Abstract
Lungs from “non-ideal,” but acceptable donors are underutilized, however organ procurement organization (OPO) metrics do not reflect the extent to which OPO-specific practices contribute to these trends. We developed a comprehensive system to evaluate non-ideal lung donor avoidance, or risk aversion among OPOs. Adult donors in the UNOS registry who donated ≥1 organ for transplantation between 2007–2018 were included. Non-ideal donors had any of age>50, smoking history ≥20 pack-years, PaO2/FiO2 ratio ≤350, donation after circulatory death, or increased risk status. OPO-level risk aversion in donor pursuit, consent attainment, lung recovery, and transplantation was assessed. Among 83,916 donors, 70,372 (83.9%) were non-ideal. Unadjusted OPO-level rates of non-ideal donor pursuit ranged from 81–100%. In a three-tier system of overall risk aversion, tier 3 OPOs (least risk-averse) had the highest rates of non-ideal donor pursuit, consent attainment, lung recovery, and transplantation. Tier 1 OPOs (most risk-averse) had the lowest rates of donor pursuit, consent attainment, and lung recovery, but higher rates of transplantation compared to tier 2 OPOs (moderately risk-averse). Risk aversion varies among OPOs and across the donation process. OPO evaluations should reflect early donation process stages to best differentiate over- and underperforming OPOs and encourage optimal OPO-specific performance.
INTRODUCTION
In the US, organ donor pursuit and utilization depend heavily on the 58 organ procurement organizations (OPOs) that oversee the donation process. Per Organ Procurement and Transplantation Network (OPTN) policy, OPOs individually define “acceptable” donor organs, resulting in 58 unique sets of criteria that could introduce considerable unmeasured variability into patterns of donor pursuit and utilization.1 To maximize organ recovery, the Centers for Medicare and Medicaid Services (CMS) evaluate OPO performance using donation and transplantation rate metrics.2 Discordance between these measures may signify underutilization by transplant centers rather than OPOs and current metrics fail to measure performance in earlier stages of the donation process, which may better reflect OPO-specific performance and identify targets for practice modification to expand the potential donor pool.3,4 Numerous recent reports have called for new OPO performance metrics to minimize reliance on the eligible death statistic, better reflect the true potential donor pool and encourage wider donor pursuit and utilization, highlighting particularly dismal procurement rates within “non-ideal” donor populations.3–7
In lung transplantation (LTx), transplant candidates outnumber available donor lungs and up to 30% of candidates die or are removed from the waitlist prior to transplantation.8 A critical limiting factor is donor selection, which has traditionally focused on healthy young donors with no smoking history, normal chest x-ray and bronchoscopy, and minimal organ ischemic time.9 As “ideal” donors remain scarce, use of “non-ideal” or “extended-criteria” donor lungs offers decreased waitlist mortality and non-inferior post-transplant survival.10–13 Nonetheless, lungs from donors with age>50, US Public Health Service (PHS) “increased risk for disease transmission” (IRD) classification, donation after circulatory death (DCD), and smoking history ≥20 pack-years remain disproportionally underutilized.5,6,8
As optimizing use of lungs from non-ideal donors is critical to provide LTx to a greater proportion of listed candidates, we characterized OPO-level variability in pursuit and utilization of non-ideal donor lungs. We sought to construct a comprehensive metric of OPO performance to characterize patterns of non-ideal donor avoidance, or risk aversion, and to determine which non-ideal donor characteristics were most associated with risk aversion in LTx. We hypothesized that risk aversion varies among OPOs and across specific categories of non-ideal donor.
METHODS
Data source
We conducted a retrospective cohort analysis using United Network for Organ Sharing (UNOS) Standard Analysis and Research data. This study was deemed exempt by our Institutional Review Board.
Study population and design
Adult (age≥18) donors who donated at least one organ for transplantation between December 1, 2007 and December 31, 2018 were included. Donors who were missing a documented date of death, had organs recovered outside the US, were missing an OPO identifier, and for whom lung disposition could not be determined were excluded. Non-ideal lung donors were defined as those with any of the following characteristics: age>50, smoking history ≥20 pack-years, PaO2/FiO2 (P/F) ratio ≤350, DCD, or IRD status. Per PHS guidelines, donors with risk factors for recent hepatitis B, hepatitis C, or human immunodeficiency virus (HIV) infection are classified as IRD.14 Non-ideal donors could have multiple non-ideal characteristics, but only one was required to meet criteria. Only 1.1% of P/F ratios were missing from our data; donors who were missing this parameter were considered to have P/F ratio >350.
Donor disposition
Unique donors were the units of analysis. The final disposition for each donor was determined based on which of the left, right, or bilateral lungs progressed farthest in the donation process (Figure 1), since both single and bilateral LTx are considered successful donor utilization.
Figure 1.
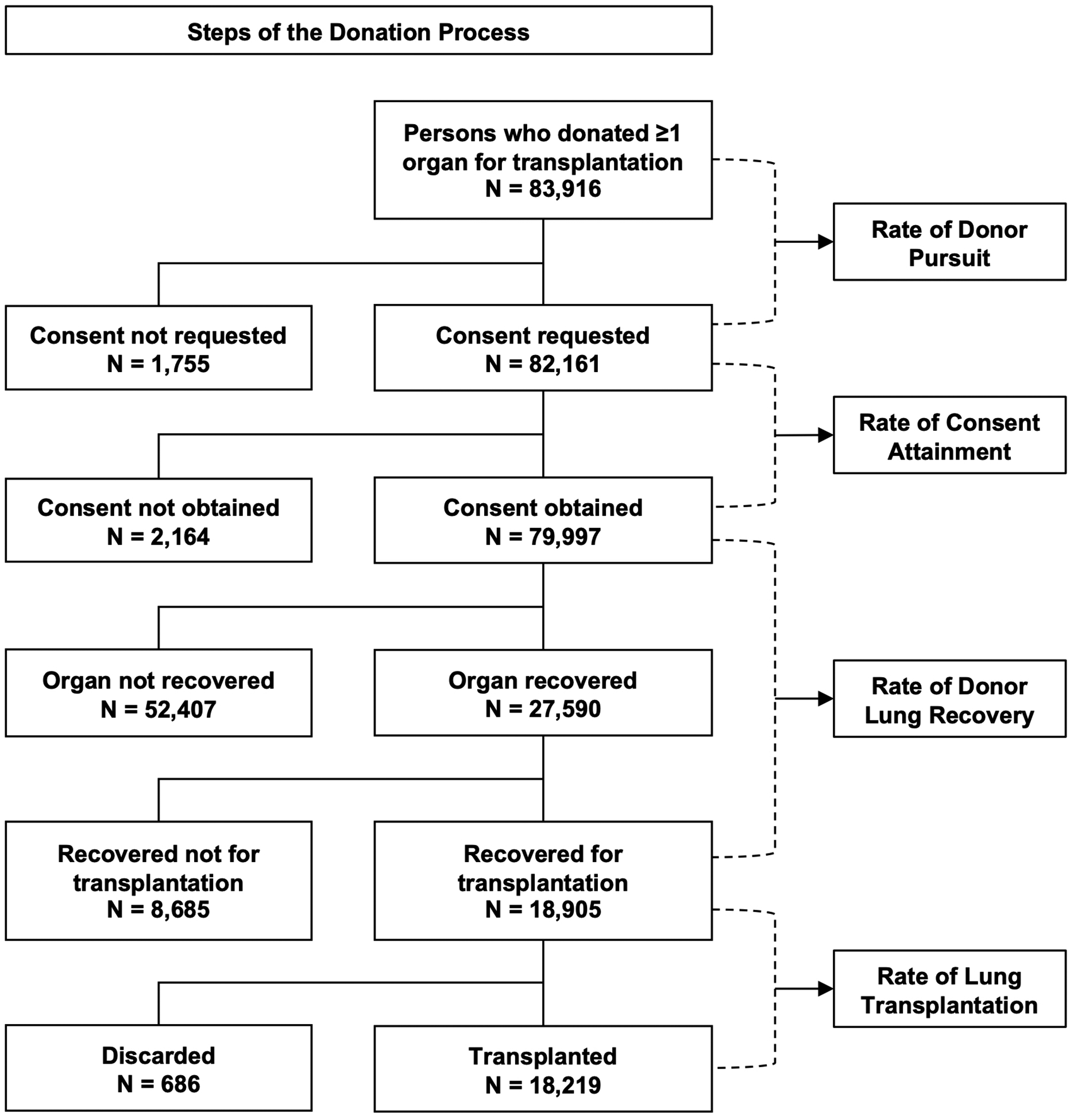
Schematic representation of the donation process and study design.
Donor pursuit
Donor pursuit was defined as an OPO requesting consent for lung donation. The rate of non-ideal donor pursuit was defined as the proportion of non-ideal donors at each OPO from whom consent for lung donation was requested with lower numbers corresponding to increased donor avoidance or increased risk aversion (Table S1). The rate of overall donor pursuit was determined as the proportion of all donors (ideal and non-ideal) at each OPO from whom consent for lung donation was requested. The correlation between non-ideal and overall donor pursuit was estimated using a Spearman correlation coefficient. Multivariable logistic regression was employed to estimate independent associations between non-ideal donor characteristics and donor pursuit.
Assessment of risk aversion throughout the donation process
In addition to non-ideal donor pursuit, OPOs were evaluated based on rates of consent attainment, lung recovery, and lung transplantation (Table S1). Multivariable logistic regression was used to estimate independent associations between non-ideal donor characteristics and the relevant outcome at each juncture.
Categorizing OPOs based on performance throughout the donation process
Overall OPO performance was evaluated based on levels of risk aversion across the donation process. Adjusted odds ratios (ORs) for non-ideal donor pursuit, consent attainment, lung recovery, and transplantation were ranked with each OPO receiving a score from 1–58 with higher scores corresponding to decreased risk aversion (Figure S1). Final OPO performance scores could range from 4–232 based on the sum of scores across the four steps of the donation process (donor pursuit, consent attainment, lung recovery, transplantation).
The distribution of individual OPO performance scores is shown in Figure S2. As natural breakpoints were observed at scores of 70 and 150, OPOs were assigned to three tiers, separating outlying under- and overperforming OPOs for performance assessment:
Tier 1 (most risk-averse): score < 70
Tier 2 (moderately risk-averse): 70 ≤ score ≤ 150
Tier 3 (least risk-averse): 150 < score
OPOs in each tier were characterized based on size (average annual lung donor volume during the study period) and rates of non-ideal donor pursuit, consent attainment, lung recovery, and transplantation. Since CMS’s OPO evaluation does not account for the number of potential donors encountered by each OPO,2,15 we did not adjust for this in our models, but present data about how potential donor volume can affect OPO performance categorization.
Statistical analysis
Donor race, sex, and OPO were included as covariates in models of donor pursuit, consent attainment, lung recovery, and transplantation to adjust for case-mix heterogeneity across OPOs. Independent associations between individual non-ideal donor characteristics and risk aversion at each step of the donation process were determined by adjusting for all other non-ideal characteristics, in addition to the covariates detailed above. Models exploring the association between any non-ideal donor characteristic, a binary indicator for the presence of at least one non-ideal characteristic, and risk aversion were adjusted for donor race, sex, and OPO, but were not adjusted for other non-ideal characteristics. OPO-specific ORs for performance ranking were determined using a fixed effects model with an interaction between OPO and the presence of at least one non-ideal donor characteristic to determine how the effect of non-ideal donor characteristic on donor pursuit, consent attainment, lung recovery, and transplantation varied by OPO. All analyses were performed using SAS version 9.4 (SAS Institute).
RESULTS
Study population
Among 83,916 unique donors, 70,372 (83.9%) were non-ideal. Donor demographics are summarized in Table S2.
Risk aversion in non-ideal donor pursuit
Among 58 OPOs, unadjusted rates of non-ideal lung donor pursuit ranged from 81% to 100% and varied both within and across UNOS regions (Figure 2A). Non-ideal lung donor pursuit was strongly correlated with overall lung donor pursuit (r=0.998, Figure 2B), corresponding to decreased risk aversion for OPOs that pursued more lung donors overall.
Figure 2. Individual organ procurement organization (OPO) lung donor pursuit rates.
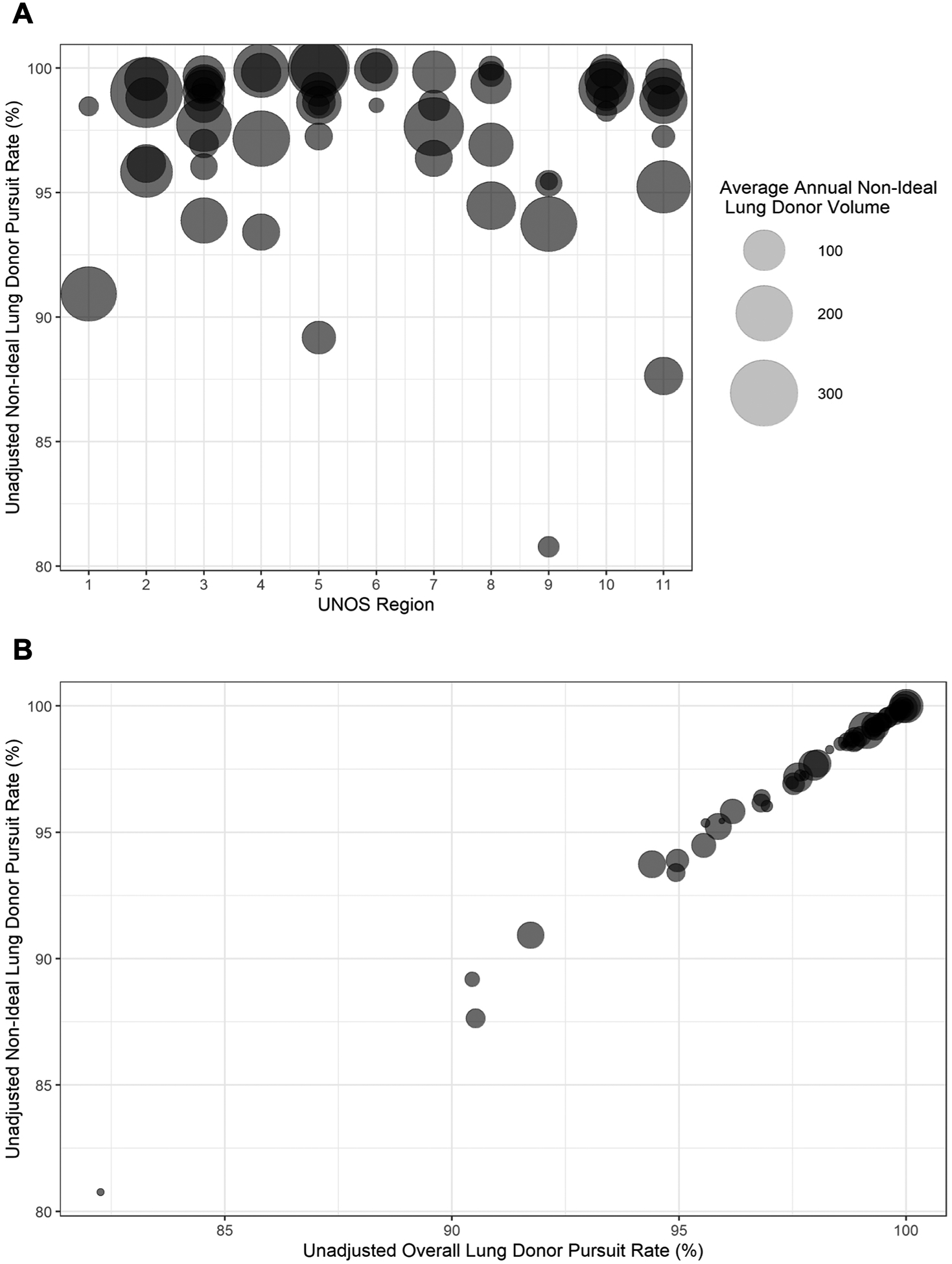
(A) Distribution of unadjusted non-ideal lung donor pursuit rates among OPOs. OPOs were stratified by United Network for Organ Sharing (UNOS) region. Circles represent unique OPOs with representative points scaled according to the average number of non-ideal lung donors at that OPO for each year of the study period. (B) Comparison of OPO-level non-ideal and total lung donor pursuit rates.
Factors associated with risk aversion in non-ideal donor pursuit
Of five non-ideal donor characteristics, DCD and IRD status were associated with the most and least risk aversion, respectively (Figure 3). On adjusted analysis, DCD, age>50, P/F ratio ≤350, and smoking history were independently associated with significant risk aversion, corresponding to significantly decreased odds of donor pursuit. IRD status was associated with increased odds of donor pursuit (Table 1A).
Figure 3.
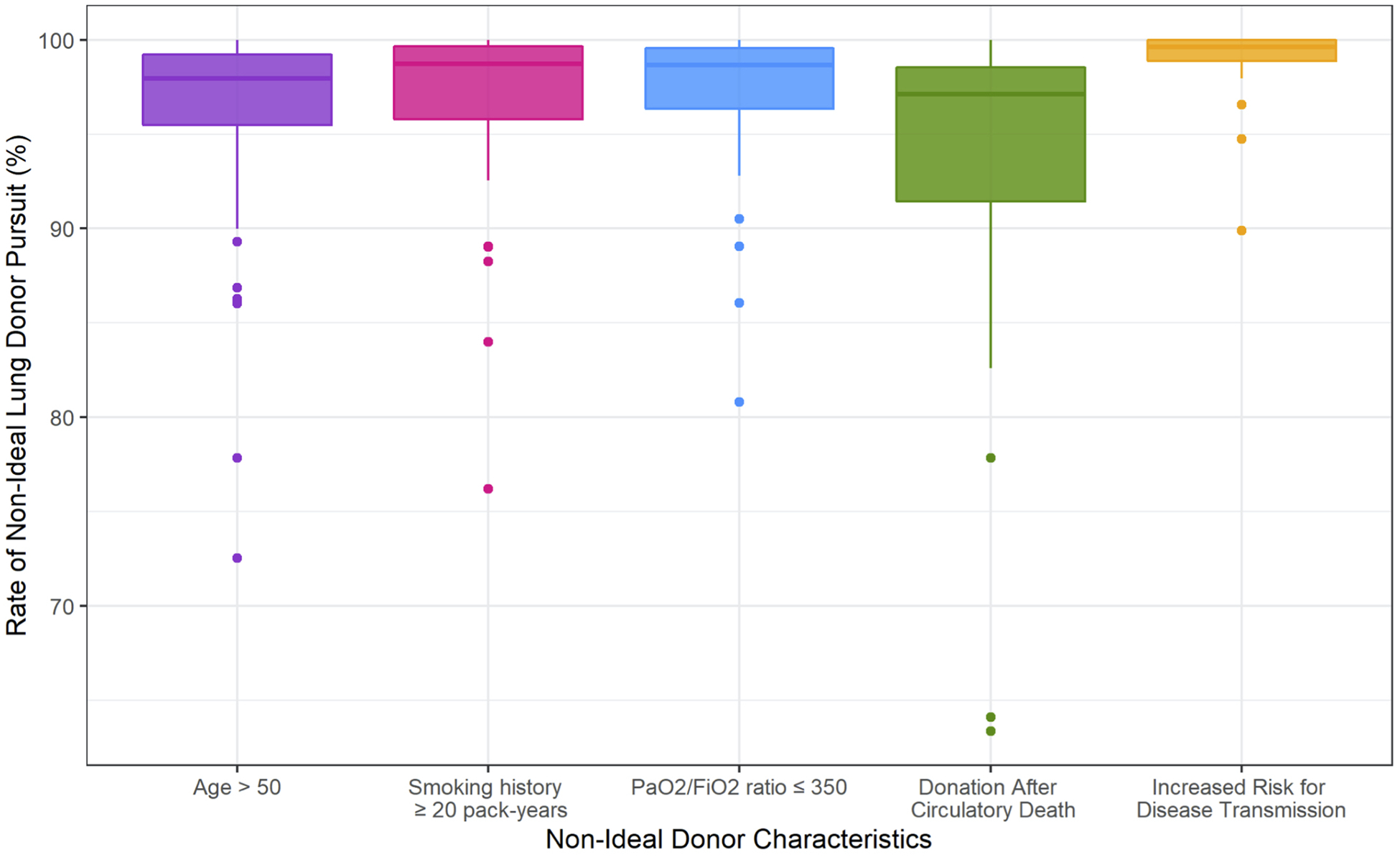
Distribution of unadjusted non-ideal lung donor pursuit rates across organ procurement organizations (OPOs) separated by non-ideal donor characteristic.
Table 1.
Unadjusted rates and adjusted odds ratios for non-ideal lung donor pursuit, consent attainment, lung recovery, and transplantation.
| Non-Ideal Characteristic | Rate (%)a | OR (95% Confidence Interval) | ||
|---|---|---|---|---|
| Adjustedb | Adjustedc | Adjustedd | ||
| A. Donor Pursuit | ||||
| Any non-ideal characteristics | 97.53 | 0.04 (0.03, 0.07) | 0.04 (0.02, 0.07) | 0.04 (0.03, 0.07) |
| Age > 50 years | 96.07 | 0.23 (0.21, 0.26) | 0.24 (0.21, 0.26) | 0.24 (0.21, 0.26) |
| Smoking history ≥ 20 pack-years | 97.08 | 0.64 (0.58, 0.71) | 0.64 (0.58, 0.71) | 0.67 (0.60, 0.74) |
| PaO2/FiO2 ≤ 350 | 97.42 | 0.48 (0.43, 0.54) | 0.48 (0.42, 0.53) | 0.49 (0.44, 0.55) |
| DCD status | 93.80 | 0.24 (0.22, 0.27) | 0.23 (0.20, 0.25) | 0.20 (0.18, 0.23) |
| IRD status | 99.06 | 2.56 (2.15, 3.06) | 2.51 (2.15, 3.06) | 2.49 (2.08, 2.98) |
| B. Consent Attainment | ||||
| Any non-ideal characteristics | 97.05 | 0.35 (0.29, 0.41) | 0.30 (0.26, 0.36) | 0.32 (0.27, 0.38) |
| Age > 50 years | 96.13 | 0.49 (0.45, 0.54) | 0.49 (0.45, 0.53) | 0.51 (0.47, 0.56) |
| Smoking history ≥ 20 pack-years | 96.83 | 0.77 (0.70, 0.84) | 0.67 (0.61, 0.74) | 0.69 (0.62, 0.76) |
| PaO2/FiO2 ≤ 350 | 96.83 | 0.52 (0.47, 0.58) | 0.47 (0.43, 0.52) | 0.48 (0.43, 0.53) |
| DCD status | 94.95 | 0.45 (0.40, 0.50) | 0.38 (0.34, 0.42) | 0.36 (0.32, 0.40) |
| IRD status | 98.57 | 2.07 (1.80, 2.39) | 1.99 (1.73, 2.30) | 2.00 (1.73, 2.30) |
| C. Lung Recovery for Transplantation | ||||
| Any non-ideal characteristics | 15.00 | 0.09 (0.09, 0.09) | 0.09 (0.09, 0.09) | 0.09 (0.08, 0.09) |
| Age > 50 years | 12.59 | 0.34 (0.33, 0.35) | 0.34 (0.32, 0.35) | 0.34 (0.32, 0.35) |
| Smoking history ≥ 20 pack-years | 8.86 | 0.24 (0.23, 0.26) | 0.25 (0.24, 0.26) | 0.25 (0.23, 0.26) |
| PaO2/FiO2 ≤ 350 | 7.40 | 0.07 (0.07, 0.08) | 0.07 (0.07, 0.08) | 0.07 (0.07, 0.07) |
| DCD status | 5.62 | 0.17 (0.16, 0.19) | 0.18 (0.16, 0.19) | 0.17 (0.15, 0.19) |
| IRD status | 21.90 | 0.89 (0.85, 0.92) | 0.90 (0.86, 0.94) | 0.90 (0.87, 0.94) |
| D. Transplantation | ||||
| Any non-ideal characteristics | 94.81 | 0.35 (0.29, 0.42) | 0.35 (0.29, 0.42) | 0.38 (0.32, 0.46) |
| Age > 50 years | 96.11 | 0.91 (0.76, 1.11) | 0.88 (0.73, 1.07) | 0.92 (0.76, 1.12) |
| Smoking history ≥ 20 pack-years | 95.72 | 0.83 (0.65, 1.06) | 0.85 (0.67, 1.09) | 0.84 (0.65, 1.08) |
| PaO2/FiO2 ≤ 350 | 92.52 | 0.34 (0.29, 0.40) | 0.34 (0.29, 0.40) | 0.36 (0.30, 0.43) |
| DCD status | 75.56 | 0.10 (0.08, 0.12) | 0.10 (0.08, 0.13) | 0.08 (0.06, 0.10) |
| IRD status | 94.87 | 0.63 (0.53, 0.76) | 0.66 (0.55, 0.79) | 0.74 (0.61, 0.89) |
OR, odds ratio; DCD, donation after circulatory death; IRD, increased risk for disease transmission.
Mean unadjusted rate among 58 OPOs.
ORs for individual non-ideal donor characteristics mutually adjusted for all other non-ideal donor characteristics.
ORs for individual non-ideal donor characteristics mutually adjusted for all other non-ideal donor characteristics. All ORs adjusted for donor race and donor sex.
ORs for individual non-ideal donor characteristics mutually adjusted for all other non-ideal donor characteristics. All ORs adjusted for donor race, donor sex, and OPO.
Risk aversion across the donation process
DCD and IRD status remained associated with the most and least risk aversion, respectively with regards to consent attainment. On adjusted analysis, DCD, P/F ratio ≤350, age>50, and smoking history were independently associated with significant risk aversion, corresponding to significantly decreased odds of consent attainment. IRD status was associated with increased odds of consent attainment (Table 1B).
The average rate of lung recovery among donors from whom consent was requested and obtained ranged from 5.6% (DCD) to 21.9% (IRD). As transplant centers become more intimately involved in the donation process at organ recovery, lung recovery rates may reflect a combination of OPO and center-level risk aversion. On adjusted analysis, all five non-ideal donor characteristics were independently associated with decreased odds of lung recovery, with the most significant effect observed for P/F ratio ≤350 (Table 1C).
On average, rates of lung transplantation among donors from whom consent was requested, obtained, and lung(s) were recovered ranged from 75.6% (DCD) to 96.1% (age>50). DCD, P/F ratio ≤350, and IRD status were independently associated with significantly decreased odds of transplantation. Age>50 and smoking history were not associated with risk aversion in transplantation (Table 1D).
Assessment of OPO performance
Overall, 4 OPOs were assigned to tier 1, 44 to tier 2, and 10 to tier 3 based on overall risk aversion throughout the donation process. Compared to all others, tier 3 OPOs (least risk-averse) had the highest rates of non-ideal donor pursuit, consent attainment, lung recovery, and transplantation; tier 3 OPOs were also larger than those in tiers 1 and 2 corresponding to a higher average annual lung donor volume. Tier 1 OPOs (most risk-averse) had the lowest rates of non-ideal donor pursuit, consent attainment, and lung recovery. However, rates of non-ideal donor lung transplantation were higher among tier 1 OPOs compared to those in tier 2 (moderately risk-averse) (Table 2).
Table 2.
Characteristics of OPOs assigned to tiers 1, 2, and 3.
| Characteristic | Tier 1 (Most Risk-Averse) | Tier 2 (Moderately Risk-Averse) | Tier 3 (Least Risk-Averse) |
|---|---|---|---|
| Average annual lung donor volumea | 100.13 (69.28) | 117.95 (75.39) | 141.81 (92.75) |
| Pursuit Rateb | 91.89% (7.48%) | 97.76% (2.78%) | 97.93% (2.43%) |
| Consent Attainment Rateb | 96.75% (1.04%) | 97.35% (2.08%) | 97.63% (2.14%) |
| Recovery for Transplantation Rateb | 13.47% (5.27%) | 14.05% (3.67%) | 14.68% (4.28%) |
| Transplant Rateb | 96.83% (1.24%) | 95.17% (6.28%) | 97.05% (1.90%) |
Overall annual lung donor volume including both ideal and non-ideal lung donors. Represented as the mean (standard deviation) among all OPOs in each group.
Restricted to non-ideal lung donors. Represented as the mean (standard deviation) unadjusted rate among all OPOs in each group.
DISCUSSION
In this review of the UNOS registry, we analyzed patterns of risk aversion in LTx among 58 US OPOs. We found that risk aversion varies among OPOs and across stages of the donation process, and is dependent upon specific non-ideal donor characteristics including donor age, smoking history, DCD, and P/F ratio. Based on a three-tier classification system to categorize OPO performance, our study suggests that measures to standardize practices across OPOs and encourage pursuit of all potential non-ideal lung donors may represent meaningful avenues by which to optimize OPO performance and increase utilization of non-ideal lung donors to expand the potential donor pool for LTx.
In both non-ideal donor pursuit and consent attainment, DCD status and age>50 were associated with significant risk aversion among OPOs, findings consistent with several prior studies of predictors of non-utilization of non-ideal donor lungs and failure of consent authorization among all solid organs.16–20 Although OPOs receive guidance on management and approach to consent for DCD donors from organizations including the International Society for Heart and Lung Transplantation and Association of Organ Procurement Organizations,21 our findings suggest that these guidelines are not sufficient to standardize pursuit of these donors and may not provide OPOs with adequate guidance regarding family education about DCD donation, one aspect that has been identified as a particular barrier in this setting.22 Based on our findings, ubiquitous education regarding acceptable lung donor standards including donor smoking history, P/F ratio, age allowance, and DCD status, and incorporation of explicit practice guidelines into OPTN deceased donor policies may standardize patterns of non-ideal donor pursuit across OPOs and may help enhance rates of consent attainment across OPOs and non-ideal lung donor categories.
Center-level risk aversion in offer acceptance and transplantation may further influence OPO-level risk aversion throughout the donation process, even with regard to donor pursuit.5 In LTx, center-level acceptance rates for offers made to the highest priority candidates vary among transplant centers and across UNOS regions, with the geographic distribution of risk aversion resembling patterns of OPO-level risk aversion in donor pursuit in our study.23 Furthermore, offers made to the highest priority candidates that are declined tend to be slightly older, prior smokers, and classified as DCD and IRD.23 Our findings of significant risk aversion in donor pursuit for donors with age>50, smoking history ≥20 pack-years, and DCD status may be understood in the context of these data, suggesting that patterns of risk aversion among OPOs are influenced by center-level risk aversion even in the early stages of the donation process. However, in our study, the degree of risk aversion toward all non-ideal donor groups increased significantly from consent attainment to lung recovery, suggesting that center-level risk aversion, which plays a more prominent role later in the donation process may be a greater determinant of which organs are ultimately recovered and transplanted.
In light of the association between OPO and center-level risk aversion in LTx, quantifying OPO performance based on measures of organ recovery and transplantation may offer OPOs little information about how they can optimize their performance as transplant center practices in these areas remain out of OPOs’ control. In our three-tier system for categorization of OPO performance, the primary determinants of classification as a tier 1 OPO (most risk-averse) were underperformance in donor pursuit and consent attainment, components of the donation process that are more reflective of OPO-specific performance and less intertwined with transplant center practices. Importantly, tier 1 OPOs demonstrated higher rates of non-ideal donor lung transplantation compared to tier 2 OPOs. In CMS’s current system, which evaluates OPO performance based on organ recovery and transplantation, tier 1 OPOs may be reassured that their performance is adequate despite modifiable shortcomings in early stages of the donation process. While newly proposed OPO performance metrics under consideration by CMS may improve objectivity of OPO performance evaluation, continued evaluation based on donation and transplantation rate metrics that focus on donors and organs that were used for transplantation may perpetuate this system in which areas for practice modification within an OPO’s jurisdiction remain undiscussed.15
Our system in which OPOs are assigned to tiers based on performance throughout the donation process may represent a particularly attractive alternative to current OPO performance metrics. In addition to providing a detailed breakdown of performance in each component of the donation process, this system closely resembles the current five-tier system of transplant program evaluation employed by the Scientific Registry of Transplant Recipients.24 As members of the transplant community are familiar with this metric, use of an equivalent system for OPO performance evaluation may not only provide OPOs with actionable information to optimize performance, but may help members of the transplant community at large better understand how their OPO partners are performing and help hold these indispensable organizations accountable for working to maximize the potential donor pool. While our specific classification scheme provides a reflection of OPO risk aversion in LTx, analogous tier classifications may be developed to evaluate OPO performance in LTx more broadly as well as across solid organs and donor subtypes, providing increasingly granular and interpretable information to OPOs and transplant centers across areas of transplantation.
There are several limitations in our study. Retrospective reviews using large national databases have the inherent limitation of unmeasured confounders that cannot be accounted for within the analysis. Such confounders include population and mortality patterns across donation service areas (DSA), specific reasons why consent was not requested or obtained, differences in OPOs’ definitions and reporting of donor pursuit, OPO-specific donor management protocols, the number of donor hospitals per DSA, and transplant center volume and offer acceptance practices. Additionally, we did not examine recipient outcomes, which may reflect donor management by OPOs and provide a more nuanced indication of “successful” transplantation beyond use of donor lungs. Using the UNOS database also meant that we were limited to the subset of potential donors from whom at least one organ was recovered for transplantation and lacked data regarding the total number of donor referrals and eligible deaths received by each OPO during the study period. However, the eligible death statistic is generally unreliable in quantifying the potential donor pool due to subjective reporting by OPOs and poor correlation with patterns of mortality across the US.3,4,6,7,15 Our methodology thus allowed us to obtain objective estimates of risk aversion throughout the donation process and likely underestimates the magnitude of OPO-level risk aversion in LTx due to use of a smaller denominator than would be afforded if the number of eligible deaths or donor referrals was known. Finally, in our OPO performance evaluation, we were unable to account for differences in protocols and definitions of “acceptable” donor and therefore cannot comment on how well OPOs performed within these boundaries.
CONCLUSIONS
In this national analysis of OPO-level risk aversion in LTx, we found that levels of risk aversion differ among OPOs and across stages of the donation process. While education and incorporation of explicit practice guidelines into deceased donor policies may standardize the approach to all non-ideal lung donor subtypes across OPOs, OPO performance evaluations should additionally consider performance in earlier stages of the donation process that best reflect OPO-specific practices. Use of a tiered system for OPO performance evaluation offers an objective and comprehensive system to elucidate specific, actionable areas in which OPOs can reasonably be expected to improve their performance to increase utilization of non-ideal lung donors and expand the potential donor pool for LTx.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (NIH) under award numbers 5T32CA093245 (VR) and 5T32HL069749 (OKJ), the National Center for Advancing Translational Sciences (NCATS) of the NIH under award numbers TL1TR002555 (SEH, AYC) and UL1TR002553 (SBP, AM), and the NIH Roadmap for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NCATS.
ABBREVIATIONS:
- CMS
Centers for Medicare and Medicaid Services
- DCD
donation after circulatory death
- DSA
donation service area
- HIV
human immunodeficiency virus
- IRD
increased risk for disease transmission
- LTx
lung transplantation
- OPO
organ procurement organization
- OPTN
Organ Procurement and Transplantation Network
- OR
odds ratio
- P/F
PaO2/FiO2
- PHS
Public Health Service
- UNOS
United Network for Organ Sharing
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Data Availability Statement
The data that support the findings of this study are available from the United Network for Organ Sharing.
SUPPORTING INFORMATION STATEMENT
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES:
- 1.OPTN Policy 2: Deceased Donor Organ Procurement. Published 2020. Accessed May 26, 2020. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf
- 2.Electronic Code of Federal Regulations. Title 42: Public Health, Part 486—Conditions for Coverage of Specialized Services Furnished by Suppliers, Subpart G—Requirements for Certification and Designation and Conditions for Coverage: Organ Procurement Organ. Published 2018. Accessed May 26, 2020. https://www.govinfo.gov/content/pkg/CFR-2018-title42-vol5/xml/CFR-2018-title42-vol5-part486.xml#seqnum486.301
- 3.Cannon RM, Jones CM, Davis EG, Franklin GA, Gupta M, Shah MB. Patterns of geographic variability in mortality and eligible deaths between organ procurement organizations. Am J Transplant. 2019;19(10):2756–2763. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg D, Kallan MJ, Fu L, et al. Changing Metrics of Organ Procurement Organization Performance in Order to Increase Organ Donation Rates in the United States. Am J Transplant. 2017;17:3183–3192. [DOI] [PubMed] [Google Scholar]
- 5.Klassen DK, Edwards LB, Stewart DE, Glazier AK, Orlowski JP, Berg CL. The OPTN Deceased Donor Potential Study: Implications for Policy and Practice. Am J Transplant. 2016;16:1707–1714. [DOI] [PubMed] [Google Scholar]
- 6.Luskin R, Nathan H. Eligible Death Statistic: Not a True Measure of OPO Performance nor the Potential to Increase Transplantation. Am J Transplant. 2015;15:2019–2020. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg D, Karp S, Shah MB, Dubay D, Lynch R. Importance of incorporating standardized, verifiable, objective metrics of organ procurement organization performance into discussions about organ allocation. Am J Transplant. 2019;19(11):2973–2978. [DOI] [PubMed] [Google Scholar]
- 8.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2018 Annual Data Report: Lung. Am J Transplant. 2020;20(s1):427–508. [DOI] [PubMed] [Google Scholar]
- 9.Chaney J, Suzuki Y, Cantu E III, van Berkel V. Lung donor selection criteria. J Thorac Dis. 2014;6(11):1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyes KG, Mason DP, Thuita L, et al. Guidelines for Donor Lung Selection: Time for Revision? Ann Thorac Surg. 2010;89(6):1756–1765. [DOI] [PubMed] [Google Scholar]
- 11.Zych B, García Sáez D, Sabashnikov A, et al. Lung transplantation from donors outside standard acceptability criteria – are they really marginal? Transpl Int. 2014;27(11):1183–1191. [DOI] [PubMed] [Google Scholar]
- 12.Cox ML, Mulvihill MS, Choi AY, et al. Implications of declining donor offers with increased risk of disease transmission on waiting list survival in lung transplantation. J Hear Lung Transplant. 2019;38(3):295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Suylen V, Luijk B, Hoek RAS, et al. A Multicenter Study on Long-Term Outcomes After Lung Transplantation Comparing Donation After Circulatory Death and Donation After Brain Death. Am J Transplant. 2017;17(10):2679–2686. [DOI] [PubMed] [Google Scholar]
- 14.Seem DL, Lee I, Umscheid CA, Kuehnert MJ. PHS guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission through organ transplantation. Public Health Rep. 2013;128(4):247–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Medicare & Medicaid Services, United States Department of Health and Human Services. Medicare and Medicaid Programs; Organ Procurement Organizations Conditions for Coverage: Revisions to the Outcome Measure Requirements for Organ Procurement Organization. Fed Regist. 2019;84(246):70628–70710. [Google Scholar]
- 16.Brown CVR, Foulkrod KH, Dworaczyk S, et al. Barriers to obtaining family consent for potential organ donors. J Trauma - Inj Infect Crit Care. 2010;68(2):447–451. [DOI] [PubMed] [Google Scholar]
- 17.Van Leiden HA, Jansen NE, Haase-Kromwijk BJJM, Hoitsma AJ. Higher refusal rates for organ donation among older potential donors in the netherlands: Impact of the donor register and relatives. Transplantation. 2010;90(6):677–682. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg DS, Halpern SD, Reese PP. Deceased organ donation consent rates among racial and ethnic minorities and older potential donors. Crit Care Med. 2013;41(2):496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi AY, Jawitz OK, Raman V, et al. Predictors of nonuse of donation after circulatory death lung allografts. J Thorac Cardiovasc Surg. Published online 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi AY, Jawitz OK, Raman V, et al. Predictors of Older Donor Lung Utilization: Are We Too Good at Saying No? Ann Thorac Surg. Published online 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gries CJ, White DB, Truog RD, et al. An Official American Thoracic Society/International Society for Heart and Lung Transplantation/Society of Critical Care Medicine/Association of Organ and Procurement Organizations/United Network of Organ Sharing Statement: Ethical and Policy Consideration. Am J Respir Crit Care Med. 2013;188(1):103–109. [DOI] [PubMed] [Google Scholar]
- 22.Squires JE, Graham N, Coughlin M, et al. Barriers and enablers to organ donation after circulatory determination of death: A qualitative study exploring the beliefs of frontline intensive care unit professionals and organ donor coordinators. Transplant Direct. 2018;4(7):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulvihill MS, Lee HJ, Weber J, et al. Variability in donor organ offer acceptance and lung transplantation survival. J Hear Lung Transplant. 2020;39(4):353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wey A, Salkowski N, Kasiske BL, Israni AK, Snyder JJ. A Five-Tier System for Improving the Categorization of Transplant Program Performance. Health Serv Res. 2018;53(3):1979–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


