Abstract
Background:
Emerging evidence suggests that opioid receptor antagonists, such as naltrexone, are effective pharmacotherapies for alcohol, opioid, and possibly stimulant use disorders. It is posited that naltrexone exerts its effects, in part, by increasing functional connectivity between neural reward circuitry and frontal systems implicated in executive function. Yet no studies had examined whether executive function moderates these effects.
Objectives:
This study examined whether a composite measure of executive function (EF) moderates the effect of naltrexone on craving for methamphetamine and subjective responses following infusion of the drug.
Methods:
Individuals with methamphetamine use disorder (N=30; 27% Female) completed baseline neurocognitive assessments of premorbid and executive function, and an executive function factor was computed. Participants then underwent a randomized, double-blind, cross-over study of titration with naltrexone and placebo. Participants then received a 30-mg intravenous methamphetamine infusion and completed subjective response questionnaires at 8 times in the 120 minutes post-infusion.
Results:
Multilevel mixed models indicated a significant EF × medication interaction, reflecting greater effects of naltrexone to decrease “desire to access the drug”, “want more of the drug”, “crave the drug”, “feel drug effects” and “feel high” in participants with low EF compared to those with high EF (Bs = .36–1.29, SEs = .14–.17, ps<0.01). These effects remained significant after controlling for premorbid cognitive functioning, baseline responses to methamphetamine, severity of methamphetamine use, and methamphetamine-related functional problems.
Conclusion:
Naltrexone may be especially effective in methamphetamine-dependent individuals with low EF. Neuropsychological assessments may also provide predictive clinical utility not captured by traditional measures of substance use severity.
Keywords: methamphetamine, executive function, naltrexone, craving, substance use disorder
Introduction
Methamphetamine use disorder remains a persistent problem in the United States, with approximately 1 million individuals over the age of 12 meeting criteria for this diagnosis in 2017 (1). Methamphetamine trafficking and abuse have also increased exponentially in East and Southeast Asia in recent years (2). In the context of these trends, and lack of an FDA-approved medication for methamphetamine use disorder, an effective methamphetamine addiction treatment is critically needed.
One promising medication for methamphetamine use disorder is naltrexone, a competitive mu-opioid receptor antagonist that has demonstrated success in moderating responses to substances of abuse and in treating alcohol and opioid use disorders in clinical trials (3–5). It is posited that naltrexone reduces GABAergic inhibition of midbrain dopaminergic neurons, thereby attenuating subjective stimulatory effects of, and craving for, substances that act upon striatolimbic circuitry (6). As cue-induced craving and subjective effects of drugs of abuse are predictive of relapse across drug classes (7, 8), laboratory studies have correspondingly utilized controlled administration paradigms to examine effects of medications on craving and subjective response to methamphetamine. In studies of non-treatment-seeking research participants, naltrexone reduced cue-induced craving for methamphetamine, and subjective effects of methamphetamine and dexamphetamine in standardized administration paradigms among adults with methamphetamine use disorder (9–11). The few clinical trials that have been conducted with individuals with methamphetamine use disorder, however, have produced mixed results; in one placebo-controlled study, extended-release naltrexone significantly reduced methamphetamine use over 3 weeks post-injection (6); another trial found no difference between the effects of extended-release naltrexone and placebo on methamphetamine use over 12 weeks (12).
In light of these discrepancies, there are growing efforts to identify those individuals who would benefit most from naltrexone treatment, thereby optimizing treatment response and improving treatment pipeline efficiency (13–15). Additionally, investigation in identifying responders to treatments may be informative; for instance, in a study of bupropion for methamphetamine use, responders were individuals who were more adherent and used methamphetamine less than once per day at baseline (16). For naltrexone, a meta-analysis examining pharmacogenetic effects of naltrexone and OPRM1 genotype for alcohol use disorder treatment, found that carriers of the G allele of the A118G polymorphism of OPRM1 may exhibit better recovery outcomes when treated with naltrexone compared to noncarriers (17). A more recent meta-analysis, however, found wide confidence intervals and significant variability in these pharmacogenetic effects across studies (18). Elucidation of other factors in these personalized medicine efforts is warranted, despite the lack of FDA approval for naltrexone as a treatment for methamphetamine use disorder.
One such factor may be executive function, a multidimensional construct that incorporates complex higher-level reasoning, response-inhibition, and cognitive flexibility, which collectively allow for planning, initiating, and monitoring complex goal-directed behaviors (19). While such diverse functions are correlated with activity in multiple brain circuits, frontal and prefrontal areas are most commonly found to underlie performance in executive function tasks (20). For populations with either alcohol use disorder or methamphetamine use disorder, naltrexone has been shown to increase functional connectivity between striatum and frontal/prefrontal areas during presentation of drug cues (21–23). Frontostriatal connectivity during reward anticipation among alcohol-dependent individuals is negatively associated with alcohol craving (24). The capacity for response-inhibition correlates negatively with amphetamine-induced euphoria and stimulation (25). Beyond this link between a measure of executive function and response to a stimulant, it is notable that gray matter integrity in a prefrontal region, specifically the right inferior frontal gyrus is related both to capacity for response inhibition and to spontaneous methamphetamine craving, which are negatively correlated with one another (26). Given these associations, it is possible that effects of naltrexone on subjective responses and craving induced by methamphetamine may be related to or involve executive function. Yet no human studies have tested whether behavioral assessments of executive function predict or moderate the effects of naltrexone on laboratory subjective response/craving paradigms.
Neurocognitive batteries may be useful in such an inquiry; the Delis-Kaplan Executive Function System (DKEFS; (27) measures multiple aspects of executive function, exhibits high external validity (28), has been co-normed on a large, representative national sample (29, 30), and is used in both research and clinical settings for myriad psychological and medical disorders (31–33). Regarding its clinical specificity, impaired DKEFS scores have been shown to be associated with damage within a distributed network of lateral prefrontal and parietal cortices and white-matter association tracts in individuals with traumatic brain injuries (34). DKEFS test performance has also been used to differentiate individuals with frontal and temporal lobe epilepsy (35). Among methamphetamine users, DKEFS and neurocognitive batteries have been used to examine potential neuropsychological effects of methamphetamine use. One meta-analysis identified neurocognitive deficits in methamphetamine users relative to controls, with the largest weaknesses in learning, executive function, memory, and processing speed (36). Overall, while there is a likely a need for better matched healthy controls for comparison (37), other meta-analyses of these assessments have provided evidence that premorbid weaknesses in cognition, such as impulsivity, are a risk factor for subsequent methamphetamine use. Further, chronic methamphetamine use is associated with neurocognitive impairment across multiple domains in animals and in humans, including executive function, attention, processing speed, verbal learning and memory, and social cognition (30, 38, 39).
While neuroimaging studies have increasingly studied the effects of naltrexone on connectivity and response to substance cues, there is a relatively sparse literature on the effects of naltrexone on general cognition, particularly in humans. In rats and mice, naltrexone has been shown to improve working memory performance (40), reverse age-associated set-shifting deficits (41), as well as mixed results in reversing deficits in working memory induced by microwave exposure (42, 43). Naltrexone also demonstrates mixed results in improving inhibitory control in delay discounting and gambling tasks in animals (42, 44). In humans, abstinent heroin users treated with naltrexone demonstrated similar performance to healthy controls in tests of cognitive flexibility, verbal memory, and visual perception (45). In clinical samples of individuals with schizophrenia or at risk for Alzheimer’s disease, as well as among a sample of overweight men, naltrexone had no effect on cognitive functioning in any assessed domain (46–48).
Within the context of this literature, this study examined whether an index of executive function moderates the effects of naltrexone on methamphetamine-induced subjective response/craving in a non-treatment-seeking sample of methamphetamine users. This assessment tapped several dimensions of executive functioning, including inhibition, planning, and set-switching. In this secondary analysis of a naltrexone experimental study (9), we hypothesized that naltrexone would attenuate methamphetamine-induced stimulation and craving to a greater extent in individuals with high executive function as compared with their counterparts with low executive function. Consistent with a review identifying that executive function deficits are associated with addiction treatment failure, we anticipated that naltrexone might not reduce drug-induced effects for individuals with potentially greater dysregulated frontostriatal circuitry.
Material and Methods
Participants
All study procedures were approved by the local medical institutional review board. Participants in this study were non-treatment-seeking methamphetamine users recruited through online and print advertisements in the greater Los Angeles area. As indicated in the parent study (9), inclusion criteria were: (a) meeting current DSM-IV diagnostic criteria for methamphetamine abuse or dependence; (b) fluency in English; (c) age between 18 and 50 years; (d) methamphetamine use verified by urine toxicology at a screening visit; (e) agreement to abstain from methamphetamine use during the study, indicated by methamphetamine-negative urine at the start of each inpatient admission and every morning during their stay. Exclusion criteria were: (a) current desire for treatment for methamphetamine use; (b) meeting DSM-IV diagnostic criteria for current (past 12 months) dependence on a drug other than methamphetamine or nicotine, lifetime diagnosis of schizophrenia, bipolar disorder, or any psychotic disorder, or current major depressive disorder with suicidal ideation; (c) current use of psychoactive drugs other than marijuana, nicotine, or methamphetamine, bioverified through urine toxicology; (d) having significant medical problems (i.e. failing physical examination, abnormal blood chemistry panel and/or liver profile, cardiovascular abnormalities in electrocardiogram or vital signs (HR<50 or >90, SBP<105 or >140, DBP<45 or >90); (e) current use of medications that could interact adversely with naltrexone; (f) testing positive for pregnancy, currently nursing, or refusing to use reliable methods of birth control; (g) reporting the intranasal route as the sole method of methamphetamine self-administration.
A total of 126 individuals (74% male) were eligible after the phone screening interview and completed an in-person screening visit. Of these, 46 individuals attended and completed the subsequent medical screening visit with the study physician. Reasons for attrition from initial laboratory screening to medical screening include (a) participant dropout (n=32), (b) methamphetamine-negative urine at initial laboratory screening (n=18), (c) positive urine for exclusionary substances at initial laboratory screening (n=7), and (d) not meeting diagnostic criteria for methamphetamine abuse/dependence, or meeting criteria for other exclusionary psychological disorders (n=14). Of the 46 participants after medical screening, 32 completed at least one experimental session. Thirty participants completed both sessions (placebo and naltrexone) and were included in the current study’s analyses.
Screening and Neurocognitive Testing Procedures
Interested individuals completed an initial telephone interview, and those eligible after the interview came into the laboratory for an in-person eligibility visit. During this visit, they completed informed consent procedures, a Breathalyzer test for breath alcohol concentration, provided a urine sample for methamphetamine bioverification, and underwent a structured clinical interview for the DSM-IV (49) to assess methamphetamine abuse/dependence, and to determine age of first methamphetamine use and regular use, and other exclusionary diagnoses. Individuals also completed questionnaires assessing demographics, psychological functioning, and medical and psychiatric history. Those eligible after this initial screening visit returned to the laboratory 4–7 days later to complete a physical exam with the study physician. To reduce potential methamphetamine effects on neurocognitive performance, individuals were required to remain methamphetamine-abstinent during this 4–7 day period, bioverified with urine toxicology. After the physical exam, participants completed a neuropsychological battery lasting approximately 2 hours and including the following: assessments for premorbid functioning (Test of Premorbid Functioning; 50) and selected tests of executive function (DKEFS – Trail Making Test, Color-Word Inhibition, and Tower tests; 27) administered by master’s-level clinicians who were supervised by a licensed psychologist. The Trail Making and Color-Word Inhibition-Switching Tests assessed mental set switching, Color-Word Inhibition assessed inhibition of automatic responses, and Tower Test assessed planning and inhibition of impulsive and perseverative responding. A study procedures flowchart is provided in Figure 1.
Figure 1.
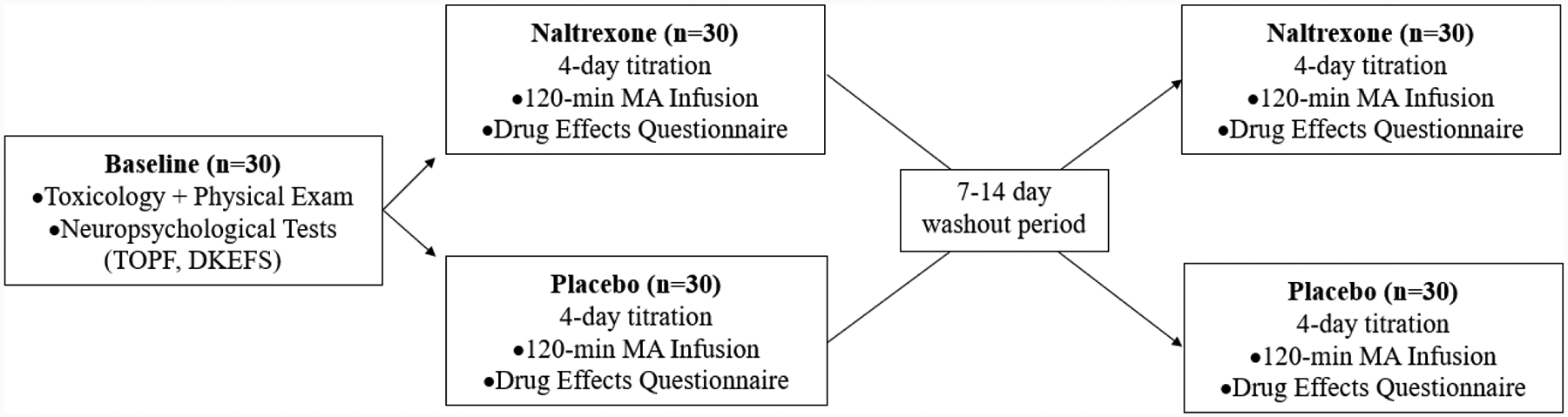
Study procedures
Medication Titration and Inpatient Procedures
Inpatient procedures followed a double-blind, randomized, counterbalanced crossover design (9). Participants eligible after the medical screening were then admitted to the UCLA Clinical and Translational Research Center (CTRC) inpatient unit. They received the first dose of their randomized study medication (either naltrexone or placebo), and took this assigned medication for 4 days under staff supervision. The naltrexone dosages were titrated to minimize adverse events, such that participants received 25 mg on day 1, and 50 mg on days 2–4; this 4-day titration period has previously been used to examine effects of naltrexone in experimental studies (21, 51). The first experimental session on medication day 4 included an intravenous methamphetamine administration paradigm that occurred 2 hours after their final study medication dose. On day 5, participants were discharged from the unit. After a 7–14-day washout period, participants were re-admitted to the inpatient unit to complete these study procedures for the second study medication (naltrexone or placebo). After participants completed the experimental paradigm on day 4 and prior to final discharge, master’s-level clinicians under the supervision of a licensed psychologist conducted a motivational interview to promote reduction in methamphetamine use and treatment seeking. Participants were compensated $50 for completing each of the screening visits, $40 for each inpatient day ($400 total) and $50 for each of the two experimental sessions. Participants who attended all study sessions also received a $100 bonus.
Methamphetamine Administration
On day 4 of medication titration, participants completed an intravenous methamphetamine paradigm. They received two 15-mg intravenous methamphetamine infusions; each lasting 2 minutes and separated by 30 minutes, for a total dose of 30 mg methamphetamine. This procedure and dosages were selected from previous pharmacology studies demonstrating safety and efficacy in inducing subjective effects (52, 53). Participants were continuously monitored by CTRC nursing staff and the study physician throughout the paradigm, including cardiac telemetry, serial EKG, and vital signs. Subjective responses to, and craving for, methamphetamine were assessed before methamphetamine administration (baseline) and at 5, 10, 15, 20, 30, 60, 90, and 120 minutes after the second 15-mg methamphetamine infusion. Subjective responses and craving were measured using the Drug Effects Questionnaire (DEQ; 54), which queries the extent to which participants experience substance effect(s), like/dislike the effects, and want more of the substance, using a Likert scale from 0 (none at all) to 10 (a lot).
Analytic Plan
DKEFS Trail Making Test, Color-Word Inhibition, and Tower test scores were calculated as scaled scores using age-corrected norms (27). To capture the shared variance between the multiple EF tests, a principal components analysis with varimax rotation was conducted on the initial screening visit sample data for the following: Color-Word Inhibition, Color-Word Inhibition-Switching, Trail Making Test Switching, and Tower Test age-corrected scaled scores; there is evidence that factor analysis with DKEFS tests that exclude verbal fluency produce a 1- or 2-factor solution (29).
Consistent with previous studies (55, 56) and to reduce the number of covariates to be examined in primary analyses, a principal components analysis with varimax rotation was conducted on the initial screening visit sample data including methamphetamine-related variables of interest: (a) DSM-IV methamphetamine abuse/dependence symptom count (55); (b) Methamphetamine Urge Questionnaire (MAUQ) total score (9); (c) Methamphetamine Withdrawal Questionnaire (MAWQ) total score (57); (d) number of years of regular methamphetamine use; (e) age of first methamphetamine use; and (f) methamphetamine use days in the past 30 days via the Timeline Follow-Back days (55, 58).
Multilevel mixed modeling analyses in SAS version 9.4 were used to examine whether executive function moderated naltrexone effects on methamphetamine-induced subjective response and craving. Linear mixed models with random intercepts were conducted, with medication (naltrexone and placebo) and time (throughout the 120 minutes) as within-subject factors, EF factor (DKEFS factor score) as a between-subject factor, and their interactions. The outcome variables were separate DEQ items, including items related to subjective drug effects (i.e. “Feel drug effects”; “Feel high”; “Like effects”; “Feel stimulated”;) and items related to craving (i.e. “Would like more drug”; “Crave more drug”; and “Want to access drug”). As baseline subjective responses prior to administration are often distinct from subjective response induced by a drug, baseline DEQ responses were included in the models as covariates (21, 59). Other examined covariates included two generated methamphetamine-related factor scores, nicotine and alcohol use disorder severity (to account for potential effects of commonly co-used substances), bioverified cannabis use status (at baseline toxicology test), and depression and anxiety symptoms. To ensure that potential executive function effects were not driven by differences in basic neurocognitive functioning, models also covaried for premorbid functioning (Test of Premorbid Functioning (TOPF); 40) and simple attention (DKEFS Trail Making Test – Visual Scanning).
Results
Characteristics for the final sample of 30 participants are indicated in Table 1. The majority of participants were Latino and/or Caucasian men who met criteria for methamphetamine Dependence (DSM-IV), attained a high school education, and reported recent cigarette smoking and/or alcohol use. All participants reported smoking as their primary methamphetamine administration method. Notably, converting the DSM-IV criteria to DSM-5 (excluding the craving symptom, which was not assessed), all participants met criteria for current DSM-5 (60) methamphetamine use disorder at the time of the assessment.
Table 1.
Sample characteristics (N = 30)
| Variable | Statistic (M(SD)) |
|---|---|
| Age | 36.93 (8.78) |
| Sex (% Female) | 26.7% |
| Ethnicity (n(%)) | |
| Latin-X | 10 (33%) |
| Caucasian | 9 (30%) |
| Bi/Multiracial | 5 (17%) |
| African-American | 4 (13%) |
| Asian/Pacific-Islander | 2 (7%) |
| Years of Education | 12.73 (2.86) |
| BAI | 8.17 (12.67) |
| BDI | 11.43 (11.60) |
| 30-day TLFB Methamphetamine Days | 20.13 (8.84) |
| MAUQ | 18.50 (10.93) |
| MAWQ | 13.63 (12.56) |
| Years of Methamphetamine Use | 12.38 (8.71) |
| DSM-IV Symptom Total | 5.83 (2.23) |
| Age of First Methamphetamine Use | 24.59 (9.88) |
| 30-day TLFB Alcohol Drinking Days | 5.56 (8.63) |
| AUDIT | 5.00 (7.23) |
| Age of First Cigarette | 15.84 (3.76) |
| SHQ Cigarettes Per Day in Past Week | 10.52 (9.47) |
| FTND | 3.97 (3.75) |
| Marijuana Use At Baseline (% positive Utox) | 23% |
| TOPF Standard Score | 92.93 (13.46) |
| DKEFS Trail Making Visual Scanning | 10.53 (2.21) |
| DKEFS Trail Making Test Number-Letter Sequencing | 9.20 (3.15) |
| DKEFS Color-Word Interference | 9.57 (2.64) |
| DKEFS Color-Word Interference Switching | 8.63 (3.50) |
| DKEFS Tower Test – Total Achievement | 9.50 (2.19) |
| Executive Function Factor | −.20 (1.05) |
Note. AUDIT = Alcohol Use Disorders Identification Test; BAI = Beck Anxiety Inventory; BDI = Beck Depression Inventory-II; DKEFS = Delis-Kaplan Executive Function System; FTND = Fagerström Test for Nicotine Dependence; MAUQ = Methamphetamine Urge Questionnaire; MAWQ = Methamphetamine Withdrawal Questionnaire; SHQ = Smoking History Questionnaire; TLFB = Timeline Follow-Back; TOPF = Test of Premorbid Functioning. DKEFS scores are presented as age-normed scaled scores.
Principal Components Analysis
The principal components analysis of DKEFS Color-Word Inhibition, Color-Word Inhibition-Switching, Trail Making Test Switching, and Tower Test, yielded one factor (Eigenvalue = 2.05) that explained 52% of the variance and with variable loadings between .57 and .81 (see Table 2). This single executive function factor was utilized for primary analyses.
Table 2.
Principal components analysis factor loadings
| Measure | Factor 1 |
|---|---|
| DKEFS Color-Word Inhibition | .81 |
| DKEFS Color-Word Inhibition Switching | .70 |
| DKEFS Trail Making Test Number-Letter Switching | .77 |
| DKEFS Tower Test Total | .57 |
| Measure | Factor 1 (‘Severity’) | Factor 2 (‘Frequency’) |
|---|---|---|
| MAWQ | .79 | −.03 |
| MAUQ | .70 | .05 |
| DSM-5 symptoms | .72 | .25 |
| 30-day TLFB Methamphetamine use days | .23 | .41 |
| Age of First Methamphetamine Use | −.05 | −.82 |
| Years of Regular Methamphetamine Use | −.03 | .82 |
Note. DKEFS = Delis-Kaplan Executive Function System; TLFB = Timeline Follow-Back; MAUQ = Methamphetamine Urge Questionnaire; MAWQ = Methamphetamine Withdrawal Questionnaire.
The principal components analysis of DSM-IV symptom count, MAUQ, MAWQ, years of regular use, age of first methamphetamine use, and 30-day methamphetamine use days, yielded two factors that explained 55% of variance and with all variables loading >.40 on at least one factor. Methamphetamine withdrawal, craving, and DSM-IV symptom count loaded strongly onto the first factor (‘Methamphetamine Severity’), while years of use, age of first use, and number of monthly methamphetamine use days loaded onto the second factor (‘Methamphetamine Frequency’). Factor loadings can be found in Table 2.
Mixed Models
As indicated in the primary manuscript from which these data are derived (9), methamphetamine administration increased subjective feelings of “feel drug effects”, “like effects”, “feel high”, “feel stimulated”, “crave more drug”, “would like drug access”, and “would like more drug” (baseline vs. 5 mins post-methamphetamine administration in placebo medication condition; ps<.01). Of note, the EF factor was not significantly correlated with baseline DEQ levels for any single item (rs=.01–.28, ps=.13–.97) except “How stimulated do you feel right now” (r=.39, p=.03). EF factor was also not associated with either of the methamphetamine frequency and severity factor scores (rs=−.12–.17, ps=.39–.53).
Subjective Drug Effects
In the final models with outcomes related to subjective drug effects (“feel drug effects”, “feel high”, and “feel stimulated”), the main effects of medication condition ranged from nonsignificant to significant (bs=−0.93–0.39, SEs=0.22–0.25, ts=−3.65–1.44, ps=.01–.15). There were also either trending (“feel drug effects” and “feel high”, bs=−0.01, SEs=0.01, ts=−1.77–1.72, ps=.08–.09) or significant effects (“feel stimulated”, b=−.02, SE=.01, t=−2.23, p =.03) of time, with these effects decreasing over the 2-hour experimental period. There was a main effect of EF, which was negatively associated with these reported effects (bs=−1.66–0.92, SEs=.50–.53, ts=−3.28–1.80, ps=.01–.08). While there were no significant EF × time interactions (ps=.41–.61), the effects of medication × time were significant for “feel drug effects” and “feel high” (bs=−.01, SEs=.01, ts=−2.68–2.41, ps=.01–.02), such that naltrexone reduced these effects more at later times in the paradigm. There were significant medication × EF interactions (bs=.36–.87, SEs=0.14–0.16, ts=2.21–6.04, ps=.001–.03); naltrexone relative to placebo elicited greater reductions in these subjective methamphetamine effects across time in low-EF compared to high-EF individuals (“feel drug effects” exemplar shown in Figure 2). There were no significant medication × time × EF interactions (ps=.16–.49). Significant covariates included baseline (pre-infusion) subjective effect ratings (bs=.58–.80, SEs=.07–.35, ts=2.28–8.47, ps=.001–.02) and premorbid functioning (bs=.06–.12, SEs=.03–.04, ts=1.69–3.27, ps=.001–.10).
Figure 2.
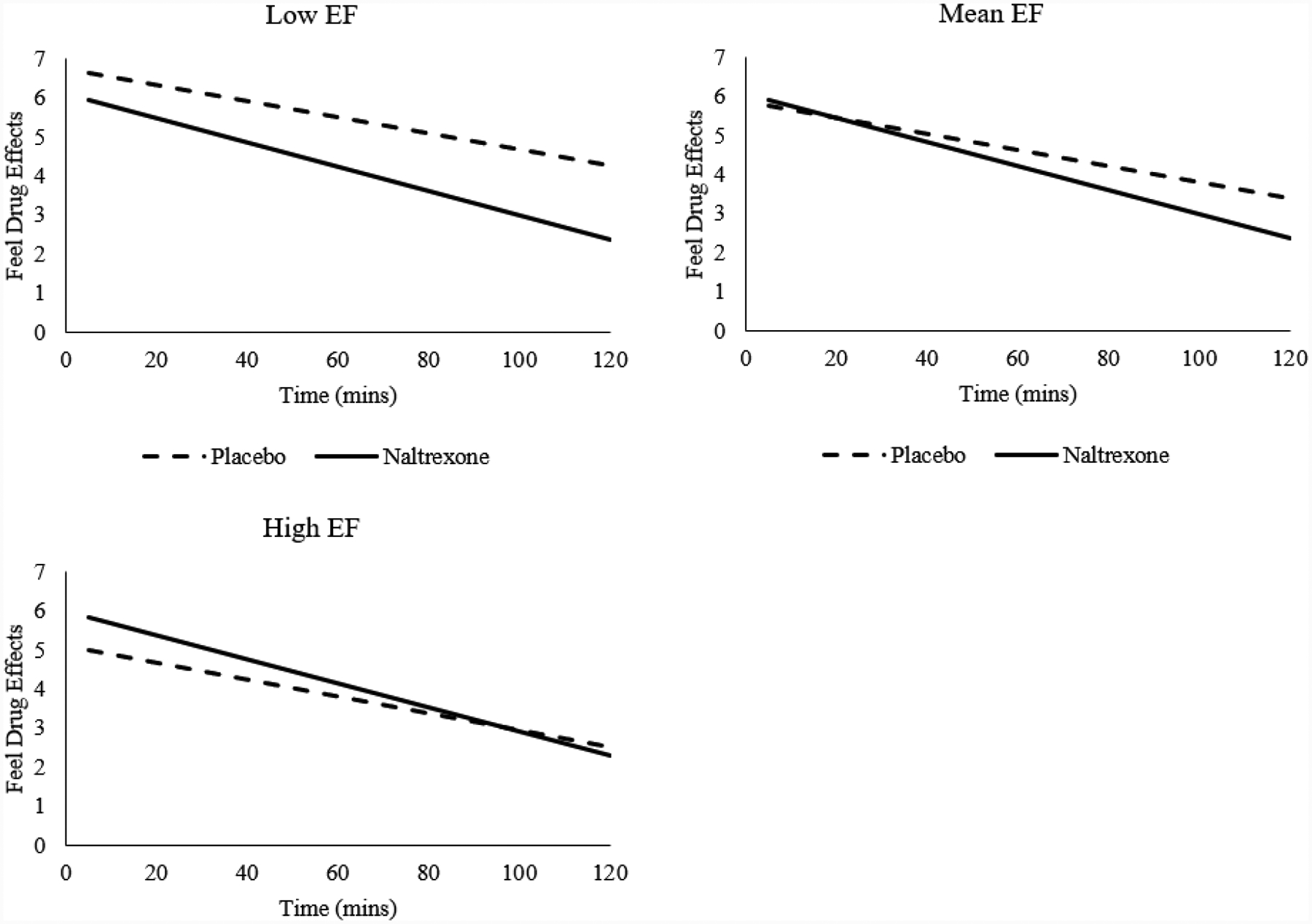
Executive function (EF) moderates the relationship between medication condition and ratings of “feel drug effects” during methamphetamine infusion; naltrexone relative to placebo induce greater reductions in methamphetamine effects among Low-EF, compared to high-EF individuals (bs=.36–.87, SEs=0.14–0.16, ps=.001–.03). Low and High EF represent −1 and +1 SD from mean EF factor, and are visualized as anchors for the continuous EF variable.
Craving
For items related to methamphetamine craving (“crave more drug”, “would like drug access”, and “would like more drug”), there were no significant main effects of medication (bs=−0.31–0.11, SEs=0.27–0.66, ts=−1.47–0.43, ps=.25–.67). EF was negatively associated with these craving items (bs=−1.68–1.64, SEs=.60–.68, ts=−2.69–2.48, ps=.01–.02). There were no significant effects of time, EF × time, or medication × time (ps=.16–.99). There were, however, significant medication × EF effects (bs=.87–1.29, SEs=.14–.17, ts=6.18–7.52, ps<.001), such that naltrexone relative to placebo induced greater craving reductions in low-EF than in high-EF individuals (“crave more drug” exemplar shown in Figure 3). The only significant covariate was baseline craving (bs=.21–.28, SEs=.04–.05, ts=3.58–4.52, ps<.001).
Figure 3.
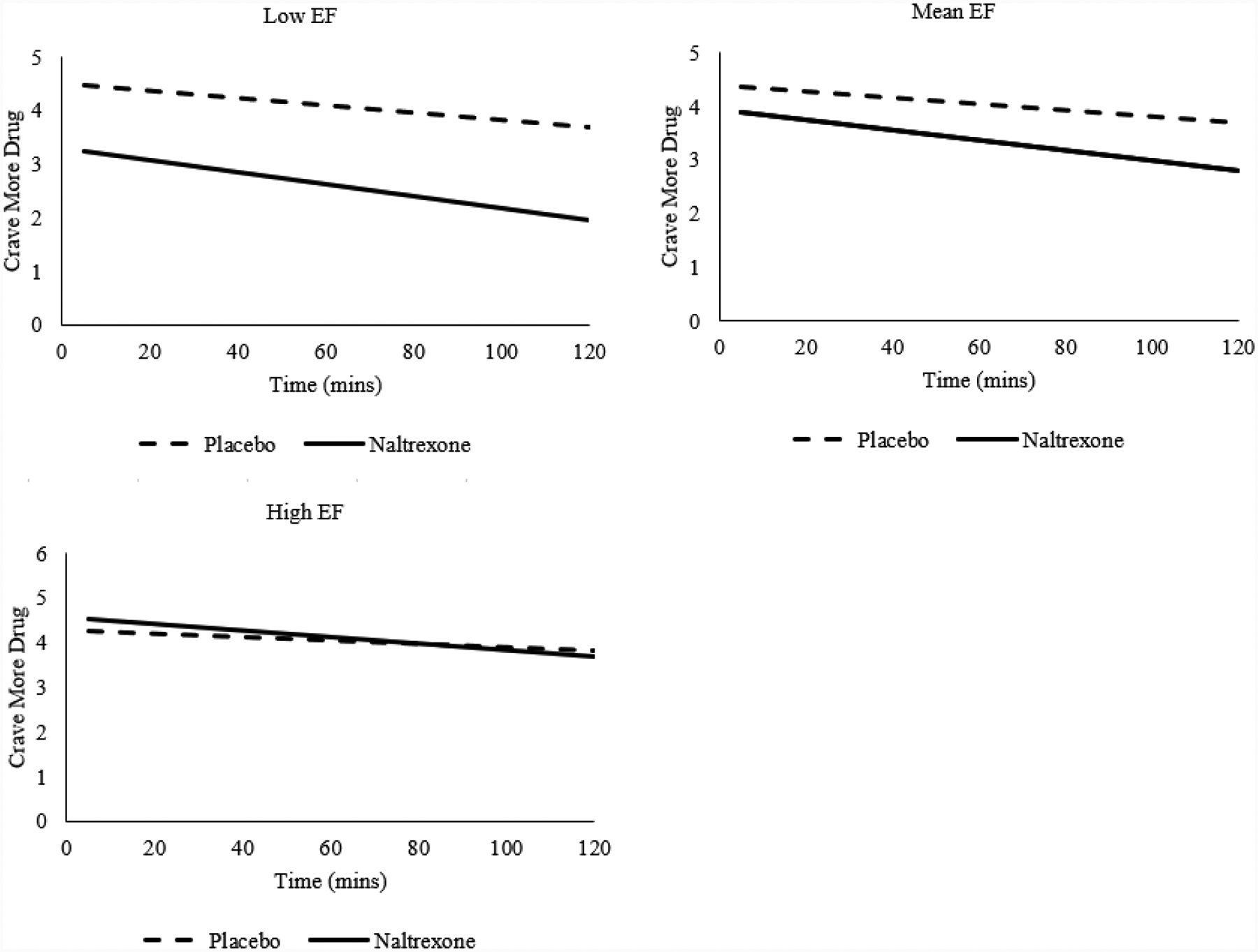
Executive function (EF) moderates the relationship between medication condition and ratings of “crave more drug” during methamphetamine infusion; naltrexone relative to placebo induce greater reductions in methamphetamine craving among Low-EF, compared to high-EF individuals (b=.87, SE=.14, p<.001). Low and High EF represent −1 and +1 SD from mean EF factor, and are visualized as anchors for the continuous EF variable.
Liking Drug Effects
For the “like effects” analysis, there were significant effects of time (b=−.02, SE=0.01, t=−2.49, p=.01). There were no significant effects of EF, medication, medication × time, time × EF, or 3-way interactions (medication × time × EF; ps=.11–.95). There was, however, a significant medication × EF effect (b=0.47, SE=0.16, t=3.04, p<.01), such that naltrexone relative to placebo decreased liking the effects to a greater extent in low EF relative to high EF individuals. Significant or trending covariates included baseline “like effects” responses (b=0.32, SE=0.08, t=4.18, p<.01) and premorbid functioning (b=0.06, SE=0.03, t=1.88, p=.07).
Discussion
This study examined whether executive function, assessed through a neurocognitive battery, would moderate naltrexone effects on methamphetamine-induced subjective responses and craving over a 2-hour experimental methamphetamine infusion. While there were largely no significant main effects of medication, the executive function factor showed an interaction with medication condition on multiple subjective responses. Contrary to our hypotheses, individuals with low executive function reported greater reductions in “feeling drug effects” and “feeling high” when titrated to naltrexone relative to placebo, as compared to their counterparts with high executive function. Similarly, lower executive function predicted overall greater reductions on multiple items related to methamphetamine craving (“crave more drug”, “would like drug access”, and “would like more drug”) when individuals were titrated to naltrexone relative to placebo. For “liking drug effects”, while there was no effect of naltrexone in reducing liking of methamphetamine infusion, executive function moderated this relationship such that naltrexone relative to placebo produced larger reductions in liking the effects among those with low, relative to high, executive function.
With recognition that naltrexone is not an FDA-approved medication for methamphetamine use disorder, these findings suggest potential value in examining whether executive function assessments capture medication-induced moderations in brain connectivity among those with substance use disorders, both in response to substance cues and at rest. Naltrexone increases functional connectivity between regions involved in reward processing (ventral and dorsal striatum, anterior cingulate cortex, and ventromedial prefrontal cortex) with prefrontal regions implicated in cognitive control and response inhibition (6, 22, 61, 62). Such naltrexone-induced changes in connectivity have also been shown to predict reductions in methamphetamine use (6). Long-term consequences for methamphetamine use include dysregulated striatal dopamine levels, oxidative stress, apoptosis, and neurocognitive deficits associated with executive function, and altered frontostriatal connectivity (63, 64). Amphetamine-induced euphoria and stimulation are also shown to be negatively associated with response inhibition capacity and negatively associated with middle frontal gyrus activation during response inhibition (25).
To the extent that neuropsychological batteries adequately assess executive function, it is perhaps not surprising that pharmacological interventions such as naltrexone may, at least partly, restore frontostriatal deficits among users who may be uniquely vulnerable to methamphetamine effects, such as those with relatively low executive function. As this study assessed executive function in the domains of response inhibition, set-switching, and planning, individuals who may struggle with decision-making in substance- or risk-related situations could theoretically benefit more from improved frontostriatal connectivity than those with stronger capabilities.
These results provide initial evidence that neuropsychological batteries may be translationally informative for addiction treatments. To date in the human methamphetamine literature, assessment batteries such as the D-KEFS have been used to characterize neuropsychological profiles of methamphetamine-using participants (37, 65, 66), predict adherence in treatment (67), and in clinical trials of cognitive-enhancing medications (e.g. modafinil; 68, 69). As the analyses in this study controlled for methamphetamine use disorder severity, which was not associated with the executive function factor, neuropsychological assessments may provide clinical utility for evaluating response to addictions pharmacotherapy that is not captured by substance use severity measures, which predominate the field. Recent efforts within the addiction medicine field identify neurocognition as an important predictor of treatment adherence across substances (70), have identified neurocognitive rehabilitation as a potential adjunct treatment for addictive disorders (71), and developed new psychoeducation procedures that consider attentional and memory abilities (72). Amidst this effort, implementation of batteries that assess multidimensional executive function capabilities in particular may be informative and are warranted for future clinical studies.
The findings of this study should be interpreted in light of its limitations. The sample size was small, limiting our ability to generate reliable effect sizes for our multilevel model, and larger sample sizes are needed to examine these effects among the full spectrum of executive function and in additional moderators of interest (e.g. comorbid substance use, sex). While the executive function factor was calculated from a larger baseline sample of methamphetamine users and a single EF factor score is consistent with previous DKEFS studies (29), additional studies corroborating the principal components analysis employed in this study are warranted. This study also did not administer the entire D-KEFS battery or an effort measure. Thus, it is not clear whether the inclusion of executive function measures steeped in verbal abilities (e.g. Twenty Questions Test, Verbal Fluency, Proverbs) would yield similar results. The study did not assess for neurodevelopmental disorders such as Attention Deficit Hyperactivity Disorder; as individuals with this disorder demonstrate significant executive function deficits (73), future consideration of ADHD within the study of naltrexone may be warranted. Separately, while there is evidence that craving is proximal to subsequent use (74), future studies might examine real-world methamphetamine consumption to improve external validity of the intravenous paradigm used in this study. Examination of these moderating effects should be tested with treatment-seeking populations to determine whether these findings apply beyond non-treatment seekers. As the acute- and long-term effects of methamphetamine abstinence on neurocognition are not entirely understood (75), additional research is needed to understand whether this study’s findings would replicate across varying durations of methamphetamine abstinence (76). Finally, naltrexone is not an FDA-approved medication for methamphetamine use disorder, and further study of naltrexone in large scale clinical trials are needed to corroborate its efficacy in methamphetamine-using populations.
In sum, the present study examined whether executive function moderates the effects of naltrexone on intravenous methamphetamine-induced subjective effects and craving. While executive function was not associated with participants’ reported liking of methamphetamine effects, individuals with low executive function reported greater naltrexone-induced reductions in subjective drug effects and methamphetamine craving throughout the 2-hour experimental paradigm. The results suggest that neuropsychological batteries may be uniquely predictive of proximal determinants of substance consumption and that these effects may extend into detection of treatment response. Based on the present findings, consideration of measures of executive function as potential moderators of clinical response to naltrexone may enhance clinical studies of methamphetamine use disorder to advance precision medicine for addiction.
Funding:
This work was supported by the National Institute on Drug Abuse under Grant R21DA029831 and UCLA’s training Grants 5T32DA024635 & 4T32DA007272; the National Institutes of Health NCATS: UCLA’s CTSI Grant UL1TR001881; the Tobacco Related Disease Research Program Grants T29DT0371 &T30DT0950; the National Institute on Alcohol Abuse and Alcoholism Grants F32AA027699, 3R01AA026190-02S1 & K24AA025704; UCLA’s Thomas P. and Katherine K. Pike Chair in Addiction Studies; UCLA’s Greene Family Trust.
Footnotes
Disclosure of Interest: LAR has received study medication from Pfizer and Medicinova and consulted for GSK. The authors report no other conflict of interest.
References
- 1.Abuse S. Mental Health Services Administration (SAMHSA), 2014. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-48, HHS Publication No.(SMA) 14–4863 Substance Abuse and Mental Health Services Administration, Rockville, MD. 2017. [Google Scholar]
- 2.Crime UNOoDa. World Drug Report 2018 (United Nations publication, Sales No. E.18.XI.9). 2019. [Google Scholar]
- 3.Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. Jama. 2014;311(18):1889–900. [DOI] [PubMed] [Google Scholar]
- 4.Sofuoglu M, DeVito EE, Carroll KM. Pharmacological and behavioral treatment of opioid use disorder. Psychiatric Research and Clinical Practice. 2018;1(1):4–15. [Google Scholar]
- 5.Stoops WW, Rush CR. Combination pharmacotherapies for stimulant use disorder: a review of clinical findings and recommendations for future research. Expert review of clinical pharmacology. 2014;7(3):363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohno M, Dennis LE, McCready H, Schwartz DL, Hoffman WF, Korthuis PT. A preliminary randomized clinical trial of naltrexone reduces striatal resting state functional connectivity in people with methamphetamine use disorder. Drug and alcohol dependence. 2018;192:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, Li W, Wang H, Wang Y, Zhang Y, Zhu J, et al. Predicting subsequent relapse by drug‐related cue‐induced brain activation in heroin addiction: an event‐related functional magnetic resonance imaging study. Addiction biology. 2015;20(5):968–78. [DOI] [PubMed] [Google Scholar]
- 8.Strong DR, Leventhal AM, Evatt DP, Haber S, Greenberg BD, Abrams D, et al. Positive reactions to tobacco predict relapse after cessation. Journal of abnormal psychology. 2011;120(4):999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray LA, Bujarski S, Courtney KE, Moallem NR, Lunny K, Roche D, et al. The effects of naltrexone on subjective response to methamphetamine in a clinical sample: a double-blind, placebo-controlled laboratory study. Neuropsychopharmacology. 2015;40(10):2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marks KR, Lile JA, Stoops WW, Glaser PE, Hays LR, Rush CR. Separate and combined effects of naltrexone and extended-release alprazolam on the reinforcing, subject-rated, and cardiovascular effects of methamphetamine. Journal of clinical psychopharmacology. 2016;36(3):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayaram-Lindström N, Konstenius M, Eksborg S, Beck O, Hammarberg A, Franck J. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2008;33(8):1856. [DOI] [PubMed] [Google Scholar]
- 12.Coffin PO, Santos GM, Hern J, Vittinghoff E, Santos D, Matheson T, et al. Extended‐release naltrexone for methamphetamine dependence among men who have sex with men: a randomized placebo‐controlled trial. Addiction. 2018;113(2):268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray LA, Chin PF, Miotto K. Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders). 2010;9(1):13–22. [DOI] [PubMed] [Google Scholar]
- 14.Ray LA, Bujarski S, Grodin E, Hartwell E, Green R, Venegas A, et al. State-of-the-art behavioral and pharmacological treatments for alcohol use disorder. The American journal of drug and alcohol abuse. 2019;45(2):124–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Stel J. Focus: Personalized Medicine: Precision in Addiction Care: Does It Make a Difference? The Yale journal of biology and medicine. 2015;88(4):415. [PMC free article] [PubMed] [Google Scholar]
- 16.Heinzerling KG, Swanson AN, Hall TM, Yi Y, Wu Y, Shoptaw SJ. Randomized, placebo‐controlled trial of bupropion in methamphetamine‐dependent participants with less than daily methamphetamine use. Addiction. 2014;109(11):1878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chamorro AJ, Marcos M, Mirón‐Canelo JA, Pastor I, González‐Sarmiento R, Laso FJ. Association of μ‐opioid receptor (OPRM1) gene polymorphism with response to naltrexone in alcohol dependence: a systematic review and meta‐analysis. Addiction biology. 2012;17(3):505–12. [DOI] [PubMed] [Google Scholar]
- 18.Jonas DE, Amick HR, Feltner C, Wines R, Shanahan E, Rowe CJ, et al. Genetic polymorphisms and response to medications for alcohol use disorders: a systematic review and meta-analysis. Pharmacogenomics. 2014;15(13):1687–700. [DOI] [PubMed] [Google Scholar]
- 19.Diamond A. Executive functions. Annual review of psychology. 2013;64:135–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowrangi MA, Lyketsos C, Rao V, Munro CA. Systematic review of neuroimaging correlates of executive functioning: converging evidence from different clinical populations. The Journal of neuropsychiatry and clinical neurosciences. 2014;26(2):114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray LA, Green R, Roche DJ, Bujarski S, Hartwell EE, Lim AC, et al. Pharmacogenetic effects of Naltrexone in individuals of East Asian descent: Human laboratory findings from a randomized trial. Alcoholism: Clinical and Experimental Research. 2018;42(3):613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Courtney KE, Ghahremani DG, Ray LA. The effects of pharmacological opioid blockade on neural measures of drug cue-reactivity in humans. Neuropsychopharmacology. 2016;41(12):2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim AC, Ghahremani DG, Grodin EN, Green R, Bujarski S, Hartwell EE, et al. Neuroimaging findings from an experimental pharmacology trial of naltrexone in heavy drinkers of East Asian descent. Drug and alcohol dependence. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker A, Kirsch M, Gerchen MF, Kiefer F, Kirsch P. Striatal activation and frontostriatal connectivity during non‐drug reward anticipation in alcohol dependence. Addiction biology. 2017;22(3):833–43. [DOI] [PubMed] [Google Scholar]
- 25.Weafer J, Gorka SM, Hedeker D, Dzemidzic M, Kareken DA, Phan KL, et al. Associations between behavioral and neural correlates of inhibitory control and amphetamine reward sensitivity. Neuropsychopharmacology. 2017;42(9):1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabibnia G, Monterosso JR, Baicy K, Aron AR, Poldrack RA, Chakrapani S, et al. Different forms of self-control share a neurocognitive substrate. Journal of Neuroscience. 2011;31(13):4805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system: technical manual: Psychological Corporation; 2001. [Google Scholar]
- 28.Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: an update. Journal of the International Neuropsychological Society. 2004;10(2):301–3. [DOI] [PubMed] [Google Scholar]
- 29.Karr JE, Hofer SM, Iverson GL, Garcia-Barrera MA. Examining the latent structure of the Delis–Kaplan executive function system. Archives of Clinical Neuropsychology. 2018;34(3):381–94. [DOI] [PubMed] [Google Scholar]
- 30.Dean AC, Groman SM, Morales AM, London ED. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology. 2013;38(2):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell M, Miller LS. Prediction of functional status in older adults: The ecological validity of four Delis–Kaplan Executive Function System tests. Journal of Clinical and Experimental Neuropsychology. 2008;30(6):683–90. [DOI] [PubMed] [Google Scholar]
- 32.Homack S, Lee D, Riccio CA. Test review: Delis-Kaplan executive function system. Journal of clinical and experimental neuropsychology. 2005;27(5):599–609. [DOI] [PubMed] [Google Scholar]
- 33.Parmenter BA, Zivadinov R, Kerenyi L, Gavett R, Weinstock-Guttman B, Dwyer MG, et al. Validity of the Wisconsin card sorting and Delis–Kaplan executive function system (DKEFS) sorting tests in multiple sclerosis. Journal of clinical and experimental neuropsychology. 2007;29(2):215–23. [DOI] [PubMed] [Google Scholar]
- 34.Heled E, Hoofien D, Margalit D, Natovich R, Agranov E. The Delis–Kaplan Executive Function System Sorting Test as an evaluative tool for executive functions after severe traumatic brain injury: A comparative study. Journal of Clinical and Experimental Neuropsychology. 2012;34(2):151–9. [DOI] [PubMed] [Google Scholar]
- 35.McDonald CR, Delis DC, Norman MA, Wetter SR, Tecoma ES, Iragui VJ. Response inhibition and set shifting in patients with frontal lobe epilepsy or temporal lobe epilepsy. Epilepsy & Behavior. 2005;7(3):438–46. [DOI] [PubMed] [Google Scholar]
- 36.Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychology review. 2007;17(3):275–97. [DOI] [PubMed] [Google Scholar]
- 37.Potvin S, Pelletier J, Grot S, Hebert C, Barr AM, Lecomte T. Cognitive deficits in individuals with methamphetamine use disorder: a meta-analysis. Addictive behaviors. 2018;80:154–60. [DOI] [PubMed] [Google Scholar]
- 38.Dean AC, Morales AM, Hellemann G, London ED. Cognitive deficit in methamphetamine users relative to childhood academic performance: link to cortical thickness. Neuropsychopharmacology. 2018;43(8):1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ersche KD, Turton AJ, Chamberlain SR, Müller U, Bullmore ET, Robbins TW. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. American Journal of Psychiatry. 2012;169(9):926–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canli T, Cook RG, Miczek KA. Opiate antagonists enhance the working memory of rats in the radial maze. Pharmacology Biochemistry and Behavior. 1990;36(3):521–5. [DOI] [PubMed] [Google Scholar]
- 41.Rodefer JS, Nguyen TN. Naltrexone reverses age-induced cognitive deficits in rats. Neurobiology of Aging. 2008;29(2):309–13. [DOI] [PubMed] [Google Scholar]
- 42.Cobb BL, Jauchem JR, Adair ER. Radial arm maze performance of rats following repeated low level microwave radiation exposure. Bioelectromagnetics. 2004;25(1):49–57. [DOI] [PubMed] [Google Scholar]
- 43.Lai H, Horita A, Guy AW. Microwave irradiation affects radial‐arm maze performance in the rat. Bioelectromagnetics: Journal of the Bioelectromagnetics Society, The Society for Physical Regulation in Biology and Medicine, The European Bioelectromagnetics Association. 1994;15(2):95–104. [DOI] [PubMed] [Google Scholar]
- 44.Oberlin BG, Bristow RE, Heighton ME, Grahame NJ. Pharmacologic dissociation between impulsivity and alcohol drinking in high alcohol preferring mice. Alcoholism: clinical and experimental research. 2010;34(8):1363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Messinis L, Lyros E, Andrian V, Katsakiori P, Panagis G, Georgiou V, et al. Neuropsychological functioning in buprenorphine maintained patients versus abstinent heroin abusers on naltrexone hydrochloride therapy. Human Psychopharmacology: Clinical and Experimental. 2009;24(7):524–31. [DOI] [PubMed] [Google Scholar]
- 46.Ralevski E, Balachandra K, Gueorguieva R, Limoncelli D, Petrakis I. Effects of naltrexone on cognition in a treatment study of patients with schizophrenia and comorbid alcohol dependence. Journal of dual diagnosis. 2006;2(4):53–69. [Google Scholar]
- 47.Pomara N, Roberts R, Rhiew HB, Stanley M, Gershon S. Multiple, single-dose naltrexone administrations fail to effect overall cognitive functioning and plasma cortisol in individuals with probable Alzheimer’s disease. Neurobiology of aging. 1985;6(3):233–6. [DOI] [PubMed] [Google Scholar]
- 48.Hatsukami DK, Mitchell JE, Morley JE, Morgan SF, Levine AS. Effect of naltrexone on mood and cognitive functioning among overweight men. Biological psychiatry. 1986;21(3):293–300. [DOI] [PubMed] [Google Scholar]
- 49.First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV-TR Axis I disorders: patient edition: Biometrics Research Department, Columbia University; New York, NY; 2005. [Google Scholar]
- 50.Pearson N. Advanced clinical solutions for WAIS-IV and WMS-IV: Administration and scoring manual. San Antonio: The Psychological Corporation. 2009. [Google Scholar]
- 51.Inagaki TK, Ray LA, Irwin MR, Way BM, Eisenberger NI. Opioids and social bonding: naltrexone reduces feelings of social connection. Social cognitive and affective neuroscience. 2016;11(5):728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newton TF, Roache JD, De La Garza R, Fong T, Wallace CL, Li S-H, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31(7):1537–44. [DOI] [PubMed] [Google Scholar]
- 53.Newton TF, Reid MS, De La Garza R, Mahoney III JJ, Abad A, Condos R, et al. Evaluation of subjective effects of aripiprazole and methamphetamine in methamphetamine-dependent volunteers. International Journal of Neuropsychopharmacology. 2008;11(8):1037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology. 2013;227(1):177–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moallem NR, Courtney KE, Ray LA. The relationship between impulsivity and methamphetamine use severity in a community sample. Drug and alcohol dependence. 2018;187:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim AC, Cservenka A, Ray LA. Effects of alcohol dependence severity on neural correlates of delay discounting. Alcohol and Alcoholism. 2017;52(4):506–15. [DOI] [PubMed] [Google Scholar]
- 57.Zorick T, Nestor L, Miotto K, Sugar C, Hellemann G, Scanlon G, et al. Withdrawal symptoms in abstinent methamphetamine‐dependent subjects. Addiction. 2010;105(10):1809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug and alcohol dependence. 1996;42(1):49–54. [DOI] [PubMed] [Google Scholar]
- 59.Bujarski S, Hutchison KE, Prause N, Ray LA. Functional significance of subjective response to alcohol across levels of alcohol exposure. Addiction biology. 2017;22(1):235–45. [DOI] [PubMed] [Google Scholar]
- 60.Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub; 2013. [DOI] [PubMed] [Google Scholar]
- 61.Morris LS, Baek K, Tait R, Elliott R, Ersche KD, Flechais R, et al. Naltrexone ameliorates functional network abnormalities in alcohol‐dependent individuals. Addiction biology. 2018;23(1):425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elton A, Dove S, Spencer CN, Robinson DL, Boettiger CA. Naltrexone acutely enhances connectivity between the ventromedial prefrontal cortex and a left frontoparietal network. Alcoholism: Clinical and Experimental Research. 2019;43(5):965–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu S, Zhu L, Shen Q, Bai X, Di X. Recent advances in methamphetamine neurotoxicity mechanisms and its molecular pathophysiology. Behavioural neurology. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Volkow ND, Chang L, Wang G-J, Fowler JS, Leonido-Yee M, Franceschi D, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. American Journal of Psychiatry. 2001;158(3):377–82. [DOI] [PubMed] [Google Scholar]
- 65.Vonmoos M, Hulka LM, Preller KH, Jenni D, Baumgartner MR, Stohler R, et al. Cognitive dysfunctions in recreational and dependent cocaine users: role of attention-deficit hyperactivity disorder, craving and early age at onset. The British Journal of Psychiatry. 2013;203(1):35–43. [DOI] [PubMed] [Google Scholar]
- 66.Bernardin F, Maheut-Bosser A, Paille F. Cognitive impairments in alcohol-dependent subjects. Frontiers in psychiatry. 2014;5:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dean AC, London ED, Sugar CA, Kitchen CM, Swanson A-N, Heinzerling KG, et al. Predicting adherence to treatment for methamphetamine dependence from neuropsychological and drug use variables. Drug and alcohol dependence. 2009;105(1–2):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dean AC, Sevak RJ, Monterosso JR, Hellemann G, Sugar CA, London ED. Acute modafinil effects on attention and inhibitory control in methamphetamine-dependent humans. Journal of studies on alcohol and drugs. 2011;72(6):943–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hester R, Lee N, Pennay A, Nielsen S, Ferris J. The effects of modafinil treatment on neuropsychological and attentional bias performance during 7-day inpatient withdrawal from methamphetamine dependence. Experimental and clinical psychopharmacology. 2010;18(6):489. [DOI] [PubMed] [Google Scholar]
- 70.Domínguez-Salas S, Díaz-Batanero C, Lozano-Rojas OM, Verdejo-García A. Impact of general cognition and executive function deficits on addiction treatment outcomes: Systematic review and discussion of neurocognitive pathways. Neuroscience & Biobehavioral Reviews. 2016;71:772–801. [DOI] [PubMed] [Google Scholar]
- 71.Rezapour T, DeVito EE, Sofuoglu M, Ekhtiari H. Perspectives on neurocognitive rehabilitation as an adjunct treatment for addictive disorders: from cognitive improvement to relapse prevention. Progress in brain research. 224: Elsevier; 2016. p. 345–69. [DOI] [PubMed] [Google Scholar]
- 72.Ekhtiari H, Rezapour T, Aupperle RL, Paulus MP. Neuroscience-informed psychoeducation for addiction medicine: A neurocognitive perspective. Progress in brain research. 235: Elsevier; 2017. p. 239–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martel M, Nikolas M, Nigg JT. Executive function in adolescents with ADHD. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46(11):1437–44. [DOI] [PubMed] [Google Scholar]
- 74.Green R, Grodin E, Lim AC, Venegas A, Bujarski S, Krull J, et al. The Interplay Between Subjective Response to Alcohol, Craving, and Alcohol Self‐Administration in the Human Laboratory. Alcoholism: Clinical and Experimental Research. 2019;43(5):907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huckans M, Fuller BE, Chalker AL, Adams M, Loftis JM. Plasma inflammatory factors are associated with anxiety, depression, and cognitive problems in adults with and without methamphetamine dependence: an exploratory protein array study. Frontiers in psychiatry. 2015;6:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ruan X, Zhong N, Yang Z, Fan X, Zhuang W, Du J, et al. Gray matter volume showed dynamic alterations in methamphetamine users at 6 and 12 months abstinence: A longitudinal voxel-based morphometry study. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2018;81:350–5. [DOI] [PubMed] [Google Scholar]


