Abstract
Background
Lung cancer is a public health problem worldwide. Small-cell lung cancer (SCLC) is the most aggressive histologic type, with a 5-year survival <10%. SCLC is closely associated with tobacco consumption and infrequent in never-smokers. We aim to describe SCLC characteristics in never-smokers recruited in a radon-prone area.
Patients and methods
We designed a multicentric case series where SCLC cases were recruited consecutively following histologic confirmation. Detailed information was obtained for indoor radon exposure, occupation and environmental tobacco smoke. We also collected different clinical characteristics such as extended or limited disease at diagnosis.
Results
We recruited 32 never-smoking SCLC cases. Median age was 75 years and 87.5% were women; 47% had extended disease. Median radon concentration was 182 Bq/m3. There were no statistically significant differences in residential radon concentration neither regarding age at diagnosis nor regarding sex. The most frequent symptoms were constitutional syndrome (23.1%) and coughing (23.1%). As much as 63% of cases had an Eastern Cooperative Oncology Group Study (ECOG) status of 0-2. The 1- and 2-year survival rates were 34.4% and 21.9%, respectively. The 2-year survival rate with a localized tumor was 26.7%, compared with 18.8% for extended disease.
Conclusions
These results show, for the first time, that indoor radon might not be associated with SCLC characteristics at diagnosis in never-smokers, and also confirms the low survival of this aggressive type of lung cancer also for never-smokers.
Key words: small-cell lung cancer, residential radon, never-smokers, survival
Highlights
-
•
Small-cell lung cancer in never-smokers is an infrequent disease.
-
•
Risk factors are poorly characterized, and we have observed that residential radon may have a role (182 Bq/m3 as average).
-
•
In this study, 1- and 2-year survival rates were 34.4% and 21.9%, respectively.
Introduction
Small-cell carcinoma (SCLC) represents ∼15% of all cases of lung cancer1 and is the histologic type most closely linked to tobacco consumption, being a very rare tumor in never-smokers.2 The incidence of microcytic lung cancer could be decreasing given the progressive abandonment of the habit of smoking in developed countries.3 SCLC is also the histologic type with the poorest prognosis, with a 5-year survival rates of 10% in patients with a localized tumor and 4.6% when the disease has spread.2 It is a tumor derived from neuroendocrine cells characterized by its fast growth and a good initial response to treatment, although it develops resistance after some time. Recent research on 5632 SCLC cases, including 100 never-smokers, has found significant differences regarding age distribution and gender between smokers and never-smokers, as well as differences in their mutational profiles.4,5
Indoor radon exposure is the second risk factor for lung cancer worldwide and the first for never-smokers.6 The link between the exposure to indoor radon and the risk of developing SCLC has been assessed by different studies and a recent systematic review seems to confirm this relationship.7 Some papers published on this topic include a subanalysis by histologic type showing that SCLC could be the type with the closest association to radon exposure.4, 5, 6 Nonetheless, the majority of SCLC cases included in these studies were ever-smokers, given its low incidence in never-smokers. It is estimated that only 2.5% of SCLC cases are diagnosed in never-smokers,8,9 even though some studies carried out in Asia reveal a greater incidence on cases from eastern Asia (13%-22.6%).10,11
The risk factors for SCLC in never-smokers are little known but it is thought that exposure to environmental tobacco smoke, some work-related fumes, and indoor radon could be contributing factors. In a study including 19 never-smokers published by our group in 2016,12 the characteristics of this disease were analyzed in relation to exposure to indoor radon. The study was carried out in a radon-prone area and it was observed that radon concentrations in these cases were higher than those found in healthy controls in the same geographical area. Nevertheless, available scientific evidence on microcytic cancer is scarce in never-smokers and studies with a larger sample size are needed. Therefore, we decided to continue the recruitment of these patients to have a wider picture of their characteristics with a higher sample size.
The main objective of this study is to expand existing available information about SCLC in never-smokers, with a special focus on exposure to indoor radon.
Materials and methods
Design and settings
We selected all diagnosed cases of SCLC in never-smokers included in several multicentric case–control studies on radon and lung cancer (LCRINS,13,14 Small Cell15 and DNA-Repair). Cases were recruited from January 2011 to September 2019 from 11 hospitals in the Northwest of Spain, six of them in Galicia, an area with a high radon exhalation rate.
All consecutive SCLC cases diagnosed in never-smokers at the participating hospitals were included, all of which had pathological confirmation of diagnosis. Participants were never-smokers according to the World Health Organization's definition: individuals who smoked (a) <100 cigarettes in their lifetime or (b) never as much as one cigarette per day during a consecutive period of 6 months. Participants were >30 years of age and with no cancer history. All studies have been approved by the corresponding Ethics Research Committees and all participants have provided written informed consent.
Data collection
All participants were interviewed by trained investigators using a specific questionnaire. They were asked about different aspects of their lifestyle, including exposure to environmental tobacco smoke, leisure time activities, occupational exposure, and characteristics related to their dwellings and radon exposure. Participants' electronic medical records were checked to identify their symptoms at the onset of the disease, to review the disease stage at diagnosis (limited or extended), according to the International Association for the Study of Lung Cancer (IASLC) classification,16 and to calculate survival after diagnosis. Performance status was assessed for all patients at diagnosis using the Eastern Cooperative Oncology Group Study (ECOG) Performance Status Scale.17
Residential radon measurements were obtained at the participants' dwelling by placing a radon detector for a minimum of 3 months, mainly in the master bedroom. Participants were given written instructions on how to place radon devices, including a descriptive picture showing where the device should be placed. They also received a prepaid and easy-to-seal envelope in order to send back the detector after the end of the 3-month measurement period. Participants were phoned twice by researchers, the first time to check that they had no queries and the detector had been placed and the second to remind them to send back the detector. Once received, devices were read at the Galician Radon Laboratory, one of the three Spanish laboratories certified by the National Accreditation Entity (ENAC), according to international standards. All participants were informed of the results obtained.
Statistical analysis
We performed a descriptive analysis to characterize the clinical characteristics of small-cell lung cancer (SCLC) cases. Median residential radon concentrations were calculated for all cases, it was considered whether SCLC was limited or extended at diagnosis, and differences by sex and age distribution, broken down by exposure to indoor radon, were analyzed. We also assessed the survival of these patients using Kaplan–Meier analysis and we broke down results by extension at diagnosis. The analysis was performed with IBM SPSS Statistics for Windows (version 22.0; IBM Corp, Armonk, NY).
Results
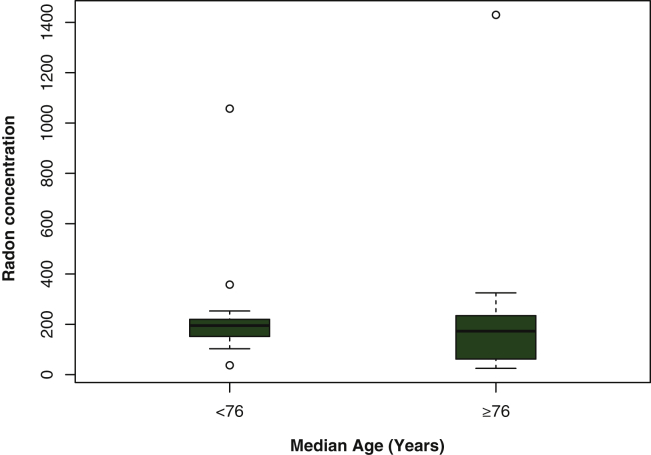
A total of 32 never-smokers with SCLC were included, of which eight patients (25%) had been exposed to environmental tobacco smoke in their home. Median age was 75 years (interquartile range [IQR] 69-80). Of the total study population, 87.5% were women, whose main occupations were housewife and agricultural worker. All cases presented a low educational level, limited to primary schooling. In 47% of cases (15 patients), the disease had spread (TNM stage IV)18 at the time of diagnosis and five of them (15.6%) presented brain metastasis. Median radon concentration was 182 Bq/m3 (IQR 82-234), ranging between 25 and 1.430 Bq/m3, and the median number of years residing in the home where measurements were taken was 43 (IQR 25-54). Table 1 presents a description of included patients. There were no statistically significant differences in radon concentration in the home neither regarding age at diagnosis, as presented in Figure 1, nor regarding sex (P = 0.311).
Table 1.
Description of cases
| Patients | Value |
|---|---|
| Sex, n (%) | |
| Male | 4 (12.5) |
| Female | 28 (87.5) |
| Age (years) | |
| Median | 75 |
| P25-P75 | 69-80 |
| Education, n (%) | |
| No formal studies | 15 (46.9) |
| Primary education | 17 (53.1) |
| Radon concentration (Bq/m3) | |
| Median | 182 |
| P25-P75 | 85-234 |
| Range | 25-1430 |
| Stage of disease, n (%) | |
| Limited | 17 (53) |
| Extended | 15 (47) |
| Environmental Tobacco Smoke exposure at home, n (%) | |
| Yes | 8 (25) |
| No | 24 (75) |
| Time living in the same dwelling (years) | |
| Median | 43 |
| P25-P75 | 25-54 |
| Occupation, n (%) | |
| Agriculture | 12 (37.5) |
| Housewife | 10 (31.2) |
| Cleaner | 4 (12.5) |
| Factory | 3 (9.4) |
| Others | 3 (9.4) |
Figure 1.
Indoor radon concentration and age at diagnosis. Circles belong to outliers.
Information about symptoms at diagnosis was available for 26 patients. The most frequent symptoms were constitutional syndrome (23.1%) and coughing (23.1%), followed by hemoptysis (15.4%), dyspnea (11.5%), and thoracic pain (7.7%). Three patients were asymptomatic at the moment of diagnosis, one presented a superior cava vein syndrome and another displayed symptoms secondary to metastasis. With regard to performance status at the time of diagnosis (ECOG), 63% of cases were at ECOG status 0-2 and 30.7% at ECOG status 3-4, without significant differences in relation to stage at diagnosis. Table 2 shows the frequency of primary symptoms and ECOG status regarding the extent of the disease at the time of diagnosis.
Table 2.
Main symptoms and ECOG status
| Symptoms | Limited disease, n (%) | Extended disease, n (%) |
|---|---|---|
| Constitutional syndrome | 2 (14.3) | 4 (33.3) |
| Cough | 4 (28.6) | 2 (16.7) |
| Hemoptysis | 2 (14.3) | 2 (16.7) |
| Dyspnea | 2 (14.3) | 2 (16.7) |
| Chest pain | 2 (14.3) | 1 (8.3) |
| Asymptomatic | 1 (7.1) | 2 (16.7) |
| Metastatic disease | 0 (0.0) | 1 (8.3) |
| Superior cava vein syndrome | 1 (7.1) | 0 (0.0) |
| ECOG status | ||
| 0 | 5 (37.5) | 3 (23.1) |
| 1 | 4 (28.6) | 6 (46.2) |
| 2 | 1 (7.1) | 0 (0) |
| 3 | 2 (14.3) | 3 (23.1) |
| 4 | 2 (14.3) | 1 (7.7) |
ECOG, Eastern Cooperative Oncology Group Study.
One-year survival was 34.4%, and 21.9% for 2 years. With regard to the stage of the tumor at diagnosis, 1-year survival with a localized tumor was 46.7% and 26.7% for 2 years, compared with 25% and 18.8% in patients on whom the disease had spread, respectively.
No significant differences were found for survival in relation to exposure to a concentration of radon over or below 200 Bq/m3 (P = 0.369). Table 3 shows survival broken down by stage at diagnosis and exposure to indoor radon.
Table 3.
Survival at 1 and 2 years from diagnosis
| Survival | 1 year, % | 2 years, % |
|---|---|---|
| Global | 34.4 | 21.9 |
| Limited disease | 46.7 | 26.7 |
| Extended disease | 25 | 18.8 |
| Radon <200 Bq/m3 | 31.3 | 12.5 |
| Radon ≥200 Bq/m3 | 37.5 | 31.3 |
Discussion
These results show that SCLC in never-smokers is an aggressive neoplasia, with a high mortality rate. A significant number of tumors are diagnosed in an advanced phase and age at diagnosis is superior to that published for other histologic types in never-smokers.13 Radon concentration is high for this subgroup of microcytic carcinomas, which could point to radon playing an important role in its etiology. This is one of the largest studies conducted on never-smoker Caucasic patients affected by SCLC cancer to date, and the only one in which exposure to radon, second risk factor for LC after tobacco, has been measured.
In general, SCLC is considered to be closely linked to smoking and, therefore, its diagnosis in never-smokers is very rare. It is estimated that only 5%-6% of LC cases diagnosed in never-smokers are SCLC,13,19 with adenocarcinoma being the most frequent. Exposure to environmental tobacco smoke has been postulated as a risk factor in the development of SCLC in never-smokers, although only 25% of participants in this study had lived with smokers in the past 20 years.
Exposure to indoor radon could be a risk factor in the development of SCLC in never-smokers. In the majority of studies that have analyzed the link between radon exposure and the different histologic types, SCLC is one of the tumors which shows the closest association with indoor radon, even though the majority of these studies include smokers and ex-smokers, given the low incidence of SCLC in never-smokers. Thus, in the European pooling study, published in 2005,20 there were 1379 cases of SCLC from a total of 7148 lung cancer cases and it was this histologic type that revealed the greatest relative risk per 100 Bq/m3 increment in radon concentration (31%), much higher than that observed for other common histologic types such as adenocarcinoma (6%). Nevertheless, the role of radon in SCLC in never-smokers was not analyzed in that study.
The results obtained per histologic type in the American pooling were similar,21 with a 23% increase per 100 Bq/m3 for SCLC. More recent studies revealed equivalent findings, which suggest that, although exposure to residential radon increases the risk for all histologic types, there is a higher risk for SCLC than for other types, such as adenocarcinoma or squamous cell carcinoma. Thus, for instance, Barros-Dios et al.22 carried out a case–control study in a radon-prone area in 2012, including 349 lung cancer cases, among which 54 (15.5%) had SCLC, and found that this histologic type carried a greater risk due to radon exposure. In a recently published systematic review, the association between exposure to indoor radon and SCLC was stronger than that for other histologic types in eight out of 11 studies.7 In our series, the median radon concentration was 182 Bq/m3, well above the action level of 100 Bq/m3 recommended by the World Health Organization (WHO),6 and is also higher than the average radon concentration observed in never-smoker controls who lived in the same area from a previous study by our group (median concentration 149 Bq/m3),14 or compared with the median concentration of the Galician population, established at 100 Bq/m3.23 Furthermore, the patients included in this case series had lived in the dwelling where the measurements took place for an extended period, a median of 43 years, and this prolonged exposure lends greater validity to potential effect due to high radon exposure. In addition, the majority of SCLC cases included in this series were women, many of them housewives or dedicated to local agricultural labor, and thus, the proportion of daily time spent in the dwelling is higher than it would be if they worked outside the home. This could have contributed to a greater intensity of exposure to indoor radon. The predominance of women in our sample, 87.5%, is in direct correlation with the results of previous lung cancer studies in never-smokers performed in different geographical areas13,24 and it is related to the fact that most older women in Galicia are never-smokers. The low level of education among the participants is also relevant.
One of the problems of lung cancer is late diagnosis, in the late stages of the disease, when patients already present symptoms related to the illness, as this is indicative of a poor prognosis. It is estimated that, at a global level, ∼20% of tumors are diagnosed in localized stages although some series suggest that the percentage of patients diagnosed with localized lung cancer has been increasing for the last two decades.25 In our series, 47% of lung cancer cases were diagnosed at stage IV, with evidence of extrapulmonary metastatic disease. Among the cases of intrathoracic localized disease, only four were at TNM stages I and II,18 whereas the majority of cases were at stages IIIA and IIIB (12% and 24%, respectively). One of the reasons for the late diagnosis of LC, despite all the technical advances of the last decades, would be related to the increase in the incidence of adenocarcinomas26 in comparison to other histological types of tumors, because of their tendency to be localized in the periphery, unlike epidermoid carcinomas or SCLC itself. The central location of SCLC could cause it to show symptoms earlier and lead to a higher percentage of diagnosis in the localized stages of the disease.
The most frequent symptoms present in our series were the constitutional syndrome and coughing. These results are similar to a recently published study from the Spanish Tumor Thoracic Registry, which included >1300 SCLC cases, and where cough was the most frequent symptom.27 As much as 10% of patients were asymptomatic at the moment of diagnosis and only one had symptoms attributable to the metastatic disease.
Survival rates for SCLC are extremely poor. Among the patients included in this survey, 1-year survival was 34.4% and 2-year survival was 21.9%. Patients presenting a localized tumor had a higher 1- and 2-year survival rates compared with those diagnosed with a metastatic disease (46.7% versus 25% for 1 year and 26.7% versus 18.8% for 2 years). Stage at diagnosis is one of the most relevant prognostic factors for all histologic lung cancer types,28 and also for SCLC.29 Existing published data on SCLC survival rates in never-smokers are scarce given the disease's low incidence; however, a recent study on Chinese population11 has compared SCLC survival in both smokers and never-smokers. This research included 22.5% never-smokers and this group showed a higher survival rate than smokers (19.36 months versus 14.40 months, P = 0.044). The mechanisms by which prognosis might be different for both groups are not known. Ogino et al.,30 in a recently published study including seven never-smoking SCLC cases analyzed through next-generation sequencing, showed that these patients might have a different molecular signature than ever-smokers mainly at MEK and mTOR pathways.
This study has a series of limitations, such as its small sample size. However, given the low incidence of SCLC on never-smokers, it constitutes an acceptable number of participants. Among its advantages are its multicentric design and it being the first study to measure residential radon in order to assess its influence on survival rate. Moreover, patient follow up is almost complete, with barely any losses.
In conclusion, SCLC in never-smokers is very rare, but an aggressive illness with a poor prognosis. Its stage at diagnosis is one of the main determining factors for survival and, therefore, it is necessary to continue working on early diagnosis, which will enable early detection and treatment in localized stages and better prognosis. Exposure to indoor radon is the first risk factor for lung cancer in never-smokers and, in the case of SCLC, it could be extremely relevant from the etiologic point of view. Survival to SCLC in never-smokers seems slightly higher than for smokers.
Acknowledgments
Funding
This work was supported by the Instituto de Salud Carlos III (ISCIII)/PI15/01211/Cofinanciado FEDER, Spanish Society of Neumology and Thoracic Surgery (Project number 848. 2019 call).
Disclosure
The authors has declared no conflict of interest.
Footnotes
Note: This paper is part of the work related to the PhD degree of María Teresa Curiel García.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Früh M., De Ruysscher D., Popat S. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi99–vi105. doi: 10.1093/annonc/mdt178. [DOI] [PubMed] [Google Scholar]
- 3.Govindan R., Page N., Morgensztern D. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 4.Cardona A.F., Rojas L., Zatarain-Barrón Z.L. Multigene mutation profiling and clinical characteristics of small-cell lung cancer in never-smokers vs. heavy smokers (Geno1.3-CLICaP) Front Oncol. 2019;9:254. doi: 10.3389/fonc.2019.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas A., Mian I., Tlemsani C. Clinical and genomic characteristics of small cell lung cancer in never smokers: results from a retrospective multicenter cohort study. Chest. 2020;158:1723–1733. doi: 10.1016/j.chest.2020.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . WHO; Geneva, Switzerland: 2009. Handbook on Indoor Radon: A Public Health Perspective. [PubMed] [Google Scholar]
- 7.Rodríguez-Martínez Á., Torres-Durán M., Barros-Dios J.M., Ruano-Ravina A. Residential radon and small cell lung cancer. A systematic review. Cancer Lett. 2018;426:57–62. doi: 10.1016/j.canlet.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Varghese A.M., Zakowski M.F., Yu H.A. Small-cell lung cancers in patients who never smoked cigarettes. J Thorac Oncol. 2014;9(6):892–896. doi: 10.1097/JTO.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ou S.-H.I., Ziogas A., Zell J.A. Prognostic factors for survival in extensive stage small cell lung cancer (ED-SCLC): the importance of smoking history, socioeconomic and marital statuses, and ethnicity. J Thorac Oncol. 2009;4(1):37–43. doi: 10.1097/JTO.0b013e31819140fb. [DOI] [PubMed] [Google Scholar]
- 10.Sun J.-M., Choi Y.-L., Ji J.H. Small-cell lung cancer detection in never-smokers: clinical characteristics and multigene mutation profiling using targeted next-generation sequencing. Ann Oncol. 2015;26(1):161–166. doi: 10.1093/annonc/mdu504. [DOI] [PubMed] [Google Scholar]
- 11.Liu X., Jiang T., Li W. Characterization of never-smoking and its association with clinical outcomes in Chinese patients with small-cell lung cancer. Lung Cancer. 2018;115:109–115. doi: 10.1016/j.lungcan.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Torres-Durán M., Ruano-Ravina A., Kelsey K.T. Small cell lung cancer in never-smokers. Eur Respir J. 2016;47(3):947–953. doi: 10.1183/13993003.01524-2015. [DOI] [PubMed] [Google Scholar]
- 13.Torres-Durán M., Ruano-Ravina A., Parente-Lamelas I. Lung cancer in never-smokers: a case-control study in a radon-prone area (Galicia, Spain) Eur Respir J. 2014;44(4):994–1001. doi: 10.1183/09031936.00017114. [DOI] [PubMed] [Google Scholar]
- 14.Torres-Durán M., Ruano-Ravina A., Parente-Lamelas I. Residential radon and lung cancer characteristics in never smokers. Int J Radiat Biol. 2015;91(8):605–610. doi: 10.3109/09553002.2015.1047985. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez-Martínez Á., Ruano-Ravina A., Torres-Durán M. Small cell lung cancer. Methodology and preliminary results of the SMALL CELL study. Arch Bronconeumol. 2017;53(12):675–681. doi: 10.1016/j.arbres.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Micke P., Faldum A., Metz T. Staging small cell lung cancer: veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer--what limits limited disease? Lung Cancer. 2002;37(3):271–276. doi: 10.1016/s0169-5002(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 17.Oken M.M., Creech R.H., Tormey D.C. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 18.Detterbeck F.C., Boffa D.J., Kim A.W., Tanoue L.T. The eighth edition lung cancer stage classification. Chest. 2017;151(1):193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Lagarde F., Axelsson G., Damber L., Mellander H., Nyberg F., Pershagen G. Residential radon and lung cancer among never-smokers in Sweden. Epidemiol Camb Mass. 2001;12(4):396–404. doi: 10.1097/00001648-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Darby S., Hill D., Auvinen A. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. Br Med J. 2005;330(7485):223. doi: 10.1136/bmj.38308.477650.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krewski D., Lubin J.H., Zielinski J.M. A combined analysis of North American case-control studies of residential radon and lung cancer. J Toxicol Environ Health A. 2006;69(7):533–597. doi: 10.1080/15287390500260945. [DOI] [PubMed] [Google Scholar]
- 22.Barros-Dios J.M., Ruano-Ravina A., Pérez-Ríos M., Castro-Bernárdez M., Abal-Arca J., Tojo-Castro M. Residential radon exposure, histologic types, and lung cancer risk. A case-control study in Galicia, Spain. Cancer Epidemiol Biomarkers Prev. 2012;21(6):951–958. doi: 10.1158/1055-9965.EPI-12-0146-T. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzo-González M., Ruano-Ravina A., Peón J., Piñeiro M., Barros-Dios J.M. Residential radon in Galicia: a cross-sectional study in a radon-prone area. J Radiol Prot. 2017;37(3):728–741. doi: 10.1088/1361-6498/aa7922. [DOI] [PubMed] [Google Scholar]
- 24.Lo Y.-L., Hsiao C.-F., Chang G.-C. Risk factors for primary lung cancer among never smokers by gender in a matched case-control study. Cancer Causes Control. 2013;24(3):567–576. doi: 10.1007/s10552-012-9994-x. [DOI] [PubMed] [Google Scholar]
- 25.Leiro-Fernández V., Mouronte-Roibás C., Ramos-Hernández C. Changes in clinical presentation and staging of lung cancer over two decades. Arch Bronconeumol. 2014;50(10):417–421. doi: 10.1016/j.arbres.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Devesa S.S., Bray F., Vizcaino A.P., Parkin D.M. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117(2):294–299. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 27.Ruano-Raviña A., Provencio M., Calvo de Juan V. Lung cancer symptoms at diagnosis: results of a nationwide registry study. ESMO Open. 2020;5(6):e001021. doi: 10.1136/esmoopen-2020-001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodard G.A., Jones K.D., Jablons D.M. Lung cancer staging and prognosis. Cancer Treat Res. 2016;170:47–75. doi: 10.1007/978-3-319-40389-2_3. [DOI] [PubMed] [Google Scholar]
- 29.Bernhardt E.B., Jalal S.I. Small cell lung cancer. Cancer Treat Res. 2016;170:301–322. doi: 10.1007/978-3-319-40389-2_14. [DOI] [PubMed] [Google Scholar]
- 30.Ogino A., Choi J., Lin M. Genomic and pathological heterogeneity in clinically diagnosed small cell lung cancer in never/light smokers identifies therapeutically targetable alterations. Mol Oncol. 2021;15(1):27–42. doi: 10.1002/1878-0261.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]