Abstract
Background
Although Idiopathic Parkinson’s disease (IPD) develops in considerable patients with drug-induced Parkinsonism (DIP), the association hasn’t been well defined. We aimed to evaluate the underlying association and risk factors of DIP and IPD.
Methods
A retrospective cohort study using National Health Insurance Claims data in 2011–2016 was conducted. New-onset DIP patients in 2012 were selected and matched with active controls having diabetes mellitus at a 1:4 ratio by age, sex, and Charlson’s Comorbidity Index score. Comorbidity, causative drugs, and prescription days were evaluated as covariates.
Results
A total of 441 DIP were selected. During the 4-year follow up, 14 IPD events in the DM group but 62 events in the DIP group were observed (adjusted hazard ratio, HR: 18.88, 95% CI, 9.09–39.22, adjusting for comorbidities and causative drugs). IPD diagnosis in DIP was observed high in males compared to females (15.58/13.24%). The event was the most within the 1st year follow-up, mean days 453 (SD 413.36). Subgroup analysis in DIP showed calcium channel blocker (verapamil, diltiazem, and flunarizine) was significantly associated with increased IPD risk (HR: 2.24, 95% CI, 1.27–3.93).
Conclusion
Increased IPD in DIP patients might not be from the causal toxicity of antidopaminergic effects but from a trigger by the causative drugs on the DIP patients who already had subclinical IPD pathology. DIP can serve as a strong proxy for IPD incidence. Subjects who develop DIP should be monitored carefully for potential IPD incidence.
Introduction
Drug-induced Parkinsonism (DIP) is the most serious iatrogenic movement disorder in the elderly as it increases the risk of gait dysfunction, falls, assisted living condition and considerably decreases the daily functioning and quality of life [1]. A recent 2016 report from the Rochester Epidemiology Project reported that the incidence of both the Idiopathic Parkinson’s disease (IPD) and Parkinsonism were increased significantly over the 30 years. The relative risk (RR) for IPD was 1.35 per decade (95% CI, 1.10–1.65), and the RR for Parkinsonism was 1.24 per decade (95% CI, 1.07–1.44) [2]. The average annual incidence rate of DIP over 30 years in Olmsted County, Minnesota was 3.3 per 100,000 person-years overall (108 patients), 2.1 in men (33 patients), and 4.3 in women (75 patients). DIP was the most common type of Parkinsonism, accounting for 11 of 15 cases (73.3%) in the youngest age group (0–39 years) [1].
The risk factors for DIP include aging, female sex, anti-dopaminergic potency and length of causative drug use, pre-existing extrapyramidal signs, and familial or genetic predisposition [3]. The main etiology of DIP is from diminished D2 receptor stimulation, which occurs primarily through D2 receptor blockade by antipsychotics and related drugs [4].
In general, distinct differences between DIP and IPD exist by molecular imaging techniques; the estimated number of presynaptic dopamine-secreting neurons is diminished in IPD, but normal in DIP [4]. In general, the symptoms of Parkinsonism resolve in 6 months in DIP [5], and 70% of patients usually recover within a few months after withdrawal of the causative drugs. However, Parkinsonism persists in the remaining DIP patients [6, 7], and a considerable proportion of DIP patients is diagnosed with IPD [8, 9]. This phenomenon has evoked concerns about whether neuroleptic harmful exposure of anti-dopaminergic drugs (presented as DIP) would cause IPD.
Kim et al presented 2 DIP patients who initially presented normal dopamine transporter (DAT) activity and reached full remission, but experienced recurrence with decreased DAT activity at a 2-year follow-up scan [10]. Burn & Brook also reported 5 patients who had normal DAT imaging at DIP diagnosis did not experience full remission until 2 years [11]. Foubert-Samier et al [12] conducted a prospective study and demonstrated a long-term neuroleptic exposure might be a risk factor for IPD as well. Based on these findings, one theory supports some dopamine receptor blockers have direct toxic effects on neurons by inhibiting mitochondrial respiratory function and contribute to the irreversible cell death [13] or pathway deficits in nigrostriatal dopamine area [14].
However, another theory argues that a significant loss of dopaminergic neurons already exists in DIP patients, and the causative drugs accelerate the development of IPD [7, 15]. Adding on this theory, a recent study showed that 6 out of 7 patients with DIP had pathological findings compatible with an underlying IPD [16], which implicates dopamine blocking agents can unmask preclinical PD.
In the context of these conflicting findings, we aimed to evaluate the association and potential risk factors of DIP diagnosis and IPD incidence using a nationwide population-based health claims database.
Materials and methods
Study design and database
A retrospective cohort design was adopted. The cohort comprised all DIP patients in the National Health Insurance Claims Database combined with pharmacy prescription claims data in 2010–2016; the year 2010 and 2011 were used for the screening, 2012 for enrollment, and 2013–2016 for the 4-year follow-up. The national health insurance program in Korea provides universal coverage for the entire population, including roughly 50 million Koreans, so the diagnosis and therapeutic trends and characteristics of IPD and DIP could be evaluated systematically.
This study was approved by Hallym University Sacred Heart Hospital Institutional Review Board (IRB No. 2016–1081). We acquired the data through a form that deleted individual identities and only received the items pertaining to this research purpose, IRB did not require us to obtain informed consent from individual patients. All procedures performed in this study were conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Study design and subject definition
DIP subjects were defined as patients who 1) were 50–100 years of age, and 2) had a diagnosis code of DIP (ICD-10: G21.1), 3) had prescriptions of causative drugs within 1 year before DIP diagnosis. DIP is regarded as a sort of drug adverse events, and the diagnosis code is used when the physicians made sure Parkinsonism related symptoms are disappeared or reduced after the probable drugs are interrupted, which makes the code having high ascertainment.
As a comparison, we adopted an active comparator (patients with Diabetes Mellitus, DM) to avoid confounding bias common in pharmacoepidemiologic studies such as the healthy initiator bias in the selection of controls where control subjects might be too healthy compared to the normal population [17–19]. Since accumulating prospective cohort studies showed a possible association between DM and IPD incidence that pre-existing DM might be a risk factor for the development of IPD [20], we chose DM as an active control. DM subjects were defined as new-onset patients who had ICD-10 codes (E11, E12, E13, E14) along with anti-diabetes prescription. DM patients were matched to the exposed group (DIP) in 4:1 ratio by a propensity score derived from sex, age, and Charlson Comorbidity Index (CCI).
To include new-onset subjects, we excluded patients who had a diagnosis code for DIP or IPD in 2010 and 2011. The index dates (cohort entry date) for the DIP and DM group were the date of the first DIP or DM diagnosis code that appeared in claim data, respectively.
IPD patients were defined as those who had v-124 code, which is used for specific beneficiary insurance claims for IPD treatment in Korea. Since the v-124 code yields a major benefit to IPD patients who only need to pay 10% of all medical expenditures, assigning this code to a patient requires careful and complicated documentation. To assign the v-124 code to the patients, diagnostic MRI findings and major Parkinsonism syndromes including tremor, bradykinesia, rigidity, and postural instability should be examined and confirmed by the neurologists. Therefore, we were able to use the v-124 code for IPD ascertainment.
The event dates for both the DIP and the DM were defined as the first diagnosed date of IPD (v-124). We evaluated all the included patients until the last date of four-year follow-up or follow-up loss or death dates whichever comes first. To assess the comparison between pure DIP and DM patients, when DIP incidence happened in the DM group, they were followed up until DIP incidence happened (censoring was applied).
Definition of confounders
Age, sex, CCI scores, and comorbidities in the previous year and DIP-inducing (causative) drugs taken within 12 months before DIP diagnosis were assessed.
Drugs known to induce Parkinsonism included typical and atypical antipsychotics, dopamine depleters, dopamine synthesis inhibitors, calcium channel blockers (CCB), antiepileptics, antiemetics, a mood stabilizer (lithium), antiarrhythmics, antidepressants, and an immunosuppressant (cyclosporin), as proven by previous research [9]. They were reclassified into four categories by their potency for D2 receptor blockade (S1 Table).
Diseases that could affect IPD incidence but were not included in the CCI score calculation were assessed as variables (comorbidities). They were dementia [21, 22], depression [23], other neurodegenerative diseases [24], and hypertension [25]. The ICD-10 codes defining these diseases are provided in S2 Table.
Statistical analysis
Baseline characteristics were compared by the t-test for quantitative and the chi-square test for qualitative variables. Cox proportional hazard regression analysis was performed to evaluate the risk of IPD incidence in both groups. The hazard ratios (HRs) and the corresponding 95% CIs were estimated, adjusting for possible confounders. A sub-group analysis in the DIP group to identify potential risk factors associated with IPD was conducted using Cox proportional hazard regression analysis. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Study subjects
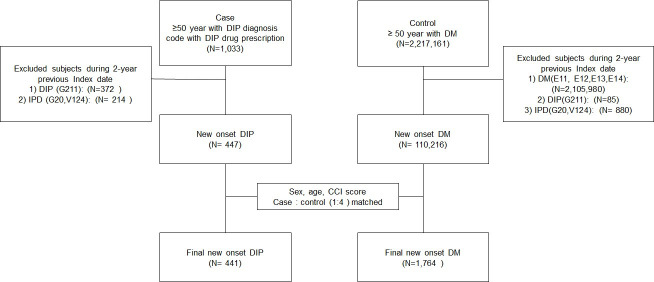
A total of 441 patients satisfied the inclusion criteria of DIP, and 1,764 DM patients were randomly matched by a propensity score calculated by age, sex, and CCI score at 1:4 ratio (Fig 1).
Fig 1. Flow diagram of subject selection and matching.
At baseline, female patients constituted 64.49% and the 70-79-years group constituted 43.67%, which was the highest proportion of the total subject. CCI score ranging 1–2 was 50.43% having the highest number of subjects. Hypertension (43.13%) was the highest comorbidity followed by depression (28.44%) and dementia (11.97%) in the total subject, which showed the same trend in both groups.
The causative drugs taken within 12 months before the DIP diagnosis were identified in 34.01% in the DM subjects. The most commonly prescribed causative drugs were class III (anti-emetics) in both groups (82.09%: DIP group, 29.76%: DM group). The next was class I (antipsychotics and others) for the DIP group (36.5%) and class IV (antidepressants and others), for the DM group (8.73%). Mean prescription days were 60.81(122.45) days for DM vs. 261.2 (190.46) days for the DIP group (p < .0001). In the DM group, the subject number who had prescription day ≥ 70% of the previous 1 year was 9.41% whereas it was 46.03% in the DIP group (Table 1).
Table 1. Baseline characteristics of DM and DIP subjects.
| Category | Subjects | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| DM, N (%) | DIP, N (%) | Total, N (%) | ||||||
| Sex | Male | 629 | 35.66 | 154 | 34.92 | 783 | 35.51 | 0.772 |
| Female | 1135 | 64.34 | 287 | 65.08 | 1422 | 64.49 | ||
| Age | 50–59 y | 275 | 15.59 | 70 | 15.87 | 345 | 15.65 | 0.995 |
| 60–69 y | 421 | 23.87 | 104 | 23.58 | 525 | 23.81 | ||
| 70–79 y | 769 | 43.59 | 194 | 43.99 | 963 | 43.67 | ||
| ≥80 y | 299 | 16.95 | 73 | 16.55 | 372 | 16.87 | ||
| Mean age (SD) | 70.46 (9.39) | 70.44 (9.43) | 70.46 (9.40) | 0.959 | ||||
| CCI score | 0 | 607 | 34.41 | 154 | 34.92 | 761 | 34.51 | 0.877 |
| 1–2 | 894 | 50.68 | 218 | 49.43 | 1112 | 50.43 | ||
| ≥3 | 263 | 14.91 | 69 | 15.65 | 332 | 15.06 | ||
| Mean CCI (SD) | 1.19 (1.31) | 1.18 (1.21) | 1.19 (1.29) | 0.863 | ||||
| Co-Morbidity | Dementia | 214 | 12.13 | 50 | 11.34 | 264 | 11.97 | 0.646 |
| Depression | 502 | 28.46 | 125 | 28.34 | 627 | 28.44 | 0.962 | |
| Hypertension | 771 | 43.71 | 180 | 40.82 | 951 | 43.13 | 0.273 | |
| Neuro-degenerative disease | 46 | 2.61 | 14 | 3.17 | 60 | 2.72 | 0.513 | |
| DIP-inducing drugs** (causative drugs) | Class I (antipsychotics) | 101 | 5.73 | 161 | 36.51 | 262 | 11.88 | < .0001 |
| Class II (calcium channel blockers) | 107 | 6.07 | 65 | 14.74 | 172 | 7.80 | < .0001 | |
| Class III (antiemetics) | 525 | 29.76 | 362 | 82.09 | 887 | 40.23 | < .0001 | |
| Class IV (antidepressants) | 154 | 8.73 | 110 | 24.94 | 264 | 11.97 | < .0001 | |
| Total | 600 | 34.01 | 441 | 100.00 | 1041 | 47.21 | < .0001 | |
| None | 1164 | 65.99 | 0 | 0.00 | 1164 | 52.79 | - | |
| Total prescription days in 1 year previous Index date | 0% | 1164 | 65.99 | 0 | 0.00 | 1164 | 52.79 | < .0001 |
| 0–30% | 263 | 14.91 | 123 | 27.89 | 386 | 17.51 | ||
| 30–70% | 171 | 9.69 | 115 | 26.08 | 286 | 12.97 | ||
| > = 70% | 166 | 9.41 | 203 | 46.03 | 369 | 16.73 | ||
| Mean days | 60.81(122.45) | 261.2 (190.46) | 100.89 (160.19) | < .0001 | ||||
| Total | 1764 | 100.00 | 441 | 100.00 | 2205 | 100.00 | ||
**DIP inducing drugs were multiple counted if subjects had different prescriptions during screening period.
Overall IPD risk between DIP and DM
DIP group had much higher IPD events than the DM group; 62/441(14.06%) in DIP vs. 14/1764 (0.79%) in DM. (p < .0001) The Cox proportional hazard regression analysis found a 17.90 times greater hazard of IPD in the DIP group than in the DM group (crude HR: 17.90, 95% CI, 10.02–31.97). When comorbidities and causative drugs were adjusted, the HR increased to 18.88 (adjusted HR: 18.88; 95 CI, 9.09–39.22).
Sex, age, and comorbidity did not significantly affect IPD incidence in both groups. Different levels of CCI scores did not significantly affect IPD incidence in either group. When the higher was the score, the more incidence of IPD was detected in both groups.
The neurodegenerative disease presented the highest IPD incidence (21.43%) but the other comorbidities showed similar incidences around 15–16% in the DIP group. In the DM group, dementia presented the highest IPD incidence yielding 2.34% similar to neurodegenerative disease (2.17%).
Among the causative drug classes, class II (CCB) was significantly associated with IPD incidence in the DIP group (p < .0001). In the DIP groups, IPD incidence was the highest in Class II (27.69%), but Class III (13.81%) and Class IV (13.64%) showed similar incidences. On the contrary, Class I (1.98%) and Class IV (1.95%) had a similarly high incidence of IPD in the DM group. The mean duration of taking causative drugs before DIP diagnosis in each group was not significantly different (Table 2).
Table 2. IPD incidence between DM and DIP subjects.
| Category | DM (N = 1,764) | DIP (N = 441) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-IPD | IPD | IPD N/row total N | p-value | Non-IPD | IPD | IPD N/row total N | p-value | ||||||
| N | % | N | % | % | N | % | N | % | % | ||||
| Total, N | 1750 | 99.21 | 14 | 0.79 | 0.79 | 379 | 85.94 | 62 | 14.06 | 14.06 | |||
| Mean follow-up days | 1,211 (484.90) | 832 (463.23) | 1,296(485) | 0.23 | 1,296(380.62) | 453 (413.53) | 1,178 (484) | < .0001 | |||||
| Sex | Male | 624 | 35.37 | 5 | 0.28 | 0.79 | 1.00 | 130 | 29.48 | 24 | 5.44 | 15.58 | 0.50 |
| Female | 1126 | 63.83 | 9 | 0.51 | 0.79 | 249 | 56.46 | 38 | 8.62 | 13.24 | |||
| Age | 50–59 y | 275 | 15.59 | 0 | 0.00 | 0.00 | 0.30 | 64 | 14.51 | 6 | 1.36 | 8.57 | 0.26 |
| 60–69 y | 418 | 23.70 | 3 | 0.17 | 0.71 | 85 | 19.27 | 19 | 4.31 | 18.27 | |||
| 70–79 y | 760 | 43.08 | 9 | 0.51 | 1.17 | 165 | 37.41 | 29 | 6.58 | 14.95 | |||
| ≥80 y | 297 | 16.84 | 2 | 0.11 | 0.67 | 65 | 14.74 | 8 | 1.81 | 10.96 | |||
| Mean (SD) | 70.43 (9.41) | 73.93 (7.05) | 70.46 (9.40) | 0.17 | 70.36 (9.62) | 70.87 (8.24) | 70.44 (9.43) | 0.70 | |||||
| CCI score, mean | 0 | 605 | 34.30 | 2 | 0.11 | 0.33 | 0.17 | 135 | 30.61 | 19 | 4.31 | 12.34 | 0.43 |
| 1–2 | 886 | 50.23 | 8 | 0.45 | 0.89 | 188 | 42.63 | 30 | 6.80 | 13.76 | |||
| ≥3 | 258 | 14.63 | 4 | 0.23 | 1.53 | 56 | 12.70 | 13 | 2.95 | 18.84 | |||
| Mean (SD) | 1.19 (1.31) | 1.64 (1.08) | 1.19 (1.31) | 0.19 | 1.17 (1.21) | 1.26 (1.21) | 1.18 (1.21) | 0.58 | |||||
| *Co-Morbidity | Dementia | 209 | 11.85 | 5 | 0.28 | 2.34 | 0.01 | 42 | 9.52 | 8 | 1.81 | 16.00 | 0.68 |
| Depression | 496 | 28.12 | 6 | 0.34 | 1.20 | 0.23 | 105 | 23.81 | 20 | 4.54 | 16.00 | 0.46 | |
| Hypertension | 763 | 43.25 | 8 | 0.45 | 1.04 | 0.31 | 152 | 34.47 | 28 | 6.35 | 15.56 | 0.45 | |
| Neuro-degenerative | 45 | 2.55 | 1 | 0.06 | 2.17 | 0.29 | 11 | 2.49 | 3 | 0.68 | 21.43 | 0.42 | |
| **Causative drugs taken 1 year before DIP diagnosis | Class I | 99 | 5.61 | 2 | 0.11 | 1.98 | 0.17 | 144 | 32.65 | 17 | 3.85 | 10.56 | 0.11 |
| Class II | 107 | 6.07 | 0 | 0.00 | 0.00 | 0.34 | 47 | 10.66 | 18 | 4.08 | 27.69 | 0.00 | |
| Class III | 518 | 29.37 | 7 | 0.40 | 1.33 | 0.10 | 312 | 70.75 | 50 | 11.34 | 13.81 | 0.75 | |
| Class IV | 151 | 8.56 | 3 | 0.17 | 1.95 | 0.09 | 95 | 21.54 | 15 | 3.40 | 13.64 | 0.88 | |
| Total defined | 592 | 33.56 | 8 | 0.45 | 1.33 | 0.07 | 379 | 85.94 | 62 | 14.06 | 14.06 | - | |
| None | 1158 | 65.65 | 6 | 0.34 | 0.34 | 0 | 0 | 0 | 0 | 0.00 | |||
| #Total prescription days in 1 year prior to the Index date | 0% | 1158 | 65.65 | 6 | 0.34 | 0.52 | 0.27 | 0 | 0.00 | 0 | 0.00 | - | 0.11 |
| <30% | 260 | 14.74 | 3 | 0.17 | 1.14 | 101 | 22.90 | 22 | 4.99 | 17.89 | |||
| 30%-70% | 168 | 9.52 | 3 | 0.17 | 1.75 | 96 | 21.77 | 19 | 4.31 | 16.52 | |||
| > = 70% | 164 | 9.30 | 2 | 0.11 | 1.20 | 182 | 41.27 | 21 | 4.76 | 10.34 | |||
| Mean days | 60.41 (122.24) | 110.5 (141.71) | 60.8 (122.5) | 0.13 | 268.67 (193.08) | 215.55 (167.84) | 268.2 (190.5) | 0.18 | |||||
*Co-morbidities were multiple counted if subjects had different ICD-10 codes during screening period.
** Dichotomous evaluation of having prescription (yes or no) were done and multiple counted if subjects had different prescriptions during screening period.
#Total sum of durations of all available prescriptions (duplicate prescriptions were allowed and overlapping period was not subtracted).
The total follow-up days for DIP and DM was 1,178.08 (SD 484.12) and 1,208.66 (SD 485.78) (p = 0.237), respectively. During the 4-year follow-up, 446 subjects died in the DM group but 77 subjects died in the DIP group (p = 0.0005) (S3 Table).
IPD risk by the year in age and sex groups
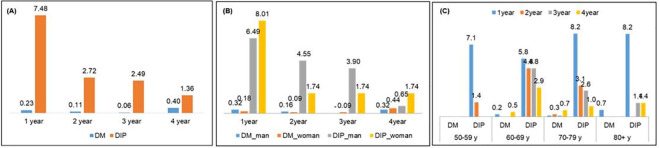
Aging is the strongest risk factor for IPD [26] and sex difference in IPD has been well characterized [27], so we evaluated IPD incidence by the year in age and sex groups. When IPD events by each year were compared in both groups, IPD incidence was the highest in the 1st year with a dramatic reduction thereafter in the DIP group. However, the DM group did not show a trend and it was the highest at the 4th year, suggesting DIP events triggered IPD diagnosis in DIP group whereas IPD diagnosis in DM group was increased with the time (aging) (Table 3, Fig 2A).
Table 3. Age and sex stratified IPD incidence by the year.
| Category | IPD, N (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | DIP | ||||||||||||
| Total DM | 1year | 2year | 3year | 4year | Total IPD | Total DIP | 1year | 2year | 3year | 4year | Total IPD | ||
| Sex | Male | 629 (100) | 2(0.32) | 1(0.16) | 0 | 2(0.32) | 5(0.79) | 154(100) | 10(6.49) | 7(4.55) | 6(3.90) | 1(0.65) | 24(15.58) |
| Female | 1135(100) | 2(0.18) | 1(0.09) | 1(0.09) | 5(0.44) | 9(0.79) | 287(100) | 23(8.01) | 5(1.74) | 5(1.74) | 5(1.74) | 38(13.24) | |
| Age | 50–59 y | 275 (100) | 0 | 0 | 0 | 0 | 0 | 70 (100) | 5 (7.14) | 1(1.43) | 0 | 0 | 6(8.57) |
| 60–69 y | 421 (100) | 1(0.24) | 0 | 0 | 2(0.48) | 3(0.71) | 104(100) | 6(5.77) | 5(4.81) | 5(4.81) | 3(2.88) | 19(18.27) | |
| 70–79 y | 769 (100) | 1(0.13) | 2(0.26) | 1(0.13) | 5(0.65) | 9(1.17) | 194(100) | 16(8.25) | 6(3.09) | 5(2.58) | 2(1.03) | 29(14.95) | |
| ≥80 y | 299 (100) | 2(0.67) | 0 | 0 | 0 | 2(0.67) | 73(100) | 6(8.22) | 0 | 1(1.37) | 1(1.37) | 8(10.96) | |
| Total | 1764 (100) | 4(0.23) | 2(0.11) | 1(0.06) | 7(0.40) | 14(0.79) | 441(100) | 33(7.48) | 12(2.72) | 11(2.49) | 6(1.36) | 62(14.06) | |
Abbreviation; IPD, Idiopathic Parkinson’s Disease; DM, Diabetes Mellitus; DIP, Drug Induced Parkinsonism.
Fig 2. Age and sex stratified IPD incidence by the year.
Both sexes presented the same proportion of IPD incidence in the DM group (0.79%). Although it was not significant, males had higher proportion of IPD incidents than females in the DIP group (15.58% vs. 13.24%) (Table 3, Fig 2B).
IPD occurred highest in the 60-69-years age category in DIP (18.27%) but 70-79- years age group in DM (1.17%) in the follow-up period. In both groups, the 50-59-years age group developed the fewest IPD events; the DIP group (8.57%) and the DM group (0%). The highest IPD events in all the age groups occurred in the first year of follow-up in the DIP group (Table 3, Fig 2C).
Subgroup analysis: Risk factors associated with IPD diagnosis in DIP
The risk factors associated with IPD development were evaluated by a Cox proportional hazard regression model, considering the death events in the DIP group. Sex, age group, and comorbid condition were not significantly associated with IPD. Only Class II (CCB) presented a significantly increased risk of IPD (HR: 2.24, 95% CI: 1.28–3.93) (Table 4).
Table 4. Subgroup analysis: Risk factors of IPD incidence in DIP subjects.
| Variables | Reference | Hazard Ratio | 95% CI | |
|---|---|---|---|---|
| Male | Female | 1.32 | 0.782 | 2.228 |
| 65–74 year | <65 year | 1.129 | 0.564 | 2.257 |
| ≥ 75 | <65 year | 1.024 | 0.487 | 2.152 |
| CCI continuous | 1.174 | 0.659 | 2.092 | |
| Dementia | No | 1.298 | 0.572 | 2.947 |
| Depression | No | 1.201 | 0.686 | 2.104 |
| Hypertension | No | 1.307 | 0.777 | 2.199 |
| Neurodegenerative disease | No | 1.516 | 0.466 | 4.937 |
| Class I | No | 0.554 | 0.271 | 1.13 |
| Class II | No | 2.238 | 1.276 | 3.927 |
| Class III | No | 0.543 | 0.245 | 1.203 |
| Class IV | No | 0.918 | 0.485 | 1.738 |
Discussion
We observed a dramatically increased risk of IPD incidence in DIP compared to DM subjects. The HR was a little bit increased to 18.88 from 17.90 after adjustment by comorbidities and the causative drugs, which means the confounders did not contribute to the high risk of IPD incidence in DIP subjects.
The mean age of DIP subjects was 70.44 (9.43) and the incidence was increased by the age except for ≥ 80 years old group which showed an abrupt decrease. Savica et al reported the median age at onset of DIP was 70.9 years (interquartile range: 54.4–79.7) but they reported the incidence of DIP kept increased with older age [1]. The decreased DIP in the oldest group (≥ 80 years) might be attributable to the stringent inclusion criteria of this study. We excluded all subjects who had either DIP or IPD diagnosis codes in the previous 2-year period of enrollment to only include pure incidence DIP and IPD cases. So, the older adults who had those incidences already could not be included in our study ended up having a low proportion of DIP incidence in the oldest group. DIP incidence in females was higher than males in this study, which is in line with the results of the previous study [1].
In the DIP group, male to female IPD incidence ratio was 1.17 (15.58/13.24), which also agrees with the previous study. A review article which examined sex difference in IPD incidence reported IPD incidence ratio of male to female ranges from around 1.3 to 2.0 in most studies, but rates as low as 0.95 have been observed in Asia [28]. IPD incidence was the highest in the 60–69 year-old group, which is slightly different from previous Korean IPD epidemiology results in 2007 where a ceiling of IPD incidence in individuals aged 70–79 years was noted with a decreasing trend thereafter [29]. This factor implicates DIP diagnosis prompted more incident IPD in the younger group disrupting the natural aging course. In the DM group, IPD incidence was increasing with aging and reduced after 80 years old.
In Korea, females are known to have a higher incidence of both DIP [30] and IPD [31] than males, unlike what has been established in studies from other countries, where a higher male prevalence has been found for IPD and a higher female prevalence for DIP [1, 32]. However, in this study, females presented higher DIP but lower IPD incidence than males, implicating DIP incidence influenced more males resulting in higher IPD incidence in males than females.
In an attempt to evaluate the risk factors associated with IPD incidence in subgroup analysis, all comorbidities (dementia, depression, hypertension, and neurodegenerative disease) showed a trend for increased risks but with insignificant results.
The most well-known drugs associated with the development of DIP were typical antipsychotics but we did not observe a strong impact of antipsychotics on DIP incidence. This might be due to a trend to prescribe more atypical antipsychotics, which have a weaker D2 blocking effect than the first-generation ‘typical’ antipsychotics [9]. We found that in this study, Class I drugs which include antipsychotics showed the lowest incidence of IPD in the DIP group and it was not much high in the DM group as well. Classes II showed the highest incidence of IPD (27.69%), and III and IV showed a similar incidence (13.81 and 13.64%) in DIP subjects. CCB has been associated with the incidence of DIP out of plausible mechanisms that neurons increase their reliance on Ca (2+) channels to maintain autonomous activity with age. Blockage of Ca(2+) channels by CCB could pose sustained metabolic stress on mitochondria, accelerating dopaminergic neuronal cell death [33], In a series of DIP in older adults, about 2/3 of DIP incidences were caused by CCB and 70% presented tremor as the initial symptom [34]. Cinnarizine and flunarizine (only flunarizine was in the market in Korea during the study period), a selective T-type calcium channel blocker of which receptors are highly expressed in the basal ganglia, are used for vertigo and motion sickness. The risk can be twice as high for flunarizine compared with cinnarizine. The prevalence of parkinsonism with these drugs was found in 280 (3%) of 9,830 patients in a population-based study in Taiwan [35]. Whereas the most common classes of drugs reported in a pharmacovigilance study from France are antidepressants and antihistamines [32].
According to these findings, DIP incidence can be presumably a strong risk factor that alters the natural course of IPD development, resulting in features unlike those established in previous epidemiological analyses. However, we doubt that a causal relationship exists between DIP and IPD, as a few previous studies have proposed [14, 36]. If the toxic effects of the dopamine blocking-drugs caused irreversible damage to the neurons of vulnerable patients, then the potency and duration of the causative drugs taken by DIP subjects should have affected IPD incidence proportionately. However, we could not find support for this causal inference in our study. Instead, patients who had the lowest number of prescriptions of the causative drugs (duration: <30%) developed IPD the most often (17.89%). The strongest dopamine blockers, class I, were not associated with the highest number of IPD patients. From these observations, we conclude that DIP has been diagnosed in vulnerable patients who already had subclinical IPD pathology, and dopamine blockers acted as triggers unmasking IPD.
We suggest that our findings provide support for the theory that DIP diagnosis is a strong proxy indicator of risk for developing IPD. The causative drugs triggered IPD development from the existing subclinical Parkinsonism, as a few previous studies have suggested [7, 15].
Age-related decline or other environmental or traumatic insults have been resulted in damaging nigral dopaminergic neurons [37] and an increase in Lewy body fibrils (alpha-synuclein) [38]. These preconditions may remain in dormant or subclinical form and triggered by dopamine blockers [39]. Moreover, DIP diagnosis hastened the diagnosis (finding) of IPD by around 1-year, which has the strong clinical implication that clinicians should be alert to monitor the elderly who were diagnosed with DIP for potential IPD development around 1-year and be cautious in prescribing dopamine blockers to older adults.
We need to mention the advantage of selecting an active comparator to overcome healthy initiator bias commonly found in observational studies. This bias can arise through the selection of control groups with healthy and health-conscious patients, who through the effects of their healthy lifestyle, are also at a decreased risk of several adverse health outcomes [18, 19]. Therefore we selected DM patients as active comparators who might have a certain degree of increased risk of developing IPD [40].
We should address a few limitations of our study. Firstly, we only included 50-100- year-old population assuming the low IPD incidence in younger age might not affect the outcome. However, if we have included the younger population with DIP diagnosis, the patients might yield a moderate IPD outcome between two groups and give additional insights. Secondly, we classified antidopaminergic drugs based on their D2 receptor blocking potency. However, summing these agents into one single drug class might have some difficulties interpreting the outcomes considering their complex pharmacology. Thirdly, other risk factors of IPD such as dairy product consumption (urate-lowering effect) [41], smoking [42, 43]. and pesticide exposure [44] could not be assessed due to the data availability issue. We attempted to assess COPD (Chronic Obstructive Pulmonary Disease) as a proxy for smoking usually known for protective to IPD risk [45], but the number of subjects was too small to evaluate. Lastly, Parkinsonism may not have been recognized or diagnosed in our general population. For example, some mild symptoms related to DIP may not have been described in the medical records and had the diagnosis code because they were considered an inevitable consequence of the treatment with certain drugs (typical antipsychotics) resulting in an under-reporting of DIP [1].
Conclusion
DIP was closely associated with the risk of IPD incidence. The association might not be causal rather DIP unmasks subclinical preexisting condition of IPD. Therefore, DIP diagnosis in older adults can be utilized as a strong proxy for IPD incidence.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This study used NHIS-NHID data (NHIS-2017-1-242)) made by National Health Insurance Service (NHIS).
Data Availability
Customized health information data is a third-party data modified by the Korea National Health Insurance Service (KNHIS) as requested in the purpose of policy and academic research. Data is available upon request to the review committee at the National Health Insurance Sharing Service (https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do) and the committee decides whether to provide the data. This data cannot be shared publicly due to legal restrictions. Secondary analysis data are provided within this manuscript and accompanying Supporting Information.
Funding Statement
HM and SJ were supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C1240).The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. KHIDI: https://www.khidi.or.kr/board;jsessionid=SRxVfMwJvhwHwP7nrQTfGJY2dV3bBDLLh6l1Shjh7Vrsh4nN2svL!2129625964?menuId=MENU00770&siteId=SITE00012.
References
- 1.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Mielke MM, Rocca WA. Incidence and time trends of drug-induced parkinsonism: A 30-year population-based study. Mov Disord. 2017;32(2):227–34. 10.1002/mds.26839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Time Trends in the Incidence of Parkinson Disease. JAMA Neurology. 2016. 10.1001/jamaneurol.2016.0947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasten M, Bruggemann N, Konig I, Doerry K, Steinlechner S, Wenzel L, et al. Risk for antipsychotic-induced extrapyramidal symptoms: influence of family history and genetic susceptibility. Psychopharmacology (Berl) 2011;214(3):729–36. 10.1007/s00213-010-2079-1 [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Sendon J, Mena M, de Yebenes J. Drug-induced parkinsonism in the elderly: incidence, management and prevention. Drugs Aging. 2012;29:105–18. 10.2165/11598540-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 5.Bower J, Maraganore D, McDonnell S, Rocca W. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976–1990. Neurology. 1999;52:1214–20. 10.1212/wnl.52.6.1214 [DOI] [PubMed] [Google Scholar]
- 6.Klawans HJ, Bergen D, Bruyn G. Prolonged drug-induced Parkinsonism. Confin Neurol 1973;35:368–77. 10.1159/000102857 [DOI] [PubMed] [Google Scholar]
- 7.Brigo F, Erro R, Marangi A, Bhatia K, Tinazzi M. Differentiating drug-induced parkinsonism from Parkinson’s disease: an update on non-motor symptoms and investigations. Parkinsonism Relat Disord. 2014;20:808–14. 10.1016/j.parkreldis.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 8.Hardie R, Lees A. Neuroleptic-induced Parkinson’s syndrome: clinical features and results of treatment with levodopa. Journal of neurology, neurosurgery, and psychiatry. 1988;51(6):850–4. 10.1136/jnnp.51.6.850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephen P, Williamson J. Drug-induced parkinsonism in the elderly. Lancet. 1984;2:Lancet. 10.1016/s0140-6736(84)91516-2 [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Oh Y, Kim Y, Yang D, Chung Y, You I, et al. Combined use of (1)(2)(3)I-metaiodobenzylguanidine (MIBG) scintigraphy and dopamine transporter (DAT) positron emission tomography (PET) predicts prognosis in drug-induced Parkinsonism (DIP): a 2-year follow-up study. Archives of gerontology and geriatrics. 2013;56(1):124–8. 10.1016/j.archger.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 11.Burn D, Brooks D. Nigral dysfunction in drug-induced parkinsonism: an 18 F-dopa PET study. Neurology. 1993;43(3 Pt 1):552–6. [DOI] [PubMed] [Google Scholar]
- 12.Foubert-Samier A, Helmer C, Perez F, Le Goff M, Auriacombe S, Elbaz A, et al. Past exposure to neuroleptic drugs and risk of Parkinson disease in an elderly cohort. Neurology. 2012;79:1615–21. 10.1212/WNL.0b013e31826e25ce [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld M, Brenner-Lavie H, Ari S, Kavushansky A, Ben-Shachar D. Perturbation in mitochondrial network dynamics and in complex I dependent cellular respiration in schizophrenia. Biol Psychiatry. 2011;69:980–8. 10.1016/j.biopsych.2011.01.010 [DOI] [PubMed] [Google Scholar]
- 14.Wenning G, Litvan I, Tolosa E. Milestones in atypical and secondary Parkinsonisms. Mov Disord. 2011;26(6):1083–95. 10.1002/mds.23713 [DOI] [PubMed] [Google Scholar]
- 15.Brugger F, Bhatia K, Besag F. Valproate-Associated Parkinsonism: A Critical Review of the Literature. CNS Drugs. 2016;30(6):527–40. 10.1007/s40263-016-0341-8 [DOI] [PubMed] [Google Scholar]
- 16.Shuaib U, Rajput A, Robinson C, Rajput A. Neurolepticinduced Parkinsonism: Clinicopathological study. Mov Disord. 2016;31:360–5. 10.1002/mds.26467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221–8. Epub 2015/09/30. 10.1007/s40471-015-0053-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brookhart M, Stürmer T, Glynn R, Rassen J, Schneeweiss S. Confounding control in healthcare database research: challenges and potential approaches. Med Care. 2010;48(6 Suppl):S114–S20. 10.1097/MLR.0b013e3181dbebe3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stürmer T, Jonsson Funk M, Poole C, Brookhart M. Nonexperimental comparative effectiveness research using linked healthcare databases. Epidemiology. 2011;22(3):298–301. 10.1097/EDE.0b013e318212640c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sergi D, Renaud J, Simola N, Martinoli M-G. Diabetes, a Contemporary Risk for Parkinson’s Disease: Epidemiological and Cellular Evidences. Frontiers in Aging Neuroscience. 2019;11:302. 10.3389/fnagi.2019.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomperts SN. Lewy Body Dementias: Dementia With Lewy Bodies and Parkinson Disease Dementia. Continuum (Minneap Minn). 2016;22(2): 435–63. 10.1212/CON.0000000000000309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Ptacek S, Kramberger MG. Parkinson Disease and Dementia. J Geriatr Psychiatry Neurol. 2016;29(5):261–70. 10.1177/0891988716654985 [DOI] [PubMed] [Google Scholar]
- 23.Dallé E, Mabandla MV. Early Life Stress, Depression And Parkinson’s Disease: A New Approach. Mol Brain. 2018;11(1):18. 10.1186/s13041-018-0356-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mhyre TR, Boyd JT, Hamill RW, Maguire-Zeiss KA. Parkinson’s disease. Subcell Biochem. 2012;65:389–455. 10.1007/978-94-007-5416-4_16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu C, Hu G, Kivipelto M, Laatikainen T, Antikainen R, Fratiglioni L, et al. Association of Blood Pressure and Hypertension With the Risk of Parkinson Disease: The National FINRISK Study. Hypertension. 2011;57:1094–100. 10.1161/HYPERTENSIONAHA.111.171249 [DOI] [PubMed] [Google Scholar]
- 26.Reeve A, Simcox E, Turnbull D. Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res Rev. 2014;14(100):19–30. Epub 2014/02/03. 10.1016/j.arr.2014.01.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith K M., Dahodwala N. Sex differences in Parkinson’s disease and other movement disorders. Experimental Neurology. 2014;259:44–56. 10.1016/j.expneurol.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 28.Taylor K, Cook J, Counsell C. Heterogeneity in male to female risk for Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:905–6. 10.1136/jnnp.2006.104695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheong H-K, Lee CS, Kim Y, Sung J-H, Yi SE, Kim JW, et al. Nationwide Survey on the Prevalence of Parkinson’s Disease in Korea. Seoul, Korea: Sungkyunkwan university, 2007. [Google Scholar]
- 30.Shin H. Movement disorder related with drugs Seoul, Korea: MEDICAL Observer; 2012. [cited 2018 September 30]. Available from: http://www.monews.co.kr/news/articlePrint.html?idxno=53885. [Google Scholar]
- 31.Lee JE, Choi J-k, Lim HS, Kim JH, Cho JH, Kim GS, et al. The Prevalence and Incidence of Parkinson′s Disease in South Korea: A 10-Year Nationwide Population–Based Study. Journal Korean Neurol Assoc. 2017;35(4):191–8. [Google Scholar]
- 32.Bondon-Guitton E, Perez-Lloret S, Bagheri H, Brefel C, Rascol O, Montastruc J-L. Drug-Induced Parkinsonism: A Review of 17 Years’ Experience in a Regional Pharmacovigilance Center in France. Movement Disorders. 2011;26(12):2011. [DOI] [PubMed] [Google Scholar]
- 33.Surmeier D. Calcium, ageing, and neuronal vulnerability in Parkinson’s disease. Lancet Neurol 2017;6:933–8. [DOI] [PubMed] [Google Scholar]
- 34.Lo´pez-Sendo´n J, Mena M, de Ye´benes J. Drug-induced parkinsonism in the elderly: incidence, management and prevention. Drugs Aging. 2012;29:105–18. 10.2165/11598540-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 35.Lin H, Lin H, Tseng Y, Chen S, Hsu C. Risk of parkinsonism induced by flunarizine or cinnarizine: a population-based study. Eur J Clin Pharmacol 2017;73(3):365–71. 10.1007/s00228-016-2181-3 [DOI] [PubMed] [Google Scholar]
- 36.Mena M, Garcia de Yebenes M, Tabernero C, Casarejos M, Pardo B, Garcia de Yébenes J. Effects of calcium antagonists on the dopamine system. Clin Neuropharmacol 1995;18(5):410–26. 10.1097/00002826-199510000-00004 [DOI] [PubMed] [Google Scholar]
- 37.Thal D, Del Tredici K, Braak H. Neurodegeneration in normal brain aging and disease. Sci Aging Knowledge Environ. 2004;23:26. 10.1126/sageke.2004.23.pe26 [DOI] [PubMed] [Google Scholar]
- 38.Wakabayashi K, Tanji K, Mori F, Takahashi H. The Lewy body in Parkinson’s disease: molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathology. 2007;27(5):494–506. 10.1111/j.1440-1789.2007.00803.x [DOI] [PubMed] [Google Scholar]
- 39.Shuaib U, Rajput A, Robinson C, Rajput A. Neuroleptic induced Parkinsonism: Clinicopathological study. Mov Disord. 2016;31:360–5. 10.1002/mds.26467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cereda E, Barichella M, Pedrolli C, Klersy C, Cassani E, Caccialanza R, et al. Diabetes and Risk of Parkinson’s Disease. Diabetes Care. 2011;34(12):2614–23. 10.2337/dc11-1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi H, Atkinson K, Karlson E, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350:1093–103. 10.1056/NEJMoa035700 [DOI] [PubMed] [Google Scholar]
- 42.Thacker E, O’Reilly E, Weisskopf M, Chen H, Schwarzschild MA, McCullough ML, et al. Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology. 2007;68:764–8. 10.1212/01.wnl.0000256374.50227.4b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ascherio A, Schwarzschild M. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15(12):1257–72. 10.1016/S1474-4422(16)30230-7 [DOI] [PubMed] [Google Scholar]
- 44.Yan D, Zhang Y, Liu L, Shi N, Yan H. Pesticide exposure and risk of Parkinson’s disease: Dose-response meta-analysis of observational studies. Regul Toxicol Pharmacol. 2018;96:57–63. 10.1016/j.yrtph.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 45.Li C, Chen W, Liao W, Tu C, Lin C, Sung F, et al. The association between chronic obstructive pulmonary disease and Parkinson’s disease: a nationwide population-based retrospective cohort study. QJM. 2015;108(1):39–45. 10.1093/qjmed/hcu136 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Customized health information data is a third-party data modified by the Korea National Health Insurance Service (KNHIS) as requested in the purpose of policy and academic research. Data is available upon request to the review committee at the National Health Insurance Sharing Service (https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do) and the committee decides whether to provide the data. This data cannot be shared publicly due to legal restrictions. Secondary analysis data are provided within this manuscript and accompanying Supporting Information.