Abstract
Background:
Anxiety is associated with aberrant patterns of cortical thickness in regions implicated in emotion regulation. However, few studies have examined cortical thickness differences between individuals with anxiety and healthy controls (HCs) across development, particularly during childhood when cortical thinning begins and anxiety risk increases. A better understanding of age-related changes in cortical thickness patterns among anxious individuals is essential to develop plausible targets for early identification.
Methods:
The current study examined how age impacted differences in cortical thickness patterns between HCs and anxious individuals. Participants included 233 individuals (ages 7–35) with a current anxiety disorder (n=149) or no lifetime history of psychopathology (n=84). Cortical thickness of regions that are implicated in emotion regulation [ventromedial prefrontal cortex (vmPFC), rostral anterior cingulate (rACC), and insula] were assessed.
Results:
All regions showed significant thinning with age, except left rACC and right insula. However, rates of thinning differed among anxious and HC participants, with anxious participants demonstrating slower rates of right vmPFC thinning. Regions of significance analyses indicated that anxious, relative to HC, participants exhibited thinner right vmPFC before age 11, but thicker right vmPFC after age 24.
Conclusions:
Current findings suggest that anxious individuals do not demonstrate normative right vmPFC cortical thinning, which may lead them to exhibit both thinner vmPFC in middle childhood and thicker vmPFC in adulthood compared to HCs. These findings may provide plausible targets for identification of anxiety risk that differ based on developmental stage.
Keywords: anxiety, cortical thickness, developmental differences, imaging, ventromedial prefrontal cortex
Introduction
Anxiety disorders impact a large proportion of the population (Bandelow & Michaelis, 2015), and are among the leading causes of disability world-wide (World Health Organization, 2017). Additionally, they are characterized by a chronic course (Yonkers et al., 2003), and often onset between childhood and later adolescence (De Lijster et al., 2017). Given high rates of lifetime prevalence and childhood onset, there is a significant need for the identification of targets that can be utilized in early identification and prevention for individuals at high risk for anxiety.
Cortical thickness of regions implicated in emotion regulation, such as the ventromedial prefrontal cortex (vmPFC), rostral anterior cingulate cortex (rACC), and insula (Etkin et al., 2011, 2015), may be promising neural markers of anxiety for two reasons. First, there is evidence that activation of these regions during task-based emotional reactivity is altered in anxiety (Duval et al., 2015; Taylor & Whalen, 2015). However, though anxious individuals consistently exhibit insula hyperactivation, both hypo- and hyperactivation of the vmPFC and rACC has been observed across studies and tasks (Duval et al., 2015; Taylor & Whalen, 2015). Therefore, though functional evidence implicates the role of these regions in anxiety, structural indices, such as cortical thickness, display greater retest reliability (Elliott et al., 2020), and therefore may be more reliable markers of risk. Second, cortical thickness is a sensitive index of age-related changes in cortical structure (Lemaitre et al., 2012). Given its sensitivity to developmental changes, deviations from typical trajectories of cortical thickness development may be more easily detected, thereby leading to early identification of at-risk individuals.
To date, research examining abnormalities in vmPFC, rACC, and insula cortical thickness in anxious adults has yielded largely mixed results. Some studies find that, compared to healthy controls (HCs), anxious participants exhibit thinner vmPFC (Carnevali et al., 2019; Syal et al., 2012), though others have failed to detect group differences (Asami et al., 2018; Maggioni et al., 2019; Molent et al., 2018). Results are also mixed for rACC, with some work demonstrating that anxious individuals exhibit thinner rACC (Carnevali et al., 2019), while others studies find thicker rACC (Brühl et al., 2014; Zhao et al., 2017), or no differences in rACC thickness (Asami et al., 2018; Maggioni et al., 2019; Molent et al., 2018; Syal et al., 2012) compared to non-disordered individuals. Similarly, anxious individuals have been shown to exhibit both thinner (Kang et al., 2017; Syal et al., 2012) and thicker (Brühl et al., 2014; Zhao et al., 2017) insula than HCs, while other studies have failed to find differences between these groups (Asami et al., 2018; Frick et al., 2013; Maggioni et al., 2019; Molent et al., 2018).
In comparison to the adult literature which, while largely mixed, has begun to highlight cortical structural abnormalities in anxiety disorders, only three studies to date have compared cortical thickness between youth (ages 8 to 18) with and without anxiety disorders. Two recent studies found that anxious youth exhibited thicker vmPFC than controls across two studies (Gold et al., 2017; Strawn et al., 2014). Findings were less consistent for rACC and insula, with one study indicating less cortical thickness in these regions for anxious youth compared to controls (Suffren et al., 2019), while two others reported null results (Gold et al., 2017; Strawn et al., 2014).
Clearly, questions remain regarding whether clinical anxiety is associated with cortical thickening or thinning in regions implicated in emotion regulation. Along with methodological differences across labs, inconsistent findings may relate to developmental change. Research indicates indices of cortical thickness demonstrate developmental changes, typically reaching their peak in early childhood (Ducharme et al., 2016) before linearly thinning until middle age (Fjell et al., 2014; Tamnes et al., 2010). However, individuals with anxiety may not demonstrate typical trajectories of cortical thinning. Preliminary evidence from community samples suggests that youth with greater anxiety symptoms demonstrate slower rates of cortical thinning, thereby leading to differential relations between anxiety and cortical thickness across development such that cortical thickness is thinner in younger anxious youth but thicker in older anxious youth (Ducharme et al., 2014; Newman et al., 2016). This deviance from normative cortical thinning may also predict future increases in symptoms in youth (Whittle et al., 2019). However, to date, studies examining differences in age-related patterns of cortical thinning have been limited to youth community samples. As the current body of literature is largely mixed regarding whether anxiety is associated with increased or decreased cortical thickness of regions implicated in emotion regulation (i.e., vmPFC, rACC, insula), developmental differences could contribute to these mixed findings. Therefore, the goal of the current study was to examine the relation between anxiety and age-related patterns of cortical thinning in a sample of individuals ranging from childhood to adulthood with and without anxiety disorders.
In addition to examining developmental differences, we expand on prior study findings by taking a Research Domains of Criteria (RDoC) Initiative (Insel et al., 2010) approach. The goal of the RDoC Initiative is to classify psychopathology utilizing a dimensional approach based on neurobiological and behavioral markers, rather than traditional categorical diagnoses (Cuthbert & Insel, 2013). Indeed, increasing research highlights transdiagnostic biological and behavioral markers that cut across categorically defined anxiety disorders and provides evidence for dimensional models of anxiety (Sharp et al., 2015; Tambs et al., 2009). Therefore, rather than examining cortical thickness within the context of specific diagnoses, the current study examined whether clinical anxiety is associated with deviations in typical age-related patterns of cortical thinning in a large sample of diagnostically diverse patients with anxiety and HCs ranging from childhood to middle adulthood. Based on preliminary evidence from community samples, we hypothesized that anxious individuals would show diminished age-related patterns of cortical thinning in regions implicated in emotion regulation (i.e., vmPFC, rACC, insula) compared to HCs, thereby leading the relation between anxiety and cortical thickness to change across age.
Materials and Methods
Participants
Participants included 233 participants between the ages of 7 and 35 (M Age=17.86, SD=5.96) from two separate studies. The first study was conducted at both University of Illinois at Chicago (UIC) and University of Michigan (UM) and included a sample of children and adolescents (ages 7–19). The second study was conducted at UIC and included a sample of adults (ages 18–35). Of note, although the original adult sample included participants up to age 65, we restricted the age range of the current study to 35 because cortical thickness data was only available for 4 HCs between the ages of 30 and 65 and rates of cortical thinning decelerate around middle age (Fjell et al., 2014).
Inclusionary criteria for the current study included either a current anxiety diagnosis (n=149) or no lifetime history of any DSM-IV Axis I disorder in the youth sample or any DSM-5 disorder in the adult sample (n=84). Comorbidity was permitted. Exclusionary criteria included treatment (i.e., psychotropic medication, psychotherapy), histories of serious mental illness (i.e., bipolar disorder, schizophrenia), intellectual disability, pervasive development disorders, current substance use disorders within the past 6 months, or contraindications to magnetic resonance imaging (e.g., pregnancy, metal implants). In the current study, 62.2% of participants were female. In terms of participants’ racial identity, 54.9% were Caucasian, 15.5% were African American, 14.2% were Asian, and the rest identified as biracial or another race. Participants in the adult and youth samples did not differ by gender, χ2(1)=3.78, p=.051, or race, χ2(1)=0.02, p=.90. However, as expected, participants in the youth sample were significantly younger than those in the adult sample, t(231)=-16.98, p<.001.
Across both studies, participants were recruited from outpatient clinics and the community using a variety of means including flyers and internet advertisements. Informed consent and assent were obtained and all participants completed assessments of clinical diagnoses and symptoms and a resting MRI scan. Study procedures were approved by the Institutional Review Boards at both UIC and UM.
Clinical Measures
Clinical diagnoses in youth and adults were assessed using the Schedule for Affective Disorders and Schizophrenia for School Age Children (Kaufman et al., 1997) and the Structured Clinical Interview for DSM–5 (First et al., 2015), respectively. Diagnoses within the anxious group are presented in Table 1. As seen, there were high levels of comorbidity and the majority of anxious participants had 2 or more current anxiety diagnoses. Within the anxious group, participants in the youth and adult samples did not differ in frequency of diagnoses of generalized anxiety disorder, social anxiety disorder, specific phobia, or agoraphobia (lowest p=.31). However, participants in the adult sample had higher frequency of diagnoses of panic disorder, χ2(1)=11.46, p=.001, and posttraumatic stress disorder, χ2(1)=7.51, p=.006. As expected, rates of separation anxiety disorder were higher in the youth sample, χ2(1)=12.20, p<.001.
Table 1.
Demographic and Clinical Characteristics
| Healthy Controls (n=84) | Anxiety Diagnosis (n=149) | t/χ2 | |
|---|---|---|---|
| Demographics | |||
| Age | 16.94 (5.08) | 18.38 (6.36) | 1.78 |
| Gender (% female) | 58.3% | 64.4% | 0.85 |
| Race (% Caucasian) | 48.8% | 58.4% | 1.99 |
| Study Site (% UIC) | 70.2% | 71.8% | 0.07 |
| Cortical Thickness (mm) | |||
| Right vmPFC | 2.73 (0.17) | 2.72 (0.16) | 0.57 |
| Left vmPFC | 2.61 (0.17) | 2.65 (0.16) | 1.64 |
| Right rACC | 3.08 (0.24) | 3.09 (0.22) | 0.40 |
| Left rACC | 3.01 (0.28) | 3.00 (0.23) | 0.54 |
| Right Insula | 2.99 (0.18) | 3.00 (0.16) | 0.30 |
| Left Insula | 2.99 (0.19) | 3.01 (0.18) | 0.35 |
| Clinical Characteristics | |||
| Anxiety Symptoms (z score) | −1.19 (0.26) | 0.63 (0.59) | 25.52*** |
| Generalized Anxiety Disorder | - | 73.2% | - |
| Social Anxiety Disorder | - | 61.1% | - |
| Panic Disorder | - | 18.1% | - |
| Specific Phobia | - | 18.1% | - |
| Separation Anxiety Disorder | - | 8.1% | - |
| Posttraumatic Stress Disorder | - | 8.7% | - |
| Agoraphobia | - | 4.0% | - |
| Two or More Anxiety Diagnoses | - | 59.1% | - |
| Number of Anxiety Diagnoses | - | 1.91 (0.95) | - |
Note. vmPFC=ventromedial prefrontal cortex. rACC=rostral anterior cingulate cortex.
p<.001.
Anxiety symptoms were assessed using the Pediatric Anxiety Rating Scale (PARS; Research Units on Pediatric Psychopharmacology Anxiety Study Group, 2002) in the youth study and the Hamilton Anxiety Rating Scale (HAMA; Hamilton, 1959) in the adult study. To allow for comparison of anxiety symptoms across the two samples, both the PARS and HAMA total scores were converted into standardized z-scores by subtracting the sample mean from the observed score and dividing by the sample standard deviation.
Cortical Thickness Acquisition
At UIC, the structural MRI scans were obtained on a 3 Tesla GE Discovery System (General Electric Healthcare, Waukesha, WI) with an 8-channel head coil. After positioning the subjects in a supine position in the scanner, structural scans were obtained with a 3D BRAVO pulse sequence with the following parameters: flip angle 13°, inversion time 450 msec, field of view 22 × 22 cm, matrix size 256 × 256, slice thickness 1 mm3, 182 axial slices of the whole brain. At UM, a 3.0 T GE Signa Scanner (General Electric; Milwaukee, Wisconsin, USA) with a GE quad head coil was used to acquire high resolution, T1-weighted volumetric anatomical scans (3D spoiled-gradient echo sequence, 9 ms repetition time, 1.8 ms echo time, 500 ms inversion time, 15° flip angle, 256 × 256 matrix, 256 mm field of view; 124 slices, 1.2 mm slice thickness).
Cortical Thickness Preprocessing
Across both studies, the cortical thickness measurements were obtained using FreeSurfer Image analysis suite version 6.0 (http://freesurfer.net/fswiki/FreeSurferWiki). Automated procedure for cortical reconstruction and volumetric segmentation has been previously documented in detail (Dale et al., 1999; Fischl et al., 2002). In short, structural images analysis involved motion correction, removal of non-brain tissue, transformation to Talairach space, segmentation of white matter and gray matter structures, intensity normalization, tessellation of gray-white matter boundary, topology correction, and surface deformation following intensity gradients for optimal placement of gray-white matter boundary and cerebrospinal fluid and gray matter boundary. The reconstructed cortical surface was automatically parcellated to cortical units with respect to gyral and sulcal structure based on probabilistic information from previously labelled atlases (Desikan et al., 2006; Fischl et al., 2004) following which, the surface area, thickness, and volume of each unit were computed (Fischl & Dale, 2000; Han et al., 2006). Here, cortical thickness of bilateral rostral ACC, vmPFC, and insula were then extracted for further analysis, wherein thickness is the average distance between the white-gray matter boundary and the pia-gray matter boundary at each vertex on the tessellated surface.
Analytic Approach
Linear regressions were used to examine whether age-related patterns of cortical thickness differed among anxious participants and HCs. In the first step of the regression, study sample (youth vs. adult study), study location (UIC vs. UM), gender, estimated total intracranial volume (ICV), age, and diagnostic group (anxiety: yes vs. no) were all entered as predictor variables. The Age × Diagnostic Group interaction was entered as a predictor variable in step two of the regression. Cortical thickness for regions of interest was entered as the dependent variable. This analysis was repeated separately for bilateral indices of vmPFC, rACC, and insula cortical thickness. A Bonferroni correction was used for all primary analyses to control for multiple comparisons (adjusted p=.017).
Results
Demographic and clinical characteristics of participants are presented in Table 1. No significant differences were observed between anxious participants and HCs on any demographic variables. As expected, participants with an anxiety diagnosis had greater levels of anxiety symptoms than HCs, t(212)=25.52, p<.001, reffect size=.87.
Primary analyses are presented in Table 2. Results indicate a main effect of age on all indices of cortical thickness indices, except left rACC and right insula, such that all other examined regions showed significant linear thinning with age (βs=-.49 – -.33, ps=<.001, reffect sizes=.23–.33) (see Figure 1). Additionally, there were group differences for left vmPFC, with anxious participants showing greater thickness than HCs, β=.15, t(226)=2.42, p=.016, reffect size=.16 (see Figure 2). The main effect of gender was also significant for left vmPFC, β=-.30, t(226)=-4.23, p<.001, reffect size =.27, and right insula, β=-.18, t(226)=-2.50, p=.013, reffect size=.16, with males (37.8%; n=88) showing greater cortical thickness.
Table 2.
Regression Analyses Examining Differences in Age-Related Patterns of Cortical Thinning Among Anxious and Control Participants
| R vmPFC | L vmPFC | R rACC | L rACC | R Insula | L Insula | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Step 1 | t | reffect size | t | reffect size | t | reffect size | t | reffect size | t | reffect size | t | reffect size |
| Study | 1.41 | .09 | 1.99* | .13 | 0.65 | .04 | −0.82 | .05 | 0.53 | .03 | 1.64 | .10 |
| Study Site | −0.54 | .04 | 0.11 | .01 | 1.75 | .12 | 3.09**a | .20 | −2.96**a | .19 | −3.89***a | .25 |
| ICV | 1.79 | .12 | −1.06 | .07 | −0.93 | .06 | −1.29 | .09 | −0.52 | .03 | 0.86 | .06 |
| Gender | −0.29 | .02 | −4.23***a | .27 | −1.15 | .08 | −1.38 | .09 | −2.50*a | .16 | −2.06* | .14 |
| Age | −5.18***a | .33 | −4.29***a | .27 | −3.74***a | .24 | −1.58 | .10 | −2.20* | .14 | −3.58***a | .23 |
| Diagnosis | −0.01 | <.001 | 2.42*a | .16 | 1.01 | .07 | −0.02 | .001 | 0.85 | .06 | 0.86 | .06 |
| Step 2 | ||||||||||||
| Age × Diagnosis | 2.48*a | .16 | −0.14 | .01 | 0.72 | .05 | 1.49 | .10 | 0.31 | .02 | 0.58 | .04 |
Note. R=right. L=left. vmPFC=ventromedial PFC. rACC=rostral anterior cingulate cortex. ICV=estimated total intracranial volume.
p < .05;
p < .01;
p < .001.
Significant after Bonferroni correction (adjusted p = .017).
Figure 1.
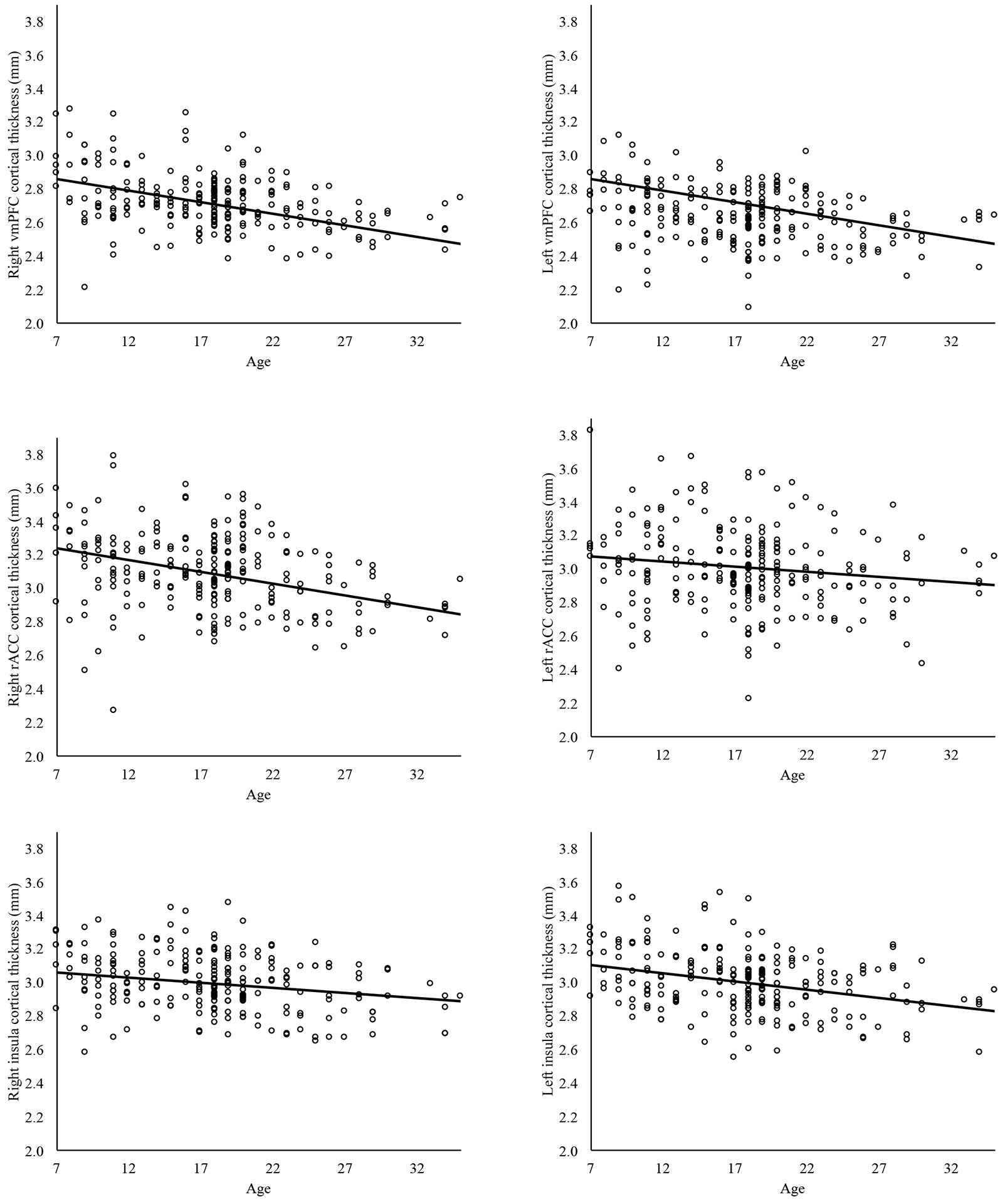
Cortical thickness across age within bilateral vmPFC, bilateral rACC, and bilateral insula. The main effect of age was significant at p<.017 for all indices of cortical thickness except left rostral anterior cingulate cortex and right insula. vmPFC=ventromedial PFC. rACC=rostral anterior cingulate cortex.
Figure 2.
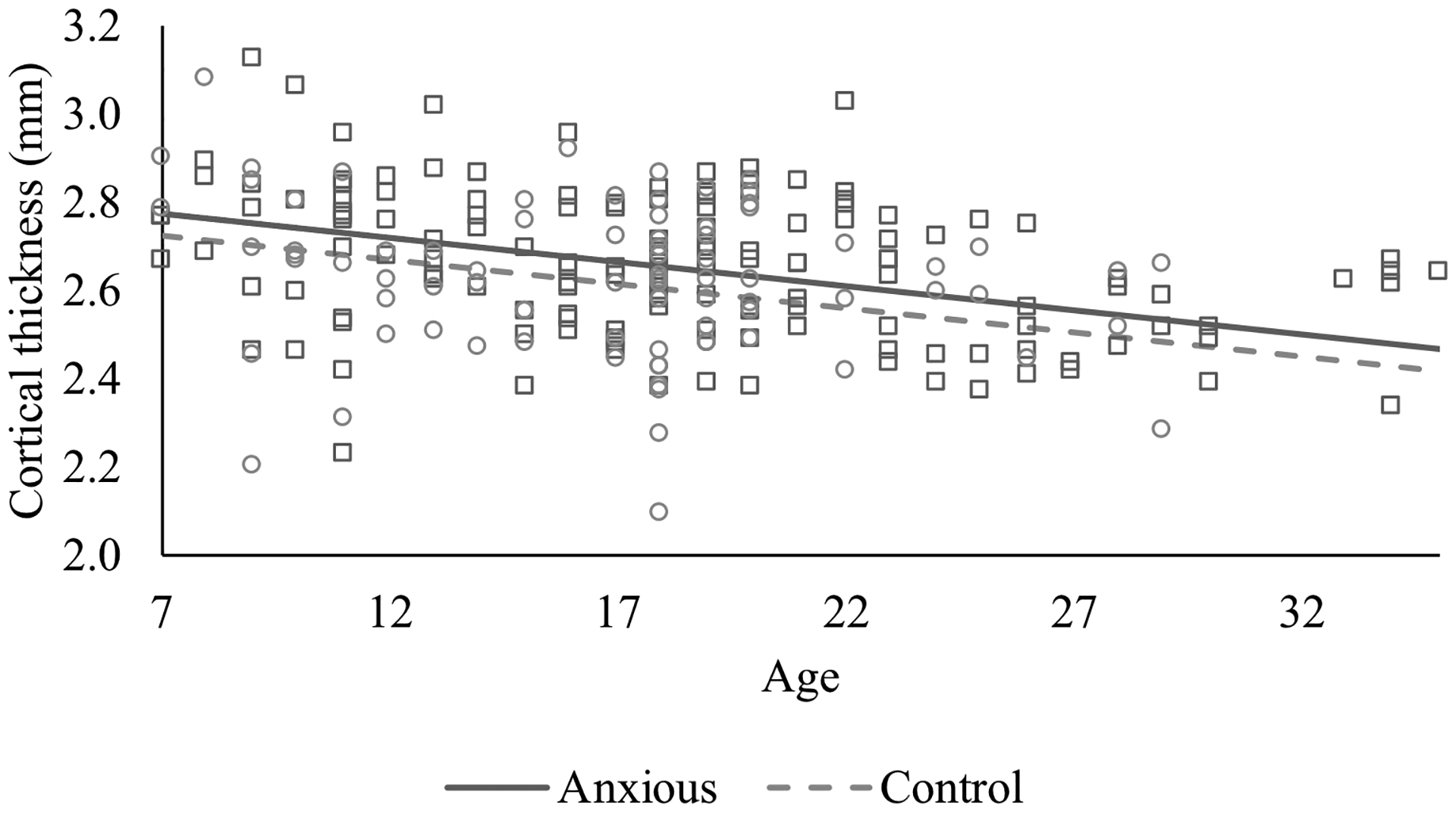
Group differences in left ventromedial prefrontal cortex thickness between anxious and control participants.
Results also revealed a significant Age × Diagnostic Group interaction for right vmPFC, β=.59, t(225)=2.48, p=.014, reffect size=.16, but none of the other regions of interest (lowest p=.14). Splitting by diagnostic group, results indicated that HCs showed greater rates of thinning, β=-.67, t(78)=-5.08, p<.001, reffect size=.50, compared to anxious participants, β=-.38, t(143)=-3.06, p=.003, reffect size=.25. A region of significance analysis utilizing the PROCESS macro (Hayes, 2017) showed that anxious patients exhibited thinner right vmPFC compared to HCs at ages 10.20 and below, but thicker right ventromedial PFC than HCs at ages 24.53 and above (see Figure 3).
Figure 3.
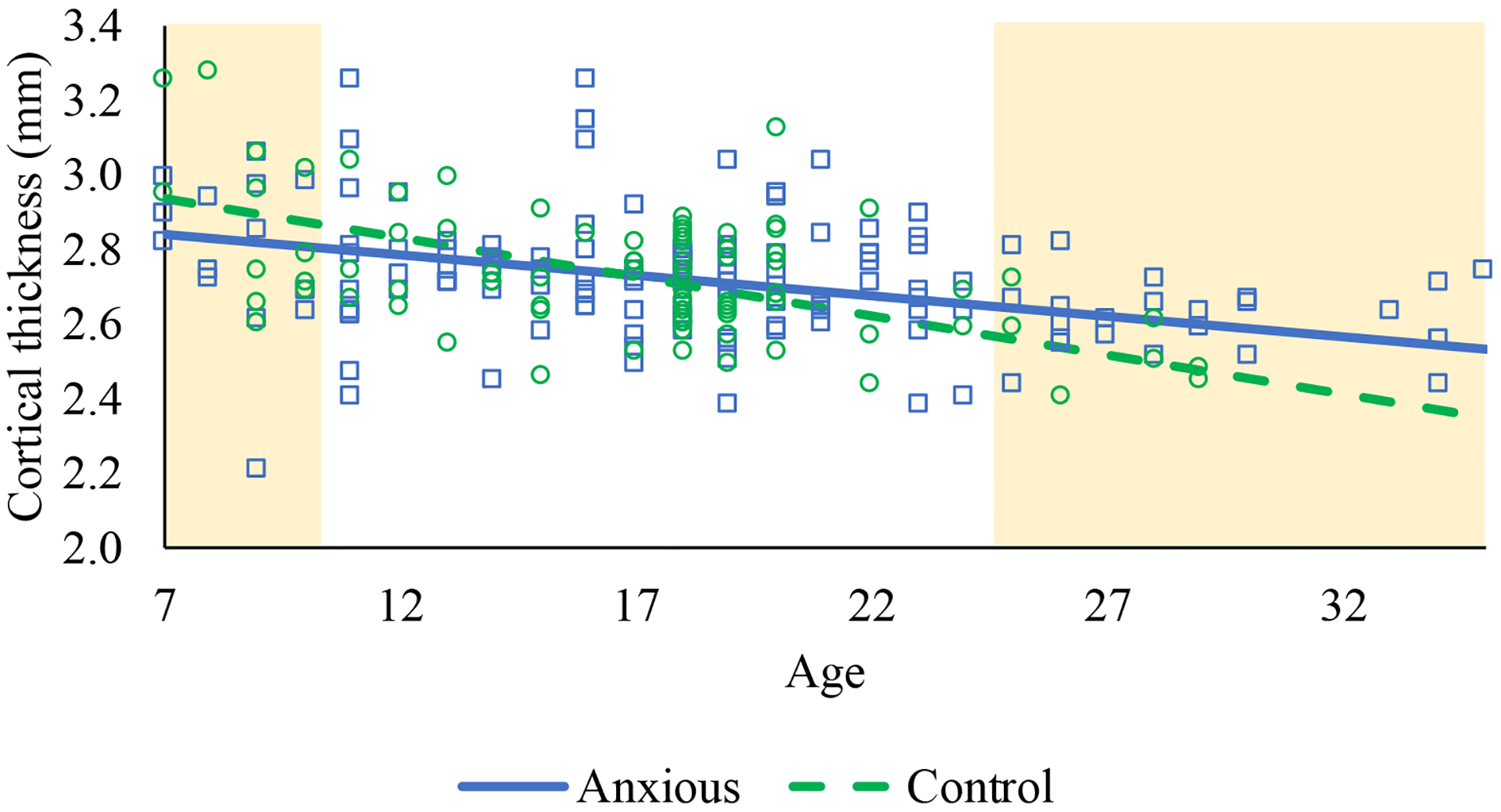
Cortical thinning across age for right ventromedial prefrontal cortex among anxious and control participants. The shaded regions are the regions at which anxious and control participants differ in right ventromedial prefrontal cortex thickness at p<.05.
Finally, information regarding additional analyses examining nonlinear effects of age and cortical thinning in anxiety diagnostic subgroups can be found in supplementary materials and Supplementary Table 1.
Discussion
The current study sought to examine whether clinical anxiety is associated with deviations in typical age-related patterns of cortical thinning in a large sample of patients with heterogeneous anxiety disorders and HCs ranging from middle childhood to middle adulthood. Consistent with hypotheses, clinically anxious individuals exhibited lesser cortical thinning of the right vmPFC across age, relative to HCs. Additionally, the relation between cortical thickness and anxiety differed across development such that, compared to HCs, anxious children between the ages of 7 and 11 exhibited thinner right vmPFC whereas relatively thicker vmPFC was observed in adulthood. Thus, results from the current study suggest that anxious individuals do not demonstrate normative cortical thinning within the right vmPFC, which may lead anxious individuals to exhibit both thinner and thicker vmPFC compared to HCs at different stages of development. Together, these findings have the potential to provide plausible targets for identification of anxiety risk that differ based on developmental stage.
In the current study, anxious and control participants only differed in age-related patterns of cortical thinning for right vmPFC, and no differences in left vmPFC or bilateral rACC or insula thickness were observed. This is partially supported by previous findings, as multiple studies have failed to detect a relation between differences in rACC and insula thickness between anxious and control individuals (Asami et al., 2018; Frick et al., 2013; Gold et al., 2017; Maggioni et al., 2019; Molent et al., 2018; Strawn et al., 2014; but see also Brühl et al., 2014; Suffren et al., 2019; Zhao et al., 2017). Additionally, the relation between symptoms and aberrant patterns of age-related cortical thinning in community samples has also been observed to be specific to right vmPFC (Ducharme et al., 2014; Whittle et al., 2019). There is debate regarding whether the lateralization of brain function relates to emotional valence or motivation (Miller et al., 2013). However, both proposed models implicate the lateralization of processes related to anxiety (i.e., negative emotion processing, avoidance behaviors) to the right hemisphere (Harmon-Jones et al., 2010; Heller et al., 1998) and are in accordance with current and previous findings that aberrant development of the right, but not left, vmPFC may be associated with anxiety. This said, as information regarding participant handedness was not systematically collected in the current study, and therefore was unable to be controlled for, interpretation of the lateralization of current findings should remain tentative.
The vmPFC is implicated in processes that may contribute to the development and maintenance of anxiety disorders, such as fear extinction and emotion regulation. Though the precise role of the vmPFC remains debated, there is increasing evidence that the vmPFC contributes to fear extinction by inhibiting conditioned fear responses during extinction recall (Sevenster et al., 2018). Similarly, the vmPFC is consistently implicated in implicit emotion regulation processes, and may serve to modulate reactivity of limbic regions (Etkin et al., 2011, 2015). Thus, as anxiety disorders are linked to deficits in both fear extinction (Duits et al., 2015) and emotion regulation (Cisler et al., 2010), it is possible that deficits in these processes may mediate the relation between aberrant age-related patterns of vmPFC thickness and anxiety. Future research is needed to confirm whether the relation between aberrant cortical thinning and anxiety is specific to right vmPFC, as well as mechanisms underlying this relation.
Current findings indicate that the relation between anxiety and vmPFC cortical thickness may vary as a function of development, with anxious children exhibiting thinner vmPFC and adults exhibiting thicker vmPFC compared to HCs. This finding is consistent with previous studies within community samples showing that cortical thickness is thinner in anxious youth during middle childhood but thicker in older adolescence/early adulthood (Ducharme et al., 2014; Newman et al., 2016). However, it conflicts with other anxious patient samples that have detected null results (Asami et al., 2018; Frick et al., 2013; Maggioni et al., 2019; Molent et al., 2018), thicker vmPFC in youth (Gold et al., 2017; Strawn et al., 2014), and thinner vmPFC in adulthood (Carnevali et al., 2019; Syal et al., 2012). Methodological differences across studies may be responsible for these contradictory findings. For instance, all but one (Gold et al., 2017) of these previous studies examining vmPFC thinning included small diagnostic samples (n<40), which may have limited their power to detect group differences in vmPFC thickness. Additionally, the majority of previous studies were limited to homogenous diagnostic groups with little comorbidity, whereas in the current study, the majority of anxious participants had multiple current anxiety diagnoses, thereby suggesting clinical differences in samples. Finally, these prior studies included samples extending to late adulthood, by which point cortical thinning is largely completed in typically developing samples (Fjell et al., 2014; Tamnes et al., 2010). If anxious individuals continue to show aberrant cortical thinning into later adulthood after thinning is typically completed, it is plausible that the relation between vmPFC thickness and anxiety could again change at this developmental switch point, thereby contributing to mixed findings in samples including older adults.
Nonetheless, results highlight trajectories of vmPFC thinning as a promising marker of anxiety, and suggest that either thinner or thicker vmPFC may be a biomarker of clinical anxiety, depending on the developmental stage. Indeed, compared to other indices, cortical thickness is particularly sensitive to age-related changes in cortical structure (Lemaitre et al., 2012), thereby allowing for the early detection of aberrant development as risk for anxiety increases. This is particularly important, as our results bolster evidence that anxiety-related aberrant cortical thinning is observable prior to adolescence in middle childhood (Ducharme et al., 2016; Newman et al., 2016; Whittle et al., 2019), when risk of anxiety onset significantly increases (Costello et al., 2005). However, it is important to note that the current study focused on individuals with and without a current diagnosis of anxiety and was not longitudinal, thereby precluding conclusions from being drawn regarding whether aberrant vmPFC cortical thinning is a predictor or correlate of clinical anxiety. Despite this, increasing evidence does point to aberrant cortical thinning as an early marker of risk which may be detected before the onset of a clinical diagnosis. For example, though environmental influences, such as abuse, may impact vmPFC thickness (e.g., Gold et al., 2016), cortical thickness is highly heritable (Panizzon et al., 2009), suggesting that this may be an important phenotype of interest when examining biological risk for anxiety. In line with this, although vmPFC thickness was not specifically examined, one study illustrated that both disordered and non-disordered offspring of anxious parents differ from controls in cortical thickness (Suffren et al., 2019). Moreover, in a separate community sample, slower rates of cortical thinning across a two-year window in middle childhood, particularly within the vmPFC region, were predictive of prospective increases in internalizing symptoms (Whittle et al., 2019). Therefore, even though future longitudinal studies are needed to test whether aberrant cortical thinning trajectories of the vmPFC predict anxiety disorder onset, preliminary evidence suggests this may be an important early biological marker of risk.
Despite the study’s many strengths including a large and clinically diverse anxious sample spanning multiple developmental periods, there are some limitations that are important to address. First, although the clinical heterogeneity of the current sample allowed for an examination of age-related patterns of cortical thickness as a transdiagnostic marker of anxiety, it is possible that the heterogeneity and high levels of comorbidity may have increased the risk of Type II error in the current study and obscured patterns of cortical thinning that are specific to subtypes of anxiety. Also, as previously noted, the current study was cross-sectional, and therefore could not assess whether abnormal vmPFC cortical thinning preceded anxiety onset. Furthermore, data on age of anxiety onset was not obtained. As anxiety typically onsets between later childhood and early adulthood (De Lijster et al., 2017), it is also possible that clinical anxiety predicts slower thinning of vmPFC after illness onset. Additionally, the current sample only examined age-related patterns of cortical thinning through middle adulthood. Future studies should examine whether abnormalities in cortical thinning trajectories observed in anxious individuals continue through later adulthood, where cortical thinning typically stabilizes. Finally, it is important to note that MRI data was obtained at two different sites, therefore, it is possible that site differences (e.g., scanner bore sizes) may have impacted results. Though site location was controlled for in the current study, the potential for a site confound cannot be completely ruled out.
Conclusion
In summary, the current study suggests that individuals with and without anxiety diagnoses differ in age-related patterns of vmPFC thinning, thereby resulting in anxiety being associated with thinner vmPFC in childhood and thicker vmPFC in adulthood. Pending replication in independent and longitudinal studies, cortical thinning of vmPFC may be a promising marker than can be used in early identification of individuals at high-risk for anxiety onset.
Supplementary Material
Acknowledgements.
This work was supported by National Institute of Mental Health grant R01-MH086517 (to C.S.M. and K.L.P), National Institute of Mental Health grant R01MH101497 (to K.L.P.), and Center for Clinical and Translational Science (CCTS) UL1RR029879. K.L.B. is supported by National Institute of Mental Health grant MH113793. J.H.S. is supported by F32-HD100075.
Footnotes
Conflicts of Interest. The authors declare no conflicts of interest.
Data Availability Statement. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Asami T, Takaishi M, Nakamura R, Yoshida H, Yoshimi A, Whitford TJ, Inoue T, & Hirayasu Y (2018). Cortical thickness reductions in the middle frontal cortex in patients with panic disorder. Journal of Affective Disorders, 240, 199–202. 10.1016/j.jad.2018.07.064 [DOI] [PubMed] [Google Scholar]
- Bandelow B, & Michaelis S (2015). Epidemiology of anxiety disorders in the 21st century. Dialogues in Clinical Neuroscience, 17(3), 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brühl AB, Hänggi J, Baur V, Rufer M, Delsignore A, Weidt S, Jäncke L, & Herwig U (2014). Increased cortical thickness in a frontoparietal network in social anxiety disorder. Human Brain Mapping, 35(7), 2966–2977. 10.1002/hbm.22378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevali L, Mancini M, Koenig J, Makovac E, Watson DR, Meeten F, Critchley HD, & Ottaviani C (2019). Cortical morphometric predictors of autonomic dysfunction in generalized anxiety disorder. Autonomic Neuroscience: Basic and Clinical, 217, 41–48. 10.1016/j.autneu.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Cisler JM, Olatunji BO, Feldner MT, & Forsyth JP (2010). Emotion regulation and the anxiety disorders: An integrative review. Journal of Psychopathology and Behavioral Assessment 10.1007/s10862-009-9161-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Egger HL, & Angold A (2005). The developmental epidemiology of anxiety disorders: Phenomenology, prevalence, and comorbidity. Child and Adolescent Psychiatric Clinics of North America, 14(4), 631–648. 10.1016/j.chc.2005.06.003 [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, & Insel TR (2013). Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Medicine, 11(1), 126. 10.1186/1741-7015-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–194. 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- De Lijster JM, Dierckx B, Utens EMWJ, Verhulst FC, Zieldorff C, Dieleman GC, & Legerstee JS (2017). The age of onset of anxiety disorders: A meta-analysis. Canadian Journal of Psychiatry, 62(4), 237–246. 10.1177/0706743716640757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, & Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Ducharme S, Albaugh MD, Hudziak JJ, Botteron KN, Nguyen TV, Truong C, Evans AC, & Karama S (2014). Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cerebral Cortex, 24(11), 2941–2950. 10.1093/cercor/bht151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S, Albaugh MD, Nguyen TV, Hudziak JJ, Mateos-Pérez JM, Labbe A, Evans AC, Karama S, Ball WS, Byars AW, Schapiro M, Bommer W, Carr A, German A, Dunn S, Rivkin MJ, Waber D, Mulkern R, Vajapeyam S, … O’Neill J (2016). Trajectories of cortical thickness maturation in normal brain development - The importance of quality control procedures. NeuroImage, 125, 267–279. 10.1016/j.neuroimage.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duits P, Cath DC, Lissek S, Hox JJ, Hamm AO, Engelhard IM, Van Den Hout MA, & Baas JMP (2015). Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depression and Anxiety, 32(4), 239–253. 10.1002/da.22353 [DOI] [PubMed] [Google Scholar]
- Duval ER, Javanbakht A, & Liberzon I (2015). Neural circuits in anxiety and stress disorders: A focused review. Therapeutics and Clinical Risk Management, 11, 115–126. 10.2147/TCRM.S48528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott ML, Knodt AR, Ireland D, Morris ML, Poulton R, Ramrakha S, Sison ML, Moffitt TE, Caspi A, & Hariri AR (2020). What is the test-retest reliability of common task-fMRI measures? New empirical evidence and a meta-analysis. BioRxiv, 681700. 10.1101/681700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Büchel C, & Gross JJ (2015). The neural bases of emotion regulation. Nature Reviews Neuroscience, 16(11), 693–700. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, & Kalisch R (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Williams J, Karg R, & Spitzer R (2015). Structured Clinical Interview for DSM-5-Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). American Psychiatric Association. [Google Scholar]
- Fischl B, & Dale AM (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11050–11055. 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, & Dale AM (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, & Dale AM (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11–22. 10.1093/cercor/bhg087 [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Dale AM, & Walhovd KB (2014). Accelerating cortical thinning: Unique to dementia or universal in aging? Cerebral Cortex, 24, 919–934. 10.1093/cercor/bhs379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A, Howner K, Fischer H, Eskildsen SF, Kristiansson M, & Furmark T (2013). Cortical thickness alterations in social anxiety disorder. Neuroscience Letters, 536, 52–55. 10.1016/j.neulet.2012.12.060 [DOI] [PubMed] [Google Scholar]
- Gold AL, Sheridan MA, Peverill M, Busso DS, Lambert HK, Alves S, Pine DS, & McLaughlin KA (2016). Childhood abuse and reduced cortical thickness in brain regions involved in emotional processing. Journal of Child Psychology and Psychiatry and Allied Disciplines, 57(10), 1154–1164. 10.1111/jcpp.12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AL, Steuber ER, White LK, Pacheco J, Sachs JF, Pagliaccio D, Berman E, Leibenluft E, & Pine DS (2017). Cortical thickness and subcortical gray matter volume in pediatric anxiety disorders. Neuropsychopharmacology, 42(12), 2423–2433. 10.1038/npp.2017.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1959). The assessment of anxiety states by rating. British Journal of Medical Psychology, 32, 50–55. 10.1111/j.2044-8341.tb00467.x.1959 [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, & Fischl B (2006). Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage, 32(1), 180–194. 10.1016/j.neuroimage.2006.02.051 [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, & Peterson CK (2010). The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biological Psychology, 84(3), 451–462. 10.1016/j.biopsycho.2009.08.010 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2017). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Publications. [Google Scholar]
- Heller W, Nitschke JB, & Miller GA (1998). Lateralization in emotion and emotional disorders. Current Directions in Psychological Science, 7(1), 26–32. 10.1111/1467-8721.ep11521823 [DOI] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, & Wang P (2010). Research Domain Criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry, 167(7), 748–751. 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Kang EK, Lee KS, & Lee SH (2017). Reduced cortical thickness in the temporal pole, insula, and pars triangularis in patients with panic disorder. Yonsei Medical Journal, 58(5), 1018–1024. 10.3349/ymj.2017.58.5.1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, & Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 980–988. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Goldman AL, Sambataro F, Verchinski BA, Meyer-Lindenberg A, Weinberger DR, & Mattay VS (2012). Normal age-related brain morphometric changes: Nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiology of Aging, 33(3), 617–e1. 10.1016/j.neurobiolaging.2010.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggioni E, Delvecchio G, Grottaroli M, Garzitto M, Piccin S, Bonivento C, Maieron M, A. SD, Giampaolo P, Balestrieri M, & Brambilla P (2019). Common and different neural markers in major depression and anxiety disorders: A pilot structural magnetic resonance imaging study. Psychiatry Research - Neuroimaging. 10.1016/j.pscychresns.2019.06.006 [DOI] [PubMed] [Google Scholar]
- Miller GA, Crocker LD, Spielberg JM, Infantolino ZP, & Heller W (2013). Issues in localization of brain function: The case of lateralized frontal cortex in cognition, emotion, and psychopathology. Frontiers in Integrative Neuroscience, 7(2). 10.3389/fnint.2013.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molent C, Maggioni E, Cecchetto F, Garzitto M, Piccin S, Bonivento C, Maieron M, D’Agostini S, Balestrieri M, Perna G, Altamura AC, & Brambilla P (2018). Reduced cortical thickness and increased gyrification in generalized anxiety disorder: A 3 T MRI study. Psychological Medicine, 48(12), 2001–2010. 10.1017/S003329171700352X [DOI] [PubMed] [Google Scholar]
- Newman E, Thompson WK, Bartsch H, Hagler DJ, Chen CH, Brown TT, Kuperman JM, McCabe C, Chung Y, Libiger O, Akshoomoff N, Bloss CS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kennedy DN, Murray SS, … Jernigan TL (2016). Anxiety is related to indices of cortical maturation in typically developing children and adolescents. Brain Structure and Function, 221(6), 3013–3025. 10.1007/s00429-015-1085-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, & Kremen WS (2009). Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex, 19(11), 2728–2735. 10.1093/cercor/bhp026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research Units on Pediatric Psychopharmacology Anxiety Study Group. (2002). The pediatric anxiety rating scale (PARS): Development and psychometric properties. Journal of the American Academy of Child and Adolescent Psychiatry, 41(9), 1061–1068. 10.1097/00004583-200209000-00006 [DOI] [PubMed] [Google Scholar]
- Sevenster D, Visser RM, & D’Hooge R (2018). A translational perspective on neural circuits of fear extinction: Current promises and challenges. Neurobiology of Learning and Memory, 155, 113–126. 10.1016/j.nlm.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PB, Miller GA, & Heller W (2015). Transdiagnostic dimensions of anxiety: Neural mechanisms, executive functions, and new directions. International Journal of Psychophysiology, 98(2), 365–377. 10.1016/j.ijpsycho.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Strawn JR, John Wegman C, Dominick KC, Swartz MS, Wehry AM, Patino LR, Strakowski SM, Adler CM, Eliassen JC, & DelBello MP (2014). Cortical surface anatomy in pediatric patients with generalized anxiety disorder. Journal of Anxiety Disorders, 28(7), 717–723. 10.1016/j.janxdis.2014.07.012 [DOI] [PubMed] [Google Scholar]
- Suffren S, Chauret M, Nassim M, Lepore F, & Maheu FS (2019). On a continuum to anxiety disorders: Adolescents at parental risk for anxiety show smaller rostral anterior cingulate cortex and insula thickness. Journal of Affective Disorders, 248, 34–41. 10.1016/j.jad.2019.01.028 [DOI] [PubMed] [Google Scholar]
- Syal S, Hattingh CJ, Fouché JP, Spottiswoode B, Carey PD, Lochner C, & Stein DJ (2012). Grey matter abnormalities in social anxiety disorder: A pilot study. Metabolic Brain Disease, 27(3), 299–309. 10.1007/s11011-012-9299-5 [DOI] [PubMed] [Google Scholar]
- Tambs K, Czajkowsky N, Røysamb E, Neale MC, Reichbom-Kjennerud T, Aggen SH, Harris JR, Ørstavik RE, & Kendler KS (2009). Structure of genetic and environmental risk factors for dimensional representations of DSM-IV anxiety disorders. British Journal of Psychiatry, 195(4), 301–307. 10.1192/bjp.bp.108.059485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due-Tønnessen P, & Walhovd KB (2010). Brain maturation in adolescence and young adulthood: Regional age-related changes in cortical thickness and white matter volume and microstructure. Cerebral Cortex, 20, 534–548. 10.1093/cercor/bhp118 [DOI] [PubMed] [Google Scholar]
- Taylor JM, & Whalen PJ (2015). Neuroimaging and anxiety: The neural substrates of pathological and non-pathological Anxiety. Current Psychiatry Reports, 17(6), 49. 10.1007/s11920-015-0586-9 [DOI] [PubMed] [Google Scholar]
- Whittle S, Vijayakumar N, Simmons JG, & Allen NB (2019). Internalizing and externalizing symptoms Are associated with different trajectories of cortical development during late childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 10.1016/j.jaac.2019.04.006 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2017). Depression and other common mental disorders: global health estimates. In World Health Organization. https://doi.org/CCBY-NC-SA3.0IGO [Google Scholar]
- Yonkers KA, Bruce SE, Dyck IR, & Keller MB (2003). Chronicity, relapse, and illness - Course of panic disorder, social phobia, and generalized anxiety disorder: Findings in men and women from 8 years of follow-up. Depression and Anxiety, 17(3), 173–179. 10.1002/da.10106 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Chen L, Zhang W, Xiao Y, Shah C, Zhu H, Yuan M, Sun H, Yue Q, Jia Z, Zhang W, Kuang W, Gong Q, & Lui S (2017). Gray matter abnormalities in non-comorbid medication-naive patients with major depressive disorder or social anxiety disorder. EBioMedicine, 21, 228–235. 10.1016/j.ebiom.2017.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


