Abstract
Autoimmune responses mediated by autoantibodies have been observed in SARS-CoV-2 infection. Herein, we evaluate the presence of rheumatic, thyroid and phospholipid autoantibodies in sera samples from 120 adult hospitalized patients with COVID-19 in comparison to pre-pandemic samples from 100 healthy individuals. In addition, to estimate the frequency of these autoantibodies in COVID-19, a meta-analysis of selected articles was conducted. Hospitalized patients with COVID-19 had latent autoimmunity characterized by a high frequency of anti-thyroid peroxidase antibodies, rheumatoid factor (RF), anti-cyclic citrullinated peptide third generation antibodies, antinuclear antibodies (ANAs), IgM anti-β2-glycoprotein I (β2GP1) and IgM anti-cardiolipin antibodies. The meta-analysis confirmed our results, with RF and ANAs being the most common autoantibodies. In addition, cluster analysis revealed that those patients with high frequency of RF, IgM anti-β2GP1 antibodies and ANAs had a longer hospital stay, required more vasopressors during hospitalization, and were more likely to develop critical disease. These data suggest that latent autoimmunity influences the severity of COVID-19, and support further post-COVID studies in order to evaluate the development of overt autoimmunity.
Keywords: COVID-19, SARS-CoV-2, Autoimmunity, Latent autoimmunity, Clusters
Highlights
-
•
Hospitalized patients with COVID-19 exhibit latent rheumatic, thyroid and antiphospholipid autoimmunity.
-
•
ANAs, RF, CCP3, antiphospholipid and anti-TPO antibodies are the most common autoantibodies in patients with COVID-19.
-
•
Levels of IgG ACA are associated with critical illness.
-
•
The presence of ANAs, RF, and IgM anti-β2GP1 antibodies is a risk factor for critical disease.
1. Introduction
The natural history of COVID-19, the disease caused by SARS-CoV-2, is beginning to be deciphered thanks to research and scientific collaboration. One of the most intriguing phenomena of COVID-19 is the presence of autoimmunity. Indeed, a) autoimmune diseases (ADs) have been associated with COVID-19, in particular Guillain-Barré syndrome, autoimmune cytopenia, and antiphospholipid syndrome [1]; b) the presence of several autoantibodies has been confirmed [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]], and c) autoantibodies against cytokines [15], and even against angiotensin-converting enzyme 2 (ACE-2) [16], the receptor for SARS-CoV-2, have been observed and associated with severity of disease.
Autoimmunity is a complex trait in which the interaction between hereditary factors and the environment plays an important role. Both are population specific, and influenced by heritability [17]. The heterogenous expression of autoantibodies in COVID-19 suggest a convoluted effect of autoantibodies on the innate and adaptive immune response of infected patients [18]. However, the role of systemic and organ specific autoimmunity in COVID-19 patients from real-world data is still unknown, and studies aimed to evaluate the role of autoantibodies in outcomes such as mortality or hospital length stay are scarce.
In the present study, latent autoimmunity (i.e., presence of autoantibodies without clinical symptoms or fulfillment of classification criteria for AD) was evaluated in a group of hospitalized patients with COVID-19 and a meta-analysis of similar studies published to date was undertaken. Our results indicate for the first time the presence of latent rheumatic and thyroid autoimmunity in hospitalized patients with COVID-19, and their association with severity of disease.
2. Methods
2.1. Study population
Patients were selected using a non-probabilistic sampling (i.e., convenience selection), from Clínica del Occidente and Hospital Universitario Mayor Méderi, in Bogota, Colombia. Hospitalized patients with COVID-19 confirmed by RT-PCR, and without evidence of overt autoimmunity were included (n: 120). As control group, 100 healthy subjects with samples collected 4 years prior the beginning of the pandemic and followed at the Center for Autoimmune Diseases Research (CREA) in Bogota, Colombia, were also involved. This was a low-risk study according to the resolution 8430 of 1993 from the Ministry of Health of Colombia.
2.2. Clinical outcomes
Medical records were reviewed using a questionnaire that sought information about demographic, clinical and immunological characteristics, including age, date of onset, symptoms at onset, comorbidities, oxygen supplementation during hospitalization (i.e., nasal cannula, high-flow nasal cannula, non-rebreather mask, or mechanical ventilation (MV)), pharmacological treatment, time to event since hospitalization (i.e., mortality, intensive care unit (ICU) admission and MV requirement). Hematological (i.e., lymphopenia, leucopenia, thrombocytopenia), renal (i.e., acute renal injury), infectious (i.e., sepsis, bacterial pneumonia), or thromboembolic (i.e., pulmonary embolism, deep vein thrombosis) events were also registered.
2.3. Autoantibodies
A panel of autoantibodies was evaluated in the sera of cases and pre-pandemic controls. Samples from patients treated with convalescent plasma, were analyzed prior transfusion. Detection of IgM rheumatoid factor (RF), IgG anti-cyclic citrullinated peptide third generation (CCP3), IgM and IgG anti-cardiolipin antibodies (ACAs), IgM and IgG anti-β2 glycoprotein-1 (β2GP1) antibodies, IgG anti-double-stranded DNA (dsDNA) antibodies, IgG anti-thyroglobulin (Tg) antibodies and anti-thyroid peroxidase (TPO) antibodies were all quantified by enzyme-linked-immunosorbent assay (ELISA), as previously reported in detail [19,20]. In addition, antinuclear antibodies (ANAs) were evaluated by using an indirect immunofluorescence assay. Positive results were considered from dilution 1/80. In case of ANA positivity, anti-SSA/Ro, anti-SSB/La, anti-ribonucleoprotein (RNP) and anti-smith (Sm) antibodies were further evaluated by a commercial ELISA. All the assay kits were from Inova Diagnostics, Inc (San Diego, CA, USA).
2.4. Statistical analysis
Univariate descriptive statistics were performed. Categorical variables were analyzed using frequencies, and quantitative continuous variables were expressed as the mean and standard deviation (SD) or the median and interquartile range (IQR). The Kruskal-Wallis, Mann–Whitney U test, or Fisher’s exact tests were used based on the results. Next, we tested the association between antibody levels and critical disease (i.e., MV or died) using multivariable logistic regression. To account for confounding factors, we included age and sex in the regression analysis. We then used a marginal probability analysis to graphically display mortality risk at a range of antibody levels.
To summarize the diverse information of frequencies of autoantibodies in COVID-19, a meta-analysis approach for selected articles was employed. The logit transformed proportion was used to derive the weighted proportion. The overall pooled prevalence and 95% confidence intervals (CIs) were obtained using a random effect model for latent autoantibodies. Statistical heterogeneity between studies was evaluated by Cochran’s Q-statistic, as well as Tau2 and I2 statistics. A P value > 0.10 in Q-statistics or <50% in I2 statistic indicated a lack of heterogeneity [21].
To determine clusters of patients with COVID-19 disclosing similar characteristics based on autoantibodies positivity, we used the mixed-cluster methodology proposed by Lebart et al. [22]. Briefly, a multiple correspondence analysis was done to obtain the representation of data based on principal components. Next, the number of clusters by a hierarchical cluster analysis was determined. Finally, a consolidation step by k-means clustering was performed. Autoantibodies with frequencies <5% were excluded since these variables with low frequencies tend to generate clusters that include only those atypical values. Then, to evaluate the clinical relevance of clusters obtained, the risk for critical disease (i.e., MV or died) was tested using a multivariable logistic regression adjusted for age and sex. A P value of <0.05 was set as significant for all type of comparisons. All analyses were done using R version 4.0.1.
3. Results
3.1. Study population
General characteristics of patients are shown in Table 1. Most of patients were men (n:85, 70.8%), with a median age of 57.5 years. The most common symptoms at onset were dyspnea, fever, malaise and fatigue, and dry cough (Table 1). Myalgias and arthralgias were present in 36.7% and 30% of patients, respectively. Other symptoms such as chest pain, diarrhea, anosmia and dysgeusia were exhibited in <30% of patients. There was no evidence of overt AD among patients. Levels of thyroid-stimulating hormone (TSH) were within the normal range. The most common comorbidities were hypertension (35.8%), type 2 diabetes mellitus (28.6%), and obesity (24.2%).
Table 1.
General characteristics of 120 hospitalized patients with COVID-19.
| Variable (%) | COVID-19 (n: 120) |
|---|---|
| Gender | |
| Female | 35 (29.2) |
| Male | 85 (70.8) |
| Age (Median – IQR) | 57.5 (51.8–66.3) |
| Symptoms on admission | |
| Fever | 95 (79.2) |
| Hemoptysis | 3 (2.5) |
| Dry cough | 91 (75.8) |
| Sore throat | 27 (22.5) |
| Anosmia | 20 (16.7) |
| Dysgeusia | 19 (15.8) |
| Rhinorrhea | 14 (11.7) |
| Wheezing | 6 (5.0) |
| Chest pain | 33 (27.5) |
| Myalgia | 44 (36.7) |
| Arthralgias | 36 (30.0) |
| Fatigue and malaise | 94 (78.3) |
| Dyspnea | 99 (82.5) |
| Inability to walk | 11 (9.2) |
| Lower chest wall indrawing | 6 (5.0) |
| Headache | 41 (34.2) |
| Seizures | 2 (1.7) |
| Abdominal pain | 8 (6.7) |
| Nausea/vomiting | 15 (12.5) |
| Diarrhea | 21 (17.5) |
| Bleeding | 2 (1.7) |
| Comorbidities | |
| Hypertension | 43 (35.8) |
| Thromboembolic disease | 2 (1.7) |
| Dyslipidemia | 14 (11.7) |
| COPD | 3 (2.5) |
| Asthma | 0 (0.0) |
| Chronic kidney disease | 9 (7.5) |
| Chronic liver disease | 0 (0.0) |
| Stroke | 3 (2.5) |
| Acid peptic disease | 6 (5.0) |
| Osteoporosis | 0 (0.0) |
| Hepatitis C | 0 (0.0) |
| Hepatitis B | 0 (0.0) |
| HIV | 1/119 (0.8) |
| Tuberculosis | 0/119 (0.0) |
| Diabetes | 34/119 (28.6) |
| Cancer | 1/119 (0.8) |
| Obesity | 29 (24.2) |
| Hypothyroidisma | 10 (8.3) |
| Current smoker | 3 (2.5) |
| Former smoker | 13 (10.8) |
| Pharmacological therapy on admission | |
| ACE inhibitors | 9 (7.5) |
| ARB II | 34 (28.3) |
| Corticosteroids | 108 (90.0) |
| Antibiotics | 117 (97.5) |
| NSAIDs | 40 (33.3) |
| Bronchodilators | 60 (50.0) |
| Anticoagulants | 114 (95.0) |
| Antimalarials | 6 (5.0) |
| Antivirals | 2 (1.7) |
| Oxygen therapy during hospitalization | |
| Pronation | 81 (67.5) |
| Nasal cannula | 100 (83.3) |
| Non-rebreather mask | 77 (64.2) |
| High-flow nasal cannula | 17 (14.2) |
| Interventions during hospitalization | |
| Dialysis | 13 (10.8) |
| ICU admission | 73 (60.8) |
| MV | 63 (52.5) |
| Vasopressors | 57 (47.5) |
| Outcomes during hospitalization | |
| Renal | 49 (40.8) |
| Infectious | 28 (23.3) |
| Hematological | 82 (68.3) |
| Thromboembolic | 8 (6.7) |
| Severe disease | 56 (46.7) |
| Critical disease (MV or death) | 66 (55.0) |
| Death | 44 (36.7) |
ACE: Angiotensin-converting enzyme; ARB II: Angiotensin receptor blockers 2; COPD: Chronic pulmonary obstructive disease; HIV: Human immunodeficiency virus; ICU: Intensive care unit; IQR: Interquartile range; MV: Mechanical ventilation; NSAIDs: Nonsteroidal anti-inflammatory drugs.
Not autoimmune
During hospitalization, all patients received supplementary oxygen, by nasal cannula (83.3%), non-rebreather mask (64.2%), MV (52.5%), or high-flow nasal cannula (14.2%). Most of the patients received antibiotics (97.5%), anticoagulation with heparins (95%), and corticosteroids (90%). Almost half of the patients required management with vasopressors (47.5%). Nine patients were treated with convalescent plasma. The most common complications during hospitalization were hematological (68.3%), and renal (40.8%). The 60.8% of patients required ICU admission, and 36.7% deceased.
3.2. Latent autoimmunity
Frequencies of autoantibodies in COVID-19 patients are shown in Table 2. Thyroid autoimmunity, given by anti-TPO antibodies, was most frequent in COVID-19 than in pre-pandemic controls. These patients showed high positivity for RF and CCP3 antibodies. Infected patients exhibited higher frequency of IgM ACA and IgM anti-β2GP1 antibodies. COVID-19 patients showed a lower frequency of ANAs than pre-pandemic controls.
Table 2.
Autoantibodies in hospitalized patients with COVID-19 and pre-pandemic controls.
| Autoantibody (%) | COVID-19 (n: 120) | Pre-Pandemic Controls (n: 100) | P valuea |
|---|---|---|---|
| TPO | 44 (36.7) | 20 (20.0) | 0.0074 |
| Tg | 2 (1.7) | 3 (3.0) | 0.6611 |
| β2GP1 IgG | 0 (0.0) | 3 (3.0) | 0.0924 |
| β2GP1 IgM | 17 (14.2) | 1 (1.0) | 0.0003 |
| ACA IgG | 2 (1.7) | 5 (5.0) | 0.2495 |
| ACA IgM | 22 (18.3) | 5 (5.0) | 0.0033 |
| ANAs | 14 (11.7) | 25 (25.0) | 0.0127 |
| RNP | 0/14 (0.0) | 1/25 (4.0) | 1.0000 |
| Sm | 2/14 (14.3) | 1/25 (4.0) | 0.2888 |
| SSB/La | 0/14 (0.0) | 1/25 (4.0) | 1.0000 |
| SSA/Ro | 0/14 (0.0) | 2/25 (8.0) | 0.5277 |
| dsDNA | 0 (0.0) | 0 (0.0) | – |
| RF | 31 (25.8) | 14 (14.0) | 0.0432 |
| CCP3 | 7 (5.8) | 0 (0.0) | 0.0168 |
P values were obtained by Fisher’s exact test. TPO: Thyroid peroxidase ; Tg: Thyroglobulin, β2GP1: β2-Glycoprotein 1; ACA: Anti-cardiolipin antibody; ANAs: Antinuclear antibodies; RNP: Ribonucleoprotein, Sm: Smith; dsDNA: Double-stranded DNA; RF: Rheumatoid factor; CCP3: Cyclic citrullinated peptide third generation.
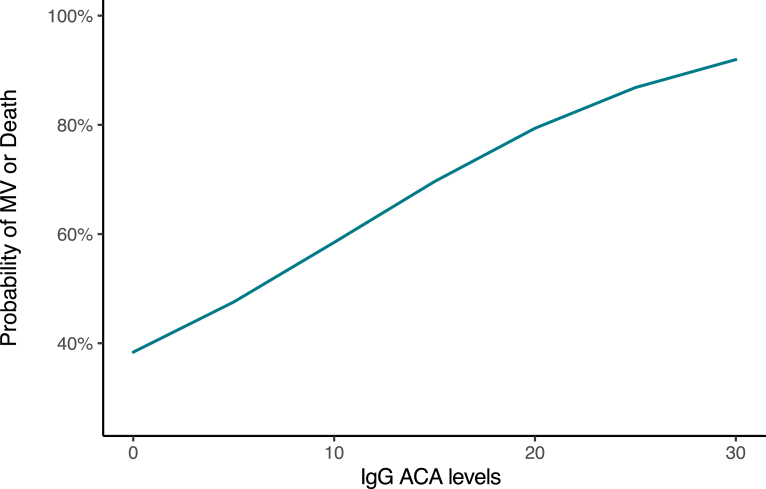
In addition, a concentration-dependent effect of IgG ACAs on the probability of critically ill disease (i.e., MV or died) in hospitalized patients with COVID-19 was observed (OR, 1.13; 95% CI, 1.01 to 1.25, P = 0.0439). High titers of these autoantibodies were associated with a higher probability for this outcome (Fig. 1). None of the other autoantibodies evaluated exhibited association with this outcome (Data not shown).
Fig. 1.
Marginal probabilities for critical disease based on IgG ACA levels. MV: Mechanical ventilation; ACA: Anti-cardiolipin antibody.
In order to estimate the prevalence of latent autoantibodies in patients with COVID-19 a meta-analysis of selected articles reporting frequencies of autoantibodies in hospitalized patients with COVID-19 was conducted [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]]. The detailed description of these manuscripts is shown in Supplementary Appendix 1. This analysis disclosed a heterogeneous autoimmune phenomenon (i.e., latent autoimmunity). ANAs and RF were the most common, whereas other autoantibodies exhibited frequencies lower than 11% (Table 3).
Table 3.
Latent autoimmunity in COVID-19 (Meta-analysis).
| Autoantibody | Number of articlesa | Cases/COVID-19b(%, 95% CI) | Q (Tau2, I2) |
|---|---|---|---|
| β2GP1 IgG | 12 | 43/848 (5.40, 3.53–8.18) | <0.01 (0.17, 32.79% |
| ACA IgG | 12 | 73/848 (8.63, 4.29–16.75) | <0.01 (1.37, 86.25%) |
| ACA IgM | 11 | 98/817 (10.56, 6.82–16) | <0.01 (0.42, 73.12%) |
| β2GP1 IgM | 11 | 69/817 (7.69, 5.21–11.21) | 0.08 (0.2, 47.53%) |
| ANAs | 8 | 109/390 (32.11, 15.6–54.71) | <0.01 (1.66, 92.47%) |
| RNP | 6 | 2/306 (1.87, 0.74–4.61) | 0.87 (0, 0%) |
| Sm | 6 | 2/306 (1.52, 0.60–3.8) | 1 (0, 0%) |
| dsDNA | 6 | 2/297 (1.92, 0.76–4.74) | 0.88 (0, 0%) |
| SSB/La | 6 | 1/306 (1.32, 0.46–3.71) | 0.97 (0, 0%) |
| SSA/Ro | 5 | 0/286 (0.99, 0.29–3.36) | 0.96 (0, 0%) |
| MPO | 4 | 2/164 (3.14, 1.1–8.65) | 0.39 (0, 0%) |
| Proteinase 3 | 4 | 5/164 (4.43, 1.82–10.40) | 0.45 (0.09,9.79%) |
| ANCA | 3 | 10/137 (4.9, 0.56–31.91) | <0.01 (2.64, 70.47%) |
| RF | 3 | 43/171 (19.9, 3.64–61.96) | 0.06 (2.26, 93.09%) |
| Ro 52 | 3 | 4/82 (6.38, 1.25–26.74) | 0.06 (1.07, 46.56%) |
| Ro 60 | 3 | 5/82 (6.6, 1.04–32.04) | 0.03 (1.65, 57.76%) |
| CCP | 2 | 8/149 (5.46, 2.75–10.54) | 0.61 (0, 0%) |
| TPO | 1 | 44/120 (36.67, 28.54–45.63) | NA |
| Tg | 1 | 2/120 (1.66, 0.42–6.42) | NA |
Tau2 is the variance of the effect size parameters across the population of studies and it reflects the variance of the true effect size (i.e., heterogeneity among studies). I2 refers to the percentage of heterogenetic among the included studies. β2GP1: β2-Glycoprotein 1; ACA: Anti-cardiolipin antibody; ANAs: Antinuclear antibodies; RNP: Ribonucleoprotein ; Sm: Smith; dsDNA: Double-stranded DNA; MPO: Myeloperoxidase; ANCA: Anti-neutrophil cytoplasmic antibody; RF: Rheumatoid factor; CCP: Cyclic citrullinated peptide; TPO: Thyroid peroxidase; Tg: Thyroglobulin; CI: Confidence interval; NA: Not applicable/available; Estimation was done assuming a random effects model.
Results of this study were included in the global analysis. Results were ordered according to the number of articles included in each meta-analysis.
COVID-19 represents the total of patients reported in selected articles.
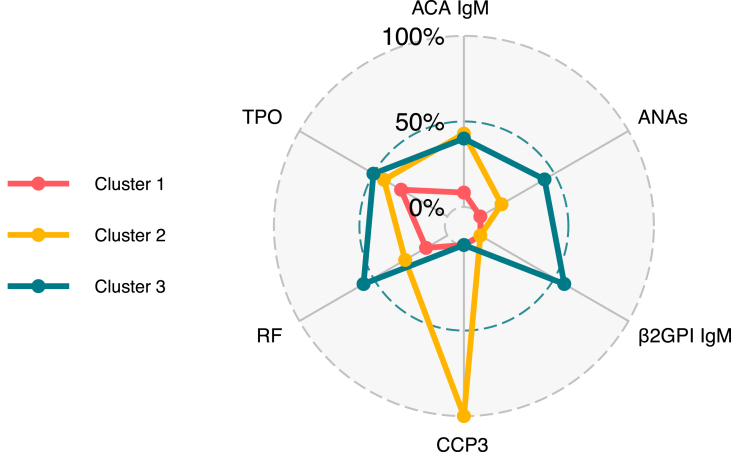
3.3. Latent autoimmune clusters
General characteristics of clusters are shown in Table 4. Three main clusters were observed. The first cluster was characterized by a low frequency of autoantibodies and included 83 patients (Fig. 2). The second cluster included 7 patients with high positivity for anti-CCP3 (P = 0.0001), and the third cluster comprised 30 patients with high positivity for multiple autoantibodies including RF (56.7%, P = 0.0001), IgM anti-β2GP1 antibodies (56.7%, P = 0.0001), and ANAs (43.3%%, P = 0.0002). Patients from the third cluster frequently required management with vasopressors during hospitalization (63.3%, P = 0.0457), had prolonged hospital stay (Kruskal-Wallis test, P = 0.0314), and were more likely to develop a critical disease (AOR, 2.75; 95% CI, 1.08 to 7.02; P = 0. 0339) (Table 5).
Table 4.
Cluster analysis of latent autoimmunity in hospitalized patients with COVID-19.
| Variable (%) | Cluster 1 (n: 83) | Cluster 2 (n: 7) | Cluster 3 (n: 30) | P valuea |
|---|---|---|---|---|
| Gender | 0.3422 | |||
| Female | 21 (25.3) | 2 (28.6) | 12 (40.0) | |
| Male | 62 (74.7) | 5 (71.4) | 18 (60.0) | |
| Age (median – IQR) | 57 (50–66) | 58 (53.5–70) | 58 (53.25–67) | 0.7084 |
| Symptoms on admission | ||||
| Fever | 67 (80.7) | 5 (71.4) | 23 (76.7) | 0.6806 |
| Hemoptysis | 1 (1.2) | 0 (0.0) | 2 (6.7) | 0.3093 |
| Dry cough | 64 (77.1) | 4 (57.1) | 23 (76.7) | 0.4974 |
| Sore throat | 16 (19.3) | 3 (42.9) | 8 (26.7) | 0.2228 |
| Anosmia | 15 (18.1) | 0 (0.0) | 5 (16.7) | 0.7566 |
| Dysgeusia | 15 (18.1) | 0 (0.0) | 4 (13.3) | 0.6297 |
| Rhinorrhea | 8 (9.6) | 0 (0.0) | 6 (20.0) | 0.2981 |
| Wheezing | 3 (3.6) | 0 (0.0) | 3 (10.0) | 0.3337 |
| Chest pain | 24 (28.9) | 1 (14.3) | 8 (26.7) | 0.8278 |
| Myalgia | 34 (41.0) | 0 (0.0) | 10 (33.3) | 0.0882 |
| Arthralgias | 24 (28.9) | 0 (0.0) | 12 (40.0) | 0.1087 |
| Fatigue and malaise | 67 (80.7) | 5 (71.4) | 22 (73.3) | 0.5840 |
| Dyspnea | 70 (84.3) | 6 (85.7) | 23 (76.7) | 0.7080 |
| Inability to walk | 8 (9.6) | 1 (14.3) | 2 (6.7) | 0.7388 |
| Lower chest wall indrawing | 4 (4.8) | 0 (0.0) | 2 (6.7) | 0.7615 |
| Headache | 29 (34.9) | 1 (14.3) | 11 (36.7) | 0.6701 |
| Seizures | 0 (0.0) | 0 (0.0) | 2 (6.7) | 0.0933 |
| Abdominal pain | 7 (8.4) | 0 (0.0) | 1 (3.3) | 0.8046 |
| Nausea/vomiting | 13 (15.7) | 0 (0.0) | 2 (6.7) | 0.4228 |
| Diarrhea | 17 (20.5) | 0 (0.0) | 4 (13.3) | 0.4121 |
| Bleeding | 0 (0.0) | 0 (0.0) | 2 (6.7) | 0.0933 |
| Comorbidities | ||||
| Hypertension | 32 (38.6) | 2 (28.6) | 9 (30.0) | 0.6765 |
| Thromboembolic disease | 2 (2.4) | 0 (0.0) | 0 (0.0) | 1.0000 |
| Dyslipidemia | 14 (16.9) | 0 (0.0) | 0 (0.0) | 0.0274 |
| COPD | 2 (2.4) | 0 (0.0) | 1 (3.3) | 1.0000 |
| Asthma | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Chronic kidney disease | 8 (9.6) | 0 (0.0) | 1 (3.3) | 0.6807 |
| Chronic liver disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Stroke | 2 (2.4) | 0 (0.0) | 1 (3.3) | 1.0000 |
| Acid peptic disease | 6 (7.2) | 0 (0.0) | 0 (0.0) | 0.4371 |
| Osteoporosis | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Hepatitis C | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Hepatitis B | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| HIV | 1/82 (1.2) | 0 (0.0) | 0 (0.0) | 1.0000 |
| Tuberculosis | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Diabetes | 25/82 (30.5) | 1 (14.3) | 8 (26.7) | 0.7360 |
| Cancer | 1/82 (1.2) | 0 (0.0) | 0 (0.0) | 1.0000 |
| Obesity | 20 (24.1) | 1 (14.3) | 8 (26.7) | 0.8750 |
| Hypothyroidism | 8 (9.6) | 0 (0.0) | 2 (6.7) | 1.0000 |
| Current smoker | 2 (2.4) | 0 (0.0) | 1 (3.3) | 1.0000 |
| Former smoker | 12 (14.5) | 0 (0.0) | 1 (3.3) | 0.2038 |
| Pharmacological therapy on admission | ||||
| ACE inhibitors | 6 (7.2) | 0 (0.0) | 3 (10.0) | 0.8273 |
| ARB II | 24 (28.9) | 4 (57.1) | 6 (20.0) | 0.1342 |
| Corticosteroids | 75 (90.4) | 6 (85.7) | 27 (90.0) | 0.8734 |
| Antibiotics | 82 (98.8) | 7 (100.0) | 28 (93.3) | 0.3093 |
| NSAIDs | 26 (31.3) | 2 (28.6) | 12 (40.0) | 0.7105 |
| Bronchodilators | 35 (42.2) | 6 (85.7) | 19 (63.3) | 0.0248 |
| Anticoagulants | 78 (94.0) | 7 (100.0) | 29 (96.7) | 1.0000 |
| Antimalarials | 3 (3.6) | 0 (0.0) | 3 (10.0) | 0.3337 |
| Antivirals | 1 (1.2) | 0 (0.0) | 1 (3.3) | 0.5234 |
| Oxygen therapy during hospitalization | ||||
| Pronation | 54 (65.1) | 5 (71.4) | 22 (73.3) | 0.7501 |
| Nasal cannula | 69 (83.1) | 5 (71.4) | 26 (86.7) | 0.5907 |
| Non-rebreather mask | 51 (61.4) | 6 (85.7) | 20 (66.7) | 0.5193 |
| High-flow nasal cannula | 13 (15.7) | 0 (0.0) | 4 (13.3) | 0.7382 |
| Interventions during hospitalization | ||||
| Dialysis | 10 (12.0) | 0 (0.0) | 3 (10.0) | 1.0000 |
| ICU admission | 49 (59.0) | 3 (42.9) | 21 (70.0) | 0.3412 |
| MV | 40 (48.2) | 3 (42.9) | 20 (66.7) | 0.2006 |
| Vasopressors | 37 (44.6) | 1 (14.3) | 19 (63.3) | 0.0457 |
| Outcomes during hospitalization | ||||
| Renal | 33 (39.8) | 4 (57.1) | 12 (40.0) | 0.6590 |
| Infectious | 19 (22.9) | 2 (28.6) | 7 (23.3) | 0.9339 |
| Hematological | 56 (67.5) | 3 (42.9) | 23 (76.7) | 0.2226 |
| Thromboembolic | 8 (9.6) | 0 (0.0) | 0 (0.0) | 0.1944 |
| Severe disease | 44 (53.0) | 3 (42.9) | 9 (30.0) | 0.0879 |
| Critically ill disease (MV or death) | 41 (49.4) | 4 (57.1) | 21 (70.0) | 0.1409 |
| Death | 31 (37.3) | 2 (28.6) | 11 (36.7) | 1.0000 |
| Days of hospital stay (median – IQR) | 13 (8–21) | 8 (6–13) | 17 (13–27.8) | 0.0314 |
| Days of ICU management (median – IQR) | 14 (9–19) | 8 (8–10) | 13 (10–20) | 0.3204 |
| Days on MV (median – IQR) | 13 (8.8–19.3) | 9 (6.5–9) | 13 (8.8–20.3) | 0.2281 |
Quantitative variables were analyzed by Kruskal-Wallis test. ACE: Angiotensin-converting enzyme; ARB II: Angiotensin receptor blockers 2; COPD: Chronic pulmonary obstructive disease; HIV: Human immunodeficiency virus; ICU: Intensive care unit; IQR: Interquartile range; MV: Mechanical ventilation; NSAIDs: Nonsteroidal anti-inflammatory drugs.
P values for categorical variables were obtained by Fisher’s exact test.
Fig. 2.
Radar plots of frequency of autoantibodies by cluster. ACA: Anti-cardiolipin antibodies; ANAs: Anti nuclear antibodies; β2GP1: β2-Glycoprotein 1; CCP3: anti-cyclic citrullinated peptide third generation; RF: Rheumatoid factor; TPO: Thyroid peroxidase antibodies.
Table 5.
Latent autoimmune clusters and critical COVID-19 (multivariate analysis).
| Variable | AOR | 95% CI | P value |
|---|---|---|---|
| Clustera | |||
| 2 | 1.19 | 0.23 to 6.21 | 0.8333 |
| 3 | 2.75 | 1.08 to 7.02 | 0.0339 |
| Age | 1.03 | 1.0 to 1.06 | 0.0326 |
| Sex (Male) | 2.11 | 0.9 to 4.95 | 0.0879 |
Cluster 1 was set as reference. AOR: Adjusted odd ratio; CI: Confidence interval.
4. Discussion
In this study, hospitalized patients with COVID-19 exhibited latent rheumatic, thyroid and antiphospholipid autoimmunity. Antiphospholipid, ANAs, RF, anti-CCP3 and anti-TPO antibodies were the most common autoantibodies, and levels of IgG ACA were associated with MV or mortality. Cluster analysis revealed that patients with ANAs, RF, and IgM anti-β2GP1 antibodies together were more prone to develop critical disease. The temporal association of autoimmunity and COVID-19 suggests that SARS-CoV-2 may be a trigger for autoimmunity.
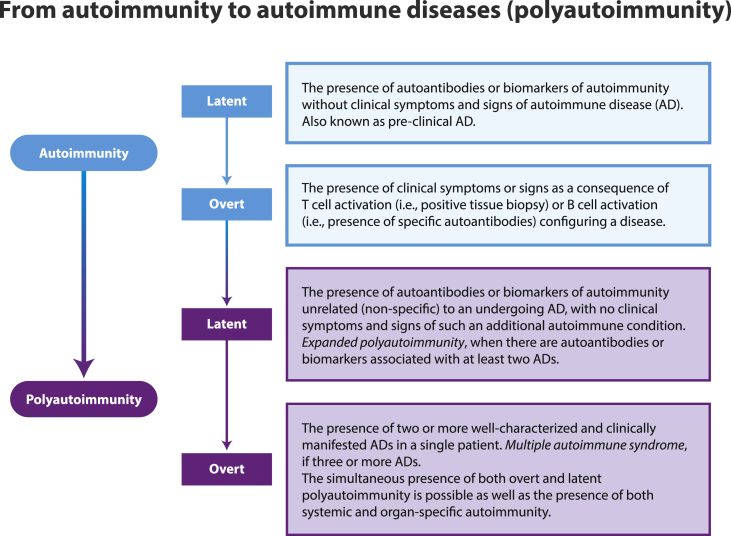
Autoimmunity is a continuum spectrum phenomenon ranging from latent autoimmunity (i.e., pre-clinical disease) to overt ADs (Fig. 3) [21,[23], [24], [25], [26]]. Little is known about the precise frequency of autoantibodies in patients with COVID-19, and their influence on clinical outcomes. Several reports have shown diverse prevalence of autoantibodies. The most reported are the antiphospholipid antibodies and ANAs [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]]. Herein, we found that adult hospitalized patients with COVID-19 exhibited higher frequency of latent phospholipid, rheumatic and thyroid autoimmunity when compared with pre-pandemic controls. In addition, meta-analysis of selected articles showed that ANAs and RF were the most common autoantibodies, followed by antiphospholipid antibodies. This suggest that COVID-19 is characterized by an autoimmune phenomenon that may influence inflammatory response. Longitudinal analysis of recovered patients will be critical to understand the persistence of these autoreactivities and the role of latencies in the development of overt ADs (Fig. 3). This is of paramount importance to personalized medicine, early pharmacological therapeutics and to establish effective long-term care protocols.
Fig. 3.
Continuum spectrum of autoimmunity. From latent to overt autoimmune diseases. AD: autoimmune disease.
Latent autoimmunity and its association with clinical outcomes in COVID-19 have been mainly associated with antiphospholipid [1], ACE-2 [16], and IFN-α [15] autoantibodies. However, recent evidence suggests that patients with COVID-19 may present other autoantibodies that influence the progression of the disease. In the study of Wang et al. [18], in addition to IFN-α autoantibodies, patients with COVID-19 exhibited a convoluted immune response secondary to the increase of antibodies against lymphocytes or the central nervous system. In our study, we demonstrated that clustering of autoantibodies allowed the recognition of patients with worse prognosis. Patients with positivity for ANAs, RF, and IgM anti-β2GP1 antibodies were more likely to develop critically ill disease, having longer clinical stay, and requiring more vasopressors during hospitalization.
Our results are in line with those of Woodruff et al. [14], who found that patients with high levels of C reactive protein (CRP) exhibited high positivity for ANAs and RF. Other studies found similar results in which ANAs and antiphospholipid antibodies were associated with mortality [14] or thromboembolic manifestations [4,11]. This is of critical relevance since clinical testing for ANAs, RF, and IgM anti-β2GP1 may allow the identification of patients that will develop deleterious outcomes during hospitalization. Altogether, data indicate that the miscellaneous clinical presentation of patients with COVID-19 is influenced by multiple pathways of autoimmunity and support the use of immunomodulatory therapies in hospitalized patients, which have been recently confirmed as modifiers of disease [27]. It would be of interest to evaluate whether early administration of immunomodulatory therapies based on antiantibodies profiling may help to lessen the inflammatory response and impact the adverse outcomes in this condition.
Several biomarkers have been tested for their reliability in clinical settings for patients with COVID-19. The CRP, IL-6, ferritin and D-Dimer have been associated with deleterious outcomes [[28], [29], [30], [31]]. However, other immunological parameters have emerged as potential biomarkers for monitoring the disease. In the study of Zuo et al. [13], levels of antiphospholipid antibodies were associated with neutrophil hyperactivity (i.e., including the release of neutrophil extracellular traps), higher platelet count, more severe respiratory disease and lower glomerular filtration rate. In other study, anti-Annexin A2 antibody levels were associated with mortality [32]. In our study, it was found that IgG ACA levels were associated with prediction of critical disease, suggesting that levels of antiphospholipid antibodies may help monitoring the disease and guide the treatment (e.g., appropriate anticoagulation and immunosuppresive regimens).
Our study has several strengths. We included hospitalized patients with COVID-19 that did not have prior history of autoimmunity, and those patients with overt autoimmunity were excluded from the study. This guaranteed that evaluation of latency was accurate allowing a precise estimation of the real clinical effect of latencies in COVID-19 from real-world data. In addition, loss of data was lower than 1%.
Limitations must be also acknowledged. This was a retrospective study that could have been susceptible for selection bias. However, grouping for this study was researcher independent given by the unsupervised machine learning approach implemented. It is highly unlikely that our results might be influenced by chance alone or the moderate sample size.
5. Conclusions
Latent autoimmunity is common in adult hospitalized patients with COVID-19. Follow-up of patients with latent autoimmunity may clarify the role of autoantibodies in post-COVID disease, or the development of overt autoimmunity. Latent autoimmunity is useful to classify patients that may develop a critical disease. IgG ACA should be considered in monitoring the disease.
Funding
The CP-COVID-19 group was supported by grants from Universidad del Rosario (ABN011), Bogota, and Grupo ISA, Medellin, Colombia.
Role of the funder/sponsor
The funders had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank all the members of the CP-COVID-19 group for their contributions and fruitful discussions during the preparation of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtauto.2021.100091.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rodríguez Y., Novelli L., Rojas M., De Santis M., Acosta-Ampudia Y., Monsalve D.M., Ramírez-Santana C., Costanzo A., Ridgway W.M., Ansari A.A., Gershwin M.E., Selmi C., Anaya J.-M. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J. Autoimmun. 2020;114:102506. doi: 10.1016/j.jaut.2020.102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou Y., Han T., Chen J., Hou C., Hua L., He S., Guo Y., Zhang S., Wang Y., Yuan J., Zhao C., Zhang J., Jia Q., Zuo X., Li J., Wang L., Cao Q., Jia E. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin. Transl. Sci. 2020;13:1077–1086. doi: 10.1111/cts.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertin D., Brodovitch A., Beziane A., Hug S., Bouamri A., Mege J.L., Heim X., Bardin N. Anticardiolipin IgG autoantibody level is an independent risk factor for COVID-19 severity. Arthritis Rheum. 2020;72:1953–1955. doi: 10.1002/art.41409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiaffino M.T., Di Natale M., García-Martínez E., Navarro J., Muñoz-Blanco J.L., Demelo-Rodríguez P., Sánchez-Mateos P. Immunoserologic detection and diagnostic relevance of cross-reactive autoantibodies in coronavirus disease 2019 patients. J. Infect. Dis. 2020;222:1439–1443. doi: 10.1093/infdis/jiaa485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borghi M.O., Beltagy A., Garrafa E., Curreli D., Cecchini G., Bodio C., Grossi C., Blengino S., Tincani A., Franceschini F., Andreoli L., Lazzaroni M.G., Piantoni S., Masneri S., Crisafulli F., Brugnoni D., Muiesan M.L., Salvetti M., Parati G., Torresani E., Mahler M., Heilbron F., Pregnolato F., Pengo M., Tedesco F., Pozzi N., Meroni P.L. Anti-phospholipid antibodies in COVID-19 are different from those detectable in the anti-phospholipid syndrome. Front. Immunol. 2020;11:2692. doi: 10.3389/fimmu.2020.584241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lerma L.A., Chaudhary A., Bryan A., Morishima C., Wener M.H., Fink S.L. Prevalence of autoantibody responses in acute coronavirus disease 2019 (COVID-19) J. Transl. Autoimmun. 2020;3:100073. doi: 10.1016/j.jtauto.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacchi M.C., Tamiazzo S., Stobbione P., Agatea L., De Gaspari P., Stecca A., Lauritano E.C., Roveta A., Tozzoli R., Guaschino R., Bonometti R. SARS-CoV-2 infection as a trigger of autoimmune response. Clin. Transl. Sci. n/a. 2021 doi: 10.1111/cts.12953. cts.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pascolini S., Vannini A., Deleonardi G., Ciordinik M., Sensoli A., Carletti I., Veronesi L., Ricci C., Pronesti A., Mazzanti L., Grondona A., Silvestri T., Zanuso S., Mazzolini M., Lalanne C., Quarneti C., Fusconi M., Giostra F., Granito A., Muratori L., Lenzi M., Muratori P. COVID-19 and immunological dysregulation: can autoantibodies be useful? Clin. Transl. Sci. 2021 doi: 10.1111/cts.12908. cts.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatto M., Perricone C., Tonello M., Bistoni O., Cattelan A.M., Bursi R., Cafaro G., De Robertis E., Mencacci A., Bozza S., Vianello A., Iaccarino L., Gerli R., Doria A., Bartoloni E. Frequency and clinical correlates of antiphospholipid antibodies arising in patients with SARS-CoV-2 infection: findings from a multicentre study on 122 cases. Clin. Exp. Rheumatol. 2020;38:754–759. [PubMed] [Google Scholar]
- 10.Vlachoyiannopoulos P.G., Magira E., Alexopoulos H., Jahaj E., Theophilopoulou K., Kotanidou A., Tzioufas A.G. Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann. Rheum. Dis. 2020;79:1661–1663. doi: 10.1136/annrheumdis-2020-218009. [DOI] [PubMed] [Google Scholar]
- 11.Amezcua-Guerra L.M., Rojas-Velasco G., Brianza-Padilla M., Vázquez-Rangel A., Márquez-Velasco R., Baranda-Tovar F., Springall R., Gonzalez-Pacheco H., Juárez-Vicuña Y., Tavera-Alonso C., Sanchez-Muñoz F., Hernández-Salas M. Presence of antiphospholipid antibodies in COVID-19: case series study. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-218100. annrheumdis-2020-218100. [DOI] [PubMed] [Google Scholar]
- 12.Pineton de Chambrun M., Frere C., Miyara M., Amoura Z., Martin-Toutain I., Mathian A., Hekimian G., Combes A. High frequency of antiphospholipid antibodies in critically ill COVID-19 patients: a link with hypercoagulability? J. Intern. Med. 2020:13126. doi: 10.1111/joim.13126. joim. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuo Y., Estes S.K., Ali R.A., Gandhi A.A., Yalavarthi S., Shi H., Sule G., Gockman K., Madison J.A., Zuo M., Yadav V., Wang J., Woodard W., Lezak S.P., Lugogo N.L., Smith S.A., Morrissey J.H., Kanthi Y., Knight J.S. Prothrombotic antiphospholipid antibodies in COVID-19. MedRxiv. 2020 doi: 10.1101/2020.06.15.20131607. [DOI] [Google Scholar]
- 14.Woodruff M.C., Ramonell R.P., Lee F.E.-H., Sanz I. Clinically identifiable autoreactivity is common in severe SARS-CoV-2 Infection. MedRxiv. 2020 doi: 10.1101/2020.10.21.20216192. [DOI] [Google Scholar]
- 15.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., Manry J., Shaw E., Haljasmägi L., Peterson P., Lorenzo L., Bizien L., Trouillet-Assant S., Dobbs K., de Jesus A.A., Belot A., Kallaste A., Catherinot E., Tandjaoui-Lambiotte Y., Le Pen J., Kerner G., Bigio B., Seeleuthner Y., Yang R., Bolze A., Spaan A.N., Delmonte O.M., Abers M.S., Aiuti A., Casari G., Lampasona V., Piemonti L., Ciceri F., Bilguvar K., Lifton R.P., Vasse M., Smadja D.M., Migaud M., Hadjadj J., Terrier B., Duffy D., Quintana-Murci L., van de Beek D., Roussel L., Vinh D.C., Tangye S.G., Haerynck F., Dalmau D., Martinez-Picado J., Brodin P., Nussenzweig M.C., Boisson-Dupuis S., Rodríguez-Gallego C., Vogt G., Mogensen T.H., Oler A.J., Gu J., Burbelo P.D., Cohen J.I., Biondi A., Bettini L.R., D’Angio M., Bonfanti P., Rossignol P., Mayaux J., Rieux-Laucat F., Husebye E.S., Fusco F., Ursini M.V., Imberti L., Sottini A., Paghera S., Quiros-Roldan E., Rossi C., Castagnoli R., Montagna D., Licari A., Marseglia G.L., Duval X., Ghosn J., Tsang J.S., Goldbach-Mansky R., Kisand K., Lionakis M.S., Puel A., Zhang S.-Y., Holland S.M., Gorochov G., Jouanguy E., Rice C.M., Cobat A., Notarangelo L.D., Abel L., Su H.C., Casanova J.-L. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;80–:370. doi: 10.1126/science.abd4585. eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casciola-Rosen L., Thiemann D.R., Andrade F., Trejo Zambrano M.I., Hooper J.E., Leonard E., Spangler J., Cox A.L., Machamer C., Sauer L., Laeyendecker O., Garibaldi B.T., Ray S.C., Mecoli C., Christopher-Stine L., Gutierrez-Alamillo L., Yang Q., Hines D., Clarke W., Rothman R.E., Pekosz A., Fenstermacher K., Wang Z., Zeger S.L., Rosen A. IgM autoantibodies recognizing ACE2 are associated with severe COVID-19. MedRxiv. 2020 doi: 10.1101/2020.10.13.20211664. [DOI] [Google Scholar]
- 17.Anaya J.-M., Shoenfeld Y., Rojas-Villarraga A., Levy R.A., Cervera R., editors. Autoimmunity: from Bench to Bedside. 2013. Bogota (Colombia) [PubMed] [Google Scholar]
- 18.Wang E.Y., Mao T., Klein J., Dai Y., Huck J.D., Liu F., Zheng N.S., Zhou T., Israelow B., Wong P., Lucas C., Silva J., Oh J.E., Song E., Perotti E.S., Fischer S., Campbell M., Fournier J.B., Wyllie A.L., Vogels C.B.F., Ott I.M., Kalinich C.C., Petrone M.E., Watkins A.E., Dela Cruz C., Farhadian S.F., Schulz W.L., Grubaugh N.D., Ko A.I., Iwasaki A., Ring A.M. Diverse functional autoantibodies in patients with COVID-19. MedRxiv Prepr. Serv. Heal. Sci. 2020 doi: 10.1101/2020.12.10.20247205. [DOI] [PubMed] [Google Scholar]
- 19.Franco J.-S., Amaya-Amaya J., Molano-González N., Caro-Moreno J., Rodríguez-Jiménez M., Acosta-Ampudia Y., Mantilla R.D., Rojas-Villarraga A., Anaya J.-M. Autoimmune thyroid disease in Colombian patients with systemic lupus erythematosus. Clin. Endocrinol. 2015;83:943–950. doi: 10.1111/cen.12662. [DOI] [PubMed] [Google Scholar]
- 20.Pacheco Y., Monsalve D.M., Acosta-Ampudia Y., Rojas C., Anaya J.-M., Ramírez-Santana C. Antinuclear autoantibodies: discordance among four different assays. Ann. Rheum. Dis. 2020;79 doi: 10.1136/annrheumdis-2018-214693. e6–e6. [DOI] [PubMed] [Google Scholar]
- 21.Botello A., Herrán M., Salcedo V., Rodríguez Y., Anaya J.-M., Rojas M. Prevalence of latent and overt polyautoimmunity in autoimmune thyroid disease: a systematic review and meta-analysis. Clin. Endocrinol. 2020;93:375–389. doi: 10.1111/cen.14304. [DOI] [PubMed] [Google Scholar]
- 22.Lebart A.L., Piron M., Dunod M. 1995. Statistique Exploratoire Multidimensionnelle. Paris. [Google Scholar]
- 23.Arbuckle M.R., McClain M.T., V Rubertone M., Scofield R.H., Dennis G.J., James J.A., Harley J.B. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 24.Molano-González N., Rojas M., Monsalve D.M., Pacheco Y., Acosta-Ampudia Y., Rodríguez Y., Rodríguez-Jimenez M., Ramírez-Santana C., Anaya J.-M. Cluster analysis of autoimmune rheumatic diseases based on autoantibodies. New insights for polyautoimmunity. J. Autoimmun. 2019;98 doi: 10.1016/j.jaut.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 25.James J.A., Chen H., Young K.A., Bemis E.A., Seifert J., Bourn R.L., Deane K.D., Demoruelle M.K., Feser M., O’Dell J.R., Weisman M.H., Keating R.M., Gaffney P.M., Kelly J.A., Langefeld C.D., Harley J.B., Robinson W., Hafler D.A., O’Connor K.C., Buckner J., Guthridge J.M., Norris J.M., Holers V.M. Latent autoimmunity across disease-specific boundaries in at-risk first-degree relatives of SLE and RA patients. EBioMedicine. 2019;42:76–85. doi: 10.1016/j.ebiom.2019.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anaya J.-M., Restrepo-Jiménez P., Rodríguez Y., Rodríguez-Jiménez M., Acosta-Ampudia Y., Monsalve D.M., Pacheco Y., Ramírez-Santana C., Molano-González N., Mantilla R.D. Sjögren’s syndrome and autoimmune thyroid disease: two sides of the same coin. Clin. Rev. Allergy Immunol. 2019;56:362–374. doi: 10.1007/s12016-018-8709-9. [DOI] [PubMed] [Google Scholar]
- 27.Sterne J.A.C., Murthy S., V Diaz J., Slutsky A.S., Villar J., Angus D.C., Annane D., Azevedo L.C.P., Berwanger O., Cavalcanti A.B., Dequin P.-F., Du B., Emberson J., Fisher D., Giraudeau B., Gordon A.C., Granholm A., Green C., Haynes R., Heming N., Higgins J.P.T., Horby P., Jüni P., Landray M.J., Le Gouge A., Leclerc M., Lim W.S., Machado F.R., McArthur C., Meziani F., Møller M.H., Perner A., Petersen M.W., Savovic J., Tomazini B., Veiga V.C., Webb S., Marshall J.C. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19. J. Am. Med. Assoc. 2020;324:1330. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velavan T.P., Meyer C.G. Mild versus severe COVID-19: laboratory markers. Int. J. Infect. Dis. 2020;95:304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leisman D.E., Ronner L., Pinotti R., Taylor M.D., Sinha P., Calfee C.S., V Hirayama A., Mastroiani F., Turtle C.J., Harhay M.O., Legrand M., Deutschman C.S. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponti G., Maccaferri M., Ruini C., Tomasi A., Ozben T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin. Lab Sci. 2020;57:389–399. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goel H., Harmouch F., Garg K., Saraiya P., Daly T., Kumar A., Hippen J.T. The liver in COVID-19: prevalence, patterns, predictors, and impact on outcomes of liver test abnormalities. Eur. J. Gastroenterol. Hepatol. Publish Ah. 2020 doi: 10.1097/MEG.0000000000002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuniga M., Gomes C., Carsons S.E., Bender M.T., Cotzia P., Miao Q.R., Lee D.C., Rodriguez A. Autoimmunity to the lung protective phospholipid-binding protein annexin A2 predicts mortality among hospitalized COVID-19 patients. MedRxiv. 2021:2020. doi: 10.1101/2020.12.28.20248807. 12.28.20248807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.