Abstract
Background
Nrf2-Bach1 antioxidant signaling pathway is considered as one of the most important mechanisms of cellular resistance to oxidative injury. The effect of hyperoside (Hyp) on the expression and distribution of Bach1, the relationship of Hyp’s antioxidative effect and the influence of Bach1 remains unclear.
Purpose
The aim of this study was to investigate the role and mechanisms of Bach1 in the protective effect of Hyp on oxidative liver injury.
Methods
The protective effect of Hyp on oxidative stress injury was observed in vivo and in vitro. Next, the influence of Hyp on Bach1 expression and distribution, and competitive combination of Nrf2-Bach1 with ARE in H2O2-induced L02 cell was studied by Western blot, RT-PCR, immunofluorescence and CHIP assay. Finally, the expressions of Crm1, ERK and p38 and their roles on Hyp mediated nuclear export of Bach1 were investigated by Western blot.
Results
Hyp ameliorated the pathological damage, reduced the liver index, AST, ALT and MDA activities, and increased SOD and GSH levels in the CCl4-induced acute liver injury mouse model. Hyp attenuated H2O2-induced oxidative stress injury in L02 cells. Hyp promoted the early rapid redistribution of Bach1 from nucleus to cytoplasm. CHIP analyses demonstrated that Hyp enhanced the levels of Nrf2-ARE complex, and weakened the levels of Bach1-ARE complex within three hours. In addition, Hyp enhanced transport protein Crm1 expression and ERK1/2 activity. And LMB, a Crm1 inhibitor, attenuated the effect of Hyp on Bach1 nuclear export and anti-oxidation. U0126, an ERK1/2 inhibitor, reduced the effect of Hyp on Crm1 expression and the Bach1 redistribution.
Conclusion
The hepatoprotective mechanism of Hyp was related to improve Bach1 nuclear export depending on ERK1/2-Crm1 to upregulate the level of Nrf2 binding to ARE.
Keywords: hyperoside, oxidative stress, Bach1, Crm1, hepatoprotective
Introduction
The ability of liver to detoxify xenobiotics makes it particularly important in the maintenance of body health. Hepatic diseases such as acute or chronic liver injury, hepatic inflammation, fibrosis, cirrhosis and hepatocellular carcinoma are a major health burden globally. Oxidative stress induced by chemicals, heavy metals and ionizing radiation is a major pathophysiological process of liver impairment, such as hepatotoxins, alcoholic liver disease, nonalcoholic steatohepatitis, hepatocellular carcinoma, viruses, liver fibrosis and ischemia-reperfusion.1–7 Oxidative stress defined as an imbalance between production of free radicals and reactive metabolites, and their elimination by antioxidant systems. Carbon tetrachloride (CCl4) is a well-known hepatotoxic compound, commonly used for animal models in liver fibrosis and acute or chronic liver injury. CCl4 is metabolized by cytochrome P450 2E1 to generate ROS and triggers lipid peroxidation damage. In addition, CCl4 could lead to the apoptosis of damaged hepatocytes through oxidative stress and cytokines. The oxidative stress and inflammatory response play an important role in CCl4-induced acute liver injury.8
Nuclear factor E2-related factor 2 (Nrf2)-ARE antioxidant signaling pathway is considered as one of the most important mechanisms of cellular resistance to oxidative injury.9 There are two major molecules affect the activation of Nrf2, one is Kelch-like ECH associated protein-1 (Keap1), and the other is exogenous BTB-CNC homolog 1 (Bach1). During oxidative injury, the mercaptan of Keap1 is oxidized to disulfide. Nrf2 is then released from the complex and transferred into the nucleus to dimerize with the small Maf proteins (including MafG, MafK and MafF) that bind to the antioxidant response element (ARE) to regulate the expression of genes of phase II detoxification enzymes.10–12 Bach1 is a transcriptional factor and a member of cap “n” collar (CNC) and basic region leucine zipper factor family. Bach1 can either transactivate or repress gene expression via binding to the ARE. During oxidative stress, Bach1 binding small Maf proteins to generate heterodimers could compete with Nrf2 for ARE-binding and inhibits antioxidant gene transcription like heme oxygenase (HO-1) and NAD(P)H dehydrogenase quinone 1 (NQO1). However, only when Bach1 dissociates from ARE and exports from the nucleus upon oxidative stress, imported nuclear Nrf2 could become accessible to ARE and initiate the transcription of Nrf2 target downstream genes. Therefore, Nrf2 and Bach1 were found to regulate the expression of antioxidant gene HO-1 with mutually antagonistic effect.13
Therapeutically effective agents developed from natural sources are particularly attractive for hepatoprotective as they are generally considered nontoxic for humans. In flavonoids, such as luteolin, baicalein, baicalin, chrysin, apigenin, naringenin, quercetin, rutin and epigallocatechin gallate, there exists obvious hepatoprotective activity which is attributed to their antioxidant properties and the ability to mobilize endogenous antioxidant defense system. Hyp, a naturally occurring flavonoid present in fruits and vegetables, is able to exert protective effects, not only free radical scavenging, but also augment expression of cytoprotective and/or antioxidant genes in cardiovascular, lung and nervous system diseases.
Our previous study showed that Hyp attenuates H2O2-induced hepatic L02 cell oxidative damage via MAPK-dependent Keap1-Nrf2-ARE signaling pathway to upregulate HO-1 expression and enhance intracellular antioxidant activity, and Hyp could protect against oxidative stress-induced liver injury by regulating the PHLPP2-AKT-GSK-3β signaling pathway in vivo and in vitro.14,15 However, rarely research reports the effect of Hyp on the oxidative liver injury model animal. The influence of Hyp on the expression and distribution of Bach1, and the relationship of Hyp’s antioxidative effect and the influence of Bach1 remain unclear. In the present study, the effect of Hyp on CCl4-induced acute liver injury mouse model was evaluated; further, the influence and regulation mechanisms of Hyp on Bach1 in oxidative damage of hepatocytes was investigated for further elucidation of its role.
Materials and Methods
Chemicals and Reagents
Hyp was purchased from Aladdin (Shanghai, China). Leptomycin B (LMB) was purchased from Beyotime Biotechnology (Shanghai, China), tert-butylhydroquinone (t-BHQ), genistein (GEN) and H2O2 were purchased from Sigma-Aldrich (St Louis, MO, USA). Mouse antibodies against Bach1 (sc-271,211), HO-1 (sc-136,960), Crm1 (sc-74,454), MafK (sc-166,548), ERK1/2 (sc-135,900), p-ERK1/2 (sc-81,492), p38 (sc-7972), p-p38 (sc-7973), PCNA (sc-25,280), β-actin (sc-130,300) and all secondary antibodies conjugated with horseradish peroxidase IgG were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). All other chemicals were reagent grade and purchased from Sigma-Aldrich, unless otherwise indicated.
Acute Liver Injury Model and Groups
Male BALB/c mice of four to six weeks of age and 18–24 g, were obtained from the Laboratory Animal Center of Army Medical University. The mice were housed in plastic cages in a room kept at a constant temperature of 22±2°C, and they had free access to tap water and food throughout the study. The study was approved by the Medical Ethics Committee of Army Medical University, China, and was conducted according to the Regulations of Experimental Animal Administration issued by the State Committee of Science and Technology of People’s Republic of China. Mice were randomly divided into five groups (n=6/group): normal control, CCl4-treated control, positive control (Bifendate treatment group), and two Hyp treatment groups, which consisted of low (Hyp-L, 50 mg/kg), and high dose (Hyp-H, 100 mg/kg) treatments. All the mice except the normal control group were given a 0.02% CCl4/peanut oil mixture (v/v) once by intraperitoneal injection (ip, 10 mL/kg), while the control mice received peanut oil only. After that, Hyp was administered intragastrically (ig) at 50 and 100 mg/kg once daily for three consecutive days. Hepatoprotective drug bifendate (Bif) at 200 mg/kg was used as a positive control. The dosage of Bif or Hyp was 0.1 mL/10 g. The animals from the normal control group and CCl4-treated group were also given the same volume of vehicle for three consecutive days.
Histopathological Study, Liver Index and MDA, GSH and SOD Measurement
At the end of the experimental period, blood and liver sample were obtained. The blood samples were centrifuged at 500 g, 10 min at 4°C to obtain serum. A portion of liver tissue were fixed in 4% paraformaldehyde and embedded in paraffin. Sections (4 μm) were prepared, stained with H&E, and examined under a light microscope. The wet weight of liver was measured to calculate the liver index (the ratio of liver wet weight to body weight in the mouse). Remaining liver samples were homogenized in PBS at 4°C with a glass homogenizer. The crude homogenates were centrifuged at 500× g for 10 min, and the supernatants were obtained for further assays. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), malondialdehyde (MDA), glutathione (GSH) and superoxide dismutase (SOD) levels were measured using commercial kits (Jiancheng Institute of Biotechnology, Nanjing, China).
Cell Culture and Treatment
Human hepatocytes L02 cells obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) were cultured in HyClone RPMI 1640 medium (GE Healthcare, Beijing, China) supplemented with 10% FBS (Biological Industries, Cromwell, CT, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere at 5% CO2. Hyp and t-BHQ stock solutions were prepared in DMSO and diluted with RPMI 1640 culture medium immediately prior to the experiment. Control cells were treated with DMSO only at a final concentration of <0.1% (v/v). Oxidative stress conditions were produced by addition of H2O2 (100 μM) to L02 cells.14
Measurement of Reactive Oxygen Species
L02 cells were pretreated with or without Hyp (200 μM) for 24 h and incubated with H2O2 as described above. The intracellular ROS level was detected using the fluorescent probe 2′,7′-dichlorofluorescin diacetate (DCFH-DA), which is a cell-permeable nonfluorescent probe and turns to highly fluorescent 2′,7′-dichlorofluorescin (DCF) upon oxidation. After incubation of DCFH-DA for 30 min, the cells were washed with PBS and adjusted to a concentration of 1×106/mL after trypsinization and analyzed by FACSAriaTM III (BD, USA) within 30 min. 10,000 cells were counted in each determination and the specific fluorescence signals were collected with a 488 nm band pass filter.
Real-time PCR
L02 cells were pretreated and incubated as described above. Total RNA was extracted from cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Equal amounts (2.0 μg) of total RNA were reverse-transcribed to cDNA by using the PrimeScript RT reagent Kit (Takara, Dalian, China) for use in PCR. The following primers synthesized by Sangon Biotech (Shanghai, China) were used for PCR experiments: Bach1, TCG CGT AAG AAA AGC CGA GT (forward) and CGC TGT CCC TCC ACA AAG AT (reverse); and β-actin, GTG GGG CGC CCA GGC ACC T (forward) and CTT CCT TAA TGT CAC ACA CGA TTG (reverse). For RT-PCR, the cDNA and primer pairs were used in KOD OneTM PCR Mix (Toyobo Life Science, Shanghai, China). The total mix was run on a 7900 Real Time System (Applied Biosystems by Life Technologies, Carlsbad, CA, USA) with the PCR conditions as follows: 94°C (20 seconds), 58°C (20 seconds) and 72°C (20 seconds) for 35 cycles. The data are presented as the relative mRNA level normalized to β-actin and then expressed as the fold increase relative to the control.
Western Blotting
L02 cells were pretreated and incubated as described above. Total cell proteins, nuclear proteins and cytoplasmic proteins from L02 cells pretreated with Hyp were extracted with ice-cold lysis buffer (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instruction. Protein concentrations were determined using the BCA assay (Pierce, Rockford, IL, USA). Protein (30 μg) from each sample was separated by 10% SDS-PAGE and then transferred to polyvinylidene fluoride (PVDF) membranes (Merck Millipore, Darmstadt, Germany). The membranes were blocked with 5% BSA for one hour at room temperature and incubated overnight at 4°C using 1:1000 dilutions of relevant primary mouse antibodies, followed by 1:1000 dilutions of goat anti-mouse secondary antibodies. The protein bands were visualized using the ECL system (Thermo Scientific, Rockford, IL, USA), and the density was determined by Image J software (NIH, USA).
Immunofluorescence
L02 cells were grown on poly-L-lysine-coated coverslips at a density of 2×104 cells/well. Following chemicals treatment, cells were washed, fixed in 4% paraformaldehyde for 20 min, and then permeabilized with 0.1% Triton X-100 for 10 min and blocked with 3% BSA for one hour. Then cells were incubated with primary antibodies at a dilution of 1:200 and incubated overnight at 4°C. Then the sections were incubated with FITC-labeled secondary antibody for one hour, and stained with DAPI (Thermo Scientific) for 15 min, and mounted in glycerol. Controls were treated by omitting the primary antibody. Sections were examined with a confocal laser scanning microscope.
Chromatin Immunoprecipitation (CHIP) Assay
CHIP assay was performed using a PierceTM Agarose CHIP Kit (Thermo Scientific, Rockford, IL, USA). Briefly, L02 cells were treated with or without Hyp (200 μM) for 24 h. DNA and proteins were cross-linked by incubating cells with 1% formaldehyde for 10 min at room temperature. Excess formaldehyde was quenched with glycine for five minutes. Cells were then lysed and nuclei were digested using micrococcal nuclease. Sheared chromatin was diluted and immunoprecipitated with 10 μg of anti-Nrf2, anti-Bach1 or control IgG antibody. DNA-protein complexes were eluted from the protein A/G agarose beads using a spin column and were reverse cross-linked by incubating with NaCl at 65°C.
Statistical Analysis
All of the experiments were performed at least three times, and the results were presented as means ± SD. The statistical significance of differences between two or more than two groups was determined by Student’s t-test or one-way ANOVA, respectively, using the Statistical Package for the Social Sciences (SPSS) (Chicago, IL, USA) 18.0 software. P-values less than 0.05 were considered as statistically significant.
Results
Hyp Ameliorated the Pathological Damage in the CCl4-induced Acute Liver Injury Mouse Model
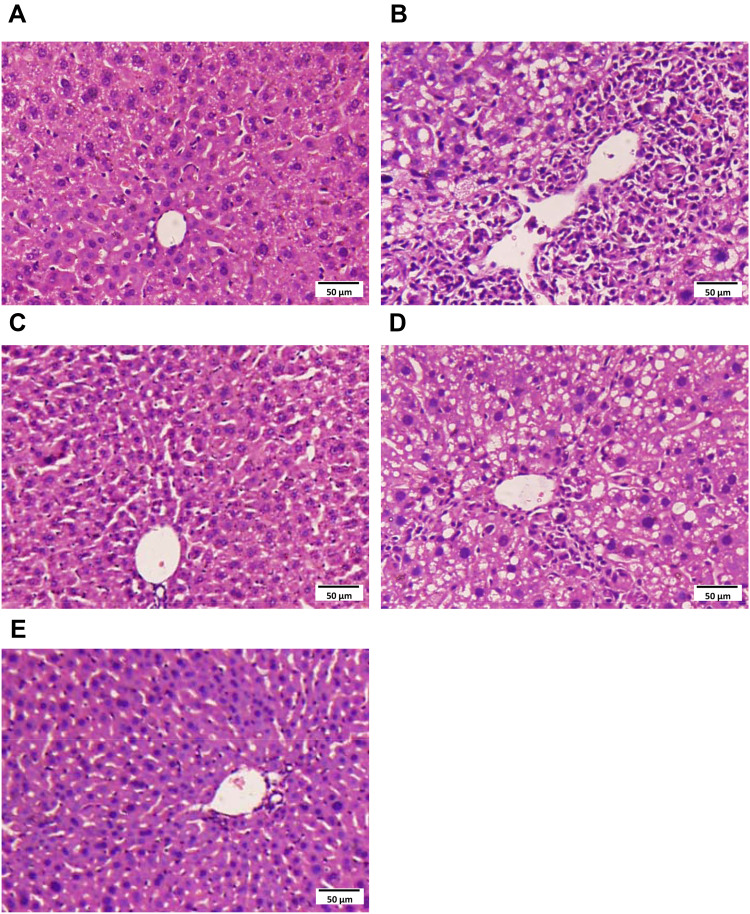
In order to investigate the effect of Hyp on pathological damage of CCl4-induced acute liver injury in mice, histopathological analysis of liver tissue was performed. H&E staining results indicated that normal liver architecture was seen in the mouse liver of normal control group (Figure 1A). In the mouse liver of CCl4 model group, extensive vacuolization, increased sinusoidal space and condensed nuclei around the central vein was seen (Figure 1B). However, CCl4-induced liver injuries were ameliorated in the liver of mice treated with Bif, 50 and 100 mg/kg Hyp (Figure 1C–E), the pathological lesions were close to normal range (Figure 1C and E).
Figure 1.
The H&E staining of representative liver tissues in experimental mice. (HE×200). (A) Normal mouse liver. (B) CCl4 treated mouse liver. (C) Bifendate treated mouse liver (200 mg/kg). (D) Hyp-L (low dose, 50 mg/kg). (E) Hyp-H (high dose, 100 mg/kg).
Hepatoprotective Effect of Hyp in CCl4-induced Acute Liver Injury Mouse Model
To investigate the hepatoprotective effect of Hyp on CCl4 induced acute liver injury mouse model, the liver index was calculated. It was shown that the liver index was markedly increased in the CCl4 model group, whereas Hyp treatment significantly reduced the liver index of acute liver injury model mice induced by CCl4. In addition, the expressions of biochemical markers serum AST, ALT and MDA, SOD, GSH in the liver homogenate were examined to measure the antioxidative and hepatoprotective effect of Hyp. The results showed that the serum AST and ALT levels and the hepatic MDA level in the CCl4-induced model mice was obviously higher than that in the mice of normal control group. And the levels of SOD and GSH in the model mice were markedly lower than in the normal control group mice (P<0.01, Table 1). Whereas Hyp treatment significantly reduced AST, ALT and MDA levels, improved SOD and GSH activity levels compared with that in the CCl4 model mice (P<0.05 or P<0.01, Table 1).
Table 1.
Effect of Hyp on Serum AST and ALT Levels and Liver MDA, SOD and GSH Activities in Acute Liver Injury Mice Induced by CCl4
| Group | ALT (karU) |
AST (karU) |
MDA (nmol/mgprot) |
SOD (U/mgprot) |
GSH (mg/gprot) |
Liver Index |
|---|---|---|---|---|---|---|
| Normal | 27.78±4.15 | 36.49±14.18 | 22.47±3.37 | 365.63±38.03 | 4.37±0.58 | 5.03±0.31 |
| CCl4 | 299.16±52.64* | 359.16±74.75* | 41.97±6.17* | 247.52±52.74* | 2.58±0.66* | 7.87±0.56* |
| Bif | 86.71±22.87*** | 151.27±36.41*** | 33.7±4.03** | 343.40±38.81** | 3.83±0.60*** | 6.77±0.45*** |
| Hyp-L | 226.04±70.23 | 221.16±64.96** | 35.87±5.10 | 315.88±43.11 | 3.28±0.65 | 7.32±0.52 |
| Hyp-H | 139.81±56.96*** | 166.63±52.25*** | 31.52±4.42*** | 338.32±42.24*** | 3.65±0.52** | 6.52±0.63*** |
Notes: *P<0.01 compared with normal control group; **P<0.05, ***P<0.01 as compared with CCl4 control group ( ± s, n=6).
± s, n=6).
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione.
Hyp Attenuated H2O2-induced Oxidative Stress Injury in L02 Cells
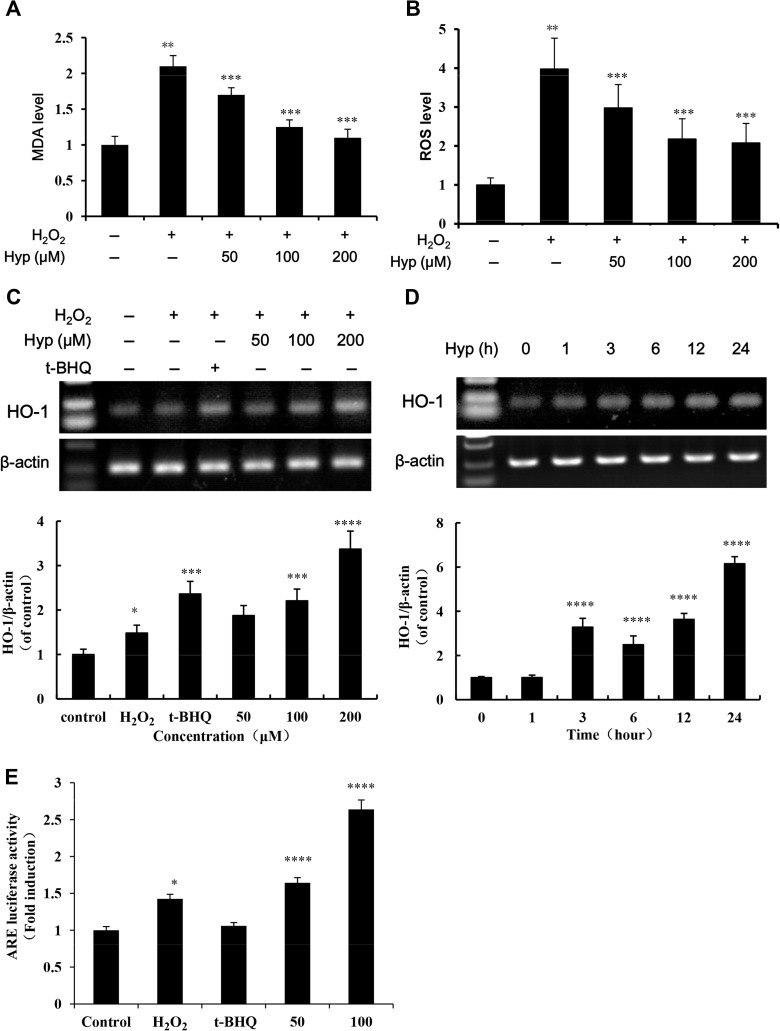
To confirm the protective effect of Hyp on oxidative liver injury in vitro and ensure the consistency of experimental conditions with our previous studies, the effect of Hyp on H2O2-induced MDA, ROS level and HO-1 mRNA expression in L02 cells was observed. The results showed that 0.1 mM H2O2 could significantly increased MDA and ROS levels in L02 cells (P<0.05). Pretreatment of L02 cells with Hyp for 24 h significantly decreased MDA and ROS levels (Figure 2A and B, P<0.05). Compared with H2O2 treated cells, pretreatment of L02 cells with Hyp for 24 h enhanced the transcript of HO-1 as determined by PCR (Figure 2C). The mRNA expression of HO-1 in L02 cells was induced after one hour Hyp pre-treatment and progressively continuously increased during incubation (Figure 2D). As shown in Figure 2E, 24 h of Hyp pretreatment increased the luciferase activity of ARE. Dual luciferase reporter assay showed that Hyp significantly enhanced the transcription activity of HO-1 promoter.
Figure 2.
The effect of Hyp on MDA, ROS and HO-1 levels in the L02 cells induced by H2O2. Liver cells were pretreated with Hyp (50, 100, 200 μM) or tBHQ (50 μM) for 24 h and then treated with or without H2O2 (100 μM) for one hour. Effect of Hyp on MDA (A) and ROS (B) levels in L02 cells treated with H2O2 (n=6); L02 cells were cultured with increasing concentrations of Hyp for 24 h or 200 μM of Hyp for 0–24 h, HO-1 mRNA levels were analyzed by RT-PCR. Dose-effect (C) and time-effect (D) of Hyp on HO-1 expression in L02 cells treated with H2O2; β-actin was used as loading control for each lane. Scanning densitometry was used for semi-quantitative analysis by comparison with control groups (graphs of A and B). (E) Relative luciferase activity was examined by the dual-luciferase reporter assay system. The luciferase activity was normalized against untreated cells with plasmid transfection. *P<0.05, **P<0.01 compared with normal control cells; ***P<0.05, ****P<0.01 compared with H2O2 treated cells.
Hyp Enhanced Nrf2 mRNA and Protein Expression Instead of Bach1 mRNA Expression
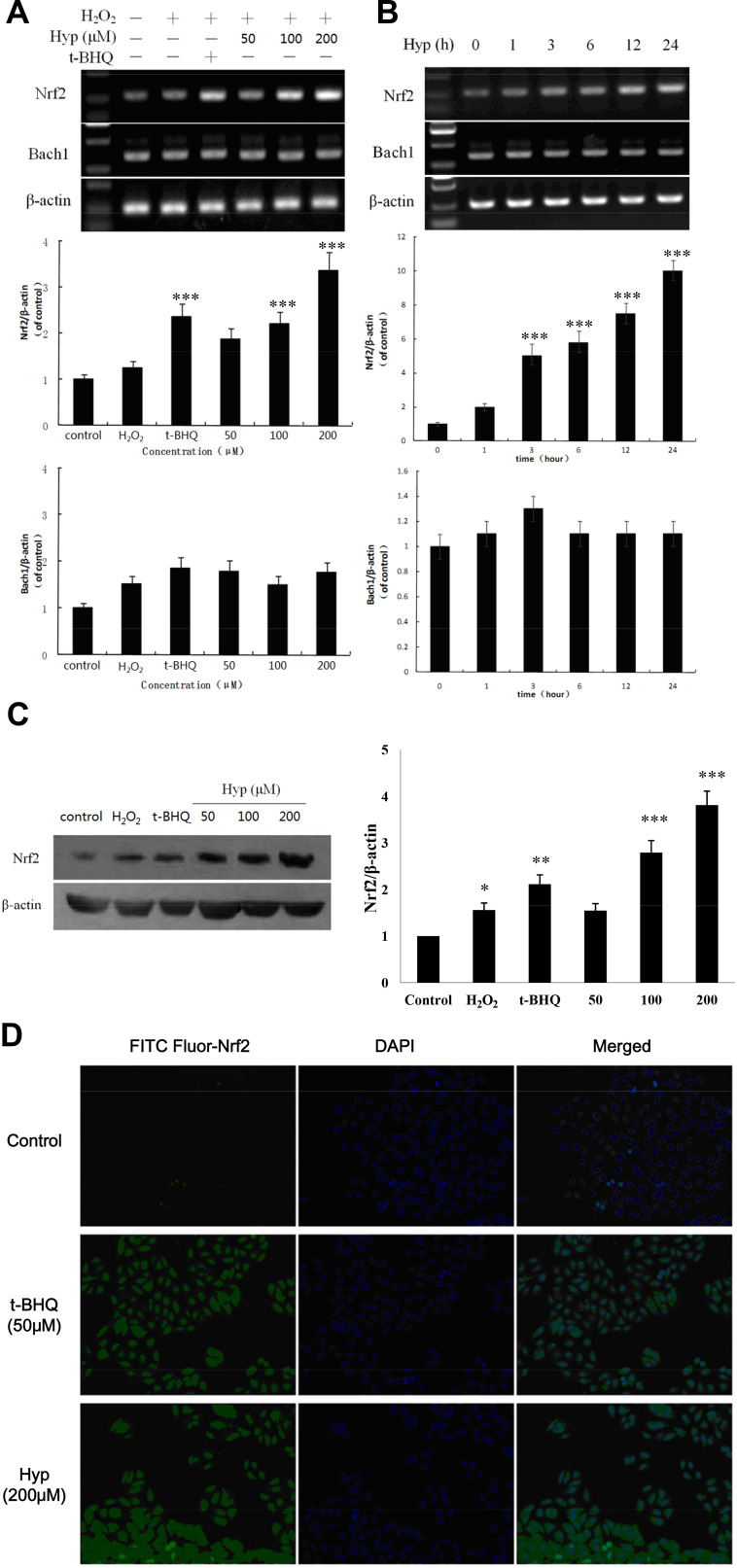
To further clarify the protective effect of Hyp on hepatic cells, we examined the expression levels of Nrf2 and Bach1. As shown in Figure 3A and B, Hyp pretreatment enhanced Nrf2 mRNA expression in a dose- or time-dependent manner. Hyp significantly enhanced Nrf2 protein expression after 24 h pretreatment (Figure 3C). Immunofluorescent staining of L02 cells also showed that Hyp or t-BHQ pretreatment induced an increasing of Nrf2 proteins expression in the cytoplasm of L02 cells (Figure 3D). The result about Nrf2 and HO-1 is consistent with our previous findings, which suggest that the experimental system is stable and reliable.14 However, both in the dose-effect and time-effect experiment, the expression of Bach1 mRNA did not obviously change in the L02 cells pretreated with Hyp.
Figure 3.
The effects of Hyp on Nrf2 and Bach1 in L02 cells induced by H2O2. (A) Dose-dependent effect of Hyp on the mRNA levels of Nrf2 and Bach1 in L02 cells. (B) Time-dependent effect of Hyp on the mRNA levels of Nrf2 and Bach1 in L02 cells. (C) The effects of Hyp on protein expression of Nrf2 in L02 cells. (D) The effects of Hyp on nuclear translocation of Nrf2 in L02 cells (without H2O2 treatment). H2O2: 100 μM, *P<0.05 compared with normal control cells; **P<0.05, ***P<0.01 compared with the H2O2 treated cells.
Hyp Promoted Bach1 Early Rapid Redistribution from Nucleus to Cytoplasm
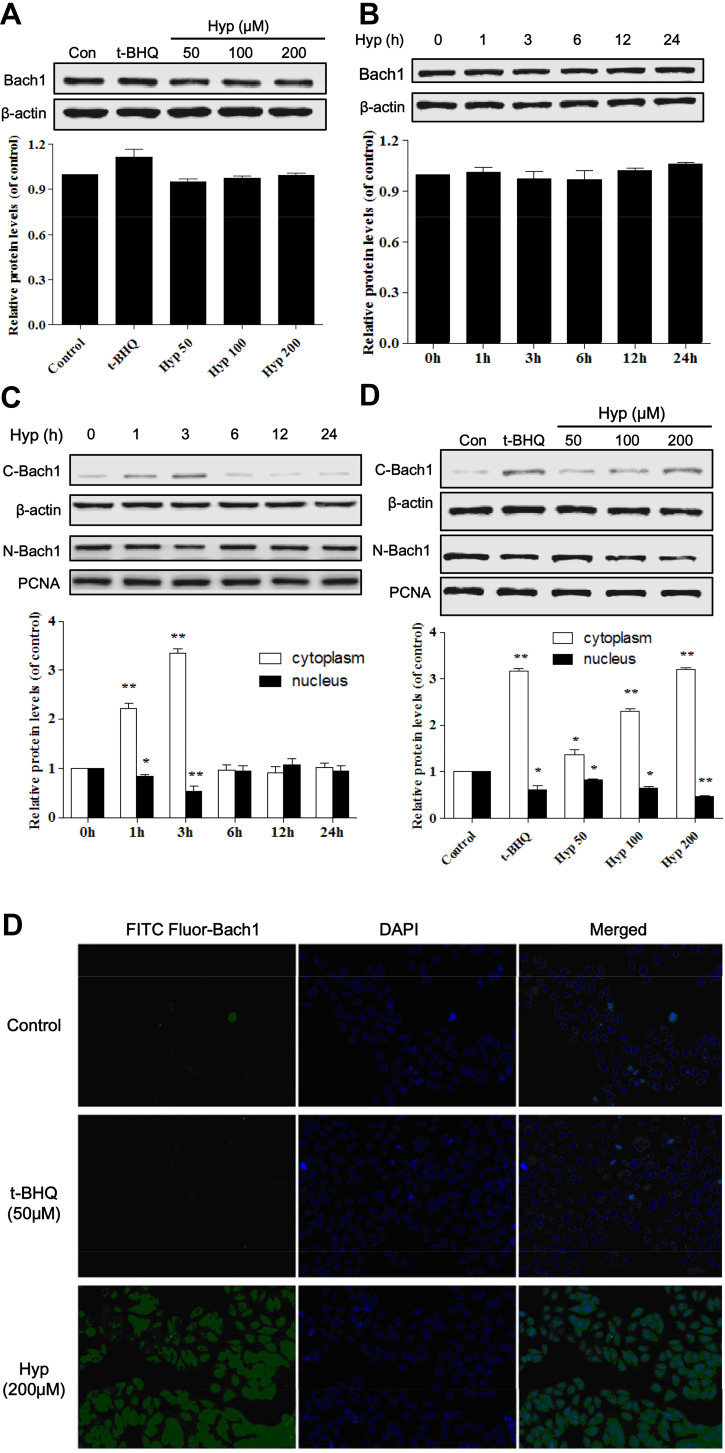
Some studies report that Bach1 can compete with Nrf2 to bind small Maf proteins to generate heterodimers to bind Maf recognition element (MARE) and inhibits antioxidant gene transcription.13 To investigate the effect of Hyp on Bach1 protein expression in the L02 cells induced by H2O2, the total protein expression and subcellular localization of Bach1 was determined by Western blotting assays. As shown in Figure 4A and B, the Bach1 total protein levels of L02 cells pretreated with Hyp or t-BHQ for 24 h had no obvious difference compared with that of control group. These findings indicated that Hyp did not affect the expression of Bach1 total protein.
Figure 4.
The effects of Hyp on Bach1 total protein expression and subcellular localization in L02 cells. Dose-dependent effect (A) and time-dependent effect (B) of Hyp on the protein expression of Bach1 in L02 cells. Time-dependent effect (C) and dose-dependent effect (D) on the subcellular localization of Bach1 in L02 cells. (E) The effects of Hyp on nuclear translocation of Bach1 in L02 cells. *P<0.05, **P<0.01 compared with control cells.
However, we further explored the influence of Hyp on the subcellular localization of Bach1 in the L02 cells. The results showed that Bach1 was predominantly located in the nucleus under normal conditions in the L02 cell. However, Bach1 level within the nucleus was gradually decreased and reached the lowest point at three hours after Hyp pretreatment. Correspondingly, Bach1 level within the cytoplasm was gradually increased, and also reached the highest at three hours (Figure 4C). Our results also showed that Hyp regulated the subcellular localization of Bach1 in a dose-dependent manner (Figure 4D). Hyp promoted the early (within three hours) rapid nuclear export of Bach1 protein from nucleus to cytoplasm in L02 cells.
To further confirm Hyp indeed promoted nuclear export of Bach1 in L02 cells, an immunofluorescence assay was used to detect the intracellular localization of Bach1. The fluorescence of Bach1 mostly concentrated in the nucleus in control cells. However, the nuclear fluorescence levels of Bach1 was marked decreased and at the same time Bach1 fluorescence mostly appeared in the cytoplasm after Hyp pretreatment for three hours in L02 cell (Figure 4E). Our results suggested that Hyp probably mediated rapid nuclear export of Bach1 protein after Hyp pretreatment.
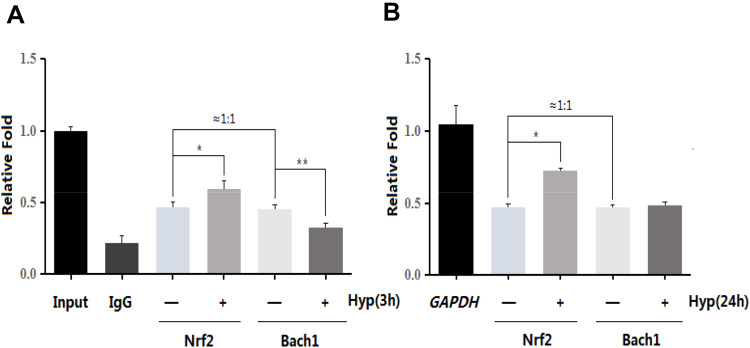
Hyp Enhanced the Levels of Nrf2 and are Complex and Weakened the Levels of Bach1 and are Complex Within three Hours
We further found that upregulation of Bach1 in L02 cells competed with Nrf2 for ARE binding and inhibited the expression of antioxidant protein genes. CHIP analysis demonstrated Hyp enhanced the levels of Nrf2 and ARE complex in three and 24 h (Figure 5A and B), and weakened the levels of Bach1 and ARE complex in three hours (Figure 5A) but had no effect on the levels of Bach1 and ARE complex in 24 h (Figure 5B, P>0.05). There was a competition between Bach1 and Nrf2 for binding to ARE.
Figure 5.
The effects of Hyp on the combination of Nrf2, Bach1 and ARE in L02 cells. (A) Hyp treatment for three hours. (B) Hyp treatment for 24 h. *P<0.05 Nrf2 compared with negative control group cells. **P<0.05 Bach1 compared with negative control group cells.
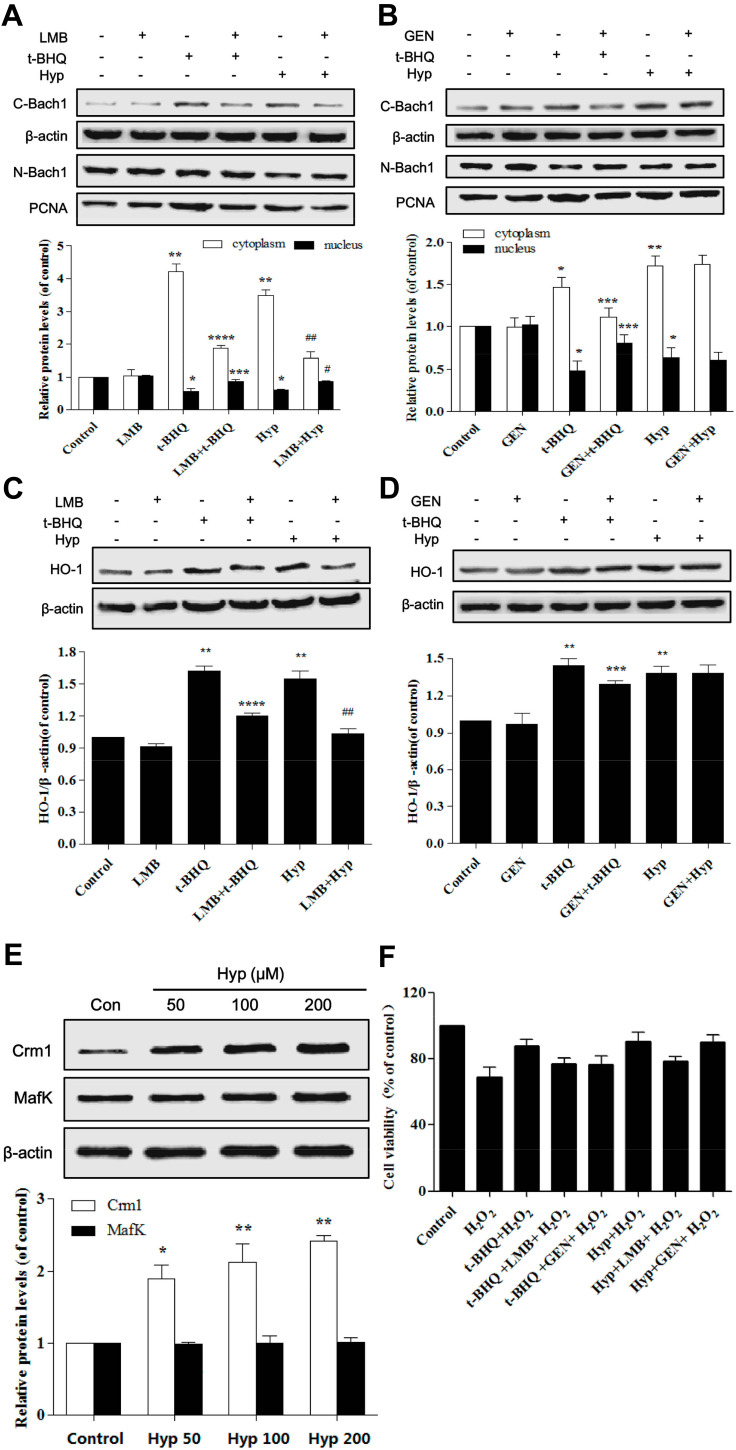
Hyp-mediated Bach1 Nuclear Export Depended on Crm1
Then we investigated the mechanism of Hyp promoting nuclear export of Bach1 in L02 cells. We first examined the effect of leptomycin B (LMB, a specific inhibitor of nuclear export protein Crm1) or genistein (GEN, a tyrosine kinase inhibitor). The result showed that Hyp-induced nuclear export of Bach1 was attenuated by LMB but not by GEN (Figure 6A and B), the similar results were also observed in HO-1 protein expression (Figure 6C and D). Next, we further confirmed this pathway using Western blotting to detect the expression of Crm1 protein directly. We observed that Crm1 protein level increased in a dose-dependent manner in L02 cell treated with Hyp (Figure 6E). These results suggested that Hyp depended on Crm1 pathway to promote nuclear export of Bach1. Furthermore, we further examined the role of LMB in Hyp-mediated protection effect. The results demonstrated Hyp significantly protected the L02 cells by increasing cell viability in the presence of LMB (Figure 6F). Bach1 forms a heterodimer with MafK, and regulates the expression of HO-1. The expression of MafK has not changed after Hyp pretreatment with different concentrations (Figure 6E). Taken together, these data confirmed that Crm1 played an important role in Hyp-mediated protection effect in H2O2-induced oxidative damage in L02 cells.
Figure 6.
The effects of Hyp on Bach1 and Crm1 in L02 cells. (A) The effect of LMB on the protein expression of Bach1 in L02 cells. (B) The effect of GEN on the protein expression of Bach1 in L02 cells. (C) The effect of LMB on the protein expression of HO-1 in L02 cells. (D) The effect of GEN on the protein expression of HO-1 in L02 cells. (E) Dose-dependent effect of Hyp on the protein expression of Crm1 and MafK in L02 cells. (F) The effect of Hyp on the cell viability of L02 cells in the presence of H2O2 and LMB or GEN. H2O2: 100 μM. *P<0.05, **P<0.01 compared with control group or LMB/GEN group, ***P<0.05, ****P<0.01 as compared with t-BHQ group, #P<0.05, ##P<0.01 as compared with Hyp group.
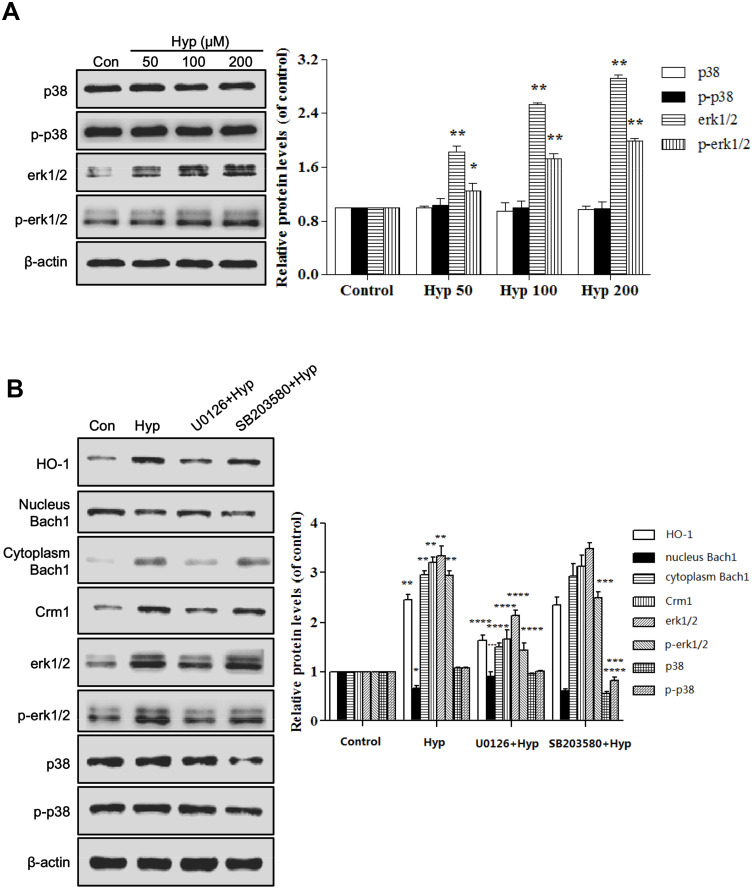
Hyp Enhanced Expression of Crm1 and HO-1 via ERK Phosphorylation
Our previous study demonstrated that the ERK and p38 signaling pathways participated in Hyp-mediated Nrf2 nuclear translocation.14 We hypothesized that the upstream signaling pathway p38 and ERK1/2 may be involved in Hyp-mediated the nuclear export of Bach1. Firstly, we investigated whether p38 and ERK1/2 activities were required for the Hyp-induced expression of Crm1 and nuclear export of Bach1 by treating cells with SB203580, an inhibitor of p38, or with U0126, an inhibitor of MEK1/2 (activators of ERK1/2). Western blot analysis revealed that Hyp treatment was associated with increased ERK1/2 and p-ERK1/2 (Figure 7A). U0126 treatment partially diminished Hyp-mediated increasing expression of Crm1 as well as nuclear export of Bach1 (Figure 7B). However, SB203580 showed no effects. To further confirm the effects of p38 and ERK1/2 in regulating redox balance through Hyp-mediated Crm1-Bach1 signaling, we measured HO-1 expression and cell viability in L02 cells treated with U0126 or SB203580 combined with Hyp, respectively. The result showed that Hyp-increased HO-1 expression and L02 cell viability were attenuated by U0126 instead of by SB203580 (Figure 7B). Taken together, these results demonstrated that Hyp activated ERK1/2-Crm1 pathway to promote the nuclear export of Bach1 to exhibition antioxidative damage in L02 cell.
Figure 7.
Relative protein levels in L02 cells pretreated with Hyp. (A) The effect of Hyp on the protein expression of p38, p-p38, erk1/2 and p-erk1/2 in L02 cells. (B) The effect of SB203580 or U0126 on Hyp-induced expression of Crm1 and nuclear export of Bach1. *P<0.05, **P<0.01 compared with control cells. ***P<0.05, ****P<0.01 compared with Hyp group cells.
Discussion
Our previous study revealed that Hyp was able to downregulate PHLPP2 expression, induce AKT phosphorylation and inactivate GSK-3β, and thereby, promoted Nrf2 nuclear translocation.15 Hyp could enhance the cellular antioxidant defense system through Nrf2 signaling pathway to upregulate HO-1 expression. In this study, we further dissect the mechanism that Hyp control Bach1 regulation. Our studied demonstrated that the hepatoprotective mechanism of Hyp was related to improve Bach1 nuclear export depending on ERK1/2-Crm1 to upregulate the level of Nrf2 binding to ARE.
Bach1 competes with Nrf2 for binding to the MAREs in oxidative stress-response genes.13 During oxidative stress, Nrf2 dissociates from Keap1 and Nrf2 degradation is inhibited, so Nrf2 will accumulate in the cytoplasm and translocate into the nucleus. Then, Nrf2 binds to MAREs as a heterodimer with small Mafs, activating HO-1 expression, while Bach1 is displaced from MAREs and exported out of the nucleus. Nrf2 has a critical role in cellular defense against xenobiotics and oxidative stress.16 Nrf2 forms heterodimers with small Maf proteins (MafF, MafG and MafK), binds to antioxidant response elements (AREs), and activates transcription of target genes. In contrast, small Maf homodimers have been reported to suppress ARE-mediated transcription.17 Other CNC molecules, including Bach1, form heterodimers with small Maf proteins and suppress ARE-mediated transcription.18 For example, the MafK-Bach1 heterodimer interacts with AREs in the enhancer region of the HO-1 gene (HMOX1) and suppresses its transcription. Heme, an inducer of HO-1, displaces Bach1 from the AREs, which is followed by binding of Nrf2 to the AREs, and increases in HO-1 expression.19–21 Although HO-1 is activated by Nrf2/small Maf heterodimers, it is repressed by Bach1/small Maf heterodimers, indicating that Bach1 and Nrf2 compete with each other.
Some study show that ultraviolet A irradiation of cultured human skin fibroblasts enhances accumulation of Bach1 mRNA and protein severalfold.22 Cadmium induces nuclear export of Bach1. Some reports suggest a role for Bach1-mediated repression in the regulation of ferritin, β-globin, and other phase II enzymes such as NQO1 and glutathione S-transferase.13,23,24 Bach1 is the critical repressor of the HO-1 enhancers under normal conditions and is required to maintain a low level of HO-1 expression.22,25
Interestingly, our data showed that Hyp did not affect the total amounts of Bach1 but resulted in a translocation from nuclear to cytoplasm. Hyp promoting the rapid nuclear export of Bach1 resulted in weakening the levels of Bach1 and ARE complex and enhancing the levels of Nrf2 and ARE complex within three hours. These results indicate that the nuclear export of Bach1 constitutes an important regulatory mechanism to relieve the Bach1-mediated repression of genes such as HO-1 (HMOX1). But how would Hyp lead to the redistribution of high-mobility group box 1?
Several pathways participate in the export of proteins from the nucleus to the cytoplasm. Chromosome region maintenance-1 (Crm1), also referred to as exportin 1, is a member of the karyopherin-b family of receptor proteins and has a central role in nuclear export of the proteins harboring a leucine-rich nuclear export signals. Previous studies have indicated that heavy metals cadmium as well as heme control Bach1-mediated repression by promoting Crm1-dependent nuclear export of Bach1. In addition, antioxidant tert-Butylhydroquinone (t-BHQ) induced rapid export of Bach1 from the nucleus is dependent on the phosphorylation of tyrosine 486 by an unknown tyrosine kinase and to allow Nrf2 to bind to the ARE and activate defensive gene expression. Recent studies also found that the nuclear export of Bach1 treated by gold nanoparticles and tetrachlorobenzoquinone is dependent on its Crm1 interaction and tyrosine phosphorylation. So we hypothesized that Crm1 and t-BHQ may be the candidate mechanisms for the nuclear export of Bach1. Our investigated showed the nuclear export of Bach1 and the elevation of HO-1 were attenuated by blockade of Crm1 with LMB but not by blockade of the phosphorylation of tyrosine 486 with t-BHQ in Hyp treated H2O2-induced L02 cell damage. We further confirmed Hyp significantly enhanced the expression of Crm1. Crm1 is a well-known factor that mediates the nuclear export of proteins bearing a leucine-rich nuclear export signal. Thus, these data support Hyp activates Crm1, allowing Bach1 to translocate from nucleus into cytoplasm. Some studies report stimulation of astrocytes with IL-1β upregulate the nuclear export protein Crm1 to enable nuclear HMGB1 to translocate from nucleus to cytoplasm before release.26 Alternatively, cadmium controls Bach1-mediated repression by promoting Crm1-dependent nuclear export of Bach1 protein and thereby removing Bach1 from the nucleus.27 Our results are consistent with previous reports describing the connection between Crm1-mediated nuclear export in various cell lines.28,29 The nuclear export of Bach1 was dependent on Crm1.
Some studies report IL-1β enhances expression of Crm1 via ERK phosphorylation.25 Our previous study demonstrated that the ERK and p38 signaling pathways participated in Hyp-mediated Nrf2 nuclear translocation. We hypothesized that the upstream signaling pathway p38 and ERK1/2 may be involved in Hyp-mediated the nuclear export of Bach1. Our results showed U0126 treatment but not SB203580 partially diminished Hyp-mediated increasing expression of Crm1 as well as nuclear export of Bach1. Further study showed increased HO-1 expression and L02 cell viability by Hyp were attenuated by U0126 but not by SB203580. Taken together, these results demonstrated that Hyp activated ERK1/2 pathway to promote the nuclear export of Bach1 to exhibition antioxidative damage in L02 cell.
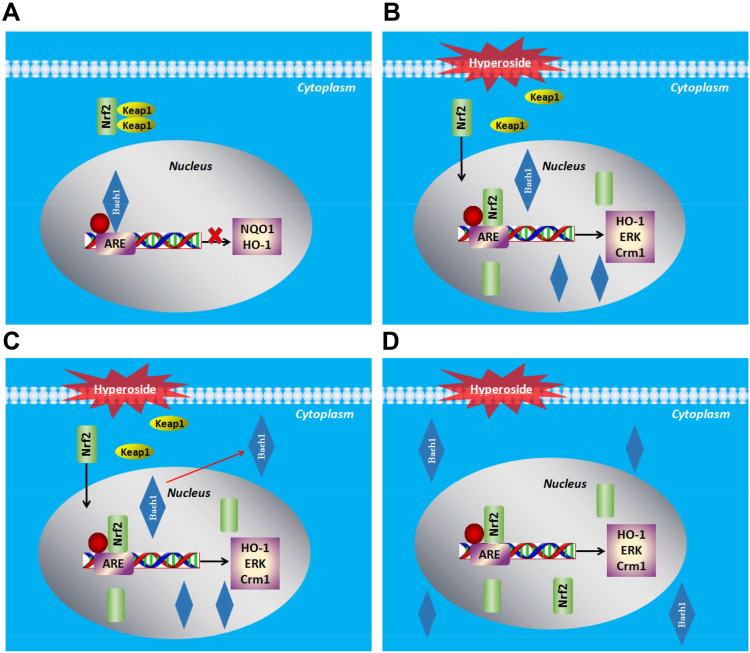
Conclusion
In summary, our results clearly indicate the importance of coordinated regulation of activators and the Bach1 repressor in the regulation of HO-1 gene expression. The regulatory program is wired as a competitive interact (Figure 8). Keap1 binds to Nrf2 and promotes its degradation by the ubiquitin proteasome pathway, and Bach1 is the critical repressor of the HO-1 enhancers under normal conditions (Figure 8A). Hyp promotes the nuclear accumulation of Nrf2 and the nuclear export of Bach1, and thus contributes to the activation of their target genes Crm1 (XPO1) and HO-1 (HMOX1) (Figure 8B–D). These results indicate that the nuclear export of Bach1 constitutes an important regulatory mechanism of Hyp to ensure that the entire system effectively responds to oxidative damage.
Figure 8.
Diagrammatic representation of effect of Hyp on Bach1 and Nrf2. (A) Normal conditions; (B–D) After oxidative stress, Hyp promotes the nuclear accumulation of Nrf2 and the nuclear export of Bach1, and thus contributes to the activation of their target genes Crm1 (XPO1) and HO-1 (HMOX1).
Acknowledgments
This study was funded by grants from Science and Technology Achievements Transformation Fund Project of Third Military Medical University (No. 2015XZH19), Application Fundamental Research Project of Army Logistics (No. BWS17J031-08) and Technology Project of Chongqing Health and Family Planning Commission (No. 2019MSXM010).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hellerbrand C, Massoumi R. Cylindromatosis–a protective molecule against liver diseases. Med Res Rev. 2016;36:342–359. doi: 10.1002/med.21381 [DOI] [PubMed] [Google Scholar]
- 2.Stefanello ST, Hartmann DD, Amaral GP, et al. Antioxidant protection by β-selenoamines against thioacetamide-induced oxidative stress and hepatotoxicity in mice. J Biochem Mol Toxicol. 2017;31(12):10. doi: 10.1002/jbt.21974 [DOI] [PubMed] [Google Scholar]
- 3.Seitz HK, Bataller R, Cortez-Pinto H, et al. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4(1):16. [DOI] [PubMed] [Google Scholar]
- 4.Masarone M, Rosato V, Dallio M, et al. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2018;2018:9547613. doi: 10.1155/2018/9547613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madide T, Somboro AM, Amoako DG, et al. Di-2-picolylamine triggers caspase-independent apoptosis by inducing oxidative stress in human liver hepatocellular carcinoma cells. Biotechnol Appl Biochem. 2020. doi: 10.1002/bab.1918 [DOI] [PubMed] [Google Scholar]
- 6.Rebbani K, Tsukiyama-Kohara K. HCV-induced oxidative stress: battlefield-winning strategy. Oxid Med Cell Longev. 2016;2016:7425628. doi: 10.1155/2016/7425628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu H, Hong S, Yan Z, et al. RAP-8 ameliorates liver fibrosis by modulating cell cycle and oxidative stress. Life Sci. 2019;229:200–209. doi: 10.1016/j.lfs.2019.04.037 [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Wang S, Liu J, et al. Sesquiterpenoids from the root of Panax Ginseng protect CCl4-induced acute liver injury by anti-inflammatory and anti-oxidative capabilities in mice. Biomed Pharmacother. 2018;102:412–419. doi: 10.1016/j.biopha.2018.02.041 [DOI] [PubMed] [Google Scholar]
- 9.Bellezza I, Giambanco I, Minelli A, et al. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865(5):721–733. doi: 10.1016/j.bbamcr.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 10.Koriyama Y, Nakayama Y, Matsugo S, et al. Protective effect of lipoic acid against oxidative stress is mediated by Keap1/Nrf2-dependent heme oxygenase-1 induction in the RGC-5 cell line. Brain Res. 2013;1499:145–157. doi: 10.1016/j.brainres.2012.12.041 [DOI] [PubMed] [Google Scholar]
- 11.Shin JM, Kim MY, Sohn KC, et al. Nrf2 negatively regulates melanogenesis by modulating PI3K/Akt signaling. PLoS One. 2014;9:e96035. doi: 10.1371/journal.pone.0096035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashita Y, Ueyama T, Nishi T, et al. Nrf2-inducing anti-oxidation stress response in the rat liver–new beneficial effect of lansoprazole. PLoS One. 2014;9:e97419. doi: 10.1371/journal.pone.0097419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhakshinamoorthy S, Jain AK, Bloom DA, et al. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H: quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem. 2005;280:16891–16900. doi: 10.1074/jbc.M500166200 [DOI] [PubMed] [Google Scholar]
- 14.Xing HY, Liu Y, Chen JH, et al. Hyperoside attenuates hydrogen peroxide-induced L02 cell damage via MAPK-dependent Keap(1)-Nrf(2)-ARE signaling pathway. Biochem Biophys Res Commun. 2011;410:759–765. doi: 10.1016/j.bbrc.2011.06.046 [DOI] [PubMed] [Google Scholar]
- 15.Xing HY, Fu RQ, Cheng CY, et al. Hyperoside protected against oxidative stress-induced liver injury via the PHLPP2-AKT-GSK-3β signaling pathway in vivo and in vitro. Front Pharmacol. 2020;11:1065. doi: 10.3389/fphar.2020.01065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyu H, Wang H, Li L, et al. Hepatocyte-specific deficiency of Nrf2 exacerbates carbon tetrachloride-induced liver fibrosis via aggravated hepatocyte injury and subsequent inflammatory and fibrogenic responses. Free Radic Biol Med. 2020;150:136–147. doi: 10.1016/j.freeradbiomed.2020.02.015 [DOI] [PubMed] [Google Scholar]
- 17.Motohashi H, O’Connor T, Katsuoka F, et al. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene. 2002;294:1–12. doi: 10.1016/S0378-1119(02)00788-6 [DOI] [PubMed] [Google Scholar]
- 18.Oyake T, Itoh K, Motohashi H, et al. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol Cell Biol. 1996;16:6083–6095. doi: 10.1128/MCB.16.11.6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa K, Sun J, Taketani S, et al. Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1. EMBO J. 2001;20:2835–2843. doi: 10.1093/emboj/20.11.2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J, Brand M, Zenke Y, et al. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc Natl Acad Sci U S A. 2004;101:1461–1466. doi: 10.1073/pnas.0308083100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J, Hoshino H, Takaku K, et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raval CM, Zhong JL, Mitchell SA, et al. The role of Bach1 in ultraviolet A-mediated human heme oxygenase 1 regulation in human skin fibroblasts. Free Radic Biol Med. 2012;52:227–236. doi: 10.1016/j.freeradbiomed.2011.10.494 [DOI] [PubMed] [Google Scholar]
- 23.Hintze KJ, Katoh Y, Igarashi K, et al. Bach1 repression of ferritin and thioredoxin reductase1 is heme-sensitive in cells and in vitro and coordinates expression with heme oxygenase1, beta-globin, and NADP(H) quinone (oxido) reductase1. J Biol Chem. 2007;282:34365–34371. doi: 10.1074/jbc.M700254200 [DOI] [PubMed] [Google Scholar]
- 24.Igarashi K, Hoshino H, Muto A, et al. Multivalent DNA binding complex generated by small Maf and Bach1 as a possible biochemical basis for beta-globin locus control region complex. J Biol Chem. 1998;273:11783–11790. doi: 10.1074/jbc.273.19.11783 [DOI] [PubMed] [Google Scholar]
- 25.Reichard JF, Motz GT, Puga A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res. 2007;35:7074–7086. doi: 10.1093/nar/gkm638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayakawa K, Arai K, Lo EH. Role of ERK map kinase and CRM1 in IL-1beta-stimulated release of HMGB1 from cortical astrocytes. Glia. 2010;58:1007–1015. doi: 10.1002/glia.20982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki H, Tashiro S, Sun J, et al. Cadmium induces nuclear export of Bach1, a transcriptional repressor of heme oxygenase-1 gene. J Biol Chem. 2003;278:49246–49253. doi: 10.1074/jbc.M306764200 [DOI] [PubMed] [Google Scholar]
- 28.Le Gallic L, Virgilio L, Cohen P, et al. ERF nuclear shuttling, a continuous monitor of Erk activity that links it to cell cycle progression. Mol Cell Biol. 2004;24:1206–1218. doi: 10.1128/MCB.24.3.1206-1218.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu M, Olsen A, Faivre E, et al. Mitogen-activated protein kinase regulates nuclear association of human progesterone receptors. Mol Endocrinol. 2003;17:628–642. doi: 10.1210/me.2002-0378 [DOI] [PubMed] [Google Scholar]