Abstract
Background
Malignant melanoma was characterized by insensitive chemotherapy, drug resistance, and high metastatic ability, which resulted in the main reason for the mortality among skin-related cancers. The current agents were not sufficient to improve the treatment status of melanoma patients, and it was still needed to develop new chemotherapeutic drugs for melanoma. Our study aimed to study the anticancer effects and potential mechanisms of ouabain on melanoma cells.
Methods
The inhibitory effects of ouabain were determined by CCK8 and colony formation assays, and the morphological changes of melanoma cells were observed by inverted microscope. The apoptosis induction and cell cycle distribution were detected by annexin V/PI double staining and PI staining, respectively. The expression of the biomarker proteins in apoptosis and G2/M phase were determined by Western blotting analysis. The effects of ouabain on the migration of melanoma cells were measured by transwell migration assay and wound closure analysis. The potential mechanisms of ouabain in melanoma cells were analyzed by transcriptome sequencing.
Results
Our present study demonstrated that ouabain exhibited strong inhibitory effects on cell proliferation and triggered dramatical morphological changes of melanoma cells. Moreover, ouabain induced significant apoptosis in A375 rather than SK-Mel-28 cells via upregulation of bax expression and downregulation of bcl-2 expression. Consistently, ouabain treatment induced cell cycle arrest at G2/M phase in both A375 and SK-Mel-28 cells via upregulation of cyclin B1 and downregulation of cdc2 and cdc25c. Importantly, ouabain suppressed the migration of A375 and SK-Mel-28 cells. Furthermore, the transcriptome sequencing demonstrated that p53 and MAPK signaling pathway might play important roles in the inhibitory effects of ouabain.
Conclusion
Our study revealed that ouabain exhibited dramatical anticancer effects, which provided a novel application for cardiac glycoside drugs in the clinical treatment of melanoma.
Keywords: melanoma, ouabain, apoptosis, cell cycle, migration
Introduction
Melanoma originates from melanin-producing cells and neural crest cell precursors, and occupies more than 75% of the mortality rate of skin cancer.1 It was characterized by high metastatic ability, insensitive chemotherapy, and drug resistance, which posed a huge threat to clinical therapy for melanoma.2 The determination of the molecular pathology of melanoma remained complex and difficult because of the heterogeneous nature, such as different histologic feature, complicated cell origin, and diverse metastasis.3
The prognosis of melanoma remains poor, and the main medical treatment approaches in the clinic of melanoma include chemotherapy, immunotherapy, and molecular targeted therapy.4,5 Although dacarbazine was the most commonly used cytotoxic drug for melanoma, previous clinical trials have demonstrated that dacarbazine showed minimal to modest anticancer activity.6 Furthermore, the inhibitors that targeted the carcinogenic gene BRAF, NRAS, MEK, and Kit had provided therapeutic benefits for melanoma patients.7 However, the drug resistance seriously hampered the clinical application of these agents.7,8 The immune checkpoint inhibitors, such as nivolumab and pembrolizumab for PD1, and ipilimumab for CTLA-4, activated the killing function of T lymphocytes to exhibit the anticancer effects. Unfortunately, the clinical activity of these targeted immune antibodies were restricted and depended on the basic expression of PD1 or CTLA-4, the degree of lymphocyte infiltration in tumor tissues, the burden of tumor mutation, and tumor microsatellite instability.9–11 As reported, the overall survival at 5 years in advanced melanoma patients was 52% in nivolumab combined with ipilimumab group and 44% in nivolumab group, as compared with 26% in the ipilimumab group.12 Immunotherapy for melanoma patients was generally safe and well tolerated. However, the endocrinopathies related with immunotherapy also required long-term medications.13 Together, the clinical benefits of the above approaches were suppressed by the cellular cytotoxicity, drug resistance, and inaccurate patient medication. Hence, these agents were not sufficient to improve the treatment status of melanoma patients, and it was still needed to develop new chemotherapeutic drugs for melanoma.
The novel application of old drugs provided a new selection for the anticancer therapy in clinic. Ouabain, a cardiotonic steroid and Na+/K+-ATPase inhibitor, was mainly used in the treatment of congestive heart failure and arrhythmia. Furthermore, the potential anticancer effects of ouabain have been widely reported in various cancers via autophagic cell death, apoptosis, and migration inhibition.14–17 However, no research regarding the effects of ouabain on melanoma cells were reported, even though ouabain had been used as a cardiotonic drug for many years. In our work, we evaluated the effects of ouabain on the proliferation, apoptosis induction, cell cycle distribution, and the migration of melanoma cells, and performed transcriptome sequencing to determine the possible mechanisms for its pharmacological activity, which prompted ouabain might be a potential anticancer agent for the clinical treatment of melanoma.
Materials and Methods
Chemicals and Reagents
The chemicals ouabain and encorafenib were purchased from MedChem Express (Princeton, NJ, USA). Cell counting kit-8 (CCK8), crystal violet staining solution, 4% paraformaldehyde fix solution, and cell cycle and apoptosis analysis kit were purchased from Beyotime Biotechnology (Shanghai, China). The apoptosis detection kit (Annexin V-PI double staining) was purchased from BD Biosciences (San Jose, CA, USA). Transwell cell culture inserts with 8 μM diameter were purchased from Millipore Corporation (Bedford, MA, USA). The antibodies against Bcl-2, Bax, and β-actin were purchased from Cell Signaling Technology (Beverly, MA, USA).
Cell Culture
The melanoma cells A375 and SK-Mel-28 were purchased from CoBioer Biotechnology (Nanjing, China). The normal skin cell line MelanA was obtained from Cell Bank of Institute for Biological Sciences. A375 cells were cultured in DMEM (Gibco, Grand Island, NY, USA), SK-Mel-28 cells were cultured in MEM (Gibco). Both cells were cultured with 10% fetal bovine serum (FBS, Gibco), 100 pg/mL streptomycin, 100 U/mL penicillin (Gibco), and grown in the humidified incubator (BB150, Thermo, Langenselbold, Germany) at 37°C with 5% CO2.
Cell Viability Assay
CCK8 assay was used in our study to determine the effects of the drugs on the cell viability of melanoma cells. A375 and SK-Mel-28 cells were seeded into 96-well plates with the density of 5,000 cells per well for 24 hours, and 2,000 cells for 48 hours and 72 hours. After incubating cells overnight, cells were then treated with different concentrations of the drugs for the indicated time. Subsequently, we added 20 μL CCK8 solution into each well, and detected at absorbance at 450 nm using a Microplate reader (Tecan, Durham, NC, USA). The cell viability and inhibition rate of the drugs were calculated according to the percentage of negative controls.
Colony Formation Assay
We used a colony formation assay to detect the inhibitory effects of ouabain on the proliferation of melanoma cells. The cells were digested and counted, and about 1,000 cells were seeded into a 6-well plate and incubated overnight. The cells were treated with indicated concentrations of ouabain for 48 hours, and cells were subsequently cultured in drug-free medium for another 7 days. The cells in the 6-well plate were then fixed by 4% paraformaldehyde for 15 minutes and stained by crystal violet staining solution for 15 minutes. After washing with three-distilled water 3-times, the images of colonies were captured and the number was counted macroscopically.
Apoptosis Detection
We analyzed the effects of drugs on the induction of apoptosis using Annexin V-PI double staining. The cells after drug treatment were digested by 0.25% trypsin without EDTA, and washed with PBS 2-times. The samples were subsequently suspended by 500 μL binding buffer, and then stained with 5 μL Annexin V and 5 μL PI for 15 minutes. The apoptotic rate were detected by FACSCalibur flow cytometry (BD Biosciences, San Jose, CA, USA).
Western Blotting Analysis
Western blotting assays were used to detect the relative expression of the cellular proteins. The samples after drug treatment were harvested with RIPA buffer (Cell Signaling Technology, Beverly, MA, USA) supplemented with protease inhibitor and phosphatase inhibitor (Cell Signaling Technology). The relative concentration of each sample was analyzed by enhanced BCA protein assay. Here, we used 40 μg total protein of each sample in the SDS-PAGE electrophoresis system, and the separated proteins in gels were subsequently transferred in 0.45 μm PVDF membranes. After blocking with 5% non-fat milk for 1 hour, the membranes were incubated in primary antibodies overnight at 4°C, the dilution ratio was based on the instruction manuals of the antibody. After washing with TBST 3-times every 15 minutes, the secondary antibodies were used to incubate the membranes for another 1 hour at room temperature. Lastly, the membranes were exposed by enhanced chemiluminescence reagents (Millipore, Bedford, MA, USA) by Bio-Rad image analyzer (Richmond, CA, USA).
Cell Cycle Distribution Analysis
The distribution of cell cycle was determined by single PI staining. Melanoma cells were treated by ouabain with indicated concentrations for 48 hours, cells were harvested and fixed using 75% cold ethanol at 4°C overnight, and subsequently stained by 25 μL PI and 5 μL RNase A for 30 minutes at 37°C. The distribution of cell cycle was detected by FACSCalibur flow cytometry (BD Biosciences, San Jose, CA, USA).
Transwell Migration Assay
Melanoma cells were harvested and 5×104 cells with 200 μL medium contained 1% FBS were seeded into the upper chamber of transwell inserts, and 500 μL medium with 10% FBS were added into the lower chamber using a 24-well plate. Cells were then treated with indicated concentrations of ouabain for 12 hours, and subsequently fixed by 4% paraformaldehyde for 10 minutes and stained by crystal violet staining solution for 10 minutes. After washing with three-distilled water 3-times, the migration of melanoma cells was captured by inverted microscopy (Olympus IX53, Tokyo, Japan).
Wound Closure Analysis
Melanoma cells were harvested and 8×105 cells were seeded into a 6-well plate and incubated overnight. The cells were evenly scratched by tips. After washing with PBS, cells were cultured in the medium with 1% FBS, and treated with indicated concentrations of ouabain for 24 hours. The closure of wound was captured by inverted microscopy at 12 hours and 24 hours. The space of wound closure was measured by Image-Pro Plus 6.0 software, and the rate of wound closure was calculated compared according to negative controls.
Transcriptome Sequencing
The global gene expression profiles of negative and ouabain-treated A375 cells were determined by RNA sequencing (RNA-seq). Total RNA of the samples were extracted using TRIzol® Reagent (Invitrogen, Carlsbad, CA), and genomic DNA were removed using DNase I (Takara Bio, Shiga, Japan). The RNA-seq transcriptome library was prepared following TruSeqTM RNA sample preparation Kit (Illumina Inc, San Diego, CA) using 1 μg of total RNA. Libraries were size selected for cDNA target fragments with 200–300 bp on 2% Low Range Ultra Agarose followed by PCR amplification. After quantified by TBS380, paired-end RNA-seq sequencing library was sequenced with the Illumina HiSeq X Ten/NovaSeq 6000 sequencer. RSEM (http://deweylab.biostat.wisc.edu/rsem/) was used to quantify gene abundances. EdgeR (http://www.bioconductor.org/packages/2.12/bioc/html/edgeR.html) was utilized for differential expression analysis. GO functional enrichment and KEGG pathway analysis were carried out by Goatools (https://github.com/tanghaibao/Goatools) and KOBAS (http://kobas.cbi.pku.edu.cn/home.do).
Statistical Analysis
The graphs and histograms were made by GraphPad Prism software (Version 5.01), and the results in our study were expressed as mean±SD using Student’s t-test (two-tailed). The P-value was taken for the statistical significance, *P<0.05, **P<0.01, and ***P<0.001.
Results
Ouabain Exhibited Strong Selective Anti-Proliferation Effects in Melanoma Cells
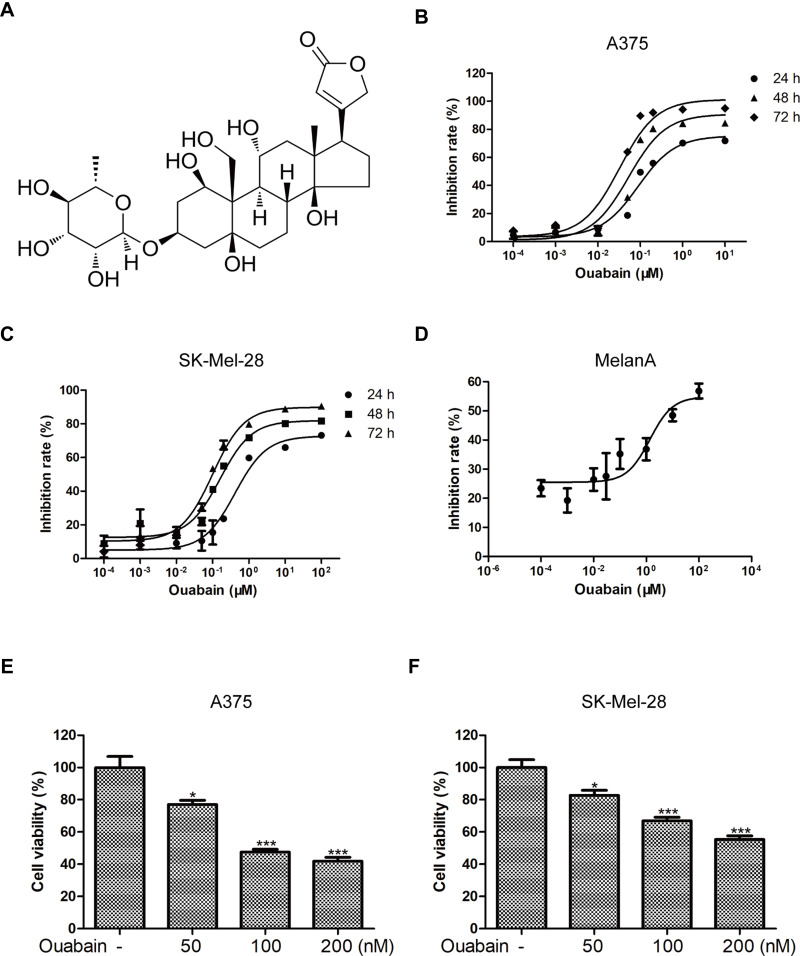
To investigate whether ouabain (Figure 1A) exerted inhibitory effects in melanoma cells, CCK8 assays were determined to detect the inhibition rate of ouabain and the cell viability of melanoma cells. Melanoma cells were treated with different concentrations of ouabain for 24 hours, 48 hours, and 72 hours. The data in Figure 1B and C showthat ouabain exhibited strong inhibitory effects on the proliferation of melanoma cells, and the IC50 values of ouabain in A375 cells were 153.11±22.69 nM (24 hours), 67.17±3.16 nM (48 hours), and 30.25±1.70 nM (72 hours), the IC50 values of ouabain in SK-Mel-28 cells were 772.14±141.48 nM (24 hours), 186.51±10.51 nM (48 hours), and 87.42±7.64 nM (72 hours), respectively. Importantly, we found that the IC50 of ouabain in MelanA cells was 9.63±3.31 μM (Figure 1D), which was much higher than that in A375 and Sk-mel-28 cells. Furthermore, ouabain greatly inhibited the cell viability of A375 and SK-Mel-28 cells in dose-dependent manner (Figure 1E and F). Above all, the results demonstrated that ouabain could selectively inhibit the proliferation of melanoma cells, and might be a potential drug for the clinical treatment of melanoma.
Figure 1.
Ouabain exerted strong inhibitory effects on the proliferation of melanoma cells. (A) The chemical structure of ouabain (C29H44O12, molecular weight: 584.66) is shown. The inhibitory effects of ouabain on A375 (B) and SK-Mel-28 (C) cells at 24 hours, 48 hours, and 72 hours were measured by CCK8 assays. (D) The inhibitory effects of ouabain on MelanA cells at 72 hours were also determined by CCK8 assays. The histograms showed the cell viability influenced by ouabain treatment in A375 (E) and SK-Mel-28 (F) cells for 48 hours. The results are showed as mean±SD by Student’s t-test (two-tailed). *P<0.05 and ***P<0.001.
Ouabain Triggered Dramatical Morphological Changes and Inhibited the Colony Formation of Melanoma Cells
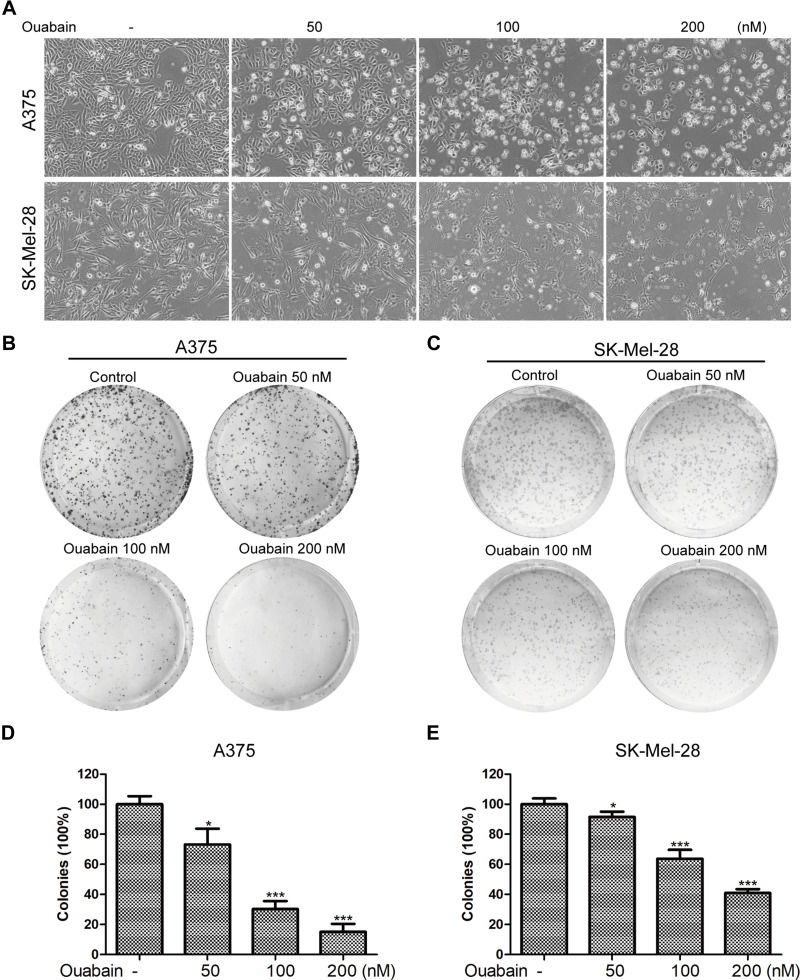
As the results in Figure 2A, we found that the morphology of A375 and SK-Mel-28 cells exhibited significant changes after the treatment of ouabain for 48 hours. A375 cells turned rounder, and too many dead cells were suspended in the medium with the increasing concentration of ouabain, while SK-Mel-28 cells turned bigger and cell edges became irregular. To investigate the effects of ouabain on the proliferation of melanoma cells, colony formation assays were performed in our study. As shown in Figure 2B–E, the results showed that ouabain treatment significantly reduced the colonies performed by A375 and SK-Mel-28 cells in a dose-dependent manner. Together, these data demonstrated that ouabain exerted significant inhibitory effects on the proliferation of melanoma cells.
Figure 2.
Ouabain influenced the morphology of melanoma cells and suppressed the colony formation of melanoma cells. (A). A375 and SK-Mel-28 cells were stimulated with indicated concentrations of ouabain for 48 hours, and the morphology of melanoma cells was observed. A375 (B) and SK-Mel-28 (C) cells were treated with indicated concentrations of ouabain for 48 hours, and melanoma cells were subsequently cultured in drug-free complete medium for another 5 days, the colonies were fixed by 4% paraformaldehyde, stained with crystal violet solution, and counted macroscopically. The histograms of A375 (D) and SK-Mel-28 (E) cells showed the colonies of each group compared with control. The results are shown as mean±SD by Student’s t-test (two-tailed). *P<0.05 and ***P<0.001.
Ouabain Induced Significant Cell Apoptosis in A375 Cells Rather Than SK-Mel-28 Cells
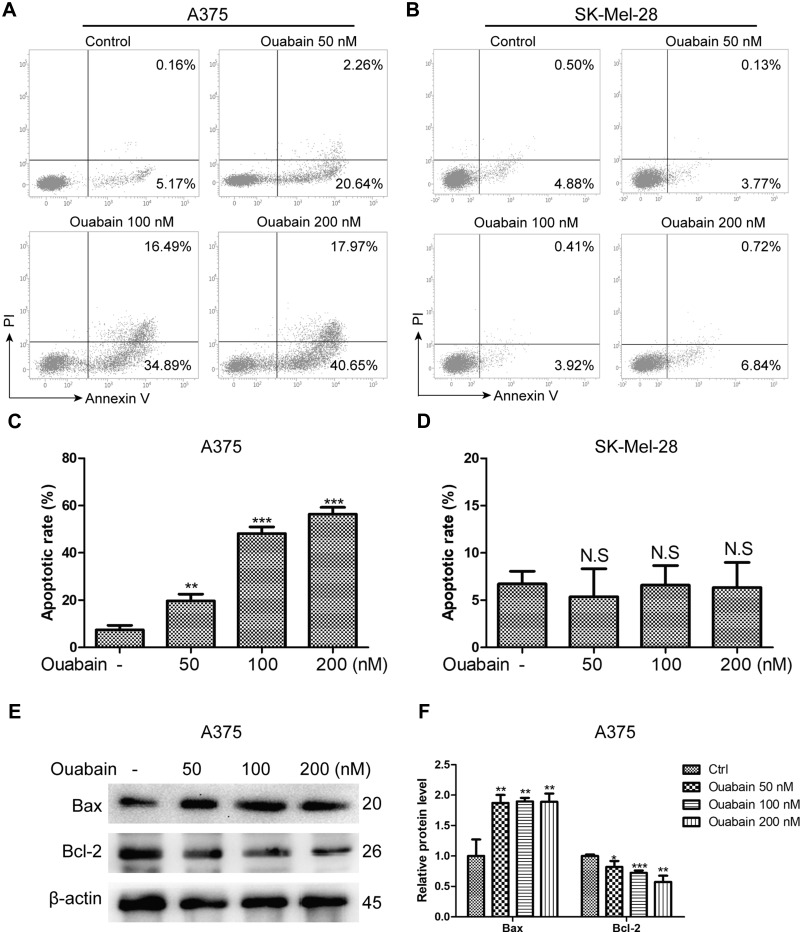
Induction of cell apoptosis was one of the main mechanisms for the majority of anti-cancer drugs. Here, we used Annexin V/PI double staining to analyze the effects of ouabain on apoptosis induction in melanoma cells. As the results in Figure 3A–D, we found that ouabain significantly induced apoptosis in A375 cells in a dose-dependent manner, while the effects of ouabain on SK-Mel-28 cells showed no significant difference after the treatment of indicated concentrations of ouabain. Furthermore, we evaluated the level of the apoptosis-related proteins after ouabain treatment. The results in Figure 3E–F showed that ouabain treatment inhibited the expression of pro-apoptosis protein bcl-2, and increased the expression of anti-apoptosis protein bax in A375 cells. Hence, these data revealed that ouabain exerted anti-cancer effects in A375 cells via induction of apoptosis rather than SK-Mel-28 cells.
Figure 3.
The effects of ouabain on apoptosis induction in A375 and SK-Mel-28 cells. Melanoma cells were treated with indicated concentrations of ouabain for 48 hours. The samples of A375 (A) and SK-Mel-28 (B) cells were stained by Annexin V/PI, the induction of apoptosis was detected by flow cytometry. The histograms of apoptotic rate in A375 (C) and SK-Mel-28 (D) cells are shown, which consisted of early apoptotic rate and late apoptotic rate. (E). The expression of bcl-2 and bax were determined by Western blotting analysis in A375 cells. (F). The histograms showed the relative protein level of each group compared with control. The results are shown as mean±SD by Student’s t-test (two-tailed). NS, no significant difference, *P<0.05, **P<0.01, and ***P<0.001.
Ouabain Induced Significant Cell Cycle Arrest at G2/M Phase in Melanoma Cells
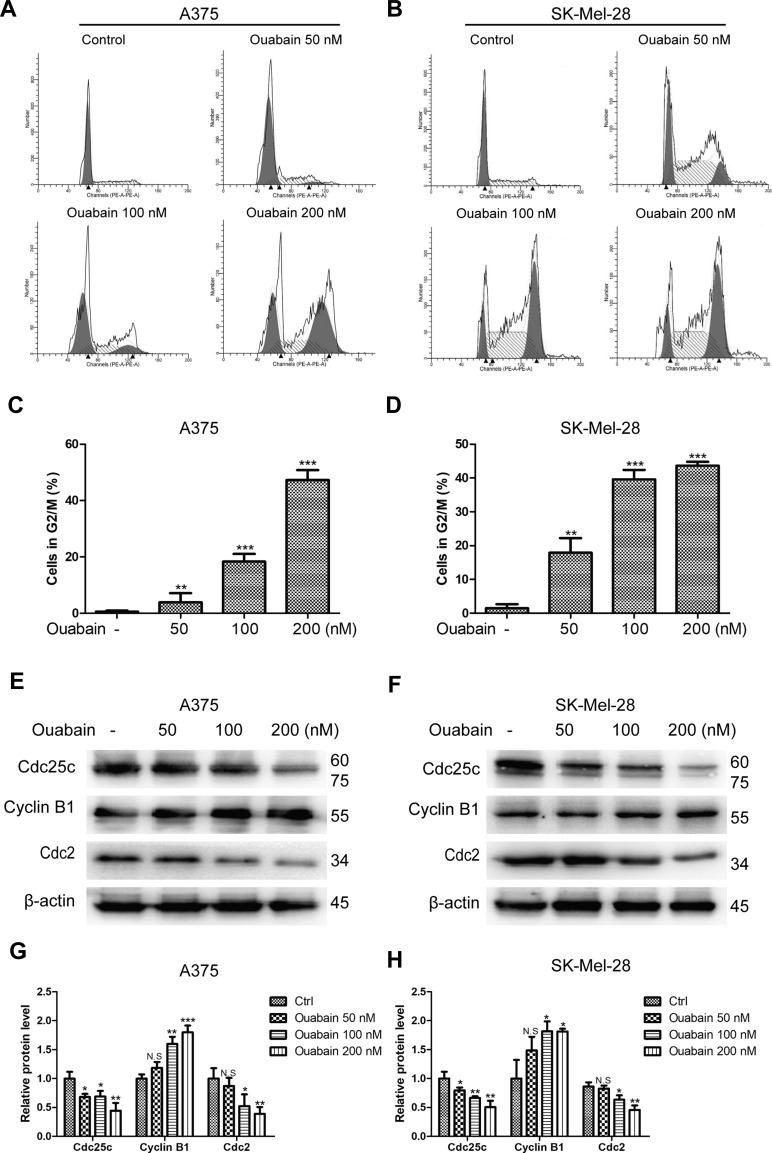
According to the special phenomenon in apoptosis induction, we furtherly evaluated the effects of ouabain on the cell cycle distribution in melanoma cells. As shown in Figure 4A–D, we found that ouabain dramatically induced cell cycle arrest at G2/M phase in both A375 and SK-Mel-28 cells. Furthermore, ouabain significantly suppressed the expression of cdc25c and cdc2, and promoted the expression of cyclin B1 in both A375 and SK-Mel-28 cells (Figure 4E–H). Taken together, ouabain exhibited strong effects on G2/M phase arrest in melanoma cells.
Figure 4.
The effects of ouabain on cell cycle distribution in A375 and SK-Mel-28 cells. Melanoma cells were treated with indicated concentrations of ouabain for 48 hours. The samples of A375 (A) and SK-Mel-28 (B) cells were stained by PI, the induction of cell cycle distribution was detected by flow cytometry. The histograms of cell cycle distribution in A375 (C) and SK-Mel-28 (D) cells are shown. The expression of cdc25c, cyclin B1, and cdc2 were determined by Western blotting analysis in A375 (E) and SK-Mel-28 (F) cells, and the histograms show the relative protein level of each group compared with control in A375 (G) and SK-Mel-28 (H) cells. The results are shown as mean±SD by Student’s t-test (two-tailed). NS, no significant difference, *P<0.05, **P<0.01, and ***P<0.001.
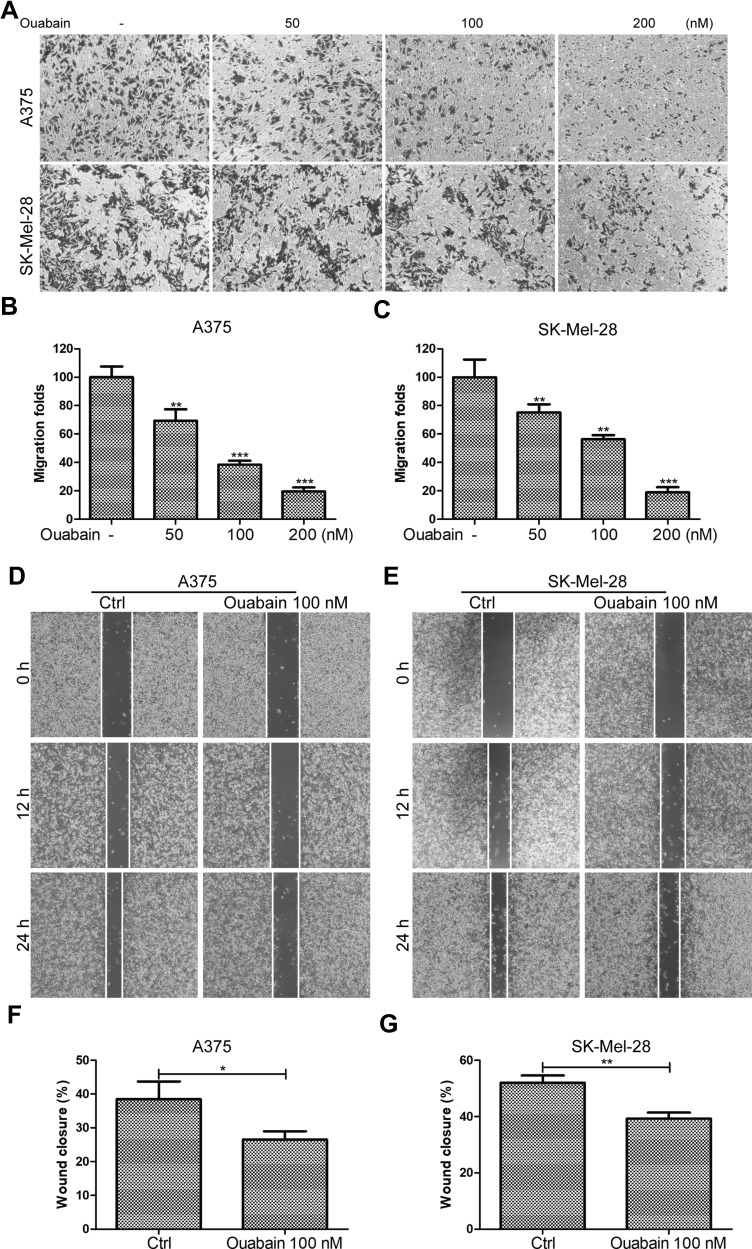
Ouabain Suppressed the Migration of Melanoma Cells
Tumor migration was an important indicator of malignant progression of melanoma. Here, we continued to study the effects of ouabain on the migration of melanoma cells. As shown in Figure 5A–C, the transwell migration assays showed that ouabain significantly inhibited the migration of A375 and SK-Mel-28 cells in a dose-dependent manner. Moreover, the wound closure analysis also demonstrated the great inhibitory effects of ouabain on the closure of melanoma cells in vitro. Above all, these data revealed that ouabain could exhibit strong inhibitory effects on the migration of melanoma cells.
Figure 5.
The effects of ouabain on cell migration in A375 and SK-Mel-28 cells. (A) Melanoma cells were treated with indicated concentrations of ouabain for 12 hours, and the samples of A375 and SK-Mel-28 cells were then fixed and stained by crystal violet, the migration of melanoma cells were captured by inverted microscopy. The histograms of migration folds in A375 (B) and SK-Mel-28 (C) cells are shown. Wound closure analysis for A375 (D) and SK-Mel-28 (E) cells were explored at 12 hours and 24 hours, and the histograms of the percentage of wound closure for A375 (F) and SK-Mel-28 (G) cells at 24 hours are shown. The results are shown as mean±SD by Student’s t-test (two-tailed). *P<0.05, **P<0.01, and ***P<0.001.
Validation of Pathway Rewiring in Ouabain-Treated Melanoma Cells Using Transcriptome Sequencing
Based on the strong inhibitory effects of ouabain on melanoma cells, we used high-throughput sequencing to analyze the gene expression of A375 cells with or without ouabain treatment. As the data in Figure 6A–B show, the heatmaps and volcano plots showed that the expression of 1398 genes were upregulated by ouabain treatment, and 1991 genes were decreased. To characterize the functional consequences of gene expression changes induced by ouabain treatment, we performed GO enrichment analysis of 3,389 differentially expressed genes (DEGs). The results in Figure 6C show regulation of the extrinsic apoptotic signaling pathway, DNA damage response, signal transduction by p53 class mediator, and regulation of stress-activated MAPK cascade were important functional groups in most of the comparisons. Furthermore, KEGG pathway enrichment analysis of DEGs showed that the p53 signaling pathway, MAPK signaling pathway, pathways in cancer, ECM-receptor interaction, and Hippo signaling pathway were significantly enriched (Figure 6D). These results might provide essential mechanisms for the anticancer effects of ouabain on melanoma cells.
Figure 6.
Transcriptome sequencing analysis of ouabain-mediated inhibitory functions in A375 cells. A375 cells were treated with 100 μM ouabain for 48 hours, and the samples were collected using TRIzol reagent for transcriptome sequencing. (A). The heatmaps of gene expression data from transcriptome analysis. (B). The volcano plots showed the DEGs between control and ouabain treatment groups. (C). The bubble diagram of top 20 ranked GO terms of DEGs. (D). KEGG pathway enrichment analysis of the genes affected by ouabain as described in (A).
Discussion
Melanoma was characterized by abnormal growth and amplification of atypical melanocytes and subsequent metastasis in the clinic. Although cytotoxic drugs, molecular targeted agents, and the antibodies against immune targets were chosen for the clinical treatment of melanoma, the benefits for melanoma patients were also restricted by several factors, such as the direct toxicity, drug resistance, or immunotherapy-related endocrinopathies. Hence, developing new agents or new anticancer applications of conventional drugs are still needed for melanoma treatment.
The novel application of old drugs provided new selection for anticancer therapy in the clinic. As a cardiotonic steroid and Na+/K+-ATPase inhibitor, ouabain has been used for the clinical treatment of several heart-related disease, such as atrial fibrillation and heart failure. Furthermore, multiple studies showed that ouabain exerted anticancer effects on lung, prostate, liver, breast cancer cells via anti-proliferative effects, autophagic cell death, apoptosis, and migration inhibition.14–16,18 Here, our study demonstrated that ouabain induced significant morphological changes and proliferation inhibition in melanoma cells. Inconsistently, Ouabain induced significant cell apoptosis in A375 cells rather than SK-Mel-28 cells. Moreover, Ouabain greatly induced cell cycle arrest at G2/M phase in both A375 and SK-Mel-28 cells, and the migration of melanoma cells was also inhibited by ouabain treatment.
Proliferation inhibition and apoptosis induction were the main features of most anticancer drugs in the clinic. As reported, ouabain exhibited double effects on cell proliferation and apoptosis. Low doses of ouabain served to prevent cell death and promote cell growth under serum deprivation condition in kidney cells via activation of the NF-κB pathway.19 On the other hand, ouabain induced apoptotic death in prostate cancer cells via caspase-dependent and mitochondria-dependent pathways.16 Our study showed ouabain exhibited strong antiproliferative effects in melanoma cells at nanomolar level, and dramatical morphological changes and colony formation inhibition were triggered by ouabain treatment. Furthermore, ouabain induced significant apoptosis in A375 cells via upregulation of anti-apoptosis protein bax and downregulation of pro-apoptosis protein bcl-2, which was consistent with the activities of ouabain in prostate cancer cells.16 Importantly, ouabain treatment induced no significant apoptosis in SK-Mel-28 cells, which might be decided by its special cell type and the genetic background of apoptosis-related protein.
Eukaryote cells were regulated by several cell cycle checkpoints via cyclin-dependent protein kinases (CDKs) and cyclins to prevent damaged and incomplete DNA in cell cycle progression. As reported, ouabain induced cell cycle arrest at S and G2/M phase in human osteosarcoma-derived U-2 OS cells,20 and G2/M phase arrest in thyroid cancer cells.21 Our study demonstrated that ouabain triggered G2/M phase arrest in both A375 and SK-Mel-28 cells. Cyclins and CDKs played important roles to regulate the progression of cell cycle. Cyclin B1 regulated the progression of G2/M transition and mitotic phase, which accumulated in the G2 phase, peaked in metaphase, and degraded at anaphase.22 We found that ouabain treatment significantly induced the expression of cyclin B1 in A375 and SK-Mel-28 cells. Moreover, cdc2 coupled with cyclin B1 promoted the production of maturation promoting factor (MPF) to regulate cell cycle transition from G2 phase to M phase.23 Cyclin B1/cdc2 complex was regulated by cdc25c via relieving the inhibitory phosphorylation of Thr14/Tyr15 residues on cdc2.24 The study showed that the expression of cdc2 and cdc25c were suppressed after ouabain treatment in A375 and SK-Mel-28 cells, which promoted the cell cycle arrest at G2/M phase induced by ouabain stimulation.
Melanoma was characterized by the highly metastatic nature, and the 5-year survival rate of melanoma patients was decreased from 91.3% to 16.6% after the formation of metastasis.25,26 Hence, the development of anti-metastasis drugs gradually received attention. In accordance with the inhibitory effects of ouabain on the migration of osteosarcoma and lung cancer cells,27,28 our study demonstrated that ouabain inhibited the migration of A375 and SK-Mel-28 cells according to wound closure analysis and transwell migration assays.
Transcriptome sequencing analysis revealed that ouabain triggered dramatic changes of the gene expression of A375 cells. GO enrichment analysis showed regulation of the extrinsic apoptotic signaling pathway, DNA damage response, signal transduction by p53 class mediator and regulation of stress-activated MAPK cascade were important functional groups in most of the comparisons, which were related with the p53 signaling pathway, MAPK signaling pathway, and pathways in cancer in KEGG pathway enrichment analysis. As reported, ouabain might induce reversible peripheral and central early DNA damage.29 Hence, the cell cycle arrested in G2/M induced by ouabain might be triggered by DNA damage. Moreover, cardiac glycoside drugs digoxin and ouabain inhibited the synthesis of p53 at nanomolar concentrations by activation of Src/MAPK signaling pathways via the binding to Na+/K+-ATPase.30 Therefore, the p53 signaling pathway and MAPK signaling pathway might play important roles in the induction of proliferation inhibition, apoptosis, and migration inhibition.
Together, our present work demonstrated that ouabain exerted strong inhibitory effects on the proliferation at nanomolar levels in both A375 and SK-Mel-28 cells, and ouabain selectively induced cell apoptosis in A375 rather than SK-Mel-28 cells via increasing the expression of pro-apoptosis protein bax and decreasing the anti-apoptosis protein bcl-2. Furthermore, ouabain significantly triggered cell cycle arrest at G2/M phase via regulating the expression of cdc25c, cdc2, and cyclin B1. Importantly, the migration of A375 and SK-Mel-28 cells were also inhibited by ouabain treatment. Transcriptome sequencing analysis revealed that the inhibitory effects of ouabain on melanoma cells might be related with the p53 signaling pathway and MAPK signaling pathway. In summary, our study showed that ouabain exhibited strong anticancer activities, which provided a novel application for cardiac glycoside drugs in the clinical treatment of melanoma.
Acknowledgment
This work was supported by the Science and Technology Development Fund of Nanjing Medical University (NMUB2018058).
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Lee C, Collichio F, Ollila D, et al. Historical review of melanoma treatment and outcomes. Clin Dermatol. 2013;31(2):141–147. doi: 10.1016/j.clindermatol.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Olbryt M. Molecular background of skin melanoma development and progression: therapeutic implications. Postepy Dermatol Alergol. 2019;36(2):129–138. doi: 10.5114/ada.2019.84590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastian BC. The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annu Rev Pathol. 2014;9(1):239–271. doi: 10.1146/annurev-pathol-012513-104658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padrik P, Valter A, Valter E, et al. Trends in incidence and survival of cutaneous malignant melanoma in Estonia: a population-based study. Acta Oncol. 2017;56(1):52–58. doi: 10.1080/0284186X.2016.1243804. [DOI] [PubMed] [Google Scholar]
- 5.Domingues B, Lopes JM, Soares P, et al. Melanoma treatment in review. Immunotargets Ther. 2018;7:35–49. doi: 10.2147/ITT.S134842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui C, Mao L, Chi Z, et al. A Phase II, randomized, double-blind, placebo-controlled multicenter trial of Endostar in patients with metastatic melanoma. Mol Ther. 2013;21(7):1456–1463. doi: 10.1038/mt.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karoulia Z, Gavathiotis E, Poulikakos PI. New perspectives for targeting RAF kinase in human cancer. Nat Rev Cancer. 2017;17(11):676–691. doi: 10.1038/nrc.2017.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gowrishankar K, Snoyman S, Pupo GM, et al. Acquired resistance to BRAF inhibition can confer cross-resistance to combined BRAF/MEK inhibition. J Invest Dermatol. 2012;132(7):1850–1859. doi: 10.1038/jid.2012.63. [DOI] [PubMed] [Google Scholar]
- 9.Buder-Bakhaya K, Hassel JC. Biomarkers for clinical benefit of immune checkpoint inhibitor treatment-A review from the melanoma perspective and beyond. Front Immunol. 2018;9:1474. doi: 10.3389/fimmu.2018.01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vilain RE, Menzies AM, Wilmott JS, et al. Dynamic changes in PD-L1 expression and immune infiltrates early during treatment predict response to PD-1 blockade in melanoma. Clin Cancer Res. 2017;23(17):5024–5033. doi: 10.1158/1078-0432.CCR-16-0698. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y, Xu J, Du C, et al. The predictive value of tumor mutation burden on efficacy of immune checkpoint inhibitors in cancers: a systematic review and meta-analysis. Front Oncol. 2019;9:1161. doi: 10.3389/fonc.2019.01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 13.Carreau NA, Pavlick AC. Nivolumab and ipilimumab: immunotherapy for treatment of malignant melanoma. Future Oncol. 2019;15(4):349–358. doi: 10.2217/fon-2018-0607. [DOI] [PubMed] [Google Scholar]
- 14.Pongrakhananon V, Chunhacha P, Chanvorachote P. Ouabain suppresses the migratory behavior of lung cancer cells. PLoS One. 2013;8(7):e68623. doi: 10.1371/journal.pone.0068623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trenti A, Grumati P, Cusinato F, et al. Cardiac glycoside ouabain induces autophagic cell death in non-small cell lung cancer cells via a JNK-dependent decrease of Bcl-2. Biochem Pharmacol. 2014;89(2):197–209. doi: 10.1016/j.bcp.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Chang YM, Shih YL, Chen CP, et al. Ouabain induces apoptotic cell death in human prostate DU 145 cancer cells through DNA damage and TRAIL pathways. Environ Toxicol. 2019;34(12):1329–1339. doi: 10.1002/tox.22834. [DOI] [PubMed] [Google Scholar]
- 17.Schneider NFZ, Cerella C, Simoes CMO, et al. Anticancer and immunogenic properties of cardiac glycosides. Molecules. 2017;22(11):22. doi: 10.3390/molecules22111932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winnicka K, Bielawski K, Bielawska A, et al. Antiproliferative activity of derivatives of ouabain, digoxin and proscillaridin A in human MCF-7 and MDA-MB-231 breast cancer cells. Biol Pharm Bull. 2008;31(6):1131–1140. doi: 10.1248/bpb.31.1131. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Zelenin S, Aperia A, et al. Low doses of ouabain protect from serum deprivation–triggered apoptosis and stimulate kidney cell proliferation via activation of NF-κB. J Am Soc Nephrol. 2006;17(7):1848–1857. doi: 10.1681/ASN.2005080894. [DOI] [PubMed] [Google Scholar]
- 20.Chou WH, Liu KL, Shih YL, et al. Ouabain induces apoptotic cell death through caspase- and mitochondria-dependent pathways in human osteosarcoma U-2 OS cells. Anticancer Res. 2018;38(1):169–178. doi: 10.21873/anticanres.12205. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, He M, Zhang Y, et al. Quantitative high-throughput drug screening identifies novel classes of drugs with anticancer activity in thyroid cancer cells: opportunities for repurposing. J Clin Endocrinol Metab. 2012;97(3):E319–28. doi: 10.1210/jc.2011-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Widrow RJ, Rabinovitch PS, Cho K, et al. Separation of cells at different times within G2 and mitosis by cyclin B1 flow cytometry. Cytometry. 1997;27(3):250–254. doi:. [DOI] [PubMed] [Google Scholar]
- 23.Grana X, Reddy EP. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene. 1995;11(2):211–219. [PubMed] [Google Scholar]
- 24.Jessus C, Ozon R. Function and regulation of cdc25 protein phosphate through mitosis and meiosis. Prog Cell Cycle Res. 1995;1:215–228. doi: 10.1007/978-1-4615-1809-9_17. [DOI] [PubMed] [Google Scholar]
- 25.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 26.Choi WI, Yim JJ, Park J, et al. Clinical characteristics and outcomes of H1N1-associated pneumonia among adults in South Korea. Int J Tuberc Lung Dis. 2011;15(2):270–275. [PubMed] [Google Scholar]
- 27.Shih YL, Au MK, Liu KL, et al. Ouabain impairs cell migration, and invasion and alters gene expression of human osteosarcoma U-2 OS cells. Environ Toxicol. 2017;32(11):2400–2413. doi: 10.1002/tox.22453. [DOI] [PubMed] [Google Scholar]
- 28.Shin HK, Ryu BJ, Choi SW, et al. Inactivation of Src-to-ezrin pathway: a possible mechanism in the ouabain-mediated inhibition of A549 cell migration. Biomed Res Int. 2015;2015:537136. doi: 10.1155/2015/537136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jornada LK, Valvassori SS, Arent CO, et al. DNA damage after intracerebroventricular injection of ouabain in rats. Neurosci Lett. 2010;471(1):6–9. doi: 10.1016/j.neulet.2009.12.072. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Zheng M, Li Z, et al. Cardiac glycosides inhibit p53 synthesis by a mechanism relieved by Src or MAPK inhibition. Cancer Res. 2009;69(16):6556–6564. doi: 10.1158/0008-5472.CAN-09-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]