Abstract
In tropical forest ecosystems leaf litter from a large variety of species enters the decomposer system, however, the impact of leaf litter diversity on the abundance and activity of soil organisms during decomposition is little known. We investigated the effect of leaf litter diversity and identity on microbial functions and the abundance of microarthropods in Ecuadorian tropical montane rainforests. We used litterbags filled with leaves of six native tree species (Cecropia andina, Dictyocaryum lamarckianum, Myrcia pubescens, Cavendishia zamorensis, Graffenrieda emarginata, and Clusia spp.) and incubated monocultures and all possible two‐ and four‐species combinations in the field for 6 and 12 months. Mass loss, microbial biomass, basal respiration, metabolic quotient, and the slope of microbial growth after glucose addition, as well as the abundance of microarthropods (Acari and Collembola), were measured at both sampling dates. Leaf litter diversity significantly increased mass loss after 6 months of exposure, but reduced microbial biomass after 12 months of exposure. Leaf litter species identity significantly changed both microbial activity and microarthropod abundance with species of high quality (low C‐to‐N ratio), such as C. andina, improving resource quality as indicated by lower metabolic quotient and higher abundance of microarthropods. Nonetheless, species of low quality, such as Clusia spp., also increased the abundance of Oribatida suggesting that leaf litter chemical composition alone is insufficient to explain variation in the abundances of soil microarthropods. Overall, the results provide evidence that decomposition and microbial biomass in litter respond to leaf litter diversity as well as litter identity (chemical and physical characteristics), while microarthropods respond only to litter identity but not litter diversity.
Keywords: Acari, Collembola, decomposition, litter quality, litterbags, metabolic quotient, microorganisms
Leaf litter identity functions as a major driver of the abundance and activity of soil organisms in tropical montane rainforests.
1. INTRODUCTION
The great majority of plant material enters the soil as litter, in the form of leaves, stems, and roots. Decomposition of these materials is an essential process for nutrient cycling and provides the basal resources of the soil food web (Berg et al., 1993; Berg & McClaugherty, 2008). In addition to providing food resources, leaf litter accumulating on the soil surface forms a variety of microhabitats for soil organisms, with more diverse litter materials increasing habitat variability, but also providing the opportunity for enhanced nutrient acquisition (Bardgett, 2005; Gessner et al., 2010). Therefore, high diversity of leaf litter in mixtures is expected to be an important determinant of the diversity and structure of decomposer communities and, consequently, litter decomposition (Gessner et al., 2010; Hättenschwiler et al., 2005; Trogisch et al., 2016).
Tropical montane rainforest ecosystems harbor an exceptional diversity of plant species (Beck & Ritcher, 2008; Homeier et al., 2008; Myers et al., 2000) and are associated with high numbers of animal species above‐ and belowground (Brehm et al., 2008; Maraun et al., 2008; Paulsch & Müller‐Hohenstein, 2008). However, the effect of plant litter diversity on decomposer communities and decomposition of litter in this ecosystems is little studied (Illig et al., 2008; Krashevska et al., 2017). Controlled experiments are needed to assess the effect of diversity and composition of litter species in mixtures on litter decomposition and microarthropod abundance.
Differences in leaf litter chemical composition are recognized as the main drivers of decomposition rates at the ecosystem level (Coûteaux et al., 1995; Hättenschwiler et al., 2005). Studies have reported positive, negative, but also no effects of litter mixtures on decomposition, with mixture effects typically related to variations in litter nutrient concentrations (Gartner & Cardon, 2004; Handa et al., 2014; Makkonen et al., 2012). However, differences in litter chemistry are not the only factors contributing to variations in litter decomposition in mixtures (Hättenschwiler, 2005; Hoorens et al., 2003). Physical leaf litter traits, such as toughness, surface structure, and shape, also contribute to microhabitat diversity and modify microenvironmental conditions of decomposer organisms, resulting in either accelerated or decelerated litter decomposition (Hansen & Coleman, 1998; Kaneko & Salamanca, 1999). Therefore, species identity, which encompasses chemical and physical characteristics, may well explain diversity effects on decomposition. Indeed, the effect of litter species identity has been found to be more powerful in explaining colonization of litter by invertebrates than litter diversity (Eissfeller et al., 2013; Korboulewsky et al., 2016; Schädler & Brandl, 2005; Vos et al., 2011; Wardle et al., 2006).
Commonly, studies investigating effects of litter diversity on litter decomposition focused on microorganisms and detritivore invertebrates (Gessner et al., 2010). Microorganisms are assumed to respond more sensitively to litter diversity than invertebrates as they directly depend on the variety of litter chemical compounds needed for metabolism and growth (Bardgett & Shine, 1999; Chapman et al., 2013). By contrast, the response of invertebrate detritivores, particularly the key decomposer groups Acari and Collembola, more strongly depends on the identity rather than diversity of leaf litter species and varies with the stage of litter decomposition (González & Seastedt, 2001; Illig et al., 2008; Kaneko & Salamanca, 1999; Korboulewsky et al., 2016; Wardle et al., 2006). Indeed, many decomposer microarthropods have the ability to select among co‐occurring leaf litter species according to litter palatability and/or the microorganisms colonizing the litter (Klironomos et al., 1992; Korboulewsky et al., 2016; Schneider & Maraun, 2005). Studies linking microbial‐dominated litter decomposition processes and colonization of litter by detritivore invertebrates are needed to uncover the mechanisms responsible for litter diversity effects on the structure and functioning of the decomposer system, particularly in tropical ecosystems characterized by high diversity of plant (tree) species.
In the present study, we investigated the effect of leaf litter diversity and identity on the colonization of litter by microorganisms and microarthropods including Acari and Collembola after 6 and 12 months of incubation in Ecuadorian montane rainforests. We hypothesized that (1) microbial growth and activity increase with litter diversity, but that the abundance of both Acari and Collembola relies more on litter identity. Additionally, assuming that microorganisms are limited by multiple nutrients (Demoling et al., 2007; Krashevska et al., 2010), we hypothesized that (2) nutrient availability increases and microbial stress conditions decrease with time and that (3) the presence of high‐quality litter benefits microorganisms. Further, assuming that Acari and Collembola prefer similar food resources and consume both leaf litter tissue and microorganisms (Dhooria, 2016; Ruess & Lussenhop, 2005; Seastedt, 1984), we hypothesized that (4) the abundance of Acari and Collembola increases as decomposition proceeds, particularly in presence of high‐quality litter.
2. MATERIALS AND METHODS
2.1. Study site
The study area is located in southern Ecuador on the eastern slopes of the Andean Cordillera. The site forms part of the Reserva Biológia San Francisco located on the northern borders of the Podocarpus National Park at 2,000 m a.s.l. (3°58′S, 79°04′W). The region is characterized by a semihumid climate with annual precipitation of about 2,200 mm and average annual temperature of 15.2°C (Bendix et al., 2006; Wullaert et al., 2009). The soil is Gley Cambisol with a soil pH of ~3.5 and a thick organic layer up to 35 cm comprised of mainly fermentation/humus material overlaid by litter material (Moser et al., 2007). The tropical rainforest is mostly undisturbed and holds an exceptionally high diversity of fauna and flora with Rubiaceae, Melastomataceae, and Piperaceae as dominant plant families (Beck & Ritcher, 2008; Brehm & Fiedler, 2005; Homeier et al., 2010; Maraun et al., 2008).
2.2. Experimental design
In September 2008, freshly fallen leaves of six common plant species at the study sites [Cecropia andina (Cuatrec.) (CA), Dictyocaryum lamarckianum (H. Wendl.) (DL), Myrcia pubescens (Humb. & Bonpl. ex Willd.) (MP), Cavendishia zamorensis (A. C. Sm.) (CZ), Graffenrieda emarginata (Ruiz & Pav.) (GE), and Clusia spp. (L.) (Cs); ordered by increasing C‐to‐N ratio, see Appendix 1] were collected, dried (60°C for 72 hr), and used to fill 20 × 20 cm and 4 mm nylon mesh litterbags. Initial chemical composition of the litter species is given in Appendix 1. The leaves used had no signs of herbivory, fungal infection or atypical texture or color. Large leaves exceeding the size of the litter bags were cut into ~5 × 5 cm pieces. Single‐species litterbags (12 g each) and mixtures with all possible two‐ (6 g per species) and four‐species combinations (3 g per species) were prepared, resulting in a total of 36 litterbag types with three levels of species diversity (1, 2, and 4 leaf litter species). Litterbags were randomly placed in the field on top of the undisturbed litter layer and fixed with nails in four blocks. Minimum distance between the blocks was 20 m. One replicate of each treatment was harvested after 6 and 12 months.
2.3. Analytical procedures
After harvest, material in each litterbag was separated into two subsamples of equal weight, disturbing the fauna as little as possible but ensuring that all litter types were present in both halves. One half was used for microarthropod extraction and the other for analysis of microbial parameters. Microarthropods were extracted by heat over one week using a modified high gradient extractor and then stored in 70% ethanol (Kempson et al., 1963; Macfadyen, 1961). Microarthropods were determined to group level [Collembola (Insecta), Oribatida, Mesostigmata, and Prostigmata (Acari)] using Schaefer (2018). The dry litter was sorted to species, weighed and used to measure litter chemical composition.
Microbial basal respiration (BR) and microbial biomass (C mic) were determined using an automated respirometer system (Scheu, 1992). BR (μl O2 g−1 dry weight hr−1) was measured at 22°C and calculated as mean of O2 consumption rates 10 to 20 hr after attachment of the samples to the respirometer system. C mic was measured by the substrate‐induced respiration method (SIR; Anderson & Domsch, 1978; Beck et al., 1997). The maximum initial respiratory response (MIRR; µl O2 g−1 dry weight hr−1) was measured at 22°C after the addition of glucose to saturate the catabolic activity of microorganisms. MIRR was calculated as the average of the lowest three readings within the first 10 hr, and C mic was calculated as C mic = 38 × MIRR (mg/g dry weight). Respiration rates between the lowest (usually 3–6 hr after glucose addition) and highest reading were taken to calculate the slope of microbial growth (+C Slope). Data were ln‐transformed, and the slope determined by linear regression. The microbial metabolic quotient (qO2; μl O2 mg−1 C mic hr−1) was calculated by dividing BR by C mic.
Leaf litter mass loss (M loss) was calculated as M loss (%) = (m 0 – m 1/m 0) × 100, where m 0 is the initial dry weight and m1 the dry weight of leaf litter at harvest. To measure chemical composition, leaves from each of the six species were dried (65°C for 72 hr) and milled to particles <1 mm. Carbon (C) and nitrogen (N) were measured using a CN elemental analyzer (Vario EL III, Elementar). Total element analysis was measured by an ICP‐OES system (ICP‐OES, Optima 5300 DV, Perkin Elmer). Lignin and cellulose concentration were measured based on the methanol–chloroform–water (2:2:1) extraction method detailed in Allen et al. (1974). For litter mixtures, the proportion of elements per litterbag was calculated by proportionally summing the amount of the respective elements in the individual litter species. The chemical concentrations of elements, lignin and cellulose, were expressed as milligram per gram litter dry weight (dw).
2.4. Statistical analyses
Analyses were performed using R version 3.6.0 (R Core Team, 2014). Data were checked for normality and homoscedasticity using Shapiro–Wilk test and Bartlett's test (package “stats”). To improve normality and homoscedasticity, data were transformed using the “bestNormalize” function (package “CRAN”). Changes in M loss, C mic, BR, qO2, +C Slope, and the abundance of microarthropod taxa (Collembola, Oribatida, Mesostigmata, and Prostigmata) were analyzed using individual linear mixed‐effects models (package “nlme”). In each model, the fixed factors litter diversity (LD; 1, 2, and 4 litter species), time of exposure (6 and 12 months), and the presence/absence all leaf litter species (litter identity; 1,0; CA, DL, MP, CZ, GE, and Cs), as well as the interactions (time × LD and time × litter identity), were fitted in a hierarchical design. Block was fitted first as random factor followed by the fixed factors litter diversity, time, interaction between litter diversity and time, and litter identity. To assess the relative importance of the six leaf litter species, analyses were repeated changing the order of fitting individual litter species and their interactions. F‐ and p‐values for individual litter species in the text and tables refer to those when fitted first (Schmid et al., 2002, 2017). Differences between means were inspected using Tukey's honestly significant difference test (package “emmeans”). Values presented in text are means ± SD of non−transformed data. Pearson correlation coefficients were calculated to investigate relationships between C‐to‐N ratio, C mic, qO2 and M loss, and the abundance of Collembola and Acari (package “stats”).
3. RESULTS
3.1. Initial litter chemistry
Initial N concentrations were highest in C. andina, followed by D. lamarckianum, M. pubescens, C. zamorensis, G. emarginata, and Clusia spp. (1.08%, 0.73%, 0.60%, 0.50%, 0.40%, and 0.40%, respectively), resulting in C‐to‐N ratios between 36.3 in C. andina and 107.2 in Clusia spp. (see Appendix 1 for details on litter chemistry). Lignin concentrations were generally high and varied between 63.9% in Clusia spp. to 42.6% in G. emarginata. By contrast, concentrations of cellulose were lowest in Clusia spp. (13.0%), low in C. andina (29.6%), but similar in the other four litter species varying between 35.8% and 40.7%. Concentrations of P and other litter elements also varied markedly between leaf litter species with P, Ca, Mg, K, and Fe being highest in C. andina, and P and Ca being lowest in G. emarginata.
3.2. Mass loss
Generally, M loss was higher after 12 than after 6 months of incubation with averages of 52.6% ± 7.1% and 41.8% ± 6.9% of initial, respectively (Table 1). M loss varied significantly with species diversity but the effect depended on time (Table 1; Figure 1a); after 6 months M loss was lower in single species (average of 29.6% ± 6.9%) compared to the two and four litter species treatments (43.1% ± 3.8 and 44.9% ± 3.6%, respectively), while after 12 months decomposition was similar in each of the litter diversity treatments. Further, M loss varied significantly with litter species identity; however, this depended on time, with the effect generally being restricted to the first sampling date and to four of the six litter species (Table 1). At the first sampling date, M loss increased in presence of C. andina from 39.7% ± 7.4% to 44.4% ± 5.1%, in presence of C. zamorensis from 40.5% ± 7.9% to 43.2% ± 5.3%, in presence of G. emarginata from 39.4% ± 7.6% to 44.8% ± 4.2%, and in presence of Clusia spp. from 39.6% ± 7.3% to 44.6% ± 5.1%. M loss positively correlated with C mic, BR, qO2, +C Slope, and the abundance of Collembola and Oribatida, but negatively with the litter C‐to‐N ratio (Pearson correlation coefficients; Table 2).
Table 1.
F‐values of linear mixed‐effects models on the effect of litter species diversity (LD), time of exposure (Time), and leaf litter species identity [Cecropia andina (CA), Dictyocaryum lamarckianum (DL), Myrcia pubescens (MP), Cavendishia zamorensis (CZ), Graffenrieda emarginata (GE), and Clusia spp. (Cs)] on mass loss (M loss), microbial biomass (C mic), basal respiration (BR), microbial metabolic quotient (qO2), and the slopes of microbial growth after C addition (+C Slope)
| df | M loss | C mic | BR | qO2 | +C Slope | |
|---|---|---|---|---|---|---|
| LD | 2, 239 | 26.32*** | 3.01* | 1.12 | 2.01 | 2.03 |
| Time | 1, 239 | 244.03*** | 31.48*** | 78.10*** | 21.15*** | 24.61*** |
| CA | 1, 239 | 0.51 | 1.63 | 1.04 | 7.76** | 1.21 |
| DL | 1, 239 | 1.09 | <0.01 | 1.78 | 1.93 | 4.59* |
| MP | 1, 239 | 2.09 | <0.01 | 3.91* | 0.46 | 0.70 |
| CZ | 1, 239 | 0.02 | 0.53 | <0.01 | 4.49* | 4.33* |
| GE | 1, 239 | 0.43 | 0.11 | 0.04 | <0.01 | 0.05 |
| Cs | 1, 239 | 0.97 | 0.05 | 0.02 | <0.01 | 0.01 |
| Time × LD | 2, 239 | 43.44*** | 4.37** | 1.43 | 1.27 | 1.73 |
| Time × CA | 1, 239 | 23.01*** | 0.12 | 0.01 | <0.01 | 2.30 |
| Time × DL | 1, 239 | 0.91 | 0.47 | 0.11 | 0.66 | 3.89* |
| Time × MP | 1, 239 | 1.76 | 0.60 | 3.13 | 0.60 | 0.59 |
| Time × CZ | 1, 239 | 7.25** | 0.71 | 0.80 | 3.76* | 2.48 |
| Time × GE | 1, 239 | 35.12*** | 6.76** | 2.29 | 0.60 | <0.01 |
| Time × Cs | 1, 239 | 21.73*** | 1.77 | 0.07 | 0.02 | 2.72 |
F‐values represent those where the respective factor was fitted first. Significant effects are given in bold (*p < .05; **p < .01; ***p < .001).
Abbreviation: df, degrees of freedom.
Figure 1.
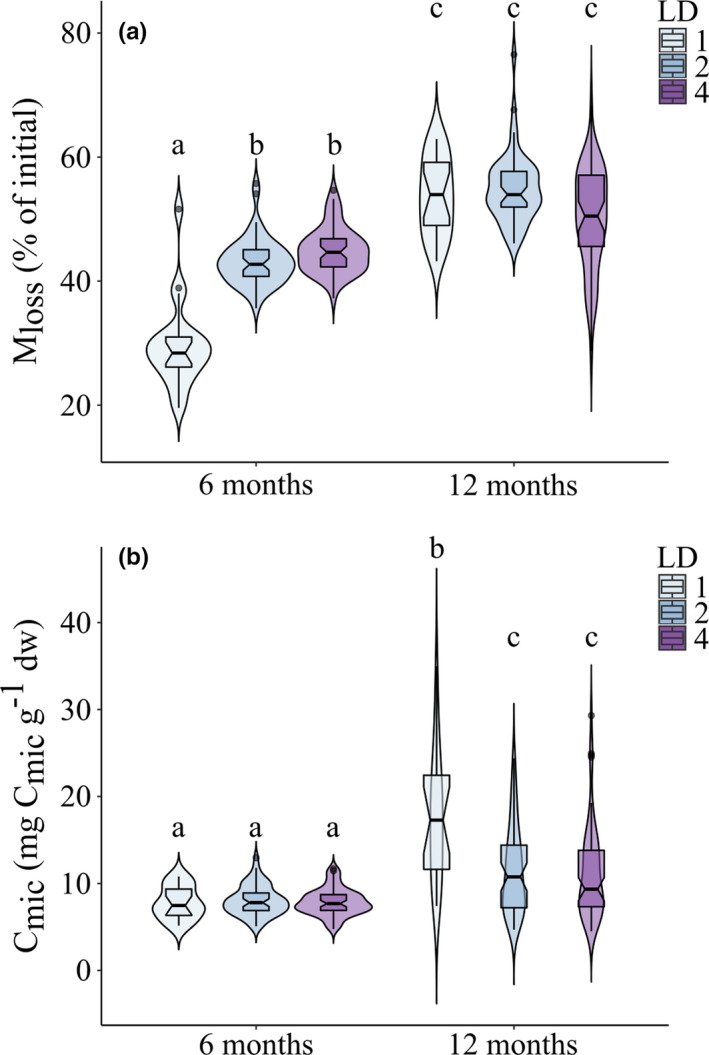
Effect of litter species diversity (LD; 1, 2, and 4 species) on (a) litter mass loss (M loss) and (b) litter microbial biomass (C mic) after 6 and 12 months of incubation in the field. Boxplots show medians and quantiles for each LD level. Violin plots illustrate kernel probability density. Different letters indicate significant differences (Tukey's HSD test, p < .05)
Table 2.
Pearson correlation coefficients between mass loss (M loss), microbial biomass (C mic), basal respiration (BR), microbial growth after C addition (+C slope), metabolic quotient (qO2), the abundance of Collembola, Oribatida, Mesostigmata, and Prostigmata, and litter C‐to‐N ratio
| M loss | C mic | BR | qCO2 | +C Slope | Collembola | Oribatida | Mesostigmata | Prostigmata | |
|---|---|---|---|---|---|---|---|---|---|
| M loss | 1 | — | — | — | — | — | — | — | — |
| C mic | 0.30*** | 1 | — | — | — | — | — | — | — |
| BR | 0.42*** | 0.53*** | 1 | — | — | — | — | — | — |
| qO2 | 0.20** | −0.16 *** | 0.50*** | 1 | — | — | — | — | — |
| +C slope | 0.20** | 0.23*** | 0.38*** | 0.07 | 1 | — | — | — | — |
| Collembola | 0.16** | 0.09 | 0.04 | −0.10 | 0.12 | 1 | — | — | — |
| Oribatida | 0.25*** | 0.08 | 0.13* | 0.04 | 0.12 | 0.50*** | 1 | — | — |
| Mesostigmata | −0.05 | −0.07 | −0.15* | −0.16* | −0.05 | 0.40*** | 0.40*** | 1 | — |
| Prostigmata | 0.05 | 0.02 | <0.01 | −0.11 | 0.07 | 0.37*** | 0.39*** | 0.48*** | 1 |
| C‐to‐N | −0.24*** | −0.16* | −0.19** | 0.05 | −0.15* | −0.15* | −0.01 | −0.07 | −0.19*** |
Significant correlations are given in bold (*p < .05; **p < .01; ***p < .001).
3.3. Microbial parameters
Parallel to M loss, the microbial parameters C mic, BR, qO2, and +C Slope significantly increased from 6 to 12 months (Table 1; for means see Appendix 2). Among microbial parameters, only C mic varied with litter diversity. Unlike M loss, the effect of litter diversity was restricted to the second sampling date, decreasing in the order one > two > four litter species (Figure 1b). Further, C mic also varied with litter species identity, but the effect was restricted to treatments with G. emarginata and depended on time. At the second sampling date, C mic decreased from 15.23 ± 11.74 to 11.58 ± 7.37 mg C mic g−1 dw in litterbags without and with G. emarginata, respectively. The other microbial parameters only were significantly affected by litter species identity, with the effects in part varying with time (Table 1). BR decreased significantly in presence of M. pubescens from an average of 157.3 ± 107.7 to 133.1 ± 69.40 μl O2 mg−1 C mic hr−1 in litterbags without and with M. pubescens, respectively. qO2 decreased from 14.90 ± 5.65 to 13.50 ± 4.18 μl O2 mg‐1 C mic hr−1 in presence of C. andina, irrespective of sampling date, but it increased from 14.44 ± 5.37 to 16.91 ± 7.45 45 μl O2 mg‐1 C mic hr−1 in presence of C. zamorensis at the second sampling date. +C Slope decreased significantly from 0.0097 ± 0.0149 to 0.0061 ± 0.0131 in presence of C. zamorensis irrespective of sampling date, but in presence of D. lamarckianum it increased from 0.0086 ± 0.0195 to 0.0151 ± 0.0180 after the second sampling.
Pearson correlation coefficients indicated that C mic positively correlated with M loss, BR and +C Slope, but negatively with qO2 and the litter C‐to‐N ratio. BR positively correlated with M loss, C mic, qO2, +C Slope, and the abundance of Oribatida, but negatively with the abundance of Mesostigmata and the litter C‐to‐N ratio. qO2 positively correlated with M loss and BR, but negatively with C mic and the abundance of Mesostigmata. +C Slope positively correlated with M loss, C mic, and BR, but negatively with the litter C‐to‐N ratio (Table 2).
3.4. Microarthropods
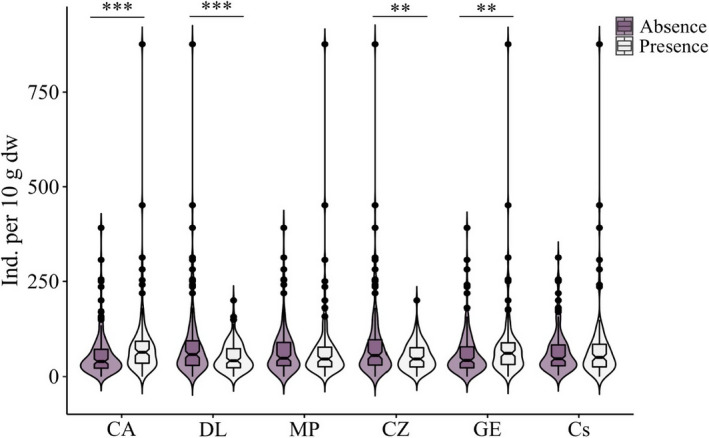
The number of Collembola, Oribatida, and Prostigmata significantly increased from 6 to 12 months, but the abundance of Mesostigmata decreased (Table 3; Figure 2; for means, see Appendix 3). None of the soil microarthropod taxa investigated varied with litter diversity, although they did vary significantly with litter species identity (Table 3). Collembola abundance (25.3% of total microarthropods; overall mean of 70 ± 80 ind. 10 g−1 litter dw) increased significantly in presence of C. andina by 43.4% and in presence of G. emarginata by 29.2%, but decreased in presence of D. lamarckianum and C. zamorensis by 39.1% and 38.1%, respectively (Appendices 3 and 4). However, the effect varied with time for D. lamarckianum and C. zamorensis (Table 3); in the presence of these species, the reduction was most pronounced after 12 months (from 60 ± 42 to 123 ± 132 and from 62 ± 38 to 124 ± 135 ind. 10 g−1 litter dw, respectively). The abundance of Oribatida (53.7% of total microarthropods; overall mean 146 ± 119 ind. 10 g−1 litter dw) increased significantly in litterbags containing G. emarginata or Clusia spp. from 133 ± 119 to 162 ± 118 and from 131 ± 99 to 163 ± 138 ind. 10 g−1 litter dw, respectively. Further, Mesostigmata abundance (11.1% of total microarthropods; overall mean of 30 ± 27 ind. 10 g−1 litter dw) decreased significantly by 24.5% from 34 ± 31 to 26 ± 21 ind. 10 g−1 litter dw in the presence of C. zamorensis. Prostigmata abundance (9.5% of total microarthropods; overall mean of 26 ± 22 ind. 10 g−1 litter dw) increased significantly in litterbags where C. andina or Clusia spp. were present. With the former, it increased by 28.1% from 23 ± 22 to 29 ± 22 ind. 10 g−1 litter dw, while in the presence of the latter the effect was restricted to the second sampling date, increasing by 23.1% from 27 ± 25 to 33 ± 26 ind. 10 g−1 litter dw.
Table 3.
F‐values of linear mixed‐effects models on the effect of litter species diversity (LD), time of exposure (Time), and leaf litter species identity [Cecropia andina (CA), Dictyocaryum lamarckianum (DL), Myrcia pubescens (MP), Cavendishia zamorensis (CZ), Graffenrieda emarginata (GE), and Clusia spp. (Cs)] on the abundance of Collembola, Oribatida, Mesostigmata, and Prostigmata
| df | Collembola | Oribatida | Mesostigmata | Prostigmata | |
|---|---|---|---|---|---|
| LD | 2, 239 | 0.15 | 1.41 | 0.75 | 0.74 |
| Time | 1, 239 | 28.08*** | 78.95*** | 4.93* | 4.22* |
| CA | 1, 239 | 15.83*** | 1.50 | 2.86 | 7.92** |
| DL | 1, 239 | 13.34*** | 0.34 | 0.05 | 0.66 |
| MP | 1, 239 | <0.01 | 0.85 | 0.37 | 2.74 |
| CZ | 1, 239 | 8.80** | 2.73 | 4.61* | 2.06 |
| GE | 1, 239 | 7.59** | 5.98** | 2.43 | 1.56 |
| Cs | 1, 239 | <0.01 | 4.24* | 0.07 | 0.02 |
| Time × LD | 2, 239 | 2.80 | 0.61 | 0.71 | 0.39 |
| Time × CA | 1, 239 | 0.14 | 0.59 | 2.26 | 3.08 |
| Time × DL | 1, 239 | 8.04** | 0.02 | 1.01 | 0.42 |
| Time × MP | 1, 239 | 0.85 | 0.30 | 0.23 | 0.03 |
| Time × CZ | 1, 239 | 4.52* | 0.01 | 0.01 | <0.01 |
| Time × GE | 1, 239 | 0.22 | 0.03 | 0.14 | 0.33 |
| Time × Cs | 1, 239 | 0.44 | 0.02 | 0.04 | 4.25* |
F‐values represent those where the respective factor was fitted first. Significant effects are given in bold (*p < .05; **p < .01; ***p < .001).
Abbreviation: df, degrees of freedom.
Figure 2.
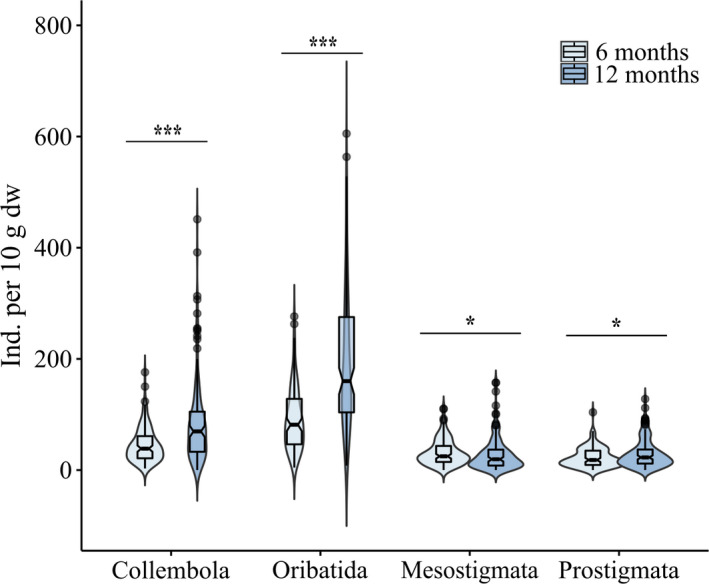
Abundance of Collembola, Oribatida, Mesostigmata, and Prostigmata in litterbags after 6 and 12 months of incubation in the field. Boxplots show medians and quantiles for each date of exposure. Violin plots illustrate kernel probability density. ***p < .001; *p < .05
Pearson correlation coefficients indicated that Collembola abundance positively correlated with M loss and the abundance of Oribatida, Mesostigmata, and Prostigmata, but negatively with the litter C‐to‐N ratio. Oribatida abundance positively correlated with M loss, BR, and the abundance of Collembola, Mesostigmata, and Prostigmata. Mesostigmata abundance positively correlated with the abundance of Collembola, Oribatida, and Prostigmata, but negatively with BR and qO2. Prostigmata abundance positively correlated with the abundance of Collembola, Oribatida, and Mesostigmata, but negatively with litter C‐to‐N ratio (Table 2).
4. DISCUSSION
4.1. Litter diversity
Contrary to our first hypothesis, C mic decreased rather than increased with increasing litter diversity after one year of exposure in the field (Figure 1). Leaves of tropical forest trees are of low nutritional quality and contain high concentrations of structural compounds and secondary metabolites, typically higher than those in trees of temperate forests (Cárdenas et al., 2015; Coley & Barone, 1996; Hallam & Read, 2006). Secondary metabolites, particularly polyphenols known to suppress microorganisms by inhibiting enzyme activity (Hättenschwiler & Vitousek, 2000; Hoorens et al., 2003), are important drivers of decomposition processes particularly in tropical rainforests (Coq et al., 2010). Potentially, secondary compounds, such as polyphenols, detrimentally affected litter microorganisms in a systemic way resulting in a decrease in C mic, thereby resulting in a negative complementarity effect in leaf litter mixtures (Chomel et al., 2016; Ristok et al., 2019). The fact that BR, qO2, and +C Slope were not significantly affected by litter diversity suggests that higher leaf litter diversity does not necessarily result in an increase in the availability of nutrient and carbon resources in this tropical rainforest. Rather, the results suggest that litter diversity increases the exposure of microorganisms to secondary leaf litter compounds, detrimentally affecting their activity. Due to the preferential decay of labile litter compounds, the concentration of secondary compounds as well as recalcitrant structural compounds, such as lignin, may increase during litter decomposition, thereby reducing litter decomposition at later stages of litter decay, as has previously been suggested for litter at our study sites (Butenschoen et al., 2014; Marian et al., 2017).
Similar to C mic, M loss significantly increased in single litter species treatments after one year of exposure underscoring the correlation between (Table 2). Changes in the chemical composition of litter material throughout the decomposition process alter the structure and functioning of microbial communities and thus affect the rate at which litter material is decomposed (Berg & McClaugherty, 2008). Notably, M loss increased with litter diversity after 6 months of exposure; however, the effect was no longer present after 12 months. Presumably, this reflects reliance of the early microbial community on labile litter compounds, which were more abundant in leaf litter mixtures (Pérez Harguindeguy et al., 2008; Rinkes et al., 2014). However, as decomposition proceeded, the remaining more recalcitrant compounds accumulated and their decomposition was independent of litter diversity.
In contrast with C mic and M loss, the abundance of microarthropods was not affected by litter diversity (Table 3). Some previous studies found mixtures to promote the abundance of microarthropods (Hansen, 2000; Hättenschwiler & Gasser, 2005; Migge et al., 1998; Schädler & Brandl, 2005), while others did not find evidence that litter diversity beneficially affects microarthropods (Bluhm et al., 2019; Ilieva‐Makulec et al., 2006; Korboulewsky et al., 2016; Patoine et al., 2020; Scheu et al., 2003). Our results agree with the latter findings and support the results of Marian et al. (2018) suggesting that litter diversity in this tropical rainforest neither improves habitat conditions nor the availability of resources for microarthropods, at least during early stages of decomposition. Indeed, detritivore microarthropods are considered to comprise predominantly generalist feeders colonizing a range of forest types and therefore are rather insensitive to changes caused by litter mixing (Ball et al., 2014; Gergócs & Hufnagel, 2016; Patoine et al., 2020; Wardle et al., 2006). However, even though litter diversity did not affect microarthropod abundance, it may still have fostered the diversity of microarthropods, as has been shown for other soil organisms, such as testate amoebae at our study site (Krashevska et al., 2017).
4.2. Exposure time
Generally, M loss increased with time parallel to microbial parameters. Litter decomposition at our study site can be divided into three phases, with the early phase lasting for about 12 months (Marian et al., 2017). This early phase of decomposition is characterized by the loss of labile C compounds via leaching and by the growth of opportunistic microorganisms that form new soluble compounds (Berg & McClaugherty, 2008), and this likely explains the close link between M loss and microbial activity and growth (Table 2). However, contrary to our second hypothesis, the increase in qO2 values between 6 and 12 months of exposure indicates that microorganisms increasingly suffered from stress conditions later during exposure. Stress conditions result in less efficient use of C compounds and increased investment into maintenance metabolism (Ndaw et al., 2009; Yan et al., 2003). Presumably, toward the end of the early litter decomposition stage microorganisms increasingly competed for resources as easily decomposable leaf litter compounds vanished (Fontaine et al., 2003; Poll et al., 2008; Rinkes et al., 2011). The parallel increase in the +C Slope with time suggests that this was associated with less efficient nutrient capture by microorganisms pointing toward a switch from predominant limitation by nutrients early during exposure to the limitation by easily available carbon resources later (Laganière et al., 2010; Sall et al., 2003). Early stages of litter decay in the studied tropical montane rainforest might be associated with high abundance of mycorrhizal fungi (Marian et al., 2017). The C input that mycorrhizal fungi obtain from plants may allow them to efficiently compete with saprotrophic fungi for nutrients, even though their enzymatic capability is typically inferior to that of saprotrophic fungi (Camenzind & Rillig, 2013; Hodge et al., 2001). Indeed, the assumption that mycorrhizal and saprotrophic fungi interact antagonistically early during litter decomposition at our study site is supported by earlier studies (Marian et al., 2019; Sánchez‐Galindo et al., 2019).
Parallel to microbial parameters, the abundance of all microarthropod taxa studied increased with time, with the exception of Mesostigmata. Mesostigmata commonly hunt in the litter for other microarthropods, particularly Collembola, Astigmata and weakly sclerotized Oribatida (Koehler, 1997; Schneider & Maraun, 2009). Although variations in the abundance of Mesostigmata were closely linked to the abundance of Collembola and Oribatida (Table 2), the fact that their abundance decreased with time likely reflects that Mesostigmata in the litterbags were not only feeding on microarthropods, but also on other organisms, presumably Nematoda, insect larvae and eggs. Indeed, some species of Mesostigmata may preferentially colonize certain microhabitats to hunt for prey such as Nematoda (Heidemann et al., 2014; Klarner et al., 2013).
The increase in the abundance of the microarthropod decomposers Collembola and Oribatida with time indicates that changes during the initial stages of decomposition influence both groups in a similar way. Surprisingly, Collembola and Oribatida abundance was not closely associated with microbial biomass (Table 2) even though microorganisms are their major food resource (Dhooria, 2016; Maraun et al., 2003; Scheu et al., 2005). Rather, the stage of litter decomposition within the early decomposition phase (i.e., 6 vs. 12 months) appears to be the more important driver of the abundance of microarthropod decomposers. Indeed, litter material that is highly colonized by microorganisms becomes more palatable for microarthropods (Bardgett, 2005; Das & Joy, 2009), which at least in part is due to the reduction in plant secondary compounds such as phenols (Asplund et al., 2013; Coulis et al., 2009). Overall, our results support earlier findings at this study site in that the role of litter resources for the nutrition of decomposer microarthropods increases with litter decomposition (Marian et al., 2018). Moreover, the parallel increase in the abundance of Prostigmata suggests that the increase in the abundance of decomposer microarthropod prey benefitted higher trophic levels.
4.3. Leaf litter identity
The presence of specific plant leaf litter species in mixtures might increase or decrease the rate at which the litter decomposes (Hector et al., 2000; Hoorens et al., 2003, 2010). Variation can be attributed predominantly to differences in litter quality among the component species in mixtures (Gartner & Cardon, 2004; Hättenschwiler et al., 2005). Indeed, litter decomposition and colonization of the litter by microarthropods in our study were related to the initial chemical composition of the litter species. Our third hypothesis was supported by the beneficial effects of high‐quality C. andina litter. Presence of this litter species significantly decreased qO2 values and increased the abundance of Collembola and Prostigmata. C. andina had high initial N and P concentrations, and low lignin content (see Appendix 1), providing readily available nutrients, reducing nutrient stress for microorganisms, and thereby contributing to an increase in C mic. Increased microbial C use efficiency may also have resulted from a shift in microbial community composition toward high‐energy‐efficient species (Dilly & Munch, 1996), for example, from opportunistic bacteria to fungi able to break down complex litter compounds (Chapman et al., 2013). Changes in microbial community composition probably were driven by increasing concentrations of recalcitrant litter compounds favoring saprotrophic fungi able to degrade these compounds, which in turn beneficially affected decomposers, such as Collembola and Oribatida, feeding on these fungi and the litter materials degraded by them.
The high qO2 and the +C Slope values after 12 months of exposure reflected the low quality of D. lamarckianum, C. zamorensis, and G. emarginata litter, and presumable scarcity of easily accessible C resources to microorganisms. All these litter species were characterized by low initial N and P concentrations, and high concentrations of lignin and cellulose (Appendix 1). The concentrations of lignin and cellulose serve as indicator of litter quality and as predictor of litter decomposition (Berg, 2014; Fioretto et al., 2005). Cellulose not entrapped in lignin degrades rapidly during early stages of decomposition, and this contributes to the release of N and P, typical elements limiting microbial growth (Berg, 2014; Berg & McClaugherty, 2008; Hobbie et al., 2012). However, during this stage, labile compounds are commonly used by opportunistic microorganisms (Cornelissen et al., 1999; Fioretto et al., 2005), impeding the growth of microorganism able to degrade recalcitrant litter compounds (Ilieva‐Makulec et al., 2006). Therefore, by the end of the early stage of litter decomposition, structural compounds become relatively more abundant and reduce resource quality, which differentially affects microorganisms and microarthropods, as indicated by the lower abundance of Collembola in litter of C. zamorensis and D. lamarckianum. Interestingly, the decrease in C mic after 12 months in litterbags containing G. emarginata was associated with high abundance of decomposer microarthropods, suggesting that there is no close relationship between decomposer microarthropods and bulk microbial biomass in litter. This conclusion is also supported by the lack of significant correlations between C mic and decomposer microarthropod abundances (Table 2).
The correlation between the abundance of Collembola and Oribatida and litter M loss presumably reflects that these microarthropods benefited from both higher quality litter and by microorganisms colonizing the litter at later stages of decay. The significant negative correlation between Collembola abundance and litter C‐to‐N ratio (Table 2) indicates that Collembola heavily rely on litter quality. However, contrary to our fourth hypothesis, the differential responses of microarthropods to litter species suggest that leaf litter chemical composition alone is insufficient to explain variations in the abundance of soil microarthropods, as has been suggested in earlier studies (González & Seastedt, 2001; Hoorens et al., 2010; Kaneko & Salamanca, 1999). This is most strongly supported by the greater abundance of Oribatida in litterbags containing Clusia spp. litter, which was of particular low quality. This indicates that physical litter characteristics such as toughness and structure might play a more important role in driving soil microarthropod abundance than litter chemistry and the degree of microbial colonization.
5. CONCLUSIONS
The results of our study showed that higher levels of litter diversity may negatively affect soil microbial biomass and mass loss in the studied tropical montane rainforest, presumably due to the accumulation of recalcitrant compounds and the generally low quality of the leaf litter material. Notably, the response of microbial parameters and microarthropod abundance to litter identity was more pronounced than to litter diversity, with the differential responses of soil biota to litter identity in part being due to differences in the initial chemical composition of litter species. Generally, the results indicate that both microarthropods and microorganisms benefit from larger amounts of easily available litter resources during early stages of decomposition, highlighting the importance of litter quality as driver of the abundance and activity of decomposer organisms. However, the results also indicate that litter traits, related to the physical structure of litter, may be more important to decomposer invertebrates than litter chemistry and gross microbial characteristics of litter such as microbial biomass. Overall, our findings indicate that litter species identity functions as major driver of the abundance and activity of soil organisms, and thereby exert distinct effects on ecosystem processes such as decomposition and nutrient mobilization.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Laura Sanchez Galindo: Data curation (equal); Formal analysis (lead); Investigation (equal); Validation (equal); Visualization (lead); Writing‐original draft (lead). Dorothee Sandmann: Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (supporting); Writing‐review & editing (equal). Franca Marian: Data curation (equal); Formal analysis (equal); Investigation (equal); Writing‐review & editing (equal). Valentyna Krashevska: Data curation (equal); Investigation (equal); Writing‐review & editing (equal). Mark Maraun: Conceptualization (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Writing‐review & editing (equal). Stefan Scheu: Conceptualization (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (lead); Resources (lead); Supervision (equal); Validation (equal); Writing‐original draft (equal).
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We thank the Deutsche Forschungsgemeinschaft (DFG; FOR816) for financial support. Further, we thank the Ministerio de Ambiente del Ecuador and the Universidad Técnico Particular de Loja (UTPL) for the research permits and the center Naturaleza y Cultura Internacional (NCI) for access to the San Francisco reserve. We thank student helpers for the establishment of the experiment in the field and Christina Lucas for her help in the laboratory.
Appendix 1.
Initial chemical composition of the litter species used in the experiment. The analyses were performed in triplicate using bulk samples. Data are given in percentages of dry mass; nd = not detected.
| Cecropia andina | Dictyocaryum lamarckianum | Myrcia pubescens | Cavendishia zamorensis | Graffenrieda emarginata | Clusia spp. | |
|---|---|---|---|---|---|---|
| C | 39.30 ± 3.65 | 41.25 ± 1.70 | 39.61 ± 3.65 | 41.74 ± 0.01 | 40.28 ± 1.68 | 42.80 ± 3.65 |
| N | 1.08 ± 0.02 | 0.73 ± 0.01 | 0.60 ± 0.02 | 0.50 ± 0.01 | 0.40 ± 0.16 | 0.40 ± 0.02 |
| C‐to‐N | 36.29 ± 2.91 | 58.59 ± 1.25 | 65.64 ± 4.62 | 84.64 ± 0.01 | 91.29 ± 1.68 | 107.21 ± 6.00 |
| Lignin | 46.67 ± 6.37 | 52.40 ± 9.83 | 50.53 ± 9.07 | 51.73 ± 13.95 | 42.60 ± 8.40 | 63.93 ± 10.10 |
| Cellulose | 29.60 ± 6.28 | 40.73 ± 4.29 | 35.80 ± 7.27 | 39.53 ± 3.33 | 40.40 ± 6.73 | 13.00 ± 3.37 |
| Al | 1.88 ± 0.65 | 0.14 ± 0.08 | 0.18 ± 0.06 | 0.23 ± 0.02 | 2.41 ± 0.33 | 0.13 ± 0.01 |
| Ca | 17.32 ± 0.82 | 1.13 ± 0.08 | 1.07 ± 0.02 | 6.11 ± 0.83 | 1.07 ± 0.02 | 3.07 ± 0.82 |
| Fe | 2.03 ± 0.05 | 1.18 ± 0.02 | 0.29 ± 0.08 | 0.09 ± 0.03 | 0.30 ± 0.03 | 0.06 ± 0.02 |
| K | 3.05 ± 0.01 | 0.37 ± 0.09 | 1.23 ± 0.09 | 1.08 ± 0.01 | 1.08 ± 0.03 | 1.65 ± 0.09 |
| Mg | 3.22 ± 0.73 | 1.25 ± 0.09 | 1.22 ± 0.09 | 1.72 ± 0.09 | 1.72 ± 0.09 | 1.55 ± 0.09 |
| Mn | 0.11 ± 0.01 | 0.31 ± 0.03 | 0.14 ± 0.09 | 0.06 ± 0.01 | 0.26 ± 0.09 | 0.48 ± 0.09 |
| Na | nd | 0.03 ± 0.02 | 0.30 ± 0.02 | nd | nd | nd |
| P | 0.48 ± 0.08 | 0.21 ± 0.08 | 0.22 ± 0.10 | 0.27 ± 0.10 | 0.12 ± 0.08 | 0.25 ± 0.08 |
Appendix 2.
Means of microbial parameters (C mic, microbial biomass carbon; BR, basal respiration, qO2, microbial specific respiration; +C Slope, the slopes of microbial growth after C addition). LD, litter diversity (LD1, one species; LD2, two species; LD4, four species); CA, Cecropia andina; DL, Dictyocaryum lamarckianum; MP, Myrcia pubescens; CZ, Cavendishia zamorensis; GE, Graffenrieda emarginata; Cs, Clusia spp. Values are means ± SD.
| C mic (mg C mic g−1 dw) | BR (μl O2 mg−1 C mic hr−1) | qO2 (μl O2 mg−1 C mic hr−1) | +C Slope | |||
|---|---|---|---|---|---|---|
| LD | 1 | 13.30 ± 8.99 | 154.57 ± 97.62 | 12.97 ± 5.55 | 0.0113 ± 0.0208 | |
| 2 | 11.01 ± 9.74 | 152.42 ± 105.28 | 14.78 ± 5.71 | 0.0066 ± 0.0111 | ||
| 4 | 10.10 ± 6.12 | 138.35 ± 79.27 | 14.27 ± 4.22 | 0.0084 ± 0.0121 | ||
| Time | 6 months | 8.28 ± 4.19 | 101.7 ± 19.5 | 12.90 ± 2.21 | 0.0042 ± 0.0035 | |
| 12 months | 13.62 ± 10.18 | 191.1 ± 114.0 | 15.65 ± 6.56 | 0.0117 ± 0.0190 | ||
| CA | Presence | 11.12 ± 6.22 | 148.50 ± 94.82 | 13.50 ± 4.18 | 0.0089 ± 0.0157 | |
| Absence | 10.81 ± 9.56 | 144.73 ± 91.97 | 14.90 ± 5.65 | 0.0075 ± 0.0114 | ||
| DL | Presence | 10.58 ± 6.98 | 150.09 ± 89.94 | 14.74 ± 4.06 | 0.0097 ± 0.0140 | |
| Absence | 11.28 ± 9.20 | 143.14 ± 96.06 | 13.85 ± 5.82 | 0.0067 ± 0.0130 | ||
| MP | Presence | 10.53 ± 9.53 | 133.07 ± 69.40 | 14.15 ± 4.14 | 0.0074 ± 0.0104 | |
| Absence | 11.29 ± 6.97 | 157.33 ± 107.72 | 14.37 ± 5.75 | 0.0088 ± 0.0156 | ||
| CZ | Presence | 10.33 ± 8.53 | 143.71 ± 89.58 | 14.94 ± 5.75 | 0.0065 ± 0.0117 | |
| Absence | 11.54 ± 7.89 | 149.02 ± 96.61 | 13.63 ± 4.27 | 0.0097 ± 0.0149 | ||
| GE | Presence | 10.31 ± 6.80 | 141.93 ± 94.72 | 14.34 ± 5.10 | 0.0079 ± 0.0121 | |
| Absence | 11.46 ± 9.17 | 149.98 ± 91.98 | 14.21 ± 5.08 | 0.0084 ± 0.0146 | ||
| Cs | Presence | 10.36 ± 6.53 | 142.19 ± 90.71 | 14.32 ± 5.05 | 0.0080 ± 0.0101 | |
| Absence | 11.42 ± 9.33 | 149.78 ± 95.13 | 14.24 ± 5.12 | 0.0083 ± 0.0157 | ||
| Time × LD | ||||||
| LD1 | 6 months | 7.79 ± 1.85 | 96.07 ± 18.97 | 12.65 ± 2.50 | 0.0045 ± 0.0031 | |
| 12 months | 18.81 ± 9.92 | 213.06 ± 109.53 | 13.29 ± 7.53 | 0.0182 ± 0.0280 | ||
| LD 2 | 6 months | 8.03 ± 1.71 | 103.05 ± 19.08 | 13.03 ± 1.91 | 0.0041 ± 0.0036 | |
| 12 months | 13.97 ± 13.06 | 201.79 ± 130.59 | 16.53 ± 7.48 | 0.0091 ± 0.0149 | ||
| LD 4 | 6 months | 8.67 ± 5.94 | 102.47 ± 20.01 | 12.86 ± 2.38 | 0.0043 ± 0.0036 | |
| 12 months | 11.52 ± 6.01 | 174.23 ± 98.28 | 15.69 ± 5.11 | 0.0126 ± 0.0158 | ||
| Time × Litter identity | ||||||
| CA | Presence | 6 months | 8.64 ± 1.66 | 103.19 ± 19.44 | 12.12 ± 2.00 | 0.0037 ± 0.0044 |
| Absence | 6 months | 7.99 ± 5.44 | 100.50 ± 19.58 | 13.53 ± 2.20 | 0.0047 ± 0.0026 | |
| Presence | 12 months | 13.61 ± 7.91 | 193.81 ± 116.56 | 14.89 ± 5.23 | 0.0142 ± 0.0206 | |
| Absence | 12 months | 13.63 ± 11.76 | 188.97 ± 112.63 | 16.27 ± 7.46 | 0.0104 ± 0.0155 | |
| DL | Presence | 6 months | 8.25 ± 5.81 | 103.55 ± 19.70 | 13.62 ± 2.31 | 0.0044 ± 0.0034 |
| Absence | 6 months | 8.31 ± 1.79 | 100.05 ± 19.29 | 12.24 ± 1.92 | 0.0041 ± 0.0037 | |
| Presence | 12 months | 12.90 ± 7.31 | 196.63 ± 107.35 | 15.86 ± 5.04 | 0.0151 ± 0.0180 | |
| Absence | 12 months | 14.29 ± 12.21 | 186.23 ± 120.22 | 15.45 ± 7.71 | 0.0094 ± 0.017 | |
| MP | Presence | 6 months | 8.27 ± 5.94 | 98.27 ± 16.49 | 13.03 ± 2.36 | 0.0041 ± 0.0033 |
| Absence | 6 months | 8.29 ± 1.74 | 104.52 ± 21.33 | 12.79 ± 2.10 | 0.0043 ± 0.0038 | |
| Presence | 12 months | 12.80 ± 11.71 | 167.88 ± 83.56 | 15.27 ± 5.14 | 0.0106 ± 0.0136 | |
| Absence | 12 months | 14.30 ± 8.76 | 210.15 ± 131.38 | 15.96 ± 7.56 | 0.0133 ± 0.0209 | |
| CZ | Presence | 6 months | 8.07 ± 1.52 | 103.63 ± 18.99 | 12.98 ± 1.81 | 0.0039 ± 0.0028 |
| Absence | 6 months | 8.48 ± 5.68 | 99.88 ± 19.92 | 12.82 ± 2.56 | 0.0046 ± 0.0041 | |
| Presence | 12 months | 12.59 ± 11.57 | 183.80 ± 112.03 | 16.91 ± 7.45 | 0.0092 ± 0.0160 | |
| Absence | 12 months | 14.60 ± 8.61 | 198.16 ± 116.24 | 14.44 ± 5.38 | 0.0149 ± 0.0194 | |
| GE | Presence | 6 months | 9.03 ± 5.98 | 105.82 ± 20.97 | 12.71 ± 2.31 | 0.0039 ± 0.0031 |
| Absence | 6 months | 7.69 ± 1.62 | 98.45 ± 17.71 | 13.04 ± 2.14 | 0.0045 ± 0.0038 | |
| Presence | 12 months | 11.58 ± 7.37 | 178.04 ± 122.51 | 15.98 ± 6.45 | 0.0119 ± 0.0159 | |
| Absence | 12 months | 15.23 ± 11.74 | 201.52 ± 106.48 | 15.39 ± 6.68 | 0.0122 ± 0.0196 | |
| Cs | Presence | 6 months | 8.40 ± 6.07 | 99.02 ± 21.32 | 12.88 ± 2.48 | 0.0056 ± 0.0042 |
| Absence | 12 months | 8.18 ± 1.60 | 103.83 ± 17.76 | 12.91 ± 1.99 | 0.0032 ± 0.0026 | |
| Presence | 6 months | 12.32 ± 6.44 | 185.36 ± 111.16 | 15.75 ± 6.41 | 0.0105 ± 0.0133 | |
| Absence | 12 months | 14.65 ± 12.31 | 195.73 ± 116.75 | 15.57 ± 6.72 | 0.0133 ± 0.0210 | |
Appendix 3.
Means of microarthropod abundance. Values are means ± SD. For legend, see Appendix 2.
| Collembola (ind. 10 g−1) | Oribatida (ind. 10 g−1) | Mesostigmata (ind. 10 g−1) | Prostigmata (ind. 10 g−1) | |||
|---|---|---|---|---|---|---|
| LD | 1 | 73 ± 79 | 132 ± 116 | 26 ± 24 | 22 ± 18 | |
| 2 | 71 ± 67 | 150 ± 128 | 32 ± 29 | 26 ± 23 | ||
| 4 | 67 ± 91 | 147 ± 113 | 30 ± 26 | 27 ± 23 | ||
| Time | 6 months | 46 ± 31 | 90 ± 55 | 32 ± 23 | 22 ± 17 | |
| 12 months | 93 ± 104 | 201 ± 137 | 29 ± 30 | 29 ± 26 | ||
| CA | Presence | 83 ± 99 | 141 ± 117 | 33 ± 27 | 29 ± 22 | |
| Absence | 58 ± 59 | 150 ± 121 | 28 ± 27 | 23 ± 22 | ||
| DL | Presence | 52 ± 37 | 141 ± 106 | 30 ± 27 | 25 ± 23 | |
| Absence | 85 ± 103 | 150 ± 130 | 30 ± 27 | 27 ± 22 | ||
| MP | Presence | 71 ± 98 | 144 ± 125 | 30 ± 28 | 24 ± 22 | |
| Absence | 69 ± 63 | 147 ± 114 | 30 ± 26 | 27 ± 22 | ||
| CZ | Presence | 53 ± 35 | 132 ± 95 | 26 ± 21 | 25 ± 23 | |
| Absence | 85 ± 105 | 159 ± 137 | 34 ± 31 | 27 ± 22 | ||
| GE | Presence | 80 ± 99 | 162 ± 118 | 34 ± 29 | 28 ± 24 | |
| Absence | 62 ± 61 | 133 ± 119 | 27 ± 25 | 24 ± 20 | ||
| Cs | Presence | 75 ± 104 | 163 ± 138 | 30 ± 28 | 27 ± 23 | |
| Absence | 65 ± 56 | 131 ± 99 | 30 ± 26 | 25 ± 22 | ||
| Time × LD | ||||||
| LD1 | 6 months | 35 ± 24 | 76 ± 59 | 27 ± 23 | 18 ± 13 | |
| 12 months | 111 ± 96 | 189 ± 132 | 25 ± 25 | 26 ± 22 | ||
| LD 2 | 6 months | 47 ± 35 | 93 ± 53 | 32 ± 26 | 21 ± 14 | |
| 12 months | 94 ± 82 | 207 ± 154 | 32 ± 32 | 32 ± 28 | ||
| LD 4 | 6 months | 48 ± 29 | 93 ± 57 | 33 ± 21 | 25 ± 21 | |
| 12 months | 87 ± 123 | 200 ± 128 | 27 ± 31 | 28 ± 25 | ||
| Time × Litter identity | ||||||
| CA | Presence | 6 months | 56 ± 34 | 88 ± 54 | 37 ± 26 | 28 ± 20 |
| Absence | 6 months | 37 ± 26 | 92 ± 57 | 27 ± 20 | 17 ± 15 | |
| Presence | 12 months | 111 ± 131 | 193 ± 137 | 28 ± 28 | 30 ± 24 | |
| Absence | 12 months | 78 ± 74 | 208 ± 140 | 29 ± 32 | 28 ± 27 | |
| DL | Presence | 6 months | 44 ± 30 | 90 ± 56 | 33 ± 25 | 23 ± 20 |
| Absence | 6 months | 47 ± 32 | 91 ± 55 | 30 ± 21 | 21 ± 15 | |
| Presence | 12 months | 60 ± 42 | 192 ± 119 | 27 ± 28 | 26 ± 25 | |
| Absence | 12 months | 123 ± 132 | 209 ± 154 | 30 ± 33 | 32 ± 27 | |
| MP | Presence | 6 months | 43 ± 27 | 87 ± 48 | 30 ± 20 | 22 ± 20 |
| Absence | 6 months | 48 ± 34 | 93 ± 61 | 33 ± 25 | 22 ± 15 | |
| Presence | 12 months | 98 ± 131 | 201 ± 151 | 31 ± 35 | 26 ± 24 | |
| Absence | 12 months | 89 ± 77 | 201 ± 129 | 27 ± 27 | 32 ± 27 | |
| CZ | Presence | 6 months | 44 ± 30 | 85 ± 54 | 28 ± 20 | 21 ± 16 |
| Absence | 6 months | 47 ± 32 | 96 ± 57 | 34 ± 26 | 23 ± 19 | |
| Presence | 12 months | 62 ± 38 | 179 ± 103 | 23 ± 22 | 28 ± 27 | |
| Absence | 12 months | 124 ± 135 | 222 ± 163 | 34 ± 37 | 30 ± 24 | |
| GE | Presence | 6 months | 50 ± 34 | 102 ± 61 | 35 ± 22 | 25 ± 20 |
| Absence | 6 months | 42 ± 29 | 81 ± 49 | 29 ± 23 | 20 ± 15 | |
| Presence | 12 months | 109 ± 131 | 221 ± 130 | 33 ± 35 | 32 ± 28 | |
| Absence | 12 months | 81 ± 76 | 185 ± 144 | 25 ± 26 | 27 ± 24 | |
| Cs | Presence | 6 months | 45 ± 27 | 100 ± 57 | 30 ± 19 | 21 ± 17 |
| Absence | 6 months | 46 ± 34 | 82 ± 53 | 33 ± 26 | 23 ± 18 | |
| Presence | 12 months | 106 ± 138 | 226 ± 165 | 31 ± 33 | 33 ± 26 | |
| Absence | 12 months | 84 ± 66 | 181 ± 111 | 27 ± 27 | 27 ± 25 | |
Appendix 4.
Abundance of Collembola as affected by the presence of leaf litter species [Cecropia andina (CA), Dictyocaryum lamarckianum (DL), Myrcia pubescens (MP), Graffenrieda emarginata (GE), Cavendishia zamorensis (CZ), and Clusia spp. (Cs)]. Boxplots show medians and quantiles of Collembola abundance for presence and absence of each leaf litter species. Violin plots illustrate kernel probability density. ***p < .001; **p < .01.
Sánchez‐Galindo LM, Sandmann D, Marian F, Krashevska V, Maraun M, Scheu S. Leaf litter identity rather than diversity shapes microbial functions and microarthropod abundance in tropical montane rainforests. Ecol Evol. 2021;11:2360–2374. 10.1002/ece3.7208
DATA AVAILABILITY STATEMENT
All data are available as electronic supplementary material.
REFERENCES
- Allen, S. E. , Grimshaw, H. M. , Parkinson, J. A. , & Quarmby, C. (1974). Chemical analysis of ecological materials. Blackwell Scientific Publications. [Google Scholar]
- Anderson, J. P. E. , & Domsch, K. H. (1978). A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biology & Biochemistry, 10, 215–221. [Google Scholar]
- Asplund, J. , Bokhorst, S. , & Wardle, D. A. (2013). Secondary compounds can reduce the soil micro‐arthropod effect on lichen decomposition. Soil Biology & Biochemistry, 66, 10–16. [Google Scholar]
- Ball, B. A. , Carrillo, Y. , & Molina, M. (2014). The influence of litter composition across the litter‐soil interface on mass loss, nitrogen dynamics and the decomposer community. Soil Biology & Biochemistry, 69, 71–82. [Google Scholar]
- Bardgett, R. D. (2005). The Biology of soils: A community and ecosystem approach. Oxford University Press. [Google Scholar]
- Bardgett, R. D. , & Shine, A. (1999). Linkages between plant litter diversity, soil microbial biomass and ecosystem function in temperate grasslands. Soil Biology & Biochemistry, 31, 317–321. [Google Scholar]
- Beck, E. , & Ritcher, M. (2008). Ecological aspects of a biodiversity hotspot in the Andes of southern Ecuador. Biodiversity and Ecology Series, 195–217. [Google Scholar]
- Beck, T. , Joergensen, R. G. , Kandeler, E. , Makeschin, F. , Nuss, E. , Oberholzer, H. R. , & Scheu, S. (1997). An inter‐laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biology & Biochemistry, 29, 1023–1032. [Google Scholar]
- Bendix, J. , Homeier, J. , Cueva Ortiz, E. , Emck, P. , Breckle, S.‐W. , Richter, M. , & Beck, E. (2006). Seasonality of weather and tree phenology in a tropical evergreen mountain rain forest. International Journal of Biometeorology, 50, 370–384. [DOI] [PubMed] [Google Scholar]
- Berg, B. (2014). Decomposition patterns for foliar litter—A theory for influencing factors. Soil Biology & Biochemistry, 78, 222–232. [Google Scholar]
- Berg, B. , Berg, M. P. , Bottner, P. , Box, E. , Breymeyer, A. , Ca de Anta, R. , Couteaux, M. , Escudero, A. , Gallardo, A. , Kratz, W. , Madeira, M. , Mälkönen, E. , McClaugherty, C. , Meentemeyer, V. , Muñoz, F. , Piussi, P. , Remacle, J. , & Vi de Santo, A. (1993). Litter mass loss rates in pine forests of Europe and Eastern United States: Some relationships with climate and litter quality. Biogeochemistry, 20, 127–159. [Google Scholar]
- Berg, B. , & McClaugherty, C. (2008). Plant litter, 2nd ed. Springer‐Verlag. [Google Scholar]
- Bluhm, C. , Butenschoen, O. , Maraun, M. , & Scheu, S. (2019). Effects of root and leaf litter identity and diversity on oribatid mite abundance, species richness and community composition. PLoS One, 14, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm, G. , & Fiedler, K. (2005). Diversity and community structure of geometrid moths of disturbed habitat in a montane area in the Ecuadorian Andes. Journal of Research on the Lepidoptera, 1999, 1–14. [Google Scholar]
- Brehm, G. , Homeier, J. , Fiedler, K. , Kottke, I. , Illig, J. , Nöske, N. M. , Werner, F. A. , & Breckle, S. W. (2008). Mountain rain forests in southern Ecuador as a hotspot of biodiversity – limited knowledge and diverging patterns. In Beck E. Bendix J. Kottke I. Makeschin F. & Mosandl R. (Eds.), Gradients in a Tropical Mountain Ecosystem of Ecuador (pp. 15–23). Berlin, Heidelberg: Springer. [Google Scholar]
- Butenschoen, O. , Krashevska, V. , Maraun, M. , Marian, F. , Sandmann, D. , & Scheu, S. (2014). Litter mixture effects on decomposition in tropical montane rainforests vary strongly with time and turn negative at later stages of decay. Soil Biology & Biochemistry, 77, 121–128. [Google Scholar]
- Camenzind, T. , & Rillig, M. C. (2013). Extraradical arbuscular mycorrhizal fungal hyphae in an organic tropical montane forest soil. Soil Biology & Biochemistry, 64, 96–102. [Google Scholar]
- Cárdenas, R. E. , Hättenschwiler, S. , Valencia, R. , Argoti, A. , & Dangles, O. (2015). Plant herbivory responses through changes in leaf quality have no effect on subsequent leaf‐litter decomposition in a neotropical rain forest tree community. New Phytologist, 207, 817–829. [DOI] [PubMed] [Google Scholar]
- Chapman, S. K. , Newman, G. S. , Hart, S. C. , Schweitzer, J. A. , & Koch, G. W. (2013). Leaf litter mixtures alter microbial community development: Mechanisms for non‐additive effects in litter decomposition. PLoS One, 8, e62671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomel, M. , Guittonny‐Larchevêque, M. , Fernandez, C. , Gallet, C. , DesRochers, A. , Paré, D. , Jackson, B. G. , & Baldy, V. (2016). Plant secondary metabolites: A key driver of litter decomposition and soil nutrient cycling. Journal of Ecology, 104, 1527–1541. [Google Scholar]
- Coley, P. D. , & Barone, J. A. (1996). Herbivory and plant defenses in tropical forests. Annual Review of Ecology and Systematics, 27, 305–335. [Google Scholar]
- Coq, S. , Souquet, J. M. , Meudec, E. , Cheynier, V. , & Hättenschwiler, S. (2010). Interspecific variation in leaf litter tannins drives decomposition in a tropical rain forest of French Guiana. Ecology, 91, 2080–2091. [DOI] [PubMed] [Google Scholar]
- Cornelissen, J. H. C. , Pérez‐Harguindeguy, N. , Díaz, S. , Grime, J. P. , Marzano, B. , Cabido, M. , Vendramini, F. , & Cerabolini, B. (1999). Leaf structure and defence control litter decomposition rate across species and life forms in regional floras on two continents. New Phytologist, 143, 191–200. [Google Scholar]
- Coulis, M. , Hättenschwiler, S. , Rapior, S. , & Coq, S. (2009). The fate of condensed tannins during litter consumption by soil animals. Soil Biology & Biochemistry, 41, 2573–2578. [Google Scholar]
- Coûteaux, M. M. , Bottner, P. , & Berg, B. (1995). Litter decomposition, climate and litter quality. Tree, 10, 63–66. [DOI] [PubMed] [Google Scholar]
- Das, S. , & Joy, V. C. (2009). Chemical quality impacts of tropical forest tree leaf litters on the growth and fecundity of soil Collembola. European Journal of Soil Biology, 45, 448–454. [Google Scholar]
- Demoling, F. , Figueroa, D. , & Bååth, E. (2007). Comparison of factors limiting bacterial growth in different soils. Soil Biology & Biochemistry, 39, 2485–2495. [Google Scholar]
- Dhooria, M. S. (2016). Soil mites. In Dhooria M. S. (Ed.), Fundamentals of applied acarology (pp. 197–206). Singapore: Springer. [Google Scholar]
- Dilly, O. , & Munch, J. C. (1996). Microbial biomass content, basal respiration and enzyme activities during the course of decomposition of leaf litter in a black alder (Alnus glutinosa (L.) Gaertn.) forest. Soil Biology & Biochemistry, 28, 1073–1081. [Google Scholar]
- Eissfeller, V. , Langenbruch, C. , Jacob, A. , Maraun, M. , & Scheu, S. (2013). Tree identity surpasses tree diversity in affecting the community structure of oribatid mites (Oribatida) of deciduous temperate forests. Soil Biology & Biochemistry, 63, 154–162. [Google Scholar]
- Fioretto, A. , Di Nardo, C. , Papa, S. , & Fuggi, A. (2005). Lignin and cellulose degradation and nitrogen dynamics during decomposition of three leaf litter species in a Mediterranean ecosystem. Soil Biology & Biochemistry, 37, 1083–1091. [Google Scholar]
- Fontaine, S. , Mariotti, A. , & Abbadie, L. (2003). The priming effect of organic matter: A question of microbial competition? Soil Biology & Biochemistry, 35, 837–843. [Google Scholar]
- Gartner, T. , & Cardon, Z. (2004). Decomposition dynamics in mixed‐species leaf litter. Oikos, 104, 230–246. [Google Scholar]
- Gergócs, V. , & Hufnagel, L. (2016). The effect of microarthropods on litter decomposition depends on litter quality. European Journal of Soil Biology, 75, 24–30. [Google Scholar]
- Gessner, M. O. , Swan, C. M. , Dang, C. K. et al (2010). Diversity meets decomposition. Trends in Ecology & Evolution, 25, 372–380. [DOI] [PubMed] [Google Scholar]
- González, G. , & Seastedt, T. R. (2001). Soil fauna and plant litter decomposition in tropical and subalpine forests. Ecology, 82, 955–964. [Google Scholar]
- Hallam, A. , & Read, J. (2006). Do tropical species invest more in anti‐herbivore defence than temperate species? A test in Eucryphia (Cunoniaceae) in eastern Australia. Journal of Tropical Ecology, 22, 41–51. [Google Scholar]
- Handa, I. T. , Aerts, R. , Berendse, F. et al (2014). Consequences of biodiversity loss for litter decomposition across biomes. Nature, 509, 218–221. [DOI] [PubMed] [Google Scholar]
- Hansen, R. A. (2000). Effects of habitat complexity and composition on a diverse litter microarthropod assemblage. Ecology, 81, 1120–1132. [Google Scholar]
- Hansen, R. A. , & Coleman, D. C. (1998). Litter complexity and composition are determinants of the diversity and species composition of oribatid mites (Acari: Oribatida) in litterbags. Applied Soil Ecology, 9, 17–23. [Google Scholar]
- Hättenschwiler, S. (2005). Effects of tree species diversity on litter quality and decomposition. In Scherer‐Lorenzen M., Körner C., & Schulze E.‐D. (Eds.), Forest diversity and function: Temperate and boreal systems (pp. 149–164). Springer Nature Switzerland. [Google Scholar]
- Hättenschwiler, S. , & Gasser, P. (2005). Soil animals alter plan litter diversity effects on decomposition. Proceedings of the National Academy of Sciences, 102, 1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hättenschwiler, S. , Tiunov, A. V. , & Scheu, S. (2005). Biodiversity and litter decomposition in terrestrial ecosystems. Annual Review of Ecology Evolution and Systematics, 36, 191–218. 10.1146/annurev.ecolsys.36.112904.151932 [DOI] [Google Scholar]
- Hättenschwiler, S. , & Vitousek, P. M. (2000). The role of polyphenols in terrestrial ecosystem nutrient cycling. Tree, 15, 238–242. 10.1016/S0169-5347(00)01861-9 [DOI] [PubMed] [Google Scholar]
- Hector, A. , Beale, A. J. , Minns, A. , Otway, S. J. , & Lawton, J. H. (2000). Consequences of the reduction of plant diversity for litter decomposition: Effects through litter quality and microenvironment. Oikos, 90, 357–371. 10.1034/j.1600-0706.2000.900217.x [DOI] [Google Scholar]
- Heidemann, K. , Ruess, L. , Scheu, S. , & Maraun, M. (2014). Nematode consumption by mite communities varies in different forest microhabitats as indicated by molecular gut content analysis. Experimental and Applied Acarology, 64, 49–60. 10.1007/s10493-014-9807-x [DOI] [PubMed] [Google Scholar]
- Hobbie, S. E. , Eddy, W. C. , Buyarski, C. R. , Adair, E. C. , Ogdahl, M. L. , & Weisenhorn, P. (2012). Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecological Monographs, 82, 389–405. 10.1890/11-1600.1 [DOI] [Google Scholar]
- Hodge, A. , Campbell, C. D. , & Fitter, A. H. (2001). An arbuscular mycorrhizal fungus accelerates decomposition and achqires nitrogen directly from organic material. Nature, 413, 297–299. [DOI] [PubMed] [Google Scholar]
- Homeier, J. , Breckle, S.‐W. , Günter, S. , Rollenbeck, R. T. , & Leuschner, C. (2010). Tree diversity, forest structure and productivity along altitudinal and topographical gradients in a species‐rich Ecuadorian montane rain forest. Biotropica, 42, 140–148. 10.1111/j.1744-7429.2009.00547.x [DOI] [Google Scholar]
- Homeier, J. , Werner, F. A. , Gradstein, S. R. et al (2008). Potential vegetation and floristic composition of Andean forests in South Ecuador, with a focus on the RBSF. In Beck E., Bendix J., & Kottke I. (Eds.), Gradients in a tropical mountain ecosystem of ecuador (pp. 87–100). Springer. [Google Scholar]
- Hoorens, B. , Aerts, R. , & Stroetenga, M. (2003). Does initial litter chemistry explain litter mixture effects on decomposition? Oecologia, 137, 578–586. 10.1007/s00442-003-1365-6 [DOI] [PubMed] [Google Scholar]
- Hoorens, B. , Coomes, D. , & Aerts, R. (2010). Neighbour identity hardly affects litter‐mixture effects on decomposition rates of New Zealand forest species. Oecologia, 162, 479–489. 10.1007/s00442-009-1454-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva‐Makulec, K. , Olejniczak, I. , & Szanser, M. (2006). Response of soil micro‐ and mesofauna to diversity and quality of plant litter. European Journal of Soil Biology, 42, 244–249. [Google Scholar]
- Illig, J. , Schatz, H. , Scheu, S. , & Maraun, M. (2008). Decomposition and colonization by micro‐arthropods of two litter types in a tropical montane rain forest in southern Ecuador. Journal of Tropical Ecology, 24, 157–167. 10.1017/S0266467407004750 [DOI] [Google Scholar]
- Kaneko, N. , & Salamanca, E. F. (1999). Mixed leaf litter effects on decomposition rates and soil microarthropod communities in an oak‐pine stand in Japan. Ecological Research, 14, 131–138. 10.1046/j.1440-1703.1999.00292.x [DOI] [Google Scholar]
- Kempson, D. , Lloyd, M. , & Ghelardi, R. (1963). A new extractor for woodland litter. Pedobiologia, 3, 1–21. [Google Scholar]
- Klarner, B. , Maraun, M. , & Scheu, S. (2013). Trophic diversity and niche partitioning in a species rich predator guild—Natural variations in stable isotope ratios (13C/12C, 15N/14N) of mesostigmatid mites (Acari, Mesostigmata) from Central European beech forests. Soil Biology & Biochemistry, 57, 327–333. 10.1016/j.soilbio.2012.08.013 [DOI] [Google Scholar]
- Klironomos, J. N. , Widden, P. , & Deslandes, I. (1992). Feeding preferences of the collembolan Folsomia candida in relation to microfungal successions on decaying litter. Soil Biology & Biochemistry, 24, 685–692. 10.1016/0038-0717(92)90047-2 [DOI] [Google Scholar]
- Koehler, H. H. (1997). Mesostigmata (Gamasina, Uropodina), efficient predators in agroecosystems. Agriculture, Ecosystems & Environment, 62, 105–117. 10.1016/S0167-8809(96)01141-3 [DOI] [Google Scholar]
- Korboulewsky, N. , Perez, G. , & Chauvat, M. (2016). How tree diversity affects soil fauna diversity: A review. Soil Biology & Biochemistry, 94, 94–106. 10.1016/j.soilbio.2015.11.024 [DOI] [Google Scholar]
- Krashevska, V. , Maraun, M. , Ruess, L. , & Scheu, S. (2010). Carbon and nutrient limitation of soil microorganisms and microbial grazers in a tropical montane rain forest. Oikos, 119, 1020–1028. [Google Scholar]
- Krashevska, V. , Sandmann, D. , Marian, F. et al (2017). Leaf litter chemistry drives the structure and composition of soil Testate Amoeba communities in a tropical montane rainforest of the Ecuadorian Andes. Microbial Ecology, 74, 681–690. [DOI] [PubMed] [Google Scholar]
- Laganière, J. , Paré, D. , & Bradley, R. L. (2010). How does a tree species influence litter decomposition? Separating the relative contribution of litter quality, litter mixing, and forest floor conditions. Canadian Journal of Forest Research, 40, 465–475. [Google Scholar]
- Macfadyen, A. (1961). Improved funnel‐type extractors for soil arthropods. Journal of Animal Ecology, 30, 171–184. [Google Scholar]
- Makkonen, M. , Berg, M. P. , Handa, I. T. et al (2012). Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecology Letters, 15, 1033–1041. [DOI] [PubMed] [Google Scholar]
- Maraun, M. , Illig, J. , Sandmann, D. et al (2008). Soil Fauna. In Beck E., Bendix J., & Kottke I. (Eds.), Gradients in a tropical mountain ecosystem of Ecuador (pp. 181–192). Springer. [Google Scholar]
- Maraun, M. , Martens, H. , Migge, S. et al (2003). Adding to “the enigma of soil animal diversity”: Fungal feeders and saprophagous soil invertebrates prefer similar food substrates. European Journal of Soil Biology, 39, 85–95. [Google Scholar]
- Marian, F. , Brown, L. , Sandmann, D. et al (2019). Roots, mycorrhizal fungi and altitude as determinants of litter decomposition and soil animal communities in tropical montane rainforests. Plant and Soil, 438, 1–18. [Google Scholar]
- Marian, F. , Sandmann, D. , Krashevska, V. et al (2017). Leaf and root litter decomposition is discontinued at high altitude tropical montane rainforests contributing to carbon sequestration. Ecology and Evolution, 7, 6432–6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marian, F. , Sandmann, D. , Krashevska, V. et al (2018). Altitude and decomposition stage rather than litter origin structure soil microarthropod communities in tropical montane rainforests. Soil Biology & Biochemistry, 125, 263–274. [Google Scholar]
- Migge, S. , Maraun, M. , Scheu, S. , & Schaefer, M. (1998). The oribatid mite community (Acarina) of pure and mixed stands of beech (Fagus sylvatica) and spruce (Picea abies) of different age. Applied Soil Ecology, 9, 115–121. [Google Scholar]
- Moser, G. , Hertel, D. , & Leuschner, C. (2007). Altitudinal change in LAI and stand leaf biomass in tropical montane forests: A transect study in ecuador and a pan‐tropical meta‐analysis. Ecosystems, 10, 924–935. [Google Scholar]
- Myers, N. , Mittermeier, R. , Mittermeier, C. et al (2000). Biodiversity hotspots for conservation priorities. Nature, 403, 853–858. [DOI] [PubMed] [Google Scholar]
- Ndaw, S. M. , Gama‐Rodrigues, A. C. , Gama‐Rodrigues, E. F. et al (2009). Relationships between bacterial diversity, microbial biomass, and litter quality in soils under different plant covers in northern Rio de Janeiro State, Brazil. Canadian Journal of Microbiology, 55, 1089–1095. [DOI] [PubMed] [Google Scholar]
- Patoine, G. , Bruelheide, H. , Haase, J. et al (2020). Tree litter functional diversity and nitrogen concentration enhance litter decomposition via changes in earthworm communities. Ecology and Evolution, 10, 6752–6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsch, A. , Müller‐Hohenstein, K. et al (2008). Bird species distribution along an altitudinal gradient in southern Ecuador and its functional relationships with vegetation structure. In Beck E., Bendix J., & Kottke I. (Eds.), Gradients in a tropical mountain ecosystem of Ecuador (pp. 149–156). Springer‐Verlag. [Google Scholar]
- Pérez Harguindeguy, N. , Blundo, C. M. , Gurvich, D. E. et al (2008). More than the sum of its parts? Assessing litter heterogeneity effects on the decomposition of litter mixtures through leaf chemistry. Plant and Soil, 303, 151–159. [Google Scholar]
- Poll, C. , Marhan, S. , Ingwersen, J. , & Kandeler, E. (2008). Dynamics of litter carbon turnover and microbial abundance in a rye detritusphere. Soil Biology & Biochemistry, 40, 1306–1321. [Google Scholar]
- R Core Team . (2014). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R‐project.org/ [Google Scholar]
- Rinkes, Z. L. , DeForest, J. L. , Grandy, A. S. et al (2014). Interactions between leaf litter quality, particle size, and microbial community during the earliest stage of decay. Biogeochemistry, 117, 153–168. [Google Scholar]
- Rinkes, Z. L. , Weintraub, M. N. , DeForest, J. L. , & Moorhead, D. L. (2011). Microbial substrate preference and community dynamics during decomposition of Acer saccharum. Fungal Ecology, 4, 396–407. [Google Scholar]
- Ristok, C. , Leppert, K. N. , Scherer‐Lorenzen, M. et al (2019). Soil macrofauna and leaf functional traits drive the decomposition of secondary metabolites in leaf litter. Soil Biology & Biochemistry, 135, 429–437. [Google Scholar]
- Ruess, L. , & Lussenhop, J. (2005). Trophic interactions of fungi and animals. In Dighton J., Oudemans P., & White J. (Eds.), The fungal community: Its organization and role in the ecosystem (pp. 581–598). CRC. [Google Scholar]
- Sall, S. N. , Masse, D. , Bernhard‐Reversat, F. et al (2003). Microbial activities during the early stage of laboratory decomposition of tropical leaf litters: The effect of interactions between litter quality and exogenous inorganic nitrogen. Biology and Fertility of Soils, 39, 103–111. [Google Scholar]
- Sánchez‐Galindo, L. M. , Camenzind, T. , Maraun, M. , & Scheu, S. (2019). Impacts of core rotation, defaunation and nitrogen addition on arbuscular mycorrhizal fungi, microorganisms and microarthropods in a tropical montane rainforest. Tropical Ecology, 60, 350–361. [Google Scholar]
- Schädler, M. , & Brandl, R. (2005). Do invertebrate decomposers affect the disappearance rate of litter mixtures? Soil Biology & Biochemistry, 37, 329–337. [Google Scholar]
- Schaefer, M. (2018). Brohmer‐Fauna von Deutschland, 25th ed. Quelle und Meyer. [Google Scholar]
- Scheu, S. (1992). Automated measurement of the respiratory response of soil microcompartments: Active microbial biomass in earthworm faeces. Soil Biology & Biochemistry, 24, 1113–1118. [Google Scholar]
- Scheu, S. , Albers, D. , Alphei, J. et al (2003). The soil fauna community in pure and mixed stands of beech and spruce of different age: Trophic structure and structuring forces. Oikos, 101, 225–238. [Google Scholar]
- Scheu, S. , Ruess, L. , & Bonkowski, M. (2005). Interactions and soil micro‐ and Mesofauna. Microorganisms in Soils: Roles in Genesis and Functions, 3, 253–275. [Google Scholar]
- Schmid, B. , Baruffo, M. , Wang, Z. , & Niklaus, P. A. (2017). A guide to analyzing biodiversity experiments. Journal of Plant Ecology, 10, 91–110. [Google Scholar]
- Schmid, B. , Hector, A. , Huston, M. et al (2002). The design and analysis of biodiversity experiments. In Loreau M., Naeem S., & Inchausti P. (Eds.), Biodiversity and ecosystem functioning: Synthesis and perspectives (pp. 61–78). Oxford University Press. [Google Scholar]
- Schneider, K. , & Maraun, M. (2005). Feeding preferences among dark pigmented fungal taxa (“Dematiacea”) indicate limited trophic niche differentiation of oribatid mites (Oribatida, Acari). Pedobiologia, 49, 61–67. [Google Scholar]
- Schneider, K. , & Maraun, M. (2009). Top‐down control of soil microarthropods ‐ Evidence from a laboratory experiment. Soil Biology & Biochemistry, 41, 170–175. [Google Scholar]
- Seastedt, T. R. (1984). The role of microarthropods in decomposition and mineralization processes. Annual Review of Entomology, 29, 25–46. [Google Scholar]
- Trogisch, S. , He, J. S. , Hector, A. , & Scherer‐Lorenzen, M. (2016). Impact of species diversity, stand age and environmental factors on leaf litter decomposition in subtropical forests in China. Plant and Soil, 400, 337–350. [Google Scholar]
- Vos, V. C. A. , van Ruijven, J. , Berg, M. P. et al (2011). Macro‐detritivore identity drives leaf litter diversity effects. Oikos, 120, 1092–1098. [Google Scholar]
- Wardle, D. A. , Yeates, G. W. , Barker, G. M. , & Bonner, K. I. (2006). The influence of plant litter diversity on decomposer abundance and diversity. Soil Biology & Biochemistry, 38, 1052–1062. [Google Scholar]
- Wullaert, H. , Pohlert, T. , Boy, J. et al (2009). Spatial throughfall heterogeneity in a montane rain forest in Ecuador: Extent, temporal stability and drivers. Journal of Hydrology, 377, 71–79. [Google Scholar]
- Yan, T. , Yang, L. , & Campbell, C. D. (2003). Microbial biomass and metabolic quotient of soils under different land use in the Three Gorges Reservoir area. Geoderma, 115, 129–138. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
All data are available as electronic supplementary material.


