy subjects. In the same way, negative LV1 and LV2 describe specific features from subjects from the asthmatic group. Although LV loadings provide useful asthmatic- versus control-regulated features, Variable Importance for the Projection (VIP) score values are principally used to select potential features. The contribution of VIPs from the PLS-DA allows a reliable selection of differential metabolites when selecting VIP values that exceed 1.0. Based on these criteria the number of relevant variables was reduced to 1338. From those, the 30 compounds with highest VIPs were selected and presented in Table 1.
Table 1.
The list of potential metabolites identified by untargeted liquid chromatography-mass spectrometry (LC-MS) analysis of all exhaled breath condensate samples.
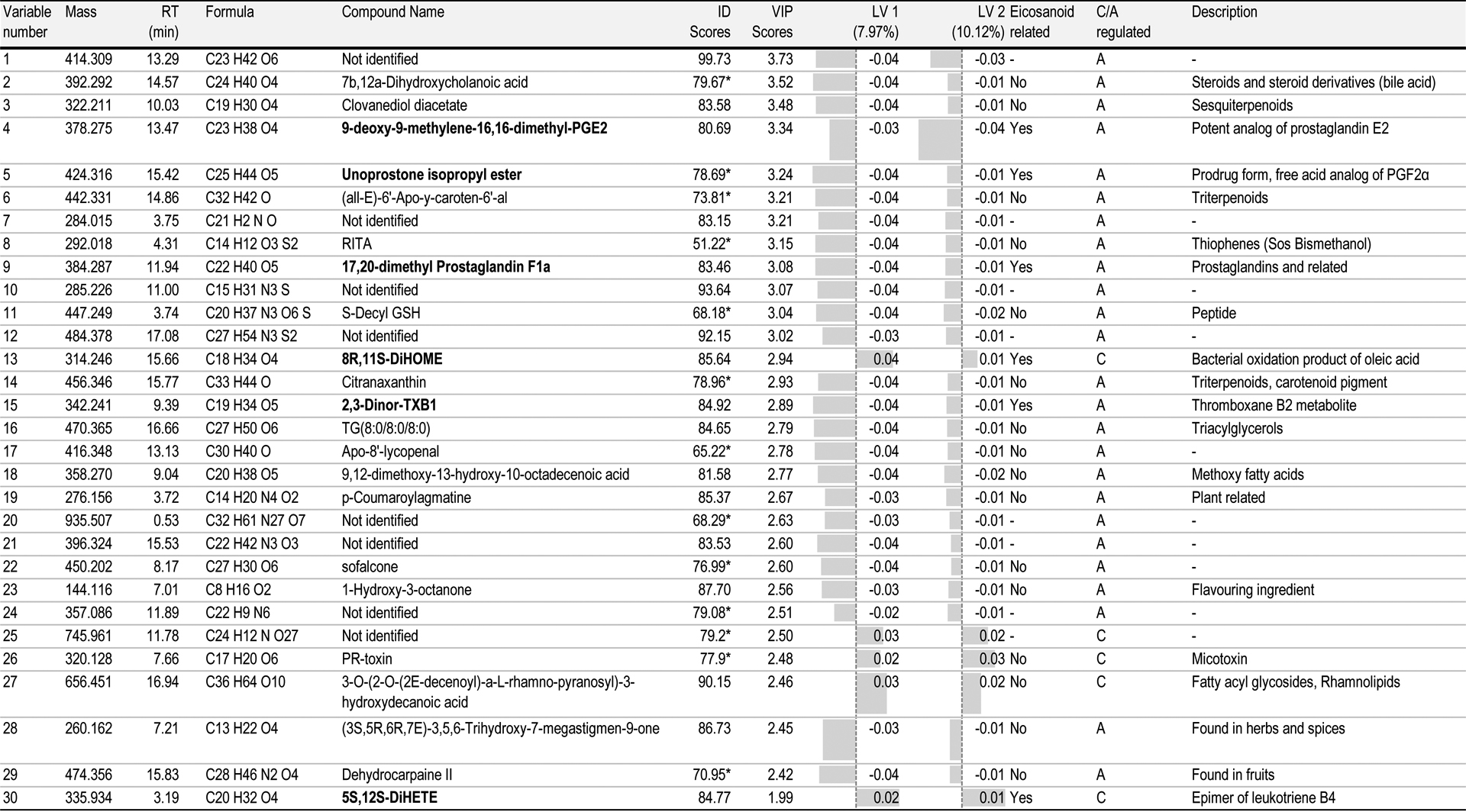
|
ID score below 80%; C: control/healthy samples; A: asthmatic samples
3.3. Potential metabolite identification
Metabolite identification of untargeted data was performed based on the MS and MS/MS spectra and the accurate masses obtained using METLIN database. In Table 1, potential biomarkers are listed with their exact molecular mass and retention time. Molecular formula and compound identification are described together with their identification (ID) score, calculated with the average values from molecular formula extraction and database ID scores. PLS-DA values from VIP scores, LV1 and LV2 loadings are also presented. Those values allow the health condition regulation. Once the features were identified, we listed them as eicosanoid or compound related to inflammation process.
Six metabolites (bolded in Table 1) were identified as eicosanoid related, four of them specific from asthmatic subjects: 9-deoxy-9-methylene-16,16-dimethyl-prostaglandin E2, unoprostone isopropyl ester, 17,20-dimethyl prostaglandin F1a and 2,3-dinor-TXB1. All of them are prostaglandin related compounds, except the last one, which is a metabolite from thromboxane B2. Also, 8R,11S-DiHOME and 5S, 12S-DiHETE were identified as regulated by control group. The first one is formed by bacterial oxidation of oleic acid; and the latter, is an epimer of leukotriene B4. Although, no asthma pro- or -anti-inflammatory studies are related to these compounds, this pilot study is presenting preliminary data from potential metabolites that can be related to asthmatic conditions. Further studies can provide more information and confirmation of these promising results.
3.4. Targeted eicosanoid metabolite analysis
The eicosanoid profile of the EBC samples was also characterized using targeted metabolomic analysis (Table 2). From the 30 compounds screened using the LC-MS method, seven were detected in more than half of the samples. We mainly detected in more than 80% of the total samples the sum of 9(S)- and 13(S)-hydroxyoctadecadienoic acids (HODE). These are oxidized derivatives of linoleic acid through 15-LOX pathway. All the compounds which formed through cytochrome P450 pathway (EpOMEs and DiOMEs) and prostaglandins B2 and E2 also appeared in most of the samples. The rest of compounds were detected in less samples, but found in more than 15% of the samples. Except for prostaglandin F2α and 8-isoPGF2α, 15(S)-HHTrE, 9-oxoODE and 8-OHdG that were rarely detected in overall the samples. Table 2 excludes undetected (zero) values in the dataset, unless if the compound was not ever detected from a subject. In this case, the value was set to zero and visualized with a white color. We found that excluding or including undetected values in the dataset did not significantly alter the metabolomic differences and patterns among subjects and between the control and asthmatic groups (data not shown).
Table 2.
Normalized average peak areas for targeted inflammatory biomarkers found in exhaled breath condensate from control and asthmatic subjects.
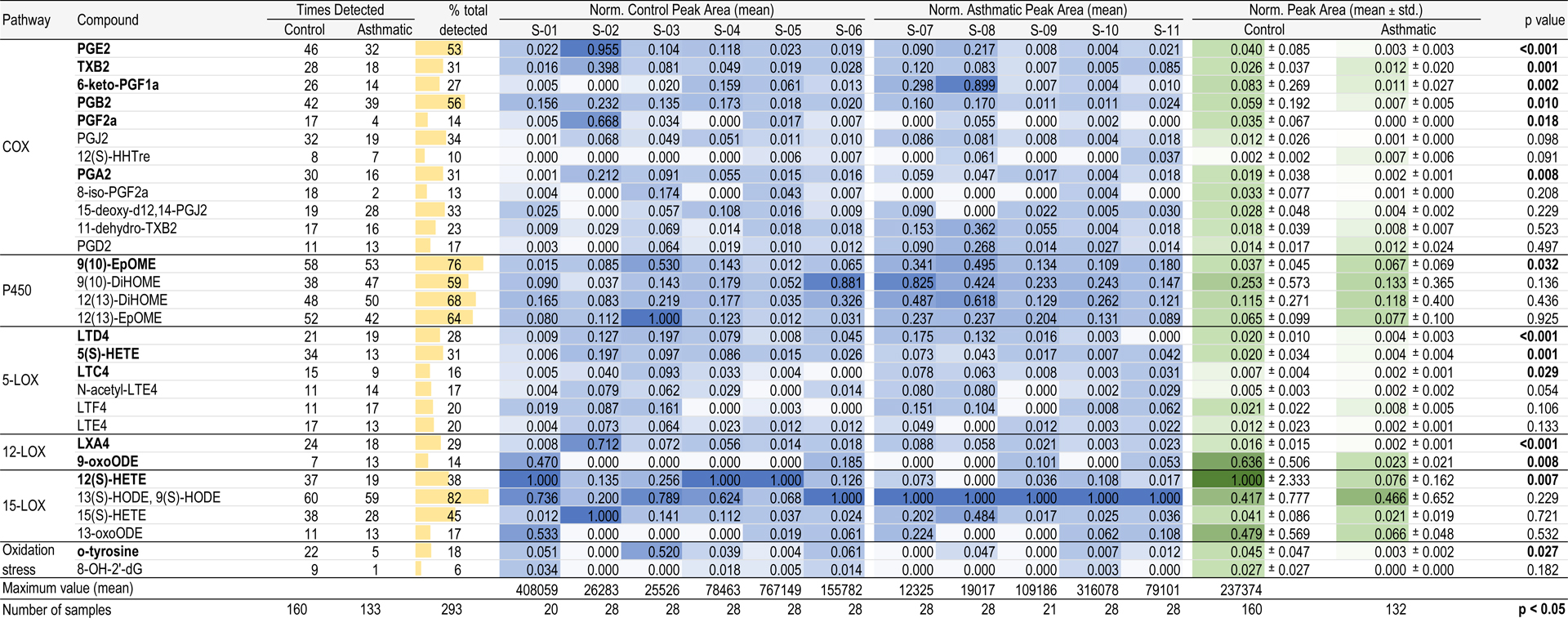
|
Table 2 shows the averaged peak signal obtained per each sample after normalization by mean and subsequently normalization by feature scaling per subject (blue-scaled columns). Here, we observe differences between individual sample batches. Maximum peak area values are listed in Table 2. When the maximum mean peak area values are multiplied by the feature scaled values (0 to 1) in its corresponding column, original average peak area (a.u.) values are obtained. While most of the compounds showed high variability, some of them appeared through all samples at high intensities, like 9(S)- and 13(S)-HODE. However, when peak areas are averaged and feature scaled by the control and asthmatic groups, these differences were enhanced (green-scaled columns). 12(S)-HETE presented high intensities for the control samples versus lower amounts for asthmatics. Surprisingly, this tendency occurs in several of the compounds, by which compounds seem to have lower intensities in asthmatic subjects than the controls.
To confirm this trend, a Wilcoxon signed rank test was applied to compare medians between the two groups. Fourteen out of thirty targeted compounds showed levels in EBC with significant differences between asthmatics and healthy control subjects (p<0.05). The targeted inflammatory biomarkers are shown for control (N=6) and asthmatic (N=5) subjects. Vertical axes are scaled non-uniformly and vary for each biomarker. n values above box plots indicate number of times the compound was detected for the control group and the asthmatic group. Biomarkers are grouped by arachidonic acid cascade metabolomic pathways. Biomarkers labelled with (+) and (–) are known to be pro-inflammatory and anti-inflammatory, respectively. Biomarkers further labelled with an asterisk (*) are likely but inconclusive in literature. The thirty targeted compounds are listed along with their CAS ID, classification and family in Supplemental Table S3. Functional descriptions of these targeted compounds are provided in Supplemental Table S4. Abundances of these fourteen compounds are summarized in Figure 4, where box-plots are also presented. Undetected (zero) values are excluded in this dataset. Box-plots allow a simple visualization of these differences between groups. We found six compounds that are formed by COX pathway: prostaglandins E2, B2, F2α and A2, thromboxane B2 and 6-keto PGF1α. All seem to be specific from the control subjects, with lower intensities in asthmatics. The same pattern appears with the metabolites generated by 5-, 12- and 15-LOX pathways, which include leukotrienes D4 and C4, lipoxin A4, 5- and 12-HETE and 9-oxoODE, as well as o-tyrosine that is formed by oxidation stress of proteins. 9(10)-EpOME presents an up-regulation towards asthmatics, although there are no studies proving the inflammatory effect of this metabolite. One plausible explanation for this pattern can be the inflammatory suppression caused by the medication prescribed for the asthmatic subjects. These medications can reduce the abundance of compounds, which are related to the asthmatic condition. Peak area distributions of the remaining sixteen compounds are summarized in Supplemental Figure S3 (p>0.05). Responses to asthma medications vary considerably among patients. Additionally, asthma is unlikely to be a single disease, but rather a series of complex, overlapping individual diseases or phenotypes, each defined by unique interactions between genetic and environmental factors [33]. Further asthmatic studies with larger sample sizes and more frequent sampling are needed to verify the effects of medicinal dosage and time of use on metabolomic profiles during breath sampling.
Figure 4.
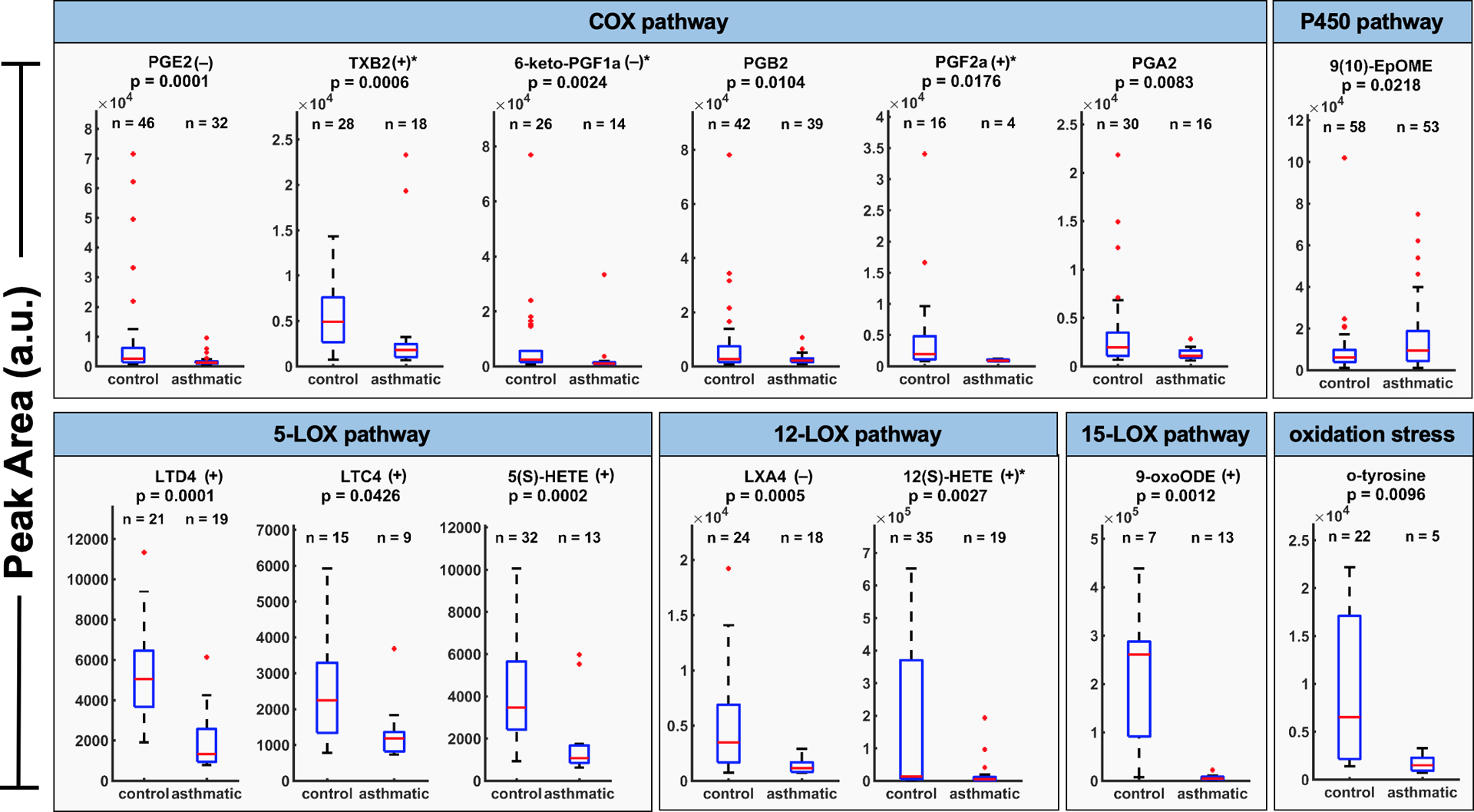
Exhaled breath condensate biomarker peak area distributions, which are significantly correlated between control and asthmatic subjects (p<0.05).
Finally, detected targeted compounds were correlated and studied considering possible confounder parameters and information collected during the study. Temperature and relative humidity considering all samples (24.7 ± 2.7 °C, 45.2 ± 10.6 %RH) showed no significant changes to the abundance of these compounds (data not shown). However, data showed interesting patterns when it was presented longitudinally (Figure 5). During the 14 days of collection EBC was sampled morning (AM) and evening (PM), and these time points were clearly differentiated for some of the compounds, such as 12(S)-HETE, 9(S)- and 13(S)-HODE. Vertical axes are scaled non-uniformly and vary for each biomarker. There is a distinct pattern of a day/night cycle with elevations of peak area values in the evening samples. Moreover, these differences were presented mainly in asthmatic subjects, which can be explained by asthma being a representation of exaggerated amplitudes in comparison to healthy circadian patterns. Average peak areas for all thirty targeted compounds separated into four groups (control/asthmatic, AM/PM) are summarized with box-plots in Supplemental Figure S4. This could help further understand the variation of asthmatic symptoms throughout the day with worsened symptoms during the night and early morning.
Figure 5.
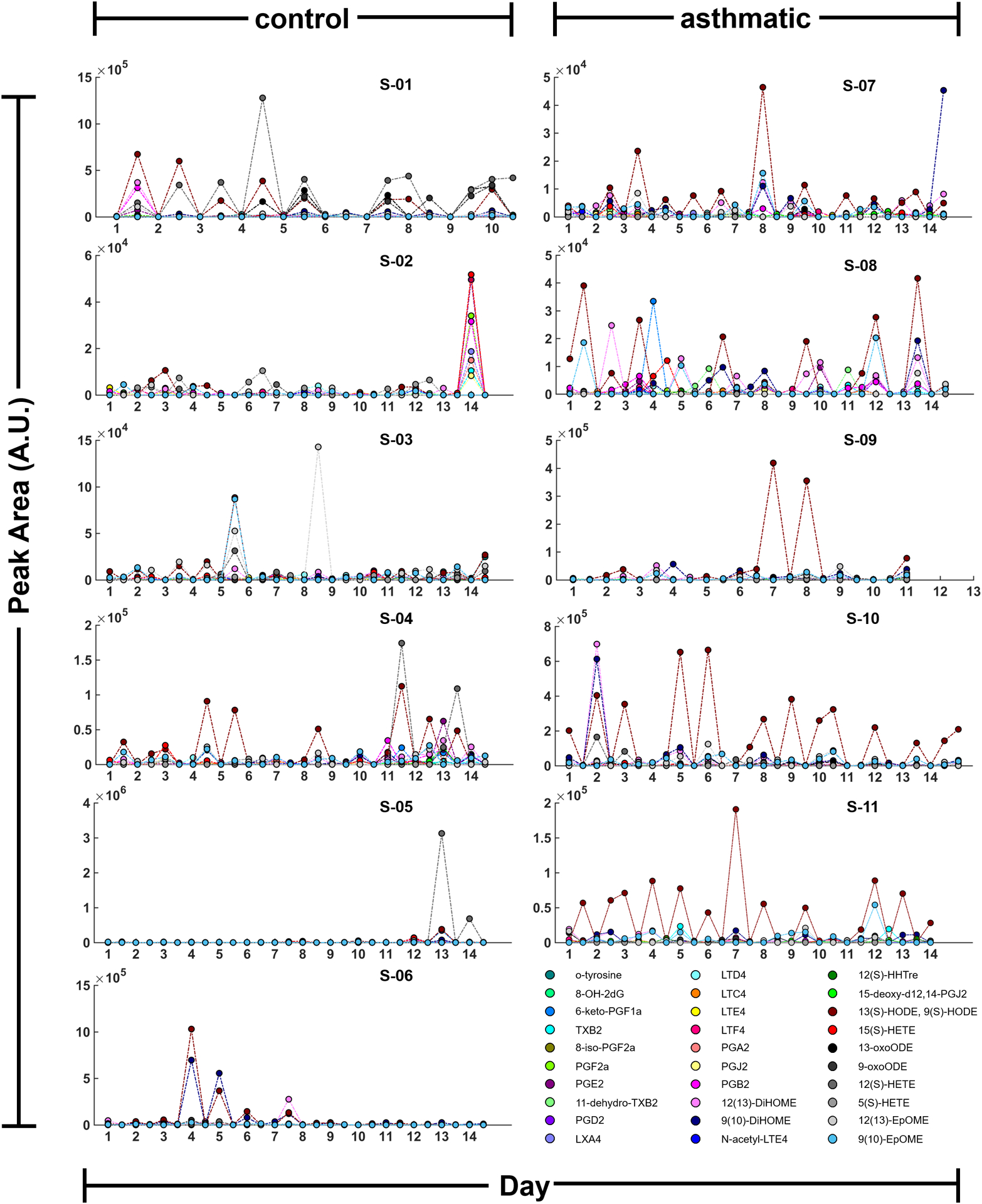
A time course of peak area values for targeted inflammatory biomarkers found in exhaled breath condensate from control and asthmatic subjects.
4. Conclusions
In future applications, this miniature breath collector may transport the collected sample from its collection surface unit into an interfaced analytical or storage unit with minimal power input. The amount of sample collection could be reduced to a few microliters and sampling time to a few minutes when collection and analysis are possible with novel microfluidic platforms. The miniaturized EBC collection device allows for minimal sample volumes needed for representative studies. This opens a non-invasive way to study metabolic effects of environmental exposure and medical interventions using measurable targets in many health conditions including asthma. Untargeted analysis measures all endogenous metabolic signals in a biological sample, which allows for the discovery of novel biomarkers that can explain differences in health conditions. Targeted analysis allows for quantifying a predefined set of selected metabolites, which are related to certain diseases and medical conditions. Further untargeted metabolite analysis may be explored, similarly done for targeted metabolites. This device is able to finely resolved time data sequence on the order of hours and can lead to a substantial amount of potential data to be collected for increasing the power of various studies. Currently, there are no other reports of EBC studies that allow for monitoring individuals longitudinally instead of cross-sectionally at study visits which can be a major advantage in personal health monitoring. Additionally, its portability is advantageous because it enables breath samples to be collected in multiple environments, including intensive care units (ICUs), outpatient clinics, workplaces, and at home.
Inflammatory airway disease such as asthma causes exaggerated fluctuations and amplitudes in metabolomic profiles in comparison to normal circadian patterns. Health conditions of interest, ones that are mostly independent on circadian rhythms, would allow for the pooling of samples to obtain more amount of sample per analysis. This would achieve lower limits of detection to more accurately quantify known biomarkers and discover novel biomarkers. Studies with larger sample sizes would also allow for determining regional differences and dependencies for metabolomic profiles. The addition of positive ionization mode or other alternative separation and detection platforms would present further opportunities to discover more metabolites correlated with other respiratory diseases including asthma.
Supplementary Material
Acknowledgements
Partial support was provided by: NIH award U01 EB0220003-01 (CED, NJK); the NIH National Center for Advancing Translational Sciences (NCATS) through grant UL1 TR000002 (CED, NJK); NIH award 1P30ES023513-01A1 (CED, NJK); NIH award UG3-OD023365 [CED]. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies. The authors also gratefully acknowledge access and use of the Food Safety and Measurement Facility in the Department of Viticulture and Enology at UCD for sample analysis. The authors thank Dr. Konstantin Zamuruyev (NASA Jet Propulsion Laboratory, Caltech) for technical discussions. The authors also thank Carolyn Doyle for lyophilization of the samples. Finally, the authors are grateful for all the participating children and their families who devoted their time and samples for the sake of conducting the research.
Definition of Abbreviations
- 8-OH-2dG
8-Oxo-2’-deoxyguanosine
- a.u.
arbitrary units
- avg.
average
- COX
cyclooxygenase
- DiHOME
dihydroxy octadecenoic acid
- ED
emergency department
- EIC
Extracted Ion Chromatogram
- EpOME
epoxy octadecenoic acid
- HETE
hydroxyl eicosatetraenoic acid
- HHTre
heptadecatrienoic acid
- HODE
hydroxyoctadecadienoic acid
- LX
lipoxin
- LOX
lypooxygenase
- LT
leukotriene
- oxoODE
oxo octadecadienoic acid
- P450
cytrochrome P450 (CYPs)
- PC
principal component
- PCA
principal component analysis
- PG
prostaglandin
- PLS-DA
partial least squares discriminant analysis
- qTOF
quadrupole time of flight
- S
subject
- std
standard deviation
- TX
thromboxane
Footnotes
Conflict of interest: The authors have a previously issued patents on a component part of the palm-sized breath sampler (US Patent #10,067,119 and US Patent #9,398,881), and one additional patent pending.
References
- 1.Horváth I, Hunt J, and Barnes PJ, Exhaled breath condensate: methodological recommendations and unresolved questions. European Respiratory Journal, 2005. 26(3): p. 523–548. [DOI] [PubMed] [Google Scholar]
- 2.Peralbo-Molina A, et al. , Development of a method for metabolomic analysis of human exhaled breath condensate by gas chromatography–mass spectrometry in high resolution mode. Analytica Chimica Acta, 2015. 887(Supplement C): p. 118–126. [DOI] [PubMed] [Google Scholar]
- 3.Brett RW, et al. , Standardization of the collection of exhaled breath condensate and exhaled breath aerosol using a feedback regulated sampling device. Journal of Breath Research, 2017. 11(4): p. 047107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pleil JD and Stiegel MA, Evolution of Environmental Exposure Science: Using Breath-Borne Biomarkers for “Discovery” of the Human Exposome. Analytical Chemistry, 2013. 85(21): p. 9984–9990. [DOI] [PubMed] [Google Scholar]
- 5.Kazani S, et al. , Exhaled breath condensate eicosanoid levels associate with asthma and its severity. J Allergy Clin Immunol, 2013. 132(3): p. 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leandro Genehr F, et al. , Profile of eicosanoids in breath condensate in asthma and COPD. Journal of Breath Research, 2012. 6(2): p. 026001. [DOI] [PubMed] [Google Scholar]
- 7.Pelclov, et al. , Leukotrienes B4, C4, D4 and E4 in the Exhaled Breath Condensate (EBC), Blood and Urine in Patients with Pneumoconiosis. Industrial Health, 2012. 50(4): p. 299–306. [DOI] [PubMed] [Google Scholar]
- 8.Esther CR, et al. , Exhaled breath condensate adenosine tracks lung function changes in cystic fibrosis. American Journal of Physiology - Lung Cellular and Molecular Physiology, 2013. 304(7): p. L504–L509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antczak A, et al. , Increased Exhaled Cysteinyl-Leukotrienes and 8-Isoprostane in Aspirin-induced Asthma. American Journal of Respiratory and Critical Care Medicine, 2002. 166(3): p. 301–306. [DOI] [PubMed] [Google Scholar]
- 10.Baraldi E, et al. , Cysteinyl leukotrienes and 8-isoprostane in exhaled breath condensate of children with asthma exacerbations. Thorax, 2003. 58(6): p. 505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zanconato S, et al. , Leukotrienes and 8-isoprostane in exhaled breath condensate of children with stable and unstable asthma. J Allergy Clin Immunol, 2004. 113(2): p. 257–63. [DOI] [PubMed] [Google Scholar]
- 12.Montuschi P and Barnes PJ, Exhaled leukotrienes and prostaglandins in asthma. Journal of Allergy and Clinical Immunology, 2002. 109(4): p. 615–620. [DOI] [PubMed] [Google Scholar]
- 13.Montuschi P, Leukotrienes, antileukotrienes and asthma. Mini Rev Med Chem, 2008. 8(7): p. 647–56. [DOI] [PubMed] [Google Scholar]
- 14.Montuschi P, et al. , Liquid chromatography/mass spectrometry analysis of exhaled leukotriene B(4 )in asthmatic children. Respiratory Research, 2005. 6(1): p. 119–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montuschi P, et al. , Ion trap liquid chromatography/tandem mass spectrometry analysis of leukotriene B4 in exhaled breath condensate. Rapid Communications in Mass Spectrometry, 2004. 18(22): p. 2723–2729. [DOI] [PubMed] [Google Scholar]
- 16.Montuschi P, LC/MS/MS analysis of leukotriene B4 and other eicosanoids in exhaled breath condensate for assessing lung inflammation. Journal of Chromatography B, 2009. 877(13): p. 1272–1280. [DOI] [PubMed] [Google Scholar]
- 17.Syslová K, et al. , Determination of cysteinyl leukotrienes in exhaled breath condensate: Method combining immunoseparation with LC–ESI-MS/MS. Journal of Chromatography B, 2011. 879(23): p. 2220–2228. [DOI] [PubMed] [Google Scholar]
- 18.Sanak M, et al. , Targeted eicosanoids lipidomics of exhaled breath condensate in healthy subjects. Journal of Chromatography B, 2010. 878(21): p. 1796–1800. [DOI] [PubMed] [Google Scholar]
- 19.Glowacka E, et al. , Exhaled eicosanoid profiles in children with atopic asthma and healthy controls. Pediatric Pulmonology, 2013. 48(4): p. 324–335. [DOI] [PubMed] [Google Scholar]
- 20.Sachs-Olsen C, et al. , Eoxins: A new inflammatory pathway in childhood asthma. Journal of Allergy and Clinical Immunology, 2010. 126(4): p. 859–867.e9. [DOI] [PubMed] [Google Scholar]
- 21.Ono E, et al. , Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma. Am J Respir Crit Care Med, 2014. 190(8): p. 886–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy BD, Resolvin D1 and Resolvin E1 Promote the Resolution of Allergic Airway Inflammation via Shared and Distinct Molecular Counter-Regulatory Pathways. Frontiers in Immunology, 2012. 3: p. 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dutta K, Prasad P, and Sinha D, Chronic low level arsenic exposure evokes inflammatory responses and DNA damage. Int J Hyg Environ Health, 2015. 218(6): p. 564–74. [DOI] [PubMed] [Google Scholar]
- 24.Syslova K, et al. , LC-ESI-MS/MS method for oxidative stress multimarker screening in the exhaled breath condensate of asbestosis/silicosis patients. J Breath Res, 2010. 4(1): p. 017104. [DOI] [PubMed] [Google Scholar]
- 25.Janicka M, et al. , Sensitive determination of isoprostanes in exhaled breath condensate samples with use of liquid chromatography–tandem mass spectrometry. Journal of Chromatography B, 2012. 893–894: p. 144–149. [DOI] [PubMed] [Google Scholar]
- 26.Sinha D, Biswas J, and Bishayee A, Nrf2-mediated redox signaling in arsenic carcinogenesis: a review. Arch Toxicol, 2013. 87(2): p. 383–96. [DOI] [PubMed] [Google Scholar]
- 27.Zamuruyev KO, et al. , Power-efficient self-cleaning hydrophilic condenser surface for portable exhaled breath condensate (EBC) metabolomic sampling. J Breath Res, 2018. 12(3): p. 036020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zamuruyev KO, et al. , Human breath metabolomics using an optimized non-invasive exhaled breath condensate sampler. J Breath Res, 2016. 11(1): p. 016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willis PA, Creamer JS, and Mora MF, Implementation of microchip electrophoresis instrumentation for future spaceflight missions. Anal Bioanal Chem, 2015. 407(23): p. 6939–63. [DOI] [PubMed] [Google Scholar]
- 30.Wulff JE and Mitchell MW, A Comparison of Various Normalization Methods for LC/MS Metabolomics Data. Advances in Bioscience and Biotechnology, 2018. 09(08): p. 339–351. [Google Scholar]
- 31.Pearson K, LIII. On lines and planes of closest fit to systems of points in space. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 2010. 2(11): p. 559–572. [Google Scholar]
- 32.Lee LC, Liong CY, and Jemain AA, Partial least squares-discriminant analysis (PLS-DA) for classification of high-dimensional (HD) data: a review of contemporary practice strategies and knowledge gaps. Analyst, 2018. 143(15): p. 3526–3539. [DOI] [PubMed] [Google Scholar]
- 33.Borish L and Culp JA, Asthma: a syndrome composed of heterogeneous diseases. Annals of Allergy, Asthma & Immunology, 2008. 101(1): p. 1–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


