Abstract
Objective:
The aim of this study was to estimate the cost-effectiveness of esketamine, a novel intranasally-dosed antidepressant, for patients in the United States with treatment-resistant depression.
Methods:
A decision-analytic model, parameterized with efficacy data from phase III randomized trials of esketamine, was used to simulate the effects of treatment with esketamine versus oral antidepressants over a 5-year horizon, from both societal and healthcare sector perspectives. Outcomes included remission and response of depression, quality-adjusted life-years (QALYs), costs, and incremental cost-effectiveness ratios (ICERs) for esketamine. In addition, value-based prices were calculated, defined as the per-dose price at which esketamine would become cost-effective given cost-effectiveness thresholds of $50,000/QALY, $100,000/QALY, and $150,000/QALY. Uncertainty in these outcomes was assessed using probabilistic sensitivity analyses. Key model parameters included the efficacy of esketamine versus oral antidepressants (relative risk 1.39 for remission, 1.32 for response) and the monthly cost of esketamine ($5,572 for month 1, $1,699–2,244 thereafter).
Results:
Over 5 years, esketamine was projected to increase time in remission from 25.3% to 31.1% of life-years, resulting in a gain of 0.07 QALYs. Esketamine increased societal costs by $16,617 and healthcare sector costs by $16,995. Base case ICERs were $237,111/QALY (societal) and $242,496/QALY (healthcare sector). In probabilistic sensitivity analysis, there was >95% likelihood that esketamine’s ICER would be above $150,000/QALY. At a cost-effectiveness threshold of $150,000/QALY, esketamine’s value-based price was approximately $140/dose (versus a current price of $240/dose).
Conclusions:
Esketamine is unlikely to be cost-effective for management of treatment-resistant depression in the United States unless its price falls by more than 40%.
INTRODUCTION
Esketamine is an intranasally-dosed antidepressant that was approved by the Food and Drug Administration (FDA) in March 2019 for use in treatment-resistant major depressive disorder (1). Its approval has sparked intense interest for several reasons.
First, esketamine has a novel mechanism of action (NMDA receptor antagonism) as compared with prior antidepressants (monoamine neurotransmitter modulation) (2). Second, esketamine’s rapid onset of effect has raised the potential for novel applications such as acute reduction of suicidality in emergency settings (3). Third, and the reason for its FDA approval, esketamine has demonstrated efficacy in patients with treatment-resistant depression (4) – that is, patients for whom 2 or more prior treatments have failed (5). Patients with treatment-resistant depression experience markedly reduced quality of life and increased healthcare costs (6) and have remission rates with further antidepressants as low as 10–15% (7). As a novel treatment option with demonstrated efficacy in this subpopulation, esketamine could thus have immense individual and societal benefits.
However, esketamine has drawbacks that may limit its use. Most notably, the FDA requires that patients be observed in a healthcare setting for 2 hours after each dose to monitor for dissociation and hypertension (1). Even without considering the costs of this monitoring, esketamine is substantially more expensive than other antidepressants. The first 2 months of esketamine treatment have been estimated to cost $7,000–11,000 (8); in contrast, a recent assessment of national price databases revealed that commonly-used oral antidepressants cost <$100 per year (9, 10). Amidst ongoing efforts to control the growth of healthcare spending in the United States (US), it’s critical to ensure that such an expensive treatment provides a good health-economic value (11, 12).
To address this issue, we used a previously-developed decision-analytic model (13) integrating data from 4 recently-published phase III clinical trials (4, 14) to assess the value of esketamine. Our analysis has two main objectives: 1) to quantify the cost-effectiveness of esketamine nasal spray compared to usual care for patients with treatment-resistant depression in the US, and 2) to determine drug price thresholds at which esketamine would be considered cost-effective by commonly-applied criteria in the US.
METHODS
Overview
We used a decision-analytic model, parameterized with data from phase III clinical trials of esketamine, to simulate the clinical and economic consequences of the following two treatment strategies for adults with major depressive disorder who have failed two prior antidepressants: 1) Esketamine: initial treatment with esketamine nasal spray plus oral antidepressants, followed by additional antidepressants and/or psychotherapy if depressive symptoms do not respond; 2) Usual care: initial treatment with oral antidepressants, followed by additional antidepressants and/or psychotherapy if depressive symptoms do not respond.
Over a five-year time horizon, we projected symptomatic outcomes, quality-adjusted life-years (QALYs), and costs, in 2015 United States dollars (USD), under each strategy. We chose this time horizon to ensure adequate time for longer-term costs and benefits to accrue without requiring excess extrapolation beyond available outcomes data (4, 15, 16); we varied the time horizon between 2 and 10 years in sensitivity analysis. Future costs and QALYs were discounted at an annual rate of 3% (17). We evaluated costs from both the healthcare sector perspective (incorporating those costs accrued within the medical system) and the societal perspective (including additional costs such as productivity losses, patient time, etc.) (table in online supplement) (17).
Using these outcomes, we calculated the incremental cost-effectiveness ratio (ICER) of esketamine as the ratio of its incremental cost to its incremental QALYs relative to usual care; a lower ICER indicates a better health-economic value. In the US, ICER thresholds between $50,000/QALY and $150,000/QALY have been advocated for designating health interventions as “cost-effective” (18, 19). To reflect this range of recommendations, we report results for distinct cost-effectiveness thresholds of $50,000/QALY, $100,000/QALY, and $150,000/QALY. For each threshold, we calculated the value-based price of esketamine, defined as the per-dose price at which its ICER is equal to the threshold (20); this value represents the highest price at which esketamine would be deemed cost-effective for a given cost-effectiveness threshold.
In reporting our methodology and findings, we adhere to the 2013 Consolidated Health Economic Evaluation Reporting Standards guidelines (21). As this study used published literature without identifiable data, it was exempt from institutional review board regulation. The study was conducted between March 2019 and December 2019.
Model description
To simulate the effects of esketamine, we used a previously-developed state-transition model of depression treatment (10, 13). The model uses a 1-month cycle length. The model is implemented in Microsoft Excel; statistical analyses were performed OpenMetaAnalyst (22).
Patients enter the model upon initiation of 3rd-line antidepressant treatment and can progress through up to 9 total treatment lines (figure in online supplement). With the exception of esketamine, these treatment lines do not simulate specific medications, psychotherapy, or other treatments; they are instead intended to capture the aggregate costs and effectiveness of the range of therapies provided to patients with treatment-resistant depression (7, 9).
Each treatment line is modeled using the following 5 health states: 1) Initiation: the first month on a new treatment; 2) Remission: near-complete resolution of depressive symptoms, as determined by a validated symptom rating scale (e.g. Quick Inventory of Depressive Symptomatology ≤5) (7); 3) Response: partial resolution of depressive symptoms, defined as ≥50% reduction in score on a validated symptom rating scale (7, 14); 4) Non-response: initial failure to achieve response or remission; 5) Relapse: recurrence of depressive symptoms after initial response or remission.
Patients start each new treatment in the initiation state and then transition to remission, response, or non-response in the next cycle based on efficacy estimates for that treatment. Patients in remission and response are then subject to a monthly probability of relapse. Patients in relapse or non-response will transition to initiation of the following treatment line during the next cycle. Note that patients who never respond to a given treatment will receive it for 8 weeks (1 month in initiation, 1 month in non-response), consistent with recommendations for the duration of an adequate antidepressant trial (23, 24).
Finally, patients in all states are subject to a monthly all-cause mortality probability; for simplicity, this probability is not shown in the model diagram (online supplement).
Model input data
Base case model input data, uncertainty analysis ranges, and sources are shown in Table 1. Where possible, input data reflect US adults with treatment-resistant, nonpsychotic major depressive disorder.
Table 1:
Model input data
| Parameter | Base case value | Uncertainty analysis range | Distribution | Sources |
|---|---|---|---|---|
| General and demographic | ||||
| Annual discount rate, percent | 3 | – | – | 17 |
| Time horizon, years | 5 | 2–10a | – | – |
| Annual mortality probability, percent | 0.40 | 0.38–0.43 | Normal | 25–27 |
| Esketamine efficacy relative to usual care | ||||
| Response, relative risk | 1.32 | 1.10–1.58 | Log-normal | 4 |
| Remission, relative risk | 1.39 | 1.09–1.79 | Log-normal | 4 |
| Relapse, hazard ratio | 1 | – | – | 4 |
| Usual care efficacy by treatment line | ||||
| Remission probability, percent | ||||
| Line 3 | 30.6 | 28.2–33.0 | Beta | 7 |
| Line 4 | 13.7 | 10.4–17.2 | Beta | 7 |
| Lines 5+ | 13.0 | 7.7–19.5 | Beta | 7 |
| Response probability, percent | ||||
| Line 3 | 28.5 | 26.2–30.9 | Beta | 7 |
| Line 4 | 16.8 | 13.4–20.8 | Beta | 7 |
| Lines 5+ | 16.3 | 10.3–23.2 | Beta | 7 |
| Monthly relapse probability, percent | ||||
| In remission, line 3 | 7.8 | 6.6–9.1 | Beta | 7 |
| In remission, line 4 | 7.7 | 4.1–12.6 | Beta | 7 |
| In remission, lines 5+ | 8.6 | 3.7–16.4 | Beta | 7 |
| In response, line 3 | 13.6 | 11.4–16.1 | Beta | 7 |
| In response, line 4 | 17.8 | 12.9–24.2 | Beta | 7 |
| In response, lines 5+ | 17.3 | 11.0–27.4 | Beta | 7 |
| Utility by health state | ||||
| Non-response/relapse/initiation | 0.58 | 0.50–0.66 | Normal | 30 |
| Response | 0.72 | 0.65–0.79 | Normal | 30 |
| Remission | 0.85 | 0.83–0.87 | Normal | 30 |
| Costs, 2015 USD | ||||
| Annual direct healthcare cost by treatment line | ||||
| Line 3 | 12,047 | 9,666–14,428 | Normal | 25 |
| Line 4 | 14,699 | 11,441–17,957 | Normal | 25 |
| Line 5 | 15,073 | 13,053–17,093 | Normal | 25 |
| Line 6 | 16,699 | 13,939–19,459 | Normal | 25 |
| Lines 7+ | 18,667 | 17,089–20,245 | Normal | 25 |
| Annual lost productivity cost by health state | ||||
| Non-response/relapse/initiation | 11,573 | 8,063–15,083 | Normal | 38, 39 |
| Response | 5,757 | 3,445–8,070 | Normal | 38, 39 |
| Remission | 2,067 | 1,338–2,796 | Normal | 38, 39 |
| Monthly costs of esketamine treatment | ||||
| Medication costs | ||||
| Month 1 | 5,572 | – | – | 4, 34 |
| Months 2+, response | 2,244 | – | – | 4, 34 |
| Months 2+, remission | 1,699 | – | – | 4, 34 |
| Months 2+, non-response/relapse | 1,966 | – | – | 4, 34 |
| Physician and medical assistant services | ||||
| Month 1 | 685 | – | – | 4, 36, 37 |
| Months 2+, response | 275 | – | – | 4, 36, 37 |
| Months 2+, remission | 218 | – | – | 4, 36, 37 |
| Months 2+, non-response/relapse | 246 | – | – | 4, 36, 37 |
| Patient time | ||||
| Month 1 | 689 | – | – | 4, 39 |
| Months 2+, response | 276 | – | – | 4, 39 |
| Months 2+, remission | 219 | – | – | 4, 39 |
| Months 2+, non-response/relapse | 248 | – | – | 4, 39 |
USD, United States dollars
Time horizon is varied between 2 years and 10 years in one-way sensitivity analyses but held constant at 5 years in probabilistic sensitivity analyses.
Demographics and mortality
Based on demographic data from an insurance claims analysis of patients with treatment-resistant depression, we simulated a population with mean age 40.5 years (SD 13.2), 64.2% female (25). We applied age/gender-specific mortality rates from the 2013 US life tables (26), along with a mortality hazard ratio of 1.58 (95% CI 1.47–1.70) for people with depression versus the general population (27); this yielded an overall annual mortality probability of 0.0040.
Esketamine efficacy
We based our estimates of esketamine’s efficacy on the results of 4 phase III clinical trials which are presented in the FDA Advisory Committee briefing document for esketamine (4).
In three short-term trials (TRANSFORM-1, TRANSFORM-2, and TRANSFORM-3), patients with major depressive disorder who had failed two or more prior treatment lines were randomized to esketamine nasal spray versus placebo; in addition, all patients received a new oral antidepressant. TRANSFORM-1 and TRANSFORM-2 included patients aged 18–64; TRANSFORM-3 included patients aged ≥65. We performed random effects, restricted maximum likelihood meta-analyses on four-week intention-to-treat remission and response rates from these trials (figures in online supplement). Of 421 total patients randomized to esketamine, 142 (33.7%) achieved remission and 199 (47.3%) achieved response; of 289 randomized to placebo, 68 (23.5%) achieved remission and 102 (35.3%) achieved response. Estimated relative risks for esketamine versus placebo were 1.39 (95% CI 1.09–1.79) for remission and 1.32 (95% CI 1.10–1.58) for response.
In the long-term SUSTAIN-1 trial, patients who achieved initial remission or response on esketamine were randomized to continuation of esketamine versus replacement with placebo (4). During follow-up extending up to 89 weeks, relapse hazard ratios for esketamine versus placebo were 0.49 (95% CI 0.29–0.84) for patients in remission at randomization and 0.30 (95% CI 0.16–0.55) for patients in response. These results indicate that continuation of esketamine reduces relapse risk compared to withdrawal of esketamine among patients who initially responded to it; they do not indicate that patients who respond to esketamine have a lower relapse risk than patients who respond to oral antidepressants. Because of this, in the base case of our analysis we assumed equal relapse rates for esketamine and oral antidepressants; we varied this assumption in sensitivity analysis.
Usual care efficacy
Estimated rates of remission (30.6–13.0%) and response (28.5–16.3%) across multiple treatment lines under usual care were drawn from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial (7). STAR*D was a pragmatic randomized trial in which outpatients with major depressive disorder were followed across four treatment “steps”. Specific treatments included citalopram, bupropion, cognitive therapy, lithium augmentation, and others. Notably, remission and response rates in the first step of STAR*D (36.8% and 48.6%) were lower than observed in multiple meta-analyses of antidepressant efficacy (28, 29), possibly due to prior treatment experience among STAR*D participants; for this reason, we derived our estimates of 3rd-line treatment efficacy from step 2 of STAR*D.
Monthly relapse probabilities (5.1–17.8%) were derived from the long-term follow-up phase of STAR*D (7). Observed relapse rates were higher for patients in response than in remission, and for patients who had received more prior treatments.
Utility
Utility values for our model’s depression health states were derived from a French study which used the Euro-Qol-5D questionnaire to assess quality of life among outpatients treated for major depressive disorder; utility estimates for remission, response, and non-response were 0.85, 0.72, and 0.58 (30).
Costs
Cost estimates are expressed in 2015 USD. We used the Medical Care Expenditure index from the Bureau of Labor Services to inflate costs from earlier years to 2015 (the most recent year for which this index is available) (31, 32). We deflated later costs to 2015 using the Personal Consumption Expenditure index from the Federal Reserve (33).
We derived estimates of the aggregate cost of depression treatment (including medications and outpatient, inpatient, and emergency department services) from a claims-based analysis of privately-insured patients with major depressive disorder in the US (25). Annual costs ranged from $12,047 to $18,667 for patients with 2 to 6+ prior treatments.
Our estimate of the total cost of esketamine provision includes costs of the medication itself, physician visits, and medical assistant observation during the two hours after each dose. We used a cost per 28 mg of esketamine of $240, drawn from the Veterans Affairs Federal Supply Schedule (34); of note, this database tends to provide a lower-bound estimate for a medication’s cost (35). Per manufacturer recommendations, esketamine is given twice weekly for the first 4 weeks and weekly or every other week thereafter; the initial dose is 56 mg, with subsequent doses either 56 mg or 84 mg (4). We used observed dose and dose-frequency data from the TRANSFORM-2 and SUSTAIN-1 trials (4) to estimate the number of doses patients would receive in the first month and subsequent months, depending on depression health state (table in online supplement); multiplying by the above unit cost yielded monthly medication costs ranging from $5,572 for month 1 to $1,699 for patients in remission after month 1.
We assumed that patients would see a physician at each presentation for esketamine dosing; we used a cost of $71 per visit, based on reimbursement from the 2019 Centers for Medicare and Medicaid Services Physician Fee Schedule for a 15-minute office visit (CPT code 99213) (36). Finally, we assumed a medical assistant would supervise 4 patients per 2-hour dosing/monitoring period, resulting in a per-patient cost of $8 for 30 minutes of medical assistant time (37).
When performing analyses from the societal perspective, we included additional non-medical costs. We used severity-dependent productivity losses of 8.4 hours/week, 4.2 hours/week, and 1.5 hours/week for patients in the non-response, response, and remission states, derived from a national survey-based study of American workers with depression (38). We applied a cost of $26/hour to these productivity losses, based on average hourly earnings in the US (39). Finally, we assumed each esketamine dose would require 3 hours of patient time (including dosing, monitoring, and travel) with a cost of $26/hour (39).
Uncertainty and sensitivity analyses
We performed several types of sensitivity analysis to quantify the impact of modeling assumptions and uncertainty in model input parameters on our results.
In one-way sensitivity analyses, we varied the values of individual model parameters (or groups of parameters) and assessed their effect on our results. All parameters in Table 1 were included in one-way sensitivity analyses except for discount rate, relapse rate with esketamine, and esketamine costs, which were assessed in separate sensitivity analyses.
In probabilistic sensitivity analyses, the model was run using parameter values drawn at random from distributions reflecting their uncertainty (Table 1). Model results were compiled across 10,000 repetitions of this process in order to quantify the aggregate uncertainty in results attributable to joint uncertainty in the model’s input parameters.
Finally, in scenario sensitivity analyses, we assessed the effects of specific alternative modeling assumptions on our results. Scenarios included: excluding TRANSFORM-3 from our meta-analysis of esketamine efficacy due to the older patient population in this trial (figures in online supplement) (4); incorporating improved relapse rates with esketamine versus usual care, based on SUSTAIN-1, which reported relapse hazard ratios of 0.49 (remitters) and 0.30 (responders) (4); providing esketamine as 4th-line or 5th-line treatment, rather than 3rd-line; providing electroconvulsive therapy after failure of 3rd-line treatment (electroconvulsive therapy efficacy parameters were 50.9% remission, 66.6% response, and 3.0% relapse per month; monthly costs were $5,077 for the first month and $846 thereafter (13)); eliminating costs of physician visits, medical assistant time, and patient time from the total cost of esketamine to assess the effect of relaxing monitoring requirements.
RESULTS
Model validation
To assess the external validity of our model, we compared model output to independent estimates from the literature. We identified two relevant validation targets for clinical outcomes. In a treatment-resistant depression cohort in the United Kingdom followed prospectively for 8–84 months (mean 39 months), patients spent an average of 39.9% of time with uncontrolled symptoms (i.e. not in remission or response) (16); in contrast, in a US treatment-resistant depression cohort followed for 24 months, patients spent 87.8% of time with uncontrolled symptoms (15). Over 32 months of usual care, our model projected that patients spend 61.7% of time with uncontrolled symptoms.
Regarding economic outcomes, a 2018 systematic review identified 7 US-based studies on the costs of treatment-resistant depression; annual healthcare costs ranged from $12,000–19,000 (6). For comparison, over 24 months of usual care (the most common follow-up duration in the included studies), our model projected an annual healthcare cost of $15,949.
Five-year health and economic outcomes
Base case results are shown in Table 2. Over 5 years, esketamine was projected to increase the fraction of time patients are in remission from 25.3% to 31.1%; this translated to a gain of 0.07 QALYs. Total costs were projected to increase by $16,617 from the societal perspective and $16,995 from the healthcare sector perspective. This cost increase was driven largely by the cost of esketamine itself ($16,352), with smaller contributions from physician/medical assistant service costs ($2,062) and patient time costs ($2,074). Other healthcare costs and lost productivity costs both declined with esketamine.
Table 2:
Base case results
| Usual care | Esketamine | Difference | |
|---|---|---|---|
| Fraction of time spent in health state, percent | |||
| Initiation/non-response/relapse | 72.3 | 66.9 | −5.4 |
| Response | 2.4 | 2.0 | −0.4 |
| Remission | 25.3 | 31.1 | 5.7 |
| Quality-adjusted life-years | 3.00 | 3.07 | 0.07 |
| Costs, 2015 USD | |||
| Total cost, societal perspective | 121,073 | 137,690 | 16,617 |
| Total cost, healthcare sector perspective | 79,786 | 96,781 | 16,995 |
| Cost components | |||
| Esketamine treatment costs | |||
| Medication cost | – | 16,352 | 16,352 |
| Physician and medical assistant services | – | 2,062 | 2,062 |
| Patient time | – | 2,074 | 2,074 |
| Other healthcare costs | 79,786 | 78,367 | −1,419 |
| Lost productivity costs | 41,287 | 38,836 | −2,451 |
| Incremental cost-effectiveness ratios, $/QALY | |||
| Societal perspective | – | – | 237,111 |
| Healthcare sector perspective | – | – | 242,496 |
USD, United States dollars; QALY, quality-adjusted life-year
Esketamine was not cost-effective under either perspective, with projected ICERs of $237,111/QALY (societal) and $242,496/QALY (healthcare sector).
Uncertainty and sensitivity analyses
In one-way sensitivity analyses, esketamine’s ICER did not fall below $150,000/QALY with variation in any individual parameter (figure in online supplement). The lowest ICER attained with any parameter variation was $182,770/QALY (societal perspective), when applying the 95% CI upper limit estimates of relative risk of remission and response with esketamine versus usual care.
In probabilistic sensitivity analyses, the probability that esketamine is cost-effective was <0.01 at cost-effectiveness thresholds of $50,000/QALY and $100,000/QALY, regardless of perspective. At a threshold of $150,000/QALY, the probability that esketamine is cost-effective was 0.042 under a societal perspective and 0.021 under a healthcare sector perspective.
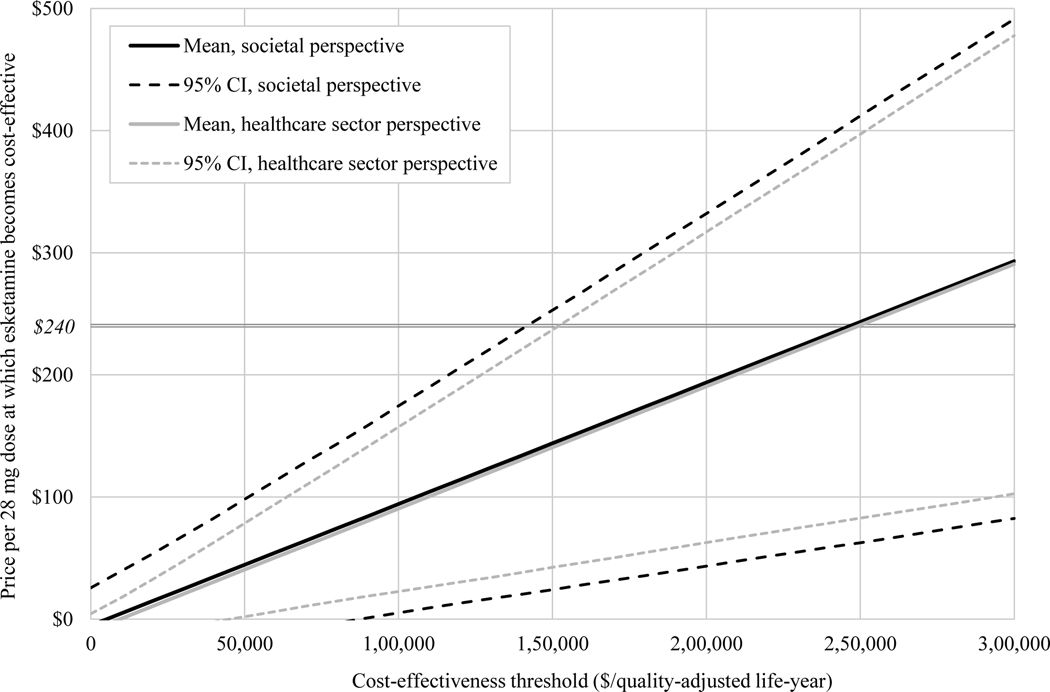
Value-based price estimates, along with confidence intervals based on probabilistic sensitivity analyses, are shown in Figure 1. To be cost-effective at a threshold of $150,000/QALY from the societal perspective, the per-dose price of esketamine would need to fall from its current value of $240 to a price of $144 or below (95% CI $24–253). From the healthcare sector perspective, the per-dose price of esketamine would need to fall to $141 or below (95% CI $42–237).
FIGURE 1. Value-based price estimates for esketaminea.
aCI, confidence interval. The horizontal axis indicates the cost-effectiveness threshold used to classify an intervention as cost-effective or not. Plotted curves show the price per 28-mg dose at which esketamine would achieve an incremental cost-effectiveness ratio equal to the threshold (that is, the value-based price) along with confidence intervals estimated via probabilistic sensitivity analysis. The horizontal line indicates the current price per 28-mg dose of esketamine ($240).
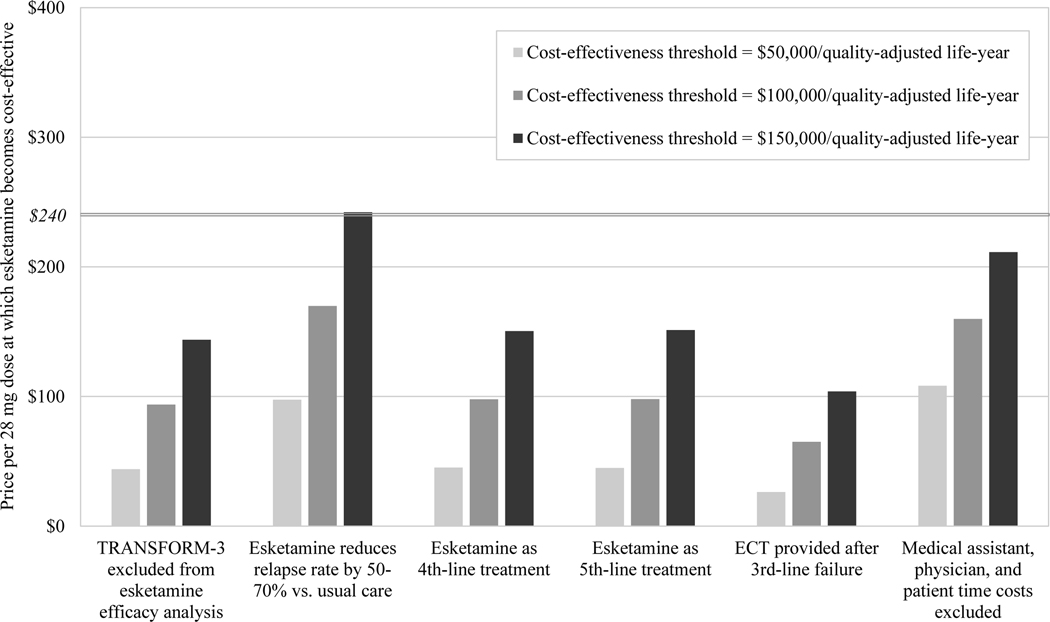
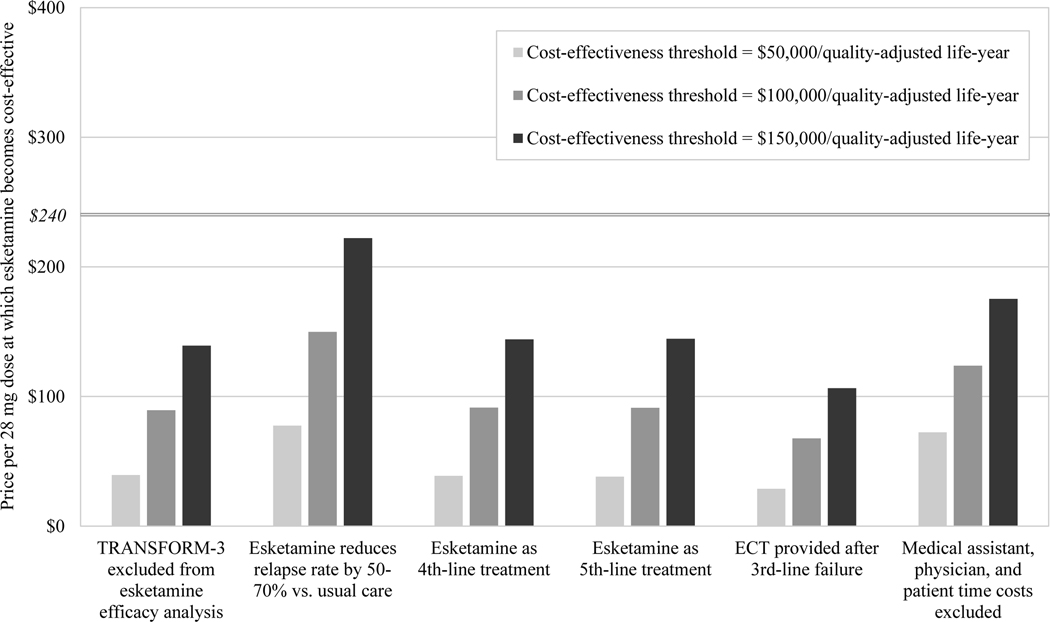
In scenario sensitivity analyses (Figure 2), esketamine required price reductions to become cost-effective under all scenarios except one. At a cost-effectiveness threshold of $150,000/QALY from the societal perspective, esketamine’s value-based price was $244 when we simulated 50–70% reductions in relapse rate with esketamine versus usual care.
FIGURE 2. Scenario sensitivity analyses for the value-based price of esketamine, from (A) the societal perspective and (B) the health care sector perspectivea.
aECT, electroconvulsive therapy. The vertical axis indicates the value-based price of esketamine, that is, the price per 28-mg dose below which esketamine would be deemed cost-effective at a given cost-effectiveness threshold. Bars indicate value-based prices at three alternative cost-effectiveness thresholds. For comparison, the horizontal line indicates the current price per 28-mg dose of esketamine ($240). The horizontal axis shows six scenarios simulated in sensitivity analysis, consisting of alternative modeling and input parameter assumptions related to treatment efficacy, treatment costs, and alternative treatments.
DISCUSSION
In this decision-analytic modeling analysis incorporating recently-released phase III clinical trial data (4), we found that esketamine nasal spray is unlikely to be a cost-effective treatment for patients with treatment-resistant depression in the United States at a cost of ~$240 per dose. Our model projected base case ICERs of $237,111/QALY (societal perspective) and $242,496/QALY (healthcare sector perspective) for esketamine compared to usual care. In uncertainty analysis, we found a >95% likelihood that esketamine’s ICER would fall above $150,000/QALY, a commonly applied upper bound for defining cost-effective medical interventions in the United States (18). To become cost-effective, we estimated that esketamine would require a price reduction of >40% from its current price of ~$240 per 28 mg dose (to ~$140 or less per dose) (34).
Our results were consistent across a wide range of deterministic and probabilistic sensitivity analyses. Of note, we did identify a single scenario under which esketamine’s ICER was below $150,000/QALY: when we assumed 50–70% reductions in depression relapse rate with esketamine versus usual care. However, as described in our Methods section, this assumption represents an inappropriate application of findings from the SUSTAIN-1 trial (4) and was included only as an exploratory analysis. Under all other sensitivity analyses, including optimistic assumptions about esketamine’s cost and efficacy, we found that esketamine cannot be considered cost-effective.
Two prior studies have evaluated the cost-effectiveness of esketamine. An evidence report released by the Institute for Clinical and Economic Review estimated esketamine’s ICER to be $198,000/QALY (40), consistent with our finding that esketamine is unlikely to be cost-effective. In contrast, an industry-supported conference abstract reported an ICER below $100,000/QALY – a more precise value was not provided in the abstract (41).
From a health policy perspective, esketamine’s current status – as a clinically effective medication that is priced well above its value-based price – is far from unique (11). For example, in a recent assessment of the 50 drugs accounting for the greatest New York state Medicaid spending, 5 had value-based price estimates; of these, 2 were priced at or below their value-based price, and the remaining 3 required discounts of up to 54% to reach their value-based price (12). Still, targeted efforts by payers and policymakers can be effective in reducing prices, even for drugs with market exclusivity. In the recent case of evolocumab and alirocumab, two novel lipid-lowering agents approved by the FDA in 2015 and initially priced at ~$14,000/year, insurers’ efforts at limiting access via restrictive prior authorizations and copayment garnered 60% reductions in the medications’ prices by 2018 (42). Subsequent analyses showed that the medications now meet cost-effectiveness criteria (42, 43). However, these price reductions came at the expense of curtailing use of these medications: prior to the price reductions, <1% of eligible patients received prescriptions for these medications (44), and a third of patients abandoned their prescriptions when confronted with the copay amount (45). In the case of esketamine, we’re hopeful that policymakers and insurers can use positive incentives and proactive negotiation to establish fair prices without making the drug inaccessible to patients with treatment-resistant depression who could benefit from it (11, 12, 46, 47).
For individual patients and providers, the cost of esketamine (in terms of out-of-pocket expenses, patient time, or costs to the healthcare system as a whole) is likely to be an important component of shared decision-making (48). Fortunately, there are multiple alternative options for treatment-resistant depression that have been shown to be cost-effective. Until esketamine’s price falls, we would recommend considering electroconvulsive therapy (13), antipsychotic augmentation (49), or cognitive-behavioral therapy (50, 51).
Our results should be interpreted in the context of several limitations. It’s important to recognize that the data used to simulate esketamine reflect samples of a few hundred clinical trial participants (4); longer-term observational data or additional randomized trials could refine our cost-effectiveness estimates. In light of these limitations in input data, our model employs a 5-year time horizon, which may fail to capture relevant longer-term health-economic consequences; reassuringly though, cost-effectiveness outcomes were robust to variation in time horizon. Next, there are several limitations to the data drawn from other sources. In instances where inadequate data were available from patients with treatment-resistant depression, we have derived model inputs from the overall population with major depressive disorder; this may result in underestimating the degree of occupational impairment and mortality in treatment-resistant individuals (52, 53). Our utility estimates for remission, response, and non-response were drawn from a French study and thus may be less applicable to the United States (30); reassuringly, though, these utility estimates are consistent with those obtained from an international meta-analysis (54). And our estimates of depression severity-dependent productivity losses are not disaggregated by gender, which may lead to some inaccuracy given the overrepresentation of women among people with major depressive disorder (25, 38). Finally, in the absence of comparative outcomes data, we were unable to directly assess esketamine’s cost-effectiveness versus alternatives such as electroconvulsive therapy or transcranial magnetic stimulation; however, indirect comparison in scenario sensitivity analysis revealed that our findings were unchanged when electroconvulsive therapy was included as a comparator.
Conclusions
In this decision-analytic modeling analysis, we found that esketamine is unlikely to be cost-effective for management of treatment-resistant depression at its current price of $240 per dose. Esketamine could become cost-effective by US standards if its price were to fall to $140 or less per dose. Achieving these price reductions while ensuring continued access for the patients who stand to benefit from esketamine will require careful and concerted efforts from payers and policymakers.
Supplementary Material
Highlights:
On the basis of several phase III randomized controlled trials demonstrating its efficacy, esketamine nasal spray was recently approved by the United States Food and Drug Administration for management of treatment-resistant depression.
Using data from these trials and a decision analytic model, the authors estimated a greater than 95% likelihood that intranasal esketamine would not be cost-effective by commonly applied United States standards.
To become cost-effective, esketamine’s price would need to fall to $140 or less per dose (a greater than 40% decline from its current price of approximately $240 per dose).
ACKNOWLEDGMENTS
Role of the funder/sponsor:
The National Institute of Mental Health did not participate in the design of the study, the analysis and interpretation of the data, or in the preparation and submission of the manuscript for publication.
Funding/support: This was study was supported by the National Institute of Mental Health (Research Training and Career Development R25 MH094612, to ELR).
Footnotes
Conflict of interest disclosures: ELR and DIS report no conflicts of interest.
REFERENCES
- 1.Kim J, Farchione T, Potter A, et al. : Esketamine for treatment-resistant depression - first FDA-approved antidepressant in a new class. New England Journal of Medicine 2019; 381:1–4 [DOI] [PubMed] [Google Scholar]
- 2.Dubovsky SL: What is new about new antidepressants? Psychotherapy and Psychosomatics 2018; 87:129–139 [DOI] [PubMed] [Google Scholar]
- 3.Canuso CM, Singh JB, Fedgchin M, et al. : Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. American Journal of Psychiatry 2018; 175:620–630 [DOI] [PubMed] [Google Scholar]
- 4.Janssen Research and Development: Advisory committee briefing document: esketamine nasal spray for patients with treatment-resistant depression [Internet]. 2019[cited 2019 Sep 6] Available from: https://www.fda.gov/media/121377/download
- 5.Conway CR, George MS, Sackeim HA: Toward an evidence-based, operational definition of treatment-resistant depression: when enough is enough. JAMA Psychiatry 2017; 74:9–10 [DOI] [PubMed] [Google Scholar]
- 6.Johnston KM, Powell LC, Anderson IM, et al. : The burden of treatment-resistant depression: a systematic review of the economic and quality of life literature. Journal of Affective Disorders 2019; 242:195–210 [DOI] [PubMed] [Google Scholar]
- 7.Rush AJ, Trivedi MH, Wisniewski SR, et al. : Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. American Journal of Psychiatry 2006; 163:1905–1917 [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson ST, Howard DH, Busch SH: Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA 2019; 1–2 [DOI] [PubMed] [Google Scholar]
- 9.Treviño LA, Ruble MW, Treviño K, et al. : Antidepressant medication prescribing practices for treatment of major depressive disorder. Psychiatric Services 2017; 68:199–202 [DOI] [PubMed] [Google Scholar]
- 10.Ross EL, Vijan S, Miller EM, et al. : The Cost-Effectiveness of Cognitive Behavioral Therapy Versus Second-Generation Antidepressants for Initial Treatment of Major Depressive Disorder in the United States: A Decision Analytic Model. [Internet]. Annals of internal medicine 2019; Available from: http://www.ncbi.nlm.nih.gov/pubmed/31658472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kesselheim AS, Avorn J, Sarpatwari A: The high cost of prescription drugs in the United States origins and prospects for reform. JAMA 2016; 316:858–871 [DOI] [PubMed] [Google Scholar]
- 12.Hwang TJ, Kesselheim AS, Sarpatwari A: Value-based pricing and state reform of prescription drug costs. JAMA 2017; 318:609–610 [DOI] [PubMed] [Google Scholar]
- 13.Ross EL, Zivin K, Maixner DF: Cost-effectiveness of electroconvulsive therapy vs pharmacotherapy/psychotherapy for treatment-resistant depression in the United States. JAMA Psychiatry 2018; 75:713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popova V, Daly EJ, Trivedi M, et al. : Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. American Journal of Psychiatry 2019; 176:428–438 [DOI] [PubMed] [Google Scholar]
- 15.Dunner DL, Rush AJ, Russell JM, et al. : Prospective, long-term, multicenter study of the naturalistic outcomes of patients with treatment-resistant depression. Journal of Clinical Psychiatry 2006; 67:688–695 [DOI] [PubMed] [Google Scholar]
- 16.Fekadu A, Rane LJ, Wooderson SC, et al. : Prediction of longer-term outcome of treatment-resistant depression in tertiary care. British Journal of Psychiatry 2012; 201:369–375 [DOI] [PubMed] [Google Scholar]
- 17.Sanders GD, Neumann PJ, Basu A, et al. : Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016; 316:1093–1103 [DOI] [PubMed] [Google Scholar]
- 18.Anderson JL, Heidenreich PA, Barnett PG, et al. : ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Journal of the American College of Cardiology 2014; 63:2304–2322 [DOI] [PubMed] [Google Scholar]
- 19.Neumann PJ, Cohen JT, Weinstein MC: Updating cost-effectiveness - the curious resilience of the $50,000-per-QALY threshold. New England Journal of Medicine 2014; 371:796–797 [DOI] [PubMed] [Google Scholar]
- 20.Kaltenboeck A, Bach PB: Value-based pricing for drugs: theme and variations. JAMA 2018; 319:2165–2166 [DOI] [PubMed] [Google Scholar]
- 21.Husereau D, Drummond M, Petrou S, et al. : Consolidated health economic evaluation reporting standards (CHEERS) - explanation and elaboration: a report of the ISPOR Health Economic Evaluations Publication Good Reporting Practices Task Force. Value in Health 2013; 16:231–250 [DOI] [PubMed] [Google Scholar]
- 22.Wallace BC, Dahabreh IJ, Trikalinos TA, et al. : Closing the gap between methodologists and end-users: R as a computational back-end. Journal of Statistical Software 2012; 49:1–15 [Google Scholar]
- 23.Kennedy SH, Lam RW, McIntyre RS, et al. : Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 3. Pharmacological treatments. Canadian Journal of Psychiatry 2016; 61:540–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McIntyre RS, Suppes T, Tandon R, et al. : Florida best practice psychotherapeutic medication guidelines for adults with major depressive disorder. Journal of Clinical Psychiatry 2017; 78:703–13 [DOI] [PubMed] [Google Scholar]
- 25.Amos TB, Tandon N, Lefebvre P, et al. : Direct and indirect cost burden and change of employment status in treatment-resistant depression: a matched-cohort study using a US commercial claims database. Journal of Clinical Psychiatry 2018; 79:1–9 [DOI] [PubMed] [Google Scholar]
- 26.Arias E, Heron M, Xu J: United States life tables, 2013. National Vital Statistics Reports 2017; 66:1–64 [PubMed] [Google Scholar]
- 27.Cuijpers P, Vogelzangs N, Twisk J, et al. : Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. American Journal of Psychiatry 2014; 171:453–462 [DOI] [PubMed] [Google Scholar]
- 28.Weinmann S, Becker T, Koesters M: Re-evaluation of the efficacy and tolerability of venlafaxine vs SSRI: meta-analysis. Psychopharmacology 2008; 196:511–520 [DOI] [PubMed] [Google Scholar]
- 29.Papakostas GI, Homberger CH, Fava M: A meta-analysis of clinical trials comparing mirtazapine with selective serotonin reuptake inhibitors for the treatment of major depressive disorder. Journal of Psychopharmacology 2008; 22:843–848 [DOI] [PubMed] [Google Scholar]
- 30.Sapin C, Fantino B, Nowicki M-L, et al. : Usefulness of EQ-5D in assessing health status in primary care patients with major depressive disorder. Health and Quality of Life Outcomes 2004; 2:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn A, Grosse SD, Zuvekas SH: Adjusting health expenditures for inflation: a review of measures for health services research in the United States. Health Services Research 2018; 53:175–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bureau of Economic Analysis: Medical care expenditure indices from MEPS [Internet]. 2019[cited 2019 Sep 5] Available from: https://www.bea.gov/national/health_care_satellite_account.htm
- 33.Federal Reserve Bank of St. Louis: Personal Consumption Expenditures [Internet]. 2019[cited 2019 Aug 19] Available from: https://fred.stlouisfed.org/series/DPCERG3A086NBEA
- 34.Veterans Affairs Office of Procurement, Acquisition and L: Veterans Affairs Federal Supply Schedule pharmaceutical pricing data [Internet]. 2019[cited 2019 Sep 6] Available from: https://www.va.gov/opal/nac/fss/pharmPrices.asp
- 35.Levy J, Rosenberg M, Vanness D: A transparent and consistent approach to assess US outpatient drug costs for use in cost-effectiveness analyses. Value in Health 2018; 21:677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Medicare and Medicaid Services: Physician Fee Schedule [Internet]. 2019[cited 2019 Sep 5] Available from: https://www.cms.gov/apps/physician-fee-schedule/
- 37.Bureau of Labor Statistics: Occupational outlook handbook, medical assistants [Internet]. 2019[cited 2019 Sep 6] Available from: https://www.bls.gov/ooh/healthcare/medical-assistants.htm
- 38.Stewart WF, Ricci JA, Chee E, et al. : Cost of lost productive work time among US workers with depression. JAMA 2003; 289:3135–3144 [DOI] [PubMed] [Google Scholar]
- 39.Bureau of Labor Statistics: Current and real (constant 1982–1984 dollars) earnings for all employees on private nonfarm payrolls, seasonally adjusted [Internet]. 2019[cited 2019 Sep 6] Available from: https://www.bls.gov/news.release/realer.t01.htm
- 40.Atlas S, Agboola F, Fazioli K, et al. : Esketamine for the treatment of treatment-resistant depression: effectiveness and value [Internet]. 2019[cited 2019 Sep 9] Available from: https://icer-review.org/material/trd-final-evidence-report-and-meeting-summary/ [Google Scholar]
- 41.Hernandez LG, Li S, Toro-Diaz H, et al. : Cost-effectiveness analysis of esketamine in treatment-resistant depression in the United States. In: 24th Annual International Meeting of the International Society for Pharmacoeconomics and Outcomes Research 2019; 18 May [Google Scholar]
- 42.Bergethon KE, Wasfy JH: Increasing the adoption and diffusion of a novel pharmacological therapy that is both mortality reducing and cost-effective. Journal of the American Heart Association 2019; 8:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fonarow GC, van Hout B, Villa G, et al. : Updated cost-effectiveness analysis of evolocumab in patients with very high-risk atherosclerotic cardiovascular disease. JAMA Cardiology 2019; 4:691–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karalis DG, Mallya UG, Ghannam AF, et al. : Prescribing patterns of proprotein convertase subtilisin-kexin type 9 inhibitors in eligible patients with clinical atherosclerotic cardiovascular disease or heterozygous familial hypercholesterolemia. American Journal of Cardiology 2018; 121:1155–1161 [DOI] [PubMed] [Google Scholar]
- 45.Navar AM, Taylor B, Mulder H, et al. : Association of prior authorization and out-of-pocket costs with patient access to PCSK9 inhibitor therapy. JAMA Cardiology 2017; 2:1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frank RG: Changing Medicare’s protected classes: history, mistrust, and budgets in the prescription drug market. Psychiatric Services 2019; 1–2 [DOI] [PubMed] [Google Scholar]
- 47.Bach PB, Pearson SD: Payer and policy maker steps to support value-based pricing for drugs. JAMA 2015; 314:2503–2504 [DOI] [PubMed] [Google Scholar]
- 48.Brown GD, Hunter WG, Hesson A, et al. : Discussing out-of-pocket expenses during clinical appointments: an observational study of patient-psychiatrist interactions. Psychiatric Services 2017; 68:610–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoon J, Zisook S, Park A, et al. : Comparing cost-effectiveness of aripiprazole augmentation with other “next-step” depression treatment strategies: a randomized clinical trial. Journal of Clinical Psychiatry 2018; 80:1–8 [DOI] [PubMed] [Google Scholar]
- 50.Wiles NJ, Thomas L, Turner N, et al. : Long-term effectiveness and cost-effectiveness of cognitive behavioural therapy as an adjunct to pharmacotherapy for treatment-resistant depression in primary care: follow-up of the CoBalT randomised controlled trial. The Lancet Psychiatry 2016; 3:137–144 [DOI] [PubMed] [Google Scholar]
- 51.Brettschneider C, Djadran H, Härter M, et al. : Cost-utility analyses of cognitive-behavioural therapy of depression: a systematic review. Psychotherapy and Psychosomatics 2015; 84:6–21 [DOI] [PubMed] [Google Scholar]
- 52.Li G, Fife D, Wang G, et al. : All-cause mortality in patients with treatment-resistant depression: A cohort study in the US population [Internet]. Annals of General Psychiatry 2019; 18:1–8Available from: 10.1186/s12991-019-0248-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rizvi SJ, Grima E, Tan M, et al. : Treatment-resistant depression in primary care across Canada. Canadian Journal of Psychiatry 2014; 59:349–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mrazek DA, Hornberger JC, Altar CA, et al. : A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatric Services 2014; 65:977–87 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.