Abstract
Purpose
Tumor size and lymph node metastasis are important factors that contribute to the progression of breast cancer. We aimed to analyze the relationship between tumor size and lymph node metastasis molecular subtype and examine the effects of nodal metastasis on overall survival (OS).
Methods
We retrospectively reviewed the data of 16,552 patients who underwent breast surgery in Samsung Medical Center between 2000 and 2015. Information on tumor size (largest diameter of the invasive component), number of positive lymph nodes, and molecular subtype were obtained. We constructed a linear regression model to evaluate the relationship between tumor size and lymph node metastasis. To determine the effect of nodal metastasis on OS, we performed a Cox proportional regression analysis with Np/T (number of metastatic lymph nodes [n]/tumor size [cm]).
Results
This study included 12,007 patients with a median follow-up of 62 months. The linear regression coefficients were 1.043 for luminal A, 1.024 for luminal B, 0.656 for HER2, and 0.435 for triple-negative breast cancer (TNBC) subtypes. No significant difference was observed in the coefficients between the luminal A and B subtypes (p = 0.797), while all other coefficients showed significant difference. After adjusting for other risk factors, the hazard ratio (HR) of Np/T for each subtype was significant for OS: luminal A (HR, 1.134; 95% confidence interval [CI], 1.097–1.171; p < 0.001), luminal B (HR, 1.049; 95% CI, 1.013–1.086; p = 0.007), HER2 (HR, 1.069; 95% CI, 1.014–1.126; p = 0.013), and TNBC (HR, 1.038; 95% CI, 1.01–1.067; p = 0.008).
Conclusion
The incidence of lymph node metastasis differed according to molecular subtype. Luminal types have higher incidence of nodal metastasis than HER2 and TNBC. The HR of Np/T was highest in luminal A subtypes and lowest in TNBC subtypes.
Keywords: Breast neoplasms, Immunohistochemistry, Lymphatic metastasis, Prognosis
INTRODUCTION
Breast cancer is the most common type of malignancy worldwide and is the second leading cause of cancer-related deaths in women [1]. Because of the development of biomolecular diagnosis, four subtypes of breast cancer have been identified (luminal A, luminal B, human epidermal growth factor receptor 2 [HER2]-neu overexpressed, and triple-negative breast cancer [TNBC]). The distinct clinical features and prognosis of each subtype have been studied. Hormonal therapy, clinical use of trastuzumab (HER2-neu targeting agent), and introduction of effective chemotherapy have improved the prognosis of breast cancer.
The American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC) stratified stages based on tumor size (T), lymph node metastasis (N), and distant metastasis (M). In evaluating the prognosis of breast cancer, tumor size and lymph node metastasis have been the most powerful prognostic factors [2,3] and comprise the most generally accepted method of evaluating prognosis [4]. With a major prognostic value, the correlation between tumor size and nodal metastasis has been evaluated by several researchers. Increasing tumor size is associated with an increasing number of metastatic lymph nodes [5]. In a retrospective study of 453 patients with stage I-II breast cancer, subtype had no significant association with the number of metastatic lymph nodes or N stage [6]. However, some researchers have reported that the size-node relationship is not concordant with specific conditions. Some studies have reported that a positive correlation between tumor size and nodal metastasis is not similar in patients with basal-like breast cancer (BLBC) or BRCA-1 [5]. The TNBC subtype is larger than the other subtypes and has a weak relationship with lymph node metastasis [6,7]. Therefore, we aimed to evaluate the relationship between tumor size and number of metastatic lymph nodes according to subtype. In addition, we aimed to compare the impact of nodal metastasis on overall survival (OS) by subtype.
METHODS
Patient population
We retrospectively reviewed the data of patients diagnosed with breast cancer who underwent breast surgery in Samsung Medical Center between January 2000 and December 2015. A total of 16,552 patients was diagnosed with primary breast cancer and underwent breast surgery. Patients who received neoadjuvant chemotherapy or were diagnosed with carcinoma in situ (with or without microinvasion) were excluded. Patients with missing or inaccurate information on tumor size, metastatic lymph node, or subtype also were excluded. In total, 12,007 of 16,522 patients were enrolled in the study (Figure 1). All patients received standardized treatment in accordance with the National Comprehensive Cancer Network guidelines. Patients' medical data were obtained from the medical records, and this study was approved by the Institutional Review Board of Samsung Medical Center in Seoul, Korea. This is a retrospective study based on the medical records, so they did not seek informed consent (2019-02-003-002).
Figure 1. Flow chart of the patient inclusion process.
Diagnosis of molecular subtypes
For diagnosis of molecular subtypes of breast cancer, immunohistochemical (IHC) staining was performed. Immunoreactivity for estrogen receptor (ER) and progesterone receptor (PR) was considered positive if greater than 1% of the tumor cell nuclei showed staining. HER2 positivity was also confirmed by IHC, and patients who showed borderline results in IHC underwent fluorescence in situ hybridization (FISH). Dissected lymph nodes were stained by hematoxylin and eosin (H&E). If H&E staining showed negative results, additional IHC staining was performed. Luminal A type of breast cancer was classified as hormone receptor (ER and PR) positive and HER2 negative, with lower levels of Ki-67 expression (14%). Meanwhile, luminal B type was defined as positive for both hormonal receptor and HER2 expression but negative for HER2 expression, with a Ki-67 expression level of >14%. HER2 type was defined as a negative for hormonal receptor and positive for HER2 expression. Meanwhile, TNBC was defined as negative for hormonal receptor and HER2 expression.
Pathologic review of patients
For evaluation of tumor size and number of metastatic lymph nodes, all pathological data were collected. Throughout the study period, patients were staged according to the AJCC 6th and 7th staging system. We excluded patients with inaccurate tumor size, re-operation, positive margin, or missing data. If patients had multiple breast cancer tumors, the diameter of the largest tumor was selected for analysis [7].
Statistical analysis
To examine the difference in lymph node metastasis between molecular subtypes of breast cancer, we created the variable “Np/T,” which is calculated as the number of metastatic lymph nodes divided by tumor size (cm). Along with this variable, multiple factors were evaluated: age, histopathology, tumor size, lymph node dissection, metastatic lymph node, multiplicity of lesions, lymphovascular invasion (LVI), nucleic grade, histologic grade, ER status, PR status, HER2 status, and history of adjuvant hormonal therapy, radiation therapy, or chemotherapy. Analysis of variance was used to identify the statistical differences between the baseline characteristics of the patients. Post hoc analysis was conducted using the Bonferroni method to distinguish the differences among multiple subtypes. The correlation between tumor size and metastatic lymph nodes was analyzed using a linear regression model. OS was defined as the period from the day of surgery to the date of death from any cause or the last follow-up. We performed a multivariable Cox regression test, including the Np/T ratio. The regression coefficients and hazard ratios (HRs) were compared by identifying the interaction effect. A 95% confidence interval (CI) with a p-value of <0.050 was considered significant. All tests were 2-ided. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, USA).
RESULTS
Patient characteristics
The clinicopathological features of the 12,007 patients enrolled are shown in Table 1. Luminal A was the most common subtype (7,621 patients, 63.5%), and HER2 was the least common subtype (1,189 patients, 9.9%). The median age of the patients was 48 years. Overall, 7,853 (65.4%) patients underwent breast-conserving surgery, while 4,153 (34.6%) underwent total mastectomy. Patients with luminal B or HER2 subtype more often underwent total mastectomy than those with other subtypes (p < 0.001). For axillary surgery, 9,204 (76.7%) patients underwent sentinel lymph node (SLN) biopsy, while 2,802 (23.3%) had axillary lymph node dissection. In addition, luminal B and HER2 patients more frequently underwent axillary lymph node dissection than those with other subtypes (p < 0.001).
Table 1. Patients' baseline characteristics, surgical features, and basic pathologic information.
| Variables | Luminal A | Luminal B | HER2 | TNBC | Total | p-value | |
|---|---|---|---|---|---|---|---|
| Number | 7,621 (63.5) | 1,510 (12.6) | 1,189 (9.9) | 1,687 (14.0) | 12,007 | ||
| Age (yr) | 47.0 (20–88) | 48.0 (23–85) | 52.0 (27–85) | 48.0 (20–90) | 48.0 (20–90) | < 0.001 | |
| Surgery | < 0.001 | ||||||
| BCS | 5,200 (68.2) | 824 (54.6) | 568 (47.8) | 1,261 (74.8) | 7,853 (65.4) | ||
| TM | 2,421 (31.8) | 686 (45.4) | 621 (52.2) | 426 (25.2) | 4,154 (34.6) | ||
| SLNB | 5,980 (78.5) | 1,052 (69.7) | 803 (67.5) | 1,369 (81,3) | 9,204 (76.7) | ||
| ALND | 1,641 (21.5) | 458 (30.3) | 386 (32.5) | 318 (18.7) | 2,803 (23.3) | ||
| Histopathology | < 0.001 | ||||||
| IDC | 6,564 (86.1) | 1,423 (94.2) | 1,136 (95.5) | 1,477 (87.6) | 10,600 (88.3) | ||
| ILC | 455 (6.0) | 34 (2.6) | 5 (0.5) | 18 (1.0) | 512 (4.3) | ||
| Mucinous carcinoma | 311 (4.1) | 20 (1.3) | 5 (0.5) | 2 (0.1) | 338 (2.8) | < 0.001 | |
| Others | 291 (3.8) | 33 (1.9) | 43 (3.5) | 190 (11.3) | 557 (4.6) | ||
| T stage | < 0.001 | ||||||
| T1 | 4,600 (60.4) | 773 (51.2) | 619 (52.1) | 715 (42.4) | 6,707 (55.9) | ||
| T2 | 2,615 (34.3) | 667 (44.2) | 485 (40.8) | 899 (53.3) | 4,666 (38.9) | ||
| T3 or T4 | 405 (5.3) | 70 (4.6) | 85 (7.1) | 74 (4.3) | 634 (5.2) | ||
| N stage | < 0.001 | ||||||
| N0 | 4,705 (61.7) | 910 (60.3) | 769 (64.7) | 1,160 (68.8) | 7,544 (62.8) | ||
| N1 | 2,048 (26.9) | 381 (25.2) | 270 (22.7) | 375 (22.2) | 3,074 (25.6) | ||
| N2 | 571 (7.5) | 137 (9.1) | 97 (8.2) | 96 (5.7) | 901 (7.5) | ||
| N3 | 297 (3.9) | 82 (5.4) | 53 (4.4) | 56 (3.3) | 488 (4.1) | ||
| Multiplicity | < 0.001 | ||||||
| Single | 5,746 (75.4) | 1,172 (77.6) | 903 (75.9) | 1,461 (86.6) | 9,282 (77.3) | ||
| Multiple | 1,875 (24.6) | 338 (22.4) | 286 (24.1) | 226 (13.4) | 2,725 (22.7) | ||
| LVI | < 0.001 | ||||||
| Positive | 2,275 (29.6) | 537 (35.6) | 335 (28.2) | 444 (26.3) | 3,591 (29.9) | ||
| Negative | 5,348 (70.4) | 971 (64.4) | 854 (71.8) | 1,243 (73.7) | 8,416 (70.1) | ||
| Histologic grade | < 0.001 | ||||||
| 1 | 1,856 (24.4) | 75 (5.0) | 6 (0.5) | 46 (2.7) | 1,983 (16.5) | ||
| 2 | 4,318 (56.7) | 684 (45.3) | 265 (22.3) | 287 (17.0) | 5,554 (46.3) | ||
| 3 | 1,447 (18.9) | 751 (49.7) | 918 (77.2) | 1,354 (80.3) | 4,470 (37.2) | ||
| Nd | 11.31 ± 4.62 | 12.74 ± 4.86 | 12.62 ± 5.09 | 12.16 ± 4.84 | 11.74 ± 4.74 | < 0.001 | |
| Np | 1.51 ± 2.03 | 1.88 ± 2.28 | 1.76 ± 2.98 | 1.21 ± 1.96 | 1.54 ± 2.10 | < 0.001 | |
Values are presented as number of patients (%), median(range), or mean ± standard deviation.
HER2 = human epidermal growth factor receptor 2; TNBC = triple-negative breast cancer; BCS = breast conserving surgery; TM = total mastectomy; SLNB = sentinel lymph node biopsy; ALND = axillary lymph node dissection; IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma; Others = papillary, medullary, apocrine, tubular, signet ring cell and micropapillary type; LVI = lymphovascular invasion; Nd = number of surgically dissected lymph node; Np = number of metastatic lymph node.
Invasive ductal carcinoma was the most common breast cancer subtype (86.1%–95.5%). A total of 512 patients (4.3%) had invasive lobular carcinoma, while 338 (2.8%) had mucinous carcinoma. T1 tumor was observed in more than half of the patients (6,707 patients, 55.9%). However, in the group with TNBC, the number of patients with a T2 tumor (899 cases) was significantly higher than that of patients with a T1 tumor (715 cases) (p < 0.001).
Over 60% of patients had an N0 status for all subtypes. Metastatic lymph nodes were found in 4,462 (37.2%) patients. Multiple lesions were found in 22.7% of patients (2,724 patients). In TNBC, patients were more often diagnosed and treated for a single lesion (86.6%, p < 0.001) compared to those of other subtypes. LVI was found in 3,591 patients (29.9%). HER2 and TNBC had higher histologic grades than luminal A and B (p < 0.001). The mean numbers of dissected lymph nodes and metastatic lymph nodes were 11.74 and 1.54, respectively. The number of dissected lymph nodes was lower in patients with luminal A than in those with other subtypes (p < 0.001), but no significant difference was observed among the other subtypes.
Correlation between tumor size and lymph node metastasis according to molecular subtype
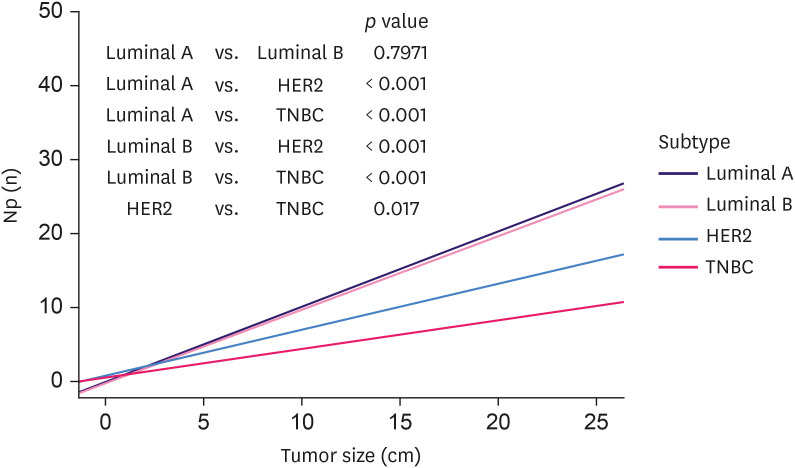
The data for tumor size and number of metastatic lymph nodes are presented in a scatter plot in Figure 2. Linear regression was used to evaluate the correlation between tumor size (cm) and the number of metastatic lymph nodes. Linear regression is conducted using least-squares estimation, which is the approximation of linear functions to data. The line calculated from the linear regression is shown in Figure 2. The slope of the line is the coefficient of linear regression. The coefficient of luminal A and B was 1.043 and 1.025, respectively. HER2 and TNBC had lower coefficients (0.656 and 0.435, respectively). For luminal A and B, the gradients were not statistically different (p = 0.797). However, all other subtypes showed significantly different gradients (p < 0.050).
Figure 2. Linear regression analysis of tumor size and metastatic lymph nodes.
Np = number of metastatic lymph node; HER2 = human epidermal growth factor receptor 2; TNBC = triple-negative breast cancer.
Effect of node metastasis adjusted by size (Np/T ratio) on survival by molecular subtype
We selected patients who survived for more than 6 months, and 11,199 patients were analyzed using the Cox regression test (Table 2). The median follow-up period was 62 months (range 6 to 186 months). For luminal A, histologic grade and Np/T were statistically significant. For luminal B, only Np/T was statistically significant. the number of patients with histologic grades I and II were extremely small, making it difficult to evaluate and compare the difference between these groups (grades 1 and 2). However, LVI and Np/T ratio showed statistical significance. For the TNBC subtype, age, multiplicity, LVI, and Np/T ratio showed statistical significance. For all patients, LVI, histologic grade, and Np/T ratio showed statistical significance. In all patients, the Np/T ratio was statistically significant (HR, 1.072; 95% CI, 1.041–1.104; p = 0.004); all subtypes had a statistically significant relationship with the Np/T ratio (HR, 1.072; 95% CI, 1.041–1.104; p = 0.004). The HR of Np/T for the subtypes were 1.134 for luminal A (95% CI, 1.097–1.171; p < 0.001), 1.049 for luminal B (95% CI, 1.013–1.086; p = 0.007), 1.069 for HER2 (95% CI, 1.014–1.126; p = 0.013), and 1.038 for TNBC (95% CI, 1.010–1.067; p = 0.008). Luminal A had the highest HR for Np/T, whereas TNBC had the lowest. Luminal B had a lower HR for Np/T than HER2 and a higher HR than TNBC. Table 3 presents a comparison of the Np/T values between subtypes. The difference between the HRs of Np/T was statistically significant between subtypes (p < 0.050).
Table 2. Results of the multivariable cox regression analysis of overall survival (n = 11,199).
| Variables | Luminal A | Luminal B | HER2 | TNBC | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age | 1.006 (0.990–1.023) | 0.452 | 0.984 (0.958–1.011) | 0.240 | 0.998 (0.971–1.027) | 0.914 | 1.019 (1.001–1.037) | 0.036 | 1.006 (0.996–1.016) | 0.249 | |
| Histopathology | |||||||||||
| IDC | 1.348 (0.723–2.511) | 0.347 | 0.768 (0.271–2.183) | 0.621 | 0.932 (0.277–3.130) | 0.909 | 0.821 (0.476–1.415) | 0.477 | 1.004 (0.703–1.433) | 0.984 | |
| Others | Ref | Ref | Ref | Ref | Ref | ||||||
| Multiplicity | |||||||||||
| Single lesion | Ref | Ref | Ref | Ref | Ref | ||||||
| Multiple lesion | 0.837 (0.565–1.242) | 0.377 | 0.658 (0.342–1.267) | 0.211 | 1.713 (0.954–3.077) | 0.071 | 0.477 (0.241–0.947) | 0.034 | 0.817 (0.630–1.061) | 0.817 | |
| LVI | |||||||||||
| No | Ref | Ref | Ref | Ref | Ref | ||||||
| Yes | 1.352 (0.969–1.886) | 0.075 | 0.901 (0.538–1.506) | 0.690 | 1.942 (1.101–3.426) | 0.021 | 2.185 (1.498–3.187) | < 0.001 | 1.499 (1.220–1.841) | < 0.001 | |
| Histologic grade | |||||||||||
| 1 | Ref | Ref | Ref | Ref | Ref | ||||||
| 2 | 3.044 (1.630–5.682) | < 0.001 | 1.861 (0.635–5.454) | 0.257 | 1.00 | 0.941 (0.348–2.546) | 0.904 | 2.853 (1.793–4.540) | < 0.001 | ||
| 3 | 5.342 (2.806–10.169) | < 0.001 | 2.331 (0.803–6.770) | 0.120 | 1.153 (0.655–2.030) | 0.621 | 1.497 (0.597–3.754) | 0.390 | 4.879 (3.091–7.702) | < 0.001 | |
| Np/size | 1.134 (1.097–1.171) | < 0.001 | 1.049 (1.013–1.086) | 0.007 | 1.069 (1.014–1.126) | 0.013 | 1.038 (1.010–1.067) | 0.008 | 1.072 (1.041–1.104) | 0.004 | |
HR = hazard ratio; CI = confidence interval; HER2 = human epidermal growth factor receptor 2; TNBC = triple-negative breast cancer; IDC = invasive ductal carcinoma; LVI = lymphovascular invasion.
Table 3. Statistical comparison of the HR of Np/T.
| Subtypes | p-value |
|---|---|
| Luminal A vs Luminal B | < 0.001 |
| Luminal A vs HER2 | < 0.001 |
| Luminal A vs TNBC | < 0.001 |
| Luminal B vs HER2 | 0.009 |
| Luminal B vs TNBC | 0.009 |
| HER2 vs TNBC | < 0.001 |
HR = hazard ratio; Np/T = number of metastatic lymph node/tumor size; HER2 = human epidermal growth factor receptor 2; TNBC = triple-negative breast cancer.
DISCUSSION
In this study, we analyzed the relationship between tumor size and lymph node metastasis according to breast cancer subtypes. Luminal types tend to have more frequent lymph node metastasis than HER2 or TNBC. In addition to this, we evaluated the effect of nodal metastasis adjusted by tumor size (Np/T) on OS. In all subtypes, nodal metastasis negatively affected the OS. In the luminal A subtype, which had the highest rate of lymphatic spread, nodal metastasis had the greatest negative effect on OS. In comparison, the TNBC subtype had the lowest rate of lymph node metastasis, with a smaller effect on OS compared to other subtypes.
In our study, the incidence of nodal metastasis differed according to the molecular subtype. Some researchers have suggested that patients with non-luminal subtypes (TNBC or HER2) have higher incidence of lymph node metastasis than those with luminal subtypes [8,9,10]. However, many researchers have reported that patients with non-luminal subtypes, including TNBC, have a lower incidence of lymph node metastases than those with luminal subtypes [11,12,13,14]. The reason for this difference in the extent of nodal metastasis according to subtype remains unclear, but genetic background and expression of molecules that determine tumor growth and lymphatic metastasis may play a role. Vascular endothelial growth factor (VEGF) stimulates the formation of blood vessels. In animal studies, VEGF-C mediated lymphangiogenesis in tumors and promoted dissemination of tumor cells to regional lymph nodes [15]. VEGF-D appears to promote angiogenesis and growth of tumors [16].
As estrogen was reported to downregulate the expression of VEGF [17], its level of expression in luminal types was relatively low, while HER2 and TNBC had higher expression of VEGF. TNBC has higher microvascular density and higher expression of VEGF [18]. From these backgrounds, patients with TNBC were presumed to have a larger tumor size and higher incidence of hematogenous metastasis [19,20,21,22,23]. However, these molecules are not the only factors that determine the risk for lymph node metastasis and tumor growth. Hence, further studies are needed to understand these phenomena and apply them in clinical practice.
In the luminal A subtype with more frequent lymph node metastasis, nodal involvement more strongly affected the OS compared with other subtypes. Despite our results, it is recognized that luminal types have a better prognosis than non-luminal subtypes. Our study demonstrated that the HR of Np/T on OS was lowest in TNBC, implying that lymph node metastasis has a weaker effect on the survival of patients with TNBC compared to those with the luminal A subtype regardless of tumor size. In accordance with our study, another research reported that tumor size and nodal metastasis are not good predictors of survival in patients with TNBC [24]. Hernandez-Aya et al. revealed significant differences in OS and recurrence-free survival between N0 and node-positive patients [12]. However, when comparing N1 with N2 and N3 patients, there were no significant differences in OS and recurrence-free survival. In a study by Dent et al. [25], a higher proportion of patients with TNBC had distant metastasis than those with other subtypes (33.9% vs. 20.4%, p < 0.001). These patients experienced distant metastasis earlier compared with those with other subtypes (2.6 vs. 5.0 years, p < 0.001). Rather than lymphatic spread, the poor prognosis of non-luminal types seemed to result from different pathways, such as distant metastasis through hematogenous spread [11,19,22,23]. TNBC is known to have more visceral and bone metastasis than other subtypes [20,21]. Therefore, the prognostic significance of lymphatic metastasis in non-luminal subtypes has little influence on the survival of breast cancer patients.
For curative treatment of breast cancer, appropriate surgical treatment and surveillance of axillary lymph node metastasis are essential [26]. Recent studies have reported that molecular subtype is associated with SLN and non-SLN metastases [14,27]. Because luminal types have higher incidence of lymph node metastasis with a larger HR of Np/T on survival compared with non-luminal subtypes, surveillance for axillary lymph nodes and additional locoregional treatments (e.g., axillary lymph node dissection or radiotherapy) may be required. In concordance with several other studies [13,28], our study found that the HER2 or TNBC subtype does not carry a higher axillary tumor burden compared with the luminal subtypes. However, due to the high locoregional recurrence rate and aggressive behavior, HER2 or TNBC patients more frequently undergo additional axillary dissection [29]. In a retrospective study, among patients with TNBC subtype potentially eligible for Z0011 criteria, axillary lymph node dissection could be avoided in 67% of node-positive patients and 84% of clinically node-negative patients [30]. For appropriate resection of axillary lymph nodes, further studies are needed to evaluate the relationship between molecular subtype and axillary lymph node metastasis.
In our study, the HRs of Np/T differed according to molecular subtype. In TNBC, the conventional nodal staging system (clinically estimated nodal status [cN]) has lesser predictive value when used in luminal A subtypes. In addition to cN, other indicators may be required to predict the risk of distant metastasis and prognosis of TNBC patients.
As this was a retrospective observational study, selection bias was present. Before performing the Cox regression test, we selected the risk factors to be included in the multivariate analysis. We analyzed and compared the incidence of nodal metastasis by tumor size in a large cohort within a longer period. In a single institution, 12,006 patients were treated using a standardized protocol, and a pathological review was conducted. For adequate evaluation of tumor size and lymph node metastasis, patients who received neoadjuvant chemotherapy were excluded. To the best of our knowledge, this is the first study to show the difference in nodal metastasis adjusted by tumor size according to molecular subtype and to compare the HR of Np/T on OS.
In conclusion, the incidence of lymph node metastasis differed according to the molecular subtype of breast cancer. Luminal types tend to have higher incidence of lymph node metastasis than HER2 or TNBC. Np/T ratio, which indicates the effect of nodal metastasis adjusted by tumor size, had a more adverse effect on the OS of patients with luminal A subtype and less effect on the OS of those with TNBC. Our findings suggest that the subtype of breast cancer has an influence on the regional management of axillary node metastasis.
ACKNOWLEDGMENTS
We would like to thank the Statistics and Data Center, Research Institute for Future Medicine, Samsung Medical Center for providing assistance with the statistical analysis.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
- Project administration: Lee SK, Nam SJ.
- Supervision: Jung SM, Ryu JM, Yu J, Lee JE, Kim SW, Chae BJ.
- Writing - original draft: Min SK.
- Writing - review & editing: Woo J.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg J, Chia YL, Plevritis S. The effect of age, race, tumor size, tumor grade, and disease stage on invasive ductal breast cancer survival in the U.S. SEER database. Breast Cancer Res Treat. 2005;89:47–54. doi: 10.1007/s10549-004-1470-1. [DOI] [PubMed] [Google Scholar]
- 3.Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:966–978. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 4.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th ed. New York (NY): Springer; 2010. [Google Scholar]
- 5.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Jones T, Neboori H, Wu H, Yang Q, Haffty BG, Evans S, et al. Are breast cancer subtypes prognostic for nodal involvement and associated with clinicopathologic features at presentation in early-stage breast cancer? Ann Surg Oncol. 2013;20:2866–2872. doi: 10.1245/s10434-013-2994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tresserra F, Rodriguez I, García-Yuste M, Grases PJ, Ara C, Fabregas R. Tumor size and lymph node status in multifocal breast cancer. Breast J. 2007;13:68–71. doi: 10.1111/j.1524-4741.2006.00365.x. [DOI] [PubMed] [Google Scholar]
- 8.Li CY, Zhang S, Zhang XB, Wang P, Hou GF, Zhang J. Clinicopathological and prognostic characteristics of triple-negative breast cancer (TNBC) in Chinese patients: a retrospective study. Asian Pac J Cancer Prev. 2013;14:3779–3784. doi: 10.7314/apjcp.2013.14.6.3779. [DOI] [PubMed] [Google Scholar]
- 9.Gülben K, Berberoğlu U, Aydoğan O, Kınaş V. Subtype is a predictive factor of nonsentinel lymph node involvement in sentinel node-positive breast cancer patients. J Breast Cancer. 2014;17:370–375. doi: 10.4048/jbc.2014.17.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howland NK, Driver TD, Sedrak MP, Wen X, Dong W, Hatch S, et al. Lymph node involvement in immunohistochemistry-based molecular classifications of breast cancer. J Surg Res. 2013;185:697–703. doi: 10.1016/j.jss.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Aya LF, Chavez-Macgregor M, Lei X, Meric-Bernstam F, Buchholz TA, Hsu L, et al. Nodal status and clinical outcomes in a large cohort of patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2628–2634. doi: 10.1200/JCO.2010.32.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattes MD, Bhatia JK, Metzger D, Ashamalla H, Katsoulakis E. Breast cancer subtype as a predictor of lymph node metastasis according to the SEER registry. J Breast Cancer. 2015;18:143–148. doi: 10.4048/jbc.2015.18.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou W, He Z, Xue J, Wang M, Zha X, Ling L, et al. Molecular subtype classification is a determinant of non-sentinel lymph node metastasis in breast cancer patients with positive sentinel lymph nodes. PLoS One. 2012;7:e35881. doi: 10.1371/journal.pone.0035881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Currie MJ, Hanrahan V, Gunningham SP, Morrin HR, Frampton C, Han C, et al. Expression of vascular endothelial growth factor D is associated with hypoxia inducible factor (HIF-1alpha) and the HIF-1alpha target gene DEC1, but not lymph node metastasis in primary human breast carcinomas. J Clin Pathol. 2004;57:829–834. doi: 10.1136/jcp.2003.015644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai H, Li H, Chun P, Avraham S, Avraham HK. Direct interaction between BRCA1 and the estrogen receptor regulates vascular endothelial growth factor (VEGF) transcription and secretion in breast cancer cells. Oncogene. 2002;21:7730–7739. doi: 10.1038/sj.onc.1205971. [DOI] [PubMed] [Google Scholar]
- 18.Liu HT, Ma R, Yang QF, Du G, Zhang CJ. Lymphangiogenic characteristics of triple negativity in node-negative breast cancer. Int J Surg Pathol. 2009;17:426–431. doi: 10.1177/1066896909337505. [DOI] [PubMed] [Google Scholar]
- 19.Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer. 2009;9(Suppl 2):S73–S81. doi: 10.3816/CBC.2009.s.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodríguez-Pinilla SM, Sarrió D, Honrado E, Hardisson D, Calero F, Benitez J, et al. Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas. Clin Cancer Res. 2006;12:1533–1539. doi: 10.1158/1078-0432.CCR-05-2281. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Shi M, Ling R, Xia Y, Luo S, Fu X, et al. Adjuvant chemotherapy and radiotherapy in triple-negative breast carcinoma: a prospective randomized controlled multi-center trial. Radiother Oncol. 2011;100:200–204. doi: 10.1016/j.radonc.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Baig A, Kasymjanova G, Kafi K, Holcroft C, Mekouar H, et al. Pattern of local recurrence and distant metastasis in breast cancer by molecular subtype. Cureus. 2016;8:e924. doi: 10.7759/cureus.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foulkes WD, Grainge MJ, Rakha EA, Green AR, Ellis IO. Tumor size is an unreliable predictor of prognosis in basal-like breast cancers and does not correlate closely with lymph node status. Breast Cancer Res Treat. 2009;117:199–204. doi: 10.1007/s10549-008-0102-6. [DOI] [PubMed] [Google Scholar]
- 25.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 26.Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reyal F, Rouzier R, Depont-Hazelzet B, Bollet MA, Pierga JY, Alran S, et al. The molecular subtype classification is a determinant of sentinel node positivity in early breast carcinoma. PLoS One. 2011;6:e20297. doi: 10.1371/journal.pone.0020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crabb SJ, Cheang MC, Leung S, Immonen T, Nielsen TO, Huntsman DD, et al. Basal breast cancer molecular subtype predicts for lower incidence of axillary lymph node metastases in primary breast cancer. Clin Breast Cancer. 2008;8:249–256. doi: 10.3816/CBC.2008.n.028. [DOI] [PubMed] [Google Scholar]
- 29.Ong CT, Thomas SM, Blitzblau RC, Fayanju OM, Park TS, Plichta JK, et al. Patient age and tumor subtype predict the extent of axillary surgery among breast cancer patients eligible for the American College of Surgeons Oncology Group Trial Z0011. Ann Surg Oncol. 2017;24:3559–3566. doi: 10.1245/s10434-017-6075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung A, Gangi A, Mirocha J, Giuliano A. Applicability of the ACOSOG Z0011 criteria in women with high-risk node-positive breast cancer undergoing breast conserving surgery. Ann Surg Oncol. 2015;22:1128–1132. doi: 10.1245/s10434-014-4090-y. [DOI] [PubMed] [Google Scholar]