Hosts with compromised or naive immune systems, such as individuals living with HIV/AIDS, transplant recipients, and fetuses, are at the highest risk for complications from cytomegalovirus (CMV) infection. Despite substantial progress in prevention, diagnostics, and treatment, CMV continues to negatively impact both solid-organ transplant (SOT) and hematologic cell transplant (HCT) recipients. In this article, we summarize important developments in the field over the past 10 years and highlight new approaches and remaining challenges to the optimal control of CMV infection and disease in transplant settings.
KEYWORDS: cytomegalovirus, antiviral agents, clinical trials, diagnostics, immune monitoring, immunocompromised hosts, transplant infectious diseases, transplantation, vaccines
SUMMARY
Hosts with compromised or naive immune systems, such as individuals living with HIV/AIDS, transplant recipients, and fetuses, are at the highest risk for complications from cytomegalovirus (CMV) infection. Despite substantial progress in prevention, diagnostics, and treatment, CMV continues to negatively impact both solid-organ transplant (SOT) and hematologic cell transplant (HCT) recipients. In this article, we summarize important developments in the field over the past 10 years and highlight new approaches and remaining challenges to the optimal control of CMV infection and disease in transplant settings.
INTRODUCTION
Human cytomegalovirus (CMV) is a member of the Betaherpesvirinae subfamily; with a 236-kb genome, it is one of the largest identified human viruses (1). CMV was originally reported in the 1950s after it was isolated from the urine of infants with disseminated disease, at that time referred to as cytomegalic inclusion disease (2). In immunocompromised hosts, the clinical presentation is likely influenced by multiple host and viral factors. Among these, the type of infection (primary versus reactivation versus superinfection), specific transplant setting (solid-organ transplant [SOT] versus hematologic cell transplant [HCT]), and degree of immunosuppression appear to be particularly important (3–5). The clinical manifestations range from mild flu-like febrile illness (especially in primary infection, such as in donor-positive/recipient-negative [D+ R−] SOT recipients) to life-threatening tissue-invasive (end-organ) disease, most commonly involving the lungs, gastrointestinal (GI) tract, liver, eye (retinitis), or central nervous system. With changing transplantation practices, the spectrum of CMV disease continues to evolve (6, 7). Reactivation from latency is often initially asymptomatic.
The CMV disease incidence and associated short-term attributable mortality have decreased with the use of various preventive strategies (3–5). Tables 1 and 2 summarize current CMV incidences among SOT and HCT recipients; they include clinical trials reported since 2010 in which the incidence of CMV disease was stratified by both D/R serological status and the type of transplant performed. CMV continues to have a significant negative impact on transplant recipients both as a consequence of direct high-grade viral replication with the associated host response and tissue injury (CMV disease) and through complex biological effects mediated by CMV that negatively impact transplant outcomes (indirect effects) (8–18).
TABLE 1.
Incidence of CMV disease in SOT patients in clinical trials with current preventative strategies
| Type of transplant (references) | D+ R− |
R+ |
||||
|---|---|---|---|---|---|---|
| Incidence range among studies (%) | Weighted avg incidence (%) (no. of patients with CMV disease/total no. of patients) | Follow-up period | Incidence range among studies (%) | Weighted avg incidence (%) (no. of patients with CMV disease/total no. of patients) | Follow-up period | |
| Kidney (23, 137, 269–273) | 0–50 | 25 (183/739) | 24 wks–1,236 daysa | 2–15 | 7 (42/603) | 3 mos–3 yrs |
| Liver (106, 150) | 8–40 | 13 (13/258) | 6–12 mos | 0–4 | 3 (1/39) | 12 mos |
| Lung (274, 275) | 10–33 | 15 (4/26) | 3–3.9b yr | 7–19c | 17 (25/150) | 3–3.9b yrs |
| Heart (86, 276, 277) | 0–25 | 10 (2/20) | 6 mos | 0–14 | 6 (7/127) | 6–12 mos |
Median.
Mean.
Includes CMV disease events/patients.
TABLE 2.
Incidence of CMV disease in HCT patients in clinical trials with current preventative strategies
| Authors (reference) | Yr | No. of patients | Incidence (%) |
Study design | Follow-up period | |
|---|---|---|---|---|---|---|
| Placebo | Vaccine or prophylaxis | |||||
| Marty et al. (131) | 2011 | 227 | 2.6 | 2.4 | Maribavir vs placebo | 100 days |
| Kharfan-Dabaja et al. (132) | 2012 | 34 | 8.8 | 7.5 | DNA vaccine vs placebo | 1 yr |
| Marty et al. (133) | 2013 | 59 | 3 | 4 | Various doses of CMX001 (brincidofovir) vs placebo | 4–8 wks following the end of drug administration |
| Chemaly et al. (134) | 2014 | 33 | 0 | 0 | Letermovir vs placebo | 96 days |
| Boeckh et al. (38) | 2015 | 89 | 2.2 | 2.1 | Valganciclovir vs placebo and PET | 270 days |
| Marty et al. (135) | 2017 | 170 | 1.8 | 1.5 | Letermovir vs placebo | 24 wks |
| Marty et al. (136) | 2019 | 149 | 3.4 | 4.3 | Brincidofovir vs placebo | 24 wks |
The goals of this review are to summarize important developments in the diagnosis, prevention, and treatment of CMV in SOT and HCT populations over the past 10 years and to identify unmet clinical and research needs. For updates on CMV biology, pathogenesis, and immunology, readers are referred to several recent updates (8, 19–22).
DIAGNOSTICS
Detection of Virus in Blood
Quantitative PCR (qPCR) for CMV DNA in blood has become the preferred diagnostic testing method due to its high sensitivity and high throughput. As such, it is widely incorporated into clinical algorithms for diagnosing CMV disease, determining when to initiate preemptive antiviral therapy (PET), and monitoring the course of infection and/or disease (22–24). In the United States, for example, commercially available platforms cleared or approved by the FDA for CMV DNA qPCR testing include the Artus CMV RGQ MDX kit by Qiagen, the Cobas AmpliPrep/Cobas TaqMan CMV test by Roche, and the RealTime CMV molecular test by Abbott (22, 25). Improved standardization and calibration of CMV PCR testing are important ongoing priorities in the field. Significant progress toward this goal was made in 2010, through the introduction of an international reference by the World Health Organization (WHO). However, issues with variability across PCR testing platforms/assays persist (26–28). Recent studies have identified multiple additional components of CMV qPCR assays that contribute to variability, including amplicon sizes and DNA extraction methods (29, 30). Variability is also significantly impacted by the sample type (e.g., plasma versus whole blood versus peripheral blood mononuclear cells) (31, 32). Newer methodologies such as droplet digital PCR show promise for decreasing the variability in measurements of CMV DNA loads, but these are not yet widely used (33–35). With the recognition that the interassay variability in viral load quantitation remains despite the integration of the WHO international standard, consensus guidelines recommend serial testing with a single sample type and the same assay to improve interpretations of changes in viral loads (22, 36, 37).
Despite the utility of blood viral load assays, several limitations constrain their use in certain clinical circumstances: compartmentalization (localized CMV replication within an anatomical site without concomitant viremia [discussed for the diagnosis of GI CMV disease below]), the absence of specific thresholds for the initiation of PET and discontinuation of therapy, the potential impact of newer antiviral agents on DNA load quantitation (e.g., letermovir, whose mechanism of action affects a target downstream of DNA replication), ambiguity about viral load thresholds for predicting disease across a range of clinical settings and disease types, and the need for standardization of viral load result reporting (actual numeric international units [IU] per milliliter versus log10 units).
Important challenges of CMV prevention strategies that employ PET (i.e., initiation of antiviral therapy on the basis of detection of early CMV replication) are the need for frequent blood-based monitoring to detect CMV replication and low adherence to these monitoring schedules, especially at later time points after transplant (38). Novel strategies are being explored to improve adherence by allowing patients to self-collect and submit blood samples for monitoring without the need for a clinic visit or standard phlebotomy. The use of dried blood spots, a methodology previously studied for the diagnosis of congenital CMV, allows the assessment of CMV viral loads using a finger stick blood sample. Dried blood spot quantitation of CMV DNA has been validated in a small study of 35 SOT patients (39) and is currently being evaluated in a multicenter NIH-supported randomized controlled trial (RCT) utilizing mobile device-assisted CMV monitoring by dried blood spots in HCT patients at risk for late CMV disease (ClinicalTrials.gov identifier NCT03910478). Devices designed for self-collection of a blood sample (without the need for a patient-performed finger stick) represent an important area of development, with products recently cleared by the FDA or in development for other blood-based diagnostic and monitoring applications (40, 41). By providing simple at-home testing for patients, this technology has the potential to improve adherence with frequent CMV monitoring (and has potential applications for additional analyses such as other blood-borne viruses or immunosuppressant levels). At-home CMV PCR testing modalities now have increased relevance given the current severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak. Currently, there are limited data on the outcomes of transplant patients with SARS-CoV-2 (42–46). At-home testing would enable these vulnerable populations to receive standard-of-care testing and monitoring while mitigating the risk of SARS-CoV-2 exposure.
Detection of Virus at Specific Sites of Disease
Although blood has been the preferred specimen for the quantitation of viral loads by PCR (discussed above), there have been important developments in the application of qPCR methods to nonblood specimens for the diagnosis of site-specific CMV disease. These methods show promise for either complementing existing gold-standard diagnostic methods (e.g., endoscopy with biopsy for histopathology) or potentially replacing more invasive diagnostic methods in the future. The two clinical situations for which site-specific CMV quantitation has been studied include GI disease and pneumonia.
For CMV GI disease, the identification of CMV in biopsy specimens by using standard or immunohistochemical stains has been considered the gold standard for the diagnosis of proven disease. However, interpretation is subjective and interpreter dependent (47). The detection of CMV in blood samples typically has a lower sensitivity in GI disease than in other forms of end-organ CMV disease: up to 50% of patients may not have detectable CMV DNA in blood despite biopsy-confirmed GI CMV disease (3, 48–50). The sensitivity of CMV plasma qPCR for GI disease varies by serological status and is reportedly higher in D+ R− than in R+ SOT recipients (48, 51). Performing CMV qPCR on stool samples has been explored as a noninvasive approach for diagnosing CMV GI disease. In two studies of adult and pediatric immunocompromised patients (including SOT and HCT recipients), qPCR for CMV DNA in stool was found to have a relatively low sensitivity (67% to 71%) but high specificity (85% to 96%) compared to a standard diagnostic methodology consisting of histopathology of a tissue biopsy specimen (52) or histopathology, endoscopy, and CMV DNA levels in a tissue biopsy specimen (53). Additionally, droplet digital PCR assays may improve the detection of CMV in PCR-inhibition-prone stool specimens compared to laboratory-developed qPCRs.
qPCR on tissue biopsy specimens is another approach with potential for the diagnosis of GI CMV disease. Current guidelines developed for clinical trial standardization consider a positive qPCR result from a GI biopsy specimen, in a compatible clinical setting, to represent possible GI disease (54). qPCR has been evaluated in both formalin‐fixed paraffin‐embedded and fresh tissue biopsy samples for this application; Table 3 summarizes studies reported since 2010 that evaluated tissue PCR for the diagnosis of GI CMV in transplant recipients (47, 55–58). Collectively, these data indicate that qPCR on either formalin‐fixed paraffin‐embedded or fresh GI biopsy specimens may have an adjunctive role for the diagnosis of GI CMV disease. Future studies are needed to standardize these assays across a range of transplant populations, define clinically meaningful thresholds, and assess the operating characteristics and clinical role of stool and biopsy PCR for the diagnosis of GI CMV disease.
TABLE 3.
PCR for GI CMV diseaseh
| Authors (reference) | Yr | Patient type(s) | Study design | Total no. of patients | No. of transplant patients | No. of samples | No. of positive/total no. of fresh tissue samples (%) | No. of positive/total no. of FFPE samples (%) | Results | Conclusion(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| Suarez-Liedo et al. (57) | 2019 | HCT | A retrospective cohort of patients who underwent endoscopy for GI symptoms was analyzed; IHC results at the time of diagnosis and PCR results were obtained from FFPE samples of the same biopsy specimen; prospective IHC and PCR analyses of fresh GI tissue from endoscopy were also performed | 123; 63 FFPE, 60 fresh | 123 | 113 FFPE, 73 fresh tissue | GI disease,a 13/13 (100) PCR+; no disease, 1/52 (2) PCR+; possible disease, symptoms, IHC−, 8/8 (100) PCR+ | GI disease,a 16/20 (80) PCR+; no disease, 3/93 (3) PCR+ | Overall, for GI disease,a CMV PCR and IHC had the same sensitivity (100%), specificity (98%), PPV (93%), and NPV (100%); with macroscopic lesions and IHC-positive biopsy specimens (n = 28), all but 1 were CMV PCR positive; without macroscopic lesions and IHC+ biopsy specimens (n = 4), only 1 was PCR+; 8 patients had CMV IHC−/CMV PCR+ gut biopsy specimens | Quantitative PCR had the same sensitivity, specificity, and positive/negative predictive values as IHC; any result of >10,000 copies/μg in tissue could be considered GI CMV disease, regardless of the result of PCR of blood samples; PCR worked better for fresh samples than for FFPE samples |
| Mills et al. (47) | 2013 | HCT, SOT, colitis, IBD, HIV | Retrospective cohort evaluation of PCR in FFPE GI biopsy specimens | 74 | 30 | 102 FFPE | NR | GI disease,b 30/33 (91) CMV PCR+; no disease (H&E−/IHC−), 47/55 (85) CMV PCR− and 8/55 (15) PCR+ (all nontransplant) | For an optimal cutoff ratio of 0.276 for CMV/β-globin, sensitivity was 60% and specificity was 87.5%; in all 3 PCR− cases with IHC+ specimens, inclusions were rare; 80% (4/5) of IHC-equivocal biopsy specimens were CMV PCR− | Taking into account the cost and time of CMV PCR on FFPE specimens, CMV PCR is recommended as an adjunct tool when IHC is negative on a sample with inflammation and clinical suspicion for CMV is high |
| Rashidi et al. (55) | 2017 | HCT | Retrospective cohort evaluation of FFPE specimens from gut and lung biopsies | 151 | 151 | 151 tissue biopsy specimensc | NR | GI diseaseb (H&E+/IHC+), 16/17 CMV PCR+; no disease (H&E−/IHC−), 83/105 (79) CMV PCR−; equivocal, 16/29 CMV PCR+ | For viremia for H&E/IHC-concordant cases, tissue CMV PCR had a sensitivity of 100%, a specificity of 50%, a PPV of 44%, and an NPV of 100%; for nonviremia for H&E/IHC-concordant cases, tissue PCR had a sensitivity of 80%, a specificity of 91%, a PPV of 36%, and an NPV of 99% | Negative FFPE tissue CMV PCR may be used to rule out CMV disease in H&E/IHC-equivocal cases |
| Mavropoulou et al. (56) | 2019 | IBD, HCT | Retrospective cohort study evaluating PCR on biopsy specimens for diagnosing CMV GI disease | 108 | 61 | 442 colon biopsy specimens in HCT | GI disease,d 20/61 (33) had biopsy proven, 19/61 (31) had CMV infection, and 22/61 (36) were CMV negative | NR | Median CMV PCR value of colonic tissue in colitis,e 6,500 copies/mg; for CMV colitis,e GI CMV PCR had a sensitivity of 80%, a specificity of 100%, a PPV of 100%, and an NPV of 91%; for CMV colitise with a CMV GI PCR result of >250 copies/mg had a sensitivity of 92%, a specificity of 88%, a PPV of 92%, and an NPV of 88% | Quantitative PCR may be performed on patients with suspected high-risk IBD and on HSCT patients for CMV colitis |
| Tsuchido et al. (278) | 2018 | Non-HIV, IC | Retrospective cross-sectional study of patients who had CMV PCR performed on GI endoscopic biopsy specimens | 195 | 68; 47 HCT, 21 SOTf | 213 biopsied organsg | NR | NR | Overall, 27/28 with GI diseaseb were CMV PCR+, and 1 nontransplant patient with gastritis was CMV PCR−; for HCT recipients, 7/47 had GI disease,b with a GI CMV PCR sensitivity of 100% and a specificity of 80%, at a cutoff of 10 copies/μg DNA; for SOT recipients, 3/21 had GI disease,b with a GI PCR sensitivity of 100% and a specificity of 94.4%, with a cutoff of 530 copies/μg DNA; for probable disease with a PCR+ result, there were 5 SOT and 8 HCT cases | Use of quantitative PCR on endoscopic biopsy specimens for non-HIV IC patients may increase the diagnostic yield when added to histopathology |
Probable and proven GI disease.
Proven GI disease.
Fifty-nine colon, 44 duodenum, 37 stomach, 7 esophagus, and 4 lung cases.
Hematoxylin and eosin, immunohistochemistry staining, and/or PCR on biopsy specimens of macroscopic lesions.
HCT recipients.
Thirteen liver, 5 lung, 2 kidney, and 1 small intestine.
Eleven esophagus, 49 stomach, 26 small intestine, 117 colon, and 10 >2 organs.
Studies were excluded if they did not differentiate SOT and HCT results from those for other medical conditions. IC, immunocompromised; NR, not reported; PPV, positive predictive value; NPV, negative predictive value; FFPE, formalin fixed and paraffin embedded; IBD, irritable bowel disease; HSCT, hematopoietic stem cell transplant; H&E−, hematoxylin and eosin negative; IHC+, immunohistochemistry positive.
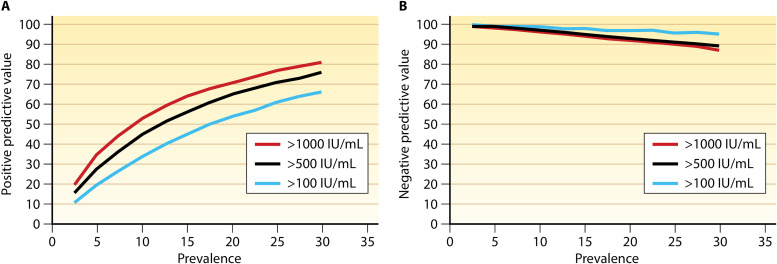
Another challenging area for the diagnosis of CMV in HCT and SOT recipients is CMV pneumonia. Historically, diagnosis relied on a positive viral culture from bronchoalveolar lavage (BAL) fluid, which has a high sensitivity for the diagnosis of CMV pneumonia by histopathology (59, 60). However, interpreting positive BAL fluid CMV culture results requires distinguishing between asymptomatic viral shedding (relatively common in this setting) and tissue-invasive infection (pneumonia). Qualitative PCR (results reported as positive or negative) is available for testing BAL fluid. However, while it may be useful to rule out the presence of CMV DNA, qualitative PCR lacks specificity and may not be as useful in discriminating between low-level viral shedding and end-organ disease (22, 61). Clinically, quantitative PCR is now widely available and preferred over qualitative PCR on BAL fluid for diagnosing CMV pneumonia. In current guidelines, the definition of probable CMV pneumonia now includes the detection of CMV DNA in BAL fluid by quantitative PCR, combined with clinical symptoms and/or signs of pneumonia in the appropriate clinical setting (54). Recent studies (Tables 4 and 5) indicate the diagnostic potential of qPCR for CMV DNA in BAL fluid but also highlight the challenges in establishing a precise diagnostic viral load threshold. Tables 4 and 5 include studies reported since 2010 that assessed the utility of BAL CMV qPCR for the diagnosis of CMV pneumonia in recipients of a lung transplant or HCT (62–69). In these studies, transplant recipients with CMV pneumonia had higher median CMV BAL fluid viral loads than did non-CMV pneumonia cases, although there was an overlap between cases and controls. In determining a viral load threshold to differentiate CMV pneumonia from pulmonary viral shedding, a key concept to consider is the predictive value. The predictive value takes into account the population prevalence of CMV pneumonia, while sensitivity and specificity provide only one half of the diagnostic equation. For example, our group used predictive models to calculate that a threshold of 500 IU/ml could have a positive predictive value of ∼60% in a population with a CMV pneumonia prevalence of 10%, but the positive predictive value of this threshold drops to <30% with a CMV pneumonia prevalence of 5% (Fig. 1). The corresponding negative predictive values of this viral threshold at each population prevalence were >90% and ∼100%, respectively (66). Limitations of studies of CMV qPCR on BAL fluid have included one or more of the following: a small sample size, a single-center design, and variable definitions of CMV pneumonia. Further studies of BAL fluid CMV PCR are warranted to standardize collection and assay techniques and reporting and to identify optimal thresholds in different clinical settings. If successful, such approaches have the potential to replace the need for biopsy and obviate viral culture for the diagnosis of CMV pneumonia in the appropriate clinical setting.
TABLE 4.
Utility of BAL fluid viral load determination for diagnosis of CMV pneumonia in SOT recipientsf
| Authors (reference) | Yr | Transplant type(s) | Study design and population | Total no. of patients | No. of CMV pneumonia cases | Median VL |
% of CMV pneumonia cases with positive blood PCR (no. of positive cases/total no. of cases) | Findings | Conclusion(s) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BAL fluid, in CMV pneumonia cases | TBB samples, in CMV pneumonia cases | BAL fluid, in non-CMV pneumonia cases | |||||||||
| Lodding et al. (62) | 2018 | Lung | Retrospective cohort study; lung transplant patients with positive CMV PCR of BAL fluid | 141; 66 CMV PCR+ | 34a | 32,940 IU/ml | NR | 1,260 IU/ml | 88 (30/34), plasma | For BAL CMV PCR, 91% sensitivity and 77% specificity at VL cutoff of 4,545 IU/ml | CMV BAL fluid PCR was a useful tool for diagnosing CMV pneumonia in lung transplant recipients |
| Costa et al. (63) | 2013 | Lung | Prospective cohort study; positive CMV PCR on matched TBB, BAL, and whole-blood specimens | 33 | 7b | 5.9 × 104 copies/mlc (4 cases) | 4.4 × 104 copies/mlc | 5.6 × 104 copies/mlc | 43 (3/7), whole blood | CMV pneumonia was associated with CMV PCR+ result for TBB samples and whole blood but not BAL fluid | CMV evaluation of TBB samples could represent a useful tool to discriminate between asymptomatic and clinical infection with consequent organ disease |
| Beam et al. (64) | 2018 | Lung, OLT, KT, HCT, nontransplant | Retrospective case-control study; comparison of CMV pneumonia vs non-CMV pneumonia cases | 38, 22d | 17, 5d | >1.82 × 107 IU/mld | NR | 7,500 IU/ml | 100 (5/5),d plasma | For all transplant patients, CMV BAL fluid PCR for pneumoniae had a 91.7% sensitivity and a 100% specificity at a VL cutoff of 34,800 IU/ml | CMV BAL fluid PCR may improve the clinician’s ability to diagnose CMV pneumonia without the need for and associated risks of lung biopsy |
| Wiita et al. (65) | 2012 | Lung | Retrospective and prospective cohort study; evaluation of CMV detection in the setting of indefinite valganciclovir prophylaxis | 128 | 2 | 650,000 copies/ml | NR | <20,000 copies/ml | NR | Compared with shell vial culture, BAL fluid PCR showed a sensitivity of only 36% and a specificity of 99%; however, results were not compared to IHC results | BAL fluid PCR was significantly more sensitive than shell vial culture; however, BAL fluid CMV VL was not predictive of subsequent disease development |
Probable and proven cases.
Documented by histopathology and immunohistochemistry.
Mean.
Lung transplant recipients, all with proven pneumonia.
Possible, probable, and proven pneumonia (n = 12).
NR, not reported; TBB, transbronchial biopsy; VL, viral load; OLT, orthotopic liver transplant; BAL, bronchoalveolar lavage.
TABLE 5.
Utility of BAL fluid viral load determination for diagnosis of CMV pneumonia in HCT recipientsm
| Author (reference) | Yr | Transplant type(s) | Study design/population | Total no. of patients | No. of CMV pneumonia cases | Median BAL VL |
% of CMV pneumonia cases with positive blood PCR result (no. of positive cases/total no. of cases) | Findings | Conclusion(s) | |
|---|---|---|---|---|---|---|---|---|---|---|
| CMV pneumonia cases | Non-CMV pneumonia cases | |||||||||
| Boeckh et al. (66) | 2017 | HCT | Case-control study; CMV pneumonia patients vs controls (asymptomatic, IPS, or non-CMV pneumonia) | 271 | 132a | 3.9 log10 IU/ml | 1.63 log10 IU/mlb ; 0 log10 IU/mlc | 86 (18/21), plasma | 84.1% sensitivity and 76.2% sensitivity at a VL cutoff of 203 IU/mld ; 90.2% sensitivity and 80.5% specificity at a VL cutoff of 99.7 IU/mle | CMV BAL PCR threshold of 500 IU/ml is suggested to differentiate between CMV pneumonia and pulmonary shedding |
| Lee et al. (67) | 2017 | Hematological malignancy, BMT | Retrospective case-control study; CMV pneumonia vs non-CMV pneumonia | 94; 59 BMT | 24,f 16g | 187,224 copies/ml | 3,055 copies/ml | NR | For HCT patients, CMV BAL fluid PCR had an 81.3% sensitivity and an 81.4% specificity at a VL cutoff of 18,900 copies/ml; for all patients, CMV BAL fluid PCR had a 75% sensitivity and an 88.6% specificity at a VL cutoff of 28,774 copies/ml | BAL fluid PCR can aid in the diagnosis of CMV pneumonia; the VL threshold for pneumonia diagnosis in BMT patients was suggested to be 18,900 copies/ml |
| Iglesias et al. (68) | 2017 | HCT | Retrospective cohort study; positive CMV PCR on BAL fluid | 56 | 6h | 53,250 copies/ml | <150 copies/mli | 83 (5/6), plasma | All CMV pneumonia patients’ plasma PCR values were lower than those from BAL fluid PCR; 75% of patients with a VL of >50,000 copies/ml died of refractory pneumonia | Any value for CMV VL in BAL fluid, especially if it is higher than that in plasma and clinically correlated, should be considered suggestive of CMV pneumonia |
| Pinana et al. (69) | 2019 | HCT | Retrospective cohort study; patients with BAL fluid data | 123 | 2,i 4j | 7,225 IU/ml | 1,210 IU/ml | NR | Increased mortality had a sensitivity of 84.2% and a specificity of 53.1% at a VL cutoff of 500 IU/ml | 500 IU/ml is unlikely to be discriminative between CMV pneumonia and viral shedding in this setting |
| Beam et al. (64) | 2018 | Lung, OLT, KT, HCT, nontransplant | Retrospective case-control study; CMV pneumonia vs non-CMV pneumonia | 38, 4g | 17, 3k | 34,800 IU/ml | 7,500 IU/ml | 100 (3/3), plasma | For all transplant patients, CMV BAL fluid PCR for pneumoniae had 91.7% sensitivity and 100% specificity at a VL cutoff of 34,800 IU/mll | CMV BAL fluid PCR may improve the clinician’s ability to diagnose CMV pneumonia without the need for and associated risks of lung biopsy; of note, none of the HCT patients underwent biopsy and histopathology to confirm CMV pneumonia |
Pneumonia defined as symptoms or signs of pneumonia with positive results by shell vial or conventional culture or by direct fluorescent-antibody (DFA) testing.
Asymptomatic CMV shedder.
Non-CMV pneumonia and idiopathic pneumonia syndrome (IPS) groups.
Compared to asymptomatic controls.
CMV pneumonia patients compared to IPS or non-CMV pneumonia patients.
Possible, probable, proven, or indeterminate by IHC of bronchial wash and TBB specimens.
HCT patients.
Probable CMV pneumonia.
Proven pneumonia.
For possible-probable CMV pneumonia, with 4 events (1 recurrence from proven), CMV PCR values for BAL fluid ranged from 1,382 to 40,048 IU/ml, and plasma PCR values ranged from 3,510 to 54,540 IU/ml, with 1 negative cytospin result.
Possible pneumonia, only HCT.
Possible, probable, and proven pneumonia (n = 12).
NR, not reported; VL, viral load; BMT, bone marrow transplant.
FIG 1.
Predictive models of positive (A) and negative (B) predictive values with thresholds of 100, 500, and 1,000 IU/ml across a range of cytomegalovirus (CMV) pneumonia prevalences in patients who underwent bronchoalveolar lavage for evaluation of pulmonary infiltrates (132 patients with CMV pneumonia and 118 controls with non-CMV pneumonia). (Reproduced from reference 66 with permission of Oxford University Press.)
Host Response to Virus (CMV-Specific Immunity)
Until recently, direct quantitation of CMV in blood and other samples, typically by qPCR, has been the focus of diagnostic methods, based on a well-established relationship between CMV viral loads and the risk for progression to CMV disease (8–17). However, the use of highly sensitive CMV qPCR assays can lead to overtreatment. Additional risk stratification is needed to individualize treatment and avoid unnecessary antiviral exposure. Multiple risk factors for significant CMV replication and/or disease in transplant recipients are known, but precise quantitation of risk in an individual patient remains challenging. Standardized methods for the detection and quantitation of CMV-specific immunity have been developed to complement existing CMV viral load assays and offer an opportunity to identify patients capable of controlling viral replication through host immune mechanisms, without the need for antiviral therapy. Several studies have evaluated CMV-specific and nonspecific immune functions (e.g., lymphopenia and CD4 counts) (70–74) as tools for individualizing CMV risk, and a recent review of studies evaluating CMV-specific cell-mediated immunity (CMI) in transplantation summarizes the available data (75). Nonspecific immune markers such as lymphopenia have also been associated with an increased risk for CMV infection or disease but do not appear to have adequate positive and negative predictive values to make them useful for clinical routine use (76, 77). A summary of the types of commercially available immune monitoring assays, and their advantages and limitations, can be found in recent guidelines for the management of CMV in SOT (22). Commercially available assays include the QuantiFERON-CMV enzyme-linked immunosorbent assay (QFN) as well as T-Track and T-Spot.CMV, which are enzyme-linked immunosorbent spot (ELISpot) assays. Most of these assays detect interferon gamma (IFN-γ) release from cells (in blood or peripheral blood mononuclear cells) stimulated with CMV-specific antigens or peptides.
Studies of CMV-specific CMI in SOT, the majority of which have been conducted in kidney transplant (KT) recipients, have demonstrated that CMV-specific CMI correlates with virologic outcomes. A positive CMV IFN-γ release assay result was associated with reduced CMV infection/disease, lower initial and peak viral loads, freedom from CMV events, and a decreased incidence of CMV recurrence (78–94). In a recent large multicenter study of KT transplant recipients (n = 368), positive CMV-specific CMI at the end of prophylaxis (by T-Spot) predicted freedom from CMV events (93). However, an important limitation of this study was the inability of the assay to predict CMV events in the highest-risk group (D+ R− patients), limiting its clinical utility in this high-need population. Indeed, both the predictive value and the optimal CMI threshold for predicting CMV infection and disease may differ for transplant recipients with specific risk factors (e.g., antithymocyte globulin induction), and assay sensitivities and specificities may vary across the available platforms (82, 85, 95).
In HCT, observational studies have evaluated CMV-specific CMI as a tool for risk stratification of CMV infection and disease posttransplantation (96–103). In these studies, CMI testing has been done both pretransplantation and posttransplantation, at various intervals up to 1-year posttransplantation. qPCR was concurrently performed to monitor for viremia. Patients with detectable CMV-specific immunity had higher rates of spontaneous viremia clearance, lower rates of CMV reactivation, and decreased peak viral loads (96–102). The sensitivities and specificities varied across testing modalities and studies. However, these studies collectively demonstrate that CMV-specific CMI has the potential to guide antiviral prophylaxis and therapy in the future.
At present, data to demonstrate the clinical utility of CMV-specific CMI assays in large, well-designed interventional studies are lacking. Currently available standardized CMV-specific CMI testing platforms are limited by low utility in the setting of profound lymphopenia and the absence of assessments of polyfunctionality and markers such as the T-box transcription factor T-bet, which may play a role in predicting CMV-specific immunity (104, 105). Neutralizing antibodies, which may have an important role in primary infection (106), are likewise not included in these CMI-based testing platforms. Conversely, an important advantage of commercially available platforms is their relative ease of use, standardized format, and suitability for comparing results across studies/populations (22).
In addition to the observational studies described above, small interventional studies have described the integration of CMV-specific CMI testing to guide antiviral therapy. With this approach, CMI testing (at one or more time points) is used to determine the duration of antiviral treatment or prophylaxis based on the predicted risk of subsequent CMV viremia. Kumar et al. assessed the use of CMV-specific CMI testing (by QFN) to guide antiviral therapy following the treatment of CMV viremia in 27 SOT patients (7 kidney transplant, 10 liver, 6 lung, and 4 combined) (92). Patients (predominantly D+ R− [44.4%] and R+ [48.1%]) were treated until the CMV viral load was undetectable by PCR at one time point or <137 IU/ml at two consecutive time points. At the end of antiviral therapy, the CMV QFN result was used to assign patients to observation without further therapy (positive QFN result, 51.9% of patients) or to additional antiviral prophylaxis for 2 months (negative QFN result, 48.1% of patients). For those with a positive QFN result (for whom antiviral therapy was discontinued), only 1/27 developed subsequent low-level viremia and required treatment, while the recurrence rate among CMV QFN-negative patients was 69.2% despite an additional 2 months of antiviral prophylaxis (92). In a second study, Westall et al. evaluated CMV-specific CMI testing (by QFN) to direct the length of antiviral prophylaxis following lung transplantation (91). Lung transplant recipients (n = 118) 5 months after transplant were randomized 1:2 to either cessation of antiviral prophylaxis or continuation of prophylaxis for a duration guided by serial CMV QFN testing (up to 11 months). CMV infection in the lung allograft within 18 months of transplantation was significantly decreased in the QFN-guided prophylaxis duration arm (37% versus 58%). Among patients who stopped antiviral prophylaxis at 5 months based on a positive QFN result, compared to patients without protective immunity, significant reductions were observed in the incidences of viremia (13% versus 67%) and high-grade viremia (defined as >10,000 copies/ml) (3% versus 50%) (91). A positive recipient CMV serostatus was associated with a positive QFN result. Among R+ patients (n = 88) at the time of lung transplantation, 72% had a positive QFN result, compared to only 7% (2/30) of D+ R− patients at study inclusion. These preliminary interventional studies suggest that the incorporation of CMV-specific CMI into clinical care is feasible and has the potential to refine current prevention and/or treatment strategies in the SOT setting. However, additional studies are needed to define the specific patient populations and indications for the use of CMV CMI-based testing in SOT recipients.
In the HCT setting, a prospective multicenter matched-control trial utilized serial CMV-specific CMI (via ELISpot assays) and viral load monitoring to guide the duration of antiviral therapy in R+ and D+ R− HCT recipients (n = 61) for CMV viremia within 100 days of transplantation (101). CMV-specific CMI was assessed on days 7, 14, 21, and 28 after the initiation of antiviral therapy. In 11 (18%) of the 61 patients, antiviral therapy was discontinued based on the fulfillment of viral and immunological criteria (a positive CMI result and clearance of CMV DNAemia). The rate of CMV viremia recurrence was significantly lower among patients who met immunological criteria for discontinuing treatment (9%) than in the comparator group that was guided solely by plasma CMV DNA loads (∼40%). The duration of antiviral therapy was also shorter in these patients, by approximately 1 week.
Collectively, these studies suggest that CMV-specific CMI shows significant promise for individualizing risk prediction and prophylaxis/therapy duration, especially with the availability of standardized commercially available platforms. However, future studies directly comparing the various platforms will be required, since each assesses different CMI parameters and appears to have different operating characteristics. Additionally, carefully designed prospective randomized interventional studies integrating CMV-specific CMI testing to guide clinical decision-making should be done to define the potential clinical utility of these assays to complement or potentially even replace the information provided by currently available CMV viral load assays. Analogous approaches might have the potential for other transplant-associated viruses (e.g., Epstein-Barr virus and BK virus).
PREVENTION: STRATEGIES
Overview
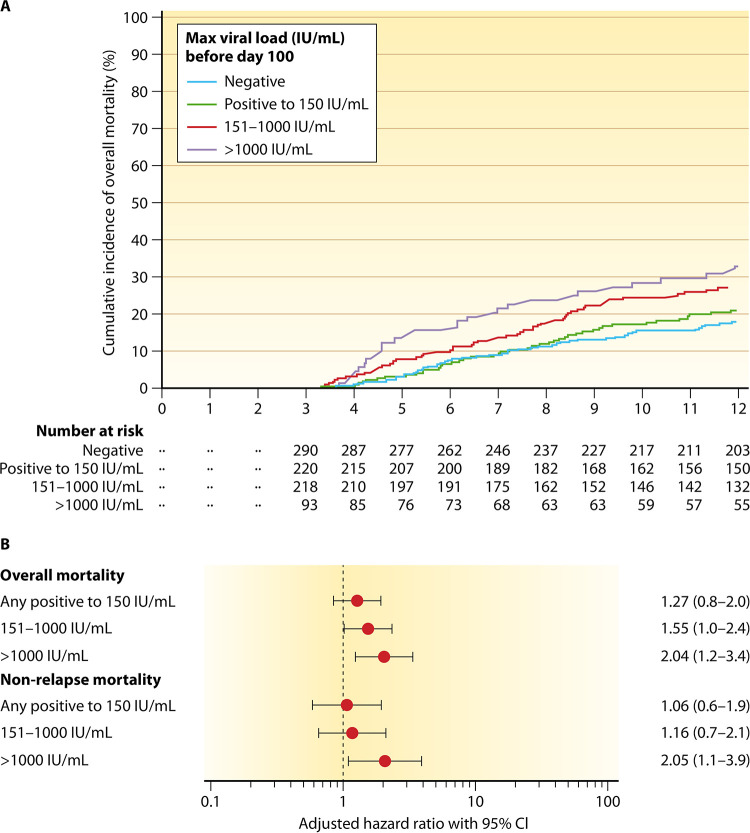
CMV infection and disease have a substantial negative impact on graft and patient outcomes in both SOT and HCT populations (10, 22, 107–110), as demonstrated by natural history studies and high rates of morbidity and mortality in the preantiviral era (111–113). Even in the context of PET, higher CMV viral loads carry an increased risk of mortality (107) (Fig. 2) and are considered an appropriate surrogate endpoint for clinical trials in HCT and SOT (114, 115). Consequently, the use of a CMV prevention strategy is considered the standard of care in all transplant patients at risk (most commonly defined on the basis of donor and recipient serological status). Major guidelines consider the use of a specific CMV prevention strategy a grade A1 recommendation. Indeed, it is no longer considered ethical to perform trials of CMV prevention in transplant recipients with a comparator group that does not receive any CMV-preventive strategy.
FIG 2.
Cumulative incidence of overall mortality in survivors at day 100 (n = 832) stratified by the maximum cytomegalovirus viral load before day 100 (A) and multivariable Cox proportional-hazard models assessing maximum cytomegalovirus viral load before day 100 as a risk factor for overall mortality (B). Covariates for overall mortality models were age, donor relation, transplantation year, underlying disease, disease risk, hemopoietic stem cell transplantation-specific comorbidity index score, neutropenia before day 100, and cytomegalovirus viremia after day 100 (time dependent). CI, confidence interval. (Reproduced from reference 107 with permission of Elsevier.)
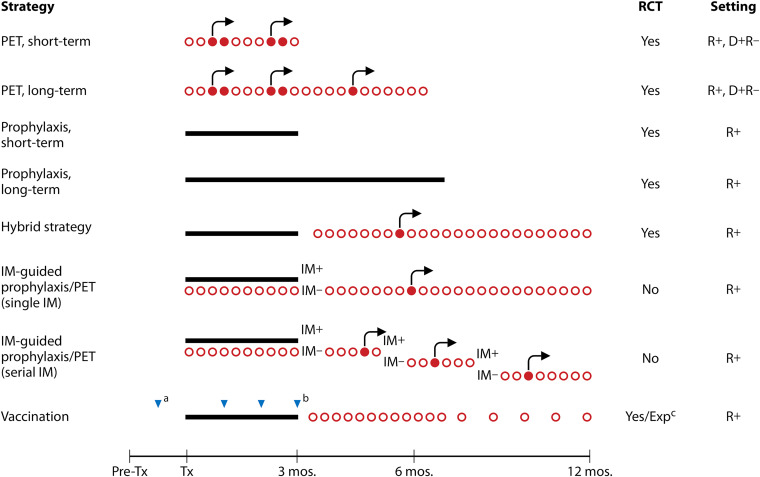
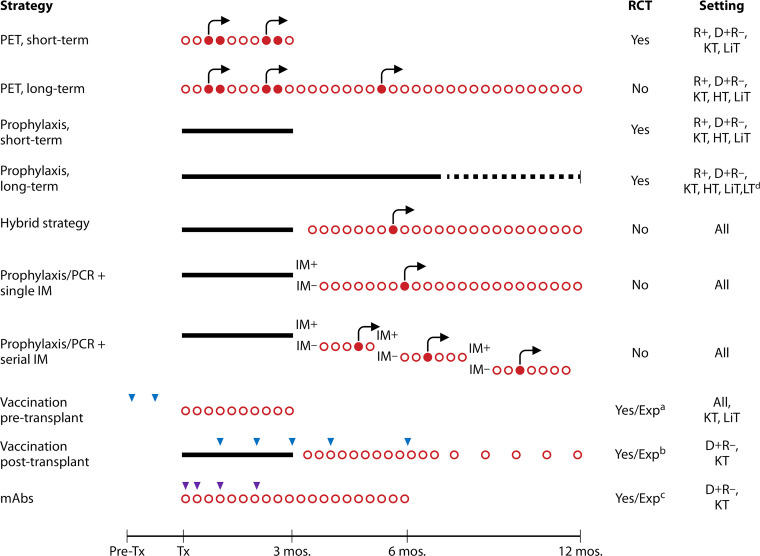
PET and antiviral prophylaxis are the two most widely used CMV prevention strategies in HCT and SOT for patients at risk for CMV infection/disease based on recipient and/or donor CMV-seropositive status. These two strategies are depicted in Fig. 3 and 4 and are described and compared below.
FIG 3.
CMV prevention strategies in HCT, including potential combined approaches. Red circles indicate weekly monitoring for CMV viremia; open circles indicate test time points that yielded viral loads below the threshold for the initiation of antiviral therapy, while filled shapes indicate test time points with values above this threshold. Black arrows indicate the administration of antivirals as preemptive therapy (PET). Black bars indicate the administration of antivirals as prophylaxis. Blue triangles indicate the administration of a dose of vaccine. All strategies include clinical surveillance. IM, immune monitoring; Exp, experimental; Tx, therapy. a, vaccination of transplant donor and/or recipient; b, various vaccination schedules have been used; c, see references 171, 175, and 267.
FIG 4.
CMV prevention strategies in SOT, including potential combined approaches. Red circles indicate weekly monitoring for CMV viremia; open circles indicate test time points that yielded viral load below the threshold for the initiation of antiviral therapy, while filled shapes indicate test time points with values above this threshold. Black arrows indicate the administration of antivirals as preemptive therapy (PET). Black bars indicate the administration of antivirals as prophylaxis. Blue and purple triangles indicate the administration of a dose of vaccine or monoclonal antibodies (mAb), respectively. All strategies include clinical surveillance. LiT, liver transplant; LT, lung transplant; PCR, CMV viral load monitoring by PCR; IM, immune monitoring; Exp, experimental. a, see reference 168; b, see reference 169; c, see reference 23; d, the dotted line represents the duration of prophylaxis for lung transplantation.
Description of the PET Strategy
PET consists of scheduled monitoring to detect early CMV replication, with the initiation of an antiviral drug at a predetermined threshold to prevent the progression of CMV replication that may ultimately result in CMV disease (Fig. 3 and 4). The availability of qPCR, a highly sensitive test, has made PET a feasible prevention strategy. qPCR is now the preferred modality for CMV monitoring in PET (11, 14, 22, 116–120). Because of the significant toxicity of previously available antivirals, and despite several challenges and limitations (Table 6), PET has until now been the preferred CMV prevention strategy in HCT recipients, primarily because of the toxicity of available antiviral drugs (e.g., ganciclovir). However, with the availability of newer antiviral drugs without significant hematological toxicity (i.e., letermovir and the investigational agent maribavir), prophylaxis has also become feasible in the HCT setting.
TABLE 6.
Challenges with the use of a PET prevention strategy
| Challenge(s) | Consideration(s) |
|---|---|
| Lack of consensus regarding the threshold for initiation of antiviral therapy | Preventing disease progression |
| Allowing sufficient antigen stimulation to prevent late episodes of viremia | |
| Unclear optimal testing frequency and duration | Rapid viral replication necessitating frequent testing |
| Logistics and cost of frequent testing | |
| Testing adherence at late time points | Frequent testing is logistically challenging at late time points after transplant |
| Choice of monitoring test | PCR is most frequently used, but antigenemia is still used by some centers |
| Variability among qPCR assays |
Description of the Prophylaxis Strategy
Antiviral prophylaxis entails the administration of antiviral medication around the time of transplantation for all at-risk patients for a defined duration posttransplantation, with the goal of maintaining viral suppression during the period of greatest risk for infection/reactivation (Fig. 3 and 4). Prophylaxis is generally effective in preventing viremia and disease during the prophylaxis period in those who can tolerate the drug but has been associated with relatively high rates of postprophylaxis late-onset CMV disease, especially among D+ R− SOT patients (73, 121, 122). D+ R− or R+ SOT patients are typically given prophylaxis for 3 to 6 months posttransplantation and up to 12 months for lung transplant recipients (22). There is a large body of evidence demonstrating the efficacy of this strategy for preventing CMV disease and beneficially impacting CMV-associated indirect effects (bacterial and fungal infections, graft function, and overall survival), and it has been the dominant strategy for CMV prevention in SOT recipients. It is also becoming more widely used in HCT recipients with the availability of less myelotoxic antiviral agents (discussed below) (123).
Combined Approaches to CMV Prevention
Because postprophylaxis delayed-onset CMV disease is a well-recognized limitation of antiviral prophylaxis, a hybrid strategy that combines a duration of prophylaxis followed by PET (i.e., monitoring for CMV replication after discontinuation of antiviral prophylaxis) has also been proposed (124–127). This approach (Fig. 3 and 4) aims to address the important clinical problem of postprophylaxis delayed-onset CMV disease but has multiple limitations, including logistical issues related to frequent blood draws and the implementation of antiviral therapy at a time point when patients are at a significant distance from the transplant center and may have less access to diagnostic testing, evidence from small studies that did not demonstrate a benefit of this approach, and the same limitations inherent to PET in general (e.g., the lack of consensus on a specific threshold for the initiation of therapy).
The landscape of CMV prevention is evolving. As depicted in Fig. 3 and 4, newer diagnostic or therapeutic modalities could be combined with existing preventative strategies (PET or prophylaxis) to further augment the suppression of CMV. For example, the duration of antiviral therapy could be guided by CMV-specific CMI, as discussed above (91, 92, 101). Alternatively, the administration of a CMV vaccine later in the posttransplant period, at a time when lower levels of immunosuppression are present, might enhance vaccine responses.
Updates in Prophylaxis
A major development in the field has been the approval of letermovir, a novel CMV-specific terminase complex inhibitor that was approved by the FDA in 2017 and the European Medicines Agency in 2018 for the prophylaxis of CMV in seropositive HCT recipients. Evidence supporting the safety and efficacy of letermovir for this application is discussed further in Prevention: Novel Agents and Approaches, below. The availability of letermovir has altered the landscape of CMV prevention in HCT; the absence of significant myelotoxicity has made prophylaxis with this drug feasible for HCT recipients (123). However, as seen in other trials of antiviral prophylaxis, delayed-onset postprophylaxis CMV infection occurred in those who received letermovir. Thus, an ongoing trial (ClinicalTrials.gov identifier NCT03930615) is evaluating the efficacy of longer-duration prophylaxis (i.e., 200 days versus 100 days of letermovir prophylaxis in R+ HCT recipients). Letermovir is also being directly compared to valganciclovir for prophylaxis in D+ R− kidney transplant recipients in an ongoing phase 3 RCT (ClinicalTrials.gov identifier NCT03443869), with the results of this study anticipated in ∼2021.
Updates in Preemptive Therapy
Current guidelines generally favor prophylaxis over PET for CMV prevention in high-risk D+ R− SOT recipients (22). However, late-onset CMV is observed frequently in the months following the cessation of antiviral prophylaxis and is associated with graft failure and mortality in SOT (128) and with mortality in HCT (129) recipients. A recently completed, NIH-supported, multicenter RCT offers new evidence suggesting that PET, compared to prophylaxis, decreases the incidence of late-onset CMV disease in D+ R− liver transplant recipients (130). The proposed underlying mechanism is preferentially enhanced CMV-specific immunity with PET compared to prophylaxis (increased multifunctional T cells and neutralizing antibody titers), as demonstrated in a small observational study and, more recently, in a multicenter RCT directly comparing PET to prophylaxis in D+ R− liver transplant recipients (106, 130). Among HCT recipients, the incidence of CMV disease is low with current PET regimens incorporating qPCR and ganciclovir/valganciclovir (38, 131–136).
Preemptive Therapy versus Prophylaxis
There are relative advantages and disadvantages of PET and prophylaxis. While PET may reduce unnecessary drug exposure and reduce the risk of drug-induced myelosuppression in HCT patients, universal prophylaxis may be preferred for higher-risk transplant recipients, including unrelated, HLA-mismatched, and umbilical cord blood transplants. In SOT, only two new RCTs since 2010 have directly compared these two approaches using valganciclovir (Table 7). Witzke et al. randomized R+ KT transplant patients (n = 299) to PET (n = 151) or valganciclovir prophylaxis (n = 148) (137, 138). Significantly fewer patients in the prophylaxis arm developed CMV disease at both 12 and 84 months of follow-up, while rates of mortality and graft loss were similar between the two strategies. An important limitation of the study that might explain the relatively high incidence of CMV disease in the PET group (15.2%) was the infrequent monitoring schedule used (weekly for 4 weeks and then every 3 weeks until 28 weeks posttransplantation). Thus, these results demonstrating an apparent reduction in CMV disease with prophylaxis over PET in R+ kidney transplant recipients should be interpreted cautiously, and future studies should incorporate weekly monitoring for 3 months to guide the initiation of antiviral therapy in PET strategies. In the single largest direct comparative study of PET versus prophylaxis in high-risk SOT recipients to date, Singh et al. randomized D+ R− liver transplant recipients to PET (n = 100) versus universal prophylaxis (n = 105) with valganciclovir (130). PET significantly reduced the incidence of CMV disease compared to prophylaxis (9.1% versus 19.1%). Rates of mortality and graft loss were similar for both arms at 12 months posttransplantation and during longer-term follow-up. The feasibility of PET across a range of clinical settings and generalizability to nonliver D+ R− SOT populations deserve further study.
TABLE 7.
Newer studies directly comparing preemptive therapy and prophylaxis for CMV prevention strategies in SOT recipientsa
| Authors (reference) | Yr | SOT type | No. of patients |
CMV serostatus | Monitoring strategy for PET | Drug regimen |
PET vs prophylaxis |
Follow-up duration (mos) | Other outcome(s)/conclusion(s) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | PET vs prophylaxis | PET | Prophylaxis | % CMV infection | % CMV disease | % biopsy-proven allograft rejection | % mortality | % graft loss | |||||||
| Witzke et al. (137) | 2018 | KT | 299 | 151 vs 148 | R+ | qPCR of plasma weekly for 1–4 wks, every 3 wks for 6–28 wks, on wk 40 and wk 52, and then twice a year | VGCV at 900 mg BID for >14 days | VGCV at 900 mg/day | 39 vs 11 | 15 vs 5 | 13 vs 18 | 1 vs 2 | 5 vs 1 | 12 | Rates of opportunistic infections were also similar between regimens, but higher rates of posttransplantation diabetes were observed with prophylaxis |
| Singh et al. (130) | 2020 | OLT | 100 vs 105 | D+ R− | qPCR of plasma weekly for 100 days | VGCV at 900 mg BID until 2 consecutive negative weekly tests | VGCV at 900 mg/day for 100 days | NR | 9 vs 19 | 28 vs 25 | 15 vs 19 | 2 vs 2 | 12 | Opportunistic infections and neutropenia were the same in both groups; PET significantly reduced the incidence of CMV disease compared to prophylaxis | |
Clinical trials comparing PET and prophylaxis. NR, not reported; VGCV, valganciclovir; BID, twice a day.
For HCT, a multicenter RCT directly compared the efficacies of real-time PET versus prophylaxis (n = 89 and 95, respectively) to prevent late-onset CMV-associated complications. The composite endpoints incorporating mortality, CMV disease, and non-CMV invasive infections were similar between the two preventative strategies at day 640 posttransplantation (38). CMV DNAemia was reduced in the prophylaxis arm, but rates of CMV disease were similarly low in both arms. Importantly, no differences in immune reconstitution (T-cell responses to CMV, varicella-zoster virus, and herpes simplex virus) or toxicity were observed between the groups. This study supports the current clinical practice of PET in HCT for CMV prevention by demonstrating that universal prophylaxis was not superior to PET in preventing late-onset CMV complications. However, prophylaxis may be an alternative if weekly surveillance is not feasible, especially in high-risk patients.
Summary
There is strong evidence to support the use of a CMV-preventive strategy for all at-risk (i.e., donor or recipient seropositive) transplant patients. The relative efficacies of PET and prophylaxis may vary by risk category and transplant setting. New studies incorporating less myelotoxic agents (i.e., letermovir) demonstrate the superiority of prophylaxis over PET in HCT. In contrast, a recent study demonstrated the superiority of PET over prophylaxis for the prevention of CMV disease in high-risk D+ R− liver transplant recipients, primarily due to a reduction in late-onset CMV disease; however, feasibility and generalizability to other high-risk SOT populations require further study. Due to the relatively low incidence of disease (as a result of the success of PET and prophylaxis strategies) in HCT settings, future studies should focus on outcomes other than CMV disease endpoints alone. The integration of CMV-specific CMI diagnostic tools for risk stratification and guidance of preventative strategies will likely refine preventative approaches in clinical practice in the future.
PREVENTION: NOVEL AGENTS AND APPROACHES
Novel Agents
Over the last decade, new antiviral medications, including letermovir, maribavir, and brincidofovir, have been clinically evaluated for CMV prophylaxis in transplant recipients.
Letermovir, a 3,4-dihydro-quinazoline-4-yl-acetic acid derivative, binds the CMV terminase complex and inhibits the processing and packaging of DNA concatemers into smaller viral particles (139, 140). Importantly, this mechanism of action disrupts a later stage in the viral life cycle (i.e., after DNA replication has occurred) than currently approved DNA replication inhibitors. This difference might have implications for assessments of CMV loads by qPCR, especially in patients receiving letermovir for established CMV infection rather than prophylaxis. In the pivotal phase 3 double-blind RCT in R+ HCT recipients, 12 weeks of letermovir significantly reduced the incidence of clinically significant CMV infection, defined as the initiation of PET for viremia or end-organ disease (37.5% in the letermovir arm versus 60.6% in the placebo arm), 24 weeks after HCT (135). Follow-up analysis showed that letermovir was effective even among patients with CMV viremia at randomization (141). Letermovir was well tolerated, with a low incidence of overall adverse events and no significant hematological toxicity. There was a nonsignificant reduction in all-cause mortality at week 48 posttransplantation: 20.9% in letermovir recipients and 25.5% in the placebo group (135). In a post hoc analysis (142), in the letermovir group, there was no difference in mortality between those with and those without clinically significant viremia (CS-CMVi). In contrast, in the placebo group, the all-cause mortality rate at week 48 post-HCT was higher in patients with than in those without CS-CMVi (31.0% versus 18.2%). These results suggest that letermovir may reduce mortality by preventing or delaying CS-CMVi in HCT recipients.
Similarly, letermovir provides the option of prophylaxis in SOT patients without the adverse hematological profile of valganciclovir or ganciclovir. A phase 3 clinical trial of letermovir prophylaxis in D+ R− KT patients is ongoing (ClinicalTrials.gov identifier NCT03443869). Limitations of letermovir include a potentially lower barrier to the emergence of resistance (143, 144) and a lack of activity against other herpesviruses, necessitating the use of additional antiviral agents for the prevention of herpes simplex virus and varicella-zoster virus infections. Letermovir is a cytochrome P450 3A (CYP3A) inducer and a CYP2C8 and organic anion-transporting polypeptide 1B inhibitor (139). Drug interactions with other commonly used transplant medications are anticipated and may have clinical consequences (145), and letermovir requires a substantial dose reduction when coadministered with cyclosporine as well as monitoring and adjustment of the tacrolimus dose when used concurrently (146, 147).
Maribavir, an oral benzimidazole l-riboside, competitively inhibits ATP binding to CMV UL97 kinase and interferes with viral packaging and egress (148). This drug was initially developed for prophylaxis, and maribavir prophylaxis in HCT recipients decreased CMV infection compared with the placebo in a phase 2 dose-escalation study (149). However, phase 3 studies evaluating twice-daily (BID) dosing at 100 mg for prophylaxis in liver transplant recipients and HCT recipients failed to prevent CMV disease (131, 150). It has been postulated that the studied dose was too low; current studies for treatment are using significantly higher doses (151). Currently, further studies for maribavir prophylaxis are not planned, and the focus of development has shifted to studies of treatment of established infection: PET and treatment of resistant or refractory infection. A recent phase 2, open-label, maribavir dose-blinded trial evaluated three doses of maribavir for PET in HCT recipients with CMV viremia (152). Subjects were randomly assigned to receive various doses of maribavir (n = 117) or a standard treatment dose of valganciclovir (n = 39) for up to 12 weeks. Maribavir at 400 mg BID or higher had rates of CMV clearance equivalent to those of valganciclovir. High rates of GI side effects but low rates of neutropenia were reported in the maribavir arm compared to the valganciclovir arm (152). A recent phase 2 trial compared maribavir treatment at different doses (400 mg BID, 800 mg BID, or 1,200 mg BID) for refractory or resistant CMV in recipients of HCT or SOT. In that trial, CMV viremia resolved by 6 weeks in 67% (n = 120) of patients (153). In regard to drug interactions, maribavir has been found to be a weak inhibitor of P‐glycoprotein activity but did not affect CYP2D6 (154). The role(s) of maribavir for the treatment of refractory/resistant infection or as PET will be better defined by the two ongoing phase 3 trials (ClinicalTrials.gov identifiers NCT02927067 and NCT02931539).
Brincidofovir (CMX001) is an oral lipid-conjugated nucleotide analog and oral prodrug of cidofovir that has been evaluated for the prevention of CMV. In phase 2 dose-ranging studies in HCT patients, brincidofovir was found to decrease CMV infection compared to the placebo (133). However, in a subsequent phase 3 trial, brincidofovir failed to prevent clinically significant CMV infection by week 24 after HCT and was associated with significant GI toxicity (136). Further development of oral brincidofovir for CMV prevention in transplant recipients has therefore been halted. An intravenous (i.v.) formulation with a more favorable toxicity profile is currently being developed and is expected to undergo clinical trials in HCT recipients in the near future (155).
Novel Approaches: Serosorting
Serosorting is a CMV prevention strategy in which grafts are preferentially directed to recipients with a matching CMV serostatus. Current guidelines for HCT recommend that, when possible, serosorting should be used for both CMV-seronegative and -seropositive recipients (36, 156). A positive donor or recipient CMV serostatus is independently associated with increased mortality (109, 157). More recently, there has been renewed interest in utilizing the serosorting approach in SOT, although this was previously deemed infeasible due to concerns about delays of life-saving transplantation (158). Despite multiple potential limitations of this approach (the applicability to seronegative patients only, the potential delay in transplantation with associated risks, the decreased pool of available donors, and the lack of incorporation of CMV serostatus into current organ allocation policies), recent studies have suggested that serosorting might be feasible and improve outcomes by reducing direct and indirect impacts of CMV, specifically in kidney transplant recipients (159, 160). The broader applicability of this approach to other transplant populations and across a range of allocation settings remains to be defined but represents an important area of future investigation.
Novel Approaches: Vaccines
Impairment in CMV-specific immunity is the primary mechanism that underlies CMV-associated complications in HCT and SOT recipients and other immunosuppressed populations. While specific immunological correlates of risk or protection have not been fully characterized, major targets of the immune response are well studied (161–163). In theory, a vaccine could directly target the host deficits that underlie CMV reactivation and disease and drive a protective immune response that could control viral reactivation and prevent disease.
The development of a CMV vaccine has been studied for decades (164), focused primarily on preventing or mitigating congenital CMV; there is now also a focus on transplant populations (165–167). In this context, several vaccine candidates have emerged and entered clinical trials, with mixed results (Table 8) (165–167). A vaccine based on recombinant CMV glycoprotein B (gB) formulated with the MF59 adjuvant was administered (3 doses pretransplantation) to kidney and liver transplant recipients in a double-blind phase 2 trial and significantly increased antibodies to gB regardless of recipient serostatus (168). Vaccinated recipients also had a shorter duration of viremia (which was inversely correlated with the magnitude of the gB antibody response) and a decrease in CMV treatment days compared with those who received a placebo (168). ASP0013, a DNA-based vaccine encoding gB and the tegument phosphoprotein 65 (pp65), recently yielded disappointing results in both a phase 2 study in SOT and a phase 3 study in HCT recipients. In the phase 2 trial, kidney transplant (D+ R−) recipients who received the vaccine showed no reduction in CMV viremia compared to unvaccinated subjects (169). In the phase 3 trial (ClinicalTrials.gov identifier NCT01877655), vaccinated CMV R+ HCT recipients showed no reduction in the primary endpoint, which was a composite of overall mortality and CMV end-organ disease through 1 year after transplantation. Secondary endpoints, including viremia, duration of antiviral therapy, and overall mortality, also showed no benefit associated with vaccination (170). A chimeric peptide vaccine targeting the well-conserved pp65 epitope HLA A*0201 pp65495–503, PepVax, was assessed in HCT recipients in a randomized phase 1b trial; a phase 2 trial in this population is ongoing (ClinicalTrials.gov identifier NCT02396134). Vaccination led to significantly increased pp65-specific CD8+ T cells expressing effector phenotypes, reduced CMV reactivation and antiviral usage, and increased relapse-free survival (171–173).
TABLE 8.
Selected CMV vaccine candidates for prevention of CMV infection in transplant recipients
| Candidate | Type of vaccine | Target(s) | Reference(s) |
|---|---|---|---|
| gB | Recombinant protein | gB | 168 |
| MVA Triplex | Vector (MVA) | pp65, IE1-exon 4, and IE2-exon 5 | 174, 279 |
| HB-101 | Vector (LCMV) | gB and pp65 | 175 |
| ASP0113 | DNA | gB and pp65 | 132, 169 |
| PepVax | Chimeric peptide | pp65 | 171, 172 |
| ALVAC-pp65 | Vector (canarypox virus) | pp65 | 280, 281 |
| Towne | Attenuated strain | Whole virus | 282 |
The Triplex vaccine, which is based on a modified vaccinia virus Ankara (MVA) strain encoding three CMV antigens (pp65, immediate early protein 1 [IE1]-exon 4, and IE2-exon 5), was well tolerated in a randomized, multisite phase 2 trial in HCT recipients (ClinicalTrials.gov identifier NCT02506933) (174). A lower-than-anticipated incidence of CMV events prevented conclusive statistical analysis of the primary endpoint, CMV reactivation events through day 100 after HCT (defined as ≥1,250 CMV DNA IU/ml, low-level reactivation prompting antiviral therapy, or CMV disease). CMV events occurred in 5 patients (9.8%) in the vaccinated group and 10 recipients (19.6%) in the placebo group. Trials are ongoing to evaluate Triplex for additional applications in transplant recipients. HB-101 is also a vector-based vaccine; it is based on attenuated recombinant lymphocytic choriomeningitis virus (LCMV) and expresses the antigens pp65 and truncated gB. HB-101 was well tolerated in a phase 1 dose-escalation trial (175) and is currently being evaluated for living donor kidney transplant recipients (D+ R−) in a phase 2 trial (ClinicalTrials.gov identifier NCT03629080). An mRNA-based vaccine (mRNA-1647) encoding gB and the CMV pentameric complex was well tolerated and immunogenic (dose-related increase in neutralizing antibodies), based on interim results from a phase 1 trial (ClinicalTrials.gov identifier NCT03382405) (176).
The approach of directly addressing the underlying host deficit(s) that predisposes to CMV infection and disease through vaccination is conceptually attractive. However, significant challenges to the eventual development of a CMV vaccine remain, including selecting the appropriate target(s), defining the optimal vaccination schedule (pre- versus posttransplantation, or donor or recipient, etc.), and selecting appropriate endpoints and populations for clinical trials. Moreover, the optimal approach to vaccination may differ for SOT versus HCT. Ultimately, there could still be a significant beneficial impact of even a partially effective vaccine, which could be combined with other interventions (prophylaxis and PET, etc.) (Fig. 3 and 4) for optimizing the control of CMV infection and disease in transplantation.
Novel Approaches: Monoclonal Antibodies
Prior to the development of antivirals, CMV hyperimmune globulin (CMV Ig) was licensed for the prevention of CMV disease after kidney transplantation, based on randomized trials showing benefit (177, 178). However, the benefit of CMV Ig in HCT recipients was less certain (179). In modern clinical practice, antivirals have largely replaced CMV Ig in preventive strategies. Progress in understanding how CMV enters cells has led to the identification of specific targets for CMV entry into primary target cell types (180–183), including epithelial and endothelial cells (pentameric complex) (106, 184). Through the use of recombinant technology, these advances have led to the development of more potent CMV Ig preparations (23, 185). In a recent study that retrospectively evaluated samples from a prior RCT (179) in D+ R− HCT recipients, patients who received i.v. CMV Ig (IVIG) prophylaxis (n = 28) showed a trend toward high weekly pentameric complex entry neutralizing antibody titers and low rates of primary CMV infection compared to the control group (n = 33) (184). Complementary preclinical data have demonstrated that strain-specific antibodies that recognize CMV are sufficient to prevent CMV reactivation in a murine model (186). As a result, there is renewed interest in the potential of CMV antibodies in transplant populations.
An initial trial of a monoclonal antibody against CMV glycoprotein H (gH) showed no significant benefit for preventing CMV infection in HCT recipients (187). This product was not developed further after a trial in HIV-infected patients with CMV retinitis was halted prematurely due to increased mortality in subjects who received the monoclonal antibody (188). More recently, a phase 2 trial in kidney transplant recipients (D+ R−) has demonstrated favorable results for RG7667, a combination of two high-affinity antibodies, each targeting a neutralizing epitope required for viral entry (on gH and the gH/gL/UL128/UL130/UL131 complex, respectively) (23). RG7667 prolonged the median time to viremia, decreased the incidence of CMV infection at 24 weeks posttransplantation, and reduced the incidence of CMV disease compared to a placebo (23). A second CMV-targeting monoclonal antibody product, CSJ148, similarly consists of two antibodies that target CMV gB and the pentameric complex (185). CSJ148 was well tolerated in a recently completed phase 2 trial in HCT recipients; however, the study did not meet its primary endpoint of reducing clinically significant CMV reactivation (ClinicalTrials.gov identifier NCT02268526) (189). Based on the phase 2 data for RG7667, potent neutralizing monoclonal CMV antibody preparations targeting the pentameric complex appear to show clinically relevant anti-CMV activity in certain transplant settings (D+ R− with primary infection). However, the need for i.v. infusions, the modest observed clinical benefit, and the availability of alternatives make it unlikely that CMV antibody preparations will be used as a stand-alone primary CMV prevention modality in transplant recipients. Future studies should assess their potential additive/synergistic effect with other CMV prevention measures (antivirals and vaccination) (Fig. 3 and 4) in the highest-risk populations and as potential adjuncts to antiviral therapy in patients with severe CMV disease in the context of primary infection. Recent preclinical data also indicate that the strain specificity of the antibody preparation may be an important consideration for effective antibody-mediated CMV control (186).
DRUG-RESISTANT/REFRACTORY CMV: IDENTIFICATION OF RESISTANCE AND ALGORITHMS FOR TREATMENT
Antiviral-resistant CMV is an uncommon but important clinical problem in transplantation and has recently been reviewed (22, 190–192). The underlying pathogenesis and risk factors appear to include severely impaired CMV-specific immunity, high viral loads, and prolonged viral replication in the presence of incompletely suppressive antiviral drug exposure, with the eventual selection and expansion of resistant mutants (193–199). The incidence of resistance is much higher among recipients of SOT than among those of HCT. In a retrospective case-control study of SOT patients, 1% of all SOT patients had genotypically confirmed ganciclovir resistance (193). The incidence of resistance ranged widely, from 0.4% in liver transplant recipients to 12% in lung transplant recipients (193). Among SOT populations, virtually all resistance occurs in the high-risk D+ R− subset, while among HCT recipients, resistance occurs in R+ populations with severe immunodeficiency (193, 198–200). Recent studies indicate that antiviral resistance is associated with significant additional attributable morbidity and mortality in SOT recipients compared with drug-susceptible CMV disease (193).
Mutations that confer resistance to antiviral drugs are typically not present at baseline but emerge and become amplified over time and eventually become the predominant viral population in the presence of an incompletely suppressive drug (11, 201). These mutations (typically substitutions or deletions) confer various degrees of fitness advantage in the presence of the drug. For long-established antiviral drugs (ganciclovir, foscarnet, and cidofovir), mutations associated with phenotypic resistance have been well characterized and include canonical mutations at specific codons (accounting for the majority of resistant strains from clinical specimens) and some newly described mutations outside these regions (190, 202). The characterization of these mutations and their impact on phenotypic drug resistance has paved the way for the development of diagnostic genotypic assays to detect mutations directly in clinical specimens (e.g., blood, cerebrospinal fluid, and ocular fluid). Direct detection of mutations from clinical specimens is advantageous because a genotypic or phenotypic assessment of viral isolates requires weeks or months and is therefore of limited clinical value. However, assays for genotypic resistance have several limitations: they have not been well standardized, might not target all resistance-encoding loci, and may variably report mutations that have not been definitively shown to confer phenotypic resistance by marker transfer experiments (polymorphisms). In addition, detection of resistance is feasible only if the viral load is above a particular threshold and the mutant virus represents at least a certain minimum proportion of the total viral population (203).
Traditionally, diagnostic assays for CMV resistance have been based on Sanger sequencing. However, deep-sequencing technologies offer improved sensitivity versus the Sanger method at low viral loads (<1,000 copies/ml) or when mutant virus represents a minority (<20%) of the total viral population (203–211). Next-generation sequencing-based detection of resistance mutations has been performed on tissue samples and for the identification of compartmentalized resistance (212). Thus, next-generation sequencing has the potential to allow earlier identification of CMV resistance and guide targeted therapy but requires further study.
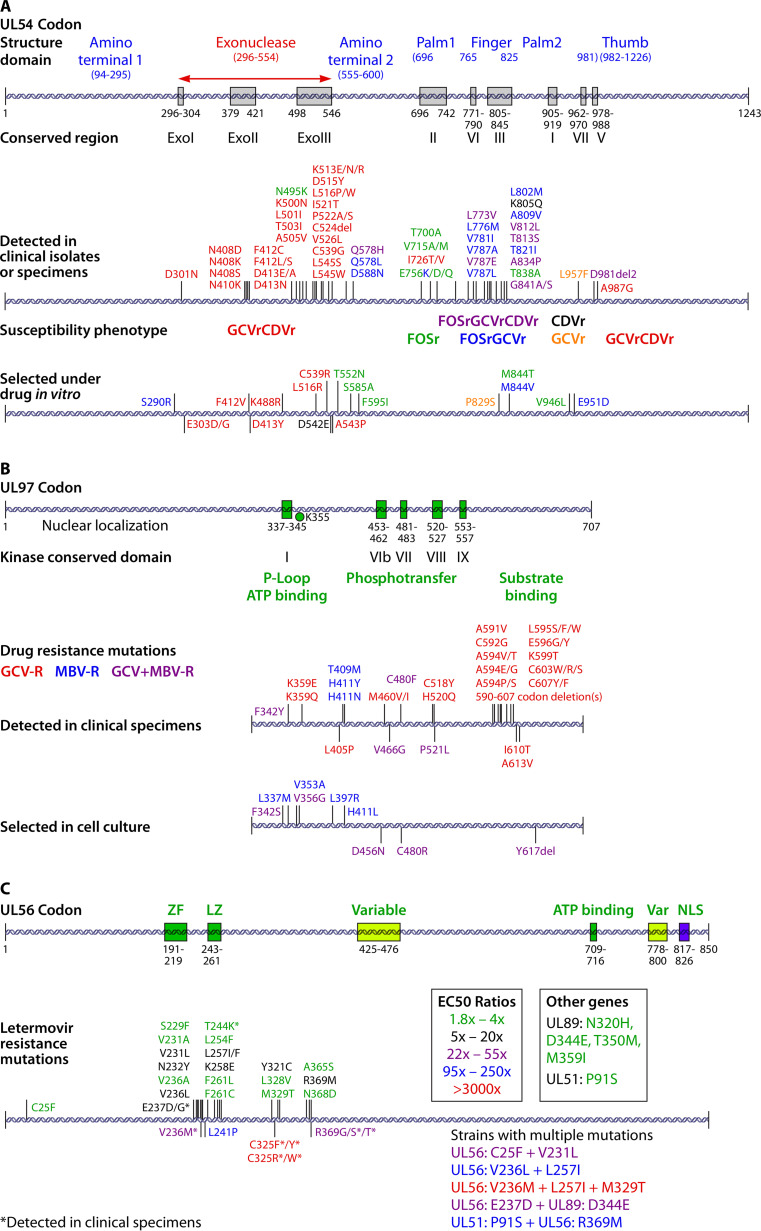
The availability of new antivirals for CMV necessitates an expansion of the targets of diagnostic assays for CMV resistance. Originally, for the detection of resistance to ganciclovir, cidofovir, or foscarnet, these assays targeted narrow regions of the UL97 kinase and UL54 viral DNA polymerase genes since all known phenotypic resistance-conferring mutations from clinical isolates occurred in these regions. Mutations in UL97 confer various degrees of phenotypic resistance to ganciclovir. In contrast, mutations in UL54 can confer higher-level resistance to ganciclovir, tend to occur as a second step after mutations in UL97 have developed, and can confer cross-resistance to cidofovir and/or foscarnet, depending on the specific mutation(s). Detailed maps of resistance-conferring mutations in UL97 and UL54 (Fig. 5) have been reported previously (22, 200) and were recently updated in a review (190). As letermovir has been evaluated in vitro and used clinically, mutations that confer phenotypic resistance to this new antiviral have been identified (Fig. 5) (134, 135, 190, 213, 214). Specific mutations that occur in multiple loci, including UL56, UL89, and UL51, and their relative impact on phenotypic resistance have been recently reviewed (143, 190, 192). Commercially available tests for detecting mutations in UL56 that are associated with phenotypic letermovir resistance are now available (215). An investigational antiviral agent (maribavir) is being studied as a therapy for established CMV infection in transplant recipients. Mutations that confer phenotypic resistance to maribavir have been identified in UL97 (216); the mutations most commonly selected in vivo do not confer significant resistance to ganciclovir.
FIG 5.
Drug resistance-associated mutations in CMV genes. CMV gene mutation maps for UL54, UL97, and UL56 show structural domains and the locations of identified resistance mutations. Color-coding indicates the resistance phenotype (A and B) and the degree of resistance conferred (C). GCVr, ganciclovir resistant; CDVr, cidofovir resistant; FOSr, foscarnet resistant; MBV-R, maribavir resistant; ZF, zinc finger; LZ, leucine zipper; NLS, nuclear localization signal; EC50, 50% effective concentration. (Reproduced from reference 190 with permission of Elsevier.)
Clinical guidelines and algorithms for the identification of potential CMV drug resistance to ganciclovir and management strategies have been reported (22, 36, 199). In patients with sight- or life-threatening CMV infection and suspected antiviral resistance, an empirical switch to an alternative antiviral is recommended while awaiting genotypic testing results, which typically take several days to weeks. Genotypic resistance should be suspected when, after >2 weeks of full-dose valganciclovir or ganciclovir treatment, there is no reduction in the viral load or if there is no significant improvement in clinical symptoms (193). Specific algorithms for suspecting resistance to either maribavir or letermovir in patients receiving these drugs for either prophylaxis or treatment have not yet been reported. Because of potentially lower barriers to resistance and different kinetics of the virologic response, the thresholds for resistance testing might need to be modified from those for ganciclovir. Until more formalized recommendations are available, resistance should be considered in cases of breakthrough infection with sustained or rising viral loads during prophylaxis (letermovir) or a failure to have a clinical or virologic response (maribavir). Based on genotype/phenotype studies, the specific genotypic results are useful for the rational selection of alternate antivirals with predicted antiviral activity, although options are limited in situations with multidrug-resistant isolates.
There are no RCTs for second- and third-line agents, which include foscarnet and cidofovir, respectively, in the setting of CMV resistance. Ganciclovir is more frequently cross-resistant with cidofovir than foscarnet, making foscarnet the drug of choice for high-level ganciclovir mutations in UL97 and UL54. Foscarnet therapy for refractory and resistant CMV infection was reviewed for 39 transplant recipients (22 SOT and 17 HCT) with 15 documented ganciclovir resistance mutations (217). Recipients were treated for a median of 32 days with foscarnet, with 13 (33%) experiencing virologic failure and 20 (51%) experiencing renal dysfunction. Data for cidofovir therapy are limited in SOT and HCT. In a retrospective study, 82 HCT recipients (47 of whom had previously received ganciclovir, foscarnet, or both drugs) received a median of 22 days of cidofovir. Response rates were reported to be 66% for primary and 68% for secondary cidofovir PET, with 25.6% nephrotoxicity (218). Smaller case series have shown some efficacy of cidofovir therapy (22). In nine SOT recipients with ganciclovir-refractory CMV disease, seven (78%) cleared CMV, and two (22%) had an incomplete response to cidofovir therapy (219). Kidney dysfunction was common, affecting seven out of nine (78%) patients, three of whom developed renal failure. In summary, the suboptimal outcomes and treatment-limiting nephrotoxicity of foscarnet and cidofovir for refractory and ganciclovir-resistant CMV treatment highlight the need for new therapies.
NEWER DRUGS AND APPROACHES FOR RESISTANT/REFRACTORY CMV
Novel Agents
As discussed above, several new antivirals for CMV either were recently approved or are in advanced clinical development: letermovir, maribavir, and brincidofovir. Key parameters for these drugs, including targets, formulations, and side effects, are summarized in Table 9.
TABLE 9.
Approved drugs for treatment of CMVd
| Treatment (reference) | Formulation(s) | Target | Drug class | Indication | Induction dosagea | Maintenance dosagea | Major side effect(s) | Note(s) |
|---|---|---|---|---|---|---|---|---|
| Ganciclovir | i.v. | UL97 kinase | Nucleoside analog | First-line treatment | 5 mg/kg of body wt i.v. BID | 5 mg/kg i.v. daily | Myelosuppression, fever, headache, liver toxicity | |
| Valganciclovir | p.o. | UL97 kinase | Nucleoside analog | First-line treatment | 900 mg p.o. BID | 900 mg p.o. daily | Myelosuppression | |
| Foscarnet | i.v. | Viral DNA polymerase | Pyrophosphate analog | Second-line treatment, resistant CMV | 90 mg/kg BID | 90 mg/kg daily | Nephrotoxicity, electrolyte disturbances | i.v. fluids are administered to mitigate nephrotoxicity; electrolyte disturbances require close monitoring |
| Cidofovir | i.v. | Viral DNA polymerase | Nucleotide analog | Third-line treatment, resistant CMV | 5 mg/kg weekly | 5 mg/kg every other wk | Nephrotoxicity | Probenecid is administered before/after to mitigate nephrotoxicity |
| Experimental treatments | ||||||||
| Maribavir (153) | p.o. | UL97 kinase | Benzimidazole riboside | Off-label, resistant CMV | 400–1,200 mg BID | 400–1,200 mg BID | GI symptoms, particularly dysgeusia | |
| Letermovir | p.o., i.v. | CMV terminase | DNA terminase complex inhibitor | Off-label, resistant CMV | NRb | NRb | Peripheral edema, headache, GI symptoms | |
| Brincidofovir (224) | p.o. | Viral DNA polymerase | Nucleotide analog | Off-label, resistant CMV | NRc | NRc | GI symptoms |
Normal renal function.
Not reported; 480 mg/daily is the prophylaxis dosing. Case series have reported this dosing in off-label use.
Not reported; 100 to 300 mg biweekly.
i.v., intravenous; p.o., oral; BID, twice daily; NR, not reported; GI, gastrointestinal.
Letermovir has been shown to be effective for CMV prophylaxis in R+ HCT recipients. However, there are limited data on its use for the treatment of established CMV infection in transplantation, although its tolerability and in vitro activity against resistant CMV (mutations in UL97 and/or UL54) make it an attractive treatment option. Early during development, a small study used significantly lower doses of letermovir than those approved for prophylaxis (40 mg BID or 80 mg once per day) for the treatment of asymptomatic viremia in kidney transplant recipients (n = 9 for each group) and reported rates of virologic response similar to those of the comparator, valganciclovir (n = 9) (220). In a more recent study of four lung transplant patients and one HCT recipient with refractory or resistant CMV, letermovir either alone (n = 2) or with foscarnet or ganciclovir (n = 3) led to a significant decrease in CMV viremia in four out of the five subjects (221). No UL56 mutations were identified, and treatment failure was attributed to a lower dose of letermovir, 240 mg daily, as opposed to the 480-mg daily dosing used for prophylaxis. In another recent case series of four SOT (two lung transplant and two heart transplant) patients, letermovir was administered either alone (n = 1) or in combination with intravitreal foscarnet or ganciclovir (n = 3) (222). Induction treatment was initiated at a dose of 720 mg and was increased to 960 mg in one patient. All four patients had resolution of retinitis upon fundoscopic examination but failed to achieve sustained viral suppression. In a third recent case series, four lung transplant patients with CMV infection/disease were treated with letermovir at 480 mg/day following treatment failure (223). Clearance of CMV viremia was observed after 17.7 ± 12.6 weeks, although 2 patients still had low-level viremia (<1,000 IU/ml) at 3 months. UL56 mutations were not assessed in this study. Quantification of CMV-specific CMI (by the T-Track assay) revealed that only one patient (of three tested) had a positive T-Track result. CMV viremia was undetectable in this patient at 3 months and did not relapse despite the discontinuation of letermovir. Based on this result, the authors suggested that CMI as well as letermovir use may have contributed to viral clearance. In all of these studies, letermovir was well tolerated. Further investigation of the optimal dosing and efficacy of letermovir for the treatment of established CMV infection is needed, particularly given the apparent lower barrier for the development of letermovir resistance in vitro.
In a phase 2 study evaluating maribavir as salvage therapy for resistant or refractory CMV infection, 63% to 70% of HCT and SOT patients receiving ≥400 mg BID achieved undetectable viral loads at 6 weeks (153). Maribavir was reasonably well tolerated, with 65% of patients reporting dysgeusia as the most commonly reported adverse event. Additionally, a phase 2 study showed similar efficacies of maribavir and valganciclovir for PET of CMV reactivation in HCT recipients (152). An advantage of maribavir appears to be the lack of any significant myelotoxicity. However, there were higher rates of attributable adverse events (mostly GI) and higher rates of emergence of resistance during maribavir therapy than with comparator agents. Phase 3 clinical trials are ongoing to evaluate the efficacy and safety of maribavir for PET of CMV in HCT (ClinicalTrials.gov identifier NCT02927067) and for the treatment of resistant or refractory CMV in SOT and HCT (ClinicalTrials.gov identifier NCT02931539).
Although brincidofovir was initially developed for the prevention of CMV, there is some experience with its use as a therapy for established CMV infection. Based on the failure of brincidofovir in a phase 3 trial of CMV prevention in HCT recipients, no further clinical development of brincidofovir for the prevention or treatment of CMV in transplant populations is planned. During development, a few case reports showed promising results for brincidofovir as a salvage therapy for resistant and refractory CMV infection/disease associated with UL97 mutations (224–226), although UL54 gene mutations were a limiting factor. A major theoretical advantage of brincidofovir over the parent compound, cidofovir, is reduced nephrotoxicity. However, Faure et al. reported potential brincidofovir-induced tubular necrosis in two SOT recipients (one kidney transplant and one heart transplant) receiving therapy for resistant CMV (227). Both patients had complicated clinical courses with prior renal failure and previous exposure to known nephrotoxic medications, but kidney function improved following brincidofovir withdrawal in both cases. This is suggestive of a possible association of brincidofovir with tubular necrosis and warrants further investigation. Brincidofovir is not currently available for compassionate use for the treatment of CMV.
Both artesunate, an antimalarial drug, and leflunomide, an immunosuppressant, demonstrate in vitro activity against CMV (228–230). Leflunomide has been used for resistant CMV in case series and case reports, but definitive evidence of a clinical benefit is lacking (231–236). Leflunomide is limited by hepatoxicity and marrow suppression (237, 238). Artesunate has had mixed outcomes for the treatment of resistant CMV in case reports (239, 240). An artesunate derivative with more potent in vitro activity against CMV is being studied (241).
Novel Approaches: Cellular Therapies
Infusion of virus-specific T cells (VSTs) has been explored as a strategy to treat or prevent CMV disease by directly reconstituting CMV-specific cellular immunity. In 1991, Riddell et al. first generated CMV-specific CD8+ T cells by ex vivo clonal expansion of cells from bone marrow donors and demonstrated that infusion of these cells could prevent CMV infections in allogeneic HCT recipients (242). Since then, small case series have demonstrated the feasibility and efficacy of this approach for the treatment of resistant or refractory CMV in HCT recipients and identified improvements in methods for generating VSTs (243–245). However, widespread utilization in HCT continues to be limited by various technical issues. Studies of VSTs for the treatment of CMV in SOT recipients are more limited.
Recently, several important advances have increased the feasibility of VSTs, leading to the development of multiple new products. Specifically, the development of off-the-shelf VSTs (246–249), an approach in which VSTs are isolated and expanded from third-party donors and banked, has the potential to allow timely treatment for a broader population of transplant recipients. Protocols for the in vitro selection and expansion of VSTs in a shorter time frame have also been developed (250–252), and progress has been made on regulatory advances such as good manufacturing practice (GMP)-compatible manufacture and scalability.
For HCT, results from small cohort trials and case-control trials have indicated that VST therapy is generally tolerated and feasible and associated with high response rates. In a prospective case-control study of haploidentical stem cell transplant patients, 27 out of 32 patients with drug-refractory CMV infection had viral clearance within 4 weeks of VST infusion (253). Similar results were reported in a single-arm prospective study of third-party VSTs designed to target multiple viruses; of 16 patients treated for CMV, 6 patients had a complete response, and 10 had a partial response, with a cumulative response rate of 94.1% (249). A recent study in which VSTs were used to treat drug-resistant CMV encephalitis in two HCT recipients suggests that VSTs may be an effective treatment option for CMV infections involving the central nervous system (254). Recent data also suggest a sustained response after VST infusion. In a prospective, multicenter trial of third-party VSTs in 30 patients (28 of whom were treated for CMV), the cumulative incidence of overall responses was 93% 12 months after VST infusion, with complete responses in 76% of subjects (255).
In the SOT setting, several case reports of VSTs as salvage therapy for ganciclovir-resistant CMV have yielded variable results. In lung transplant recipients, autologous VST infusion resulted in the clearance of ganciclovir-resistant CMV following four infusions in one case (256). A second lung transplant recipient, who had CMV pneumonia, had complete clinical recovery after VST infusion (257) but developed recurrent low-level viremia and died a few weeks later due to allograft rejection (257). In a kidney transplant recipient, a third‐party partially HLA‐matched VST infusion preceded a significant decrease in CMV viral load and disease upon renal biopsy (258), with sustained low-level viremia (73 copies/ml) at 1 year (258). In another report, a multivisceral transplant recipient with ganciclovir-resistant CMV received HLA-matched VSTs (259). The infused VSTs showed proliferation in vivo; however, the patient died on postoperative day 214 from multiorgan failure (259). Recently, a prospective study of VSTs in SOT recipients with recurrent or resistant CMV showed improvements in symptoms in 11 of 13 patients treated, including complete resolution, a reduction of CMV DNA in blood, and/or reduction or cessation of antiviral drugs (260).
The use of VSTs to treat or prevent CMV is an active area of research with multiple ongoing trials (see Table S1 in the supplemental material), mostly in the HCT setting (261, 262). Data from controlled studies are necessary to define the optimal role(s) of VSTs for the treatment and/or prevention of CMV disease in the transplant setting. Infusions of natural killer (NK) cells are another potential immunotherapeutic approach for controlling CMV infections (263). NK cells have activity against a diverse range of pathogens and are currently being investigated in multiple trials for anticancer effects (264). Recent studies suggest that NK cells have a role in protection against CMV reactivation following HCT (265, 266); further investigation of this approach is needed.
ONGOING AND RECENTLY COMPLETED TRIALS
This is an exciting time for CMV research, with numerous ongoing or recently completed trials for improving the diagnosis, prevention, and treatment of CMV (Table S1). In diagnostics, two trials (ClinicalTrials.gov identifiers NCT02538172 and NCT03699254) are assessing the utility of measuring CMV immunity to guide antiviral prophylaxis following kidney, liver, and lung transplantations. Another trial (ClinicalTrials.gov identifier NCT03910478) aims to address a major logistical hurdle of PET at late time points after HCT by using mobile-device-supported, self-collected dried blood spots for monitoring CMV viral loads. Following the approval of letermovir for prophylaxis in HCT recipients through 100 days after transplantation, one ongoing phase 3 trial (ClinicalTrials.gov identifier NCT03443869) is evaluating the efficacy of extending this to 200 days, while another (ClinicalTrials.gov identifier NCT03930615) is testing letermovir for prophylaxis in kidney transplant recipients. Two phase 3 trials (ClinicalTrials.gov identifiers NCT02927067 and NCT02931539) are ongoing to determine the role of maribavir for PET and the treatment of refractory or resistant CMV in the HCT setting. A phase 3 trial of the ASP0113 vaccine recently failed to meet its primary endpoint of overall mortality and CMV end-organ disease (267). However, efforts to develop a CMV vaccine continue, with large phase 2 trials of the PepVax (ClinicalTrials.gov identifier NCT02396134), Triplex (ClinicalTrials.gov identifier NCT04060277), and HB-101 (ClinicalTrials.gov identifier NCT03629080) vaccines ongoing. Numerous early-stage trials involve VST infusions as treatment for persistent or refractory CMV, prophylaxis, or treatment of initial infection or reactivation. Currently, a multinational, placebo-controlled, phase 3 clinical trial (TRACE [EudraCT identifier 2018-000853-29]) is planned to evaluate the role of VSTs for the treatment of refractory CMV in HCT recipients (268). The results of these trials will be eagerly anticipated and will guide the future of CMV diagnosis, prevention, and treatment.
CONCLUSIONS AND FUTURE DIRECTIONS
Over the past decade, there have been important advances in the prevention, diagnosis, and treatment of CMV in the transplant setting. The advent of preventative strategies has reduced the prevalence of early CMV infection, shifting the epidemiology of CMV toward late-onset disease. Highly sensitive diagnostic testing has improved pretransplant risk stratification and posttransplant screening of transplant patients. New treatments, including letermovir and VST infusions, expand the repertoire for managing CMV infection and disease.
However, many challenges remain. First, a positive CMV donor and/or recipient serostatus is persistently associated with worse clinical outcomes, indicating that new advances will be necessary to eliminate the impact of CMV on patient outcomes after transplantation. Available antivirals have several limitations, toxicity, resistance, and/or cost, as do other, nondrug therapies such as VST infusions. Likewise, diagnostic tools are currently imprecise for optimizing risk stratification, leading to overtreatment and associated complications. Finally, the last decade has shown limited progress in vaccine development, including the failure of a promising DNA vaccine candidate.
Within the next 10 years, several key advancements are likely to further diminish the impact of CMV on transplantation. Interventional studies of diagnostic tools such as CMV immune monitoring assays may facilitate increasingly targeted prevention/treatment strategies through more precise risk stratification. Similarly, rigorous assessments of novel prevention (e.g., vaccines) and treatment (e.g., VSTs) strategies will help to define their potential role in transplantation and potentially other clinical settings. Drug discovery efforts remain a high priority due to current treatment toxicities and resistance, while vaccine development continues to be a key goal for CMV prevention efforts.
ACKNOWLEDGMENTS
This work was supported by National Institute of Allergy and Infectious Diseases, National Institutes of Health, grants HHSN272201100041C to A.P.L., K24HL093294 to M.B., and HHSN272201600015C to M.B. and A.P.L. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
T.M.B. has no conflicts of interest to declare. A.P.L. has been on an advisory board for Merck; has been a site investigator for Merck, Astellas, Abbott, and Roche; has been involved in contracted research for Merck; and has been an adjudication committee member for Gilead. M.B. has received research support and has served as a consultant for Chimerix Inc., Gilead Sciences, Takeda (previously Shire), Merck, and Astellas; has served as a consultant for Moderna; and has served as an advisory board member for Helocyte. These individuals and organizations have no role in the overview and perspectives presented in this article.
We thank Ashley Sherrid for editorial assistance.
Biographies

Ajit P. Limaye is Professor of Medicine, Division of Infectious Diseases, and Director of the Organ Transplant Infectious Disease Program at the University of Washington, Seattle, WA. He completed a research fellowship as a Howard Hughes Scholar in residence at the NIH, internal medicine residency at Massachusetts General Hospital (Boston, MA), and chief residence and infectious diseases fellowship at the University of Washington, Seattle, WA. His clinical and research interests are focused on viral infections (CMV, BK virus, and respiratory viruses) in transplantation.

Tara M. Babu is Adjunct Faculty at the University of Rochester Medical Center, Rochester, NY. She is also clinical staff at Overlake Hospital, Bellevue, WA. She completed a clinical and research infectious diseases fellowship at the University of Rochester, followed by a fellowship in transplant and the immunocompromised host at Massachusetts General Hospital, Boston, MA. She trained in internal medicine at the University of Massachusetts, Worcester, MA. Her research interests include virology and clinical outcomes in transplantation.

Michael Boeckh received his medical degrees from the Freie Universität Berlin, Germany, where he also received training in internal medicine. He completed a fellowship in infectious diseases at the Fred Hutchinson Cancer Research Center and the University of Washington. He is currently a Professor at the Fred Hutchinson Cancer Research Center and the University of Washington as well as Head of the Infectious Disease Sciences Program of the Vaccine and Infectious Disease Division at Fred Hutch. His major areas of interest are cytomegalovirus and respiratory viruses. He conducts laboratory research, observational studies, as well as clinical trials of all phases.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Dolan A, Cunningham C, Hector RD, Hassan-Walker AF, Lee L, Addison C, Dargan DJ, McGeoch DJ, Gatherer D, Emery VC, Griffiths PD, Sinzger C, McSharry BP, Wilkinson GW, Davison AJ. 2004. Genetic content of wild-type human cytomegalovirus. J Gen Virol 85:1301–1312. doi: 10.1099/vir.0.79888-0. [DOI] [PubMed] [Google Scholar]
- 2.Craig JM, Macauley JC, Weller TH, Wirth P. 1957. Isolation of intranuclear inclusion producing agents from infants with illnesses resembling cytomegalic inclusion disease. Proc Soc Exp Biol Med 94:4–12. doi: 10.3181/00379727-94-22841. [DOI] [PubMed] [Google Scholar]
- 3.Green ML, Leisenring W, Stachel D, Pergam SA, Sandmaier BM, Wald A, Corey L, Boeckh M. 2012. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant 18:1687–1699. doi: 10.1016/j.bbmt.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erard V, Guthrie KA, Seo S, Smith J, Huang M, Chien J, Flowers ME, Corey L, Boeckh M. 2015. Reduced mortality of cytomegalovirus pneumonia after hematopoietic cell transplantation due to antiviral therapy and changes in transplantation practices. Clin Infect Dis 61:31–39. doi: 10.1093/cid/civ215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodson EM, Ladhani M, Webster AC, Strippoli GF, Craig JC. 2013. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev 2013:CD003774. doi: 10.1002/14651858.CD003774.pub4. [DOI] [PubMed] [Google Scholar]
- 6.Yan CH, Wang Y, Mo XD, Sun YQ, Wang FR, Fu HX, Chen Y, Han TT, Kong J, Cheng YF, Zhang XH, Xu LP, Liu KY, Huang XJ. 2020. Incidence, risk factors, and outcomes of cytomegalovirus retinitis after haploidentical hematopoietic stem cell transplantation. Bone Marrow Transplant 55:1147–1160. doi: 10.1038/s41409-020-0790-z. [DOI] [PubMed] [Google Scholar]
- 7.Oltolini C, Greco R, Galli L, Clerici D, Lorentino F, Xue E, Stanghellini MTL, Giglio F, Uhr L, Ripa M, Scarpellini P, Bernardi M, Corti C, Peccatori J, Castagna A, Ciceri F. 2020. Infections after allogenic transplant with post-transplant cyclophosphamide: impact of donor HLA matching. Biol Blood Marrow Transplant 26:1179–1188. doi: 10.1016/j.bbmt.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Kaminski H, Fishman JA. 2016. The cell biology of cytomegalovirus: implications for transplantation. Am J Transplant 16:2254–2269. doi: 10.1111/ajt.13791. [DOI] [PubMed] [Google Scholar]
- 9.Freeman RB, Jr. 2009. The ‘indirect’ effects of cytomegalovirus infection. Am J Transplant 9:2453–2458. doi: 10.1111/j.1600-6143.2009.02824.x. [DOI] [PubMed] [Google Scholar]
- 10.Kotton CN. 2013. CMV: prevention, diagnosis and therapy. Am J Transplant 13(Suppl 3):24–40; quiz, 40. doi: 10.1111/ajt.12006. [DOI] [PubMed] [Google Scholar]
- 11.Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. 2000. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet 355:2032–2036. doi: 10.1016/S0140-6736(00)02350-3. [DOI] [PubMed] [Google Scholar]
- 12.Weinberg A, Hodges TN, Li S, Cai G, Zamora MR. 2000. Comparison of PCR, antigenemia assay, and rapid blood culture for detection and prevention of cytomegalovirus disease after lung transplantation. J Clin Microbiol 38:768–772. doi: 10.1128/JCM.38.2.768-772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong CY, Cuevas LE, Williams H, Bakran A. 2000. Prediction and diagnosis of cytomegalovirus disease in renal transplant recipients using qualitative and quantitative polymerase chain reaction. Transplantation 69:985–991. doi: 10.1097/00007890-200003150-00054. [DOI] [PubMed] [Google Scholar]
- 14.Razonable RR, van Cruijsen H, Brown RA, Wilson JA, Harmsen WS, Wiesner RH, Smith TF, Paya CV. 2003. Dynamics of cytomegalovirus replication during preemptive therapy with oral ganciclovir. J Infect Dis 187:1801–1808. doi: 10.1086/375194. [DOI] [PubMed] [Google Scholar]
- 15.Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D, Stevens-Ayers T, Flowers ME, Cunningham T, Corey L. 2003. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood 101:407–414. doi: 10.1182/blood-2002-03-0993. [DOI] [PubMed] [Google Scholar]
- 16.Einsele H, Hebart H, Kauffmann-Schneider C, Sinzger C, Jahn G, Bader P, Klingebiel T, Dietz K, Loffler J, Bokemeyer C, Muller CA, Kanz L. 2000. Risk factors for treatment failures in patients receiving PCR-based preemptive therapy for CMV infection. Bone Marrow Transplant 25:757–763. doi: 10.1038/sj.bmt.1702226. [DOI] [PubMed] [Google Scholar]
- 17.Camargo JF, Kimble E, Rosa R, Shimose LA, Bueno MX, Jeyakumar N, Morris MI, Abbo LM, Simkins J, Alencar MC, Benjamin C, Wieder E, Jimenez A, Beitinjaneh A, Goodman M, Byrnes JJ, Lekakis LJ, Pereira D, Komanduri KV. 2018. Impact of cytomegalovirus viral load on probability of spontaneous clearance and response to preemptive therapy in allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant 24:806–814. doi: 10.1016/j.bbmt.2017.11.038. [DOI] [PubMed] [Google Scholar]
- 18.van Delden C, Stampf S, Hirsch HH, Manuel O, Meylan P, Cusini A, Hirzel C, Khanna N, Weisser M, Garzoni C, Boggian K, Berger C, Nadal D, Koller M, Saccilotto R, Mueller NJ, Swiss Transplant Cohort Study. . 9 January 2020. Burden and timeline of infectious diseases in the first year after solid organ transplantation in the Swiss Transplant Cohort Study. Clin Infect Dis doi: 10.1093/cid/ciz1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carbone J. 2016. The immunology of posttransplant CMV infection: potential effect of CMV immunoglobulins on distinct components of the immune response to CMV. Transplantation 100(Suppl 3):S11–S18. doi: 10.1097/TP.0000000000001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camargo JF, Komanduri KV. 2017. Emerging concepts in cytomegalovirus infection following hematopoietic stem cell transplantation. Hematol Oncol Stem Cell Ther 10:233–238. doi: 10.1016/j.hemonc.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 21.L’Huillier AG, Ferreira VH, Ku T, Bahinskaya I, Kumar D, Humar A. 2019. Improving our mechanistic understanding of the indirect effects of CMV infection in transplant recipients. Am J Transplant 19:2495–2504. doi: 10.1111/ajt.15371. [DOI] [PubMed] [Google Scholar]
- 22.Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, Humar A, Transplantation Society International CMV Consensus Group . 2018. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 102:900–931. doi: 10.1097/TP.0000000000002191. [DOI] [PubMed] [Google Scholar]
- 23.Ishida JH, Patel A, Mehta AK, Gatault P, McBride JM, Burgess T, Derby MA, Snydman DR, Emu B, Feierbach B, Fouts AE, Maia M, Deng R, Rosenberger CM, Gennaro LA, Striano NS, Liao XC, Tavel JA. 2017. Phase 2 randomized, double-blind, placebo-controlled trial of RG7667, a combination monoclonal antibody, for prevention of cytomegalovirus infection in high-risk kidney transplant recipients. Antimicrob Agents Chemother 61:e01794-16. doi: 10.1128/AAC.01794-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, Humar A, Transplantation Society International CMV Consensus Group . 2013. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 96:333–360. doi: 10.1097/TP.0b013e31829df29d. [DOI] [PubMed] [Google Scholar]
- 25.US Food and Drug Administration. 2019. Nucleic acid based tests. US Food and Drug Administration, Silver Spring, MD. https://www.fda.gov/medical-devices/vitro-diagnostics/nucleic-acid-based-tests. Accessed 31 January 2020. [Google Scholar]
- 26.Hayden RT, Preiksaitis J, Tong Y, Pang X, Sun Y, Tang L, Cook L, Pounds S, Fryer J, Caliendo AM. 2015. Commutability of the first World Health Organization international standard for human cytomegalovirus. J Clin Microbiol 53:3325–3333. doi: 10.1128/JCM.01495-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preiksaitis JK, Hayden RT, Tong Y, Pang XL, Fryer JF, Heath AB, Cook L, Petrich AK, Yu B, Caliendo AM. 2016. Are we there yet? Impact of the first international standard for cytomegalovirus DNA on the harmonization of results reported on plasma samples. Clin Infect Dis 63:583–589. doi: 10.1093/cid/ciw370. [DOI] [PubMed] [Google Scholar]
- 28.Meesing A, Germer JJ, Yao JD, Gartner ML, Digmann BJ, Razonable RR. 2020. Differences in duration and degree of cytomegalovirus DNAemia observed with two standardized quantitative nucleic acid tests and implications for clinical care. J Infect Dis 221:251–255. doi: 10.1093/infdis/jiz452. [DOI] [PubMed] [Google Scholar]
- 29.Hayden RT, Sun Y, Tang L, Procop GW, Hillyard DR, Pinsky BA, Young SA, Caliendo AM. 2017. Progress in quantitative viral load testing: variability and impact of the WHO quantitative international standards. J Clin Microbiol 55:423–430. doi: 10.1128/JCM.02044-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayden RT, Tang L, Su Y, Cook L, Gu Z, Jerome KR, Boonyaratanakornkit J, Sam S, Pounds S, Caliendo AM. 2020. Impact of fragmentation on commutability of Epstein-Barr virus and cytomegalovirus quantitative standards. J Clin Microbiol 58:e00888-19. doi: 10.1128/JCM.00888-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dioverti MV, Lahr BD, Germer JJ, Yao JD, Gartner ML, Razonable RR. 2017. Comparison of standardized cytomegalovirus (CMV) viral load thresholds in whole blood and plasma of solid organ and hematopoietic stem cell transplant recipients with CMV infection and disease. Open Forum Infect Dis 4:ofx143. doi: 10.1093/ofid/ofx143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razonable RR, Brown RA, Wilson J, Groettum C, Kremers W, Espy M, Smith TF, Paya CV. 2002. The clinical use of various blood compartments for cytomegalovirus (CMV) DNA quantitation in transplant recipients with CMV disease. Transplantation 73:968–973. doi: 10.1097/00007890-200203270-00025. [DOI] [PubMed] [Google Scholar]
- 33.Hayden RT, Gu Z, Sam SS, Sun Y, Tang L, Pounds S, Caliendo AM. 2016. Comparative performance of reagents and platforms for quantitation of cytomegalovirus DNA by digital PCR. J Clin Microbiol 54:2602–2608. doi: 10.1128/JCM.01474-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sedlak RH, Cook L, Cheng A, Magaret A, Jerome KR. 2014. Clinical utility of droplet digital PCR for human cytomegalovirus. J Clin Microbiol 52:2844–2848. doi: 10.1128/JCM.00803-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sedlak RH, Kuypers J, Jerome KR. 2014. A multiplexed droplet digital PCR assay performs better than qPCR on inhibition prone samples. Diagn Microbiol Infect Dis 80:285–286. doi: 10.1016/j.diagmicrobio.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Ljungman P, de la Camara R, Robin C, Crocchiolo R, Einsele H, Hill JA, Hubacek P, Navarro D, Cordonnier C, Ward KN, European Conference on Infections in Leukaemia Group . 2019. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis 19:e260–e272. doi: 10.1016/S1473-3099(19)30107-0. [DOI] [PubMed] [Google Scholar]
- 37.Grossi PA, Baldanti F, Andreoni M, Perno CF. 2020. CMV infection management in transplant patients in Italy. J Clin Virol 123:104211. doi: 10.1016/j.jcv.2019.104211. [DOI] [PubMed] [Google Scholar]
- 38.Boeckh M, Nichols WG, Chemaly RF, Papanicolaou GA, Wingard JR, Xie H, Syrjala KL, Flowers ME, Stevens-Ayers T, Jerome KR, Leisenring W. 2015. Valganciclovir for the prevention of complications of late cytomegalovirus infection after allogeneic hematopoietic cell transplantation: a randomized trial. Ann Intern Med 162:1–10. doi: 10.7326/M13-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Limaye AP, Santo Hayes TK, Huang ML, Magaret A, Boeckh M, Jerome KR. 2013. Quantitation of cytomegalovirus DNA load in dried blood spots correlates well with plasma viral load. J Clin Microbiol 51:2360–2364. doi: 10.1128/JCM.00316-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fierce Biotech. 2017. FDA clears 7SBio’s ‘virtually painless’ blood collection device. Fierce Biotech, Framingham, MA. https://www.fiercebiotech.com/medical-devices/fda-clears-7sbio-s-virtually-painless-blood-collection-device. Accessed 25 November 2019. [Google Scholar]
- 41.Tasso. 2019. Tasso raises $6.1M to bring effortless blood collection into the home. Tasso, Seattle, WA. https://www.tassoinc.com/press-releases/2019/3/5/tasso-raises-61m-to-bring-self-collection-of-blood-samples-into-pharma-trials. Accessed 25 November 2019. [Google Scholar]
- 42.Fernández-Ruiz M, Andrés A, Loinaz C, Delgado JF, López-Medrano F, San Juan R, González E, Polanco N, Folgueira MD, Lalueza A, Lumbreras C, Aguado JM. 2020. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant 20:1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gandolfini I, Delsante M, Fiaccadori E, Zaza G, Manenti L, Degli Antoni A, Peruzzi L, Riella LV, Cravedi P, Maggiore U. 2020. COVID-19 in kidney transplant recipients. Am J Transplant 20:1941–1943. doi: 10.1111/ajt.15891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu L, Xu X, Ma K, Yang J, Guan H, Chen S, Chen Z, Chen G. 2020. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant 20:1859–1863. doi: 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, Ross M, Forest S, Goldstein YD, Ajaimy M, Liriano-Ward L, Pynadath C, Loarte-Campos P, Nandigam PB, Graham J, Le M, Rocca J, Kinkhabwala M. 2020. Covid-19 and kidney transplantation. N Engl J Med 382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kates OS, Fisher CE, Stankiewicz-Karita HC, Shepherd AK, Church EC, Kapnadak SG, Lease ED, Riedo FX, Rakita RM, Limaye AP. 2020. Earliest cases of coronavirus disease 2019 (COVID-19) identified in solid organ transplant recipients in the United States. Am J Transplant 20:1885–1890. doi: 10.1111/ajt.15944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mills AM, Guo FP, Copland AP, Pai RK, Pinsky BA. 2013. A comparison of CMV detection in gastrointestinal mucosal biopsies using immunohistochemistry and PCR performed on formalin-fixed, paraffin-embedded tissue. Am J Surg Pathol 37:995–1000. doi: 10.1097/PAS.0b013e31827fcc33. [DOI] [PubMed] [Google Scholar]
- 48.Fisher CE, Alexander J, Bhattacharya R, Rakita RM, Kirby KA, Boeckh M, Limaye AP. 2016. Sensitivity of blood and tissue diagnostics for gastrointestinal cytomegalovirus disease in solid organ transplant recipients. Transpl Infect Dis 18:372–380. doi: 10.1111/tid.12531. [DOI] [PubMed] [Google Scholar]
- 49.Nagata N, Kobayakawa M, Shimbo T, Hoshimoto K, Yada T, Gotoda T, Akiyama J, Oka S, Uemura N. 2011. Diagnostic value of antigenemia assay for cytomegalovirus gastrointestinal disease in immunocompromised patients. World J Gastroenterol 17:1185–1191. doi: 10.3748/wjg.v17.i9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mori T, Mori S, Kanda Y, Yakushiji K, Mineishi S, Takaue Y, Gondo H, Harada M, Sakamaki H, Yajima T, Iwao Y, Hibi T, Okamoto S. 2004. Clinical significance of cytomegalovirus (CMV) antigenemia in the prediction and diagnosis of CMV gastrointestinal disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 33:431–434. doi: 10.1038/sj.bmt.1704369. [DOI] [PubMed] [Google Scholar]
- 51.Durand CM, Marr KA, Arnold CA, Tang L, Durand DJ, Avery RK, Valsamakis A, Neofytos D. 2013. Detection of cytomegalovirus DNA in plasma as an adjunct diagnostic for gastrointestinal tract disease in kidney and liver transplant recipients. Clin Infect Dis 57:1550–1559. doi: 10.1093/cid/cit521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prachasitthisak N, Tanpowpong P, Lertudomphonwanit C, Treepongkaruna S, Boonsathorn S, Angkathunyakul N, Sornmayura P, Chantratita W. 2017. Short article: stool cytomegalovirus polymerase chain reaction for the diagnosis of cytomegalovirus-related gastrointestinal disease. Eur J Gastroenterol Hepatol 29:1059–1063. doi: 10.1097/MEG.0000000000000906. [DOI] [PubMed] [Google Scholar]
- 53.Ganzenmueller T, Kluba J, Becker JU, Bachmann O, Heim A. 2014. Detection of cytomegalovirus (CMV) by real-time PCR in fecal samples for the non-invasive diagnosis of CMV intestinal disease. J Clin Virol 61:517–522. doi: 10.1016/j.jcv.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, Pikis A, Razonable RR, Miller V, Griffiths PD, Disease Definitions Working Group of the Cytomegalovirus Drug Development Forum . 2017. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 64:87–91. doi: 10.1093/cid/ciw668. [DOI] [PubMed] [Google Scholar]
- 55.Rashidi A, Vij KR, Buller RS, Wylie KM, Storch GA, DiPersio JF. 2017. Tissue polymerase chain reaction for the diagnosis of cytomegalovirus disease after allogeneic hematopoietic cell transplantation. Am J Hematol 92:E19–E20. doi: 10.1002/ajh.24609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mavropoulou E, Ternes K, Mechie NC, Bremer SCB, Kunsch S, Ellenrieder V, Neesse A, Amanzada A. 2019. Cytomegalovirus colitis in inflammatory bowel disease and after haematopoietic stem cell transplantation: diagnostic accuracy, predictors, risk factors and disease outcome. BMJ Open Gastroenterol 6:e000258. doi: 10.1136/bmjgast-2018-000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suarez-Lledo M, Marcos MA, Cuatrecasas M, Bombi JA, Fernandez-Aviles F, Magnano L, Martinez-Cibrian N, Llobet N, Rosinol L, Gutierrez-Garcia G, Jorge S, Martinez C, Rovira M, Urbano-Ispizua A. 2019. Quantitative PCR is faster, more objective, and more reliable than immunohistochemistry for the diagnosis of cytomegalovirus gastrointestinal disease in allogeneic stem cell transplantation. Biol Blood Marrow Transplant 25:2281–2286. doi: 10.1016/j.bbmt.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 58.Burston J, van Hal S, Dubedat S, Lee A. 2017. Inclusions or bystanders? CMV PCR sensitivity and specificity in tissue samples. J Clin Virol 90:38–39. doi: 10.1016/j.jcv.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 59.Crawford SW, Bowden RA, Hackman RC, Gleaves CA, Meyers JD, Clark JG. 1988. Rapid detection of cytomegalovirus pulmonary infection by bronchoalveolar lavage and centrifugation culture. Ann Intern Med 108:180–185. doi: 10.7326/0003-4819-108-2-180. [DOI] [PubMed] [Google Scholar]
- 60.Cathomas G, Morris P, Pekle K, Cunningham I, Emanuel D. 1993. Rapid diagnosis of cytomegalovirus pneumonia in marrow transplant recipients by bronchoalveolar lavage using the polymerase chain reaction, virus culture, and the direct immunostaining of alveolar cells. Blood 81:1909–1914. doi: 10.1182/blood.V81.7.1909.1909. [DOI] [PubMed] [Google Scholar]
- 61.Buffone GJ, Frost A, Samo T, Demmler GJ, Cagle PT, Lawrence EC. 1993. The diagnosis of CMV pneumonitis in lung and heart/lung transplant patients by PCR compared with traditional laboratory criteria. Transplantation 56:342–347. doi: 10.1097/00007890-199308000-00017. [DOI] [PubMed] [Google Scholar]
- 62.Lodding IP, Schultz HH, Jensen JU, Kirkby N, Perch M, Andersen C, Lundgren JD, Iversen M. 2018. Cytomegalovirus viral load in bronchoalveolar lavage to diagnose lung transplant associated CMV pneumonia. Transplantation 102:326–332. doi: 10.1097/TP.0000000000001927. [DOI] [PubMed] [Google Scholar]
- 63.Costa C, Curtoni A, Sidoti F, Balloco C, Simeone S, Mantovani S, Piasentin Alessio E, Libertucci D, Delsedime L, Solidoro P, Baldi S, Cavallo R. 2013. Detection of human cytomegalovirus in transbronchial biopsies from lung transplant recipients. Arch Virol 158:1461–1465. doi: 10.1007/s00705-013-1607-9. [DOI] [PubMed] [Google Scholar]
- 64.Beam E, Germer JJ, Lahr B, Yao JDC, Limper AH, Binnicker MJ, Razonable RR. 2018. Cytomegalovirus (CMV) DNA quantification in bronchoalveolar lavage fluid of immunocompromised patients with CMV pneumonia. Clin Transplant 32:e13149. doi: 10.1111/ctr.13149. [DOI] [PubMed] [Google Scholar]
- 65.Wiita AP, Roubinian N, Khan Y, Chin-Hong PV, Singer JP, Golden JA, Miller S. 2012. Cytomegalovirus disease and infection in lung transplant recipients in the setting of planned indefinite valganciclovir prophylaxis. Transpl Infect Dis 14:248–258. doi: 10.1111/j.1399-3062.2012.00723.x. [DOI] [PubMed] [Google Scholar]
- 66.Boeckh M, Stevens-Ayers T, Travi G, Huang ML, Cheng GS, Xie H, Leisenring W, Erard V, Seo S, Kimball L, Corey L, Pergam SA, Jerome KR. 2017. Cytomegalovirus (CMV) DNA quantitation in bronchoalveolar lavage fluid from hematopoietic stem cell transplant recipients with CMV pneumonia. J Infect Dis 215:1514–1522. doi: 10.1093/infdis/jix048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee HY, Rhee CK, Choi JY, Lee HY, Lee JW, Lee DG. 2017. Diagnosis of cytomegalovirus pneumonia by quantitative polymerase chain reaction using bronchial washing fluid from patients with hematologic malignancies. Oncotarget 8:39736–39745. doi: 10.18632/oncotarget.14504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iglesias L, Perera MM, Torres-Minana L, Pena-Lopez MJ. 2017. CMV viral load in bronchoalveolar lavage for diagnosis of pneumonia in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 52:895–897. doi: 10.1038/bmt.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pinana JL, Gimenez E, Gomez MD, Perez A, Gonzalez EM, Vinuesa V, Hernandez-Boluda JC, Montoro J, Salavert M, Tormo M, Amat P, Moles P, Carretero C, Balaguer-Rosello A, Sanz J, Sanz G, Solano C, Navarro D. 2019. Pulmonary cytomegalovirus (CMV) DNA shedding in allogeneic hematopoietic stem cell transplant recipients: implications for the diagnosis of CMV pneumonia. J Infect 78:393–401. doi: 10.1016/j.jinf.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hakki M, Riddell SR, Storek J, Carter RA, Stevens-Ayers T, Sudour P, White K, Corey L, Boeckh M. 2003. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood 102:3060–3067. doi: 10.1182/blood-2002-11-3472. [DOI] [PubMed] [Google Scholar]
- 71.Almyroudis NG, Jakubowski A, Jaffe D, Sepkowitz K, Pamer E, O’Reilly RJ, Papanicolaou GA. 2007. Predictors for persistent cytomegalovirus reactivation after T-cell-depleted allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis 9:286–294. doi: 10.1111/j.1399-3062.2007.00235.x. [DOI] [PubMed] [Google Scholar]
- 72.Jaskula E, Dlubek D, Duda D, Bogunia-Kubik K, Mlynarczewska A, Lange A. 2009. Interferon gamma 13-CA-repeat homozygous genotype and a low proportion of CD4(+) lymphocytes are independent risk factors for cytomegalovirus reactivation with a high number of copies in hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant 15:1296–1305. doi: 10.1016/j.bbmt.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 73.Ozdemir E, Saliba RM, Champlin RE, Couriel DR, Giralt SA, de Lima M, Khouri IF, Hosing C, Kornblau SM, Anderlini P, Shpall EJ, Qazilbash MH, Molldrem JJ, Chemaly RF, Komanduri KV. 2007. Risk factors associated with late cytomegalovirus reactivation after allogeneic stem cell transplantation for hematological malignancies. Bone Marrow Transplant 40:125–136. doi: 10.1038/sj.bmt.1705699. [DOI] [PubMed] [Google Scholar]
- 74.Gardiner BJ, Nierenberg NE, Chow JK, Ruthazer R, Kent DM, Snydman DR. 2018. Absolute lymphocyte count: a predictor of recurrent cytomegalovirus disease in solid organ transplant recipients. Clin Infect Dis 67:1395–1402. doi: 10.1093/cid/ciy295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Girmenia C, Lazzarotto T, Bonifazi F, Patriarca F, Irrera G, Ciceri F, Aversa F, Citterio F, Cillo U, Cozzi E, Gringeri E, Baldanti F, Cavallo R, Clerici P, Barosi G, Grossi P. 2019. Assessment and prevention of cytomegalovirus infection in allogeneic hematopoietic stem cell transplant and in solid organ transplant: a multidisciplinary consensus conference by the Italian GITMO, SITO, and AMCLI societies. Clin Transplant 33:e13666. doi: 10.1111/ctr.13666. [DOI] [PubMed] [Google Scholar]
- 76.Manuel O, Husain S, Kumar D, Zayas C, Mawhorter S, Levi ME, Kalpoe J, Lisboa L, Ely L, Kaul DR, Schwartz BS, Morris MI, Ison MG, Yen-Lieberman B, Sebastian A, Assi M, Humar A. 2013. Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study. Clin Infect Dis 56:817–824. doi: 10.1093/cid/cis993. [DOI] [PubMed] [Google Scholar]
- 77.Meesing A, Razonable RR. 2018. Absolute lymphocyte count thresholds: a simple, readily available tool to predict the risk of cytomegalovirus infection after transplantation. Open Forum Infect Dis 5:ofy230. doi: 10.1093/ofid/ofy230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lucia M, Crespo E, Melilli E, Cruzado JM, Luque S, Llaudo I, Niubo J, Torras J, Fernandez N, Grinyo JM, Bestard O. 2014. Preformed frequencies of cytomegalovirus (CMV)-specific memory T and B cells identify protected CMV-sensitized individuals among seronegative kidney transplant recipients. Clin Infect Dis 59:1537–1545. doi: 10.1093/cid/ciu589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Costa C, Balloco C, Sidoti F, Mantovani S, Ritta M, Piceghello A, Fop F, Messina M, Cavallo R. 2014. Evaluation of CMV-specific cellular immune response by ELISpot assay in kidney transplant patients. J Clin Virol 61:523–528. doi: 10.1016/j.jcv.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 80.Ritta M, Costa C, Sidoti F, Ballocco C, Ranghino A, Messina M, Biancone L, Cavallo R. 2015. Pre-transplant assessment of CMV-specific immune response by Elispot assay in kidney transplant recipients. New Microbiol 38:329–335. [PubMed] [Google Scholar]
- 81.Kim SH, Lee HJ, Kim SM, Jung JH, Shin S, Kim YH, Sung H, Lee SO, Choi SH, Kim YS, Woo JH, Han DJ. 2015. Diagnostic usefulness of cytomegalovirus (CMV)-specific T cell immunity in predicting CMV infection after kidney transplantation: a pilot proof-of-concept study. Infect Chemother 47:105–110. doi: 10.3947/ic.2015.47.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee H, Park KH, Ryu JH, Choi AR, Yu JH, Lim J, Han K, Kim SI, Yang CW, Chung BH, Oh EJ. 2017. Cytomegalovirus (CMV) immune monitoring with ELISpot and QuantiFERON-CMV assay in seropositive kidney transplant recipients. PLoS One 12:e0189488. doi: 10.1371/journal.pone.0189488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schachtner T, Stein M, Reinke P. 2017. CMV-specific T cell monitoring offers superior risk stratification of CMV-seronegative kidney transplant recipients of a CMV-seropositive donor. Transplantation 101:e315–e325. doi: 10.1097/TP.0000000000001825. [DOI] [PubMed] [Google Scholar]
- 84.De Gracia-Guindo MDC, Ruiz-Fuentes MDC, Galindo-Sacristan P, Osorio-Moratalla JM, Ruiz-Fuentes N, Rodriguez Granger J, Osuna-Ortega A. 2018. Cytomegalovirus infection monitoring based on interferon gamma release assay in kidney transplantation. Transplant Proc 50:578–580. doi: 10.1016/j.transproceed.2017.09.052. [DOI] [PubMed] [Google Scholar]
- 85.Gliga S, Korth J, Krawczyk A, Wilde B, Horn PA, Witzke O, Lindemann M, Fiedler M. 2018. T-Track-CMV and QuantiFERON-CMV assays for prediction of protection from CMV reactivation in kidney transplant recipients. J Clin Virol 105:91–96. doi: 10.1016/j.jcv.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 86.Chiereghin A, Potena L, Borgese L, Gibertoni D, Squarzoni D, Turello G, Petrisli E, Piccirilli G, Gabrielli L, Grigioni F, Lazzarotto T. 2018. Monitoring of cytomegalovirus (CMV)-specific cell-mediated immunity in heart transplant recipients: clinical utility of the QuantiFERON-CMV assay for management of posttransplant CMV infection. J Clin Microbiol 56:e01040-17. doi: 10.1128/JCM.01040-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sood S, Haifer C, Yu L, Pavlovic J, Gow PJ, Jones RM, Visvanathan K, Angus PW, Testro AG. 2018. Early viral-specific T-cell testing predicts late cytomegalovirus reactivation following liver transplantation. Transpl Infect Dis 20:e12934. doi: 10.1111/tid.12934. [DOI] [PubMed] [Google Scholar]
- 88.Deborska-Materkowska D, Perkowska-Ptasinska A, Sadowska A, Gozdowska J, Ciszek M, Serwanska-Swietek M, Domagala P, Miszewska-Szyszkowska D, Sitarek E, Jozwik A, Kwiatkowski A, Durlik M. 2018. Diagnostic utility of monitoring cytomegalovirus-specific immunity by QuantiFERON-cytomegalovirus assay in kidney transplant recipients. BMC Infect Dis 18:179. doi: 10.1186/s12879-018-3075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Páez-Vega A, Poyato A, Rodriguez-Benot A, Guirado L, Fortún J, Len O, Abdala E, Fariñas MC, Cordero E, de Gracia C, Hernández D, González R, Torre-Cisneros J, Cantisán S, Spanish Network for Research in Infectious Diseases, Spanish Renal Disease Network . 2018. Analysis of spontaneous resolution of cytomegalovirus replication after transplantation in CMV-seropositive patients with pretransplant CD8+IFNG+ response. Antiviral Res 155:97–105. doi: 10.1016/j.antiviral.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 90.Thompson G, Boan P, Baumwol J, Chakera A, MacQuillan G, Swaminathan S, Lavender M, Flexman J, James I, John M. 2018. Analysis of the QuantiFERON-CMV assay, CMV viraemia and antiviral treatment following solid organ transplantation in Western Australia. Pathology 50:554–561. doi: 10.1016/j.pathol.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 91.Westall GP, Cristiano Y, Levvey BJ, Whitford H, Paraskeva MA, Paul E, Peleg AY, Snell GI. 2019. A randomized study of QuantiFERON CMV-directed versus fixed-duration valganciclovir prophylaxis to reduce late CMV after lung transplantation. Transplantation 103:1005–1013. doi: 10.1097/TP.0000000000002454. [DOI] [PubMed] [Google Scholar]
- 92.Kumar D, Mian M, Singer L, Humar A. 2017. An interventional study using cell-mediated immunity to personalize therapy for cytomegalovirus infection after transplantation. Am J Transplant 17:2468–2473. doi: 10.1111/ajt.14347. [DOI] [PubMed] [Google Scholar]
- 93.Kumar D, Chin-Hong P, Kayler L, Wojciechowski D, Limaye AP, Osama Gaber A, Ball S, Mehta AK, Cooper M, Blanchard T, MacDougall J, Kotton CN. 2019. A prospective multicenter observational study of cell-mediated immunity as a predictor for cytomegalovirus infection in kidney transplant recipients. Am J Transplant 19:2505–2516. doi: 10.1111/ajt.15315. [DOI] [PubMed] [Google Scholar]
- 94.Jarque M, Crespo E, Melilli E, Gutierrez A, Moreso F, Guirado L, Revuelta I, Montero N, Torras J, Riera L, Meneghini M, Taco O, Manonelles A, Paul J, Seron D, Facundo C, Cruzado JM, Gil-Vernet S, Grinyó JM, Bestard O. 20 February 2020. Cellular immunity to predict the risk of cytomegalovirus infection in kidney transplantation: a prospective, interventional, multicenter clinical trial. Clin Infect Dis doi: 10.1093/cid/ciz1209. [DOI] [PubMed] [Google Scholar]
- 95.Fernandez-Ruiz M, Rodriguez-Goncer I, Parra P, Ruiz-Merlo T, Corbella L, Lopez-Medrano F, Polanco N, Gonzalez E, San Juan R, Folgueira MD, Andres A, Aguado JM. 2020. Monitoring of CMV-specific cell-mediated immunity with a commercial ELISA-based interferon-gamma release assay in kidney transplant recipients treated with antithymocyte globulin. Am J Transplant 20:2070–2080. doi: 10.1111/ajt.15793. [DOI] [PubMed] [Google Scholar]
- 96.Yong MK, Cameron PU, Slavin M, Morrissey CO, Bergin K, Spencer A, Ritchie D, Cheng AC, Samri A, Carcelain G, Autran B, Lewin SR. 2017. Identifying cytomegalovirus complications using the QuantiFERON-CMV assay after allogeneic hematopoietic stem cell transplantation. J Infect Dis 215:1684–1694. doi: 10.1093/infdis/jix192. [DOI] [PubMed] [Google Scholar]
- 97.Paouri B, Soldatou A, Petrakou E, Theodosaki M, Tsentidis C, Kaisari K, Oikonomopoulou C, Matsas M, Goussetis E. 2018. Quantiferon-cytomegalovirus assay: a potentially useful tool in the evaluation of CMV-specific CD8+ T-cell reconstitution in pediatric hematopoietic stem cell transplant patients. Pediatr Transplant 22:e13220. doi: 10.1111/petr.13220. [DOI] [PubMed] [Google Scholar]
- 98.Nesher L, Shah DP, Ariza-Heredia EJ, Azzi JM, Siddiqui HK, Ghantoji SS, Marsh LY, Michailidis L, Makedonas G, Rezvani K, Shpall EJ, Chemaly RF. 2016. Utility of the enzyme-linked immunospot interferon-gamma-release assay to predict the risk of cytomegalovirus infection in hematopoietic cell transplant recipients. J Infect Dis 213:1701–1707. doi: 10.1093/infdis/jiw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krawczyk A, Ackermann J, Goitowski B, Trenschel R, Ditschkowski M, Timm J, Ottinger H, Beelen DW, Gruner N, Fiedler M. 2018. Assessing the risk of CMV reactivation and reconstitution of antiviral immune response post bone marrow transplantation by the QuantiFERON-CMV-assay and real time PCR. J Clin Virol 99–100:61–66. doi: 10.1016/j.jcv.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 100.El Haddad L, Ariza-Heredia E, Shah DP, Jiang Y, Blanchard T, Ghantoji SS, El Chaer F, El-Haddad D, Prayag A, Nesher L, Rezvani K, Shpall E, Chemaly RF. 2019. The ability of a cytomegalovirus ELISpot assay to predict outcome of low-level CMV reactivation in hematopoietic cell transplant recipients. J Infect Dis 219:898–907. doi: 10.1093/infdis/jiy592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Navarro D, Amat P, de la Camara R, Lopez J, Vazquez L, Serrano D, Nieto J, Rovira M, Pinana JL, Gimenez E, Solano C. 2016. Efficacy and safety of a preemptive antiviral therapy strategy based on combined virological and immunological monitoring for active cytomegalovirus infection in allogeneic stem cell transplant recipients. Open Forum Infect Dis 3:ofw107. doi: 10.1093/ofid/ofw107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bono P, Orlandi A, Zoccoli A, Salvatore A, Annaloro C, Tagliaferri E, Lunghi G. 2016. Quantiferon CMV assay in allogenic stem cell transplant patients. J Clin Virol 79:10–11. doi: 10.1016/j.jcv.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 103.Wagner-Drouet E, Teschner D, Wolschke C, Janson D, Schafer-Eckart K, Gartner J, Mielke S, Schreder M, Kobbe G, Kondakci M, Hilgendorf I, von Lilienfeld-Toal M, Klein S, Heidenreich D, Kreil S, Verbeek M, Grass S, Ditschkowski M, Gromke T, Koch M, Lindemann M, Hunig T, Schmidt T, Rascle A, Guldan H, Barabas S, Deml L, Wagner R, Wolff D. 26 December 2019. Standardized monitoring of cytomegalovirus-specific immunity can improve risk stratification of recurrent cytomegalovirus reactivation after hematopoietic stem cell transplantation. Haematologica doi: 10.3324/haematol.2019.229252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pipeling MR, John ER, Orens JB, Lechtzin N, McDyer JF. 2011. Primary cytomegalovirus phosphoprotein 65-specific CD8+ T-cell responses and T-bet levels predict immune control during early chronic infection in lung transplant recipients. J Infect Dis 204:1663–1671. doi: 10.1093/infdis/jir624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Snyder LD, Chan C, Kwon D, Yi JS, Martissa JA, Copeland CA, Osborne RJ, Sparks SD, Palmer SM, Weinhold KJ. 2016. Polyfunctional T-cell signatures to predict protection from cytomegalovirus after lung transplantation. Am J Respir Crit Care Med 193:78–85. doi: 10.1164/rccm.201504-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Limaye AP, Green ML, Edmison BC, Stevens-Ayers T, Chatterton-Kirchmeier S, Geballe AP, Singh N, Boeckh M. 2019. Prospective assessment of cytomegalovirus immunity in high-risk donor-seropositive/recipient-seronegative liver transplant recipients receiving either preemptive therapy or antiviral prophylaxis. J Infect Dis 220:752–760. doi: 10.1093/infdis/jiz181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Green ML, Leisenring W, Xie H, Mast TC, Cui Y, Sandmaier BM, Sorror ML, Goyal S, Ozkok S, Yi J, Sahoo F, Kimball LE, Jerome KR, Marks MA, Boeckh M. 2016. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol 3:e119–e127. doi: 10.1016/S2352-3026(15)00289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Teira P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, Green JS, Saad A, Antin JH, Savani BN, Lazarus HM, Seftel M, Saber W, Marks D, Aljurf M, Norkin M, Wingard JR, Lindemans CA, Boeckh M, Riches ML, Auletta JJ. 2016. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood 127:2427–2438. doi: 10.1182/blood-2015-11-679639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ljungman P, Brand R, Hoek J, de la Camara R, Cordonnier C, Einsele H, Styczynski J, Ward KN, Cesaro SInfectious Diseases Working Party of the European Group for Blood and Marrow Transplantation ,. 2014. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European Group for Blood and Marrow Transplantation. Clin Infect Dis 59:473–481. doi: 10.1093/cid/ciu364. [DOI] [PubMed] [Google Scholar]
- 110.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. 2002. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis 185:273–282. doi: 10.1086/338624. [DOI] [PubMed] [Google Scholar]
- 111.Hartmann A, Sagedal S, Hjelmesaeth J. 2006. The natural course of cytomegalovirus infection and disease in renal transplant recipients. Transplantation 82:S15–S17. doi: 10.1097/01.tp.0000230460.42558.b0. [DOI] [PubMed] [Google Scholar]
- 112.Sagedal S, Hartmann A, Nordal KP, Osnes K, Leivestad T, Foss A, Degre M, Fauchald P, Rollag H. 2004. Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney Int 66:329–337. doi: 10.1111/j.1523-1755.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- 113.Sagedal S, Nordal KP, Hartmann A, Sund S, Scott H, Degre M, Foss A, Leivestad T, Osnes K, Fauchald P, Rollag H. 2002. The impact of cytomegalovirus infection and disease on rejection episodes in renal allograft recipients. Am J Transplant 2:850–856. doi: 10.1034/j.1600-6143.2002.20907.x. [DOI] [PubMed] [Google Scholar]
- 114.Duke ER, Williamson BD, Borate B, Golob JL, Wychera C, Stevens-Ayers T, Huang M-L, Cossrow N, Wan H, Mast C, Marks MA, Flowers M, Jerome KR, Corey L, Gilbert PB, Schiffer JT, Boeckh M.. 24 September 2020. Cytomegalovirus viral load kinetics as surrogate endpoints after allogeneic transplantation. J Clin Invest doi: 10.1172/JCI133960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Natori Y, Alghamdi A, Tazari M, Miller V, Husain S, Komatsu T, Griffiths P, Ljungman P, Orchanian-Cheff A, Kumar D, Humar A, CMV Consensus Forum . 2018. Use of viral load as a surrogate marker in clinical studies of cytomegalovirus in solid organ transplantation: a systematic review and meta-analysis. Clin Infect Dis 66:617–631. doi: 10.1093/cid/cix793. [DOI] [PubMed] [Google Scholar]
- 116.Caliendo AM, St George K, Allega J, Bullotta AC, Gilbane L, Rinaldo CR. 2002. Distinguishing cytomegalovirus (CMV) infection and disease with CMV nucleic acid assays. J Clin Microbiol 40:1581–1586. doi: 10.1128/JCM.40.5.1581-1586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Humar A, Gregson D, Caliendo AM, McGeer A, Malkan G, Krajden M, Corey P, Greig P, Walmsley S, Levy G, Mazzulli T. 1999. Clinical utility of quantitative cytomegalovirus viral load determination for predicting cytomegalovirus disease in liver transplant recipients. Transplantation 68:1305–1311. doi: 10.1097/00007890-199911150-00015. [DOI] [PubMed] [Google Scholar]
- 118.Humar A, Kumar D, Boivin G, Caliendo AM. 2002. Cytomegalovirus (CMV) virus load kinetics to predict recurrent disease in solid-organ transplant patients with CMV disease. J Infect Dis 186:829–833. doi: 10.1086/342601. [DOI] [PubMed] [Google Scholar]
- 119.Lao WC, Lee D, Burroughs AK, Lanzini G, Rolles K, Emery VC, Griffiths PD. 1997. Use of polymerase chain reaction to provide prognostic information on human cytomegalovirus disease after liver transplantation. J Med Virol 51:152–158. doi:. [DOI] [PubMed] [Google Scholar]
- 120.Rollag H, Sagedal S, Kristiansen KI, Kvale D, Holter E, Degre M, Nordal KP. 2002. Cytomegalovirus DNA concentration in plasma predicts development of cytomegalovirus disease in kidney transplant recipients. Clin Microbiol Infect 8:431–434. doi: 10.1046/j.1469-0691.2002.00449.x. [DOI] [PubMed] [Google Scholar]
- 121.Limaye AP, Bakthavatsalam R, Kim HW, Randolph SE, Halldorson JB, Healey PJ, Kuhr CS, Levy AE, Perkins JD, Reyes JD, Boeckh M. 2006. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation 81:1645–1652. doi: 10.1097/01.tp.0000226071.12562.1a. [DOI] [PubMed] [Google Scholar]
- 122.Arthurs SK, Eid AJ, Pedersen RA, Kremers WK, Cosio FG, Patel R, Razonable RR. 2008. Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis 46:840–846. doi: 10.1086/528718. [DOI] [PubMed] [Google Scholar]
- 123.Chen K, Cheng MP, Hammond SP, Einsele H, Marty FM. 2018. Antiviral prophylaxis for cytomegalovirus infection in allogeneic hematopoietic cell transplantation. Blood Adv 2:2159–2175. doi: 10.1182/bloodadvances.2018016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lisboa LF, Preiksaitis JK, Humar A, Kumar D. 2011. Clinical utility of molecular surveillance for cytomegalovirus after antiviral prophylaxis in high-risk solid organ transplant recipients. Transplantation 92:1063–1068. doi: 10.1097/TP.0b013e31822fa4b7. [DOI] [PubMed] [Google Scholar]
- 125.Montejo M, Montejo E, Gastaca M, Valdivieso A, Fernandez JR, Testillano M, Gonzalez J, Bustamante J, Ruiz P, Suarez MJ, Ventoso A, Rubio MC, de Urbina JO. 2009. Prophylactic therapy with valgancyclovir in high-risk (cytomegalovirus D+/R−) liver transplant recipients: a single-center experience. Transplant Proc 41:2189–2191. doi: 10.1016/j.transproceed.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 126.van der Beek MT, Berger SP, Vossen AC, van der Blij-de Brouwer CS, Press RR, de Fijter JW, Claas EC, Kroes AC. 2010. Preemptive versus sequential prophylactic-preemptive treatment regimens for cytomegalovirus in renal transplantation: comparison of treatment failure and antiviral resistance. Transplantation 89:320–326. doi: 10.1097/TP.0b013e3181bc0301. [DOI] [PubMed] [Google Scholar]
- 127.Boillat Blanco N, Pascual M, Venetz JP, Nseir G, Meylan PR, Manuel O. 2011. Impact of a preemptive strategy after 3 months of valganciclovir cytomegalovirus prophylaxis in kidney transplant recipients. Transplantation 91:251–255. doi: 10.1097/TP.0b013e318200b9f0. [DOI] [PubMed] [Google Scholar]
- 128.Hakimi Z, Aballea S, Ferchichi S, Scharn M, Odeyemi IA, Toumi M, Saliba F. 2017. Burden of cytomegalovirus disease in solid organ transplant recipients: a national matched cohort study in an inpatient setting. Transpl Infect Dis 19:e12732. doi: 10.1111/tid.12732. [DOI] [PubMed] [Google Scholar]
- 129.Kim JM, Kim SJ, Joh JW, Kwon CH, Shin M, Kim EY, Moon JI, Jung GO, Choi GS, Lee SK. 2010. Early and delayed onset cytomegalovirus infection of liver transplant recipients in endemic areas. Transplant Proc 42:884–889. doi: 10.1016/j.transproceed.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 130.Singh N, Winston DJ, Razonable RR, Lyon GM, Silveira FP, Wagener MM, Stevens-Ayers T, Edmison B, Boeckh M, Limaye AP. 2020. Effect of preemptive therapy vs antiviral prophylaxis on cytomegalovirus disease in seronegative liver transplant recipients with seropositive donors: a randomized clinical trial. JAMA 323:1378–1387. doi: 10.1001/jama.2020.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marty FM, Ljungman P, Papanicolaou GA, Winston DJ, Chemaly RF, Strasfeld L, Young JA, Rodriguez T, Maertens J, Schmitt M, Einsele H, Ferrant A, Lipton JH, Villano SA, Chen H, Boeckh M, Maribavir 1263-300 Clinical Study Group . 2011. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis 11:284–292. doi: 10.1016/S1473-3099(11)70024-X. [DOI] [PubMed] [Google Scholar]
- 132.Kharfan-Dabaja MA, Boeckh M, Wilck MB, Langston AA, Chu AH, Wloch MK, Guterwill DF, Smith LR, Rolland AP, Kenney RT. 2012. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 12:290–299. doi: 10.1016/S1473-3099(11)70344-9. [DOI] [PubMed] [Google Scholar]
- 133.Marty FM, Winston DJ, Rowley SD, Vance E, Papanicolaou GA, Mullane KM, Brundage TM, Robertson AT, Godkin S, Mommeja-Marin H, Boeckh M, CMX001-201 Clinical Study Group . 2013. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med 369:1227–1236. doi: 10.1056/NEJMoa1303688. [DOI] [PubMed] [Google Scholar]
- 134.Chemaly RF, Ullmann AJ, Stoelben S, Richard MP, Bornhauser M, Groth C, Einsele H, Silverman M, Mullane KM, Brown J, Nowak H, Kolling K, Stobernack HP, Lischka P, Zimmermann H, Rubsamen-Schaeff H, Champlin RE, Ehninger G, AIC246 Study Team . 2014. Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Engl J Med 370:1781–1789. doi: 10.1056/NEJMoa1309533. [DOI] [PubMed] [Google Scholar]
- 135.Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, Haider S, Ullmann AJ, Katayama Y, Brown J, Mullane KM, Boeckh M, Blumberg EA, Einsele H, Snydman DR, Kanda Y, DiNubile MJ, Teal VL, Wan H, Murata Y, Kartsonis NA, Leavitt RY, Badshah C. 2017. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med 377:2433–2444. doi: 10.1056/NEJMoa1706640. [DOI] [PubMed] [Google Scholar]
- 136.Marty FM, Winston DJ, Chemaly RF, Mullane KM, Shore TB, Papanicolaou GA, Chittick G, Brundage TM, Wilson C, Morrison ME, Foster SA, Nichols WG, Boeckh MJ, SUPPRESS Trial Clinical Study Group . 2019. A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 25:369–381. doi: 10.1016/j.bbmt.2018.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Witzke O, Nitschke M, Bartels M, Wolters H, Wolf G, Reinke P, Hauser IA, Alshuth U, Kliem V. 2018. Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: long-term results after 7 years of a randomized clinical trial. Transplantation 102:876–882. doi: 10.1097/TP.0000000000002024. [DOI] [PubMed] [Google Scholar]
- 138.Witzke O, Hauser IA, Bartels M, Wolf G, Wolters H, Nitschke M, VIPP Study Group . 2012. Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: 1-year results of a randomized clinical trial. Transplantation 93:61–68. doi: 10.1097/TP.0b013e318238dab3. [DOI] [PubMed] [Google Scholar]
- 139.Wang YH, Chen D, Hartmann G, Cho CR, Menzel K. 2019. PBPK modeling strategy for predicting complex drug interactions of letermovir as a perpetrator in support of product labeling. Clin Pharmacol Ther 105:515–523. doi: 10.1002/cpt.1120. [DOI] [PubMed] [Google Scholar]
- 140.Melendez DP, Razonable RR. 2015. Letermovir and inhibitors of the terminase complex: a promising new class of investigational antiviral drugs against human cytomegalovirus. Infect Drug Resist 8:269–277. doi: 10.2147/IDR.S79131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Marty FM, Ljungman PT, Chemaly RF, Wan H, Teal VL, Butterton JR, Yeh WW, Leavitt RY, Badshah CS. 2020. Outcomes of patients with detectable CMV DNA at randomization in the phase III trial of letermovir for the prevention of CMV infection in allogeneic hematopoietic cell transplantation. Am J Transplant 20:1703–1711. doi: 10.1111/ajt.15764. [DOI] [PubMed] [Google Scholar]
- 142.Ljungman P, Schmitt M, Marty FM, Maertens J, Chemaly RF, Kartsonis NA, Butterton JR, Wan H, Teal VL, Sarratt K, Murata Y, Leavitt RY, Badshah C. 2020. A mortality analysis of letermovir prophylaxis for cytomegalovirus (CMV) in CMV-seropositive recipients of allogeneic hematopoietic-cell transplantation. Clin Infect Dis 70:1525–1533. doi: 10.1093/cid/ciz490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chou SW. 2015. Rapid in vitro evolution of human cytomegalovirus UL56 mutations that confer letermovir resistance. Antimicrob Agents Chemother 59:6588–6593. doi: 10.1128/AAC.01623-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goldner T, Hempel C, Ruebsamen-Schaeff H, Zimmermann H, Lischka P. 2014. Geno- and phenotypic characterization of human cytomegalovirus mutants selected in vitro after letermovir (AIC246) exposure. Antimicrob Agents Chemother 58:610–613. doi: 10.1128/AAC.01794-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Duong A, Sweet A, Jain R, Hill JA, Pergam SA, Boeckh M, Liu C. 2020. Clinically significant drug interaction: letermovir and voriconazole. J Antimicrob Chemother 75:775–777. doi: 10.1093/jac/dkz499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McCrea JB, Macha S, Adedoyin A, Marshall W, Menzel K, Cho CR, Liu F, Zhao T, Levine V, Kraft WK, Yoon E, Panebianco D, Stoch SA, Iwamoto M. 2019. Pharmacokinetic drug-drug interactions between letermovir and the immunosuppressants cyclosporine, tacrolimus, sirolimus, and mycophenolate mofetil. J Clin Pharmacol 59:1331–1339. doi: 10.1002/jcph.1423. [DOI] [PubMed] [Google Scholar]
- 147.Kropeit D, von Richter O, Stobernack HP, Rubsamen-Schaeff H, Zimmermann H. 2018. Pharmacokinetics and safety of letermovir coadministered with cyclosporine A or tacrolimus in healthy subjects. Clin Pharmacol Drug Dev 7:9–21. doi: 10.1002/cpdd.388. [DOI] [PubMed] [Google Scholar]
- 148.Biron KK, Harvey RJ, Chamberlain SC, Good SS, Smith AA, III, Davis MG, Talarico CL, Miller WH, Ferris R, Dornsife RE, Stanat SC, Drach JC, Townsend LB, Koszalka GW. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-riboside with a unique mode of action. Antimicrob Agents Chemother 46:2365–2372. doi: 10.1128/AAC.46.8.2365-2372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Winston DJ, Young JA, Pullarkat V, Papanicolaou GA, Vij R, Vance E, Alangaden GJ, Chemaly RF, Petersen F, Chao N, Klein J, Sprague K, Villano SA, Boeckh M. 2008. Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem cell transplant recipients: a multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. Blood 111:5403–5410. doi: 10.1182/blood-2007-11-121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Winston DJ, Saliba F, Blumberg E, Abouljoud M, Garcia-Diaz JB, Goss JA, Clough L, Avery R, Limaye AP, Ericzon BG, Navasa M, Troisi RI, Chen H, Villano SA, Uknis ME, 1263-301 Clinical Study Group . 2012. Efficacy and safety of maribavir dosed at 100 mg orally twice daily for the prevention of cytomegalovirus disease in liver transplant recipients: a randomized, double-blind, multicenter controlled trial. Am J Transplant 12:3021–3030. doi: 10.1111/j.1600-6143.2012.04231.x. [DOI] [PubMed] [Google Scholar]
- 151.Marty FM, Boeckh M. 2011. Maribavir and human cytomegalovirus—what happened in the clinical trials and why might the drug have failed? Curr Opin Virol 1:555–562. doi: 10.1016/j.coviro.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 152.Maertens J, Cordonnier C, Jaksch P, Poire X, Uknis M, Wu J, Wijatyk A, Saliba F, Witzke O, Villano S. 2019. Maribavir for preemptive treatment of cytomegalovirus reactivation. N Engl J Med 381:1136–1147. doi: 10.1056/NEJMoa1714656. [DOI] [PubMed] [Google Scholar]
- 153.Papanicolaou GA, Silveira FP, Langston AA, Pereira MR, Avery RK, Uknis M, Wijatyk A, Wu J, Boeckh M, Marty FM, Villano S. 2019. Maribavir for refractory or resistant cytomegalovirus infections in hematopoietic-cell or solid-organ transplant recipients: a randomized, dose-ranging, double-blind, phase 2 study. Clin Infect Dis 68:1255–1264. doi: 10.1093/cid/ciy706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Song IH, Ilic K, Murphy J, Lasseter K, Martin P. 2020. Effects of maribavir on P-glycoprotein and CYP2D6 in healthy volunteers. J Clin Pharmacol 60:96–106. doi: 10.1002/jcph.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wire MB, Morrison M, Anderson M, Arumugham T, Dunn J, Naderer O. 2017. Pharmacokinetics (PK) and safety of intravenous (IV) brincidofovir (BCV) in healthy adult subjects. Open Forum Infect Dis 4:S311. doi: 10.1093/ofid/ofx163.725. [DOI] [Google Scholar]
- 156.Ljungman P. 2014. The role of cytomegalovirus serostatus on outcome of hematopoietic stem cell transplantation. Curr Opin Hematol 21:466–469. doi: 10.1097/MOH.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 157.Schmidt-Hieber M, Labopin M, Beelen D, Volin L, Ehninger G, Finke J, Socie G, Schwerdtfeger R, Kroger N, Ganser A, Niederwieser D, Polge E, Blau IW, Mohty M. 2013. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood 122:3359–3364. doi: 10.1182/blood-2013-05-499830. [DOI] [PubMed] [Google Scholar]
- 158.Narra A, Strasfeld L, Basuli D, Stack M, Langewisch E, Olyaei A, Norman D, Lockridge J. 2018. Kidney transplant allocation with CMV seromatching reduces CMV infection without affecting wait times, abstr 185. Abstr 2018 Am Transpl Congr, Philadelphia, PA, 30 May to 3 June 2018.
- 159.Lockridge J, Langewisch E, Basuli D, Olyaei A, Shaut C, Norman D. 2017. Kidney allocation using CMV matching optimizes low and high risk profiles for prevention of CMV infection in kidney transplant recipients, abstr A192. Abstr 2017 Am Transpl Congr.
- 160.Lockridge J, Langewisch E, Basuli D, Olyaei A, Shaut C, Norman D. 2017. Kidney transplant allocation with CMV matching reduces CMV infection and related costs, abstr A190. Abstr 2017 Am Transpl Congr.
- 161.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Manley TJ, Luy L, Jones T, Boeckh M, Mutimer H, Riddell SR. 2004. Immune evasion proteins of human cytomegalovirus do not prevent a diverse CD8+ cytotoxic T-cell response in natural infection. Blood 104:1075–1082. doi: 10.1182/blood-2003-06-1937. [DOI] [PubMed] [Google Scholar]
- 163.Elkington R, Walker S, Crough T, Menzies M, Tellam J, Bharadwaj M, Khanna R. 2003. Ex vivo profiling of CD8+-T-cell responses to human cytomegalovirus reveals broad and multispecific reactivities in healthy virus carriers. J Virol 77:5226–5240. doi: 10.1128/JVI.77.9.5226-5240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Plotkin S. 2015. The history of vaccination against cytomegalovirus. Med Microbiol Immunol 204:247–254. doi: 10.1007/s00430-015-0388-z. [DOI] [PubMed] [Google Scholar]
- 165.Diamond DJ, La Rosa C, Chiuppesi F, Contreras H, Dadwal S, Wussow F, Bautista S, Nakamura R, Zaia JA. 2018. A fifty-year odyssey: prospects for a cytomegalovirus vaccine in transplant and congenital infection. Expert Rev Vaccines 17:889–911. doi: 10.1080/14760584.2018.1526085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Anderholm KM, Bierle CJ, Schleiss MR. 2016. Cytomegalovirus vaccines: current status and future prospects. Drugs 76:1625–1645. doi: 10.1007/s40265-016-0653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schleiss MR. 2016. Cytomegalovirus vaccines under clinical development. J Virus Erad 2:198–207. doi: 10.1016/S2055-6640(20)30872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Griffiths PD, Stanton A, McCarrell E, Smith C, Osman M, Harber M, Davenport A, Jones G, Wheeler DC, O’Beirne J, Thorburn D, Patch D, Atkinson CE, Pichon S, Sweny P, Lanzman M, Woodford E, Rothwell E, Old N, Kinyanjui R, Haque T, Atabani S, Luck S, Prideaux S, Milne RS, Emery VC, Burroughs AK. 2011. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet 377:1256–1263. doi: 10.1016/S0140-6736(11)60136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vincenti F, Budde K, Merville P, Shihab F, Ram Peddi V, Shah M, Wyburn K, Cassuto-Viguier E, Weidemann A, Lee M, Flegel T, Erdman J, Wang X, Lademacher C. 2018. A randomized, phase 2 study of ASP0113, a DNA-based vaccine, for the prevention of CMV in CMV-seronegative kidney transplant recipients receiving a kidney from a CMV-seropositive donor. Am J Transplant 18:2945–2954. doi: 10.1111/ajt.14925. [DOI] [PubMed] [Google Scholar]
- 170.Astellas. 2018. Astellas and Vical announce top-line results for phase 3 trial of cytomegalovirus vaccine ASP0113 in hematopoietic stem cell transplant recipients. Astellas, Northbrook, IL. https://www.astellas.com/en/news/10206. Accessed 31 January 2020. [Google Scholar]
- 171.Nakamura R, La Rosa C, Longmate J, Drake J, Slape C, Zhou Q, Lampa MG, O’Donnell M, Cai J-L, Farol L, Salhotra A, Snyder DS, Aldoss I, Forman SJ, Miller JS, Zaia JA, Diamond DJ. 2016. Viraemia, immunogenicity, and survival outcomes of cytomegalovirus chimeric epitope vaccine supplemented with PF03512676 (CMVPepVax) in allogeneic haemopoietic stem-cell transplantation: randomised phase 1b trial. Lancet Haematol 3:e87–e98. doi: 10.1016/S2352-3026(15)00246-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.La Rosa C, Longmate J, Lingaraju CR, Zhou Q, Kaltcheva T, Hardwick N, Aldoss I, Nakamura R, Diamond DJ. 2019. Rapid acquisition of cytomegalovirus-specific T cells with a differentiated phenotype, in nonviremic hematopoietic stem transplant recipients vaccinated with CMVPepVax. Biol Blood Marrow Transplant 25:771–784. doi: 10.1016/j.bbmt.2018.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Boeckh M, Gilbert PB. 2016. Search continues for a CMV vaccine for transplant recipients. Lancet Haematol 3:e58–e59. doi: 10.1016/S2352-3026(15)00286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Aldoss I, La Rosa C, Baden LR, Longmate J, Ariza-Heredia EJ, Rida WN, Lingaraju CR, Zhou Q, Martinez J, Kaltcheva T, Dagis A, Hardwick N, Issa NC, Farol L, Nademanee A, Al Malki MM, Forman S, Nakamura R, Diamond DJ, TRIPLEX VACCINE Study Group . 2020. Poxvirus vectored cytomegalovirus vaccine to prevent cytomegalovirus viremia in transplant recipients: a phase 2, randomized clinical trial. Ann Intern Med 172:306–316. doi: 10.7326/M19-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kotton CN, Schwendinge M, Thiry G, DeVos B, Boever FD, Leroux-Roels G, Lilja A. 2018. A CMV vaccine based on non-replicating lymphocytic choriomeningitis virus vectors expressing gB and pp65 is safe and immunogenic in healthy volunteers, allowing for development of a phase II clinical trial in living donor kidney transplant recipients. Open Forum Infect Dis 5:S475. doi: 10.1093/ofid/ofy210.1360. [DOI] [Google Scholar]
- 176.Moderna. 2020. Moderna announces additional positive phase 1 data from cytomegalovirus (CMV) vaccine (mRNA-1647) and first participant dosed in phase 2 study. Moderna, Cambridge, MA. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-additional-positive-phase-1-data. Accessed 7 February 2020. [Google Scholar]
- 177.Snydman DR, Werner BG, Dougherty NN, Griffith J, Rubin RH, Dienstag JL, Rohrer RH, Freeman R, Jenkins R, Lewis WD, Hammer S, O’Rourke E, Grady GF, Fawaz K, Kaplan MM, Hoffman MA, Katz AT, Doran M, Boston Center for Liver Transplantation CMVIG Study Group . 1993. Cytomegalovirus immune globulin prophylaxis in liver transplantation. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 119:984–991. doi: 10.7326/0003-4819-119-10-199311150-00004. [DOI] [PubMed] [Google Scholar]
- 178.Snydman DR, Werner BG, Heinze-Lacey B, Berardi VP, Tilney NL, Kirkman RL, Milford EL, Cho SI, Bush HL, Jr, Levey AS, Harmon WE, Zimmerman CE, Shapiro ME, Steinman T, LoGerfo F, Idelson B, Schroter GPJ, Levin MJ, McIver J, Leszczynski J, Grady GF. 1987. Use of cytomegalovirus immune globulin to prevent cytomegalovirus disease in renal-transplant recipients. N Engl J Med 317:1049–1054. doi: 10.1056/NEJM198710223171703. [DOI] [PubMed] [Google Scholar]
- 179.Bowden RA, Fisher LD, Rogers K, Cays M, Meyers JD. 1991. Cytomegalovirus (CMV)-specific intravenous immunoglobulin for the prevention of primary CMV infection and disease after marrow transplant. J Infect Dis 164:483–487. doi: 10.1093/infdis/164.3.483. [DOI] [PubMed] [Google Scholar]
- 180.Hahn G, Revello MG, Patrone M, Percivalle E, Campanini G, Sarasini A, Wagner M, Gallina A, Milanesi G, Koszinowski U, Baldanti F, Gerna G. 2004. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol 78:10023–10033. doi: 10.1128/JVI.78.18.10023-10033.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang D, Shenk T. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A 102:18153–18158. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang D, Shenk T. 2005. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J Virol 79:10330–10338. doi: 10.1128/JVI.79.16.10330-10338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhou M, Lanchy JM, Ryckman BJ. 2015. Human cytomegalovirus gH/gL/gO promotes the fusion step of entry into all cell types, whereas gH/gL/UL128-131 broadens virus tropism through a distinct mechanism. J Virol 89:8999–9009. doi: 10.1128/JVI.01325-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zamora D, Krantz EM, Green ML, Joncas-Schronce L, Blazevic R, Edmison BC, Huang ML, Stevens-Ayers T, Jerome KR, Geballe AP, Boeckh M. 2020. The cytomegalovirus humoral response against epithelial cell entry-mediated infection in the primary infection setting after hematopoietic cell transplantation. J Infect Dis 221:1470–1479. doi: 10.1093/infdis/jiz596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dole K, Segal FP, Feire A, Magnusson B, Rondon JC, Vemula J, Yu J, Pang Y, Pertel P. 2016. A first-in-human study to assess the safety and pharmacokinetics of monoclonal antibodies against human cytomegalovirus in healthy volunteers. Antimicrob Agents Chemother 60:2881–2887. doi: 10.1128/AAC.02698-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Martins JP, Andoniou CE, Fleming P, Kuns RD, Schuster IS, Voigt V, Daly S, Varelias A, Tey SK, Degli-Esposti MA, Hill GR. 2019. Strain-specific antibody therapy prevents cytomegalovirus reactivation after transplantation. Science 363:288–293. doi: 10.1126/science.aat0066. [DOI] [PubMed] [Google Scholar]
- 187.Boeckh M, Bowden RA, Storer B, Chao NJ, Spielberger R, Tierney DK, Gallez-Hawkins G, Cunningham T, Blume KG, Levitt D, Zaia JA. 2001. Randomized, placebo-controlled, double-blind study of a cytomegalovirus-specific monoclonal antibody (MSL-109) for prevention of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 7:343–351. doi: 10.1016/S1083-8791(01)80005-7. [DOI] [PubMed] [Google Scholar]
- 188.Borucki MJ, Spritzler J, Asmuth DM, Gnann J, Hirsch MS, Nokta M, Aweeka F, Nadler PI, Sattler F, Alston B, Nevin TT, Owens S, Waterman K, Hubbard L, Caliendo A, Pollard RB, AACTG 266 Team . 2004. A phase II, double-masked, randomized, placebo-controlled evaluation of a human monoclonal anti-cytomegalovirus antibody (MSL-109) in combination with standard therapy versus standard therapy alone in the treatment of AIDS patients with cytomegalovirus retinitis. Antiviral Res 64:103–111. doi: 10.1016/j.antiviral.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 189.Maertens J, Logan AC, Jang J, Long G, Tang JL, Hwang WYK, Koh LP, Chemaly R, Gerbitz A, Winkler J, Yeh SP, Hiemenz J, Christoph S, Lee DG, Wang PN, Holler E, Mielke S, Akard L, Yeo A, Ramachandra S, Smith K, Pertel P, Segal F. 2020. Phase 2 study of anti-human cytomegalovirus monoclonal antibodies for prophylaxis in hematopoietic cell transplantation. Antimicrob Agents Chemother 64:e02467-19. doi: 10.1128/AAC.02467-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chou S. 2020. Advances in the genotypic diagnosis of cytomegalovirus antiviral drug resistance. Antiviral Res 176:104711. doi: 10.1016/j.antiviral.2020.104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Khawaja F, Batista MV, El Haddad L, Chemaly RF. 2019. Resistant or refractory cytomegalovirus infections after hematopoietic cell transplantation: diagnosis and management. Curr Opin Infect Dis 32:565–574. doi: 10.1097/QCO.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 192.Piret J, Boivin G. 2019. Clinical development of letermovir and maribavir: overview of human cytomegalovirus drug resistance. Antiviral Res 163:91–105. doi: 10.1016/j.antiviral.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 193.Fisher CE, Knudsen JL, Lease ED, Jerome KR, Rakita RM, Boeckh M, Limaye AP. 2017. Risk factors and outcomes of ganciclovir-resistant cytomegalovirus infection in solid organ transplant recipients. Clin Infect Dis 65:57–63. doi: 10.1093/cid/cix259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Boivin G, Goyette N, Rollag H, Jardine AG, Pescovitz MD, Asberg A, Ives J, Hartmann A, Humar A. 2009. Cytomegalovirus resistance in solid organ transplant recipients treated with intravenous ganciclovir or oral valganciclovir. Antivir Ther 14:697–704. [PubMed] [Google Scholar]
- 195.Limaye AP, Corey L, Koelle DM, Davis CL, Boeckh M. 2000. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet 356:645–649. doi: 10.1016/S0140-6736(00)02607-6. [DOI] [PubMed] [Google Scholar]
- 196.Limaye AP, Raghu G, Koelle DM, Ferrenberg J, Huang ML, Boeckh M. 2002. High incidence of ganciclovir-resistant cytomegalovirus infection among lung transplant recipients receiving preemptive therapy. J Infect Dis 185:20–27. doi: 10.1086/338143. [DOI] [PubMed] [Google Scholar]
- 197.Sandkovsky U, Qiu F, Kalil AC, Florescu A, Wilson N, Manning C, Florescu DF. 2018. Risk factors for the development of cytomegalovirus resistance in solid organ transplantation: a retrospective case-control study. Transplant Proc 50:3763–3768. doi: 10.1016/j.transproceed.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 198.Boeckh M, Ljungman P. 2009. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood 113:5711–5719. doi: 10.1182/blood-2008-10-143560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.El Chaer F, Shah DP, Chemaly RF. 2016. How I treat resistant cytomegalovirus infection in hematopoietic cell transplantation recipients. Blood 128:2624–2636. doi: 10.1182/blood-2016-06-688432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lurain NS, Chou S. 2010. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev 23:689–712. doi: 10.1128/CMR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Emery VC, Griffiths PD. 2000. Prediction of cytomegalovirus load and resistance patterns after antiviral chemotherapy. Proc Natl Acad Sci U S A 97:8039–8044. doi: 10.1073/pnas.140123497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Drew WL. 2010. Cytomegalovirus resistance testing: pitfalls and problems for the clinician. Clin Infect Dis 50:733–736. doi: 10.1086/650463. [DOI] [PubMed] [Google Scholar]
- 203.Chou S, Ercolani RJ, Sahoo MK, Lefterova MI, Strasfeld LM, Pinsky BA. 2014. Improved detection of emerging drug-resistant mutant cytomegalovirus subpopulations by deep sequencing. Antimicrob Agents Chemother 58:4697–4702. doi: 10.1128/AAC.03214-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Houldcroft CJ, Bryant JM, Depledge DP, Margetts BK, Simmonds J, Nicolaou S, Tutill HJ, Williams R, Worth AJ, Marks SD, Veys P, Whittaker E, Breuer J. 2016. Detection of low frequency multi-drug resistance and novel putative maribavir resistance in immunocompromised pediatric patients with cytomegalovirus. Front Microbiol 7:1317. doi: 10.3389/fmicb.2016.01317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sahoo MK, Lefterova MI, Yamamoto F, Waggoner JJ, Chou S, Holmes SP, Anderson MW, Pinsky BA. 2013. Detection of cytomegalovirus drug resistance mutations by next-generation sequencing. J Clin Microbiol 51:3700–3710. doi: 10.1128/JCM.01605-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schnepf N, Dhedin N, Mercier-Delarue S, Andreoli A, Mamez AC, Ferry C, Deback C, Ribaud P, Robin M, Socie G, Simon F, Mazeron MC. 2013. Dynamics of cytomegalovirus populations harbouring mutations in genes UL54 and UL97 in a haematopoietic stem cell transplant recipient. J Clin Virol 58:733–736. doi: 10.1016/j.jcv.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 207.Kampmann SE, Schindele B, Apelt L, Buhrer C, Garten L, Weizsaecker K, Kruger DH, Ehlers B, Hofmann J. 2011. Pyrosequencing allows the detection of emergent ganciclovir resistance mutations after HCMV infection. Med Microbiol Immunol 200:109–113. doi: 10.1007/s00430-010-0181-y. [DOI] [PubMed] [Google Scholar]
- 208.Garrigue I, Moulinas R, Recordon-Pinson P, Delacour ML, Essig M, Kaminski H, Rerolle JP, Merville P, Fleury H, Alain S. 2016. Contribution of next generation sequencing to early detection of cytomegalovirus UL97 emerging mutants and viral subpopulations analysis in kidney transplant recipients. J Clin Virol 80:74–81. doi: 10.1016/j.jcv.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 209.Schindele B, Apelt L, Hofmann J, Nitsche A, Michel D, Voigt S, Mertens T, Ehlers B. 2010. Improved detection of mutated human cytomegalovirus UL97 by pyrosequencing. Antimicrob Agents Chemother 54:5234–5241. doi: 10.1128/AAC.00802-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lopez-Aladid R, Guiu A, Mosquera MM, Lopez-Medrano F, Cofan F, Linares L, Torre-Cisneros J, Vidal E, Moreno A, Aguado JM, Cordero E, Martin-Gandul C, Carratala J, Sabe N, Niubo J, Cervera C, Capon A, Cervilla A, Santos M, Bodro M, Munoz P, Farinas MC, Anton A, Aranzamendi M, Montejo M, Perez-Romero P, Len O, Marcos MA. 2019. Improvement in detecting cytomegalovirus drug resistance mutations in solid organ transplant recipients with suspected resistance using next generation sequencing. PLoS One 14:e0219701. doi: 10.1371/journal.pone.0219701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Guermouche H, Burrel S, Mercier-Darty M, Kofman T, Rogier O, Pawlotsky JM, Boutolleau D, Rodriguez C. 2020. Characterization of the dynamics of human cytomegalovirus resistance to antiviral drugs by ultra-deep sequencing. Antiviral Res 173:104647. doi: 10.1016/j.antiviral.2019.104647. [DOI] [PubMed] [Google Scholar]
- 212.Hage E, Wilkie GS, Linnenweber-Held S, Dhingra A, Suarez NM, Schmidt JJ, Kay-Fedorov PC, Mischak-Weissinger E, Heim A, Schwarz A, Schulz TF, Davison AJ, Ganzenmueller T. 2017. Characterization of human cytomegalovirus genome diversity in immunocompromised hosts by whole-genome sequencing directly from clinical specimens. J Infect Dis 215:1673–1683. doi: 10.1093/infdis/jix157. [DOI] [PubMed] [Google Scholar]
- 213.Lischka P, Michel D, Zimmermann H. 2016. Characterization of cytomegalovirus breakthrough events in a phase 2 prophylaxis trial of letermovir (AIC246, MK 8228). J Infect Dis 213:23–30. doi: 10.1093/infdis/jiv352. [DOI] [PubMed] [Google Scholar]
- 214.Douglas CM, Barnard R, Holder D, Leavitt R, Levitan D, Maguire M, Nickle D, Teal V, Wan H, van Alewijk D, van Doorn LJ, Chou S, Strizki J. 2020. Letermovir resistance analysis in a clinical trial of cytomegalovirus prophylaxis for hematopoietic stem cell transplant recipients. J Infect Dis 221:1117–1126. doi: 10.1093/infdis/jiz577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Grantham J, Nutt J, Tyler A, Bixler E, Altrich M, Kleiboeker S. 2019. Detection of CMV antiviral resistance mutations to letermovir in patients using a validated clinical sequencing assay. Biol Blood Marrow Transplant 25:S344–S345. doi: 10.1016/j.bbmt.2018.12.558. [DOI] [Google Scholar]
- 216.Schubert A, Ehlert K, Schuler-Luettmann S, Gentner E, Mertens T, Michel D. 2013. Fast selection of maribavir resistant cytomegalovirus in a bone marrow transplant recipient. BMC Infect Dis 13:330. doi: 10.1186/1471-2334-13-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Avery RK, Arav-Boger R, Marr KA, Kraus E, Shoham S, Lees L, Trollinger B, Shah P, Ambinder R, Neofytos D, Ostrander D, Forman M, Valsamakis A. 2016. Outcomes in transplant recipients treated with foscarnet for ganciclovir-resistant or refractory cytomegalovirus infection. Transplantation 100:e74–e80. doi: 10.1097/TP.0000000000001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ljungman P, Deliliers GL, Platzbecker U, Matthes-Martin S, Bacigalupo A, Einsele H, Ullmann J, Musso M, Trenschel R, Ribaud P, Bornhauser M, Cesaro S, Crooks B, Dekker A, Gratecos N, Klingebiel T, Tagliaferri E, Ullmann AJ, Wacker P, Cordonnier C. 2001. Cidofovir for cytomegalovirus infection and disease in allogeneic stem cell transplant recipients. The Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Blood 97:388–392. doi: 10.1182/blood.V97.2.388. [DOI] [PubMed] [Google Scholar]
- 219.Bonatti H, Sifri CD, Larcher C, Schneeberger S, Kotton C, Geltner C. 2017. Use of cidofovir for cytomegalovirus disease refractory to ganciclovir in solid organ recipients. Surg Infect (Larchmt) 18:128–136. doi: 10.1089/sur.2015.266. [DOI] [PubMed] [Google Scholar]
- 220.Stoelben S, Arns W, Renders L, Hummel J, Muhlfeld A, Stangl M, Fischereder M, Gwinner W, Suwelack B, Witzke O, Durr M, Beelen DW, Michel D, Lischka P, Zimmermann H, Rubsamen-Schaeff H, Budde K. 2014. Preemptive treatment of cytomegalovirus infection in kidney transplant recipients with letermovir: results of a phase 2a study. Transpl Int 27:77–86. doi: 10.1111/tri.12225. [DOI] [PubMed] [Google Scholar]
- 221.Phoompoung P, Ferreira VH, Tikkanen J, Husain S, Viswabandya A, Kumar D, Humar A. 2020. Letermovir as salvage therapy for cytomegalovirus infection in transplant recipients. Transplantation 104:404–409. doi: 10.1097/TP.0000000000002785. [DOI] [PubMed] [Google Scholar]
- 222.Turner N, Strand A, Grewal DS, Cox G, Arif S, Baker AW, Maziarz EK, Saullo JH, Wolfe CR. 2019. Use of letermovir as salvage therapy for drug-resistant cytomegalovirus retinitis. Antimicrob Agents Chemother 63:e02337-18. doi: 10.1128/AAC.02337-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Veit T, Munker D, Kauke T, Zoller M, Michel S, Ceelen F, Schiopu S, Barton J, Arnold P, Milger K, Behr J, Kneidinger N. 2020. Letermovir for difficult to treat cytomegalovirus infection in lung transplant recipients. Transplantation 104:410–414. doi: 10.1097/TP.0000000000002886. [DOI] [PubMed] [Google Scholar]
- 224.El-Haddad D, El Chaer F, Vanichanan J, Shah DP, Ariza-Heredia EJ, Mulanovich VE, Gulbis AM, Shpall EJ, Chemaly RF. 2016. Brincidofovir (CMX-001) for refractory and resistant CMV and HSV infections in immunocompromised cancer patients: a single-center experience. Antiviral Res 134:58–62. doi: 10.1016/j.antiviral.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 225.Camargo JF, Morris MI, Abbo LM, Simkins J, Saneeymehri S, Alencar MC, Lekakis LJ, Komanduri KV. 2016. The use of brincidofovir for the treatment of mixed dsDNA viral infection. J Clin Virol 83:1–4. doi: 10.1016/j.jcv.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 226.Vial R, Zandotti C, Alain S, Decourt A, Jourde-Chiche N, Purgus R, Bornet C, Daniel L, Moal V, Legris T. 2017. Brincidofovir use after foscarnet crystal nephropathy in a kidney transplant recipient with multiresistant cytomegalovirus infection. Case Rep Transplant 2017:3624146. doi: 10.1155/2017/3624146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Faure E, Galperine T, Cannesson O, Alain S, Gnemmi V, Goeminne C, Dewilde A, Bene J, Lasri M, Lessore de Sainte Foy C, Lionet A. 2016. Case report: brincidofovir-induced reversible severe acute kidney injury in 2 solid-organ transplant for treatment of cytomegalovirus infection. Medicine (Baltimore) 95:e5226. doi: 10.1097/MD.0000000000005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kaptein SJ, Efferth T, Leis M, Rechter S, Auerochs S, Kalmer M, Bruggeman CA, Vink C, Stamminger T, Marschall M. 2006. The anti-malaria drug artesunate inhibits replication of cytomegalovirus in vitro and in vivo. Antiviral Res 69:60–69. doi: 10.1016/j.antiviral.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 229.Waldman WJ, Knight DA, Blinder L, Shen J, Lurain NS, Miller DM, Sedmak DD, Williams JW, Chong AS. 1999. Inhibition of cytomegalovirus in vitro and in vivo by the experimental immunosuppressive agent leflunomide. Intervirology 42:412–418. doi: 10.1159/000053979. [DOI] [PubMed] [Google Scholar]
- 230.Chacko B, John GT. 2012. Leflunomide for cytomegalovirus: bench to bedside. Transpl Infect Dis 14:111–120. doi: 10.1111/j.1399-3062.2011.00682.x. [DOI] [PubMed] [Google Scholar]
- 231.Avery RK, Mossad SB, Poggio E, Lard M, Budev M, Bolwell B, Waldman WJ, Braun W, Mawhorter SD, Fatica R, Krishnamurthi V, Young JB, Shrestha R, Stephany B, Lurain N, Yen-Lieberman B. 2010. Utility of leflunomide in the treatment of complex cytomegalovirus syndromes. Transplantation 90:419–426. doi: 10.1097/TP.0b013e3181e94106. [DOI] [PubMed] [Google Scholar]
- 232.Chon WJ, Kadambi PV, Xu C, Becker YT, Witkowski P, Pursell K, Kane B, Josephson MA. 2015. Use of leflunomide in renal transplant recipients with ganciclovir-resistant/refractory cytomegalovirus infection: a case series from the University of Chicago. Case Rep Nephrol Dial 5:96–105. doi: 10.1159/000381470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Morita S, Shinoda K, Tamaki S, Kono H, Asanuma H, Nakagawa K, Oya M. 2016. Successful low-dose leflunomide treatment for ganciclovir-resistant cytomegalovirus infection with high-level antigenemia in a kidney transplant: a case report and literature review. J Clin Virol 82:133–138. doi: 10.1016/j.jcv.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 234.Verkaik NJ, Hoek RA, van Bergeijk H, van Hal PT, Schipper ME, Pas SD, Beersma MF, Boucher CA, Jedema I, Falkenburg F, Hoogsteden HC, van den Blink B, Murk JL. 2013. Leflunomide as part of the treatment for multidrug-resistant cytomegalovirus disease after lung transplantation: case report and review of the literature. Transpl Infect Dis 15:E243–E249. doi: 10.1111/tid.12156. [DOI] [PubMed] [Google Scholar]
- 235.Fu L, Santhanakrishnan K, Al-Aloul M, Jones NP, Steeples LR. 17 October 2019. Management of ganciclovir resistant cytomegalovirus retinitis in a solid organ transplant recipient: a review of current evidence and treatment approaches. Ocul Immunol Inflamm doi: 10.1080/09273948.2019.1645188. [DOI] [PubMed] [Google Scholar]
- 236.Czarnecka P, Czarnecka K, Tronina O, Durlik M. 2018. Cytomegalovirus disease after liver transplant—a description of a treatment-resistant case: a case report and literature review. Transplant Proc 50:4015–4022. doi: 10.1016/j.transproceed.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 237.Williams JW, Mital D, Chong A, Kottayil A, Millis M, Longstreth J, Huang W, Brady L, Jensik S. 2002. Experiences with leflunomide in solid organ transplantation. Transplantation 73:358–366. doi: 10.1097/00007890-200202150-00008. [DOI] [PubMed] [Google Scholar]
- 238.Silva JT, Perez-Gonzalez V, Lopez-Medrano F, Alonso-Moralejo R, Fernandez-Ruiz M, San-Juan R, Branas P, Folgueira MD, Aguado JM, de Pablo-Gafas A. 2018. Experience with leflunomide as treatment and as secondary prophylaxis for cytomegalovirus infection in lung transplant recipients: a case series and review of the literature. Clin Transplant 32:e13176. doi: 10.1111/ctr.13176. [DOI] [PubMed] [Google Scholar]
- 239.Germi R, Mariette C, Alain S, Lupo J, Thiebaut A, Brion JP, Epaulard O, Saint Raymond C, Malvezzi P, Morand P. 2014. Success and failure of artesunate treatment in five transplant recipients with disease caused by drug-resistant cytomegalovirus. Antiviral Res 101:57–61. doi: 10.1016/j.antiviral.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 240.Shapira MY, Resnick IB, Chou S, Neumann AU, Lurain NS, Stamminger T, Caplan O, Saleh N, Efferth T, Marschall M, Wolf DG. 2008. Artesunate as a potent antiviral agent in a patient with late drug-resistant cytomegalovirus infection after hematopoietic stem cell transplantation. Clin Infect Dis 46:1455–1457. doi: 10.1086/587106. [DOI] [PubMed] [Google Scholar]
- 241.Oiknine-Djian E, Weisblum Y, Panet A, Wong HN, Haynes RK, Wolf DG. 2018. The artemisinin derivative artemisone is a potent inhibitor of human cytomegalovirus replication. Antimicrob Agents Chemother 62:e00288-18. doi: 10.1128/AAC.00288-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. 1992. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science 257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 243.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med 333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 244.Peggs KS, Verfuerth S, Pizzey A, Khan N, Guiver M, Moss PA, Mackinnon S. 2003. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet 362:1375–1377. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 245.Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Löffler J, Grigoleit U, Moris A, Rammensee H-G, Kanz L, Kleihauer A, Frank F, Jahn G, Hebart H. 2002. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 99:3916–3922. doi: 10.1182/blood.V99.11.3916. [DOI] [PubMed] [Google Scholar]
- 246.Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, Kapoor N, Pai SY, Rowley SD, Kebriaei P, Dey BR, Grilley BJ, Gee AP, Brenner MK, Rooney CM, Heslop HE. 2013. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 121:5113–5123. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Papadopoulou A, Gerdemann U, Katari UL, Tzannou I, Liu H, Martinez C, Leung K, Carrum G, Gee AP, Vera JF, Krance RA, Brenner MK, Rooney CM, Heslop HE, Leen AM. 2014. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med 6:242ra83. doi: 10.1126/scitranslmed.3008825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Neuenhahn M, Albrecht J, Odendahl M, Schlott F, Dossinger G, Schiemann M, Lakshmipathi S, Martin K, Bunjes D, Harsdorf S, Weissinger EM, Menzel H, Verbeek M, Uharek L, Kroger N, Wagner E, Kobbe G, Schroeder T, Schmitt M, Held G, Herr W, Germeroth L, Bonig H, Tonn T, Einsele H, Busch DH, Grigoleit GU. 2017. Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after allo-HSCT. Leukemia 31:2161–2171. doi: 10.1038/leu.2017.16. [DOI] [PubMed] [Google Scholar]
- 249.Tzannou I, Papadopoulou A, Naik S, Leung K, Martinez CA, Ramos CA, Carrum G, Sasa G, Lulla P, Watanabe A, Kuvalekar M, Gee AP, Wu MF, Liu H, Grilley BJ, Krance RA, Gottschalk S, Brenner MK, Rooney CM, Heslop HE, Leen AM, Omer B. 2017. Off-the-shelf virus-specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, Epstein-Barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J Clin Oncol 35:3547–3557. doi: 10.1200/JCO.2017.73.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Schmitt A, Tonn T, Busch DH, Grigoleit GU, Einsele H, Odendahl M, Germeroth L, Ringhoffer M, Ringhoffer S, Wiesneth M, Greiner J, Michel D, Mertens T, Rojewski M, Marx M, von Harsdorf S, Dohner H, Seifried E, Bunjes D, Schmitt M. 2011. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion 51:591–599. doi: 10.1111/j.1537-2995.2010.02940.x. [DOI] [PubMed] [Google Scholar]
- 251.Feuchtinger T, Opherk K, Bethge WA, Topp MS, Schuster FR, Weissinger EM, Mohty M, Or R, Maschan M, Schumm M, Hamprecht K, Handgretinger R, Lang P, Einsele H. 2010. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood 116:4360–4367. doi: 10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- 252.Peggs KS, Thomson K, Samuel E, Dyer G, Armoogum J, Chakraverty R, Pang K, Mackinnon S, Lowdell MW. 2011. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis 52:49–57. doi: 10.1093/cid/ciq042. [DOI] [PubMed] [Google Scholar]
- 253.Pei XY, Zhao XY, Chang YJ, Liu J, Xu LP, Wang Y, Zhang XH, Han W, Chen YH, Huang XJ. 2017. Cytomegalovirus-specific T-cell transfer for refractory cytomegalovirus infection after haploidentical stem cell transplantation: the quantitative and qualitative immune recovery for cytomegalovirus. J Infect Dis 216:945–956. doi: 10.1093/infdis/jix357. [DOI] [PubMed] [Google Scholar]
- 254.Ke P, Bao X, Zhou J, Li X, Zhuang J, He X, Wu D, Zhang X, Ma X. 2020. Donor CMV-specific cytotoxic T lymphocytes successfully treated drug-resistant cytomegalovirus encephalitis after allogeneic hematopoietic stem cell transplantation. Hematology 25:43–47. doi: 10.1080/16078454.2019.1710945. [DOI] [PubMed] [Google Scholar]
- 255.Withers B, Blyth E, Clancy LE, Yong A, Fraser C, Burgess J, Simms R, Brown R, Kliman D, Dubosq MC, Bishop D, Sutrave G, Ma CKK, Shaw PJ, Micklethwaite KP, Gottlieb DJ. 2017. Long-term control of recurrent or refractory viral infections after allogeneic HSCT with third-party virus-specific T cells. Blood Adv 1:2193–2205. doi: 10.1182/bloodadvances.2017010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Holmes-Liew CL, Holmes M, Beagley L, Hopkins P, Chambers D, Smith C, Khanna R. 2015. Adoptive T-cell immunotherapy for ganciclovir-resistant CMV disease after lung transplantation. Clin Transl Immunol 4:e35. doi: 10.1038/cti.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Brestrich G, Zwinger S, Fischer A, Schmuck M, Rohmhild A, Hammer MH, Kurtz A, Uharek L, Knosalla C, Lehmkuhl H, Volk HD, Reinke P. 2009. Adoptive T-cell therapy of a lung transplanted patient with severe CMV disease and resistance to antiviral therapy. Am J Transplant 9:1679–1684. doi: 10.1111/j.1600-6143.2009.02672.x. [DOI] [PubMed] [Google Scholar]
- 258.Macesic N, Langsford D, Nicholls K, Hughes P, Gottlieb DJ, Clancy L, Blyth E, Micklethwaite K, Withers B, Majumdar S, Fleming S, Sasadeusz J. 2015. Adoptive T cell immunotherapy for treatment of ganciclovir-resistant cytomegalovirus disease in a renal transplant recipient. Am J Transplant 15:827–832. doi: 10.1111/ajt.13023. [DOI] [PubMed] [Google Scholar]
- 259.Ambrose T, Sharkey LM, Louis-Auguste J, Rutter CS, Duncan S, English S, Gkrania-Klotsas E, Carmichael A, Woodward JM, Russell N, Massey D, Butler A, Middleton S. 2016. Cytomegalovirus infection and rates of antiviral resistance following intestinal and multivisceral transplantation. Transplant Proc 48:492–496. doi: 10.1016/j.transproceed.2015.09.070. [DOI] [PubMed] [Google Scholar]
- 260.Smith C, Beagley L, Rehan S, Neller MA, Crooks P, Solomon M, Holmes-Liew CL, Holmes M, McKenzie SC, Hopkins P, Campbell S, Francis RS, Chambers DC, Khanna R. 2019. Autologous adoptive T-cell therapy for recurrent or drug-resistant cytomegalovirus complications in solid organ transplant recipients: a single-arm open-label phase I clinical trial. Clin Infect Dis 68:632–640. doi: 10.1093/cid/ciy549. [DOI] [PubMed] [Google Scholar]
- 261.Fatic A, Zhang N, Keller MD, Hanley PJ. 2020. The pipeline of antiviral T-cell therapy: what’s in the clinic and undergoing development. Transfusion 60:7–10. doi: 10.1111/trf.15501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Jiang W, Withers B, Sutrave G, Clancy LE, Yong MI, Blyth E. 2019. Pathogen-specific T cells beyond CMV, EBV and adenovirus. Curr Hematol Malig Rep 14:247–260. doi: 10.1007/s11899-019-00521-z. [DOI] [PubMed] [Google Scholar]
- 263.Boeckh M, Murphy WJ, Peggs KS. 2015. Recent advances in cytomegalovirus: an update on pharmacologic and cellular therapies. Biol Blood Marrow Transplant 21:24–29. doi: 10.1016/j.bbmt.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 264.Schmidt S, Tramsen L, Rais B, Ullrich E, Lehrnbecher T. 2018. Natural killer cells as a therapeutic tool for infectious diseases—current status and future perspectives. Oncotarget 9:20891–20907. doi: 10.18632/oncotarget.25058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kheav VD, Busson M, Scieux C, Peffault de Latour R, Maki G, Haas P, Mazeron MC, Carmagnat M, Masson E, Xhaard A, Robin M, Ribaud P, Dulphy N, Loiseau P, Charron D, Socie G, Toubert A, Moins-Teisserenc H. 2014. Favorable impact of natural killer cell reconstitution on chronic graft-versus-host disease and cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation. Haematologica 99:1860–1867. doi: 10.3324/haematol.2014.108407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Davis ZB, Cooley SA, Cichocki F, Felices M, Wangen R, Luo X, DeFor TE, Bryceson YT, Diamond DJ, Brunstein C, Blazar BR, Wagner JE, Weisdorf DJ, Horowitz A, Guethlein LA, Parham P, Verneris MR, Miller JS. 2015. Adaptive natural killer cell and killer cell immunoglobulin-like receptor-expressing T cell responses are induced by cytomegalovirus and are associated with protection against cytomegalovirus reactivation after allogeneic donor hematopoietic cell transplantation. Biol Blood Marrow Transplant 21:1653–1662. doi: 10.1016/j.bbmt.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Maertens J, Bermúdez A, Logan A, Kharfan-Dabaja MA, Chevallier P, Martino R, Wulf G, Selleslag D, Kakihana K, Langston A, Lee D, Solano C, Okamoto S, Smith LR, Boeckh M, Wingard JR, Cywin B, Fredericks C, Lademacher C, Wang X, Young J, Ljungman P. 2019. A randomised, placebo-controlled phase 3 study to evaluate the efficacy and safety of ASP0113, a first-in-class, DNA-based vaccine in CMV-seropositive allogeneic haematopoietic cell transplant recipients. Bone Marrow Transplant 54:103–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kaeuferle T, Krauss R, Blaeschke F, Willier S, Feuchtinger T. 2019. Strategies of adoptive T-cell transfer to treat refractory viral infections post allogeneic stem cell transplantation. J Hematol Oncol 12:13. doi: 10.1186/s13045-019-0701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Reischig T, Kacer M, Hruba P, Hermanova H, Hes O, Lysak D, Kormunda S, Bouda M. 2018. Less renal allograft fibrosis with valganciclovir prophylaxis for cytomegalovirus compared to high-dose valacyclovir: a parallel group, open-label, randomized controlled trial. BMC Infect Dis 18:573. doi: 10.1186/s12879-018-3493-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Luan FL, Samaniego M, Kommareddi M, Park JM, Ojo AO. 2010. Choice of induction regimens on the risk of cytomegalovirus infection in donor-positive and recipient-negative kidney transplant recipients. Transpl Infect Dis 12:473–479. doi: 10.1111/j.1399-3062.2010.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Humar A, Lebranchu Y, Vincenti F, Blumberg EA, Punch JD, Limaye AP, Abramowicz D, Jardine AG, Voulgari AT, Ives J, Hauser IA, Peeters P. 2010. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant 10:1228–1237. doi: 10.1111/j.1600-6143.2010.03074.x. [DOI] [PubMed] [Google Scholar]
- 272.Bataille S, Moal V, Gaudart J, Indreies M, Purgus R, Dussol B, Zandotti C, Berland Y, Vacher-Coponat H. 2010. Cytomegalovirus risk factors in renal transplantation with modern immunosuppression. Transpl Infect Dis 12:480–488. doi: 10.1111/j.1399-3062.2010.00533.x. [DOI] [PubMed] [Google Scholar]
- 273.Halim MA, Al-Otaibi T, Gheith O, Adel H, Mosaad A, Hasaneen AA, Zakaria Z, Makkeya Y, Said T, Nair P. 2016. Efficacy and safety of low-dose versus standard-dose valganciclovir for prevention of cytomegalovirus disease in intermediate-risk kidney transplant recipients. Exp Clin Transplant 14:526–534. doi: 10.6002/ect.2015.0305. [DOI] [PubMed] [Google Scholar]
- 274.Finlen Copeland CA, Davis WA, Snyder LD, Banks M, Avery R, Davis RD, Palmer SM. 2011. Long-term efficacy and safety of 12 months of valganciclovir prophylaxis compared with 3 months after lung transplantation: a single-center, long-term follow-up analysis from a randomized, controlled cytomegalovirus prevention trial. J Heart Lung Transplant 30:990–996. doi: 10.1016/j.healun.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 275.Ghassemieh B, Ahya VN, Baz MA, Valentine VG, Arcasoy SM, Love RB, Seethamraju H, Alex CG, Bag R, DeOliveira NC, Vigneswaran WT, Charbeneau J, Garrity ER, Bhorade SM. 2013. Decreased incidence of cytomegalovirus infection with sirolimus in a post hoc randomized, multicenter study in lung transplantation. J Heart Lung Transplant 32:701–706. doi: 10.1016/j.healun.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 276.Kobashigawa J, Ross H, Bara C, Delgado JF, Dengler T, Lehmkuhl HB, Wang SS, Dong G, Witte S, Junge G, Potena L. 2013. Everolimus is associated with a reduced incidence of cytomegalovirus infection following de novo cardiac transplantation. Transpl Infect Dis 15:150–162. doi: 10.1111/tid.12007. [DOI] [PubMed] [Google Scholar]
- 277.Vigano M, Dengler T, Mattei MF, Poncelet A, Vanhaecke J, Vermes E, Kleinloog R, Li Y, Gezahegen Y, Delgado JF, RAD A2411 Study Investigators . 2010. Lower incidence of cytomegalovirus infection with everolimus versus mycophenolate mofetil in de novo cardiac transplant recipients: a randomized, multicenter study. Transpl Infect Dis 12:23–30. doi: 10.1111/j.1399-3062.2009.00448.x. [DOI] [PubMed] [Google Scholar]
- 278.Tsuchido Y, Nagao M, Matsuura M, Nakano S, Yamamoto M, Matsumura Y, Seno H, Ichiyama S. 2018. Real-time quantitative PCR analysis of endoscopic biopsies for diagnosing CMV gastrointestinal disease in non-HIV immunocompromised patients: a diagnostic accuracy study. Eur J Clin Microbiol Infect Dis 37:2389–2396. doi: 10.1007/s10096-018-3387-3. [DOI] [PubMed] [Google Scholar]
- 279.Walsh SR, Wilck MB, Dominguez DJ, Zablowsky E, Bajimaya S, Gagne LS, Verrill KA, Kleinjan JA, Patel A, Zhang Y, Hill H, Acharyya A, Fisher DC, Antin JH, Seaman MS, Dolin R, Baden LR. 2013. Safety and immunogenicity of modified vaccinia Ankara in hematopoietic stem cell transplant recipients: a randomized, controlled trial. J Infect Dis 207:1888–1897. doi: 10.1093/infdis/jit105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Adler SP, Plotkin SA, Gonczol E, Cadoz M, Meric C, Wang JB, Dellamonica P, Best AM, Zahradnik J, Pincus S, Berencsi K, Cox WI, Gyulai Z. 1999. A canarypox vector expressing cytomegalovirus (CMV) glycoprotein B primes for antibody responses to a live attenuated CMV vaccine (Towne). J Infect Dis 180:843–846. doi: 10.1086/314951. [DOI] [PubMed] [Google Scholar]
- 281.Berencsi K, Gyulai Z, Gonczol E, Pincus S, Cox WI, Michelson S, Kari L, Meric C, Cadoz M, Zahradnik J, Starr S, Plotkin S. 2001. A canarypox vector-expressing cytomegalovirus (CMV) phosphoprotein 65 induces long-lasting cytotoxic T cell responses in human CMV-seronegative subjects. J Infect Dis 183:1171–1179. doi: 10.1086/319680. [DOI] [PubMed] [Google Scholar]
- 282.Plotkin SA, Higgins R, Kurtz JB, Morris PJ, Campbell DA, Jr, Shope TC, Spector SA, Dankner WM. 1994. Multicenter trial of Towne strain attenuated virus vaccine in seronegative renal transplant recipients. Transplantation 58:1176–1178. doi: 10.1097/00007890-199412150-00006. [DOI] [PubMed] [Google Scholar]