Abstract
Background
Pertussis can lead to serious disease and even death in infants. Older adults are more vulnerable to complications as well. In high-income countries, acellular pertussis vaccines are used for priming vaccination. In the administration of booster vaccinations to different age groups and target populations there is a substantial between-country variation. We investigated the effect of age on the response to acellular pertussis booster vaccination in three European countries.
Methods
This phase IV longitudinal intervention study performed in Finland, the Netherlands and the United Kingdom between October 2017 and January 2019 compared the vaccine responses between healthy participants of four age groups: children (7–10y), adolescents (11–15y), young adults (20–34y), and older adults (60–70y). All participants received a three-component acellular pertussis vaccine. Serum IgG and IgA antibody concentrations to pertussis antigens at day 0, 28, and 1 year were measured with a multiplex immunoassay, using pertussis toxin concentrations at day 28 as primary outcome. This trial is registered with ClinicalTrialsRegister.eu (2016–003,678–42).
Findings
Children (n = 109), adolescents (n = 121), young adults (n = 74), and older adults (n = 75) showed high IgG antibody concentrations to pertussis toxin at day 28 with GMCs of 147 (95% CI 120–181), 161 (95% CI 132–196), 103 (95% CI 80–133), and 121 IU/ml (95% CI 94–155), respectively. A significant increase in GMCs for vaccine antigens in all age groups by 28 days was found which had decreased by 1 year. Differences in patterns of IgG GMCs at 28 days and 1 year post-vaccination did not have a consistent relationship to age. In contrast, IgA antibodies for all antigens increased with age at all timepoints.
Interpretation
Acellular pertussis booster vaccination induces significant serum IgG responses to pertussis antigens across the age range which are not uniformly less in older adults. Acellular boosters could be considered for older adults to reduce the health and economic burden of pertussis.
Keywords: Vaccination, Pertussis, IGG, IgA, Children, Adults
Research in context.
Evidence before this study
Pertussis is a severe and life-threatening respiratory disease caused by Bordetella pertussis. Unvaccinated or incomplete vaccinated infants are most vulnerable to serious complications of pertussis, but older adults have a higher chance of complications and hospitalisation as well. In most European countries the acellular pertussis vaccine is used with different infant priming vaccination schedules and booster vaccinations at different ages. Despite a high vaccination coverage in European countries, notification rates are increasing indicating an increasing circulation of Bordetella pertussis. We searched PubMed for clinical trials and reviews up to August 14, 2017, with no language restrictions, using the search terms (older adults OR elderly) AND pertussis AND (vaccination OR vaccine OR Tdap) in the title and/or abstract. This search revealed that older adults do show a response to an acellular pertussis booster vaccination but did not yield any clinical trials that investigated possible differences in immunogenicity between older adults and other age groups who regularly received booster vaccinations, like school aged children, adolescents, and young adults. Therefore, we performed this prospective clinical trial, comparing four different age groups (children 7–10 years old, adolescents 11–15 years old, young adults 20–34 years old, and older adults 60–70 years old) in three different countries (Finland, the Netherlands, and the United Kingdom) with different epidemiological and vaccination background.
Added value of this study
This phase IV longitudinal intervention study is conducted as a multicentre trial in Finland, the Netherlands, and the UK and compares the effect of an acellular pertussis booster vaccination in four different age groups and three European countries. Here we present the IgG and IgA immune responses directed against the pertussis vaccine components of a Tdap-IPV booster vaccination administered to children, adolescents, and young and older adults with a follow-up to one year. Our study shows that IgG responses to the pertussis vaccine antigens do not have a consistent relationship to age up to one year post-vaccination and thus that the influence of age, and vaccination and epidemiological background on the IgG responses is limited. The IgA responses on the other hand seem to be higher at baseline in older adults, who also show greater increase upon vaccination and thus age influences the IgA responses.
Implications of all the available evidence
This study emphasizes that despite considerable differences in age, country-specific vaccination schedules and history, and epidemiological background, the IgG responses on the three-component acellular pertussis vaccine in older adults is not uniformly less compared to children, adolescents, and young adults. The widespread circulation of B. pertussis in high-income countries underscores the need for vigilant surveillance of whooping cough. Maternal immunisation to prevent pertussis in young infants at high risk for serious disease and death has been implemented in many countries and has been found highly effective from birth until the primary vaccinations. Besides the young infants also older adults are at increased risk of complications and hospitalisation due to whooping cough. This study shows that a booster vaccination could be considered for older adults in order to prolong their protection and reduce the epidemiological pressure on the circulation of pertussis in the population.
Alt-text: Unlabelled box
1. Introduction
Pertussis is an acute respiratory disease caused by the gram-negative bacterium Bordetella pertussis [1]. It can lead to serious disease and even death, especially in infants [2]. Complications due to pertussis are also associated with patients with chronic diseases and older age [3]. The incidence of clinical pertussis cases dropped with the introduction of whole cell (wP) pertussis vaccines in the 1940/1950s [4]. However, due to reported high reactogenicity rates and consequent decrease in vaccine coverage, acellular pertussis (aP) vaccines were developed in the late 1970/1980s. These vaccines proved to be less reactogenic and the short-term efficacy seemed to be similar to wP vaccines [5,6]. However, long-term efficacy of aP vaccines appeared to be inferior compared with wP vaccines and natural infection although whole cell vaccines were reported to have a broad range of efficacy, some had significantly lower efficacy than aP vaccines [6], [7], [8]. While natural infection, acellular and whole cell vaccination all three induce antibodies and protect against disease, the ability of aP vaccines to protect against colonisation in comparison with wP vaccines or natural infection seems limited. In the baboon model it has been shown that natural infection and wP vaccination also protect against newly acquired colonisation and consequent transmission of B. pertussis. Therefore aP vaccines may be less effective than wP vaccines in generating herd immunity [9].
The switch from wP to aP vaccines was made since 1990 in many developed countries [6,[10], [11], [12], [13]]. Several aP vaccines are available: two-component aP (aP2) vaccines contain pertussis toxin (Ptx) and filamentous haemagglutinin (FHA), three-component aP (aP3) vaccines also contain pertactin (Prn) and five-component aP (aP5) vaccines additionally contain Fimbriae 2 and 3 (Fim2/3). The aP vaccines have proven to induce high IgG antibody levels against the various vaccine antigens that play an important role in protection against pertussis [14,15]. Specific antibody levels against pertussis vary per vaccine antigen and the lack of an internationally established correlate of protection makes the interpretation of the data challenging [16], [17], [18], [19].
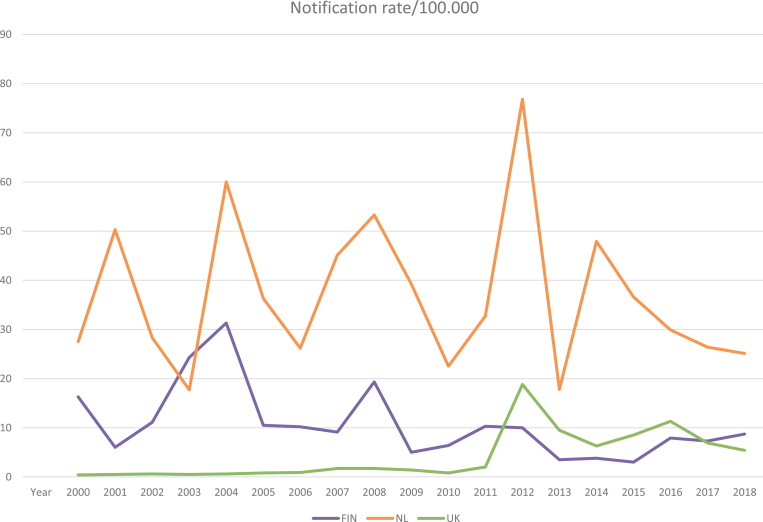
Despite high vaccination coverage of wP/aP vaccines, pertussis has re-emerged worldwide, usually with cyclic outbreaks [20]. These cyclic outbreaks of pertussis occurred every three to four years in Finland before the onset of aP vaccinations in 2005 [21]. Since 1996 a similar pattern was seen in the Netherlands with cyclic outbreaks every three to five years. Introduction of aP vaccines seemed to disrupt this cyclic pattern in Finland, but also after the introduction of aP vaccines in 2005, the Netherlands still faced outbreaks in 2008, 2012, and 2014 (Fig. 1) [22], [23], [24]. In the United Kingdom (UK), a cyclic pattern was observed in the wP era despite low incidence, and following the switch to aP in 2004, there was a major outbreak in 2012 and since then a cyclical pattern of outbreaks every three to four years [25,26]. Notification rates are dependant on the use of diagnostics, reporting and ascertainment per country and therefore do not reflect the real incidence numbers. In response to continued pertussis disease, many countries have adopted an accelerated schedule of primary series vaccinations and all western countries have implemented booster vaccinations in childhood [27,28]. In addition, some countries introduced extra aP boosters for adolescents and adult target populations, including pregnant women, military conscripts, healthcare workers, and older adults [29], [30], [31], [32], [33], [34].
Fig. 1.
Notification rates from the ECDC Surveillance Atlas for pertussis. 2019 [24].
From the pertussis incidence data in Europe, it can be deduced that the proportion of the observed pertussis cases in young children is rather constant over the years, while that of the adolescents is declining and the proportion of cases in adults 30 years and older is rising [24]. These European trends in pertussis incidences are also seen for the three individual countries involved in this study. In the study countries, the pertussis vaccination coverage at around age one in 2017 was 94% in the Netherlands and the UK, and 99% in Finland [35].
The aim of this study was to investigate the effect of age on aP booster vaccination when administered in school aged children, adolescents, and young and older adults. The study was performed in three European countries with differences in vaccination schedules and pertussis epidemiology which allowed additional investigation of possible country-specific effects.
2. Methods
2.1. Study design and participants
This phase IV longitudinal intervention study was conducted as a multicentre trial in Finland, the Netherlands, and the UK. In all three countries, children aged 7–10 years, adolescents aged 11–15 years, young adults aged 20–34 years, and older adults aged 60–70 years old were recruited. Finnish children were informed at local schools in Turku after permission was received from the school authorities. Adult participants were recruited by information in the newspapers and by University of Turku and Turku University Hospital web pages. The Finnish arm of this study was conducted by the Department of Microbiology and Immunology (University of Turku, Turku, Finland) in Turku University Hospital. Dutch participants were recruited by mail-outs to a sex balanced sample in the region of Hoofddorp, attained via the Municipal Administration (adolescents, young adults, and older adults) or through the NIP (children). The Dutch arm of the study was conducted by the Spaarne Academy (Spaarne Hospital, Hoofddorp, the Netherlands). In the UK, age appropriate participants in the Oxford region, identified via National Health Service databases, received mail-outs, also posters and media advertisings were used. The UK arm of the study was conducted by the Oxford Vaccine Group (University of Oxford, Oxford, UK). All participants should have been healthy and vaccinated according to the country-specific NIPs, which are presented in Supplementary Table 1. Most important exclusion criteria were the presence of serious immune modulating illnesses, previous administration of serum products, occurrence of serious adverse events after previous vaccinations, pregnancy, and for adults administration of pertussis containing vaccines in the last five years. A full list of inclusion and exclusion criteria can be found in the Supplementary Panel. All participants received a Tdap3-IPV vaccine (Boostrix™-IPV - GlaxoSmithKline (GSK), Wavre, Belgium) at baseline. Serum samples were collected at day 0 and 28 (± 4 days), and 1 year (± 4 weeks) post-vaccination and subsequently stored at −20 °C until analysis. As part of a broader set of immunological studies not reported here additional samples were drawn at day 7 and day 14 in all groups and day 1 in the 11–15year age group. All laboratory analyses were performed at a single laboratory in Bilthoven the Netherlands. This study was initiated by the IMI2 PERISCOPE Consortium [36].
2.2. Ethics statement
This human clinical study was designed and conducted in accordance with the provisions of the Declaration of Helsinki (1996) and the International Conference on Harmonisation Guidelines for Good Clinical Practice. The trial was registered at the EU Clinical Trial database (EudraCT number 2016-003678-42) and was approved by the Medical Research Ethics Committees United (MEC-U, NL60807.100.17-R17.039) in the Netherlands, the South Central - Hampshire B Research Ethics Committee (REC, 19/SC/0368) in the UK, and the MREC UTU (ETMK Dnro: 129/1800/2017) in Finland. Written informed consent was obtained from all adult participants, and parents or legal guardian of minors, at the start of the study.
2.3. Serological analysis
Serum IgG concentrations against Ptx (GSK), FHA (SP), Prn (GSK), and Fim2/3 (SP) were quantified in independent duplicate using the fluorescent-bead-based multiplex immunoassay (MIA) as previously described [37], [38], [39], and serum IgA concentrations for the pertussis antigens were measured. In brief, the conjugated fluorescent microbeads were incubated with serum samples in two dilutions (200 and 4000), a reference serum in a dilution series and control sera, on each plate. The measurement of the IgG and IgA antibody levels was performed with a Bioplex LX200 in combination with BioPlex Manager 6.2 (Bio-Rad Laboratories). For IgG an in-house standard, calibrated on the Pertussis Antiserum (human) 1st WHO International Standard (IS) was used to express IgG antibody concentrations in IU/ml. For IgA the Pertussis Antiserum (human) 1st WHO IS itself was used. For each analyte, the MFI was converted to IU/ml by interpolation from a five-parameter logistic standard curve. The in-house standard reference for IgG-Fim2/3 was calibrated against U.S. reference pertussis antiserum (human) lot 3 and arbitrarily set at 100 AU/ml as previously described [38], and for IgA-Fim2/3 the Pertussis Antiserum (human) 1st WHO IS was arbitrarily set at 100 AU/ml. The lower limits of quantification (LLOQs) for IgG were 0•214 IU/ml for Ptx and 0•092 AU/ml for Fim2/3. The LLOQs for IgA were 0•032 IU/ml for Ptx and FHA, 0•021 IU/ml for Prn, and 0•049 AU/ml for Fim2/3. For IgG-Ptx, 20 IU/ml was defined as arbitrary cut-off of protection against clinical disease [40], [41], [42], and 100 IU/ml was defined to be indicative for a recent infection in absence of a vaccination in the last few years [39]. All the Luminex antibody data have been deposited in the central database of the PERISCOPE Consortium and can be accessed by a request to the PERISCOPE management team.
2.4. Outcomes
The primary outcome of the study is the specific Ptx-IgG serological antibody level at 28 days after vaccination in the four age groups of the three countries. Secondary outcomes are IgG levels at 0 and 28 days, and one year of Ptx (only day 0 and one year), FHA, Prn, and Fim2/3, and pertussis-specific IgA levels at those timepoints for the same antigens.
2.5. Statistical analysis
In each of the three countries the study aimed to include 36 children aged 7–10 years, 36 adolescents aged 11–15 years, 25 young adults aged 20–34 years, and 25 older adults aged 60–70 years, with equal distribution between aP and wP primed adolescents in early infancy in the Netherlands and Finland. The large number in the school children and adolescent groups also allowed sufficient additional samples to be collected at day 1, day 7 or day 14, for immunological end-points not reported here. Unlike adult participants not each child could be bled at all timepoints. A sample size of 108 per child cohort, across all countries was estimated to give 80% power to detect a standardised difference in log-anti-Ptx-IgG at one month post booster of 0•42 IU/ml between age cohorts, allowing 15% loss to follow-up or sample loss. The data obtained from the participants in each country will be combined and analysed per age cohort. From all participants successful blood samples at day 0 and 28 should have been taken, otherwise they could be replaced.
Concentrations below the LLOQ were replaced by LLOQ/2. For each antibody and antigen combination a linear mixed model was fitted to the log-transformed concentrations. A linear mixed model can be considered as a generalisation of a paired t-test [43]. This model describes the log geometric mean concentrations (GMCs) while accounting for the longitudinal structure of the measurements. Timepoint of blood sampling and age group were included in the model as a two-way interaction as fixed effects. Participant ID was included as a random intercept in the model and by the random intercept the baseline concentration of each participant was taken into account. An additional analysis with country included in the model was performed as a three-way interaction as fixed effects, to reveal differences within age groups between countries.
To explore the effect of previous vaccinations with or without Prn and/or Fim2/3, children and adolescents were subdivided in a group exclusively vaccinated with aP2 vaccines (without Prn and Fim2/3)(n = 10), a group exclusively vaccinated with aP3 vaccines (with Prn, without Fim2/3) (n = 7), a group at least three times vaccinated with aP5 vaccines (with Prn and Fim2/3)(n = 65), and a group primed at least three times with a wP vaccine (n = 41). A similar model, where age group was replaced by vaccination group, was used to analyse the differences between aP and wP priming background, and between participants who previously received only aP2, only aP3, at least three times aP5 or at least three times wP vaccines.
Overall significance of the fixed effect terms was assessed by a type III ANOVA. GMCs and their corresponding 95% confidence intervals (95% CI), as well as their mutual GMC ratios, corresponding 95% CI and p-values were obtained by post hoc analysis using Satterthwaite's method [44,45]. P-values were adjusted by applying the Benjamini-Hochberg procedure for multiple comparisons, controlling the false discovery rate [46]. Non-relevant comparisons were excluded.
To investigate whether seasonal, diurnal or sex effects were present, month (12-level categorical variable), hour (ten-level categorical variable, based on quantiles), and sex (two-level categorical variable) were included as fixed effects in the model as well. These, however, were not significant and were excluded in further analyses.
All statistical analysis were done in R [47], using the lme4 package [48], and lmerTest package [49].
2.6. Role of the funding source
The EFPIA partners from the PERISCOPE Consortium had some input on the study design, e.g. the determination of the age of the four groups of participants. They had no role in the data collection, analysis, interpretation of the data, nor in the writing of the report. The corresponding authors had full access to all data from the study, and final responsibility for the decision to submit for publication was by consensus of all co-authors.
3. Results
3.1. Participant and data overview
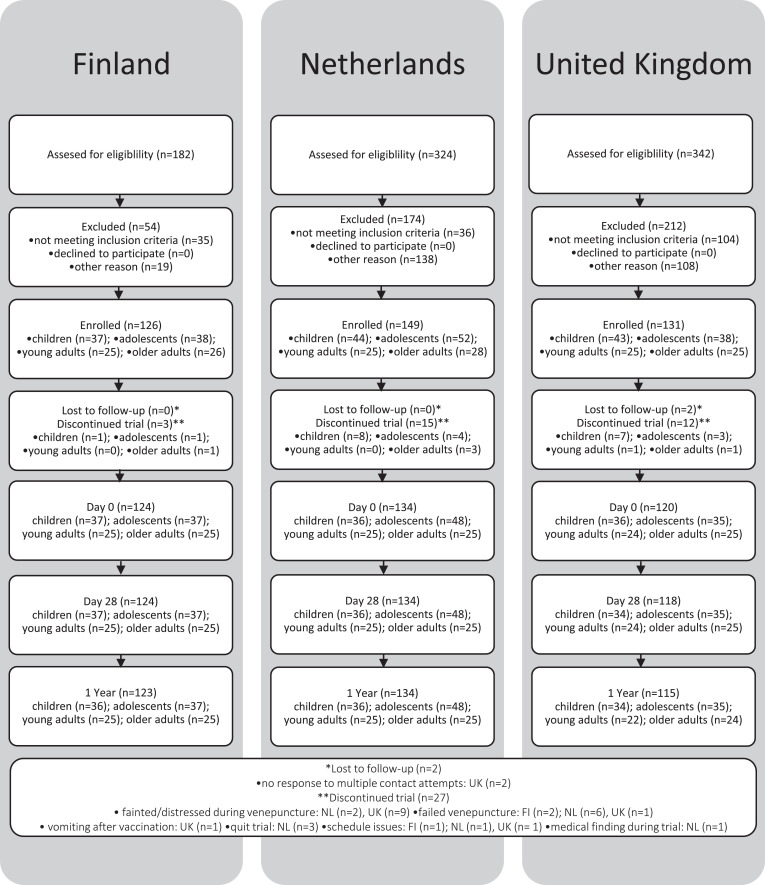
In Finland, participants were enroled in the study between August 2018 and January 2019. In total 123 study participants completed the study, 36 children, 37 adolescents, 25 young adults, and 25 older adults (Fig. 2). In the Netherlands, participants were enroled in the study between October 2017 and March 2018. Since the distribution between aP and wP primed adolescents was distorted, 12 extra participants were enroled in October 2018. A total of 134 participants completed the study subdivided in 36 children, 48 adolescents, 25 young adults, and 25 older adults (Fig. 2). In the UK participants were enroled in the study between April 2018 and January 2019. A total of 117 participants completed the study, 36 children, 35 adolescents, 22 young adults, and 24 older adults (Fig. 2). Characteristics of all 379 participants (53% female) from whom serological data were available and used for analyses, are shown in Table 1.
Fig. 2.
Flow diagram BERT-study.
Table 1.
Participant characteristics.
| Characteristics | Country | No. of children aged 7–10 yrs [%] | No. of adolescents aged 11–15 yrs [%] | No. of young adults aged 20–34 yrs [%] | No. of older adults aged 60–70 yrs [%] | |
|---|---|---|---|---|---|---|
| No. of participants | Total | FI | 37 | 37 | 25 | 25 |
| NL | 36 | 48 | 25 | 25 | ||
| UK | 36 | 36 | 24 | 25 | ||
| Age in years | Mean (95% CI) | FI | 9•0 (8•7–9•3) | 13•7 (13•2–14•2) | 30•2 (28•7–31•7) | 64•2 (63•2–65•2) |
| NL | 8•5 (8•4–8•6) | 13•6 (13•2–13•9) | 28•6 (27•0–30•2) | 65•9 (64•8–67•0) | ||
| UK | 9•2 (8•9–9•6) | 12•8 (12•5–13•1) | 26•1 (24•2–28•0) | 65•7 (64•5–67•0) | ||
| Gender | Female | FI | 18 [48•6] | 19 [51•4] | 21 [84•0] | 21 [84•0] |
| NL | 18 [50•0] | 17 [35•4] | 10 [40•0] | 14 [56•0] | ||
| UK | 16 [44•4] | 18 [50•0] | 16 [66•7] | 13 [52•0] | ||
| Pertussis priming background | aP | FI | 37 [100•0] | 19 [51•4] | 0 [0•0] | 0 [0•0] |
| NL | 36 [100•0] | 25 [52•1] | 0 [0•0] | 0 [0•0] | ||
| UK | 36 [100•0] | 36 [100•0] | 0 [0•0] | 0 [0•0] | ||
| wP / unknown | FI | 0 [0•0] | 18 [48•6] | 25 [100•0] | 25 [100•0] | |
| NL | 0 [0•0] | 23 [47•9] | 25 [100•0] | 25 [100•0] | ||
| UK | 0 [0•0] | 0 [0•0] | 24 [100•0] | 25 [100•0] |
FI: Finland; NL: Netherlands; UK: United Kingdom. CI: confidence interval.
3.2. Pertussis-specific IgG concentrations per age at day 0
The baseline GMCs were low, though several significant differences between age groups were observed as summarised in Table 2. The baseline GMCs of IgG-Ptx antibodies in all age groups were below 20 IU/ml, though six participants from the Netherlands, three from Finland, and one from the UK showed antibody concentrations above 100 IU/ml suggestive of a recent infection (Fig. 3). Baseline IgG-Ptx and FHA GMCs in young adults (n = 74) were significantly lower than in children (n = 109), adolescents (n = 120), and older adults (n = 75), while this group had higher GMCs for Fim2/3 compared to the three other age groups. Older adults showed a lower GMC for IgG-Ptx compared to adolescents, a lower GMC for IgG-Prn but a higher GMC for IgG-Fim2/3 compared to children.
Table 2.
IgG GMCs per age group.
| Antigen | timepoint | Children | Adolescents | Young adults | Older adults |
|---|---|---|---|---|---|
| Ptx GMC (CI) in IU/ml | Day 0 | 11 (9–14)a | 14 (12–18)a,b | 4 (3–5)b,c,d | 9 (7–12)a,d |
| Day 28 | 147 (120–181)e | 161 (132–196)e | 103 (80–133)f,g | 121 (94–155) | |
| 1 year | 35 (28–43)h | 49 (40–59)i,j | 26 (20–34)h,k | 43 (34–56)j | |
| FHA GMC (CI) in IU/ml | Day 0 | 28 (24–32)a | 35 (30–40)a | 20 (17–24)b,c,d | 31 (25–37)a |
| Day 28 | 290 (248–340) | 313 (269–364) | 299 (247–361) | 255 (211–308) | |
| 1 year | 88 (76–104)h | 121 (104–141)i | 113 (93–137) | 109 (90–132) | |
| Prn GMC (CI) in IU/ml | Day 0 | 16 (13–22)b | 12 (10–16) | 13 (9–18) | 8 (6–11)c |
| Day 28 | 293 (223–386)l | 318 (245–414)l | 331 (237–463)l | 171 (123–239) e,f,g | |
| 1 year | 85 (64–111)j | 114 (88–149) | 151 (108–212)i,k | 78 (56–109)j | |
| Fim2/3 GMC (CI) in AU/ml | Day 0 | 2•1 (1•5–2•9)a,b | 3•3 (2•4–4•4)a | 8•0 (5•4–12•0)b,c,d | 4•0 (2•7–5•9)a,c |
| Day 28 | 3•5 (2•5–4•8)e | 4•4 (3•2–6•0)e | 9•0 (6•0–13•4)f,g,l | 4•2 (2•8–6•2)e | |
| 1 year | 2•6 (1•9–3•6)j | 3•4 (2•5–4•6)j | 6•3 (4•2–9•4)h,i | 4•0 (2•7–5•9) |
Ptx: pertussis toxin; FHA: filamentous haemagglutinin; Prn: pertactin; Fim2/3: fimbriae 2 and 3; GMC: geometric mean concentration; CI: confidence interval; IU/ml: international units per millilitre; AU/ml: arbitrary units per millilitre. Significance per antigen has been tested between age groups within a timepoint; p ≤ 0•05, p ≤ 0•01, p ≤ 0•001. Significantly different from:
young adults at day 0.
older adults at day 0.
children at day 0.
adolescents at day 0.
young adults at day 28.
children at day 28.
adolescents at day 28.
adolescents at 1 year.
children at 1 year.
young adults at 1 year.
older adults at 1 year.
older adults at day 28.
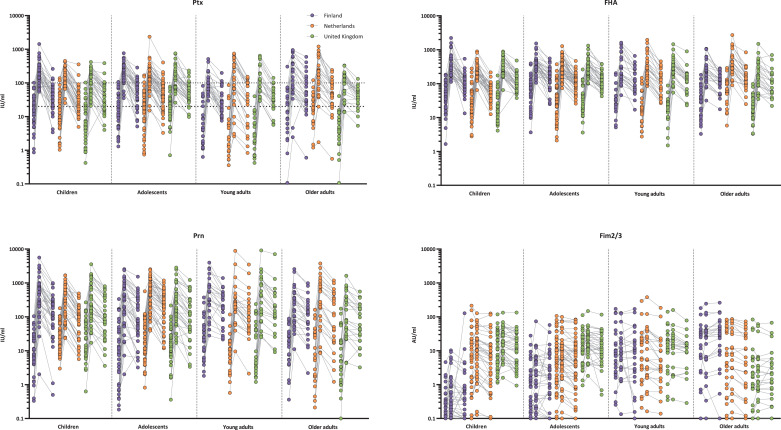
Fig. 3.
Individual IgG responses per age and country. Ptx: pertussis toxin; FHA: filamentous haemagglutinin; Prn: pertactin; Fim2/3: Fimbriae 2 and 3; IU/ml: international units per millilitre; AU/ml: arbitrary units per mililitre. Ptx 20 IU/ml: arbitrary cut-off of protection; Ptx 100 IU/ml: indication of recent infection in absence of a recent vaccination. Values below LLOQ are shown as 0•1 IU/ml or AU/ml. Concentrations from day 0, day 28 and 1 year connected per individual.
3.3. Vaccine-specific IgG concentrations per age at day 28
The pertussis antigen specific GMCs 28 days after the Tdap-IPV booster vaccination increased significantly in all age groups and for all antigens except for Fim2/3 which antigen was not in the vaccine (Table 2). For IgG-Ptx, the young adults (n = 74) still had the lowest GMC (103 IU/ml), significantly lower than children (n = 107) and adolescents (n = 120) (147 and 161 IU/ml, respectively). In contrast young adults had the highest GMC (331 IU/ml) for Prn which along with the children and adolescents (293, 318 IU/ml respectively) was significantly higher than the older adults (n = 75) (171 IU/ml).
3.4. Vaccine-specific IgG concentrations per age at one year
All pertussis antibody levels decreased significantly one year after vaccination compared with levels at day 28, except for Fim2/3 (Table 2), but had remained significantly above baseline levels. The group of young adults (n = 71) still had the lowest GMC for IgG-Ptx, significantly lower than both paediatric groups. By comparison we observed the highest GMC for IgG Prn in the young adults group, significantly higher compared with the children (n = 106) and older adults (n = 74). For both Ptx and FHA, children showed a lower antibody concentration compared with the adolescents (n = 120).
3.5. Pre- and post-vaccination IgA concentrations
In contrast to IgG antibodies the IgA antibodies to all pertussis antigens tended to be higher with increasing age at day 0, day 28, and at 1 year (Table 3). For IgA-Ptx and IgA-Prn, the fold increase from day 0 to day 28 was also clearly age dependant (varying from 4-fold for Ptx and Prn in children to 12-fold for Ptx and 21-fold for Prn in older adults). The fold change decrease from day 28 to 1 year was also slightly age dependant (varying from 1•3-fold for Ptx in children to 2•3-fold for Prn in older adults). For FHA such a pattern could not be observed.
Table 3.
IgA GMCs per age group.
| Antigen | timepoint | Children | Adolescents | Young adults | Older adults |
|---|---|---|---|---|---|
| Ptx GMC (CI) in IU/ml | Day 0 | 0•28 (0•23–0•35)a,b,c | 0•46 (0•37–0•57)c,d | 0•6 (0•46–0•78)c,d | 1•01 (0•77–1•31)a,b,d |
| Day 28 | 1•05 (0•84–1•31)e,f,g | 1•93 (1•56–2•38)g,h | 2•6 (1•98–3•4) g,h | 12•16 (9•3–15•91)e,f,h | |
| 1 year | 0•78 (0•63–0•97)i,j,k | 1•29 (1•05–1•6)k,l | 1•71 (1•3–2•25)k,l | 5•43 (4•15–7•11)i,j,l | |
| FHA GMC (CI) in IU/ml | Day 0 | 0•12 (0•08–0•19)a,b,c | 0•45 (0•3–0•68)c,d | 0•41 (0•24–0•7)c,d | 3•3 (1•96–5•57)a,b,d |
| Day 28 | 2•17 (1•41–3•35)e,f,g | 5•49 (3•63–8•3)f,g,h | 10•89 (6•44–18•42)e,g,h | 98•18 (58•18–165•69)e,f,h | |
| 1 year | 0•25 (0•16–0•39)i,j,k | 1•29 (0•85–1•96)k,l | 2•45 (1•44–4•16)k,l | 28•55 (16•89–48•28)i,j,l | |
| Prn GMC (CI) in IU/ml | Day 0 | 1•15 (0•84–1•6)b,c | 1•78 (1•3–2•42)b,c | 4•14 (2•79–6•14)a,d | 6•19 (4•17–9•17)a,d |
| Day 28 | 4•62 (3•34–6•39)e,f,g | 8•77 (6•43–11•95)f,g,h | 45•55 (30•69–67•61)e,g,h | 131•96 (89•02–195•62)e,f,h | |
| 1 year | 4•31 (3•13–5•95)i,j,k | 6•82 (4•99–9•32)j,k,l | 20•92 (14•04–31•15)i,k,l | 57•21 (38•55–84•89)i,j | |
| Fim2/3 GMC (CI) in AU/ml | Day 0 | 1•05 (0•73–1•51)a,b,c | 2•25 (1•59–3•18)b,c,d | 7•3 (4•67–11•41)a,d | 10•14 (6•49–15•84)a,d |
| Day 28 | 1•79 (1•24–2•57)f,g | 2•78 (1•97–3•93)f,g | 8•64 (5•52–13•51)e,h | 13•17 (8•43–20•58)e,h | |
| 1 year | 1•99 (1•39–2•86)i,j,k | 3•68 (2•59–5•21)j,k,l | 8•19 (5•22–12•84)i,l | 14•21 (9•09–22•22)i,l |
Ptx: pertussis toxin; FHA: filamentous haemagglutinin; Prn: pertactin; Fim2/3: fimbriae 2 and 3; GMC: geometric mean concentration; CI: confidence interval; IU/ml: international units per millilitre; AU/ml: arbitrary units per millilitre. Significance per antigen has been tested between age groups within a timepoint; p ≤ 0•05, p ≤ 0•01, p ≤ 0•001. Significantly different from:
adolescents at day 0.
young adults at day 0.
older adults at day 0.
children at day 0.
adolescents at day 28.
young adults at day 28.
older adults at day 28.
children at day 28.
adolescents at 1 year.
young adults at 1 year.
older adults at 1 year.
children at 1 year.
3.6. Vaccine-specific antibody concentration differences between countries
At baseline several country-specific differences could be observed (Supplementary Table 2). Notable was the higher baseline IgG-FHA GMC in Dutch older adults (n = 25) compared with the older adults from Finland (n = 25) and the UK (n = 25). Also, IgG-Ptx is higher at baseline in the Dutch older adults compared with their peers from the UK. Children and adolescents in the Finnish groups showed significantly higher baseline IgG-FHA GMCs compared with those groups from the Netherlands and the UK but showed lower baseline IgG-Prn and IgG-Fim2/3 levels.
At day 28 post-vaccination, country-specific differences were observed in Dutch older adults who still showed a higher GMC for IgG-FHA (425 IU/ml) than their peers in Finland and the UK (225 and 174 IU/ml, respectively) (Supplementary Table 2). The UK young adults (n = 24) had a higher GMC for IgG-Prn compared with their older adults. IgG-Ptx antibody concentrations below the arbitrary cut-off of protection of 20 IU/ml were observed in two older adults per country. This was also the case in four Dutch young adults and one Dutch adolescent (Fig. 3).
One year post-vaccination most country-specific differences had disappeared (Supplementary Table 2). In all age groups and in all three countries 3 to 13 participants per group had antibody concentrations below the arbitrary protective cut-off of 20 IU/ml (Fig. 3).
IgA concentrations in older adults were generally highest in the Netherlands, followed by Finland, and lowest in the UK at all three timepoints and for all antigens (Fig. 4 and Supplementary Table 3).
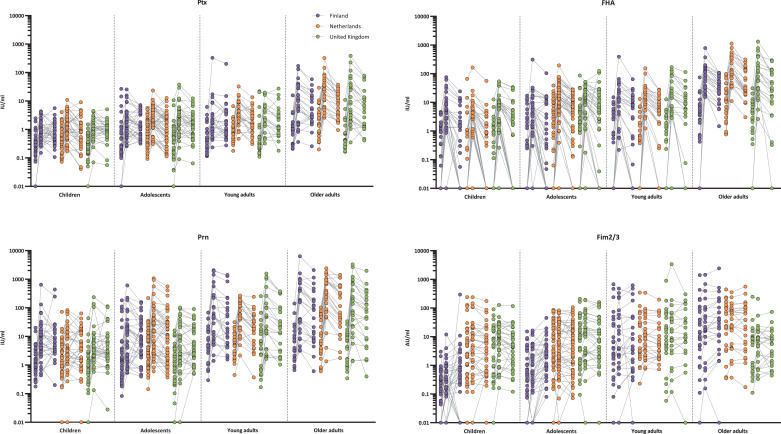
Fig. 4.
Individual IgA responses per age and country. Ptx: pertussis toxin; FHA: filamentous haemagglutinin; Prn: pertactin; Fim2/3: Fimbriae 2 and 3; IU/ml: international units per millilitre; AU/ml: arbitrary units per mililitre. Values below LLOQs for are shown as 0•01 IU/ml or AU/ml. Concentrations from day 0, day 28 and 1 year connected per individual.
3.7. Pertussis antibody concentrations in children and adolescents in relation to vaccination background
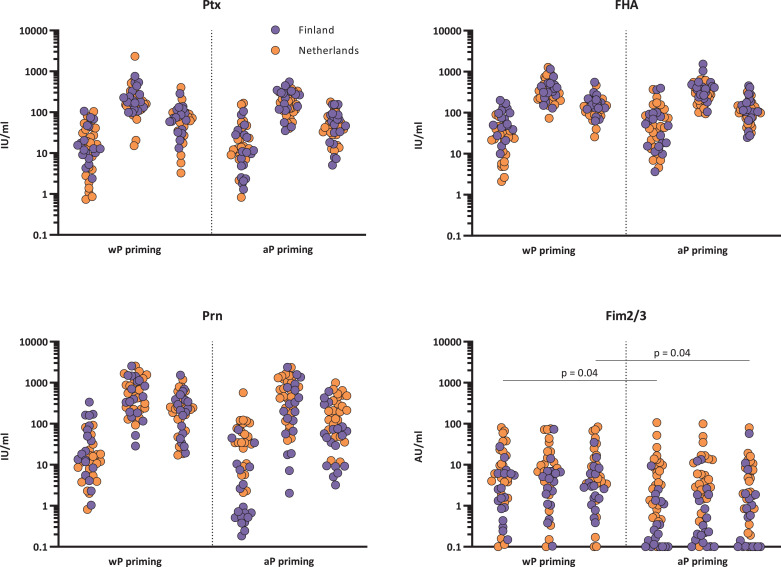
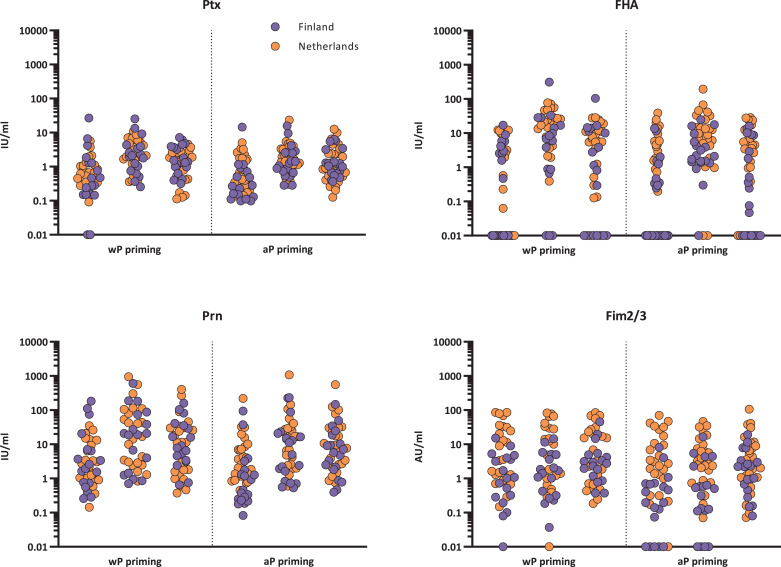
When the results from adolescents from Finland and the Netherlands were divided in two groups, one group with wP (n = 41) and the other with aP (n = 44) priming vaccine history (Fig. 5, Fig. 6), no pre- or post-vaccination differences between the aP and wP group were observed against Ptx, FHA, and Prn. The observed differences between Dutch and Finnish aP primed adolescents for IgG-Fim2/3 and Prn were in line with the different vaccination history regarding antigens included in the different aP vaccines. The subanalyses between the different aP groups showed that the aP2 group (n = 10) had lower IgG-Prn GMCs at all timepoints compared with children and adolescents previously vaccinated with aP3 (n = 7) and wP vaccines (n = 41) (Supplementary Table 4). For Fim2/3 a significant difference between aP3, aP5 (n = 65), and wP was observed at all timepoints, moreover, the aP3 group did not show a significant increase and subsequent decrease, where the aP5 group did show this pattern (Supplementary Table 5).
Fig. 5.
IgG concentrations of pertussis vaccine components per wP and aP background. Ptx: pertussis toxin; FHA: filamentous haemagglutinin; Prn: pertactin; Fim2/3: Fimbriae 2 and 3; IU/ml: international units per millilitre AU/ml: arbitrary units per mililitre; aP priming: participants received exclusively acellular pertussis components containing vaccines in the primary series; wP priming: participants received exclusively whole cell pertussis containing vaccines in the primary series. values below LLOQ are shown as 0•1 IU/ml or AU/ml. Significance has been tested at day 0, day 28 and 1 year between wP and aP priming.
Fig. 6.
IgA concentrations of pertussis vaccine components per wP and aP background. Ptx: pertussis toxin; FHA: filamentous haemagglutinin; Prn: pertactin; Fim2/3: Fimbriae 2 and 3; IU/ml: international units per millilitre; AU/ml: arbitraty units per mililitre; aP priming: participants received exclusively acellular pertussis components containing vaccines in the primary series; wP priming: participants received exclusively whole cell pertussis containing vaccines in the primary series. values below LLOQ are shown as 0•1 IU/ml or AU/ml. Significance has been tested at day 0, day 28 and 1 year between aP and wP priming.
4. Discussion
Here we present the serological data after a Tdap-IPV booster vaccination in four age groups of three European countries. IgG concentrations for all antigens demonstrated a booster pattern with considerable increases of pertussis-specific antibodies observed in all age groups after the first month. Although there were significant decreases at one year the GMCs remained at least 3-fold higher than baseline for all antigens and age groups. There were some significant differences between age groups which varied by antigen but antibody responses were not consistently worse with increasing age. These data emphasise that at least in terms of antibody quantity, responses are not uniformly less in older adults compared with the other age groups. When analysed with the countries separately, the IgG antibody concentrations were overall not significantly different in 90% (81/90 at 28 days) and 97% (87/90 at 1 year) of the comparisons. For IgA increases for all antigens were relatively smaller and decreases varied in significance. Moreover, the IgA antibody concentrations showed pronounced increase with age for all antigens and timepoints especially notable in older adults.
Age seemed to influence the specific IgA responses to vaccination. It is already known that pre-vaccination IgA concentrations increase with age [50], [51], [52]. It is also previously described that greater IgA-antibody increase due to vaccination is positively correlated to high pre-vaccination concentrations [53]. In this study we see a similar pattern of higher IgA-antibody concentrations at baseline with higher age. One month post-vaccination a clear age dependant increase for IgA-Ptx and Prn is observed. Interestingly the antibody decay of these IgA antibodies was also age dependant, with greater fold decrease in older age groups. IgG responses on the other hand did not show a consistent relationship with age. In contrast a booster vaccine study in the USA previously described higher post-vaccination IgG concentrations for Ptx in adolescents compared with adults until three years post-vaccination. They hypothesized that this was either due to greater immune responsiveness at adolescent age or due to a shorter time interval since the paediatric vaccinations in the adolescents compared to the adults [54]. The post-vaccination GMCs for IgG-Ptx of young adults in our study was lower compared with the adolescents, but the older adults on the other hand, had a significantly higher GMC compared with young adults one year post-vaccination. Therefore, with respect to IgG, we did not observe less or better immune responsiveness, or a positive or negative effect with more recent vaccinations. Although the fold increase and subsequent fold decrease in IgG responses was greater than in IgA, the degree of change in IgG responses did not seem to be influenced by age. Differences in antibody kinetics between specific IgG and IgA responses can possibly be explained by the route of primary activation. Usually specific IgA responses result after the person encounters B. pertussis in life [55], and IgA production is therefore considered activated via the mucosal route, while IgG production has mostly been parenterally activated after vaccination in early infancy. It is plausible to assume that these two routes lead to different kinetics [56]. Both IgG and IgA responses will be boosted by revaccination and reinfection. It is highly likely that constant circulation of B. pertussis in the population makes that the number of encounters increase with age. Therefore, our results imply that IgA antibody responses are more influenced by this constant circulation than the IgG responses. This is supported by the Dutch data, given that the circulation of B. pertussis is higher in the Netherlands and the Dutch older adults had highest IgA concentrations for all antigens at all timepoints. The difference in IgA concentrations between young and older adults might not only be explained by the amount of encounters, but also the route of primary and secondary activation is likely to influence the IgA response since the older adults will not all have been primed by vaccination but by natural infection and also later the natural boosting might have had a more pronounced effect to IgA mediated immunity.
Based on the reported cases with pertussis the circulation of B. pertussis might be different between the three countries participating in this study. The notification rate (N/100,000, all ages) in the last decade was highest in the Netherlands, followed by Finland, and lowest numbers in the UK (Fig. 1) [24]. These differences in circulation between the countries appear to be reflected in some findings in this study. The Dutch older adults had a significantly higher IgG and IgA baseline GMC for Ptx, and FHA compared with UK older adults, while the GMC of older Finnish adults were in between these two. For FHA this difference still exists one month post-vaccination. The effect of pertussis circulation is probably best reflected in older adults, since their vaccination history is comparable between the countries and previous vaccinations were administered long ago or not at all. Additionally, in this study the number of recent pertussis infections defined as ≥ 100 IU/ml for IgG-Ptx at baseline [39], were most observed in the Dutch cohort, followed by the Finnish cohort, and then the UK, which is also in line with the national notification rates. Nevertheless, we should bear in mind that the notification rates in the European countries as illustrated on the ECDC website are fluctuating heavily per country and are strongly dependant on the use of diagnostic methods (PCR and/or serology) and the accuracy of the pertussis surveillance system in each country. Thus the reports might therefore not reflect the real incidence numbers. Seroepidemiological studies could provide more information about the seroprevalence of pertussis antibodies in the population and thereby on the degree of circulation of the pathogen, but these studies have been performed in only a few European countries [57,58].
Differences due to vaccination background, especially between wP and aP priming were anticipated since higher responses in aP primed 4-year-olds compared with their wP primed peers have been described [38]. Two studies in children of respectively 9 years of age and 11 and 12 years of age, on the other hand, described better responses in wP primed individuals [59,60]. In this study however, the two priming backgrounds did not reveal any differences in IgG levels against the vaccine antigens pre- or post-vaccination, which might be explained by the limited number of participants for the comparison between wP and aP priming. Participants previously vaccinated with aP2 vaccines (and thus lacking Prn) show a significantly lower baseline and a lower Prn antibody increase upon ‘booster’ vaccination, reflecting a more primary response to Prn compared with participants previously aP3 or wP vaccinated who show a real booster response (Supplementary Table 4) [61]. Participants previously vaccinated with aP3 vaccines (and thus lacking Fim2/3) had lower Fim2/3 concentrations at baseline and did not show a significant increase where aP5 and wP primed participants did show a significant increase although Fim2/3 was not even in the vaccine, indicating polyclonal activation of non-vaccine antigens (Supplementary Table 5). Polyclonal activation of unrelated antigens to which the donor was previously immunised has been observed before [62].
It is questionable if a booster vaccination induces sufficient pertussis antibody levels on the long term (i.e. five years) for protection considering the decrease in pertussis antibody levels one year after the booster vaccination found in this study. However Ward et al. found a vaccine efficacy of 92% in adolescents and adults for 22 months, Zöldi et al. found a decrease in pertussis notifications in young adults due to military conscripts vaccination, and Liu et al. found a protective effect in older adults even after five years [29,63,64]. The study from Taranger et al. found a correlation between the height of the post-vaccination antibody concentrations and protection against pertussis in toddlers up to 33 months post-vaccination [65]. The one month post-vaccination GMCs from our groups correspond most with the GMCs of the toddler group that developed mild pertussis disease within 33 months indicating that our study population might be susceptible to contract mild pertussis within three years after the booster vaccination. The implementation of additional pertussis boosters in the (older) adults should be discussed not only in the context of the persistence of antibody levels on the long term but also on the epidemiological pertussis situation in each individual country. Moreover, a systematic literature review from Kandeil et al. states that the pertussis disease burden is considerably underestimated in older adults. Older adults are more likely to have (pulmonary) comorbidities and are therefore more prone to serious complications and hospitalisation. With the ageing population, health and economic burden of pertussis is expected to rise [3].
A strength of the study is that serological antibody results of all participants could reliably be compared between the three countries and the four age groups, since all antibody measurements were performed in the same lab with a highly standardised and validated multiplex assay. In addition, 375 participants in total completed this pertussis booster vaccination study based on inclusion of 22–48 participants per age group per country.
A limitation of the study is that the vaccination background of the participants appeared to be quite divergent between countries and even within countries, thereby making analysis based on vaccination background quickly underpowered.
In conclusion, the participants from all four age groups in the three countries responded well to the aP booster vaccination as measured after one month and one year. The influence of age and epidemiological background of pertussis in the countries seems limited on the IgG vaccine responses, however increasing age does seem to have a positive effect on IgA responses. Therefore, acellular pertussis booster vaccination might also be considered for older adults and individuals with pulmonary morbidities in order to reduce the health and economic burden of pertussis in the population.
Declaration of Competing Interest
None of the authors received payment or service from a third part at any time, nor does anyone have a financial relationship with entities in the bio-medical arena. None of the authors have any patents relevant to the work. MVP is a member of the Portuguese National Immunisation Technical Advisory Group (Comissão Técnica de Vacinação da Direcção Geral de Saúde).
Acknowledgments
Acknowledgements
We thank all participants who made this work possible, and all study nurses and clinical trial staff, especially Jacqueline Zonneveld and Greetje van Asselts, at the Spaarne Gasthuis Hospital, Hoofddorp, Netherlands for their help in the management of clinical data and performing home visits. We are also grateful to Inge Pronk and Eleonora Lambert, who both work at the Centre for Infectious Disease Control, for their assistance in clinical trial management.
PERISCOPE has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 115910. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA and BMGF.
DFK receives salary from support from the NIHR Oxford Biomedical Research Centre.
Data Sharing Statement
Individual participant data that underlie the results reported in this article, have been de-identified and deposited in the central database of the PERISCOPE Consortium and can be accessed by a request to the PERISCOPE management team.
Contributors
GAMB, DFK, and JM designed the trial with input from QH, EAMS, RdG, DAD, A-MB, PV, MVP, and AMB. Trial coordination, clinical trial management and clinical data collection was performed by PV, MVP, AMB, MAvH, RL, and SB. Samples were processed by PGMvG, SB, MVP, AMB and PV. Luminex data were generated by PGMvG and PV. Underlying data were verified by PV, PGMvG, GAMB, DFK, JM. Data analysis was performed by JvdK with input from PV, GAMB, DFK, and JM. PV and GAMB wrote the first draft of the manuscript and all co-authors contributed to subsequent drafts. All authors read and approved the final manuscript.
Footnotes
Funding: PERISCOPE has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 115910. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme, EFPIA and BMGF.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103247.
Appendix. Supplementary materials
References
- 1.Mattoo S., Cherry J.D. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18(2):326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cherry J.D. Pertussis in young infants throughout the world. Clin Infect Dis. 2016;63(suppl 4):S119–Ss22. doi: 10.1093/cid/ciw550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandeil W., Atanasov P., Avramioti D., Fu J., Demarteau N., Li X. The burden of pertussis in older adults: what is the role of vaccination? A systematic literature review. Expert Rev Vaccines. 2019;18(5):439–455. doi: 10.1080/14760584.2019.1588727. [DOI] [PubMed] [Google Scholar]
- 4.Cherry J.D. Historical review of pertussis and the classical vaccine. J Infect Dis. 1996;174(Suppl 3):S259–S263. doi: 10.1093/infdis/174.supplement_3.s259. [DOI] [PubMed] [Google Scholar]
- 5.David S., Vermeer-de Bondt P.E., van der Maas N.A.T. Reactogenicity of infant whole cell pertussis combination vaccine compared with acellular pertussis vaccines with or without simultaneous pneumococcal vaccine in the Netherlands. Vaccine. 2008;26(46):5883–5887. doi: 10.1016/j.vaccine.2008.07.105. [DOI] [PubMed] [Google Scholar]
- 6.Lambert L.C. Pertussis vaccine trials in the 1990s. J Infect Dis. 2014;209(Suppl 1):S4–S9. doi: 10.1093/infdis/jit592. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheridan S.L., Ware R.S., Grimwood K., Lambert S.B. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA. 2012;308(5):454–456. doi: 10.1001/jama.2012.6364. [DOI] [PubMed] [Google Scholar]
- 8.Klein N.P., Bartlett J., Rowhani-Rahbar A., Fireman B., Baxter R. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med. 2012;367(11):1012–1019. doi: 10.1056/NEJMoa1200850. [DOI] [PubMed] [Google Scholar]
- 9.Warfel J.M., Zimmerman L.I., Merkel T.J. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A. 2014;111(2):787–792. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell H., Amirthalingam G., Andrews N. Accelerating control of pertussis in England and Wales. Emerg Infect Dis. 2012;18(1):38–47. doi: 10.3201/eid1801.110784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Maas N.A.T., David S., Kemmeren J.M., Vermeer-de Bondt P.E. [Safety surveillance in the National Vaccination Programme; fewer adverse events with the DTP-IPV-Hib vaccine after the transition to an acellular pertussis component in 2005] Ned Tijdschr Geneeskd. 2007;151(49):2732–2737. [PubMed] [Google Scholar]
- 12.Elomaa A., He Q., Minh N.N., Mertsola J. Pertussis before and after the introduction of acellular pertussis vaccines in Finland. 2009; 27(40): 5443–9. [DOI] [PubMed]
- 13.Berbers G.A.M., de Greeff S.C., Mooi F.R. Improving pertussis vaccination. Hum Vaccin. 2009;5(7):497–503. doi: 10.4161/hv.8112. [DOI] [PubMed] [Google Scholar]
- 14.Kapil P., Merkel T.J. Pertussis vaccines and protective immunity. Curr Opin Immunol. 2019;59:72–78. doi: 10.1016/j.coi.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcellini V., Piano Mortari E., Fedele G. Protection against pertussis in humans correlates to elevated serum antibodies and memory B cells. Front Immunol. 2017;8:1158. doi: 10.3389/fimmu.2017.01158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amirthalingam G., Andrews N., Campbell H. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014;384(9953):1521–1528. doi: 10.1016/S0140-6736(14)60686-3. [DOI] [PubMed] [Google Scholar]
- 17.Cherry J.D., Gornbein J., Heininger U., Stehr K. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine. 1998;16(20):1901–1906. doi: 10.1016/s0264-410x(98)00226-6. [DOI] [PubMed] [Google Scholar]
- 18.Dabrera G., Amirthalingam G., Andrews N. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012-2013. Clin Infect Dis. 2015;60(3):333–337. doi: 10.1093/cid/ciu821. [DOI] [PubMed] [Google Scholar]
- 19.Storsaeter J., Hallander H.O., Gustafsson L., Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine. 1998;16(20):1907–1916. doi: 10.1016/s0264-410x(98)00227-8. [DOI] [PubMed] [Google Scholar]
- 20.Broutin H., Viboud C., Grenfell B.T., Miller M.A., Rohani P. Impact of vaccination and birth rate on the epidemiology of pertussis: a comparative study in 64 countries. Proc Biol Sci. 2010;277(1698):3239–3245. doi: 10.1098/rspb.2010.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elomaa A., Advani A., Donnelly D. Strain variation among Bordetella pertussis isolates in Finland, where the whole-cell pertussis vaccine has been used for 50 years. J Clin Microbiol. 2005;43(8):3681–3687. doi: 10.1128/JCM.43.8.3681-3687.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Maas N.A.T., Mooi F.R., de Greeff S.C., Berbers G.A.M., Spaendonck M.A., de Melker H.E. Pertussis in the Netherlands, is the current vaccination strategy sufficient to reduce disease burden in young infants? Vaccine. 2013;31(41):4541–4547. doi: 10.1016/j.vaccine.2013.07.060. [DOI] [PubMed] [Google Scholar]
- 23.Schurink-van't Klooster T.M., de Melker H.E. The national immunisation programme in the Netherlands: surveillance and developments in 2018-2019. 2019.
- 24.ECDC (2019). Disease data from ECDC surveillance Atlas for pertussis. http://ecdc.europa.eu/en/pertussis/surveillance-and-disease-data/atlas (accessed 20-09-2019.
- 25.Carvalho C.F.A., Andrews N., Dabrera G. National Outbreak of pertussis in England, 2011-2012: a case-control study comparing 3-component and 5-component acellular vaccines with whole-cell pertussis vaccines. Clin Infect Dis. 2020;70(2):200–207. doi: 10.1093/cid/ciz199. [DOI] [PubMed] [Google Scholar]
- 26.England P.H. Public Health England; 2019. Pertussis: laboratory confirmed cases reported in England. 2020. [Google Scholar]
- 27.Ramsay M.E., Rao M., Begg N.T., Redhead K., Attwell A.M. Antibody response to accelerated immunisation with diphtheria, tetanus, pertussis vaccine. Lancet. 1993;342(8865):203–205. doi: 10.1016/0140-6736(93)92298-8. [DOI] [PubMed] [Google Scholar]
- 28.Forsyth K.D., Tan T., von Konig C.W., Heininger U., Chitkara A.J., Plotkin S. Recommendations to control pertussis prioritized relative to economies: a Global Pertussis Initiative update. Vaccine. 2018;36(48):7270–7275. doi: 10.1016/j.vaccine.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 29.Zoldi V., Sane J., Nohynek H., Virkki M., Hannila-Handelberg T., Mertsola J. Decreased incidence of pertussis in young adults after the introduction of booster vaccine in military conscripts: epidemiological analyses of pertussis in Finland, 1995-2015. Vaccine. 2017;35(39):5249–5255. doi: 10.1016/j.vaccine.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Barug D., Pronk I., van Houten M.A. Maternal pertussis vaccination and its effects on the immune response of infants aged up to 12 months in the Netherlands: an open-label, parallel, randomised controlled trial. Lancet Infect Dis. 2019;19(4):392–401. doi: 10.1016/S1473-3099(18)30717-5. [DOI] [PubMed] [Google Scholar]
- 31.Sandmann F., Jit M., Andrews N. Infant hospitalisations and fatalities averted by the maternal pertussis vaccination programme in England, 2012-2017: post-implementation economic evaluation. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa165. [DOI] [PubMed] [Google Scholar]
- 32.Cassimos D.C., Effraimidou E., Medic S., Konstantinidis T., Theodoridou M., Maltezou H.C. Vaccination programs for adults in Europe, 2019. Vaccines (Basel) 2020;8(1) doi: 10.3390/vaccines8010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Government A. 2019. Australian immunisation handbook. In: Health Do, editor. Last updated: 4 June 2018 Last reviewed: 4 June 2018 ed: Australian Government. [Google Scholar]
- 34.Haviari S., Bénet T., Saadatian-Elahi M., André P., Loulergue P., Vanhems P. Vaccination of healthcare workers: a review. Hum Vaccin Immunother. 2015;11(11):2522–2537. doi: 10.1080/21645515.2015.1082014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.OECD (2020). Child vaccination rates (indicator). doi: 10.1787/b23c7d13-en (accessed on 21 August 2020).
- 36.PERISCOPE Consortium PERISCOPE: road towards effective control of pertussis. Lancet Infect Dis. 2019;19(5):e179. doi: 10.1016/S1473-3099(18)30646-7. -e86. [DOI] [PubMed] [Google Scholar]
- 37.van Gageldonk P.G.M., van Schaijk F.G., van der Klis F.R., Berbers G.A.M. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J Immunol Methods. 2008;335(1–2):79–89. doi: 10.1016/j.jim.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Hendrikx L.H., Berbers G.A.M., Veenhoven R.H., Sanders E.A.M., Buisman A.M. IgG responses after booster vaccination with different pertussis vaccines in Dutch children 4 years of age: effect of vaccine antigen content. Vaccine. 2009;27(47):6530–6536. doi: 10.1016/j.vaccine.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 39.van der Lee S., Stoof S.P., van Ravenhorst M.B. Enhanced Bordetella pertussis acquisition rate in adolescents during the 2012 epidemic in the Netherlands and evidence for prolonged antibody persistence after infection. Euro surveill: Bull Euro Maladies Transm = Euro Commun Dis Bull. 2017;22(47):17. doi: 10.2807/1560-7917.ES.2017.22.47.17-00011. -00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long S.S., Welkon C.J., Clark J.L. Widespread silent transmission of pertussis in families: antibody correlates of infection and symptomatology. J Infect Dis. 1990;161(3):480–486. doi: 10.1093/infdis/161.3.480. [DOI] [PubMed] [Google Scholar]
- 41.Sato Y., Sato H. Anti-pertussis toxin IgG and anti-filamentous hemagglutinin IgG production in children immunized with pertussis acellular vaccine and comparison of these titers with the sera of pertussis convalescent children. Dev Biol Stand. 1985;61:367–372. [PubMed] [Google Scholar]
- 42.Olin P., Hallander H.O., Gustafsson L., Reizenstein E., Storsaeter J. How to make sense of pertussis immunogenicity data. Clin Infect Dis. 2001;33(Suppl 4):S288–S291. doi: 10.1086/322564. [DOI] [PubMed] [Google Scholar]
- 43.Guo B., Yuan Y. A comparative review of methods for comparing means using partially paired data. Stat Methods Med Res. 2017;26(3):1323–1340. doi: 10.1177/0962280215577111. [DOI] [PubMed] [Google Scholar]
- 44.Giesbrecht F.G., Burns J.C. Two-stage analysis based on a mixed model: large-sample asymptotic theory and small-sample simulation results. Int Biometr Soc. 1985;41(2):477–486. [Google Scholar]
- 45.Hrong-Tai Fai A., Cornelius P.L. Approximate F-tests of multiple degree of freedom hypotheses in generalized least squares analyses of unbalanced split-plot experiments. J Stat Comput Simul. 1996;54(4):363–378. [Google Scholar]
- 46.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 47.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: a language and environment for statistical computing. 2020 ed. [Google Scholar]
- 48.Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;1(Issue 1):2015. [Google Scholar]
- 49.Kuznetsova A., Brockhoff P.B., Christensen R.H.B. lmerTest Package: tests in Linear Mixed Effects Models. J Stat Softw. 2017;1(Issue 13):2017. [Google Scholar]
- 50.Fumimoto R., Otsuka N., Kamiya H. Seroprevalence of IgA and IgM antibodies to Bordetella pertussis in healthy Japanese donors: assessment for the serological diagnosis of pertussis. PLoS ONE. 2019;14(7) doi: 10.1371/journal.pone.0219255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.May M.L., Evans J., Holgate T., Doi S.A., Ross P., Robson J.M. Pertussis toxin IgA testing over-diagnoses recent pertussis infection. Pathology. 2017;49(7):770–775. doi: 10.1016/j.pathol.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Prince H.E., Lieberman J.M., Cherry J.D. Age-related differences in patterns of increased Bordetella pertussis antibodies. Clin Vaccine Immunol. 2012;19(4):545–550. doi: 10.1128/CVI.05725-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le T., Cherry J.D., Chang S.J. Immune responses and antibody decay after immunization of adolescents and adults with an acellular pertussis vaccine: the APERT Study. J Infect Dis. 2004;190(3):535–544. doi: 10.1086/422035. [DOI] [PubMed] [Google Scholar]
- 54.Pool V., Tomovici A., Johnson D.R., Greenberg D.P., Decker M.D. Humoral immunity 10years after booster immunization with an adolescent and adult formulation combined tetanus, diphtheria, and 5-component acellular pertussis vaccine in the USA. Vaccine. 2018;36(17):2282–2287. doi: 10.1016/j.vaccine.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 55.Hendrikx L.H., Ozturk K., de Rond L.G. Serum IgA responses against pertussis proteins in infected and Dutch wP or aP vaccinated children: an additional role in pertussis diagnostics. PLoS ONE. 2011;6(11):e27681. doi: 10.1371/journal.pone.0027681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Engels N., Wienands J. Memory control by the B cell antigen receptor. Immunol Rev. 2018;283(1):150–160. doi: 10.1111/imr.12651. [DOI] [PubMed] [Google Scholar]
- 57.de Greeff S.C., de Melker H.E., van Gageldonk P.G.M. Seroprevalence of pertussis in The Netherlands: evidence for increased circulation of Bordetella pertussis. PLoS ONE. 2010;5(12):e14183. doi: 10.1371/journal.pone.0014183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barkoff A.M., Gröndahl-Yli-Hannuksela K., He Q. Seroprevalence studies of pertussis: what have we learned from different immunized populations. Pathog Dis. 2015;73(7) doi: 10.1093/femspd/ftv050. [DOI] [PubMed] [Google Scholar]
- 59.van der Lee S., Hendrikx L.H., Sanders E.A.M., Berbers G.A.M., Buisman A.M. Whole-cell or acellular pertussis primary immunizations in infancy determines adolescent cellular immune profiles. Front Immunol. 2018;9:51. doi: 10.3389/fimmu.2018.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Decker M.D., Greenberg D.P., Johnson D.R., Pool V. Randomized study of immune responses to two Tdap vaccines among adolescents primed with DTaP and comparison with results among adolescents primed with DTwP. Vaccine. 2019;37(35):5003–5008. doi: 10.1016/j.vaccine.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 61.Nicholson L.B. The immune system. Essays Biochem. 2016;60(3):275–301. doi: 10.1042/EBC20160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bernasconi N.L., Traggiai E., Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298(5601):2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 63.Ward J.I., Cherry J.D., Chang S.J. Efficacy of an acellular pertussis vaccine among adolescents and adults. N Engl J Med. 2005;353(15):1555–1563. doi: 10.1056/NEJMoa050824. [DOI] [PubMed] [Google Scholar]
- 64.Liu B., He W.Q., Newall A.T. Effectiveness of acellular pertussis vaccine in older adults: nested matched case-control study. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz821. [DOI] [PubMed] [Google Scholar]
- 65.Taranger J., Trollfors B., Lagergård T. Correlation between pertussis toxin IgG antibodies in post vaccination sera and subsequent protection against pertussis. J Infect Dis. 2000;181(3):1010–1013. doi: 10.1086/315318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.