Abstract
Background and Aims: The impact of proteinuria and its severity on the incidence of inflammatory bowel disease (IBD) has not yet been studied. We aimed to determine the association between proteinuria measured by urine dipstick tests and the development of IBD. Methods: This nationwide population-based study was conducted using the Korean National Health Insurance Service (NHIS) database. A total of 9,917,400 people aged 20 years or older who had undergone a national health examination conducted by the NHIS in 2009 were followed up until 2017. The study population was classified into four groups—negative, trace, 1+, and ≥ 2+—according to the degree of proteinuria measured by the urine dipstick test. The primary endpoint was newly diagnosed IBD, Crohn’s disease (CD), or ulcerative colitis (UC) during the follow-up period. Results: Compared with the dipstick-negative group, the incidence of CD significantly increased according to the degree of proteinuria (adjusted hazard ratio [aHR] with 95% confidence interval [CI], 1.01 [0.703–1.451], 1.515 [1.058–2.162], and 2.053 [1.301–3.24] in the trace, 1+, and ≥ 2+ dipstick groups, respectively; p for trend 0.007). However, there was no significant difference in the incidence of UC according to the degree of proteinuria (aHR with 95% CI, 1.12 [0.949–1.323], 0.947 [0.764–1.174], and 1.009 [0.741–1.373] in the trace, 1+, and ≥ 2+ dipstick groups, respectively; p for trend 0.722). In the subgroup analysis, dipstick-positive proteinuria independently increased the incidence of CD regardless of the subgroup. However, dipstick-positive proteinuria was associated with the risk of UC in those with diabetes mellitus and not in those without diabetes mellitus (aHR, 1.527 vs. 0.846; interaction p-value 0.004). The risk of CD was increased or decreased according to proteinuria changes but not associated with the risk of UC. Conclusion: Proteinuria, measured by the dipstick test, is strongly associated with the development of CD.
Keywords: claims data, incidence, proteinuria, Crohn’s disease
1. Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic, immune-related, progressive disease characterized by repeated improvements and deteriorations. Complex interactions between genetic elements, environmental factors, and the gut microbiome lead to the pathogenesis of IBD [1]. The prevalence and incidence of IBD are high in North America and Europe and relatively low in Asia [2] but, due to rapid socioeconomic changes, its incidence has more than doubled in recent decades in East Asia, including Japan, Korea, and Hong Kong [3]. Accordingly, IBD is becoming a global disease and its direct and indirect costs are also increasing enormously, which will become a major health problem in future societies [4]. Therefore, it is necessary to identify and screen for risk factors that can be modified before the onset of IBD.
Proteinuria is a marker of kidney damage that is associated with a rapid deterioration in renal function as the proteinuria severity increases [5]. Proteinuria also predicts patients at increased risk of adverse clinical outcomes regardless of the estimated glomerular filtration rate (eGFR) [6]. In addition, proteinuria has been regarded as a manifestation of endothelial dysfunction and systemic inflammation [7] and has recently been linked to the risk of chronic systemic inflammatory diseases [8,9,10,11,12,13]. However, there is no evidence on the impact of proteinuria on the incidence of IBD. Therefore, using a nationwide database, we examined the association of proteinuria with the risk of IBD.
2. Material and Methods
2.1. Data Source and Study Population
This nationwide cohort study was performed using data from the National Health Insurance Service (NHIS), which is mandatory for all healthcare service providers and citizens in South Korea. The NHIS is a single payment program supported by the Korean government and, because the NHIS database covers the entire Korean population, it is suitable for population-based nationwide studies [14]. The NHIS database includes demographic information, disease codes, procedural codes, inpatient and outpatient care details, and information on prescribed drugs. These data are encrypted to prevent the recognition of personal information. The NHIS provides standardized health checkups to detect diseases early and provide subsequent medical benefits to the insured and recommends that patients undergo a health checkup at least once every 2 years. The health checkup includes height, weight, body mass index (BMI), and blood pressure measurements and basic laboratory tests, including urinalysis, fasting blood glucose, total cholesterol, creatinine, and eGFR. Past medical history, social history including alcohol consumption and smoking status, and physical activity are collected from self-reported questionnaires. In the above database, we evaluated data from Koreans older than 19 years old (range 20–110 years) who received at least one health checkup provided by the NHIS from January 2009 to December 2009. Those diagnosed with IBD from January 2004 to the time of the health checkup (index date) and those diagnosed with IBD within 1 year after the index date (lag period) were excluded. Individuals with missing data for any variable were also excluded. The remaining individuals were then tracked from the index date until December 2017. This study was approved by Seoul National University College of Medicine-Seoul National University Hospital Institutional Review Board (SNUCM-SNUH IRB) on 24 March 2017 (H-1703-107-840).
2.2. Dipstick Urinalysis and Study Endpoints
Proteinuria was measured by the urine dipstick test. Although the urine protein-to-creatinine ratio (PCR) and urine albumin-to-creatinine ratio (ACR) are used as quantitative tests for proteinuria, they are expensive to use for public health screening and require a long time for result confirmation. The urine dipstick test is a relatively simple, fast, and inexpensive method for screening proteinuria in public healthcare systems. In the urine dipstick test, the degree of proteinuria is measured as negative, trace, 1+, 2+, 3+, or 4+ according to the proteinuria concentration-dependent color difference. The study population was classified into four groups according to the degree of proteinuria: negative, trace, 1+, and ≥ 2+.
The primary outcome of this study was newly developed IBD. In 2006, the Korean government established the Rare and Intractable Disease (RID) registration system to improve medical security by exempting patients from the burden of medical treatment for severe RIDs that require expensive and high-level procedures. Patients enrolled in the RID registration system are only charged 10% of the medical fees associated with the disease. CD and UC are classified as RIDs in Korea. Patients enrolled in the RID registration system are assigned a special code, V. The RID code is highly reliable because the strict diagnostic standards based on the various examination items and criteria provided by the NHIS must be verified by a qualified Korean physician [15]. In this study, we identified incident IBD using both International Classification of Disease, Tenth Revision (ICD-10) codes (CD, K50; UC, K51) and V codes (CD, V130; UC, V131), as in previous studies [16,17,18,19]. To confirm the accuracy of the incident IBD diagnosed by both V and ICD-10 codes, the medical records of IBD patients identified with both V and ICD-10 codes from January 2010 to March 2013 were retrospectively reviewed at Seoul National University Hospital, a tertiary referral hospital in Korea, and sensitivity analysis was performed. The diagnostic sensitivity of CD and UC was 94.5% and 96.4%, respectively.
2.3. Covariates
Information on demographics (age, sex, and residential area) and health checkup items (height, weight, BMI, blood pressure measurements, drinking behavior, smoking history, and exercise and baseline laboratory findings, including fasting blood glucose and total cholesterol) in the study population was evaluated as baseline characteristics. Individuals were classified as nonsmokers, former smokers, and current smokers according to smoking status. Subjects who consumed at least 30 g of alcohol per day were defined as heavy drinkers. The regular exercise group performed high-intensity exercise for at least 20 min or moderate-intensity exercise for at least 30 min at a time at least once a week. Baseline comorbidities, including diabetes mellitus (DM), hypertension, and hyperlipidemia, were also defined as in previous studies [16,17,18,19,20]. DM was defined as fasting blood glucose ≥ 126 mg/dL or as ICD-10 code E11-14 with a prescription for anti-diabetic medications. Hypertension was defined as a systolic/diastolic blood pressure ≥ 140/90 mmHg or as ICD-10 code I10-13 or I15 with a prescription of anti-hypertensive agents. Dyslipidemia was defined as fasting serum total cholesterol ≥ 240 mg/dL or as ICD-10 code E78 and a prescription for lipid-lowering drugs. eGFR was estimated using the Modification of Diet in Renal Disease study equation [21]. Dipstick-positive proteinuria was defined as a score of 1+ or more in the urine dipstick test.
2.4. Statistical Analysis
Data are presented as mean with standard deviation for continuous variables and as number with percentage for categorical variables. The incidence rate of IBD according to the degree of proteinuria was calculated as the total number of incident IBD cases divided by the total person-years and expressed as number per 100,000 person-years. The cumulative incidences of IBD according to the degree of proteinuria were compared using the Kaplan–Meier method and the log-rank test. To examine the association between proteinuria and the risk of IBD, we used the Cox proportional hazards model and presented the results as hazard ratios (HRs) with 95% confidence intervals (CIs). p < 0.05 was considered statistically significant. SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 3.2.3 (The R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analysis.
3. Results
3.1. Baseline Characteristics of the Study Population
A total of 9,917,400 individuals were enrolled in this study. They were divided into four groups according to the degree of proteinuria: negative (n = 9,444,344, 95.2%), trace (n = 224,793, 2.3%), 1+ (167,324, 1.7%), and ≥ 2+ (80,939, 0.8%). The baseline characteristics of the study population by proteinuria are shown in Table 1. Severity of proteinuria was positively correlated with age, smoking history, and heavy drinking, as well as with BMI, weight, and creatinine level (all p < 0.001). In addition, this group had higher blood pressure, higher fasting blood glucose and total cholesterol levels, and more co-morbidities including hypertension, DM, and dyslipidemia (all p < 0.001). However, the rates of current smoking and regular exercise were significantly lower, in addition to height and eGFR levels, in people with higher proteinuria levels (all p < 0.001).
Table 1.
Baseline characteristics of the study population.
| Characteristics | Proteinuria Measured by Dipstick | p-Value | |||
|---|---|---|---|---|---|
| Negative | Trace (±) | 1+ | ≥ 2+ | ||
| No. (%) | 9,444,344 (95.2) | 224,793 (2.3) | 167,324 (1.7) | 80,939 (0.8) | |
| Age, years † | 46.9 ± 14.0 | 48.9 ± 14.2 | 51.1 ± 14.5 | 53.2 ± 14.3 | <0.001 |
| 20–64 | 8,243,115 (87.3) | 189,309 (84.2) | 133,790 (80.0) | 61,528 (76.0) | <0.001 |
| 65–110 | 1,201,229 (12.7) | 35,484 (15.8) | 33,534 (20.0) | 19,411 (24.0) | |
| Male | 5,177,903 (54.8) | 121,349 (54.0) | 90,214 (53.9) | 45,386 (56.1) | <0.001 |
| Urban residence | 4,340,165 (46.0) | 109,178 (48.6) | 80,618 (48.2) | 37,299 (46.1) | <0.001 |
| Smoking status | <0.001 | ||||
| Nonsmokers | 5,614,771 (59.5) | 134,436 (59.8) | 100,081 (59.8) | 47,878 (59.2) | |
| Former smokers | 1,351,140 (14.3) | 33,116 (14.7) | 25,809 (15.4) | 13,498 (16.7) | |
| Current smokers | 2,478,433 (26.2) | 57,241 (25.5) | 41,434 (24.8) | 19,563 (24.2) | |
| Heavy drinker ‡ | 643,540 (6.8) | 16,784 (7.5) | 13,153 (7.9) | 6518 (8.1) | <0.001 |
| Regular exercise § | 4,870,116 (51.6) | 114,758 (51.1) | 81,648 (48.8) | 37,829 (46.7) | <0.001 |
| BMI, kg/m2 † | 23.7 ± 3.2 | 24.0 ± 3.4 | 24.3 ± 3.5 | 24.6 ± 3.7 | <0.001 |
| Height, cm † | 163.9 ± 9.2 | 163.6 ± 9.1 | 163.0 ± 9.1 | 162.6 ± 9.1 | <0.001 |
| Weight, kg † | 63.9 ± 11.6 | 64.4 ± 11.9 | 64.7 ± 12.1 | 65.3 ± 12.5 | <0.001 |
| Systolic blood pressure, mmHg † | 122.2 ± 14.8 | 124.1 ± 16.1 | 126.5 ± 17.0 | 129.8 ± 18.1 | <0.001 |
| Diastolic blood pressure, mmHg† | 76.2 ± 9.9 | 77.2 ± 10.6 | 78.43 ± 11.0 | 79.9 ± 11.4 | <0.001 |
| Fasting glucose, mg/dL † | 96.70 ± 21.9 | 101.7 ± 29.5 | 107.7 ± 37.0 | 115.2 ± 44.8 | <0.001 |
| Total cholesterol, mg/dL † | 194.8 ± 36.4 | 197.6 ± 38.3 | 199.6 ± 40.2 | 202.4 ± 43.4 | <0.001 |
| Creatinine, mg/dL † | 1.0 ± 0.9 | 1.1 ± 1.1 | 1.1 ± 1.0 | 1.2 ± 1.0 | <0.001 |
| eGFR, mL/min/1.73 m2 † | 88.6 ± 44.5 | 85.0 ± 31.5 | 82.8 ± 33.9 | 77.4 ± 35.4 | <0.001 |
| Comorbidity | |||||
| Diabetes mellitus | 2,353,771 (24.9) | 75,690 (33.7) | 72,603 (43.4) | 45,808 (56.6) | <0.001 |
| Hypertension | 767,049 (8.1) | 32,227 (14.3) | 36,117 (21.6) | 25,807 (31.9) | <0.001 |
| Dyslipidemia | 1,683,466 (17.8) | 51,939 (23.1) | 47,233 (28.2) | 29,879 (36.9) | <0.001 |
BMI, body mass index; eGFR, estimated glomerular filtration rate; No., number. † Mean ± standard deviation. ‡ Defined as a person who consumed at least 30 g of alcohol per day. § Defined as high-intensity exercise for at least 20 min or moderate-intensity exercise for at least 30 min at a time at least once a week.
3.2. Incidence and Risk of IBD According to Proteinuria Degree on Dipstick
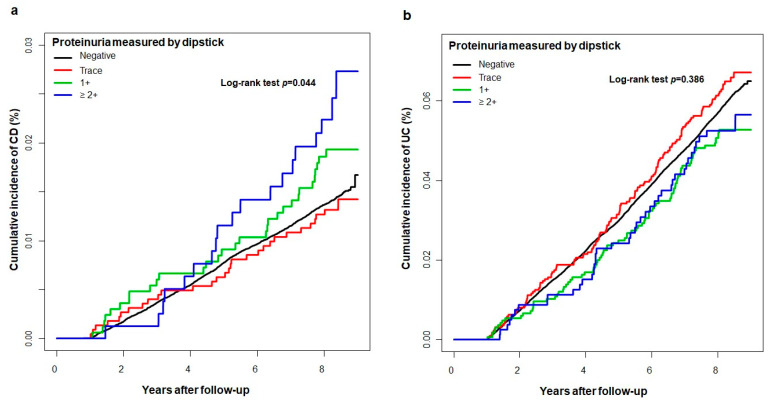
During a median follow-up of 9.3 years (interquartile range 9.1–9.6), the incidence rates of CD were 1.7, 1.6, 2.3, and 2.9 per 100,000 person-years in the negative, trace, 1+, and ≥ 2+ proteinuria groups, respectively. A higher degree of proteinuria on the dipstick test was associated with an increased risk of CD development (crude HR, 1.72; 95% CI, 1.094–2.706 in the ≥ 2+ proteinuria group, p = 0.034; Table 2 and Figure 1a). After adjustment for age, sex, income level, smoking status, alcohol consumption status, exercise level, body mass index, eGFR, and comorbidities (DM, hypertension, and dyslipidemia), the risk of CD development was also significantly higher as the degree of proteinuria increased compared with the negative proteinuria group (adjusted HRs [aHRs], 1.01, 1.515, and 2.053 in the trace, 1+, and ≥ 2+ proteinuria groups, respectively, p for trend 0.007; Model 3 in Table 2). The incidence rates of UC were 7.2, 7.7, 6.2, and 6.3 per 100,000 person-years in the negative, trace, 1+, and ≥ 2+ proteinuria groups, respectively. However, the incidence and risk of UC development were not significantly different according to the degree of proteinuria (Table 2 and Figure 1b).
Table 2.
Incidence and risk of Crohn’s disease and ulcerative colitis according to the degree of proteinuria measured by dipstick.
| IBD | Proteinuria Measured by Dipstick | p for Trend | |||
|---|---|---|---|---|---|
| Negative | Trace(±) | 1+ | ≥ 2+ | ||
| Crohn’s disease | |||||
| CD cases (n) | 1343 | 30 | 31 | 19 | |
| Person-years | 78,055,660 | 1,846,270 | 1,360,989 | 645,798 | |
| CD incidence (per 100,000 person-years) | 1.7 | 1.6 | 2.3 | 2.9 | |
| Crude HR (95% CI) | 1 (reference) | 0.946 (0.659–1.358) | 1.328 (0.93–1.895) | 1.72 (1.094–2.706) | 0.095 |
| p-value | 0.593 | 0.288 | 0.034 | ||
| Model 1 † HR (95% CI) | 1 (reference) | 0.99 (0.689–1.421) | 1.454 (1.018–2.077) | 1.949 (1.238–3.066) | 0.016 |
| p-value | 0.778 | 0.049 | 0.021 | ||
| Model 2 ‡ HR (95% CI) | 1 (reference) | 1.01 (0.704–1.451) | 1.515 (1.061–2.164) | 2.056 (1.306–3.235) | 0.006 |
| p-value | 0.879 | 0.039 | 0.011 | ||
| Model 3 § HR (95% CI) | 1 (reference) | 1.01 (0.703–1.451) | 1.515 (1.058–2.162) | 2.053 (1.301–3.24) | 0.007 |
| p-value | 0.871 | 0.035 | 0.013 | ||
| Ulcerative colitis | |||||
| UC cases (n) | 5634 | 143 | 85 | 41 | |
| Person-years | 78,040,796 | 1,845,890 | 1,360,805 | 645,715 | |
| UC incidence (per 100,000 person-years) | 7.2 | 7.7 | 6.2 | 6.3 | |
| Crude HR (95% CI) | 1 (reference) | 1.074 (0.91–1.268) | 0.868 (0.701–1.075) | 0.886 (0.652–1.204) | 0.16 |
| p-value | 0.367 | 0.115 | 0.277 | ||
| Model 1 † HR (95% CI) | 1 (reference) | 1.085 (0.919–1.281) | 0.882 (0.712–1.093) | 0.897 (0.66–1.22) | 0.172 |
| p-value | 0.339 | 0.128 | 0.268 | ||
| Model 2 ‡ HR (95% CI) | 1 (reference) | 1.1 (0.932–1.299) | 0.911 (0.735–1.128) | 0.938 (0.689–1.275) | 0.363 |
| p-value | 0.256 | 0.223 | 0.412 | ||
| Model 3 § HR (95% CI) | 1 (reference) | 1.12 (0.949–1.323) | 0.947 (0.764–1.174) | 1.009 (0.741–1.373) | 0.722 |
| p-value | 0.185 | 0.370 | 0.682 | ||
CD, Crohn’s disease; CI, confidence interval; HR, hazard ratio; UC, ulcerative colitis. † Model 1: age and sex adjusted. ‡ Model 2: model 1 + income level, smoking status, alcohol consumption status, exercise level, body mass index, and estimated glomerular filtration rate adjusted. § Model 3: model 2 + diabetes mellitus, hypertension, and dyslipidemia adjusted.
Figure 1.
Kaplan-Meier curve for the cumulative incidence of Crohn’s disease (a) and (b) ulcerative colitis according to the degree of proteinuria measured by dipstick. CD, Crohn’s disease; UC, ulcerative colitis.
3.3. Subgroup Analysis of the IBD Risk in Dipstick-Positive Proteinuria
In the subgroup analysis, we identified the effects of dipstick-positive proteinuria (≥ 1+ proteinuria) on the development of CD and UC according to subgroups classified by age, sex, eGFR, regular exercise, and presence of obesity, DM, and hypertension (Table 3).
Table 3.
Subgroup analysis of the risk of Crohn’s disease and ulcerative colitis among people with dipstick-positive proteinuria.
| Subgroup | Crohn’s Disease | Ulcerative Colitis | ||
|---|---|---|---|---|
| Adjusted HR † (95% CI) | p for Interaction | Adjusted HR † (95% CI) | p for Interaction | |
| Age, years | ||||
| 20–64 | 1.732 (1.27–2.361) | 0.828 | 0.903 (0.741–1.101) | 0.068 |
| 65–110 | 1.459 (0.708–3.003) | 1.33 (0.886–1.996) | ||
| Sex | ||||
| Male | 1.515 (1.032–2.224) | 0.099 | 0.984 (0.794–1.221) | 0.489 |
| Female | 2.099 (1.38–3.194) | 0.899 (0.657–1.231) | ||
| eGFR, mL/min/1.73 m2 | ||||
| ≥ 90 | 1.703 (1.063–2.728) | 0.340 | 0.945 (0.693–1.287) | 0.552 |
| 60–90 | 1.46 (0.952–2.238) | 0.923 (0.721–1.181) | ||
| < 60 | 2.217 (1.109–4.431) | 1.303 (0.818–2.078) | ||
| Regular exercise ‡ | ||||
| No | 1.726 (1.162–2.564) | 0.938 | 1.102 (0.869–1.397) | 0.143 |
| Yes | 1.633 (1.083–2.462) | 0.831 (0.635–1.086) | ||
| Obese § | ||||
| No | 1.765 (1.255–2.452) | 0.711 | 0.987 (0.794–1.228) | 0.912 |
| Yes | 1.482 (0.863–2.546) | 0.922 (0.68–1.251) | ||
| Diabetes mellitus | ||||
| No | 1.532 (1.099–2.135) | 0.171 | 0.846 (0.687–1.041) | 0.004 |
| Yes | 2.28 (1.281–4.057) | 1.527(1.073–2.174) | ||
| Hypertension | ||||
| No | 1.617 (1.111–2.355) | 0.572 | 0.936(0.738–1.188) | 0.720 |
| Yes | 1.751 (1.127–2.719) | 0.987(0.755–1.288) | ||
CI, confidence interval; HR, hazard ratio; eGFR, estimated glomerular filtration rate. † Age, sex, income level, smoking status, alcohol consumption status, exercise level, body mass index, estimated glomerular filtration rate, diabetes mellitus, hypertension, and dyslipidemia adjusted. ‡ Defined as high-intensity exercise for at least 20 min or moderate-intensity exercise for at least 30 min at a time at least once a week. § Defined as a body mass index ≥ 25 kg/m2.
The effect of dipstick-positive proteinuria on the development of CD was not significantly different among all subgroups. Dipstick-positive proteinuria increased the risk of CD regardless of age, sex, eGFR, regular exercise, obesity, DM, or hypertension. However, dipstick-positive proteinuria was associated with the increased risk of UC in individuals with DM, but not in those without DM (aHR, 1.527 vs. 0.846; interaction p-value 0.004). The effect of dipstick-positive proteinuria on the risk of UC was not significantly different in the other subgroups except the DM subgroup.
3.4. Change in Proteinuria on the Dipstick Test and Risk of IBD
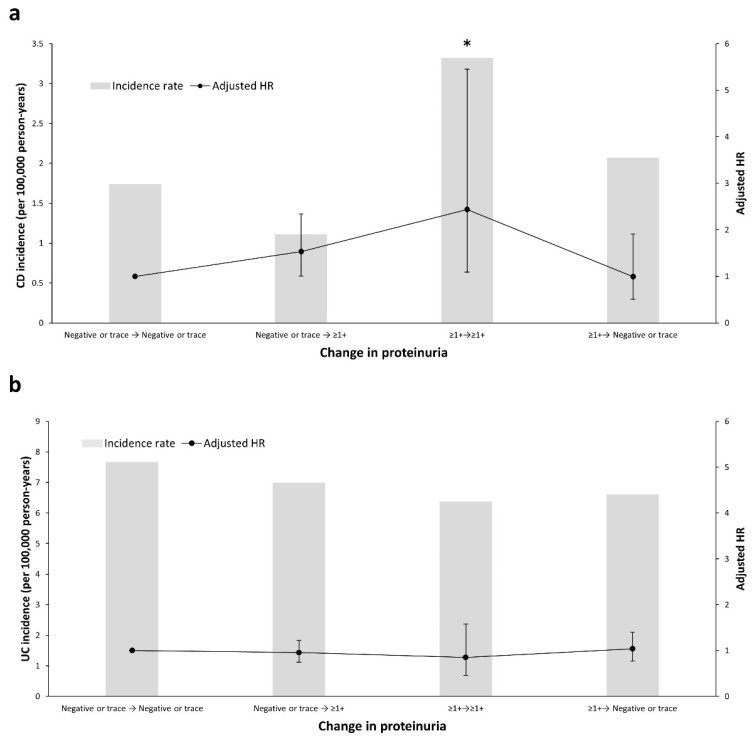
Of a total of 9,917,400 participants, 6,078,732 (61.3%) underwent another health checkup within 2 years after the baseline checkup. Most of the people (n = 5,809,318, 95.6%) who initially had dipstick-negative or trace results showed the same results at follow-up. Compared with these, there was an increased tendency of CD development among individuals who had dipstick-positive results during follow-up, although not statistically significant (aHR, 1.513; 95% CI, 0.989–2.316, negative or trace → ≥ 1+ proteinuria) (Figure 2a). In addition, the risk of developing CD was highest in people with persistent dipstick-positive proteinuria during follow-up (aHR, 2.364; 95% CI, 1.053–5.306, ≥ 1+ → ≥ 1+ proteinuria). However, the reduced dipstick-positive proteinuria at follow-up was not accompanied by a reduced risk of developing CD during follow-up (aHR, 0.977; 95% CI, 0.506–1.886, ≥ 1+ → negative or trace). In contrast to CD, the association between the change in proteinuria and UC risk was not significant (Figure 2b).
Figure 2.
Incidence and risk of (a) Crohn’s disease and (b) ulcerative colitis according to changes in proteinuria measured by dipstick. Hazard ratios were adjusted for age, sex, income level, smoking status, alcohol consumption status, exercise level, body mass index, estimated glomerular filtration rate, diabetes mellitus, hypertension, and dyslipidemia. The left Y axis and columns indicate the incidence rate, the right Y axis and line indicate the adjusted HR, and the error bars indicate the 95% confidence interval. * p < 0.05. CD, Crohn’s disease; HR, hazard ratio; UC, ulcerative colitis.
4. Discussion
This is the first study to demonstrate the effect of proteinuria measured by dipstick tests on the development of IBD. In this nationwide population-based cohort study using health screening data on approximately 10,000,000 members of the general population, we found that dipstick-positive proteinuria was significantly associated with increased risk of CD development, but not UC development, after adjusting for potential confounding factors. As the degree of proteinuria increased, the HR of CD development also increased, and individuals with persistent proteinuria during follow-up had the highest risk of CD development. Therefore, dipstick-positive proteinuria may be a predictive risk factor for the development of CD.
ACR or PCR measurement in a spot urine sample is recommended as an appropriate method for the detection of proteinuria. However, these tests are time-consuming and costly. Accordingly, the urine dipstick test is widely accepted as an initial screening tool in the general population [22,23]. Compared with ACR or PCR, the urine dipstick test shows high sensitivity and specificity in detecting proteinuria. In particular, 1+ or greater dipstick results identify ACR ≥ 300 mg/g with a sensitivity of 95% to 98% and specificity of 92% [22,24]. Therefore, dipstick-positive proteinuria, defined as 1+ or greater in this study, can reliably identify significant proteinuria.
In previous studies, proteinuria was identified as a sensitive marker of progressive renal dysfunction [25]. However, it is not only a reflection of renal pathology, but it is also associated with increased vascular permeability [7,26]. When endothelial dysfunction is caused by generalized damage to the endothelial glycocalyx covering the luminal surface of the vascular endothelium, it appears as an increase in systemic microvascular permeability with proteinuria at the glomerular level [27]. Isolated proteinuria might be an early reflection of increased systemic microvascular permeability and a marker of endothelial dysfunction, which may be associated with chronic low-grade systemic inflammation [28,29]. Prior studies have demonstrated that low-grade systemic inflammation, as measured by circulating C-reactive protein in patients with asymptomatic proteinuria, is increased compared to controls and negatively correlated with microvascular endothelial function determined by acetylcholine iontophoresis [7]. In addition, proteinuria has been identified to be associated with the development of many inflammatory conditions, including DM, infectious diseases [13,27], Parkinson’s disease [8], metabolic syndrome [12], and nonalcoholic fatty liver disease [9].
Endothelial dysfunction and increased vascular permeability play crucial roles in the pathogenesis of IBD [30,31,32]. This may result in the upregulation of cellular adhesion molecules, impaired barrier integrity, increased leukocyte diapedesis, vascular smooth muscle tone, and pro-coagulant status, leading to an inflammatory process in the gastrointestinal tract [33,34]. In IBD, the expressions of intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and mucosal addressin cell adhesion molecule 1 (MAdCAM-1) are upregulated, increasing leukocyte recruitment. MAdCAM-1 interacts with the α4β7 integrins of naive CD4+ T cells and recruits these cells [33,35]. In addition, pro-inflammatory cytokines such as interleukin (IL)-1, tumor necrosis factor (TNF)-α, nitric oxide, vascular endothelial growth factor (VEGF), and IL-6 are increased in IBD, altering vascular permeability [33,36,37]. These findings suggest that chronic inflammation and endothelial dysfunction might contribute to potential mechanisms underlying the relationship between proteinuria and IBD. Dietary factors have also been linked to the pathogenesis of IBD via alterations in the microbiome, gut barrier function, and immunity [38]. Dietary patterns that can affect proteinuria, such as the Western diet and high animal protein, have also been related to increased risk of IBD [39]. Further study to evaluate the role of diet in the association between proteinuria and IBD is required.
Meanwhile, we found that a higher degree of proteinuria increased the risk of CD development but did not increase that of UC. Although the mechanism by which proteinuria affects the development of CD and UC in different ways is unknown, the inflammation in UC is typically restricted to the mucosa, whereas CD shows extensive transmural inflammation. Therefore, the systemic inflammatory response reflected by proteinuria appears to be more related to the development of CD than UC. In previous studies comparing inflammatory markers between patients with CD and UC, the higher production of C-reactive protein was observed in patients with CD than in patients with UC [40,41,42]. Serum IL-6 [43] and TNF-α [44] concentrations and TNF-α protein [45] and mRNA [46] expression in mucosal tissues were also significantly higher in patients with CD than in patients with UC. In addition, vascular etiology seems to be more involved in the development of CD. VEGF, which plays a role in increasing vascular permeability, is distinctly increased in patients with CD compared with UC patients and controls [47]. Therefore, dipstick-positive proteinuria, which is thought to reflect vascular permeability and systemic inflammation, may be more related to the development of CD than UC. In this study, dipstick-positive proteinuria increased the risk of CD as the severity of proteinuria increased. The effect did not differ according to age, sex, and co-morbidities, and recent studies have also reported a higher risk of cardiovascular diseases and DM even in young patients with CD [17,48]. Our results suggest that proteinuria can independently predict the risk of CD, apart from previously known risk factors. Further studies are needed to clarify the link between proteinuria and IBD subtypes.
Although we could not accurately account for the cause of the changes in proteinuria in this study, we found that the risk of CD decreased with a reduction in dipstick-positive proteinuria. In addition, we also found that the risk of CD development increased 1.5-fold when dipstick-positive proteinuria occurred, even with negative or trace results at baseline. Furthermore, the risk of CD development increased 2.3-fold when dipstick-positive proteinuria was repeatedly detected. Our results indicate that measurement of not only baseline proteinuria but also its serial change is important in clinical practice.
This study has several limitations. A major limitation is that because of its retrospective study design, it was not possible to determine the causal relationship accurately. Second, because information on the severity of incident IBD was unavailable in the NHIS database, we could not assess how the degree of proteinuria affects the severity of intestinal inflammation. In addition, we could not acquire data regarding dietary patterns and family history of individuals in the IBD database, as the NHI provides only non-identifiable information. Third, data on prescription drug use (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and statins) that could affect proteinuria could not be evaluated in this study. Therefore, further study is mandatory to determine the risk of IBD development according to changes in medications known to cause proteinuria. Despite these limitations, considering the increased prevalence and incidence of IBD in Korea [49], and anticipating a future burden of disease, our study has implications toward examining modifiable risk factors of IBD.
5. Conclusions
In conclusion, dipstick-positive proteinuria predicted an increased risk of CD development, but not that of UC. In addition, the risk of CD development was modified by changes in proteinuria. Therefore, our data suggest that dipstick-positive proteinuria has the potential to be used as a surrogate marker of CD development.
Abbreviations
aHR, adjusted hazard ratio; ACR, albumin-to-creatinine ratio; BMI, body mass index; CI, confidence interval; CD, Crohn’s disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HR, hazard ratio; IBD, inflammatory bowel disease; IL, interleukin; ICD-10, International Classification of Disease, Tenth Revision; MAdCAM, mucosal addressin cell adhesion molecule; NHIS, National Health Insurance Service; PCR, protein-to-creatinine ratio; RID, rare and intractable disease; TNF, tumor necrosis factor; UC, ulcerative colitis; VEGF, vascular endothelial growth factor.
Author Contributions
S.P., H.J.L. and K.-D.H.: conception and design of the study; S.P., K.-D.H., H.S., J.M.M. and S.W.H.: acquisition of data; S.P., H.J.L. and K.-D.H.: analysis and interpretation of data; K.-D.H.: statistical analysis; S.P.: drafting the article; H.J.L., E.A.K., J.P.I. and J.S.K.: critical revision of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the SK Chemical Research Fund of The Korean Society of Gastroenterology (2020).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Seoul National University College of Medicine-Seoul National University Hospital Institutional Review Board (protocol code H-1703-107-840 and date of approval 24 March 2017).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ananthakrishnan A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015;12:205–217. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 2.Molodecky N.A., Soon I.S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G., Benchimol E.I., Panaccione R., Ghosh S., Barkema H.W., et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Ng W.K., Wong S.H., Ng S.C. Changing epidemiological trends of inflammatory bowel disease in Asia. Intestig. Res. 2016;14:111–119. doi: 10.5217/ir.2016.14.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015;12:720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 5.Turin T.C., James M., Ravani P., Tonelli M., Manns B.J., Quinn R., Jun M., Klarenbach S., Hemmelgarn B.R. Proteinuria and Rate of Change in Kidney Function in a Community-Based Population. J. Am. Soc. Nephrol. 2013;24:1661–1667. doi: 10.1681/ASN.2012111118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemmelgarn B.R., Manns B.J., Lloyd A., James M.T., Klarenbach S., Quinn R.R., Wiebe N., Tonelli M., Network F.T.A.K.D. Relation Between Kidney Function, Proteinuria, and Adverse Outcomes. JAMA. 2010;303:423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 7.Paisley K.E., Beaman M., Tooke J.E., Mohamed-Ali V., Lowe G.D., Shore A.C. Endothelial dysfunction and inflammation in asymptomatic proteinuria. Kidney Int. 2003;63:624–633. doi: 10.1046/j.1523-1755.2003.00768.x. [DOI] [PubMed] [Google Scholar]
- 8.Nam G.E., Kim N.H., Han K., Choi K.M., Chung H.S., Kim J.W., Han B., Cho S.J., Jung S.J., Yu J.H., et al. Chronic renal dysfunction, proteinuria, and risk of Parkinson’s disease in the elderly. Mov. Disord. 2019;34:1184–1191. doi: 10.1002/mds.27704. [DOI] [PubMed] [Google Scholar]
- 9.Sun K., Lin D., Li F., Qi Y., Feng W., Yan L., Chen C., Ren M., Liu D. Fatty liver index, albuminuria and the association with chronic kidney disease: A population-based study in China. BMJ Open. 2018;8:e019097. doi: 10.1136/bmjopen-2017-019097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang A., Liu X., Su Z., Chen S., Zhang N., Wu S., Wang Y., Wang Y. Two-Year Changes in Proteinuria and the Risk of Stroke in the Chinese Population: A Prospective Cohort Study. J. Am. Hear. Assoc. 2017;6:6. doi: 10.1161/JAHA.117.006271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hara M., Ando M., Maeda Y., Tsuchiya K., Nitta K. Proteinuria is a simple sign of systemic inflammation that leads to a poor prognosis in individuals affected with non-Hodgkin lymphoma. Clin. Nephrol. 2014;82:51–57. doi: 10.5414/CN108132. [DOI] [PubMed] [Google Scholar]
- 12.Okada R., Yasuda Y., Tsushita K., Wakai K., Hamajima N., Matsuo S. Trace proteinuria by dipstick screening is associated with metabolic syndrome, hypertension, and diabetes. Clin. Exp. Nephrol. 2018;22:1387–1394. doi: 10.1007/s10157-018-1601-3. [DOI] [PubMed] [Google Scholar]
- 13.Wei X.-B., Liu Y.-H., He P.-C., Yu D.-Q., Zhou Y.-L., Tan N., Chen J.-Y. The relationship between albuminuria and poor clinical outcomes in patients with infective endocarditis. Clin. Chim. Acta. 2016;462:28–32. doi: 10.1016/j.cca.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Song S.O., Jung C.H., Song Y.D., Park C.-Y., Kwon H.-S., Cha B.S., Park J.-Y., Lee K.-U., Ko K.S., Lee B.-W. Background and Data Configuration Process of a Nationwide Population-Based Study Using the Korean National Health Insurance System. Diabetes Metab. J. 2014;38:395–403. doi: 10.4093/dmj.2014.38.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H.J., Hann H.J., Hong S.N., Kim K.H., Ahn I.M., Song J.Y., Lee S.H., Ahn H.S. Incidence and Natural Course of Inflammatory Bowel Disease in Korea, 2006–2012. Inflamm. Bowel Dis. 2015;21:623–630. doi: 10.1097/MIB.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 16.Choi K., Chun J., Han K., Park S., Soh H., Kim J., Lee J., Lee H.J., Im J.P., Kim J.S. Risk of Anxiety and Depression in Patients with Inflammatory Bowel Disease: A Nationwide, Population-Based Study. J. Clin. Med. 2019;8:654. doi: 10.3390/jcm8050654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang E.A., Han K., Chun J., Soh H., Park S., Im J.P., Kim J.S. Increased Risk of Diabetes in Inflammatory Bowel Disease Patients: A Nationwide Population-based Study in Korea. J. Clin. Med. 2019;8:343. doi: 10.3390/jcm8030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park S., Chun J., Han K.-D., Soh H., Choi K., Kim J.H., Lee J., Lee C., Im J.P., Kim J.S. Increased end-stage renal disease risk in patients with inflammatory bowel disease: A nationwide population-based study. World J. Gastroenterol. 2018;24:4798–4808. doi: 10.3748/wjg.v24.i42.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soh H., Chun J., Han K., Park S., Choi G., Kim J.H., Lee J., Im J.P., Kim J.S. Increased Risk of Herpes Zoster in Young and Metabolically Healthy Patients with Inflammatory Bowel Disease: A Nationwide Population-Based Study. Gut Liver. 2019;13:333–341. doi: 10.5009/gnl18304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S., Chun J., Han K.-D., Soh H., Kang E.A., Lee H.J., Im J.P., Kim J.S. Dose-response relationship between cigarette smoking and risk of ulcerative colitis: A nationwide population-based study. J. Gastroenterol. 2019;54:881–890. doi: 10.1007/s00535-019-01589-3. [DOI] [PubMed] [Google Scholar]
- 21.Levey A.S., Bosch J.P., Lewis J.B., Greene T., Rogers N., Roth D.R. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann. Intern. Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 22.White S.L., Yu R., Craig J.C., Polkinghorne K.R., Atkins R.C., Chadban S.J. Diagnostic Accuracy of Urine Dipsticks for Detection of Albuminuria in the General Community. Am. J. Kidney Dis. 2011;58:19–28. doi: 10.1053/j.ajkd.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Bailie G.R., Uhlig K., Levey A.S. Clinical Practice Guidelines in Nephrology: Evaluation, Classification, and Stratification of Chronic Kidney Disease. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2005;25:491–502. doi: 10.1592/phco.25.4.491.61034. [DOI] [PubMed] [Google Scholar]
- 24.Lim D., Lee D.-Y., Cho S.H., Kim O.Z., Cho S.W., An S.K., Kim H.W., Moon K.H., Lee M.H., Kim B. Diagnostic accuracy of urine dipstick for proteinuria in older outpatients. Kidney Res. Clin. Pr. 2014;33:199–203. doi: 10.1016/j.krcp.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bello A.K., Hemmelgarn B., Lloyd A., James M.T., Manns B.J., Klarenbach S., Tonelli M., Network F.T.A.K.D. Associations among Estimated Glomerular Filtration Rate, Proteinuria, and Adverse Cardiovascular Outcomes. Clin. J. Am. Soc. Nephrol. 2011;6:1418–1426. doi: 10.2215/CJN.09741110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen J.S., Borch-Johnsen K., Jensen G., Feldt-Rasmussen B. Microalbuminuria Reflects a Generalized Transvascular Albumin Leakiness in Clinically Healthy Subjects. Clin. Sci. 1995;88:629–633. doi: 10.1042/cs0880629. [DOI] [PubMed] [Google Scholar]
- 27.Salmon A.H.J., Satchell S.C. Endothelial glycocalyx dysfunction in disease: Albuminuria and increased microvascular permeability. J. Pathol. 2012;226:562–574. doi: 10.1002/path.3964. [DOI] [PubMed] [Google Scholar]
- 28.Festa A., Dagostino R.B., Howard G.S., Mykkänen L., Tracy R.P., Haffner S.M. Inflammation and microalbuminuria in nondiabetic and type 2 diabetic subjects: The Insulin Resistance Atherosclerosis Study. Kidney Int. 2000;58:1703–1710. doi: 10.1046/j.1523-1755.2000.00331.x. [DOI] [PubMed] [Google Scholar]
- 29.Stenvinkel P. Endothelial dysfunction and inflammation-is there a link? Nephrol. Dial. Transplant. 2001;16:1968–1971. doi: 10.1093/ndt/16.10.1968. [DOI] [PubMed] [Google Scholar]
- 30.Cibor D., Domagala-Rodacka R., Rodacki T., Jurczyszyn A., Mach T., Owczarek D. Endothelial dysfunction in inflammatory bowel diseases: Pathogenesis, assessment and implications. World J. Gastroenterol. 2016;22:1067–1077. doi: 10.3748/wjg.v22.i3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roifman I., Sun Y.C., Fedwick J.P., Panaccione R., Buret A.G., Liu H., Rostom A., Anderson T.J., Beck P.L. Evidence of endothelial dysfunction in patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2009;7:175–182. doi: 10.1016/j.cgh.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Deban L., Correale C., Vetrano S., Malesci A., Danese S. Multiple pathogenic roles of microvasculature in inflammatory bowel disease: A jack of all trades. Am. J. Pathol. 2008;172:1457–1466. doi: 10.2353/ajpath.2008.070593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gravina A.G., Dallio M., Masarone M., Rosato V., Aglitti A., Persico M., Loguercio C., Federico A. Vascular Endothelial Dysfunction in Inflammatory Bowel Diseases: Pharmacological and Nonpharmacological Targets. Oxidative Med. Cell. Longev. 2018;2018:1–12. doi: 10.1155/2018/2568569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steyers C.M., III, Miller F.J., Jr. Endothelial dysfunction in chronic inflammatory diseases. Int. J. Mol. Sci. 2014;15:11324–11349. doi: 10.3390/ijms150711324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danese S. Role of the vascular and lymphatic endothelium in the pathogenesis of inflammatory bowel disease: Brothers in arms. Gut. 2011;60:998–1008. doi: 10.1136/gut.2010.207480. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y.-H., Ding Y., Gao C.-C., Li L.-S., Wang Y.-X., Xu J.-D. Functional macrophages and gastrointestinal disorders. World J. Gastroenterol. 2018;24:1181–1195. doi: 10.3748/wjg.v24.i11.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danese S., Scaldaferri F., Vetrano S., Stefanelli T., Graziani C., Repici A., Ricci R., Straface G., Sgambato A., Malesci A., et al. Critical role of the CD40 CD40-ligand pathway in regulating mucosal inflammation-driven angiogenesis in inflammatory bowel disease. Gut. 2007;56:1248–1256. doi: 10.1136/gut.2006.111989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine A., Boneh R.S., Wine E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut. 2018;67:1726–1738. doi: 10.1136/gutjnl-2017-315866. [DOI] [PubMed] [Google Scholar]
- 39.Jantchou P., Morois S., Clavel-Chapelon F., Boutron-Ruault M.-C., Carbonnel F. Animal Protein Intake and Risk of Inflammatory Bowel Disease: The E3N Prospective Study. Am. J. Gastroenterol. 2010;105:2195–2201. doi: 10.1038/ajg.2010.192. [DOI] [PubMed] [Google Scholar]
- 40.Fagan E.A., Dyck R.F., Maton P.N., Hodgson H.J., Chadwick V.S., Petrie A., Pepys M.B. Serum levels of C-reactive protein in Crohn’s disease and ulcerative colitis. Eur. J. Clin. Investig. 1982;12:351–359. doi: 10.1111/j.1365-2362.1982.tb02244.x. [DOI] [PubMed] [Google Scholar]
- 41.Henriksen M., Jahnsen J., Lygren I., Stray N., Sauar J., Vatn M.H., Moum B., the IBSEN Study Group C-Reactive Protein A predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57:1518–1523. doi: 10.1136/gut.2007.146357. [DOI] [PubMed] [Google Scholar]
- 42.Saverymuttu S.H., Hodgson H.J., Chadwick V.S., Pepys M.B. Differing acute phase responses in Crohn’s disease and ulcerative colitis. Gut. 1986;27:809–813. doi: 10.1136/gut.27.7.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gross V., Andus T., Caesar I., Roth M., Scholmerich J. Evidence for continuous stimulation of interleukin-6 production in Crohn’s disease. Gastroenterology. 1992;102:514–519. doi: 10.1016/0016-5085(92)90098-J. [DOI] [PubMed] [Google Scholar]
- 44.Komatsu M., Kobayashi D., Saito K., Furuya D., Yagihashi A., Araake H., Tsuji N., Sakamaki S., Niitsu Y., Watanabe N. Tumor Necrosis Factor-α in Serum of Patients with Inflammatory Bowel Disease as Measured by a Highly Sensitive Immuno-PCR. Clin. Chem. 2001;47:1297–1301. doi: 10.1093/clinchem/47.7.1297. [DOI] [PubMed] [Google Scholar]
- 45.Murch S.H., Braegger C.P., A Walker-Smith J., Macdonald T.T. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705–1709. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dionne S., Hiscott J., D’Agata I., Duhaime A., Seidman E.G. Quantitative PCR analysis of TNF-alpha and IL-1 beta mRNA levels in pediatric IBD mucosal biopsies. Dig. Dis. Sci. 1997;42:1557–1566. doi: 10.1023/A:1018895500721. [DOI] [PubMed] [Google Scholar]
- 47.Bousvaros A., Leichtner A., Zurakowski D., Kwon J., Law T., Keough K., Fishman S. Elevated serum vascular endothelial growth factor in children and young adults with Crohn’s disease. Dig. Dis. Sci. 1999;44:424–430. doi: 10.1023/A:1026635308127. [DOI] [PubMed] [Google Scholar]
- 48.Choi Y.J., Lee D.H., Shin D.W., Han K.-D., Yoon H., Shin C.M., Park Y.S., Kim N. Patients with inflammatory bowel disease have an increased risk of myocardial infarction: A nationwide study. Aliment. Pharmacol. Ther. 2019;50:769–779. doi: 10.1111/apt.15446. [DOI] [PubMed] [Google Scholar]
- 49.Park S.H., Kim Y.-J., Rhee K.H., Kim Y.K., Hong S.N., Seo S.I., Cha J.M., Park S.Y., Jeong S.K., Lee J.H., et al. A 30-year Trend Analysis in the Epidemiology of Inflammatory Bowel Disease in the Songpa-Kangdong District of Seoul, Korea in 1986–2015. J. Crohn’s Colitis. 2019;13:1410–1417. doi: 10.1093/ecco-jcc/jjz081. [DOI] [PubMed] [Google Scholar]