Abstract
This study was designed to evaluate whether severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) can directly target the central nervous system (CNS). We present four patients suffering from the loss of consciousness and seizure during the clinical course of COVID-19 infection. In addition to positive nasopharyngeal swab tests, SARS-CoV-2 has been detected in their cerebrospinal fluid. This report indicates the neuroinvasive potential of SARS-CoV-2, suggesting the ability of this virus to spread from the respiratory tract to the CNS.
Supplementary information
The online version contains supplementary material available at 10.1007/s13365-020-00938-w.
Keywords: Neuroinvasion, Convulsion, SARS-CoV-2, Brain, CNS
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) can affect various organs (Paramasivam et al. 2020 ; Sepehrinezhad et al. 2020; Tian and Ye 2020). Coronavirus disease 2019 (COVID-19) has been reported to be associated with a wide range of neurological symptoms (Ellul et al. 2020). However, whether the neurological manifestations reflect a direct effect of SARS-CoV-2 on the central nervous system (CNS) or indirect effects of para- or post-infectious immune-mediated response needs to be elucidated (Ellul et al. 2020; Wood 2020). The neuroinvasive propensity of coronaviruses has been described in humans in previous outbreaks (Hung et al. 2003; Li et al. 2020). The brain autopsy findings in a patient with SARS-CoV-2 have suggested that this virus may gain access to the CNS by infecting endothelial cells via transcytosis to neural tissue (Paniz‐Mondolfi et al. 2020). Furthermore, the post-mortem findings in patients who died of COVID-19 have revealed the presence of SARS-CoV-2 in the cortical neurons associated with minimal immune cell infiltrates in brain tissues (Song et al. 2020). Despite several reports of neurological manifestations and neuroinvasiveness of SARS-CoV-2 during infection (Guan et al. 2020; Li et al. 2020; Mao et al. 2020), additional evidence of neuroinvasiveness of SARS-CoV-2 is warranted. To evaluate the potential of SARS-CoV-2 to spread from the respiratory tract to the CNS, the present study was designed to assess SARS-CoV-2 in the cerebrospinal fluid (CSF) of patients with COVID-19 infection, who suffers from severe neurological manifestations. Here, we report the detection of SARS-CoV-2 in the CSF of four patients with loss of consciousness and seizure during the course of COVID-19 infection.
Case presentation
Patient 1
A 56-year-old female presented to the emergency department with a loss of consciousness. Seven days before, she developed myalgia, stomachache, and mild headache, which were superimposed with behavioral disturbances, hallucinations, and altered mental status later (Table 1). There was a history of upward gaze and left deviation of the face. Except for migraine headaches, her previous medical history was unremarkable. On the day of admission, she was unconscious with an eye-opening response only to painful stimulations. She was unable to move the left extremities with the impairment of the left plantar reflex. The pupils were midsized and reactive to light.
Table 1.
Clinical characteristics, serum biochemical parameters, hematological laboratory values, CSF cellular components, herpes PCR results, and culture of CSF in different patients
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Normal value |
|---|---|---|---|---|---|
| Demographic information and clinical symptoms | |||||
| Age (years) | 56 | 24 | 65 | 71 | - |
| Gender | Female | Male | Female | Male | - |
| Neurologic complaint | Altered mental status-seizure | Altered mental status-seizure | Loss of consciousness and seizure | Loss of consciousness and seizure | - |
| Primary presentation | Myalgia-mild headaches | Myalgia | Malaise-flu-like syndrome | Malaise, cough and fever | - |
| Time from onset of the disease to neurologic presentation | 7 days | 3 days | 3 days | 10 days | - |
| Fever on admission | Yes | No | Yes | No | - |
| Outcome | ICU admission | Good | Good | Good | - |
| Nasopharynx for COVID-19 PCR | Positive | Negative | Positive | Positive | - |
| CSF for COVID-19 PCR | Positive | Positive | Positive | Positive | - |
| Serum biochemical analysis | |||||
| Blood sugar (mg/dl) | 101 | 107 | 119 | 193 | < 200 |
| Urea (mg/dl) | 25 | 52 | 52 | 45 | 18–55 |
| Creatinine (mg/dl) | 0.7 | 1 | 1.3 | 1.1 | 0.7–1.4 |
| Sodium (mg/dl) | 132 | 142 | 140 | 137 | 135–145 |
| Potassium (mg/dl) | 3.3 | 4.4 | 4.1 | 5.2 | 3.5–5.3 |
| Calcium (mg/dl) | 9.0 | 9.2 | - | 9.8 | 8.6–10.3 |
| Magnesium (mg/dl) | 2.1 | 2.5 | - | 2.1 | 1.2–2.6 |
| Aspartate transaminase (U/L) | - | 31 | 44 | - | 5–40 |
| Alanine transaminase (U/L) | - | 43 | 24 | - | 5–40 |
| Hematological laboratory values | |||||
| White blood cell ( 103 mcl) | 11.9 | 9.5 | 9.5 | 10.3 | 4–10 |
| Hemoglobin (g/dl) | 13.3 | 13.6 | 13.7 | 13.7 | 13–17 |
| Hematocrit (%) | 40.5 | 41.3 | 43.1 | 42.2 | 40–50 |
| Red blood cell ( 106 mcl) | 4.77 | 4.02 | 5.09 | 5.04 | 4.5–5.5 |
| Polymorphonuclear leukocytes (%) | 88.4 | 67 | 70.8 | 80 | 30–70 |
| Lymphocyte (%) | 8 | 26.8 | 16.3 | 15 | 20–50 |
| ESR mm | 34 | 27 | 44 | 34 | < 15 |
| Platelet ( 103 mcl) | 213 | 263 | 230 | 282 | 150–450 |
| C-reactive protein (mg/dl) | 61.3 | 19.7 | 19.1 | 20.1 | < 6 |
| CSF parameters analysis | |||||
| Sugar (mg/dl) | 63 | 64 | 66 | 133 | > 2/3 concurrent BS |
| Protein (mg/l) | 130 | 85 | 124 | 64 | 15–45 |
| White blood cell (/mm3) | 70 | 10 | 90 | 0 | 0–1 |
| Polymorphonuclear leukocytes (%) | 5 | 10 | 10 | 0 | 0 |
| Mononuclear (%) | 95 | 90 | 90 | 0 | - |
| LDH (U/l) | 197 | 42 | 57 | 82 | < 100 |
| Red blood cell (/mm3) | 70 | 20 | 20 | 0 | 0–100 |
| Herpes-PCR | Positive | Negative | Negative | Negative | (Negative) |
| Bacterial culture | Negative | Negative | Negative | Negative | (Negative) |
BS blood sugar, ESR erythrocyte sedimentation rate, g/dl gram/deciliter, mcl microliter, mg/dl milligram/deciliter, mm3 millimeter cubed, U/l Unites/liter
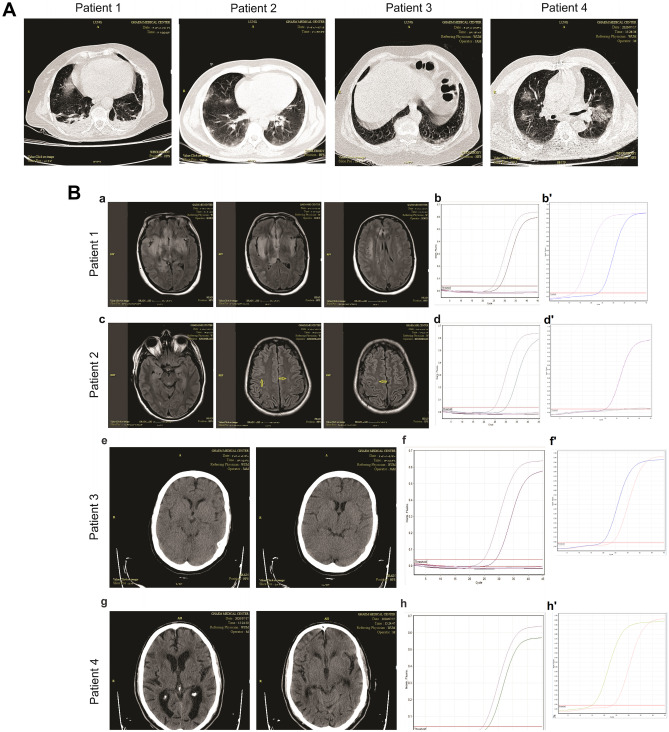
Due to the progressive loss of consciousness, increased level of PCO2, and pulmonary secretions, the patient underwent mechanical ventilation after 24 h. Biochemical and hematological parameters were normal, except for a decreased lymphocyte count (Table 1). A lung high-resolution computed tomography (HRCT) has shown peripheral ground-glass consolidations in the apical zone of the right lung. Besides, a mild pleural effusion of both lungs accompanied by subsegmental collapse consolidations in the basal segments was noted (Fig. 1A).
Fig. 1.
Lung CT scan, Neuroimaging, and RT-PCR findings in different patients. A Ground-glass consolidation is seen in the lung HRCT of all patients. B Brain MRI, brain CT scan, and RT-PCR of patients. B-a Bilateral asymmetrical areas of the hyper-signal intensity of the temporal lobes, the inferior area of the frontal lobes, the insula, and the right parietal lobe in T2 and FLAIR images, with patchy restricted areas, were seen in patient 1. B-b RT-PCR amplification plot on the CSF sample of the patient 1 (reactions include sample, negative control, positive control, and negative control of extraction). B-b' RT-PCR amplification plot on the nasopharyngeal (NP) swab specimen of patient 1. B-c Small size hyper-intense foci in the subcortical white matter of the parietal and temporal lobes in T2 and FLAIR images of brain MRI (yellow arrow). B-d RT-PCR amplification plot on the CSF sample of patient 2 (reactions include sample, negative control, positive control, and negative control of extraction). B-d' RT-PCR amplification plot on the NP swab samples of the patient 2. B-e Brain CT scan of patient 3 did not show remarkable changes. B-f RT-PCR amplification plot of the CSF sample of patient 3 (reactions include sample, negative control, positive control, and negative control of extraction). B-f' RT-PCR amplification plot on the NP swab specimens of the patient 3. B-g A brain CT scan revealed senile atrophies and chronic small vessel changes.B-h RT-PCR amplification plot of the CSF sample of patient 4 (reactions include sample, negative control, positive control, and negative control of extraction). B-h' RT-PCR amplification plot on the NP swab sample of the patient 4
MRI revealed bilateral asymmetrical areas of hyper signal intensity of the temporal lobes, inferior area of the frontal lobes, insula, and right parietal lobe (Fig. 1B: a). There was no absorption in post-contrast imaging.
The specimen of the nasopharynx and the CSF sample were both positive for SARS-CoV-2 (Fig. 1B: b-b'). In parallel, the expression of HSV was detected in the CSF. The value of protein and cellular counts were abnormal in the CSF analysis (Table 1).
Patient 2
The second patient was a 24-year-old man, who developed progressive dizziness and incoherent responses after 3 days of generalized weakness and myalgia. A few hours later, he had an attack of a generalized tonic-clonic seizure lasting for a few minutes (Table 1). No previous history of seizures or other medical conditions was reported.
The lung HRCT revealed multifocal and multi-lobar patchy ground-glass consolidations with peripheral distribution (Fig. 1A). The brain MRI showed small -size hyperintense foci in the subcortical regions of the parietal and temporal lobes in T2 and FLAIR images (Fig. 1B: c).
In this case, the specimen of the nasopharynx for COVID-19 was negative (Fig. 1B: d'). The PCR test for HSV in the CSF was also negative. However, the RNA of SARS-CoV-2 was detected in CSF (Fig. 1B: d). Analysis of the CSF showed abnormal protein levels and cellular components (Table 1).
Patient 3
A 65-year-old woman, after a few days of malaise and flu-like symptoms, was admitted with loss of consciousness followed by a seizure attack (lower limb shaking and urinary incontinence; Table 1). Lung HRCT revealed a few patchy areas of peripheral ground-glass consolidations and hilar lymphadenopathy (Fig. 1A). A CT scan of the brain was normal (Fig. 1B: e).
The nasopharyngeal swab and CSF samples were positive for COVID-19 (Fig. 1B: f-f'). The RT-PCR test for HSV in the CSF was negative. Elevated levels of protein and white blood cell count (with mononuclear predominance) in the CSF were reported (Table 1).
Patient 4
A 71-year-old man was hospitalized with loss of consciousness and seizure (Table 1). One week before, he was diagnosed with COVID-19 infection after 3 days of malaise, cough, and fever and received azithromycin (250 mg/day). No other symptoms were reported. On the day of admission, he was lethargic and afebrile. Lung HRCT revealed peripheral multifocal and multi-lobar patchy ground-glass consolidations in both lungs (Fig. 1A). A CT scan of the brain showed only senile atrophies and small vessel disease (Fig. 1B: g).
The nasopharyngeal swab and CSF samples were positive for COVID-19 (Fig. 1B: h-h'). The RT-PCR test for HSV in the CSF was negative. Increased levels of protein and white blood cell count were observed in the CSF (Table 1).
Discussion
This report described four COVID-19-positive patients with seizures and loss of consciousness, who were positive for SARS-CoV-2 in their CSF. Our findings suggest the ability of SARS-CoV-2 to spread from the respiratory tract to the CNS. These patients did not have any significant risk factors for neurological diseases and none of them had a history of neurological disorders.
During the COVID-19 pandemic, several studies have reported neurological manifestations in patients with COVID-19 (Farhadian et al. 2020; Mao et al. 2020; Moriguchi et al. 2020). Recently, several research groups have focused on the explanation of neurological manifestations of COVID-19 via its neuroinvasive potential (Farhadian et al. 2020; Li et al. 2020; Moriguchi et al. 2020; Sepehrinezhad et al. 2020). An increasing amount of evidence suggests the neuro-invading potential of SARS-CoV-2, which may lead to clinical symptoms and brain damage (Najjar et al. 2020; Wood 2020; Zangbar et al., 2021). The hypothesis of the SARS-CoV-2 invasion into the CNS has been supported by studies on the coronaviruses that caused previous outbreaks (Sepehrinezhad et al. 2020). The neuroinvasion of SARS-CoV-2 has been detected in human brain autopsy specimens (Paniz‐Mondolfi et al. 2020; Song et al. 2020). The present study confirmed the neuroinvasive propensity of SARS-CoV-2 in patients who suffer from severe neurological symptoms.
Taken together, we demonstrated the ability of SARS-CoV-2 to attack the CNS, which was associated with the loss of consciousness and seizure. Although this study demonstrated the neuro-invading properties of SARS-CoV-2, further research will be required to determine the mechanisms underlying SARS-CoV-2-mediated neurological manifestation and brain injury.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank the entire Emam Reza University Hospital staff for their sincere support.
Authors’ contributions
F.R. contributed to the interpretation of the neurological results, worked on the manuscript, and performed CSF collection. S.A.J and S.H. performed RT-PCR tests and contributed substantially to the conception of the analysis and interpretation of PCR results. F.R, A.R, F.M, F.S.E.F, and M.E.R. processed the clinical data, contributed to the case presentation. A.S. conceived the study, designed the tables, and contributed to the writing of the manuscript. A.G. critically revised the manuscript and provided the final outlines of the version to publish. S.S.N. conceived the study, wrote the manuscript, and supervised the work. All authors provided critical feedback and helped shape the research and manuscript.
Funding
This study was supported by the Student Research Committee, Mashhad University of Medical Sciences, Mashhad, Iran.
Data availability
The data collection method and RT-PCR protocol are available in the supplementary materials. Additional data and materials can be available from the corresponding authors upon request.
Compliance with ethical standards
Ethics approval and consent to participate
The ethics committee of Mashhad University of Medical Sciences approved the study. Written informed consent has been obtained from the patients in accordance with the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ali Gorji, Email: gorjial@uni-muenster.de.
Sajad Sahab Negah, Email: sahabnegahs@mums.ac.ir.
References
- Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Solomon T. Neurological associations of COVID-19. The Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadian S, Glick LR, Vogels CBF, Thomas J, Chiarella J, Casanovas-Massana A, Barakat LA. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. 2020;20(1):248. doi: 10.1186/s12883-020-01812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W-J, Ni Z-y, Hu Yu, Liang W-H, Ou C-Q, He J-X, Zhong N-S. Clinical characteristics of coronavirus disease 2019 in China. New England J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung EC, Chim SS, Chan PK, Tong YK, Ng EK, Chiu RW, Lo YM. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem. 2003;49(12):2108–2109. doi: 10.1373/clinchem.2003.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infectious Diseases. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar S, Najjar A, Chong DJ, Pramanik BK, Kirsch C, Kuzniecky RI, Azhar S. Central nervous system complications associated with SARS-CoV-2 infection: integrative concepts of pathophysiology and case reports. J Neuroinfl. 2020;17(1):231. doi: 10.1186/s12974-020-01896-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, Fowkes M (2020) Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J med virol 92(7):699–702 [DOI] [PMC free article] [PubMed]
- Paramasivam A, Priyadharsini JV, Raghunandhakumar S, Elumalai P. A novel COVID-19 and its effects on cardiovascular disease. Hypertens Res. 2020;43(7):729–730. doi: 10.1038/s41440-020-0461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehrinezhad A, Shahbazi A, NegahSajad. S. COVID-19 virus may have neuroinvasive potential and cause neurological complications: a perspective review. J NeuroVirol. 2020;26(3):324–329. doi: 10.1007/s13365-020-00851-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E, Zhang C, Israelow B, Lu-Culligan A, Sprado A, Skriabine A, Dai Y (2020) Neuroinvasion of SARS-CoV-2 in human and mouse brain. bioRxiv. [DOI] [PMC free article] [PubMed]
- Tian D, Ye Q (2020) Hepatic complications of COVID-19 and its treatment. J Med Virol n/a(n/a). 10.1002/jmv.26036 [DOI] [PMC free article] [PubMed]
- Wood H. New insights into the neurological effects of COVID-19. Nature Reviews Neurology. 2020;16(8):403–403. doi: 10.1038/s41582-020-0386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangbar HS, Gorji Al, Ghadiri T. A Review on the Neurological Manifestations of COVID-19 Infection: a Mechanistic View. Molecular Neurobiology. 2021;58:536–549. doi: 10.1007/s12035-020-02149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data collection method and RT-PCR protocol are available in the supplementary materials. Additional data and materials can be available from the corresponding authors upon request.