Abstract
Aphthous ulcers are painful sores that may occur in the mouth’s mucous membrane and are the most common type of oral lesions. The present research is aimed to develop in-situ gel of hydrocortisone for the treatment of aphthous ulcers. Temperature induced in-situ gels were prepared by using various concentrations of methylcellulose. The prepared formulations were evaluated for the conversion of sol-gel transition temperature or gelation temperature, gelling capacity, pH, viscosity, syringeability, spreadability, drug content, In vitro and ex vivo studies. The gelation temperatures of the prepared formulations were found to be in the range of 32–39 °C. The formulations exhibited fairly uniform drug content (76.40–94.7%) and pH was found to be 6.8. In vitro drug release was carried out for 8 h using phosphate buffer as a diffusion medium. In-situ gel formulation containing 1% w/v of methylcellulose as a gel base prolonged the drug release up to 8 h and showed sustained release behaviour. Via these, in-situ gel formulations, the release kinetics of the drug was first order. Finally, it can be inferred that in-situ gel formulation containing 1% w/v of methylcellulose facilitates prolonged drug release, extended drug residence period, which in turn improves the bioavailability of drugs. The short-term stability studies were carried out and no substantial changes were observed.
Keywords: Ex vivo studies, Gelation time, In-situ, Syringeability, Spreadability
1. Introduction
The National Oral Health Program was drafted by the Indian Dental Associations (IDA) to address the burden of dental illnesses and to achieve ‘optimal oral health’ for all by 2020 which has been recognized in recent years as an important component of general health.1 Food being a significant modifiable parameter can influence oral health considerably. An imbalance in nutritional status can negatively affect oral health, where, bad oral health can affect food intake, resulting in malnutrition, thus, playing a central role in maintaining good nutritional status. Interdisciplinary teams including general physicians, dentists, nurses and dietitians have recognized the importance of oral health contributing to overall wellbeing and quality of life and ensure that the patients have a healthy oral health record and achieve sufficient nutrition.2
Aphthous ulcers oral mucosal inflammatory ulcer that occurs in the oral cavity and frequently affects everyday tasks including feeding, drinking and speaking. The expression “aphthous” had been derived from the Greek word “aphtha,” meaning ulceration, where, the aphthous ulcer is also sometimes called an aphthosis. Aphthous ulcer causing canker sores are more prevalent in females than in males. These ulcers are round or oval, with a greyish yellow, crateriform base surrounded by an erythematous halo of inflamed mucosa, usually occurs on non-keratinized oral mucosa including the lips, buccal mucosa, mouth floor and tongue ventral surface.3
Aphthous ulcer is classified as a minor, major and herpetiform. Minor Aphthous ulcer involves the development of one to five ulcers each with a diameter less than 1 cm. There 10–100 ulcers at a time in the herpetiform, the ulcer scale is typically 1–3 cm, and ulceration lasting 7–10 days. These ulcers are also called herpetiform ulcers, as they appear similar to lesions caused during primary infection of herpes simplex.4
Higher prevalence of recurrent aphthous ulcer in young adults and the severity decreased with increasing age. The etiology of aphthous ulcer remains unclear. Other possible factors include trauma, drug use, deficiency in vitamin B12, folic acid, iron, stress, hormonal changes and metabolic diseases.3 Many topical agents such as local and systemic antibiotics, local antiseptics, topical NSAIDs, and topical corticosteroids are generally prescribed for symptomatic relief. Several approved drug formulations such as pills, mouthwash, sprays and paste such as vitamin B12, chlorhexidine mouthwash, steroid lozenges and local anesthetics are primarily suggested for the treatment of aphthous ulcer.
Herein, we have developed the hydrocortisone in -situ gel for the treatment of aphthous ulcers. In-situ, Latin term meaning “In Position”. In-situ gels are in solution form before getting in the body.5 Over the past few years, in-situ gel systems have received a significant attention as mucoadhesive drug delivery system suitable for the sustained drug release mechanism.6 This in situ gel formation is achieved by one or a combination of different stimuli such as pH change, temperature modulation and ionic cross-linking.
Both natural and synthetic polymers can be utilized in the formulation of in situ gels.7 In-situ; therefore, gels are administered through ocular, rectal, vaginal, injectable, and intraperitoneal routes. The advantages of these gel delivery systems include ease of administration, lower dose and frequency, and improved local bioavailability, patient compliance and comfort. The composition is also less complex and lowers production and manufacturing.8
Corticosteroids are a class of chemicals that includes steroid hormones, both glucocorticoids and mineralocorticoids. Glucocorticoid as an anti-inflammatory and immunosuppressive agent represents the application of physiological effects to disease treatment and this corticosteroid can directly affect T lymphocytes or modify the reaction of effectors cells to immunopathogenic precipitants.9 Hydrocortisone that belongs to the glucocorticoid class is used for the treatment of aphthous ulcer and is meant to reduce the inflammatory cycle involved with the development of aphthae and this hydrocortisone has a brief biological half-life of 8–12 h and is the first drug option for anti-inflammatory activity compared to other drug types.10 Hydrocortisone (1% w/v) was prescribed as an effective therapeutic dose based on literature about evidence-based medicine (EBM) in dental practice.11
The present research was devised as an attempt to fabricate and investigate a sustained release of hydrocortisone in-situ gel for the treatment of aphthous ulcers by using various concentrations of methyl cellulose. The study thus aims to improve the bioavailability and control the release of drugs using a biodegradable polymer network.
2. Materials and methods
Hydrocortisone, Yarrow Chem. Products, Mumbai; methyl cellulose, triethanolamine and sodium citrate were procured from S.D. Fine Chemicals, Mumbai.
2.1. Preparation of in-situ gelling system
For the preparation of in-situ gelling system, sodium citrate was added to the distilled water with continuous stirring until a clear solution was obtained for the preparation of hydrocortisone containing in-situ gel formulations. Methyl cellulose was selected as polymer and applied with continuous stirring to the above solution and allowed to hydrate overnight. The calculated amount of hydrocortisone (1% w/v) and triethanolamine was added separately (Table 1) under constant stirring to the polymer solution. The formulation configuration of hydrocortisone in situ gel was tabulated and the formulations designed for various characterization studies were further evaluated.12
Table 1.
Formulation design of in-situ gel.
| Formulation Code |
Hydrocortisone (%w/v) | Methyl cellulose (%w/v) | Sodium citrate (%w/v) | Triethanolamine | Distilled water |
|---|---|---|---|---|---|
| F1 | 1 | 0.25 | 0.25 | Q. S | Q. S |
| F2 | 1 | 0.50 | 0.25 | Q. S | Q. S |
| F3 | 1 | 0.75 | 0.25 | Q. S | Q. S |
| F4 | 1 | 1.00 | 0.25 | Q. S | Q. S |
2.2. FT-IR studies
The potential for drug-polymers interactions was assessed using FTIR (Jasco M 4100, Mumbai, India). KBr pellet method was used to spectroscopically analyze hydrocortisone, methyl cellulose and formulation these pellets were scanned from 4000 to 400 cm−1 wavenumbers, and characteristic peaks were observed.
2.3. Appearance, clarity, and pH
Appearance and clarity were visually recorded in the light before and after gelling against the white and black backgrounds. The pH is one of the most important parameters for in-situ gel formulation was directly measured using a digital pH meter (Deluxe pH meter, India).
2.4. Viscosity
Brookfield optical viscometer (RVDV2 T model) was used to assess the viscosity and rheo-related properties of in-situ gel hydrocortisone using T-96 spindle. Taking 50 g of the gel in a beaker, the spindle was dipped inside. The gel viscosity was measured at varying angular velocities at 25 °C. A normal course was for the angular velocity to change from 5 to 25 rpm.
2.5. Syringeability
All prepared formulations were transferred to the constant volume (2 ml) of a 5 ml syringe placed with a 20-gauge needle. The solutions, which were quickly passed from the syringe, were called passing and difficult to pass, as failed.
2.6. Spreadability
Approximately 1 g of the gel was loaded in the middle of the glass plate (20 mm or 20 inches) to assess the spreadability of the gel. Each piece of glass was covered with another layer of the same thickness. First, the weight of 1000 g on the upper side of the plate was gently applied, resulting in the gel spreading out over the plates. The weight was removed after 1 min of application and the diameter of the spread area (cm) was then determined.
2.7. Gelling capacity
To determine the compositions suitable for in-situ gelling systems, all the prepared formulations were tested for appearance and gelling ability. A visual approach was used to assess the gelling potential with which colored formulations solution was stored. The gelling potential was estimated by inserting 2 ml of 6.8 pH phosphate buffer in a 10 ml test tube and holding a temperature of 37 ± 1 °C. The phosphate buffer was supplemented with 1 ml of colored formulating solution. As the formulation comes into contact with the phosphate buffer, it was converted to a rigid gel-like structure in a mimetic way. Baswd on the stiffness of the formed gel and the period for which the formed gel remains as such, the formulation capacity of the gel was evaluated. The in vitro gelling ability was divided into three groups based on the freezing time and the time required to dissolve the gel.
2.8. Scanning electron microscopy
Scanning electron microscopy (SEM) was used to assess surface composition, structure, and to investigate the broken and sectioned surface morphologies. SEM studies were conducted using dry formulation (Hitachi – s-3700N, Japan) on an ion sputter-coated electron microscope brush stub.
2.9. Differential scanning calorimetry
Differential scanning calorimeter (DSC) (DSC-60 -Shimadzu, Japan) was used to assess stability tests of drug excipients and also to detect further phase shifts, such as glass transformations, crystallization, amorphous shapes of drugs and polymers. Samples of 10 mg were weighed and sealed in standard aluminum panels and then scanned at a heating rate of 10 °C/min over a temperature range from 50 °C to 300 °C.
2.10. Drug content analysis
The weighed amount of gel equivalent to 2 mg of drug was accurately taken and dissolved in phosphate buffer (pH 6.8). The product content was measured against phosphate buffer at 242 nm (pH 6.8) using UV-Visible Spectrophotometer-1800 (Shimadzu, Japan) and determined from the calibration curve.
2.11. In vitro drug release studies
The study of hydrocortisone in vitro drug release from the in-situ gel formulations was conducted using cellophane membrane for a period of 8 h. Phosphate buffer was the dissolution medium of pH 6.8. Tied to one end of a glass cylinder was the cellophane membrane, previously soaked overnight in the dissolution medium. Then 1 ml of the formulated formulation was wrapped in cellophane membrane and placed in phosphate buffer. The dissolution medium was stirred with a magnetic stirrer at 50 rpm. The sample was collected at regular intervals and replaced by a receptor medium volume similar to that. At the time interval predetermined, one ml of the sample was taken and spectrophotometrically analyzed at 242 nm.
2.12. Drug release kinetics
To understand the drug release kinetics of in situ gel formulation of hydrocortisone, the data on drug release were treated with zero order, first order kinetics, and equation of Higuchi. The release mechanism was understood by fitting the data into Korsmeyer-peppas equation Mt/Ma = Ktn, where ‘Mt/Ma’ is the fraction of the drug released at the time t,’ ‘K’ is kinetic constant, and ‘n’ is release exponent that defined the process for releasing the drug. If the value of ‘n’ is less than 0.45 then it is considered a Fickian release, values greater than 0.45 and less than 0.89 are considered anomalous (non-Fickian) transport and finally, the value of ‘n’ greater than 0.89 follows super case- II release mechanism.
2.13. Stability study
Stability analysis of optimal formulation was performed at 25 °C/60% and 40 °C/75% RH according to the State Guidelines of the International Conference of Harmonization (ICH). Stable formulations were evaluated for pH, gelling ability, product quality and rheological properties in vitro dissolution for three months.
2.14. Ex vivo studies
Ex vivo release analysis was performed with fresh chicken skin which was soaked for 5–6 h in the sodium bromide solution and washed with water to extract the adhering fat tissue. The skin was then placed in the phosphate buffered diffusion cell (pH 6.8). The medium was thermostatically controlled at temperature 37±1 °C and 5 ml of the sample was collected at fixed intervals and spectrophotometrically measured at 242 nm against their respective blank formulation.13
3. Results
3.1. FT-IR studies
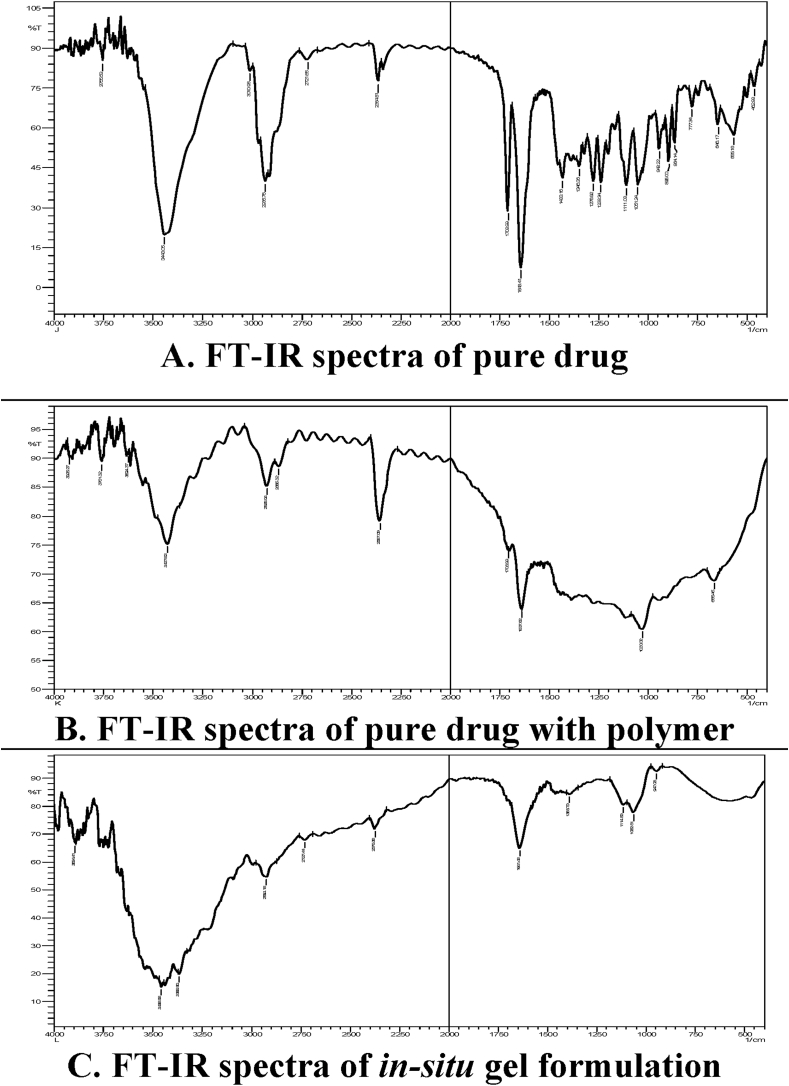
Formulations displayed typical peaks of drugs and polymers while no new bands or point changes existed suggesting no interaction between drug and polymers. FT-IR studies showed the hydrocortisone peaks at 3443 cm−1 (OH stretching), 2936 cm−1 (CH stretching), 1708 cm−1 (COOH), 1643 cm−1 (carbonyl group), 1433 cm−1 (OH bending) and 1275 cm−1 (C–O stretching).
Characteristic peaks at 3427 cm−1 (OH Stretching), 2928 cm−1 (CH Stretching), 1708 cm−1 (COOH), 1637 cm−1 (Carbonyl) and 1033 cm−1 (C–O–C Stretching) in both drug and polymer.
Peaks at 3456 cm−1 (OH Stretching) and 3365 cm−1 (NH stretching), 2924 cm−1 (CH Stretching), 1388 cm−1 (OH Bending), 1641 cm−1 carbonyl functional group and 1114 cm−1 (C–O–C stretching). This spectrum showed some drug functional groups were masked due to the encapsulation of polymer. Formulations displayed typical peaks of drug and polymers while no new bands or point changes existed suggesting no association between drugs and polymers (Fig. 1).
Fig. 1.
FT-IR studies of pure drug with excipients in A, B and C.
3.2. pH
The pH of the formulations (F1–F4) was found to be pH 6.7, which concluded suitable for aphthous ulcer as well as pH of formulation equal to the pH of gingival crevicular fluid (GCF), which suggests the prepared hydrocortisone loaded in-situ gel formulations as an optimal dosage type to be delivered in a mouth cavity without any possible inflammation and improved patient compliance (Table 3).14
Table 3.
Characterization of in situ gel.
| Batch code | Clarity | pH | Viscosity (cps) | Drug content (%) | Syringeability | Spreadability (g.cm/s) |
|---|---|---|---|---|---|---|
| F1 | Passes | 5.8 ± 0.008 | 290 ± 10 | 80.3 ± 0.012 | Passes | 12 ± 0.03 |
| F2 | Passes | 5.9 ± 0.057 | 310 ± 40 | 76.4 ± 0.051 | Passes | 13.6 ± 0.15 |
| F3 | Passes | 6.7 ± 0.044 | 395 ± 30 | 85.1 ± 0.076 | Passes | 15.2 ± 0.11 |
| F4 | Passes | 6.8 ± 0.072 | 440 ± 10 | 94.7 ± 0.082 | Fails | 17 ± 0.09 |
3.3. Viscosity
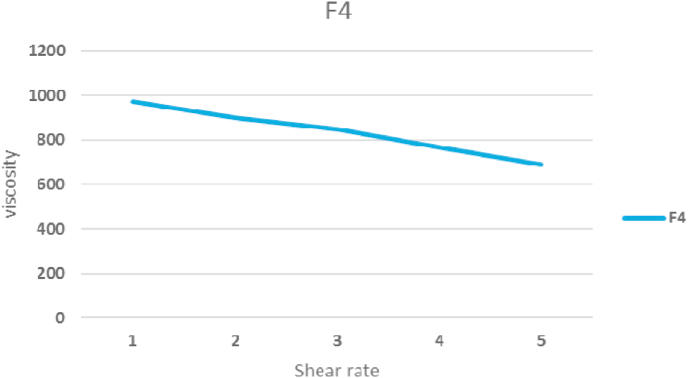
The typically formulated viscosity (F1–F4) was observed to be 290–440 cps. Methyl cellulose has an influence on the viscosity as a hydrophilic polymer. Methyl cellulose concentration ranged in formulas (0.25–1%). Higher levels of formulated methyl cellulose (F-4) showed the highest viscosity of 440 cps at 25 °C than the lowest amount of methyl cellulose used (F-1) (Fig. 4). Higher viscosity is beneficial to quickly inject the in-situ gel into the oral cavity at a lower temperature, whereas higher viscosity is beneficial for gels to stay in the oral mucosa for longer periods (Table 3).
Fig. 4.
Rheological profile of the in-situ gelling systems of F-4.
3.4. Spreadability
Evaluation of spreadability in terms of area covered by in situ gels per unit time and it was related to in-situ gel viscosity. An increase in mucoadhesive polymer concentration restricted the distance traveled by in-situ gel owing to the increased viscosity of the mucoadhesive polymer of in-situ gel. An optimal in-situ gel must have the correct gel strength so that it can be easily administered and stored at the mucosa without leakage after administration (Table 3).15
3.5. Syringeability
Ideally, the in-situ gel formulations are of low viscosity when being inserted into the oral cavity, as the formulation required less force to remove the formulation from the needle-equipped syringe. After application, the in-situ gel formulation should have a high viscosity to bind to the mucosal surface with a higher retention period in the mucosal mouth cavity to allow a sustained release of the medication for the treatment of aphthous ulcers. All formulations were found to be at preferable viscosity to be conveniently inserted into the mouth cavity as the formulations were passed to the syringeability examination with a 20-gauge needle (Table 3).16
3.6. In vitro gelling capacity
Concentrations of gelling and bioadhesive polymers were found to impact the gel strength, where the gel composition with appropriate tolerance to water is critical. Table 2 shows the measurement of the gel strength data. After 15 min, formulation F-1 and F-2 containing lower polymer concentration displayed the poorest gelation and dispersed rapidly while shaking. Formulation F-3 and F-4 containing higher polymer concentration demonstrated immediate gelation effect and the gels formed were rigid and continued to be stable for a prolonged period. This study showed that the fluid intensity of the formulation of the in-situ gel was 33–34 °C, which increased as the methyl cellulose concentration increased.
Table 2.
In vitro gelling capacity.
| Methyl cellulose concentration (%) | Gelling capacity | Gelation temperature (°c) | Gelation time (m) |
|---|---|---|---|
| 0.25 | + | No gelation up to 45 °C temperature | – |
| 0.50 | + | No gelation up to 45 °C temperature | – |
| 0.75 | ++ | 39 | 6 |
| 1.00 | ++ | 32 | 4 |
(+), gels after few minutes, dispersed rapidly.
(++), gelation immediate, remains for few hours.
3.7. Scanning electron microscopy
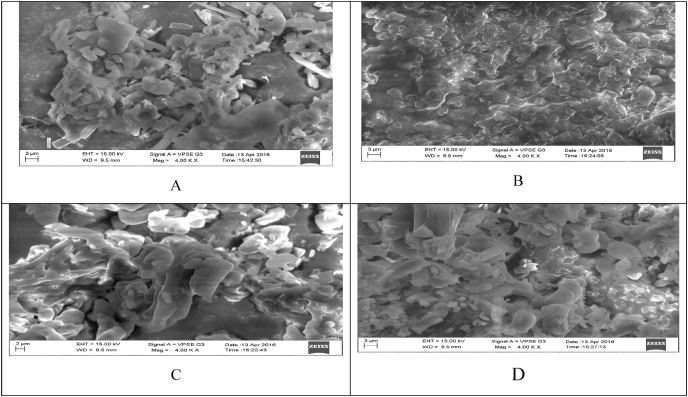
SEM (Fig. 2) was used to analyze the composition and surface texture of the in-situ gel. Drug filled in-situ gel with both formulations (F1–F4) revealed common signs of homogeneous mixing of polymers with drugs, where, no drug particles on the surface of sample gels were observed.
Fig. 2.
A. SEM of formulation (F-1) B. SEM of formulation (F-2) C. SEM of formulation (F-3) D. SEM of formulation (F-4).
3.8. Differential scanning calorimetry
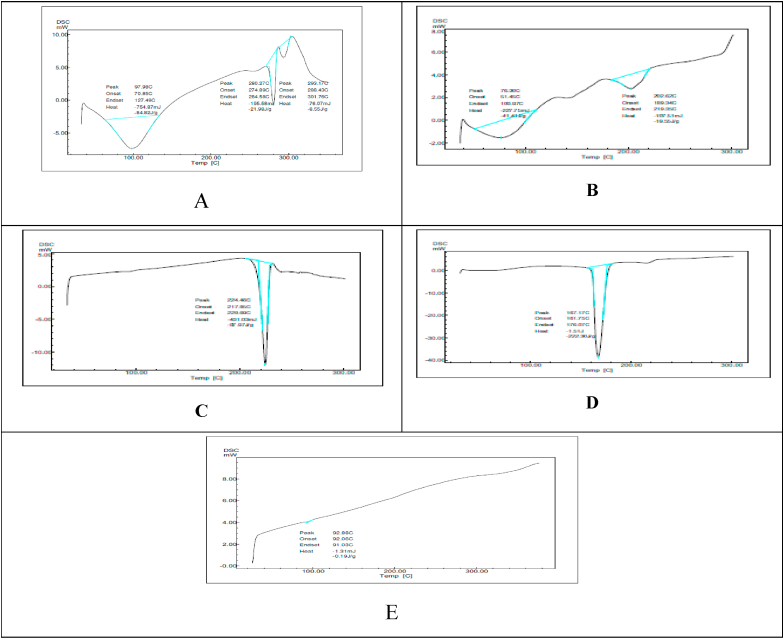
The thermogram of hydrocortisone and formulation (F-4) is shown in Fig. 3. The hydrocortisone had a long and distinctive endothermic peak at 280.27 °C. Formulation of the in-situ gel revealed product endothermic plateau at 224.40 °C. It is evident from this finding that the endothermic formulation in-situ showed the lowest melting point at 224.40 °C of the drug. Compared to pure medication (hydrocortisone), in-situ gel formulation thermogram melting point appeared to be reduced and the appearance of sharp and long endothermic peak confirmed the amorphous nature of hydrocortisone.
Fig. 3.
A. DSC of pure drug B. DSC of formulation (F-1) C. DSC of formulation (F-2) D. DSC of formulation (F-3) E. DSC of formulation (F-4).
3.9. Drug content
Product content indicates that the amount of product content was contained in the range of 76.4–94.7% for all product material formulations (Table 2). The experiments were performed in triplicate.
3.10. In vitro drug release study
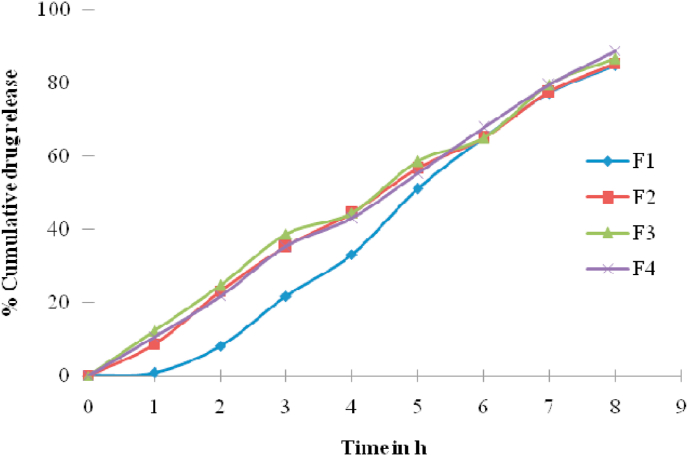
Drug loaded in-situ gel formulations were conducted for in vitro release experiments was carried out using pH 6.8 phosphate buffer in the diffusion medium for 8 h. The F1–F4 formulations containing polymer concentration (0.25–1%) displayed up to 4–8 h of the drug release (Fig. 5). The Formulations F-1 and F-2 released 100% of the drug within 5 h. The immediate release of the drug that was observed in formulations was due to the low concentration of methylcellulose. These observations indicated that the drug’s immediate release is attributable to the weak gelling capacity at low concentrations of methyl cellulose. Due to higher amounts of methyl cellulose than other formulations, the formulation containing 1% w/v of methyl cellulose (F-4) showed about 50% of the drug was released within 3 h. Study of the release of drugs in vitro revealed that the release rate was dependent on concentration of methyl cellulose. The greater the concentration of methyl cellulose, the lower the drug release rate.
Fig. 5.
In vitro release study.
3.11. Release kinetics
The resulting data were fitted into the following mathematical models (Table 4) to determine the release pattern and release mechanism. The drug release data from the in situ gel (F-4) was plotted using various kinetic models such as zero order, first order, Higuchi and Korsmeyer-Peppas. The R2 value and ‘n’ value of the drug loaded in-situ gel formulation is summarized in Table 4.
Table 4.
Kinetics release studies.
| Batch code | KINETIC MODELS |
||||
|---|---|---|---|---|---|
| Zero order |
First order |
Higuchi |
Korsmeyer-Peppas |
||
| R2 | R2 | R2 | n | R2 | |
| F1 | 0.956 | 0.436 | 0.730 | 1.538 | 0.452 |
| F2 | 0.995 | 0.377 | 0.879 | 1.611 | 0.492 |
| F3 | 0.993 | 0.254 | 0.898 | 1.530 | 0.350 |
| F4 | 0.995 | 0.236 | 0.867 | 1.583 | 0.387 |
The best fit model of release sequence was considered with zero and first-order maximum regression value (R2). Higuchi model regression values showing diffusion function were similar to defining the release mechanism. The slope value ‘n’ of the Korsmeyer-Peppas model further confirms the release mechanism.
The results suggest that the in-situ gel formulation release profile value of the first order kinetics and Higuchi kinetic model have the highest linearity. The release exponent n was found to be 0.432, where, n < 0.49 indicates the Fickian diffusion mechanism. From these results, it is concluded that the release of hydrocortisone from the in-situ gel follows first order kinetics and Fickian diffusion mechanism.
3.12. Stability studies
At 25 °C/60% and 40 °C/75% RH, the selected formulation F-4 was subjected to accelerated stability studies for three months. Formulation (F-4) remained unchanged in appearance, pH, consistency, percentage drug content, and viscosity.
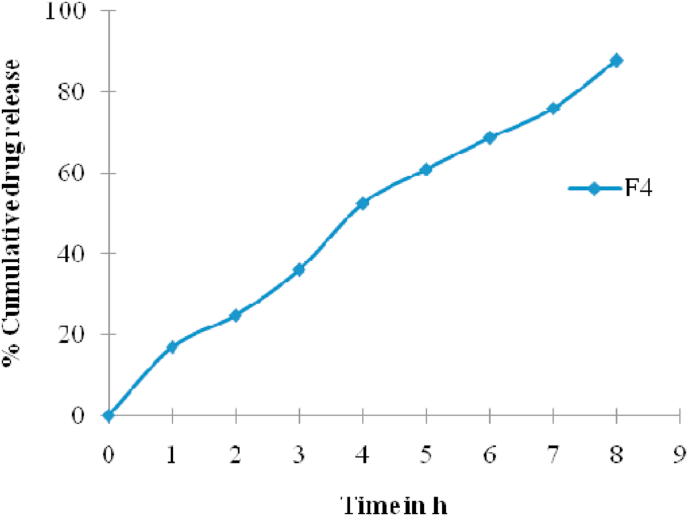
3.13. Ex vivo studies
The best stable formulation (F-4) was selected and subjected to a test of ex vivo release using diffusion cell through the chicken skin. The ex vivo release studies reveal a better estimate of the characteristics of drug permeation through animal skin. It was observed that at the end of 8 h, the permeation of optimized formulation F-4 showed a release of 88.33% as seen in Fig. 6.
Fig. 6.
Ex vivo studies of F-4.
4. Discussion
Aphthous ulcer is an oral mucosal inflammatory ulcer that predominantly occurs in the oral cavity that affects everyday tasks such as feeding, drinking, and speaking. In the present study, a sustained release hydrocortisone loaded in-situ gel was developed for the treatment of aphthous ulcers using various concentrations of methyl cellulose. Visually formulated in-situ gel formulation presented as a very simple and clear gel and was observed to have a pH of 6.7 which is suitable for gingival crevicular fluid (GCF). This characteristic is very important for oral formulations and does not irritate the gingiva, which is considered safe to administer in a mouth mucosal without any possible irritation and improves patient compliance.17
Higher amounts of formulated methyl cellulose (F-4) displayed a maximum viscosity of 440 cps at 25 °C than the lowest volume of methyl cellulose reported (F-1). Upon application, the in-situ gel formulation should have a high viscosity to bind to the mucosal surface for a prolonged period and ensure that the medication is absorbed consistently for the successful treatment of aphthous ulcer.
Evaluation of spreadability in terms of area covered by in-situ nasal gels per unit time showed that spreadability was related to the in-situ gel’s viscosity. Increasing mucoadhesive polymer content reduces the distance traveled by in-situ gels since mucoadhesive polymer increases the viscosity of in-situ gels. The optimal in-situ gel should have the precise gel strength such that it can be effectively applied and stored at the mucosa without leaking after administration, where, the escape of the drug from the preparation depends on the formulation viscosity. In formulation, the highest concentration (1.0% w/v) of methyl cellulose was used indicating strong gelation (++) time, where, the length of floating time remains 6–8 h. This could be due to the methylcellulose reaction with sodium citrate to form suitable gelling properties.18
FT-IR compatibility analysis was used to determine potential drug-polymer interactions. Formulation revealed typical peaks of drug and polymer and no new bands or peak changes were suggesting no association between drug and polymer. SEM observations confirmed the distribution of drug molecules in the polymer matrix and cross-linked into a gel. The SEM micrographs of F-3 & F-4 showed that hydrogel with visible porosity.19
The percentage of drug content was observed to be in the range of 76.4–94.7% in all the formulations (F1–F4). The drug release pattern was the true measure of the drug successfully loaded into the in-situ gel matrix. The release studies were meant to replicate the conditions in vivo.20 The formulation containing 1% w/v of methyl cellulose (F-4) showed that approximately 50% of the drug was released within 3 h due to higher levels of methyl cellulose than other formulations.
Among all the formulations, the F-4 (1% w/v) formulation exhibited a sustained drug release mechanism up to the end of 8 h. The decreased release rate following an enhancement in methyl cellulose concentration can be due to the relative improvement in gelling ability and gelling intensity.21 F-4 formulation demonstrated first-order release pattern as the observed higher R2 value for first-order. The formula for the in-situ gel is stable at varying temperature and humidity levels. After evaluating the results, F-4 was further tested for its ex vivo tests.14
The ex vivo release studies revealed a better estimate of the characteristics of drug permeation through animal skin. It has been observed that at the end of 8 h the permeation of optimized F4 formulation showed a release of 88.33%. Therefore, an attempt was made in this study to develop a sustained release of hydrocortisone in-situ gel for the treatment of aphthous ulcer by using different concentrations of methyl cellulose.22
5. Conclusion
In the present analysis, the in-situ gel of hydrocortisone was prepared using a specific concentration of methylcellulose (0.25–1% w/v) as the base of the gel. To assess the suitability for the formulation, all the formulated formulation was evaluated. All the prepared formulations provide intimate contact between the drug and the absorbing tissue that can lead to a high concentration of drugs in the local area. It can be established based on the findings obtained that the formulated formulation is appropriate for the treatment of aphthous ulcers. The formulation containing 1% w/v of methyl cellulose is known as an optimal formulation based on the measurement criterion from this four-different formulation. The formulation F4 (1% w/v) showed desirable gelling capacity and 6.8 pH which is suitable for the treatment of aphthous ulcer. The in vitro release of 100% of drug release up to the end of 8 h which offers a consistent means of releasing drugs and prolongs the residency duration. This can also be inferred that the formulated formulation increases patient compliance with improved bioavailability appropriate for the diagnosis of aphthous ulcer.
Acknowledgement
The authors are sincerely thankful to the Principal, Sri Adichunchanagiri College of Pharmacy, B.G. Nagara, for provided infrastructure facilities and moral support to carry out this research work.
Contributor Information
Sanjana A, Email: sanjana@yenepoya.edu.in.
Mohammed Gulzar Ahmed, Email: mohammedgulzar1@gmail.com.
Jaswanth Gowda BH, Email: jashgowda20@gmail.com.
References
- 1.Shwethashree M., George P.S., Prakash B. Prevalence of oral diseases among school children of mysuru and chamarajanagar districts, Karnataka, India. Clin Epidemiol Glob Health. 2020;8(3):725–727. [Google Scholar]
- 2.Gondivkar S.M., Gadbail A.R., Gondivkar R.S. Top cited articles on ameloblastoma: a bibliometric analysis. Transl Res Oral Oncol. 2019;4:1–7. [Google Scholar]
- 3.Gomes M.A.G., Zaroni F.M., Martins M.C., Lima A.A.S. Major recurrent aphthous stomatitis in mother and son with HIV/AIDS infection-Case report. Pediatr Pol. 2015;90(3):256–259. [Google Scholar]
- 4.Chiang C.P., Chang J.Y.F., Wang Y.P., Wu Y.H., Wu Y.C., Sun A. Recurrent aphthous stomatitis - etiology, serum autoantibodies, anemia, hematinic deficiencies, and management. J Formos Med Assoc. 2019;118(9):1279–1289. doi: 10.1016/j.jfma.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhary B., Verma S. Preparation and evaluation of novel in situ gels containing acyclovir for the treatment of oral herpes simplex virus infections. Sci World J. 2014:280928. doi: 10.1155/2014/280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nirmal H.B., Bakliwal S.R., Pawar S.P. In-situ gel: new trends in controlled & sustained drug delivery system. Int J PharmTech Res. 2010;2(2):1398–1408. [Google Scholar]
- 7.Ahmed M.G., Acharya A., Chaudhari R., Panicker K., Reddy R. Formulation and evaluation of in situ gel containing rosuvastatin in the treatment of periodontal diseases. J Pharm Res. 2015;14(2):45–50. [Google Scholar]
- 8.Nayak N.S., Bharani S.S., Thakur R.S. Formulation and evaluation of pH triggered in situ ophthalmic gel of Moxifloxacin hydrochloride. Int J Pharm Pharmaceut Sci. 2012;4(2):452–459. [Google Scholar]
- 9.Grover V.K., Babu R., Bedi S.P.S. Steroid therapy-current indications in practice. Indian J Anaesth. 2007;51(5):389–393. [Google Scholar]
- 10.Gupta P., Bhatia V. Corticosteroid physiology and principles of therapy. Indian J Pediatr. 2008;75(10):1039–1044. doi: 10.1007/s12098-008-0208-1. [DOI] [PubMed] [Google Scholar]
- 11.Mays J.W., Sarmadi M., Moutsopoulos N.M. Oral manifestations of systemic autoimmune and inflammatory diseases: diagnosis and clinical management. J Evid Base Dent Pract. 2012;12(3):265–282. doi: 10.1016/S1532-3382(12)70051-9. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed M.G., Choudhari R., Acharya A. Formulation and evaluation of in-situ gel containing Atrovastatin for the treatment of periodontal diseases. RGUHS J Pharm Sci. 2015;5(2):55–62. [Google Scholar]
- 13.Harish N.M., Prabhu P., Charyulu R.N., Gulzar M.A., Subrahmanyam E.V. Formulation and evaluation of in situ gels containing clotrimazole for oral candidiasis. Indian J Pharmaceut Sci. 2009;71(4):421–427. doi: 10.4103/0250-474X.57291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bansal M., Mittal N., Yadav S.K. Periodontal thermoresponsive, mucoadhesive dual antimicrobial loaded in-situ gel for the treatment of periodontal disease: preparation, in-vitro characterization and antimicrobial study. J Oral Biol Craniofac Res. 2018;8(2):126–133. doi: 10.1016/j.jobcr.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galgatte U.C., Kumbhar A.B., Chaudhari P.D. Development of in situ gel for nasal delivery: design, optimization, in vitro and in vivo evaluation. Drug Deliv. 2014;21(1):62–73. doi: 10.3109/10717544.2013.849778. [DOI] [PubMed] [Google Scholar]
- 16.Sheshala R., Quah S.Y., Tan G.C., Meka V.S., Jnanendrappa N., Sahu P.S. Investigation on solution-to-gel characteristic of thermosensitive and mucoadhesive biopolymers for the development of moxifloxacin-loaded sustained release periodontal in situ gels. Drug Deliv Transl Res. 2019;9(2):434–443. doi: 10.1007/s13346-018-0488-6. [DOI] [PubMed] [Google Scholar]
- 17.Garala K., Joshi P., Shah M., Ramkishan A., Patel J. Formulation and evaluation of periodontal in situ gel. Int J Pharm Investig. 2013;3(1):29–41. doi: 10.4103/2230-973X.108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wamorkar V., Varma M.M., Manjunath S.Y. Formulation and evaluation of stomach specific in situ gel of metoclopramide using natural, bio-degradable polymers. Int J Res Pharm Biomed Sci. 2011;2(1):193–201. [Google Scholar]
- 19.Swain G.P., Patel S., Gandhi J., Shah P. Development of Moxifloxacin Hydrochloride loaded in-situ gel for the treatment of periodontitis: in-vitro drug release study and antibacterial activity. J Oral Biol Craniofac Res. 2019;9(3):190–200. doi: 10.1016/j.jobcr.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Maghraby G.M., Elzayat E.M., Alanazi F.K. Development of modified in situ gelling oral liquid sustained release formulation of dextromethorphan. Drug Dev Ind Pharm. 2012;38(8):971–978. doi: 10.3109/03639045.2011.634811. [DOI] [PubMed] [Google Scholar]
- 21.Itoh K., Hatakeyama T., Shimoyama T., Miyazaki S., D’Emanuele A., Attwood D. In situ gelling formulation based on methylcellulose/pectin system for oral-sustained drug delivery to dysphagic patients. Drug Dev Ind Pharm. 2011;37(7):790–797. doi: 10.3109/03639045.2010.541465. [DOI] [PubMed] [Google Scholar]
- 22.Parekh H.B., Jivani R., Jivani N.P., Patel L.D., Makwana A., Sameja K. Novel in-situ polymeric drug delivery system: a review. J Drug Deliv Therapeut. 2012;2(5):136–145. [Google Scholar]