Abstract
Purpose
This study investigated the effects of hyaluronic acid (HA) on peri-implant clinical variables and crevicular concentrations of the proinflammatory biomarkers interleukin (IL)-1β and tumor necrosis factor (TNF)-α in patients with peri-implantitis.
Methods
A randomized controlled trial was conducted in peri-implantitis patients. Patients were randomized to receive a 0.8% HA gel (test group), an excipient-based gel (control group 1), or no gel (control group 2). Clinical periodontal variables and marginal bone loss after 0, 45, and 90 days of treatment were assessed. IL-1β and TNF-α levels in crevicular fluid were measured by enzyme-linked immunosorbent assays at baseline and after 45 days of treatment. Clustering analysis was performed, considering the possibility of multiple implants in a single patient.
Results
Sixty-one patients with 100 dental implants were assigned to the test group, control group 1, or control group 2. Probing pocket depth (PPD) was significantly lower in the test group than in both control groups at 45 days (control 1: 95% CI, −1.66, −0.40 mm; control 2: 95% CI, −1.07, −0.01 mm) and 90 days (control 1: 95% CI, −1.72, −0.54 mm; control 2: 95% CI, −1.13, −0.15 mm). There was a trend towards less bleeding on probing in the test group than in control group 2 at 90 days (P=0.07). Implants with a PPD ≥5 mm showed higher levels of IL-1β in the control group 2 at 45 days than in the test group (P=0.04).
Conclusions
This study demonstrates for the first time that the topical application of a HA gel in the peri-implant pocket and around implants with peri-implantitis may reduce inflammation and crevicular fluid IL-1β levels.
Trial Registration
ClinicalTrials.gov Identifier: NCT03157193
Keywords: Clinical trial, Dental implants, Hyaluronic acid, Peri-implantitis
Graphical Abstract
INTRODUCTION
Hyaluronic acid (HA) is a non-sulfated glycosaminoglycan that is highly abundant in the extracellular matrix of periodontal tissues [1]. Its capacity to absorb water and increase its dry weight >50-fold (as one of the most hygroscopic substances known) gives the extracellular matrix a high degree of elasticity and tissue lubrication, favoring gas and molecule exchange and enabling it to act as barrier against macromolecules, viruses, and bacteria [2]. HA has a wide range of mechanisms of action, including interaction with nociceptive pain pathways, modulation of extracellular matrix homeostasis, and cell apoptosis [3]. With regard to its role in extracellular homeostasis, HA intervenes in tissue repair and healing (inflammation, granulation tissue formation, epithelium formation, and tissue remodeling) in both mineralized and non-mineralized tissues and has been proposed as an anti-inflammatory and anti-edematous agent with no adverse effects [4].
Peri-implantitis is a plaque-associated pathological condition occurring in tissues around dental implants, characterized by inflammation in the peri-implant mucosa and subsequent progressive loss of supporting bone [5]. Gingival and peri-implant inflammation destroys the extracellular matrix of connective tissue by enzymatic heterolysis and increases gingival fluid levels of glycosaminoglycans: mainly chondroitin-4-sulfate, which is used as marker of active periodontitis, but also low-molecular-weight (LHW)-HA (0.5–2×106 Da) [6]. LHW fragments play a role in signaling tissue damage and mobilizing immune cells, while high-molecular-weight (HMW)-HA suppresses the immune response, preventing excessive inflammation [7].
In addition to clinical and radiological examinations, the measurement of cytokines in peri-implant crevicular fluid (PICF) may represent a further non-invasive method of diagnosing implant health and peri-implantitis. A recent systematic review reported that PICF concentrations of proinflammatory cytokines (interleukin [IL]-1β, IL-6, IL-12, IL-17, and tumor necrosis factor [TNF]-α) were higher in cases of peri-implantitis than in healthy implants [8].
Topical treatment of non-keratinized sulcular epithelium was found to deliver high concentrations of pharmacological agents to the periodontal tissue, gingiva, periodontal ligament, alveolar bone, and cementum [9]. To our knowledge, the only evidence on the effects of HMW-HA in peri-implantitis is from a previous publication from our group regarding a microbiome analysis performed on the same patients analyzed in this study [10], and a pilot study that evaluated the application of HA as a nebulizing spray [11]. The study involving the nebulizing spray was performed only in 5 patients and after 15 days of follow-up, without considering criteria for the diagnosis of peri-implantitis or radiologic assessments. We therefore designed a randomized controlled clinical trial to evaluate the effects of an HMW-HA gel on peri-implant clinical variables and on PICF IL-1β and TNF-α concentrations 1 year after implant loading.
MATERIALS AND METHODS
Subject population
A double-blinded, controlled, randomized clinical trial was designed with 3 parallel groups, including 104 implants placed in 63 patients and diagnosed with peri-implantitis at a private oral surgery clinic in Granada, Spain. All patients who met the inclusion criteria were asked to provide written informed consent, and the study was approved by the Ethics Committee in Human Research of the University of Granada (reference No. 589, 28/04/2011). All methods used were in accordance with the Helsinki Declaration of 1975, as revised in 2013. The study protocol was registered at clinicaltrials.gov (NCT03157193; May 17, 2017) and was designed following CONSORT guidelines [12].
Inclusion and exclusion criteria
The inclusion criteria for the study were: age >18 years and diagnosis of peri-implantitis in implants at ≥1 year since loading according to the criteria of the Association of Dental Implantology (ADI) (probing pocket depth [PPD] ≥4 mm, bleeding on probing, and radiological marginal bone loss (MBL) >2 mm) [13]. The exclusion criteria were consumption of antibiotics or anti-inflammatories in the previous 4 weeks, pregnancy or breastfeeding, the presence of cancer, and previous treatment for peri-implantitis. All implants were 1-stage surgery Tapered Swiss Plus® models (Zimmer Dental, Barcelona, Spain) with a conical design, 2.6-mm machined neck, inner connection, and microtextured surface, which were placed at the bone crest level by the same surgeon (Elena Sánchez-Fernández).
Sample size calculation
Implants were the unit of analysis. The sample size (of implants) per group was calculated using Sample Power 2.0 (SPSS Inc., Chicago, IL, USA) to detect a standardized difference of 0.80 with 80% power and 5% alpha error (a substantial difference according to the Cohen scale [14]) using the Student's t-test for independent samples. This approach is useful when there is no clear or definitive gold standard to define a clinically significant change, and the reference is therefore the Cohen scale for the standardized difference between 2 groups for any output variable of interest. A sample size of 25 implants per group was estimated for some of the quantitative outcome variables (e.g., cytokine concentrations and PPD), which was increased to 30 implants/group after applying a coefficient (design effect) of 1.2 to account for the clustering of implants in patients. Assuming an average of 2 implants per patient, this was considered equivalent to 15 patients per group, and a minimum final patient sample size of 20 per group was established to compensate for possible missing data. It should be kept in mind that these variables should all be considered as proxy variables for the primary outcome of periodontal inflammation.
Randomization and group allocation
Patients meeting the eligibility criteria were consecutively enrolled and randomly assigned to 1 of the following 3 groups by using a computer-assisted block randomization method until the sample size was reached. Sequentially numbered containers with the study products were provided by Ricerfarma srl (Milan, Italy). Random allocation of the containers was performed using a randomization list balanced for the 3 groups created with SAS (SAS Institute, Cary, NC, USA). The examiner who gathered all clinical data and the patients from the test group and control group 1 were blinded in this process, but the patients in control group 2 were not, since they did not receive any product.
Test group: These patients received, in the dental office, a single application by syringe of 0.8% HMW-HA gel (crosslinked HA, 6–7×106 Da, Ricerfarma srl) in the peri-implant pocket and around the implant, followed by application of the same gel (at a 0.2% concentration) by the patient at home, massaging the gingiva around the affected implant(s) 3 times per day for 45 days, always after tooth-brushing, followed by a 20-minute period without eating or drinking.
Control group 1: The patients applied an exopolysaccharide (galactomannan) hydroxypropyl guar gel 3 times per day for 45 days only at home, without application in the dental office. This product is a viscous and insipid gel similar to that used in the test group.
Control group 2: These patients received no topical application of any compound, either in the dental office or at home.
Clinical, radiological, and biochemical variables
Data were gathered on the age, sex, smoking habit (cigarettes per day), and alcohol consumption (g/day) of patients and on the length, diameter, location, and type (unitary or abutment) of implants, as well as the time since implant loading (in months).
A PCPUNC 15 manual probe (Hu-Friedy, Chicago, IL, USA) was used to determine the PPD at 4 implant sites (mesial, vestibular, distal, and palatal/lingual), clinical attachment (CA) loss, and peri-implant bleeding [15]. The reference point for measuring CA loss was the most coronal transmucosal portion of the implant (machined neck), at the boundary between the implant and the prosthesis, until the deepest point of the exposed intra-osseous rough surface. The examiner (Elena Sánchez-Fernández) was blinded to the group membership of patients. An inter-examiner agreement of 80% was obtained for the agreement betweenElena Sánchez-Fernández and the reference researcher (Francisco Mesa) in a sample of 10 implants, accepting a variability of ±1 mm for PPD and CA loss.
Periapical radiovisiography was used to determine the MBL, employing a radiological positioner (XCP Endokit, Dentsply Rinn, Long Island City, NY, USA) to achieve parallelism. The mesial and distal distances between the marginal bone crest and the implant rough surface (under the 2.6-mm machined neck) were obtained from X-rays using a digital image analysis program (Image J© v.1.48, National Institutes of Health, Bethesda, MD, USA) [16]. The image analysis software was calibrated using the known implant width, and calibration of the examiner (Antonio Magán-Fernández) was based on the repetition of 10 measurements (mesial and distal in 5 implants) after a 15-day interval, obtaining within-examiner agreement of >80%.
Crevicular fluid samples were gathered by Mombelli's technique [17], placing 4 paper tips (No. 30) in the deepest peri-implant pocket for 20 seconds. The paper tips were then introduced into an Eppendorf tube with 200 μL of phosphate-buffered saline (PBS) and a protease inhibitor (Sigma-Aldrich, St. Louis, MO, USA) and frozen at −70ºC for subsequent quantification of the proinflammatory cytokines IL-1β and TNF-α in units of pg/mL by the enzyme-linked immunosorbent assay (ELISA) technique (Quantikine ELISA Kit, R&D Systems, Minneapolis, MN, USA), following the manufacturer's recommendations. For this purpose, 100 μL of PBS solution at pH 7.4 containing supernatant from each sample were placed into a sterile multiwell with 20–200 µL micropipettes (Finnpipette, Thermo Fisher Scientific, Waltham, MA, USA) at room temperature and shaken for analysis by ELISA. Two replicates per sample were established, and to extrapolate the densitometric values to pg/mL, a standard curve was established for each cytokine. The optical density of each well was determined after 30 minutes, using a microplate reader at 450 nm (Vircell, Granada, Spain). The values were expressed as pg/mL in the samples obtained after 20 seconds.
Clinical and radiological variables were evaluated at baseline, 45 days, and 90 days, while biochemical variables were assessed only at baseline and 45 days. At 90 days, implants with bleeding on probing were treated by removing biofilm from the peri-implant pocket with Teflon curettes and performing subgingival irrigation with 0.2% chlorhexidine, followed by mouthwashes with chlorhexidine every 12 hours [18]. The most severe cases were treated with flap surgery and bone regeneration combined with systemic antibiotic treatment with 250 mg of metronidazole and 500 mg of amoxicillin 3 times/day for 7 days [19].
Statistical analysis
SPSS version 20 (IBM Corp., Armonk, NY, USA) was used for descriptive analyses (means, standard deviation, and percentages) and to analyze patient data, while SUDAAN version 7.0 (RTI, Research Triangle Park, NC) was used to analyze data on implants through the DESCRIPT procedure (t-test), in order to correct for clustering (multiple implants within a single mouth). The specific tests used are reported in the footnotes to the Tables. We used the a priori contrast approach, which in this context implies performing 3 paired comparisons. This approach made it possible to avoid penalizing the reader interested only in a single comparison [20]. We did not test for normality or homoscedasticity, since our sample size (number of implants) per group was larger than 30, and the t-test is known to be robust (i.e., it is not very strongly affected by non-normality or non-homoscedasticity) [21]. This allowed us to present data in their original scales, which are interpreted more easily.
Although the ADI criteria were followed for the case definition of peri-implantitis, more strict criteria of peri-implantitis, according to Renvert et al. [22], were also applied (PPD ≥5 mm). The resulting subgroup was also analyzed in order to focus on moderate and severe cases.
Samples were collected from 100 implants for cytokine analyses, but data were missing for 12 implants at baseline and for 13 other implants at 45 days. For the missing values, the mean±standard deviation concentration of the cytokine in question was calculated for each group (test, control 1, and control 2), and SPSS was used to generate 3 series of random normally distributed data with the same means and standard deviations, which were then sequentially adopted as the missing values.
RESULTS
Of the 63 patients (104 implants) initially enrolled in the study, 2 were lost to follow-up (1 patient from control group 1, who forgot to use the placebo gel, and 1 patient from control group 2, who discontinued the study voluntarily without performing follow-up analyses). The study period lasted from September 2014 to January 2016, leaving a final study sample of 61 patients with 100 dental implants: 21 (32 implants) in the test group, 20 (32 implants) in control group 1, and 20 (36 implants) in control group 2. Table 1 reports data on sociodemographic variables, oral habits, and baseline implant variables. The test group included only 1 smoker (10 cigarettes per day), control group 1 contained 3 smokers (20, 25, and >45 cigarettes per day), and control group 2 included 3 smokers (15, 20, and 20 cigarettes per day), although there was no statistically significant difference in consumption of cigarettes per day among the groups (P=0.18).
Table 1. Baseline characteristics of patients (n=61) and implants (n=100).
| Variable | Test (n=21) | Control 1 (n=20) | Control 2 (n=20) | P value | |
|---|---|---|---|---|---|
| Patient variables | 21 | 20 | 20 | ||
| Female (%) | 66.7 | 55.0 | 65.0 | 0.71a) | |
| Age range (years) | 43–81 | 54–79 | 29–78 | ||
| Age | 60±9 | 64±7 | 58±13 | 0.20b) | |
| Implants | 1.52±0.60 | 1.60±0.50 | 1.80±0.62 | 0.29b) | |
| Smokers ≥10 cigarettes/day | 1 (4.8) | 3 (15.0) | 3 (15.0) | 0.18a) | |
| Cigarettes/day | 0.48±2.18 | 4.50±11.80 | 2.25±5.57 | 0.24b) | |
| Brushing (times/day) | 2.6±0.5 | 2.3±0.6 | 2.4±0.8 | 0.37b) | |
| Mouthwash (% yes) | 42.9 | 40.0 | 40.0 | 0.98a) | |
| Bruxism (% yes) | 19.0 | 10.0 | 15.0 | 0.72a) | |
| Splint for bruxism (% yes) | 4.8 | 0.0 | 0.0 | 0.38a) | |
| Clenching (% yes) | 23.8 | 20.0 | 20.0 | 0.94a) | |
| Splint for clenching (% yes) | 4.8 | 0.0 | 0.0 | 0.38a) | |
| Diabetes (% yes) | 4.8 | 10.0 | 5.0 | 0.75a) | |
| Hypertension (% yes) | 28.6 | 20.0 | 5.0 | 0.14a) | |
| Osteoporosis (% yes) | 14.3 | 20.0 | 5.0 | 0.36a) | |
| Implant variables | 32 | 32 | 36 | ||
| Months since loading | 89±51 | 101±45 | 76±41 | 0.21c) | |
| Unitary (%) | 34.4 | 18.8 | 19.4 | 0.33d) | |
| Prosthesis pillar (% yes) | 65.6 | 81.3 | 80.6 | 0.33d) | |
| Diameter (%; 3.7–4.1–4.8 mm) | 69–28–3 | 47–50–3 | 58–25–17 | 0.24e) | |
| Length (%; 8–10–11–12–14 mm) | 6–50–3–41–0 | 0–44–0–50–6 | 0–53–0–39–8 | 0.45e) | |
| Upper jaw (%) | 56.3 | 81.3 | 83.3 | 0.11d) | |
Values are presented as mean±standard deviation or number (%).
a)The χ2 test; b)Analysis of variance; c)P values corrected for clustering (multiple implants within a single patient) with the REGRESS procedure in SUDAAN version 7.0; d)P values with the LOGISTIC procedure in SUDAAN version 7.0; e)P values with the CROSSTAB procedure in SUDAAN version 7.0.
Table 2 lists data on clinical and radiological peri-implant variables. All implants showed bleeding on probing at some site at baseline (a peri-implantitis diagnostic criterion); the greatest reduction in bleeding was observed in the test group at 45 and 90 days, and the difference with control group 2 was borderline significant at 90 days. A statistically significant difference in PPD between the test group and control group 1 was found at 45 and 90 days (P<0.01). At both 45 and 90 days, the MBL decreased in the test group and increased in the 2 control groups, but the difference was not statistically significant.
Table 2. Clinical and radiological variables of implants (n=100) from patients (n=61).
| Variable | Test (n=32) | Control 1 (n=32) | Control 2 (n=36) | Contrasts (P value)a) | |||
|---|---|---|---|---|---|---|---|
| A–B | A–C | B–C | |||||
| Bleeding on probing (%) | |||||||
| Baseline | 100 | 100 | 100 | - | - | - | |
| 45 days | 15.6 | 28.1 | 33.3 | 0.30 | 0.12 | 0.68 | |
| 90 days | 15.6 | 28.1 | 38.9 | 0.29 | 0.07 | 0.43 | |
| Probing depth (mm) | |||||||
| Baseline | 3.62±0.83 | 4.21±1.14 | 3.67±0.72 | 0.05 | 0.78 | 0.08 | |
| 45 days | 3.08±0.85 | 3.79±1.00 | 3.35±0.72 | 0.01 | 0.22 | 0.10 | |
| 90 days | 2.97±0.64 | 3.63±1.01 | 3.25±0.78 | 0.01 | 0.16 | 0.18 | |
| Clinical attachment loss (mm) | |||||||
| Baseline | 4.06±1.40 | 4.37±1.32 | 3.74±0.76 | 0.44 | 0.29 | 0.06 | |
| 45 days | 3.52±1.45 | 3.95±1.17 | 3.41±0.74 | 0.24 | 0.74 | 0.06 | |
| 90 days | 3.45±1.49 | 3.79±1.24 | 3.29±0.75 | 0.39 | 0.61 | 0.11 | |
| Marginal bone loss (mm) | |||||||
| Baseline | 3.41±1.66 | 3.56±2.01 | 3.40±0.91 | 0.77 | 0.98 | 0.71 | |
| 45 days | 3.40±1.63 | 3.65±1.99 | 3.41±0.87 | 0.61 | 0.97 | 0.57 | |
| 90 days | 3.39±1.70 | 3.66±2.04 | 3.43±0.89 | 0.60 | 0.92 | 0.60 | |
Values are presented as mean±standard deviation.
a)P values corrected for clustering (multiple implants within a single patient) with the DESCRIPT procedure in SUDAAN version 7.0.
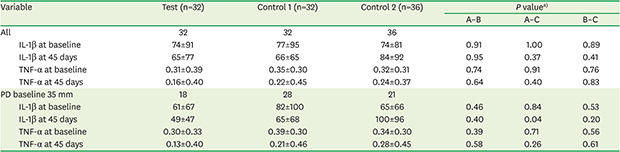
Table 3 exhibits a comparison of IL-1β and TNF-α concentrations for all studied implants and for those with a PPD ≥5 mm at baseline. The mean baseline concentration of both cytokines was very similar among the study groups. Among those with a PPD ≥5 mm, a significantly greater reduction (P=0.04) in IL-1β concentrations was observed at 45 days in the test group than in control group 2. The mean difference in IL-1β concentrations at 45 days between the test group and control group 2 was −51 pg/mL (95% CI, −98, −3 pg/mL). No other statistically significant difference in IL-1β or TNF-α concentrations was observed among the groups.
Table 3. Peri-implant crevicular fluid cytokine levels (pg/mL in a 20-second sample) of implants (n=100) from patients (n=61).
| Variable | Test (n=32) | Control 1 (n=32) | Control 2 (n=36) | P valuea) | |||
|---|---|---|---|---|---|---|---|
| A–B | A–C | B–C | |||||
| All | 32 | 32 | 36 | ||||
| IL-1β at baseline | 74±91 | 77±95 | 74±81 | 0.91 | 1.00 | 0.89 | |
| IL-1β at 45 days | 65±77 | 66±65 | 84±92 | 0.95 | 0.37 | 0.41 | |
| TNF-α at baseline | 0.31±0.39 | 0.35±0.30 | 0.32±0.31 | 0.74 | 0.91 | 0.76 | |
| TNF-α at 45 days | 0.16±0.40 | 0.22±0.45 | 0.24±0.37 | 0.64 | 0.40 | 0.83 | |
| PD baseline 35 mm | 18 | 28 | 21 | ||||
| IL-1β at baseline | 61±67 | 82±100 | 65±66 | 0.46 | 0.84 | 0.53 | |
| IL-1β at 45 days | 49±47 | 65±68 | 100±96 | 0.40 | 0.04 | 0.20 | |
| TNF-α at baseline | 0.30±0.33 | 0.39±0.30 | 0.34±0.30 | 0.39 | 0.71 | 0.56 | |
| TNF-α at 45 days | 0.13±0.40 | 0.21±0.46 | 0.28±0.45 | 0.58 | 0.26 | 0.61 | |
Values are presented as mean±standard deviation.
IL: interleukin, TNF: tumor necrosis factor.
a)P values corrected for clustering (multiple implants within a single patient) with the DESCRIPT procedure in SUDAAN version 7.0.
DISCUSSION
In this study, the topical application of HMW-HA in patients with peri-implantitis reduced inflammation and PICF concentrations of the proinflammatory cytokine IL-1β in comparison to a non-placebo control group (control group 2).
We were unable to identify any earlier publications on the topical application of HMW-HA in peri-implantitis except for the study by de Araújo Nobre et al. [23], who compared the effects of HA and chlorhexidine during the first 6 months on immediate-loading implants, following the “all on four” technique. They reported that better bleeding scores were obtained with HMW-HA in the short term, but better plaque and bleeding scores were achieved with chlorhexidine in the long-term. Regarding the microbiological effects of HMW-HA, recent results from a microbiome study performed in samples from our project have shown for the first time that HMW-HA reduced the relative abundance and diversity of peri-implantitis-related microorganisms, with special focus on early colonizing bacteria, suggesting that HMW-HA exerts a preventive action against bacterial colonization in the early stages of the disease [10]. Topical HMW-HA treatment has been more widely studied in the setting of periodontitis, and a systematic review by Casale et al. [9] of 3 studies on gingivitis and 13 on chronic periodontitis (including application alone or in association with scaling and root planing, flap surgery, and/or bone regeneration) concluded that the topical use of HA has beneficial effects in patients with chronic inflammatory gingival and periodontal disease.
HMW-HA is known to possess anti-inflammatory activity, which is well documented in osteoarthritis, with reported effects including a dose-dependent reduction in IL-1α-induced levels of prostaglandin E2 (a potent vasodilator molecule) [24], a reduction of TNF-α levels in atrophic canine cartilage [3], inhibition of IL-1β and matrix metalloproteinase protein (MMP)-3 expression in an animal model of osteoarthritis [25], and inhibition of the expression and production of MMP-1 and MMP-3 in IL-1β-stimulated synoviocytes in humans [26]. HMW-HA has been reported not to penetrate the stratum corneum of skin; however, the gingival mucosa differs from the skin in 2 key ways: the stratum corneum is thinner in keratinized and/or parakeratinized oral mucosa (2–3 cell layers vs. 25–30 in skin), and the soft wall of the peri-implant pocket is not keratinized. Hence, the topical application of HA has proven more effective to achieve high concentrations in the oral mucosa in comparison to its systemic administration [9].
Bleeding on probing was recorded in all participants at baseline, as an inclusion criterion. A greater reduction was observed in the test group than in control group 2 at 45 and 90 days, and the difference at 90 days was close to statistical significance (P=0.07). Fibrinolysis is closely related to the pericellular proteolysis observed in inflammation. The intra-articular administration of HA was found to attenuate the fibrinolytic activity mediated by urokinase-type plasminogen activator factor and its receptor [27]. Wang et al. [28] studied the influence of HMW-HA on the gene expression of various inflammatory cytokines by human fibroblast-like synoviocytes in a wide sample of patients with early-stage osteoarthritis. They reported the downregulation of TNF-α, IL-8, and inducible nitric oxide synthase in fibroblast-like synoviocytes and proposed that these effects may be mediated by the interaction of CD44 and HMW-HA. CD44, a cell-adhesion molecule that is ubiquitously expressed in leukocytes and parenchymal cells, has been implicated, together with its ligand hyaluronan, in several inflammatory diseases. In addition, blocking the CD44 receptor with anti-CD44 antibody inhibited the downregulatory effects of HMW-HA on gene expression [29]. Isoforms of the CD44 receptor are involved in the initial binding of leukocytes to endothelial cells activated by inflammatory processes [30] and in the extravasation of lymphocytes from blood vessels [31]. This may suggest that the CD44 signaling pathway plays a major role in inflammation regulation by HMW-HA. In a previous paper, our group found a significant reduction in inflammatory infiltrate in topical HMW-HA-treated versus placebo-treated quadrants in periodontal patients. This effect was explained by 2 mechanisms: reduction of the expression of proliferation antigen Ki-67 in inflammatory cells in the lamina propria, and blocking of CD44 receptors via their HA ligand [32]. A further possible explanation of the effect of HMW-HA on PPD and CA may be the increased volume of the lamina propria due to the hydration that results from greater water retention. Similar results have been reported in non-surgical periodontal therapy by a recent meta-analysis, which found greater improvements in PPD, CA, and bleeding on probing when patients were treated with HA as a coadjutant. However, a high risk of bias in most studies and great heterogeneity were found [33].
Radiological MBL measurements remained stable at 45 and 90 days in the test group, but increased in the control groups, although the differences did not reach statistical significance. This may be attributable to the effects of HMW-HA on inflammation and IL-1β or to the length of the follow-up period, which may have been too short for the observation of bone formation. Therefore, the product may need to be applied for a longer follow-up period. A prior study found that the application of HMW-HA in extraction sockets yielded higher bone formation in those treated with HMW-HA at 45 days, but this difference disappeared at 90 days [34]. Furthermore, in this study, HMW-HA was not directly applied to bone defects, but to the peri-implant pocket and gingival tissue, potentially making it more difficult for HMW-HA to arrive to the defect.
Proinflammatory cytokines (IL-1β, TNF-α, IL-6, IL-17, and IL-12) and matrix metalloproteinases (MMP-8 and MMP-9) play an important role in the onset and progression of inflammatory diseases either directly by causing tissue destruction, or indirectly by activating immunoinflammatory cells. IL-1β and TNF-α are the most important cytokines in osteoclast formation and bone resorption. Cytokine detection in PICF has become a frequently used non-invasive method to diagnose peri-implantitis [35]. A recent systematic review comparing PICF cytokine concentrations between healthy implants and those with peri-implantitis reported that concentrations of proinflammatory cytokines (IL-1β, IL-6, IL-12, IL-17, and TNF-α) were consistently higher in peri-implantitis cases, while the concentrations of anti-inflammatory cytokines and RANKL/osteoprotegerin showed inconsistent patterns. The authors concluded that proinflammatory cytokines, especially IL-1β, are the most promising proteins for utilization as PICF markers to diagnose peri-implantitis [8]. Among the present patients with more severe lesions (PPD ≥5 mm), PICF IL-1β concentrations were significantly lower at 45 days in the test group than in control group 2. A higher reduction in TNF-α concentrations was also observed in the test group (0.17 pg/mL) than in control group 2 (0.06 pg/mL) at 45 days, but statistical significance was not reached. These differences were not observed among less severe cases (PPD <5 mm), suggesting that HA has a stronger effect in more severe inflammatory lesions. Duarte et al. [8] described PPD as a key parameter of disease severity that may directly influence proinflammatory cytokine concentrations. Recent case definitions of peri-implantitis do not include PPD as a criterion, such as the one from the Eighth European Workshop on Periodontology [36], and there is considerable heterogeneity in the PPD thresholds used in the literature [37]. Our results contribute to increasing evidence that peri-implant PPD >5 mm is also a sign of peri-implantitis, as recently shown by the latest case definition of peri-implantitis by the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions [5].
Although there were fewer smokers in the test group than in the control groups in this trial, the difference was not statistically significant. The role of tobacco as a confounder is not clear in this context, with recent studies finding that smoking has no effects on the volume of crevicular fluid or on its cytokine concentrations [38]. The baseline PICF IL-1β and TNF-α concentrations were very similar in our 3 study groups (Table 3). PICF concentrations (in pg/mL) rather than volumes were analyzed in the present study, as recommended [8].
The limitations of the present study include its short follow-up, which did not allow us to observe potential effects on the MBL, and the difficulty of generalizing the results outside the studied population. All implants in the present study were of the same type. For this reason, further research using different implants is warranted to validate our findings. The reduction in bleeding in the control groups may be attributable to the Hawthorne effect, through improvements in hygiene habits in all patients caused by the awareness of being enrolled in the trial.
It is possible that the act of massaging the peri-implant gingiva offered a benefit in addition to the effect of the HA gel, given that some improvements, although not significant, were also observed in patients using the placebo. This fact may also be explained by the physical barrier effect exerted by the gel, which acted as a coating agent and prevented reinfection of the peri-implant site. The compound used, hydroxypropyl guar, is frequently utilized as a thickening vehicle in cosmetics and pharmacy and has no known biological effects on skin or mucosa. Data were missing for cytokine levels in some samples due to contamination or technical failure, although a statistical technique was applied to assign values in these cases. After a sensitivity analysis, significant differences were still shown in the levels of IL-1β at 45 days between the test group and control group 2 (P<0.01) (data not shown).
This study demonstrates for the first time that the topical application of HA in peri-implantitis may reduce inflammation and PICF levels of IL-1β. This is the first report to indicate that HMW-HA may be an effective therapeutic option, alongside plaque removal measures, to control the progression of this disease.
ACKNOWLEDGEMENTS
The authors are grateful to Richard Davies for editorial assistance.
Footnotes
- Conceptualization: Elena Sánchez-Fernández, Francisco O'Valle, Francisco Mesa.
- Formal analysis: Manuel Bravo.
- Funding acquisition: Elena Sánchez-Fernández, Francisco O'Valle, Francisco Mesa.
- Investigation: Elena Sánchez-Fernández, Antonio Magán-Fernández.
- Methodology: Antonio Magán-Fernández, Francisco O'Valle.
- Project administration: Francisco Mesa.
- Supervision: Francisco Mesa.
- Writing - original draft: Antonio Magán-Fernández, Manuel Bravo, Francisco Mesa.
- Writing - review & editing: Elena Sánchez-Fernández, Antonio Magán-Fernández, Francisco O'Valle, Manuel Bravo, Francisco Mesa.
Conflict of Interest: This study was supported by Ricerfarma srl. (Milan, Italy) in collaboration with Research Group #CTS 583 (Junta de Andalucía, Spain) (reference: OTRI-3300). The authors declare that they have no conflict of interest, either directly or indirectly, with any of the companies or products listed in the study.
References
- 1.Dahiya P, Kamal R. Hyaluronic acid: a boon in periodontal therapy. N Am J Med Sci. 2013;5:309–315. doi: 10.4103/1947-2714.112473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutherland IW. Novel and established applications of microbial polysaccharides. Trends Biotechnol. 1998;16:41–46. doi: 10.1016/S0167-7799(97)01139-6. [DOI] [PubMed] [Google Scholar]
- 3.Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther. 2003;5:54–67. doi: 10.1186/ar623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7:79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 5.Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 2018;45(Suppl 20):S286–S291. doi: 10.1111/jcpe.12957. [DOI] [PubMed] [Google Scholar]
- 6.Smith AJ, Addy M, Embery G. Gingival crevicular fluid glycosaminoglycan levels in patients with chronic adult periodontitis. J Clin Periodontol. 1995;22:355–361. doi: 10.1111/j.1600-051x.1995.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 7.Manzanares D, Monzon ME, Savani RC, Salathe M. Apical oxidative hyaluronan degradation stimulates airway ciliary beating via RHAMM and RON. Am J Respir Cell Mol Biol. 2007;37:160–168. doi: 10.1165/rcmb.2006-0413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duarte PM, Serrão CR, Miranda TS, Zanatta LC, Bastos MF, Faveri M, et al. Could cytokine levels in the peri-implant crevicular fluid be used to distinguish between healthy implants and implants with peri-implantitis? A systematic review. J Periodontal Res. 2016;51:689–698. doi: 10.1111/jre.12354. [DOI] [PubMed] [Google Scholar]
- 9.Casale M, Moffa A, Vella P, Sabatino L, Capuano F, Salvinelli B, et al. Hyaluronic acid: Perspectives in dentistry. A systematic review. Int J Immunopathol Pharmacol. 2016;29:572–582. doi: 10.1177/0394632016652906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soriano-Lerma A, Magán-Fernández A, Gijón J, Sánchez-Fernández E, Soriano M, García-Salcedo JA, et al. Short-term effects of hyaluronic acid on the subgingival microbiome in peri-implantitis: a randomized controlled clinical trial. J Periodontol. 2020;91:734–745. doi: 10.1002/JPER.19-0184. [DOI] [PubMed] [Google Scholar]
- 11.Lopez MA, Manzulli N, D'Angelo A, Lauritano D, Papalia R, Candotto V. The use of hyaluronic acid as an adjuvant in the management of peri-implantitis. J Biol Regul Homeost Agents. 2017;31:123–127. [PubMed] [Google Scholar]
- 12.Schulz KF, Altman DG, Moher D, CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63:834–840. doi: 10.1016/j.jclinepi.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Coli P, Christiaens V, Sennerby L, Bruyn H. Reliability of periodontal diagnostic tools for monitoring peri-implant health and disease. Periodontol 2000. 2017;73:203–217. doi: 10.1111/prd.12162. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J. Statistical power analysis for the behavioural sciences. 2nd ed. New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 15.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229–235. [PubMed] [Google Scholar]
- 16.Zweers J, van Doornik A, Hogendorf EA, Quirynen M, Van der Weijden GA. Clinical and radiographic evaluation of narrow- vs. regular-diameter dental implants: a 3-year follow-up. A retrospective study. Clin Oral Implants Res. 2015;26:149–156. doi: 10.1111/clr.12309. [DOI] [PubMed] [Google Scholar]
- 17.Mombelli A, McNabb H, Lang NP. Black-pigmenting gram-negative bacteria in periodontal disease. I. Topographic distribution in the human dentition. J Periodontal Res. 1991;26:301–307. doi: 10.1111/j.1600-0765.1991.tb02067.x. [DOI] [PubMed] [Google Scholar]
- 18.Crespi R, Marconcini S, Crespi G, Giammarinaro E, Menchini Fabris GB, Barone A, et al. Nonsurgical treatment of peri-implantitis without eliminating granulation tissue: a 3-year study. Implant Dent. 2019;28:4–10. doi: 10.1097/ID.0000000000000832. [DOI] [PubMed] [Google Scholar]
- 19.Astasov-Frauenhoffer M, Braissant O, Hauser-Gerspach I, Weiger R, Walter C, Zitzmann NU, et al. Microcalorimetric determination of the effects of amoxicillin, metronidazole, and their combination on in vitro biofilm. J Periodontol. 2014;85:349–357. doi: 10.1902/jop.2013.120733. [DOI] [PubMed] [Google Scholar]
- 20.Klockars AJ, Sax G. Multiple comparisons. Beverly Hills: Sage Publications Inc.; 1986. [Google Scholar]
- 21.Altman DG. Practical statistics for medical research. London: Chapman & Hall; 1991. [Google Scholar]
- 22.Renvert S, Lindahl C, Roos Jansåker AM, Persson GR. Treatment of peri-implantitis using an Er:YAG laser or an air-abrasive device: a randomized clinical trial. J Clin Periodontol. 2011;38:65–73. doi: 10.1111/j.1600-051X.2010.01646.x. [DOI] [PubMed] [Google Scholar]
- 23.de Araújo Nobre M, Cintra N, Maló P. Peri-implant maintenance of immediate function implants: a pilot study comparing hyaluronic acid and chlorhexidine. Int J Dent Hyg. 2007;5:87–94. doi: 10.1111/j.1601-5037.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 24.Goto M, Hanyu T, Yoshio T, Matsuno H, Shimizu M, Murata N, et al. Intra-articular injection of hyaluronate (SI-6601D) improves joint pain and synovial fluid prostaglandin E2 levels in rheumatoid arthritis: a multicenter clinical trial. Clin Exp Rheumatol. 2001;19:377–383. [PubMed] [Google Scholar]
- 25.Takahashi K, Goomer RS, Harwood F, Kubo T, Hirasawa Y, Amiel D. The effects of hyaluronan on matrix metalloproteinase-3 (MMP-3), interleukin-1β (IL-1β), and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression during the development of osteoarthritis. Osteoarthritis Cartilage. 1999;7:182–190. doi: 10.1053/joca.1998.0207. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki A, Sasaki K, Konttinen YT, Santavirta S, Takahara M, Takei H, et al. Hyaluronate inhibits the interleukin-1β-induced expression of matrix metalloproteinase (MMP)-1 and MMP-3 in human synovial cells. Tohoku J Exp Med. 2004;204:99–107. doi: 10.1620/tjem.204.99. [DOI] [PubMed] [Google Scholar]
- 27.Nonaka T, Kikuchi H, Ikeda T, Okamoto Y, Hamanishi C, Tanaka S. Hyaluronic acid inhibits the expression of u-PA, PAI-1, and u-PAR in human synovial fibroblasts of osteoarthritis and rheumatoid arthritis. J Rheumatol. 2000;27:997–1004. [PubMed] [Google Scholar]
- 28.Wang CT, Lin YT, Chiang BL, Lin YH, Hou SM. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthritis Cartilage. 2006;14:1237–1247. doi: 10.1016/j.joca.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Bourguignon LY, Wong G, Earle CA, Xia W. Interaction of low molecular weight hyaluronan with CD44 and toll-like receptors promotes the actin filament-associated protein 110-actin binding and MyD88-NFκB signaling leading to proinflammatory cytokine/chemokine production and breast tumor invasion. Cytoskeleton (Hoboken) 2011;68:671–693. doi: 10.1002/cm.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson P, Maiti A, Brown KL, Li R. A role for the cell adhesion molecule CD44 and sulfation in leukocyte-endothelial cell adhesion during an inflammatory response? Biochem Pharmacol. 2000;59:455–465. doi: 10.1016/s0006-2952(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 31.Siegelman MH, Stanescu D, Estess P. The CD44-initiated pathway of T-cell extravasation uses VLA-4 but not LFA-1 for firm adhesion. J Clin Invest. 2000;105:683–691. doi: 10.1172/JCI8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mesa FL, Aneiros J, Cabrera A, Bravo M, Caballero T, Revelles F, et al. Antiproliferative effect of topic hyaluronic acid gel. Study in gingival biopsies of patients with periodontal disease. Histol Histopathol. 2002;17:747–753. doi: 10.14670/HH-17.747. [DOI] [PubMed] [Google Scholar]
- 33.Eliezer M, Imber JC, Sculean A, Pandis N, Teich S. Hyaluronic acid as adjunctive to non-surgical and surgical periodontal therapy: a systematic review and meta-analysis. Clin Oral Investig. 2019;23:3423–3435. doi: 10.1007/s00784-019-03012-w. [DOI] [PubMed] [Google Scholar]
- 34.Alcântara CE, Castro MA, Noronha MS, Martins-Junior PA, Mendes RM, Caliari MV, et al. Hyaluronic acid accelerates bone repair in human dental sockets: a randomized triple-blind clinical trial. Braz Oral Res. 2018;32:e84. doi: 10.1590/1807-3107bor-2018.vol32.0084. [DOI] [PubMed] [Google Scholar]
- 35.Faot F, Nascimento GG, Bielemann AM, Campão TD, Leite FR, Quirynen M. Can peri-implant crevicular fluid assist in the diagnosis of peri-implantitis? A systematic review and meta-analysis. J Periodontol. 2015;86:631–645. doi: 10.1902/jop.2015.140603. [DOI] [PubMed] [Google Scholar]
- 36.Sanz M, Chapple IL Working Group 4 of the VIII European Workshop on Periodontology. Clinical research on peri-implant diseases: consensus report of Working Group 4. J Clin Periodontol. 2012;39(Suppl 12):202–206. doi: 10.1111/j.1600-051X.2011.01837.x. [DOI] [PubMed] [Google Scholar]
- 37.Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42(Suppl 16):S158–S171. doi: 10.1111/jcpe.12334. [DOI] [PubMed] [Google Scholar]
- 38.Ata-Ali J, Flichy-Fernández AJ, Alegre-Domingo T, Ata-Ali F, Peñarrocha-Diago M. Impact of heavy smoking on the clinical, microbiological and immunological parameters of patients with dental implants: a prospective cross-sectional study. J Investig Clin Dent. 2016;7:401–409. doi: 10.1111/jicd.12176. [DOI] [PubMed] [Google Scholar]


