Abstract
Introduction
A functional tandem repeat polymorphism in the promoter of the serotonin transporter (SERT) gene (SLC6A4) has been studied for association to neuropsychiatric conditions, including substance use disorders. Short (S) forms of this repeat result in reduced transcription, and presumably greater synaptic levels of serotonin, which are involved in opioid and cocaine-induced reward. Dual exposure to heroin and cocaine is a common pattern of poly-drug use and is associated with considerable morbidity. We hypothesize that SLC6A4 variants are associated with cocaine exposure in subjects with an opioid dependence diagnosis (OD), and also in non-dependent opioid users (NOD). Other single nucleotide polymorphisms (SNPs) of SLC6A4 may also be likewise associated.
Materials and Methods
This study determined whether variants of the SLC6A4 promoter repeats and two intronic SNPs, rs16965628 and rs2066713, are associated with categorical diagnoses of opioid dependence (DSM-IV criteria) and with dimensional aspects of cocaine use, in a Caucasian cohort (n=591). Three groups of subjects were examined: (1) 276 subjects with opioid dependence diagnosis (OD); (2) 163 subjects who had used opioids for non-medical reasons but never had an opioid dependence diagnosis (NOD); (3) 152 healthy controls (HC).
Results
Aside from high exposure to heroin in the OD group, relatively high exposure to cocaine was detected in both OD and NOD groups. The SERT repeat genotype (classified as “long-long” [LL] versus ”short-long” plus “short-short” [SL+SS]) was not associated with categorical opioid dependence diagnoses. A nominally significant association was identified with the [SL+SS] genotype of SLC6A4 and cocaine KMSK scores ≥“cutpoint” for a cocaine dependence diagnosis (p=0.026). The [SL+SS] genotype was associated with more rapid cocaine escalation than the LL genotype. No significant associations of rs16965628 and rs2066713 SNPs were found overall.
Conclusion
The functional SERT promoter tandem repeat genotype may be associated to heavy cocaine exposure and more rapid escalation of cocaine use, in persons with and without opioid dependence diagnosis.
Keywords: cocaine, serotonin transporter, SLC6A4, KMSK scale, escalation
Introduction
The trajectory from initial drug use to addiction is influenced by vulnerability based on genetic, neurobiological, behavioral, and environmental factors. In addition to its impact on other neuropsychiatric diseases, dysregulation of the serotonin (5-HT) system may also be involved in the progression of substance use disorders.1 The serotonergic system is composed of 15 types of receptors, and there is one major transporter, SERT (encoded by the SLC6A4 gene), which is responsible for the reuptake of released 5-HT into presynaptic neurons. Cocaine’s rewarding effect is generally associated with inhibition of the dopamine transporter (DAT); however, this drug is also active at the serotonin transporter.2–4
Drugs of abuse affect the serotonergic system directly and indirectly. Mu-opioid agonists (eg, heroin, morphine) lead to increased extracellular 5-HT levels,4 whereas withdrawal from chronic mu-opioid agonist exposure is associated with reduced 5-HT levels.1,5 Serotonergic neurotransmission is also affected by exposure to cocaine, which acts as a monoamine reuptake inhibitor.1,4,6,7 More generally, pharmacological modulation of SERT results in robust changes in expression of several opioid genes, including prodynorphin (Pdyn), known to modulate the consequences of exposure to cocaine.8–10
Previous studies have identified a functional tandem repeat polymorphism in the 5ʹ promoter region of the SLC6A4 gene (5-HTTLPR) yielding two main alleles: a short (“S” allele, 14 repeats of 20–23 nucleotides) and a long (“L” allele, 16 repeats) variant of the allele.11,12 The presence of the S allele (in homozygous or heterozygote genotypes, SS or SL) has been reported to result in lower transcriptional activity than the L allele,11,13 leading to a relative reduction in mRNA levels, serotonin binding, and reuptake and higher synaptic 5-HT levels.14 There is considerable variability in the prevalence of the S- and L-allele across different human populations, depending on ethnicity, and drug use comorbidity.15 The 5-HTTLPR (ie, 5-HTT length promoter polymorphic region) has been investigated in numerous genetic association studies,16–21 including systematic reviews on the role of 5-HTTLPR on substance use disorders and their pharmacotherapy.20,22
The frequency of the short–short (SS) genotype was higher among male Caucasians with heroin dependence compared to controls.23 A meta-analyses of the 5-HTTLPR allele and genotype frequencies in Caucasians showed that heroin dependence diagnosis was significantly associated with the S allele.20 The 5-HTTLPR polymorphism has also been examined in several studies of cocaine use disorders. There were no differences in the reduction in cocaine use across the LL, LS and SS genotypes, in African-American patients with cocaine dependence.20 Studies of cocaine dependence diagnosis as a categorical endpoint did not detect a robust association with 5-HTTLPR genotype, either in Spanish24 or African-American cohorts.25 In another study, carriers of the S allele reported greater “desire” and other subjective effects after challenge with intravenous cocaine injection.16 Therefore, the association of the 5-HTTLPR with cocaine use and cocaine use disorders may depend on the type of variable studied (eg, categorical diagnoses, or particular dimensional phenotypes) and ethnic/cultural group. Inconsistencies in the association of the 5-HTTLPR variants with cocaine dependence in previous studies can be explained by a high level of poly-drug use of cocaine use with other substances. Dual use of cocaine and heroin is frequently reported and is associated with considerable morbidity.26–28
Some recent genetic association studies have focused on dimensional aspects of drug use on a continuum, as opposed to only categorical substance use disorder (SUD) diagnoses.29,30 In this study, we use two major dimensional measures: 1) level of exposure to cocaine, and 2) time of escalation of cocaine use, from initiation to the onset of heaviest use.31–33 In this study, we examined the association of 5-HTTLPR tandem repeats with opioid dependence (OD) in a sample of Caucasian volunteers from the Netherlands, and also with dimensional measures of cocaine exposure and escalation, using the Kreek–McHugh–Schluger–Kellogg (KMSK) scale. To our knowledge, this is among the first studies to examine the impact of this promoter repeat polymorphism on such cocaine-related measures in a Caucasian cohort.
Materials and Methods
Subjects
In this case-control study of 591 subjects, three subject groups were recruited in the Netherlands as previously described (Table 1),34–37 including:
Persons with opioid dependence diagnosis (OD, n=276, by DSM IV criteria), who had been heroin dependent for at least 5 years and who had participated in methadone maintenance treatment for at least 12 months, without (termed MMT) or with heroin-assisted treatment (termed HAT). These persons also had a range of cocaine and alcohol exposure (characterized with KMSK scales).
Persons who used illicit opioids (ie, 5–100 times in their lifetime), but who never qualified for an opioid dependence diagnosis (NOD, n=163). These persons also had a range of cocaine and alcohol exposure (characterized with KMSK scales).
Healthy controls without a history of any illicit opioid use and with no history of any dependence diagnosis to other drugs, including cocaine, alcohol or cannabis (HC, n=152). Lifetime nicotine dependence diagnosis was not an exclusion criterion from the HC category. These persons had no opioid or cocaine exposure, but they did have a range of alcohol exposure (characterized with KMSK scales).
Table 1.
Demographics (Total n=591)
| Characteristics | Healthy Controls (HC) | Not Opioid Dependent (NOD) | Opioid Dependent (OD; MMT+HAT) | Comparison |
|---|---|---|---|---|
| n | 152 | 163 | 276 | |
| Male | 89 (59%) | 107 (66%) | 213 (77%) | Chi2=17.29, df=2 p=0.0002 |
| Female | 63 (41%) | 56 (34%) | 63 (23%) | |
| Age, years (±SD) | 39.7±10.0 | 40.1±8.8 | 43.8±6.8 | Kruskal–Wallis ANOVA=28.14 P<0.0001 |
The study was conducted in accordance with the Declaration of Helsinki. The Central Committee on Research Involving Human Subjects in the Netherlands (protocol number P04.0156C) approved the study of heroin-assisted and methadone maintenance treatments, and the human molecular genetics study for all study groups. The genetics study was also approved by the Rockefeller University’s Institutional Review Board. All subjects signed an informed consent for the study, including the genetics research.
Diagnoses and Measurements
All subjects were interviewed by trained clinical investigators. Standard questionnaires were used for collection of data on age, gender and country of origin and clinical characteristics.35 The categorical diagnosis of opioid dependence was based on DSM-IV criteria. Additionally, the Kreek–McHugh–Schluger–Kellogg (KMSK) scale was used for a dimensional assessment of a subject’s exposure to opioids, cocaine, alcohol and nicotine.38,39 The KMSK scales characterize maximal exposure to a specific substance, based on the period in a subject’s life when use is at its heaviest.
Each KMSK scale measures maximal exposure to a specific substance, on an ordinal integer scale. A KMSK score=0 indicates that the participant had never used the substance of interest, and this ordinal score increases up to a maximum (13 for alcohol, tobacco/nicotine, and heroin, and 16 for cocaine). KMSK scales have also been used as a dimensional phenotype in genetic association studies.40,41 As shown in Table 2, heroin, cocaine and alcohol exposure in all these volunteers was characterized by KMSK scores. KMSK scores ≥“cutpoint” have high concurrent validity with the respective DSM-IV dependence diagnoses.38,39 For example, the area under the receiver operating curve (AUC-ROC) for the cocaine KMSK score and the DSM-IV diagnosis of cocaine dependence was 0.97.38
Table 2.
KMSK Scores: Maximal Exposure to Specific Substances
| KMSK Scorea | Opioid Dependence Diagnosis (OD) | Non-Opioid Dependent (NOD) | Healthy Controls (HC) | Kruskal–Wallis Test of KMSK Scores (Dunn’s Tests) | |||
|---|---|---|---|---|---|---|---|
| Median (±IQR) | Subjects ≥ Cutpoint/Total (%)b | Median (±IQR) | Subjects ≥ Cutpoint/Total (%)b | Median (±IQR) | Subjects ≥ Cutpoint/Total (%)b | ||
| Heroinb Range: 0–13 |
9 (8–10) | 158/173 (91%) | 5 (4–6) | 80/150 (57%) | 0 (0–0) | 0/107 (0%) | Kruskal–Wallis statistic= 323.0; p<0.0001 (OD>NOD>HC) |
| Cocaine Range: 0–16 |
10 (12–15) | 147/172 (86%) | 6 (8–11) | 69/149 (46%) | 0 (0–0) | 0/107 (0%) | Kruskal–Wallis statistic= 236.6; p<0.0001 (OD>NOD>HC) |
| Alcohol KMSK score Range: 0–13 |
12 (10–13) | 132/157 (84%) | 11 (10–12) | 113/141 (80%) | 10 (9–11) | 65/94 (69%) | Kruskal–Wallis statistic= 46.02; p<0.0001 (OD>NOD>HC) |
Notes: Total N differs from Table 1, due to some missing KMSK scores for each drug. aKMSK scores are on an ordinal integer scale for each drug, focusing on the period in a subject’s life when use is the heaviest. A score=0 indicates no lifetime use of the substance, and greater scores indicate progressively greater exposure. bCutpoint KMSK scores with optimal concurrent validity for the respective DSM-IV Dependence Diagnosis. Heroin cutpoint score ≥6; Cocaine cutpoint score≥9; Alcohol cutpoint score ≥10.
The KMSK instrument also collects the ages of first use and age of onset of heaviest use (in whole years). “Time of escalation” for a drug was calculated as age of onset of heaviest use minus age of first use (in whole years).33 Time of escalation of cocaine use was therefore also used as phenotypes in this study.
Assessment of Percentage of European Ancestry
There is considerable variability in frequency of the promoter repeat 5-HTTLPR S- and L-alleles across different human populations.15 For this study, ethnicity was initially assigned based on self-reported family origin data, with 628 self-identified Caucasian volunteers.36,42 Biographic Ancestry Scores (eg, fractions of genetic affiliation of the individual in each cluster) were estimated by STRUCTURE 2.2 with seven clusters (K), based on 155 ancestral informative markers (AIMs). Each AIM is a single nucleotide polymorphism that differed in allele frequency by at least 70% between continental populations (Europeans, Africans, and Asians). The effectiveness of Ancestral Informative Markers (AIMs) to distinguish ethnic substructures was demonstrated by PCA analyses (Ducci et al, 2009). The AIMs were genotyped using an Illumina GoldenGate Assay array (Illumina, San Diego). Each volunteer was “anchored” against 1051 samples from 51 worldwide populations in the Human Genome Diversity Cell Line Panel.43 To minimize population stratification for the current study, the inclusion criterion was set at ≥70% European ancestry contribution. Several subjects were excluded from analysis due to the low quantity and/or poor quality of the DNA; therefore, there are 591 subjects available for these association analyses.
Genotyping of the SLC6A4 Promoter Tandem Repeat Polymorphism
Genomic DNA was extracted from blood cells using a salt-precipitation method. Genotyping of the 44-base pair (bp) insertion/deletion polymorphism in the 5ʹ promoter region of the SLC6A4 gene was performed using the following two sets of primers: The first set was (Forward) 5ʹ-ATGCCAGCA CCTAA CCCCTAATG-3ʹ and (Reverse) 5ʹ- GAGGGACTGAGCTGGACAACCA-3ʹ for PCR DNA of 513 bp for the L allele (16 repeats) and PCR DNA of 469 bp for the S allele (14 repeats). The second set was (Forward) 5ʹ-ATC GCTCCTGCATCCCCCATTAT-3ʹ and (Reverse) 5ʹ-GAGGTGCAGGGGGATGCTGGAA for size discrimination for the S (103 bp) and L (146 bp) alleles.19 PCR was performed for 40 cycles of 30 seconds at 94°C, 30 seconds at 58°C, 30 seconds at 72°C, followed by 6 minutes at 72°C using either a TaqPCRx DNA Polymerase Kit (Invitrogen) or AccuPrime GC-rich DNA Polymerase Kit (Invitrogen, catalog # 12337–016), both designed for amplification of GC-rich regions. PCR products were electrophoresed on a 2.0% agarose gel. SERT genotypes were determined according to the size and number of PCR DNA fragments. To confirm SERT specificity of the PCR fragments, DNA from 30 subjects having S allele (n=13) and L allele (n=17) was sequenced in both orientations. Genotyping and analysis of the SERT promoter repeats were performed in Laboratory of the Biology of Addictive Diseases, at the Rockefeller University.
Genotyping of the intronic SNPs rs16965628 and rs2066713 was performed at the Rockefeller University Genomics Resource Center using a custom Illumina Golden Gate Panel (GS0013101-OPA) (Illumina, Inc., CA, USA).44 Data analysis was performed using BeadStudio v2.3.43 (Illumina, Inc.). The genotype data were visually inspected for quality.
Statistical Analysis
The p=0.05 level was used as the criterion for significance. Analyses were carried out with GraphPad Prism (V.8); Hardy–Weinberg equilibrium was examined as described in a prior publication.45 Demographic variables and KMSK scores were analyzed with t-test or Mann–Whitney tests, Kruskal–Wallis tests (with Dunn’s post hoc tests), or Fisher’s tests, as appropriate. Contingency analysis for categorical OD diagnoses and 5-HTTLPR SLC6A4 genotype was carried out with Fisher’s exact test for the more commonly studied S-dominant model (ie, SL+SS, versus LL), and also for an L-dominant model (ie, LL+SL versus SS). A contingency analysis for cocaine KMSK scores ≥ cutpoint was also carried out with Fisher’s exact test. A multiple logistic regression for cocaine KMSK scores ≥ cutpoint was also carried out, adjusting for gender. Time of escalation of cocaine use was also examined as a phenotype, using a survival analysis and the Log rank test. Similar analyses were also carried out for the two intronic variants SNPs (rs16965628 and rs2066713), whenever relevant.
Results
Sample Characteristics and Demographics
After exclusion of volunteers with <70% of Caucasian AIMs markers or poor DNA quality, 591 volunteers were included for analysis (Table 1). There were relatively more females in the opioid dependent, non-opioid dependent, and healthy controls (OD, NOD and HC groups, respectively), compared to males. The mean ages at the time of ascertainment were also significantly different among groups.
Available KMSK scores for all genotyped subjects are summarized in Table 2. KMSK scores for the full cohort, including subjects who could not be genotyped here, have been previously published.36 As expected, the OD group had high heroin KMSK scores, typically ≥cutpoint with optimal concurrent validity for an OD diagnosis, as calculated from a separate cohort.38 As previously reported,36 the OD group also had relatively high exposure to both cocaine and alcohol (Table 2). The NOD group, as expected, had some exposure to heroin, but lower than that of the OD group. We note that the NOD group also had a range of exposure to both cocaine and alcohol. The HC group, as expected, had primarily “0” scores for both heroin and cocaine (ie, denoting no exposure). KMSK alcohol scores in the HC group had a broad range, but as a group they were smaller than both the OD and NOD groups (Kruskal–Wallis ANOVA; Table 2). As previously reported, all three groups (OD, NOD and HC) had considerable exposure to nicotine.36
Contingency Analysis of Genotype of SLC6A4 Variants with Categorical Diagnoses of Opioid Dependence
Our previous studies with this cohort found very few genetic differences between the HC and NOD subject groups.46–48 Therefore, the HC and NOD groups have been previously combined into a control group of subjects without opioid dependence diagnosis for comparison to the OD group. In this study, we first performed a Fisher’s exact test of the SERT promoter tandem repeat frequency between HC and NOD (Table 3, section A). We did not find significant differences in frequency between these groups, neither allelic (p=0.72) nor genotypic (p=0.84). Therefore, these two groups, HC and NOD, were combined into a control group of subjects without opioid dependence diagnosis (see Table 3, sections B and C).
Table 3.
Frequency and Fisher’s Exact Test of the Distribution of SERT Variants in Subjects with Opioid Dependence (OD) and Control Subjects (HC +NOD)
| Genotype | Cases | Controls | Model (Dominant/Recessive) | p | Odds Ratio (95% CI) |
|---|---|---|---|---|---|
| A) Promoter tandem repeats | |||||
| NOD | HC | ||||
| LL | 53 | 46 | [SS+SL] vs LL | 0.31 | 1.29 (0.782–2.15) |
| SL | 80 | 49 | |||
| SS | 17 | 16 | SS vs [SL+LL] | 0.46 | 1.32 (0.634–2.74) |
| B) Promoter tandem repeats | |||||
| OD | NOD | ||||
| LL | 55 | 53 | [SS+SL] vs LL | 0.48 | 1.18 (0.744–1.88) |
| SL | 90 | 80 | |||
| SS | 29 | 17 | SS vs [SL+LL] | 0.17 | 0.639 (0.336–1.22) |
| C) Promoter tandem repeats) | |||||
| OD | HC | ||||
| LL | 55 | 46 | [SS+SL] vs LL | 0.091 | 1.53 (0.934–2.51) |
| SL | 90 | 49 | |||
| SS | 29 | 16 | SS vs [SL+LL] | 0.61 | 0.84 (0.434–1.63) |
| D) Promoter tandem repeats | |||||
| OD | HC+NOD | ||||
| LL | 55 | 99 | [SS+SL] vs LL | 0.176 | 1.32 (0.88–1.98) |
| SL | 90 | 129 | |||
| SS | 29 | 33 | SS vs [SL+LL] | 0.24 | 0.74 (0.42–1.24) |
| E) rs16965628 (intronic SNP) | |||||
| OD | HC+NOD | ||||
| CC | 0 | 1 | [CC+CG] vs GG | 0.18 | 1.44 (0.84–2.47) |
| CG | 33 | 26 | |||
| GG | 234 | 276 | CC vs [CG+GG] | 0.35 | 2.65 (0.11–65.39) |
| F) rs2066713 (intronic SNP) | |||||
| OD | HC+NOD | ||||
| CC | 37 | 49 | [CC+CT] vs TT | 0.94 | 1.01 (0.73–1.41) |
| CT | 133 | 144 | |||
| TT | 106 | 122 | CC vs [CT+TT] | 0.46 | 1.19 (0.75–1.89) |
There was no significant deviation from Hardy–Weinberg equilibrium (HWE) in the distribution of the genotypes of the promoter tandem repeat polymorphism (p=0.21, Pearson test) and the SNPs rs1696528 and rs2066713 in the combined control group (p=0.28 and 0.64, respectively). The distribution frequencies of the SERT variant genotypes in the treatment groups did not deviate from HWE: MMT (p=016), HAT (p=0.97), and combined OD group (MMT+HAT, p=0.44).
Fisher’s exact test conducted for an S-dominant of 5-HTTLPR (ie, SL+SS vs LL) or an L-dominant model (ie, SS versus SL+LL) did not show significant association of the SERT repeat genotype with categorical OD dependence diagnosis, p=0.18, see Table 3 (section D). Fisher’s exact test for the frequency of male and female volunteers with OD across genotype was also not significant.
Neither the intronic rs1696528 or rs2066713 genotypes showed an association of SL+SS vs LL in the comparison of the combined controls with the opioid dependent group, p=0.18 and p=0.94, respectively, see Table 3 (sections E and F).
Dimensional Analyses of Drug Exposure (KMSK Scores)
We first examined whether KMSK scores differed according to tandem repeat genotype in the whole cohort, focusing on a dominant model for the “S” allele (ie, comparing [LL] versus [SL+SS] genotypes), which is typically used for this polymorphism. We found that in the whole cohort, heroin KMSK scores did not differ by genotype (Mann–Whitney test; ns). Using a similar approach, we also found that alcohol KMSK scores in the whole cohort did not differ by genotype, using the “S” dominant model (Mann–Whitney test NS, not shown). Neither rs1696528 or rs2066713 genotypes showed an association with different KMSK scores for cocaine.
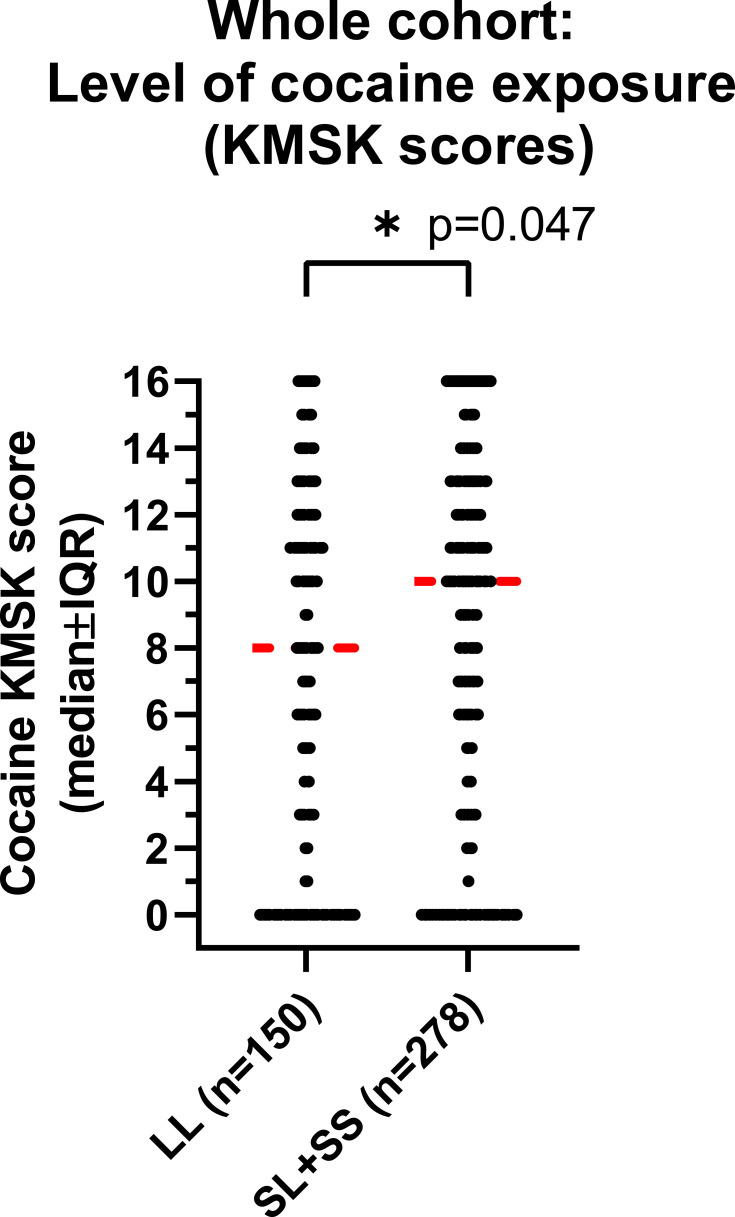
However, cocaine KMSK scores were higher in participants with the [SL+SS] versus [LL] genotype (Mann–Whitney test, p=0.047) (Figure 1). As a follow-up, we stratified cocaine KMSK scores by gender and found that this effect of genotype was primarily due to the larger group of males (Mann–Whitney test; p=0.044), whereas no difference of genotype was detected in females, which were a smaller group (Mann–Whitney test, ns).
Figure 1.
Cocaine KMSK scores in the whole cohort, by genotype. KMSK scores are on an ordinal integer scale, where “0” denotes no lifetime exposure (never used drug; see Methods), and greater scores denote increasing exposure (maximum score=16). Cocaine KMSK scores measure exposure at the point in a volunteer’s life when use was at its heaviest. A cocaine KMSK value ≥9 has optimal concurrent validity with a cocaine dependence diagnosis (DSM-IV criteria). Individual data points are shown in the scatter plot. The width of the data shown for each score is proportional to the number of subjects with that score. The red line shows the median score. The data were analyzed with a Mann–Whitney test (* denotes p=0.047).
Analysis of Cocaine KMSK “Cutpoint” Scores by Genotype
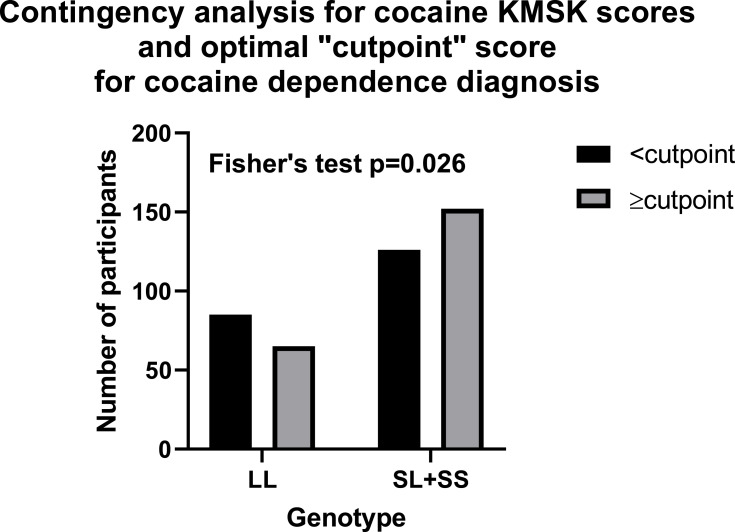
We found above that cocaine, but not heroin or alcohol KMSK scores differed by tandem repeat genotype. We therefore focused further analyses on the association of the 5HTTPLR genotype with cocaine. We did not have available formal DSM-IV diagnoses for cocaine dependence. However, as mentioned above, cocaine KMSK scores have high concurrent validity with the DSM-IV cocaine dependence diagnosis.49–51 Therefore, based on the most recent evaluation, cocaine KMSK scores ≥ cutpoint (ie, score ≥9) can be a useful surrogate for the cocaine dependence diagnosis.38 We carried out a contingency analysis to determine if scores ≥ cutpoint for cocaine are associated with tandem repeat genotype. Fisher’s exact test (p=0.026) shows that the [SL+SS] genotype was associated with greater odds of cocaine KMSK scores ≥ cutpoint versus the [LL] genotype (Figure 2). Thus, the odds ratio for the [SL+SS] genotype was 1.58 (95% CI: 1.07–2.36).
Figure 2.
Contingency analysis in the whole cohort by tandem repeat genotype, for cocaine KMSK scores < or ≥ cutpoint (ie, for a score of 9) for the cocaine dependence diagnosis; see Figure 1 and Table 2.
Since there had been an apparent effect of gender in the above analysis of cocaine KMSK scores, we carried out a multiple logistic regression for this analysis, adjusting for gender. The predicted event was the presence of a cocaine score ≥ cutpoint. The [LL] genotype was used as the reference group, and females were used as the reference group for gender. In this regression, there was a significant effect of genotype, with an odds ratio of 1.52 (95% CI: 1.01–2.28; p=0.043) for the [SL+SS] versus the LL genotype. An effect of gender was also detected, with an odds ratio of 1.96 (95% CI: 1.27–30.03; p=0.0025) of males versus females. This regression did not violate the Hosmer–Lemeshow test. The area under the receiver operating curve (ROC) was relatively modest (0.60; 95% CI:0.55–0.65), but significantly above chance. Neither rs1696528 or rs2066713 genotypes showed an association with cutpoint scores for cocaine (not shown).
Analysis of Time of Cocaine Escalation by Tandem Repeat Genotype
As mentioned above and previously reported,36 the OD and NOD groups had a range of exposure to cocaine, as measured by KMSK scales. We therefore examined whether the time of escalation of cocaine use differed by SLCA4 genotype, focusing on the OD+NOD groups combined. The HC group was excluded from this analysis since its members typically reported no lifetime use of cocaine. As mentioned in the methods, time of escalation is calculated as age of onset of heaviest use minus age of first use (in whole years).33 Therefore, an escalation time of “0” indicates that first use and onset of heaviest use of cocaine occurred in the same year (ie, the most rapid escalation that can be detected with this method of measurement).33 Larger escalation scores thus indicate a longer period elapsing between first use and onset of heaviest use.
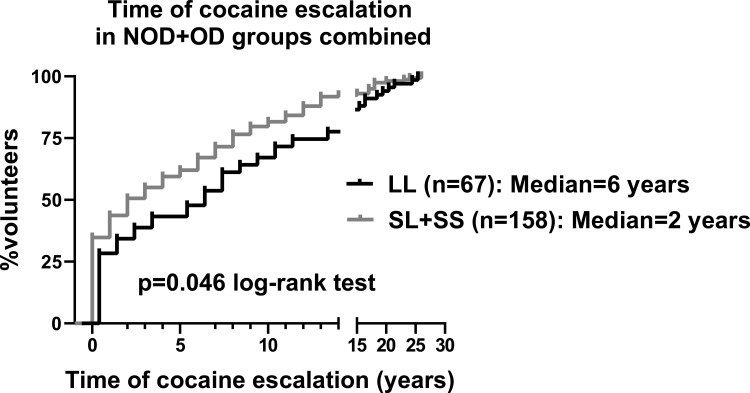
We found that the time of cocaine escalation was significantly faster in the SL+SS genotype, compared to the [LL] genotype, with median times of escalation of 2 and 6 years, respectively (Log Rank test; p=0.046) (Figure 3). Intriguingly, there was a significant correlation between cocaine KMSK scores, and time of cocaine escalation (Spearman r=+0.14; p=0.01). We found that cocaine escalation values did not differ by gender (not shown).
Figure 3.
Kaplan–Meier survival curve for time of cocaine escalation by tandem repeat genotype, in the NOD+OD groups. Time of escalation is defined as: age of onset of heaviest use – age of first use (in years). Data are presented for all the volunteers in the NOD+OD groups who had cocaine escalation values. Values were analyzed with the log-rank (Mantel-Cox) test.
Discussion
Identification of the subpopulations at elevated risk for rapid escalation of cocaine use is important for the development of dynamic models of drug addiction and treatment. Meta-analysis of twin studies showed that the heritability of all addictive diseases ranges from 40% to 60%.52,53
Although there are likely multiple contributing factors in developing substance dependences, including multiple genes with subtle effects, this study was focused on the tandem repeats in the functional polymorphism in the SERT promoter (5-HTTLPR) in a cohort of subjects of European ancestry from the Netherlands, including subjects with current opioid dependence, lifetime opioid use without opioid dependence, and healthy controls. In contrast to some previous findings showing a significant association of heroin dependence with the S allele of 5-HTTLPR in Caucasians,20,22,23 we did not find a significant association of short (S) or long (L) forms of the repeats with the categorical opioid dependence diagnosis. Reasons for this discrepancy include methodological differences. For example, one prior study in Caucasians included only males.23 Also, the difference was found only between the homozygous genotypes (SS versus LL), comparing persons with heroin dependence versus healthy controls23 (ie, without including persons exposed to opioids but never becoming opioid dependent: NOD).
Earlier studies reported that a haplotype, including 5-HTTLPR and rs16965628, was associated with obsessive compulsive disorder.54 Also, rs16965628 was found to modulate task-related activation in ventral prefrontal cortex in patients with posttraumatic stress disorder.55 In this study, we did not find any significant association of the rs16965628 or rs2066713 genotype with the categorical opioid dependence diagnosis. The limited sample size could explain the lack of associations of these intronic SNPs with opioid dependence here. However, it is also possible that associations of these intronic SNPs with opioid dependence can only be detected in populations of specific genetic background.
Other studies have shown that stratification into early and late onset subgroups may better define the role of SLC6A4 variants in the development of alcohol dependence.56 Overall, this suggests that the role of SLC6A4 variants may be examined more broadly with respect to age-related phenotypes or disease trajectory (as carried out here), rather than only by the presence of a diagnosis.
We did not have available categorical diagnoses of cocaine dependence in this cohort. However, the cocaine KMSK scores allowed us to examine if subjects had scores ≥cutpoint (score=9; shown to have high concurrent validity with the diagnosis, in a different cohort).38 We first determined that in the whole cohort, subjects with the [SL+SS] genotype had greater cocaine exposure than those with the [LL] genotype, and this effect was due primarily to the males (the larger group). We then found a greater proportion of subjects with the [SL+SS] genotype had cocaine KMSK scores ≥cutpoint for a cocaine dependence diagnosis, compared to the LL genotype. This effect of genotype also survived adjustment for gender, in a logistic regression. A prior study did not find an association of this genotype with cocaine dependence, but this was in an African American cohort,25 rather than a Caucasian cohort, as studied here. A recent study shows that cocaine users with the [SL+SS] genotype have increased subjective responses to i.v. cocaine challenge, compared to users with the LL genotype.16
Escalation of drug use has emerged as an important phenotype in disease trajectory and has been extensively studied preclinically (primarily in rodents).32,57,58 We found here that in the two groups which had cocaine exposure (ie, OD+NOD), subjects with the [SL+SS] genotype exhibited a more rapid time of cocaine escalation than the LL genotype. The magnitude of this difference of time of cocaine escalation was considerable, with median times of 2 and 6 years, for the [SL+SS] and LL genotypes, respectively. To our knowledge, this is the first demonstration that the 5-HTTLPR polymorphism is a genetic risk factor for rapid escalation of cocaine use in humans. More rapid escalation may be associated with more severe bio-behavioral consequences59–61 and allows less time for preventive clinical intervention. Intriguingly, rats with a genetic knockout in the SLC6A4 gene show enhanced cocaine self-administration.7 Selective knockdown of SERT in the dorsal raphe (the main location of serotonergic cell bodies in the CNS) also affected cocaine intake in rats, depending on high versus low exposure.3 Taken together, these findings suggest that the genetic status of SERT does affect responsiveness to cocaine, as well as vulnerability to rapid escalation of cocaine intake, in a Caucasian population.
Strength, Limitations, and Design Considerations
This study has some strengths. First, we have used more stringent criteria for heroin addiction and analyzing SERT polymorphisms in subjects participating in methadone maintenance treatment (MMT), while other studies rely on DSM-IV criteria that may be less stringent. Second, to minimize an effect of population stratification, we have analyzed 5-HTTLPR variants in subjects with 70% of European ancestry, based on 155 ancestral informative markers (AIMs) [42].
However, this study has also some limitations. Firstly, the relatively small number of females in all groups could have limited our capacity to detect effects in this gender. Also, recall bias cannot be excluded with this type of design, which is commonly used in the SUD field. However, it is unlikely that such potential recall bias would vary systematically with 5HTTLPR genotype. Future larger studies with larger “n” in each of the NOD and OD groups would also be helpful, as it may be that cocaine-related genotypic associations are stronger in one of the groups.
Conclusions
This study suggests that the SL+SS serotonin promoter repeat genotype is associated with increased vulnerability to development of heavy cocaine use in Caucasians with opioid dependence or those repeatedly exposed to non-medical opioids. We also provide initial evidence that the SL+SS genotype is associated with more rapid escalation of cocaine use, and may thus be associated with less available time for clinical intervention. Due to the relatively small sample size, our findings need replication in a larger independent sample.
Acknowledgments
We gratefully acknowledge support from Dr. Miriam & Sheldon G. Adelson Medical Research Foundation [MJK] and Central Committee on the Treatment of Heroin Addicts (CCBH) and ZonMw 310000465 [PB, JVR, & WVDB]. The Authors are grateful to Dr. Orna Levran (Laboratory on the Biology of Addictive Diseases, the Rockefeller University) for determination of Ancestral Informative Markers (AIMs), and expert advice.
Abbreviations
5-HTTLPR, serotonin-transporter-linked polymorphic region; 95% CI, 95% conference interval; AIMs markers, Ancestral informative markers; HAT, heroin-assisted therapy subjects (had opioid dependence diagnosis); IQR, inter-quartile range; KMSK scale, Kreek–McHugh–Schluger–Kellogg (KMSK) scale, measuring exposure to specific drugs dimensionally; MMT, methadone-maintained subjects (had opioid dependence diagnosis); N/A, not applicable; NOD, Non-opioid dependent group; subjects who were exposed to non-medical opioids, but did not have a lifetime opioid dependence diagnosis; OD, opioid dependence diagnosis (DSM IV criteria); OR, odds ratio; SERT, serotonin transporter; SLC6A4, serotonin transporter gene; SUD, substance use disorders.
Disclosure
The authors have no conflicts of interest to declare.
References
- 1.Kirby LG, Zeeb FD, Winstanley CA. Contributions of serotonin in addiction vulnerability. Neuropharmacology. 2011;61(3):421–432. doi: 10.1016/j.neuropharm.2011.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237(4819):1219–1223. doi: 10.1126/science.2820058 [DOI] [PubMed] [Google Scholar]
- 3.Verheij MMM, Contet C, Karel P, et al. Median and Dorsal Raphe Serotonergic Neurons Control Moderate Versus Compulsive Cocaine Intake. Biol Psychiatry. 2018;83(12):1024–1035. doi: 10.1016/j.biopsych.2017.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tao R, Ma Z, Auerbach SB. Alteration in regulation of serotonin release in rat dorsal raphe nucleus after prolonged exposure to morphine. J Pharmacol Exp Ther. 1998;286(1):481–488. [PubMed] [Google Scholar]
- 6.Mash DC, Staley JK, Izenwasser S, Basile M, Ruttenber AJ. Serotonin transporters upregulate with chronic cocaine use. J Chem Neuroanat. 2000;20(3–4):271–280. doi: 10.1016/S0891-0618(00)00102-2 [DOI] [PubMed] [Google Scholar]
- 7.Caffino L, Verheij MMM, Que L, Guo C, Homberg JR, Fumagalli F. Increased cocaine self-administration in rats lacking the serotonin transporter: a role for glutamatergic signaling in the habenula. Addict Biol. 2019;24(6):1167–1178. doi: 10.1111/adb.12673 [DOI] [PubMed] [Google Scholar]
- 8.Oliva JM, Urigüen L, Pérez-Rial S, Manzanares J. Time course of opioid and cannabinoid gene transcription alterations induced by repeated administration with fluoxetine in the rat brain. Neuropharmacology. 2005;49(5):618–626. doi: 10.1016/j.neuropharm.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 9.Valenza M, Butelman ER, Kreek MJ. Effects of the novel relatively short-acting kappa opioid receptor antagonist LY2444296 in behaviors observed after chronic extended-access cocaine self-administration in rats. Psychopharmacology. 2017;66(6):2621–2624. doi: 10.1007/s00213-017-4647-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valenza M, Windisch KA, Butelman ER, Reed B, Kreek MJ. Effects of Kappa opioid receptor blockade by LY2444296 HCl, a selective short-acting antagonist, during chronic extended access cocaine self-administration and re-exposure in rat. Psychopharmacology. 2020;237(4):1147–1160. doi: 10.1007/s00213-019-05444-4 [DOI] [PubMed] [Google Scholar]
- 11.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527 [DOI] [PubMed] [Google Scholar]
- 12.Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Hum Genet. 1997;101(2):243–246. doi: 10.1007/s004390050624 [DOI] [PubMed] [Google Scholar]
- 13.Heils A, Teufel A, Petri S, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66(6):2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x [DOI] [PubMed] [Google Scholar]
- 14.Little KY, McLaughlin DP, Zhang L, et al. Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. Am J Psychiatry. 1998;155(2):207–213. doi: 10.1176/ajp.155.2.207 [DOI] [PubMed] [Google Scholar]
- 15.Odgerel Z, Talati A, Hamilton SP, Levinson DF, Weissman MM. Genotyping serotonin transporter polymorphisms 5-HTTLPR and rs25531 in European- and African-American subjects from the National Institute of Mental Health’s Collaborative Center for Genomic Studies. Transl Psychiatry. 2013;3(9):e307. doi: 10.1038/tp.2013.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patriquin MA, Hamon SC, Harding MJ, et al. Genetic moderation of cocaine subjective effects by variation in the TPH1, TPH2, and SLC6A4 serotonin genes. Psychiatr Genet. 2017;27(5):178–186. doi: 10.1097/ypg.0000000000000178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vergne DE, Nemeroff CB. The interaction of serotonin transporter gene polymorphisms and early adverse life events on vulnerability for major depression. Curr Psychiatry Rep. 2006;8(6):452–457. doi: 10.1007/s11920-006-0050-y [DOI] [PubMed] [Google Scholar]
- 18.Gelernter J. SLC6A4 polymorphism, population genetics, and psychiatric traits. Hum Genet. 2014;133(4):459–461. doi: 10.1007/s00439-013-1412-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovallo WR, Enoch MA, Yechiam E, et al. Differential impact of serotonin transporter activity on temperament and behavior in persons with a family history of alcoholism in the Oklahoma Family Health Patterns Project. Alcohol Clin Exp Res. 2014;38(6):1575–1581. doi: 10.1111/acer.12412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin PY, Wu YS. Association between serotonin transporter gene polymorphisms and heroin dependence: a meta-analytic study. Neuropsychiatr Dis Treat. 2016;12:3061–3067. doi: 10.2147/NDT.S120786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer IE, Graham DP, Soares JC, Nielsen DA. Serotonergic gene variation in substance use pharmacotherapy: a systematic review. Pharmacogenomics. 2015;16(11):1307–1314. doi: 10.2217/pgs.15.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao J, Hudziak JJ, Multi-Cultural LD. Association of the Serotonin Transporter Gene (SLC6A4) with Substance Use Disorder. Neuropsychopharmacol. 2013;38(9):1737–1747. doi: 10.1038/npp.2013.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerra G, Garofano L, Santoro G, et al. Association between low-activity serotonin transporter genotype and heroin dependence: behavioral and personality correlates. Comparative Study. Am j Med Genetics Part B Neuropsychiatric Genetics. 2004;126B(1):37–42. doi: 10.1002/ajmg.b.20111 [DOI] [PubMed] [Google Scholar]
- 24.Tristan-Noguero A, Fernandez-Castillo N, Roncero C, et al. Lack of association between the LPR and VNTR polymorphisms of the serotonin transporter gene and cocaine dependence in a Spanish sample. Psychiatry Res. 2013;210(3):1287–1289. doi: 10.1016/j.psychres.2013.09.004 [DOI] [PubMed] [Google Scholar]
- 25.Patkar AA, Berrettini WH, Hoehe M, et al. No association between polymorphisms in the serotonin transporter gene and susceptibility to cocaine dependence among African-American individuals. Psychiatr Genet. 2002;12(3):161–164. doi: 10.1097/00041444-200209000-00007 [DOI] [PubMed] [Google Scholar]
- 26.Leri F, Stewart J, Fischer B, et al. Patterns of opioid and cocaine co-use: a descriptive study in a Canadian sample of untreated opioid-dependent individuals. Exp Clin Psychopharmacol. 2005;13(4):303–310. doi: 10.1037/1064-1297.13.4.303 [DOI] [PubMed] [Google Scholar]
- 27.McCall Jones C, Baldwin GT, Compton WM. Recent Increases in Cocaine-Related Overdose Deaths and the Role of Opioids. Am J Public Health. 2017;107(3):430–432. doi: 10.2105/ajph.2016.303627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Min JE, Krebs E, et al. Polydrug use and its association with drug treatment outcomes among primary heroin, methamphetamine, and cocaine users. Int J Drug Policy. 2017;49:32–40. doi: 10.1016/j.drugpo.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sloan ME, Gowin JL, Yan J, et al. Severity of alcohol dependence is associated with the fatty acid amide hydrolase Pro129Thr missense variant. Addict Biol. 2017;23(1):474–484. doi: 10.1111/adb.12491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson CB, Varon J, Ho A, Marks KM, Talal AH, Kreek MJ. Identification of substance use and dependence among patients with viral hepatitis. Digestive Liver Dis. 2010;42(9):650–656. doi: 10.1016/j.dld.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Comparative Study Research Support, U.S. Gov’t, P.H.S. Psychopharmacology. 2004;175(1):26–36. doi: 10.1007/s00213-004-1778-x [DOI] [PubMed] [Google Scholar]
- 32.Zernig G, Ahmed SH, Cardinal RN, et al. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology. 2007;80(2–3):65–119. doi: 10.1159/000103923 [DOI] [PubMed] [Google Scholar]
- 33.Butelman ER, Chen CY, Brown KG, Kreek MJ. Escalation of drug use in persons dually diagnosed with opioid and cocaine dependence: gender comparison and dimensional predictors. Drug Alcohol Depend. 2019;205:107657. doi: 10.1016/j.drugalcdep.2019.107657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanken P, van den Brink W, Hendriks VM, et al. Heroin-assisted treatment in the Netherlands: history, findings, and international context. Eur neuropsychopharmacol. 2010;20(Suppl 2):S105. doi: 10.1016/S0924-977X(10)70001-8 [DOI] [PubMed] [Google Scholar]
- 35.Zaaijer ER, Bruijel J, Blanken P, et al. van den Brink W. Personality as a risk factor for illicit opioid use and a protective factor for illicit opioid dependence. Drug Alcohol Depend. 2014;145:101–105. doi: 10.1016/j.drugalcdep.2014.09.783 [DOI] [PubMed] [Google Scholar]
- 36.Randesi M, van den Brink W, Levran O, et al. Variants of opioid system genes are associated with non-dependent opioid use and heroin dependence. Drug Alcohol Depend. 2016;168:164–169. doi: 10.1016/j.drugalcdep.2016.08.634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuferov V, Randesi M, Butelman ER, et al. Association of variants of prodynorphin promoter 68-bp repeats in caucasians with opioid dependence diagnosis: effect on age trajectory of heroin use. Neurosci Lett. 2019;704:100–105. doi: 10.1016/j.neulet.2019.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butelman ER, Chen CY, Fry RS, Kimani R, Levran O, Ott J. Correa da Rosa J, Kreek MJ. Re-evaluation of the KMSK scales, rapid dimensional measures of self-exposure to specific drugs: gender-specific features. Drug Alcohol Depend. 2018;190:179–187. doi: 10.1016/j.drugalcdep.2018.05.028 [DOI] [PubMed] [Google Scholar]
- 39.Kellogg SH, McHugh PF, Bell K, et al. The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend. 2003;69(2):137–150. doi: 10.1016/S0376-8716(02)00308-3 [DOI] [PubMed] [Google Scholar]
- 40.Crystal HA, Hamon S, Randesi M, et al. A C17T polymorphism in the mu opiate receptor is associated with quantitative measures of drug use in African American women. Research Support, N.I.H., Extramural. Addict Biol. 2012;17(1):181–191. doi: 10.1111/j.1369-1600.2010.00265.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuferov V, Butelman ER, Kreek MJ. Gender-specific association of functional prodynorphin 68 bp repeats with cannabis exposure in an African American cohort. Neuropsychiatr Dis Treat. 2018;14:1025–1034. doi: 10.2147/NDT.S159954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levran O, Awolesi O, Shen PH, Adelson M, Kreek MJ. Estimating ancestral proportions in a multi-ethnic US sample: implications for studies of admixed populations. Hum Genomics. 2012;6(1):2. doi: 10.1186/1479-7364-6-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ducci F, Roy A, Shen PH, et al. Association of substance use disorders with childhood trauma but not African genetic heritage in an African American cohort. Am J Psychiatry. 2009;166(9):1031–1040. doi: 10.1176/appi.ajp.2009.08071068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levran O, Londono D, O’Hara K, et al. Genetic susceptibility to heroin addiction: a candidate gene association study. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. Genes Brain Behav. 2008;7(7):720–729. doi: 10.1111/j.1601-183X.2008.00410.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasieni PD. From genotypes to genes: doubling the sample size. Biometrics. 1997;53(4):1253–1261. doi: 10.2307/2533494 [DOI] [PubMed] [Google Scholar]
- 46.Randesi M, van den Brink W, Levran O, et al. VMAT2 gene (SLC18A2) variants associated with a greater risk for developing opioid dependence. Pharmacogenomics. 2019;20(5):331–341. doi: 10.2217/pgs-2018-0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Randesi M, Levran O, den Brink WV, et al. Further evidence for the association of GAL, GALR1 and NPY1R variants with opioid dependence. Pharmacogenomics. 2020;21(13):903–917. doi: 10.2217/pgs-2020-0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Randesi M, van den Brink W, Levran O, et al. Dopamine gene variants in opioid addiction: comparison of dependent patients, nondependent users and healthy controls. Pharmacogenomics. 2018;19(2):95–104. doi: 10.2217/pgs-2017-0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kellogg SH, McHugh PF, Bell K, et al. The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend. 2003;69(2):137–150. doi: 10.1016/s0376-8716(02)00308-3 [DOI] [PubMed] [Google Scholar]
- 50.Tang YL, Khoury L, Bradley B, Gillespie CF, Ressler KJ, Cubells JF. Substance use disorders assessed using the Kreek-McHugh-Schluger-Kellogg (KMSK) scale in an urban low-income and predominantly African American sample of primary care patients. Am j Addictions. 2011;20(3):292–299. doi: 10.1111/j.1521-0391.2011.00121.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butelman ER, Chen CY, Fry RS, Kimani R, Levran O, Ott J. Re-evaluation of the KMSK scales, rapid dimensional measures of self-exposure to specific drugs: gender-specific features. Drug Alcohol Depend. 2018;190:179–187. doi: 10.1016/j.drugalcdep.2018.05.028 [DOI] [PubMed] [Google Scholar]
- 52.Ducci F, Goldman D. The genetic basis of addictive disorders. Psychiatr Clin North Am. 2012;35(2):495–519. doi: 10.1016/j.psc.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6(7):521–532. doi: 10.1038/nrg1635 [DOI] [PubMed] [Google Scholar]
- 54.Wendland JR, Moya PR, Kruse MR, et al. A novel, putative gain-of-function haplotype at SLC6A4 associates with obsessive-compulsive disorder. Hum Mol Genet. 2008;17(5):717–723. doi: 10.1093/hmg/ddm343 [DOI] [PubMed] [Google Scholar]
- 55.Morey RA, Hariri AR, Gold AL, et al. Serotonin transporter gene polymorphisms and brain function during emotional distraction from cognitive processing in posttraumatic stress disorder. BMC Psychiatry. 2011;11(1):76. doi: 10.1186/1471-244x-11-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen YC, Prescott CA, Walsh D, et al. Different phenotypic and genotypic presentations in alcohol dependence: age at onset matters. J Stud Alcohol Drugs. 2011;72(5):752–762. doi: 10.15288/jsad.2011.72.752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mantsch JR, Ho A, Schlussman SD, Kreek MJ. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Research Support, U.S. Gov’t, P.H.S. Psychopharmacology. 2001;157(1):31–39. doi: 10.1007/s002130100744 [DOI] [PubMed] [Google Scholar]
- 58.Koob GF, Ahmed SH, Boutrel B, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27(8):739–749. doi: 10.1016/j.neubiorev.2003.11.007 [DOI] [PubMed] [Google Scholar]
- 59.Fagergren P, Smith HR, Daunais JB, Nader MA, Porrino LJ, Hurd YL. Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. Eur J Neurosci. 2003;17(10):2212–2218. doi: 10.1046/j.1460-9568.2003.02636.x [DOI] [PubMed] [Google Scholar]
- 60.Valenza M, Picetti R, Yuferov V, Butelman ER, Kreek MJ. Strain and cocaine-induced differential opioid gene expression may predispose Lewis but not Fischer rats to escalate cocaine self-administration. Neuropharmacology. 2016;105:639–650. doi: 10.1016/j.neuropharm.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calipari ES, Beveridge TJ, Jones SR, Porrino LJ. Withdrawal from extended-access cocaine self-administration results in dysregulated functional activity and altered locomotor activity in rats. Eur J Neurosci. 2013;38(12):3749–3757. doi: 10.1111/ejn.12381 [DOI] [PMC free article] [PubMed] [Google Scholar]