Abstract
COVID-19 (Coronavirus disease 2019) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has reached a pandemic level, spreading across the globe by affecting over 33 million people and causing over 1,009,270 deaths. SARS-CoV-2 is highly infectious with a high basic reproduction number (R0) of 2.2–5.7 that has led to its exponential spread and very little is known about it in terms of immunogenicity and its molecular targets. SARS-CoV-2 causes acute respiratory distress syndrome, followed by multiple organ failure and death in a small percentage of individuals. Cardiac injury has emerged as another dreaded outcome of COVID-19 complications. However, a thorough understanding of the pathogenesis of SARS-CoV-2 is lacking. In this review, we discuss the virus, possible mechanisms of COVID-19-induced cardiac injury, potential therapeutic strategies, and explore if exosomes could be targeted to treat symptoms of COVID-19. Furthermore, we discuss the virus-induced sepsis, which may be the cause of multiple organ failure, including myocardial injury.
Keywords: COVID-19, SARS-CoV-2, Sepsis, Exosomes, Cardiovascular disease
Introduction
Coronavirus disease 2019 (COVID-19), caused by the SARS-CoV-2 virus, was first reported in December 2019, with the first likely case recorded in Wuhan, China (Lescure et al., 2020). Since this time, the virus has spread to more than 200 countries, with over 33 million confirmed cases and 1,009,270 deaths as of October 1, 2020 (https://covid19.who.int/). COVID-19 is thought to spread mainly through respiratory droplets and close contact, and displays a relatively high R0 value estimated between 2.2–5.7, and this high infection potential combined with a delay in visible symptoms for up to 2 weeks has enabled this virus to spread rapidly into pandemic proportions (Cui et al., 2019; Sanche et al., 2020).
Due to these extreme circumstances, great effort has been made into developing diagnostic tools and treatment options for the virus. At the time of this writing, RT-qPCR-based assays are the diagnostic standard for coronavirus testing; however, immunoassays and other technologies are rapidly being developed and deployed (Cheng et al., 2020). Data from testing suggest that age and the presence of comorbidities, which include cardiovascular disease, obesity, cancer, and diabetes are major risk factors in COVID-19 fatality (Onder et al., 2020). These risk factors pose major challenges to COVID-19 treatment, as increased isolation and more stringent testing and therapeutics are necessary in the case of comorbidity (Wu et al., 2020).
The virus itself poses major clinical challenges. It has created a large patient load that threatens to overwhelm healthcare systems and treatment demands, the use of critical supplies, such as ventilators, large scale PPE usage, and basic medical supplies (Ranney et al., 2020). The concurrent disruption of global supply chains furthers this critical need. Along with the equipment challenges, the immediate patient burden stretches all normal supplies thin and limits healthcare availability for the general population and prevents non-critical surgeries from being performed due to the risk of contamination (Cohen et al., 2020). These challenges make it critical for effective clinical treatments to be developed, to lessen the burden on stressed healthcare systems (Hatswell, 2020). In this review, we describe in brief about the virus properties, COVID-19 pathogenesis specifically focusing on sepsis and heart, and available treatment options.
SARS-CoV-2 infectivity characteristics
SARS-CoV-2 is an enveloped, single-stranded, positive-sense RNA virus (Figure 1) that belongs to the beta-coronavirus family of viruses and is capable of infecting humans and animals (Shereen et al., 2020). Several members of this virus family have been known to infect humans with mild symptoms and are self-limiting (Andersen et al., 2020). Interestingly, SARS-CoV-2 is closely related to SARS-CoV (severe acute respiratory syndrome virus-82% homology) and MERS-CoV (Middle Eastern respiratory syndrome virus-50% homology) that cause respiratory disease and were responsible for outbreaks in 2003 (China) and 2012 (Middle East), respectively. While the evolution of these viruses has become a very hot topic, these viruses undergo gene recombination, insertions, and deletions, making them easy to mutate, manipulate, and transmit from one species to another (Luo et al., 2018). Consistent with the above report, several studies using in vitro cell culture and mouse models have shown the potential for the emergence of COVID-like viruses (Menachery et al., 2015).
Figure 1. SARS-CoV-2 Virus.
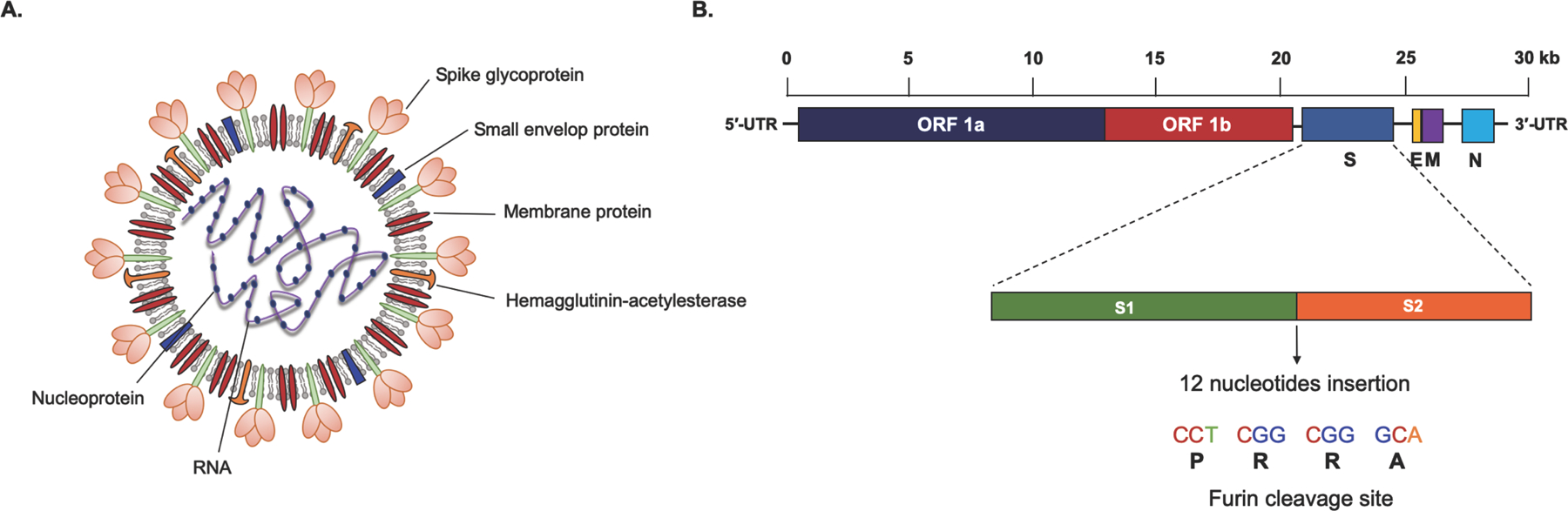
Structure and genomic organization of SARS-CoV-2. (A) A SARS-CoV-2 particle comprises of spike glycoprotein (S), small envelop protein (E), and membrane glycoprotein (M) embedded in a lipid bilayer that encloses a single-stranded RNA genome and nucleocapsid protein (N). (B) SARS-CoV-2 genome is a positive-sense single-stranded RNA of approximately 30 kb size. The viral genome consists of 5′-untranslated region (5′-UTR) at the N-terminal, ORF 1a and ORF 1b encoding for non-structural proteins, structural proteins include spike (S), envelop (E), membrane (M), and nucleocapsid (N), as well as 3′-UTR at the C-terminal. Expanded view of spike protein shows S1 and S2 subunits with a 12-nucleotides insertion at S1-S2 junction, which is targeted by host furins/ transmembrane proteases.
Virus gains entry inside the cell through Angiotensin-converting enzyme 2 (ACE2) receptors (Lan et al., 2020; Wan et al., 2020). Once inside the cells, its RNA is released, transcribed to virus proteins (mainly nonstructural proteins). In the late phase, virus structural proteins are transcribed that are used for virus repackaging and release (Figure 2) (Shereen et al., 2020). There are several important characteristics of this virus that make it unique. (1) It is an enveloped virus with a lipid bilayer, therefore, making it very stable in the environment (72 hours on plastic and 3 hours in aerosols) (van Doremalen et al., 2020) and also easy to inactivate using routine sanitizers; (2) The virus is highly infectious. This increase in infectivity is due to unique sequences in the spike protein that enhances its affinity to its receptor by several-fold (discussed below); (3) Virus is present in the saliva of infected individuals and therefore present in the droplets while talking and singing aloud, coughing, and sneezing; (4) It is less pathogenic, thus 25–50% of people are asymptomatic; (5) This virus can infect animals (mainly cats) as well. Therefore, it poses a major challenge for preventing virus spread.
Figure 2. SARS-CoV-2 Replication.
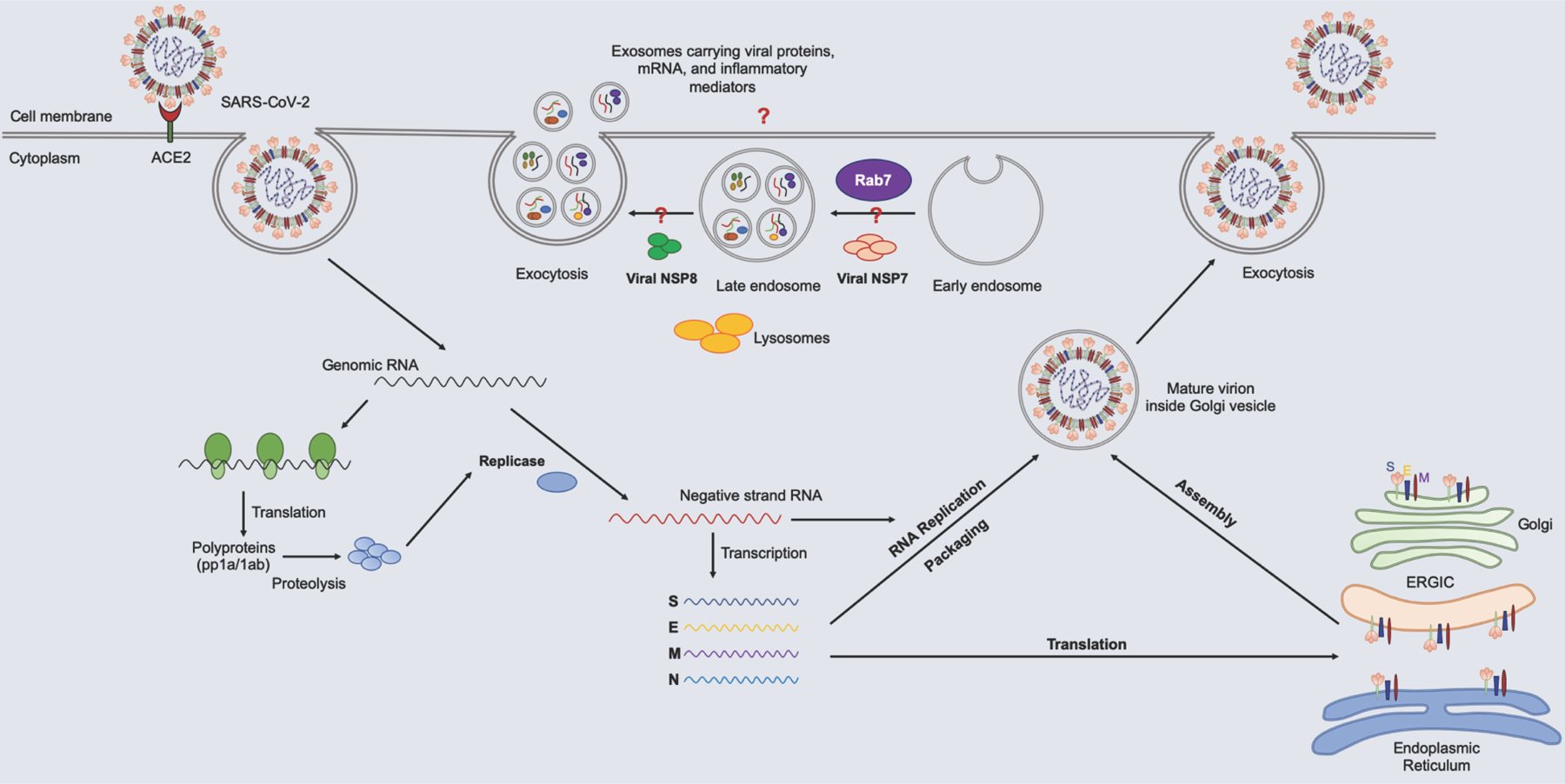
Lifecycle of SARS-CoV-2. SARS-CoV-2 enters cells via the interaction of spike protein with ACE2 present on the surface of cells. Once inside the cells, it releases its genomic RNA into the cytoplasm. The viral genome uses the host machinery to translate into polyproteins that are further proteolyzed into smaller proteins by viral proteinases. Discontinuous transcription of the positive-strand RNA results in the synthesis of subgenomic negative-strand RNA, which is translated into viral structural proteins and serves as a template for the replication of genomic RNA. Genomic RNA and nucleocapsid protein together form a nucleoprotein complex in the cytoplasm and assembled with other structural proteins, such as spike (S), envelop (E), and, membrane (M) proteins into the ER-Golgi intermediated compartment (ERGIC). New virus particles are released through exocytosis. Link between viral non-structural proteins and the Rab pathway may lead to exosome-mediated dissemination of the viral modulators, inflammatory mediators which needs to be explored.
As mentioned above, virus spike protein is very key to its infectivity. Virus genomic sequence analysis has identified several insertions and mutations in the spike gene, therefore, it may have diverged from other related viruses, such as SARS-CoV, MERS-CoV, RaTG13, and Pangolin coronavirus (Andersen et al., 2020). Interestingly, structure-function analysis by crystallography and binding studies has identified that SARS-CoV-2 spike protein has a very high affinity for ACE2 receptor (10–20 fold higher than SARS-CoV) (Wrapp et al., 2020). Also, insertion of 4 amino acids (RRAR) in the spike gene at S1-S2 junction is targeted by host furins and transmembrane protease serine 2 (Figure 1), and other proteases that promote virus fusion, and therefore increase the infectivity of the virus (Coutard et al., 2020; Hoffmann et al., 2020). Interestingly, the presence of these motifs has been associated with increased pathogenicity in several viruses, including H5N1, MERS, and others (Coutard et al., 2020). It is also intriguing that even with high sequence homology, similar structure, and affinity for the ACE2 receptor between SARS-CoV and SARS-CoV-2 spike protein, none of the antibodies available for the SARS spike protein can neutralize SARS-CoV-2 virus (NIH). This could be largely due to the insertion and several other mutations in the SARS-CoV-2.
Pathogenesis of COVID-19: The sepsis link
COVID-19 is a highly contagious respiratory syndrome and can cause multi-organ failure that can lead to death in a small percentage of infections. It is transmitted from person to person by direct contact, through droplet infection, fecal-oral transmission, and aerosol. The virus can replicate in a wide range of cells that express ACE2, including nasal epithelium, nasopharynx, upper respiratory tract, type II pneumocytes in the lung, gastrointestinal (GI) tract, immune cells, and endothelium (Kumar et al., 2020; Sungnak et al., 2020). Because of the wide range of target cells, pathological symptoms and lesions are spread across different organs (Table 1). The severity of the disease also depends on risk factors and pre-existing health conditions. Advanced age is a major risk factor followed by hypertension, diabetes, obesity, chronic respiratory conditions, including chronic obstructive pulmonary disease (COPD) and asthma, heart diseases, and immune status (Fang et al., 2020; CDC). In the United States, African Americans are affected disproportionately compared to other races. However, underlying mechanisms are unknown. A recent study identified extensive pulmonary thrombosis, microcoagulopathy in small vessels, hemorrhage, diffuse alveolar damage accompanied by intracardial necrosis and right ventricle dilation among African Americans during autopsies (Fox et al., 2020). These findings were consistent with other studies, therefore pre-existing cardiac risk factors have been suggested to be the possible causes (McGonagle et al., 2020). However, African Americans have higher incidence of several health conditions such as hypertension, obesity and diabetes, which are known risk factors for heart diseases and therefore might be prone to severity of the disease. Interestingly, A proarrhythmic variant p.Ser1103Tyr-SCN5A is highly prevalent among African Americans, which is associated with ventricular arrhythmia sudden cardiac death under hypoxic conditions may also be responsible for increased fatalities (Giudicessi et al., 2020). Also, human leukocyte antigen (HLA) gene and ACE2 gene polymorphisms (Hussain et al., 2020) have been suggested to affect the severity of the disease.
Table 1:
Organs affected, and clinical pathology associated with SARS-CoV-2
| Organ/System | Symptoms | Pathological changes/Lesions | References |
|---|---|---|---|
| Respiratory tract | Dry cough, sneezing, dyspnea, Shortness of breath |
Interstitial pneumonia with infiltration of immune cells, hypoxemia, metabolic acidosis | (Li et al., 2020; Zhou et al., 2020) |
| Gastrointestinal tract | Diarrhea | Dehydration | (Song et al., 2020) |
| Immune system | Fewer, viral sepsis | Lymph node atrophy, lymphopenia, cytokine storm | (Tan et al., 2020) |
| Heart | Rapid heart rate, fatigue, cardiac arrest | Cardiac dilatation, heart failure, Myocardial infarcts | (Libby, 2020) |
| Blood vessels | Rashes on foot | Microcirculation dysfunction, Inflamed blood vessels |
(Varga et al., 2020) |
| Kidney | Blood in urine | Acute kidney injury, focal hemorrhages | (Batlle et al., 2020) |
| Liver | Increased ALT and AST, liver enlargement, infiltration of immune cells | (Zhang et al., 2020; Zhou et al., 2020) | |
| Nervous system | Loss of taste and smell, Dizziness, headache | Edema and scattered degeneration | (Xydakis et al., 2020) |
| Blood clotting system | Increased blood clotting | Coagulopathy, deep vein thrombosis, stroke, D-Dimer | (Varga et al., 2020; Wang et al., 2020) |
ACE2 signaling has also attracted a lot of attention given the fact that the ACE2/Ang1–7/Mas axis is crucial in regulating blood pressure, inflammation, fibrosis, and thrombosis, etc. (Santos et al., 2003; Simões e Silva et al., 2013). As a key mode of internalization, downregulation or shedding of ACE2 after the virus entry has been reported in SARS-CoV (Glowacka et al., 2010; Kuba et al., 2005), NL63 (Dijkman et al., 2012), and H5N1 (Zou et al., 2014), and a similar outcome is speculated in the SARS-CoV-2 infection. Downregulation of ACE2 in the infected organs could interfere with the ACE2/Ang1–7/Mas axis, resulting in activated AngII and Renin-angiotensin-aldosterone system (RAAS), which is supposedly one of the plausible causes of COVID-19-associated alveolar inflammation and lung injury (Kai & Kai, 2020; Verdecchia et al., 2020).
After entry through ACE2 receptors, the virus sheds its genome into the cytoplasm, which is transcribed to early viral proteins that play a critical role in the suppression of host immune response, tissue damage, and enhance viral genome replication. Structural proteins are transcribed in the late phase of viral replication to repackage and release virus particles (Figure 2). Interactome analysis of all the viral proteins has revealed that viral proteins target host cell nuclear export, integrated stress response system, RNA processing, mitochondrial functions, and cell death signaling (Gordon et al., 2020). During the virus replication, host cells activate anti-viral immune response through MHC class I antigen presentation. This is followed by either an effective immune response to clear the virus infection or immune dysregulation that leads to a severe form of the disease. An effective anti-viral response involves activation of both (i) cell-mediated antiviral immunity through activation of CD8+ T cells, natural killer (NK) cells, and monocytes that target virus-infected cells and (ii) humoral immunity mediated by production of virus-neutralizing antibodies, such as IgG and IgM by CD27hiCD38hi cells, activated ICOS + PD-1+ follicular helper T cells- TFH cells (CD4+ and CXCR5+ cells) (Figure 3) (Thevarajan et al., 2020).
Figure 3. SARS-CoV-2 Pathogenesis.
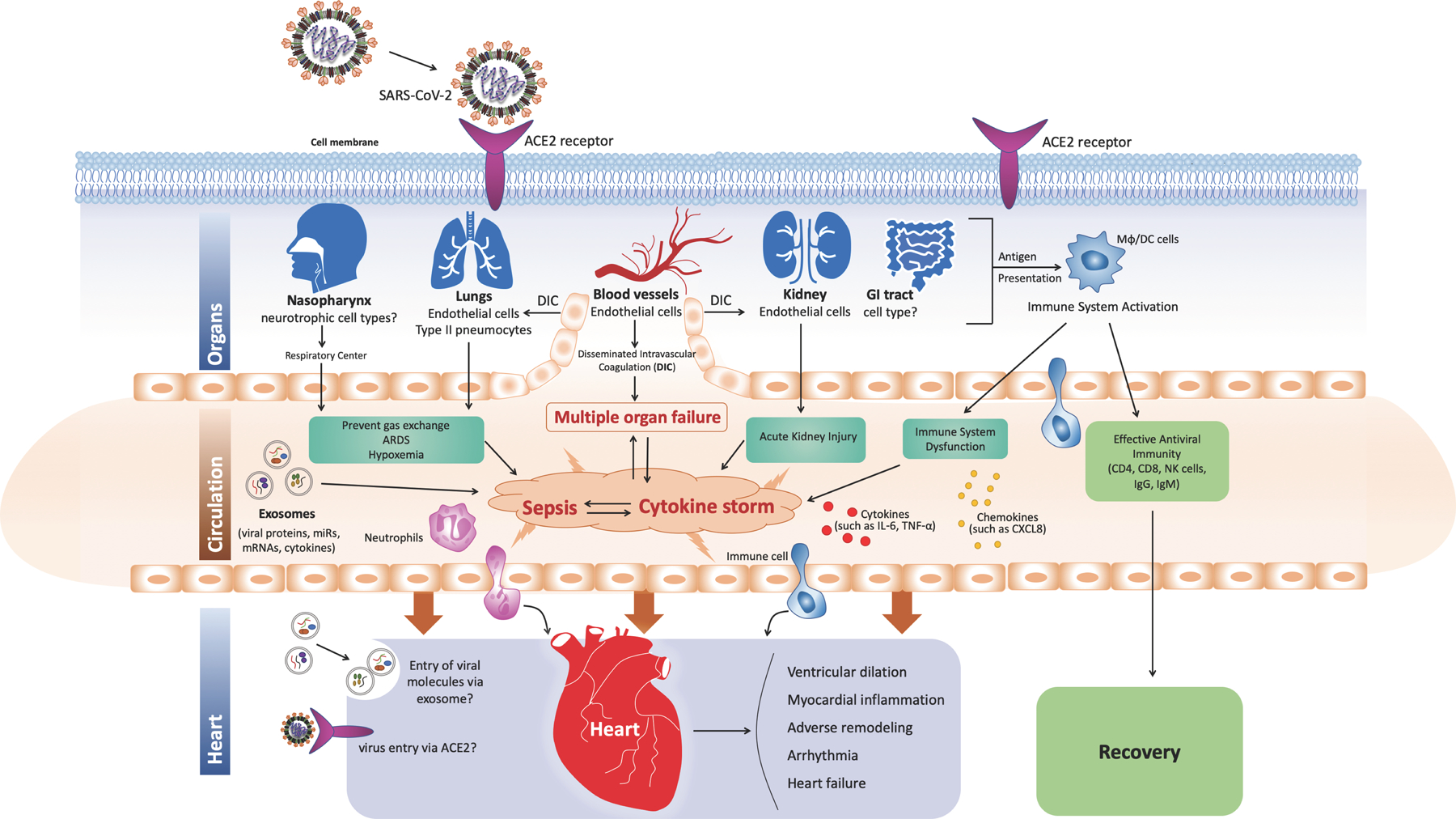
Mechanism of SARS-CoV-2-induced cardiovascular disease, role of sepsis, and exosomes. SARS-CoV-2 is capable of infecting multiple organs due to the widespread expression of ACE2 receptors. During viral replication inside cells, the immune system is activated through MHC class I antigen presentation, which is followed by effective antiviral immunity leading to recovery. However, in certain individuals, immune dysregulation results in cytokine storm and dissemination of virus in the body that might lead to sepsis. The virus can also infect endothelial cells that line the blood vessels leading to endothelialitis and disseminated intravascular coagulation, which limits gas exchange in the lungs and causes metabolic acidosis. Sepsis caused by cytokine storm, virus, exosomes, hypoxemia, and disseminated intravascular coagulation may lead to multiple-organ failure and death.
In individuals who do not recover, come down with acute respiratory syndrome, hypotension, and multiple organ failure (Xu et al., 2020). Laboratory findings showed high levels of fibrin degradation product D-dimer (indicative of abnormal clotting) (Zhou et al., 2020), lymphopenia (decrease in the number of lymphocytes) (Chan et al., 2020), increased neutrophil count (Liu et al., 2020), and cytokine storm (Mehta et al., 2020) that is suggestive of sepsis. Interestingly, the culture of lung fluids did not yield bacterial growth (Fox et al., 2020; Li et al., 2020). Therefore, sepsis is likely caused by the virus itself (Li et al., 2020) that might leads to (i) immune dysregulation leading to cytokine storm, (ii) respiratory dysfunction leading to hypoxemia, and (iii) metabolic acidosis due to circulatory dysfunction (Figure 3). Cytokine storm is characterized by increased production of cytokines, mainly IL-6, C-reactive protein (CRP), TNF-α, IL-1β, IL-33, IFNγ, GMCSF, and others (Mehta et al., 2020). In addition, virus-infected cells (type II pneumocytes, endothelial cells, etc.) could be the source of cytokines and toxins. Virus particles have also been demonstrated in endothelial cells from blood vessels (Varga et al.,2020) that may be responsible for microvascular dysfunction. Therefore, it is hypothesized that virus-induced endothelial dysfunction may be promoting disseminated intravascular coagulation that limits blood flow and prevent oxygenation in the lungs (Figure 3). Hypoxia, due to an acute respiratory syndrome, along with metabolic acidosis due to poor circulation and microvascular dysfunction, may partly explain the cause of multiple organ dysfunction (such as heart, kidney, and liver) (Figure 3). However, the cause and sources of cytokine storm, lymphopenia, and abnormal clotting are not known; although activated immune cells, lymphocyte exhaustion has been suggested (Zhou et al., 2020). However, it should be noted that cytokine storm is also observed in SARS and MERS infections. While in SARS it is attributed to exaggerated cytokine production by virus-infected alveolar endothelial cells, dendritic cells, and macrophages; in MERS it is attributed to lung infiltrating neutrophils, macrophages, and peripheral blood mononuclear cells (Channappanavar & Perlman, 2017). Interestingly, transcriptomic analysis of bronchial alveolar fluid, peripheral blood mononuclear cells from COVID-19 human patients and ferret models and invitro cell lines revealed poor antiviral responses lacking IFNI and III responses (Blanco-Melo et al., 2020; Gardinassi et al., 2020), which may partly explain asymptomatic and prolonged infection. These studies also revealed interferon-specific gene signatures, activation of neutrophils, and poor response from dendritic cells and macrophages. Furthermore, recent studies by different groups showed presence of reactive T cells to SARS-CoV-2 peptide antigens in people who have not been infected with virus, and has been attributed to exposure to corona viruses that cause common cold (Grifoni et al., 2020; Moreno et al., 2020; Premkumar et al., 2020). However, their role in pathogenesis and development of immunity remains to be seen (Sette & Crotty, 2020). In addition, future research identifying the root cause of the cytokine storm will help treat COVID-19 complications. Likewise, the cause of the severity of the disease in the presence of other comorbidities is unknown. However, it is well known from the literature that inflammation is upregulated in most of these cardiovascular and metabolic diseases characterized by an increase in C-reactive protein, TNF-α, and IL-6 levels. Therefore, we speculate that the immune system is primed for overactivation under COVID-19 infection in these individuals.
Exosome link to COVID-19 Pathogenesis
Exosomes are nanoscale extracellular double-membrane vesicles secreted by cells that have emerged as novel intercellular communicators. Exosomes are actively secreted by endolysosomal system and carry messages in the form of proteins, enzymes, cytokines, lipids, and RNA from donor cells to the target cells. Extensive research has shown that exosomes play critical role in organ cross-talk, maintaining tissue homeostasis, host-pathogen interactions, and pathophysiology of various diseases including sepsis (Dykes, 2017; Kita et al., 2019; Sahoo & Douglas, 2014; Schorey et al., 2015). Likewise, virus infections exploit exosome pathway to gain entry, spread virus infection, virus packaging, evade host immune system, and pathogenesis (shown in Figure 4; and virus pathogenesis using exosomes is summarized in Table 2) (Alenquer & Amorim, 2015; Anderson et al., 2016; Urbanelli et al., 2019; Wurdinger et al., 2012). Due to similarities in pathways of exosome biogenesis (ESCRT-dependent and independent), their fate (actively taken up by target cells by endocytosis, pinocytosis, and receptor-mediated uptake) and virus uptake, packaging, and release; they were likened to be relatives (Nolte-’t Hoen et al., 2016). Exosome-mediated host immune modulation by viral infections has been extensively studied and has been reviewed elsewhere in detail (Schorey et al., 2015). Virus infections stimulate host cells to secrete exosomes that function as pathogen-associated molecular patterns (PAMPS), carry inflammatory mediators, cause inflammation (Schorey et al., 2015). For example, exosomes from EBV-infected cells that are enriched in dUTPase induce activation of NF-κB pathway and stimulate macrophage cytokine release (Ariza et al., 2013). Likewise, HCV mRNA in exosomes induce secretion of IFN alpha from macrophages and exosomes from C3/36 cells infected with Zika virus induces expression TNF alpha from monocytes, cause endothelial damage to induce intravascular coagulation and inflammation (Martínez-Rojas et al., 2020). Exosomes from Kaposi sarcoma associated herpes virus also cause endothelial damage and induce expression of IL6 (Chugh et al., 2013). Exosomes from virus-infected cells also cause apoptosis of immune cells. For example, HIV infection induces secretion of exosomes that are enriched in viral Nef protein, which cause apoptosis of endothelial cells and CD4 T-helper cells (Lenassi et al., 2010). Likewise, EBV-infected cells secrete exosomes enriched with galactin9 that cause apoptosis of cytotoxic T cells specific to EBV-infected cells (Dukers et al., 2000). In summary exosomes from virus-infected cells can cause tissue injury by activating inflammation and cytotoxicity.
Figure 4. Hypothesized Role of Exosomes in SARS-CoV-2 Pathogenesis.
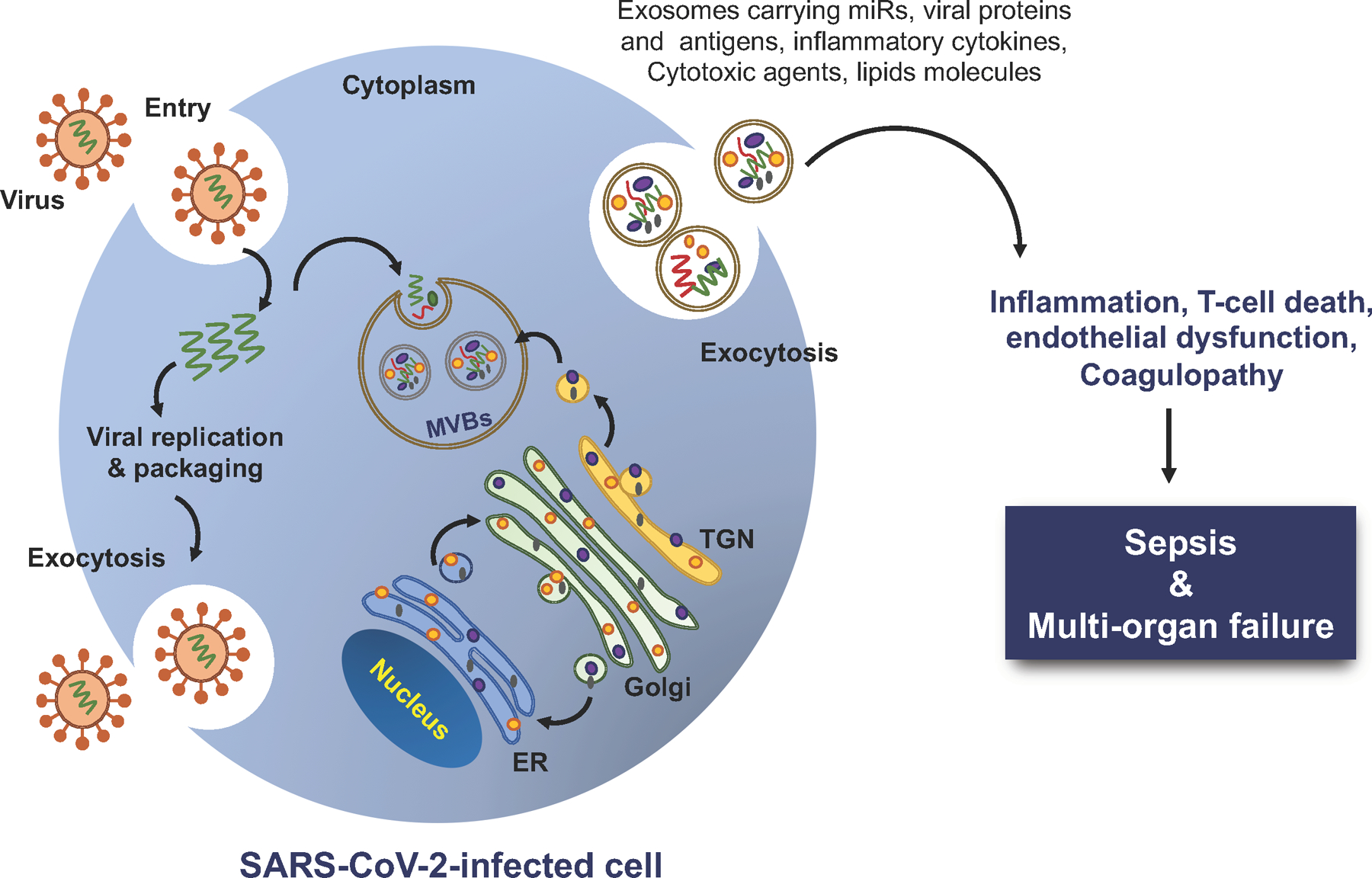
Exosomes derived from virus-infected cells promote sepsis and tissue injury. Exosomes from virus-infected cells are packaged with bioactive molecules, including miRs, viral proteins, inflammatory cytokines, cytotoxic agents, and lipids that incite inflammation, activate endothelium, and affect intravascular coagulation leading to sepsis-like condition.
Table 2:
Exosomes in virus infection pathogenesis
| Virus | Exosome component | Functions | References |
|---|---|---|---|
| HIV | Nef | Susceptibility to infection, Apoptosis of CD4 cells | (Arenaccio et al., 2015; Lenassi et al., 2010) |
| HIV | CD81 | Virus budding and spread, cholesterol metabolism | (Arenaccio et al., 2015; Grigorov et al., 2009) |
| HIV | C19MC miRNA | Resistance to virus infection | (Delorme-Axford et al., 2013) |
| HIV | HIF1α-lncRNA BACE-1AS long non-coding RNA | Neuropathogenesis | (Sil et al., 2020) |
| Zika virus | Viral RNA and protein | Virus spread to neighboring cells | (Zhou et al., 2019) |
| Zika virus | unknown | Endothelialitis and blood clots | (Martínez-Rojas et al., 2020) |
| EV-A71 (hand-foot-and-mouth disease) | Viral protein and nucleic acid | Virus spread | (Huang et al., 2020) |
| Rabies Virus | unknown | Virus spread | (Wang et al., 2019) |
| EBV | LMP1 | Inhibit cytotoxic T cells Transformation of cells |
(Dukers et al., 2000) |
| EBV | miRNA | Virus latency | (Cai et al., 2006) |
| KSHV | miRNA and others | IL6 production, cellular metabolism | (Chugh et al., 2013; Meckes et al., 2013) |
| HSV1 | HLA-DR | Immune Evasion | (Temme et al., 2010) |
| HCV | Viral genome | Virus spread to neighboring cells | (Ramakrishnaiah et al., 2013b) |
| HTLV-1 | Tax protein | IL6, TNFα production and immune cell recognition | (Jaworski et al., 2014) |
| Avian Influenza (H5N1) | miR-483–3P | Increased production of proinflammatory cytokines in vascular endothelial cells | (Maemura et al., 2020) |
Several important features of SARS-CoV-2 infection, mainly hyper-activated immune system to induce sepsis-like disease characterized by cytokine storm and lymphopenia raises the question if exosomes are involved (Figure 4). This idea is further strengthened by TGN pathway (Trans-Golgi network, which is part of sorting system in endolysosomal pathway) involvement in replication of SARS-CoV-2. In addition, recent data showing involvement of lipid metabolism including cholesterol metabolism (Zhang et al., 2020) in the pathogenesis COVID complications poses the question if exosomes are involved in pathogenesis of SARS-CoV-2 infection. Consistent with this idea, SARS-CoV-2 protein interactome analysis revealed interaction with Rab proteins that are part of ESCRT pathway involved in exosome biogenesis. Interestingly, several viruses that exploit exosomes for pathogenesis interact with Rab proteins (Bello-Morales et al., 2012; Fraile-Ramos et al., 2010, p.; Gerber et al., 2015). Moreover, high throughput lipidomics of sera from human patients revealed exosome-specific lipid profiles that were enriched with sphingomyelins, gangliosides, and deficient in Di-acyl glycerols (DAG). Interestingly, exosome enrichment with gangliosides (GM3) was strongly associated with severity of the disease and likely cause of lymphopenia, since immune cells have preference for GM3-enriched exosomes which is cytotoxic (Song et al., 2020). It should also be noted that SARS-CoV-2 barely eight-month-old and its understanding is evolving; and given the lack of strong anti-viral immune response as discussed before, role of epigenetics mechanisms including miRs and other non-coding RNAs needs full investigation. Moreover, extensive literature suggests that exosomes play an important role in shuttling of these non-coding RNAs between different cell types and have been implicated in development of cardiovascular diseases. Interestingly, in a recent in-vitro study, transduction of lung epithelial A549 cells with SARS-CoV-2 structural and non-structural genes (excluding viral Spike protein) resulted in secretion of exosomes enriched with viral RNAs. These exosomes were successfully taken up by the human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs), which resulted in elevated inflammatory markers in hiPSC-CMs along with presence of viral genes (Kwon et al., 2020), allowing us to speculate the possible role of exosomes in the SARS-CoV-2 pathogenesis. This may also explain the possible mechanism of myocardial inflammation in COVID-19 patients without direct viral infection that has puzzled the researchers. Consistent with this, given the extensive activation and inhibition of protein kinases by SARS-CoV-2 infection in cells (Bouhaddou et al., 2020), it is also possible that exosomes from virus-infected cells may also carry proteins that can activate inflammatory response and cause tissue injury in distant organs. Therefore, it will be interesting to see if exosomes can be targeted for therapy, and future research using the exosome research tools will be helpful in addressing these possibilities.
COVID-19 and Heart
Cardiac complications associated with COVID-19 infection
Although the lungs and the respiratory tract are the most vulnerable tissues for SARS-CoV-2 infection (Zou et al., 2020), the virus also severely affects the pathophysiology of the heart. Several cardiac complications are associated with SARS-CoV-2 infection, which is summarized in Table 3 and Figure 5. Here we discuss acute and chronic cardiac manifestations of COVID-19.
Table 3:
Cardiovascular complications associated with SARS-CoV-2 infection
| Pathological manifestation | Features | References |
|---|---|---|
| Acute myocardial injury | Elevated Troponin I and NT-proBNP levels | (Wang et al., 2020) |
| Cardiac Arrhythmia | Sinus tachycardia, malignant and atrial arrhythmia, Hypokalemia | (Goyal et al., 2020; Guo et al., 2020; D. Wang et al., 2020) |
| Viral cardiomyopathy | Cytokine storm, fulminant myocarditis | (Hu et al., 2020; Hua et al., 2020) |
| Myocardial infarction | Myocardial ischemia, imbalance between oxygen demand and supply, hypotension, ST-segment elevation | (Bangalore et al., 2020; Inciardi et al., 2020; Zhou et al., 2020) |
| Cardiogenic shock | Cardiorespiratory arrest, ST-segment elevation, dysrhythmias | (Sánchez-Recalde et al., 2020; Tavazzi et al., 2020) |
| Vascular complications | Venous thromboembolic events (VTEs), coagulopathy, elevated D-dimer | (Klok et al., 2020; Lodigiani et al., 2020; Tang et al., 2020; Zhou et al., 2020a) |
Figure 5. COVID-19 and Heart.
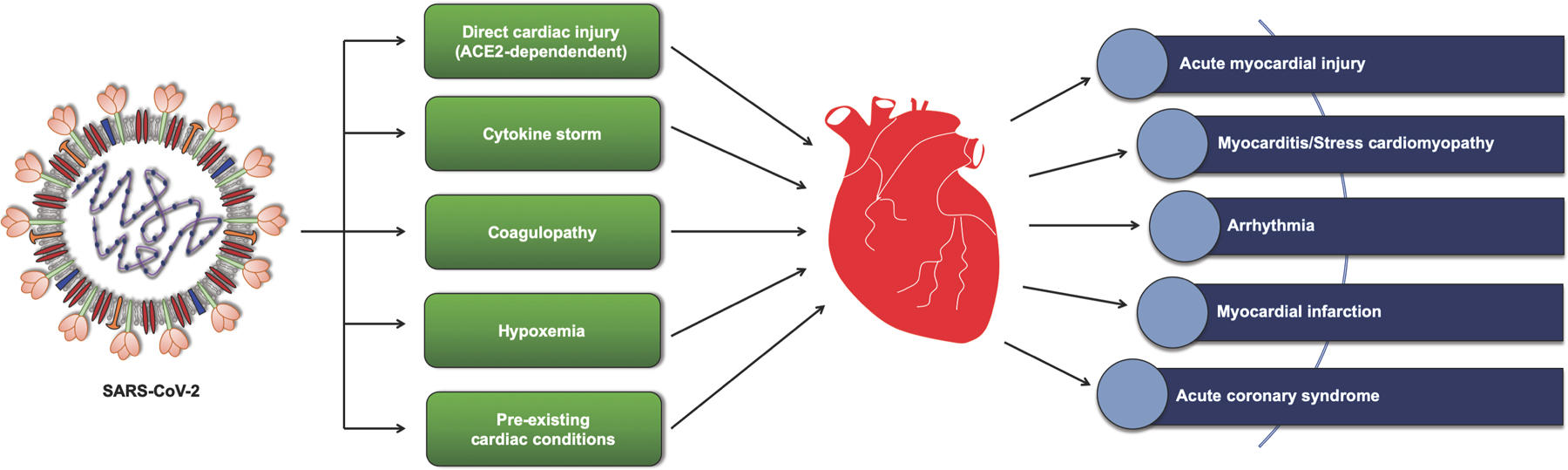
Association between SARS-CoV-2 and heart pathophysiology. SARS-CoV-2 could affect cardiac physiology either directly via its interaction with ACE2 receptors or through other indirect mechanisms, including immune response, vascular coagulation, and oxygen deprivation. SARS-CoV-2 infection has been associated with cardiogenic shock, dysrhythmias, viral myocarditis, and acute myocardial injuries, leading to cardiac damage and fatal outcomes.
Direct myocardial injury: Myocardial localization of SARS-CoV-2
Due to the high abundance of ACE2, the heart is among high-risk organs (Chen et al., 2020; Zou et al., 2020) affected by COVID-19 and speculated to harbor SARS-CoV-2 RNA possibly due to extra-pulmonary dissemination of the virus. Reduced ACE2 expression has been negatively correlated with various cardiac pathologies, such as hypertension, maladaptive cardiac remodeling, heart failure, and cardiomyopathies (Zamaneh et al., 2009; Oudit et al., 2009; Patel et al., 2014; Patel et al., 2016), and as it is postulated that SARS-CoV-2 infection could result in ACE2 downregulation, this might affect the cardiac pathophysiology via differential regulation of ACE2/Ang1–7/Mas axis. The relationship between SARS-CoV-2, ACE2, and cardiovascular outcomes has been reviewed recently and could help to expend the knowledge horizon (Junyi et al., 2020; South et al., 2020)
Direct cardiac injury by SARS-CoV-2 is debatable; however, the presence of ACE2 in the heart poses a strong possibility of internalization of COVID-19 by ACE2-expressing cells in the heart. Out of 44 patients that died from SARS, a study examined the presence of the SARS-CoV genome in the 20 autopsied heart tissues, and 7 of the samples (35%) were found positive for the viral RNA. Moreover, myocardial localization of the viral particles was attributed to the expression of ACE2 in the heart (Oudit et al., 2009). Concerning the current coronavirus pandemic, very few reports have been published to confirm the myocardial infiltration of the SARS-CoV-2. Tavazzi et al. (Tavazzi et al., 2020) reported the first case of myocardial localization of SARS-CoV-2 in a 69-year-old patient who was diagnosed with acute myocardial injury, hypotension, and cardiogenic shock. Endomyocardial biopsy of the patient showed a low-grade interstitial and endocardial inflammation along with virus particles present in the interstitial cells; however, the biopsy did not confirm the presence of coronavirus particles in cardiomyocytes or endothelial cells. Myocardial localization of COVID-19 could imply the viremic phase or migration of infected macrophages to the heart and possibly other tissues. Exosome-mediated dissemination of SARS-CoV-2 and viral genome/protein could also be of scientific interest and requires further exploration. As discussed in the previous section, recent evidence also pointed towards the exosomal transfer of SARS-CoV-2 genes to cardiomyocytes, which resulted in increased inflammation in these cells (Kwon et al., 2020). Many viruses share common endocytic signaling mechanisms and have been shown to exploit the exosomal machinery for their transmission and infection (Alenquer & Amorim, 2015; Izquierdo-Useros et al., 2010; Ramakrishnaiah et al., 2013a). The field of SARS viruses is evolving and exploring the involvement of exosomes could help better understand the pathological mechanisms and develop therapeutics.
In another study, the postmortem pathological examination of the heart biopsies of COVID-19 patients (Tian et al., 2020) revealed focal edema, myocardial hypertrophy, and interstitial fibrosis; however, these features were linked to pre-existing cardiac conditions rather than acute injury due to COVID-19 infection. Even though no apparent infiltration of inflammatory cells was observed in the heart, the real-time PCR analysis showed the SARS-CoV-2 genome in one of the two heart biopsies. Overall, these findings indicate the existence of the SARS-CoV-2 (or its genome) in the heart, either through direct infection or disseminated by migrating cells or through exosomes, which might ultimately exert pathological changes in the myocardium. However, the lack of conclusive evidence necessitates further investigations to understand the direct effects of SARS-CoV-2 in the heart.
Role of inflammation in COVID-19 associated myocardial injury
Although direct myocardial injury via SARS-CoV-2 and ACE2 interaction is a strong possibility, COVID-19-associated cardiac damage is widely attributed to cytokine-inflicted systemic and tissue inflammation. Dissemination of the virus into circulation through infected macrophages and other immune cells could lead to an exaggerated immune response and multi-organ dysfunction. One of the early reports describing myocardial inflammation in SARS-CoV-2 infection reported fulminant myocarditis with elevated IL-6 levels along with other cardiac injury markers (troponin I, myoglobin, and n-terminal brain natriuretic peptide) (Zeng et al., 2020). Various cohort-based studies also showed increased cytokine production during COVID-19 infection, and cytokine storm in those patients was found to be associated with the disease severity and patient survival (Huang et al., 2020; Zhou et al., 2020a). Previously, it was found that immunological response in SARS-patients is mainly mediated through Th1-cell activity (Wong et al., 2004) as opposed to SARS-CoV-2 infection, where an imbalance between both Th1 and Th2 activity was found to aggravate the inflammatory surge (Huang et al., 2020). Overall, evidence from the published studies so far implies that the SARS-CoV-2-induced inflammatory surge is the plausible cause of organ damage in patients and could be targeted for therapeutic interventions.
Acute myocardial injury
In SARS-CoV-2 patients, myocardial injury is evident by several factors, such as an increase in myocardial injury markers, echo- and electrocardiographic abnormalities, cytokine storm, and myocarditis.
Acute myocardial injury has been a critical and persistent feature in COVID-19 patients. An earlier report showed that among 138 patients (Wuhan, China) admitted for SARS-CoV-2 infection, 7.2% of patients had acute cardiac injury (Wang et al., 2020), and cardiac injury was more prominent in the patients who needed ICU care than non-ICU patients. In another case, 82 out of 416 hospitalized COVID-19 patients (19.7%) had cardiac injury (Shi et al., 2020) with elevated high-sensitivity troponin I levels (median interquartile range 0.19 vs <0.006 μg/L in patients without cardiac injury). Cardiac injury patients also had a higher mortality rate than those without cardiac injury (51.2% vs 4.5%). A retrospective cohort study of 191 patients from Wuhan, China showed that 46% of non-survivors had high-sensitivity cardiac troponin I level above the 99th percentile upper reference limit as compared to 1% of survivors (Zhou et al., 2020). Increased levels of high-sensitivity troponin are reported in most of the COVID-19 patients with cardiac injury (Guo et al., 2020; Inciardi et al., 2020; Sala et al., 2020), making it a crucial diagnostic marker of myocardial injury in COVID-19 patients.
In addition to high-sensitivity cardiac troponin, N-terminal pro-brain natriuretic peptide (NT-proBNP) is another important biomarker for myocardial stress in patients infected with SARS-CoV-2. Brain natriuretic peptide (BNP) and NT-proBNP concentration increase in the circulation in response to cardiac impairment and changes in ventricle wall tension, and these molecules are widely used as biomarkers of heart failure (Bay et al., 2003; Hunt et al., 1997; Yasue et al., 1994). In the patients infected with coronavirus, increased concentration of NT-proBNP in circulation manifests myocardial injury and cardiac complications. A rise in NT-proBNP has been reported in severe COVID-19 cases associated with adverse clinical outcomes and poor prognosis (Gao et al., 2020; Guo et al., 2020; Inciardi et al., 2020; Zeng et al., 2020).
Laboratory findings also showed an elevation in other cardiac injury markers, such as creatine kinase, lactate dehydrogenase, and C-reactive protein in COVID-19 patients (Du et al., 2020; Inciardi et al., 2020; Sala et al., 2020).
Chronic cardiac damage in COVID-19 patients
There is a scarcity of data on the long-term implications of respiratory viruses associated with epidemics. Metabolic profiling of 25 SARS-CoV survivors in a 12-years follow-up study showed dyslipidemia, altered glucose metabolism, and cardiovascular abnormalities (Wu et al., 2017). Another cohort-based 10-years follow-up study showed an increased risk of cardiovascular complications in patients hospitalized for pneumonia (Corrales-Medina et al., 2015). Structural similarity between SARS-CoV and SARS-CoV-2 could predict long-term cardiovascular damage. The long-term effect of SARS-CoV-2 on the heart is addressed in two recently published German cohorts-based studies (Lindner et al., 2020; Puntmann et al., 2020). One study showed a high viral load of SARS-CoV-2 in the myocardium (above 1000 copies per μg RNA) of 41.0% of the patients (16 of 39 autopsied samples). However, this high viral load was not attributed to an inflammatory reaction as no inflammatory cell infiltration was observed (Lindner et al., 2020). Similarly, an unselected cohort of 100 recovered patients revealed that 78% of the recovered patients had myocardial abnormalities, including myocardial inflammation, regional scars, and elevated injury markers (Puntmann et al., 2020). These findings necessitate the urgency of large cohort-based follow-up studies on recovered patients to evaluate the long-term effect of SARS-CoV-2 on the cardiovascular system.
Potential therapeutic strategies against SARS-CoV-2 or its complications
For COVID-19 being an infectious disease, vaccination is the best choice to prevent infection. However, this virus is just 10-months old, therefore, vaccine production and their validation, in terms of safety and protection may take longer than expected time. Fortunately, several vaccines are in production, and early testing is in humans and macaques with RNA-1273 (Moderna), ChAdOx1 (Oxford), BNT162b2 (Pfizer), Ad26.COV2-S (Johnson and Johnson), and many others have shown promising results and are in advanced stages of clinical trials. Also, very little is known about the immunogenic antigen from SARS-CoV-2 that is important for activating protective immunity. Therefore, given the pandemic nature of COVID-19, current strategies involve repurposing of existing drugs to control infection in the body, and symptomatic treatments to mitigate the complications.
Antiviral therapy
SARS-CoV-2 emerged in December 2019; it is barely 10-months old, and there is a scarcity of data about the virus. Therefore, the current strategy has been repurposing of existing drugs on compassionate grounds to identify a drug that could help mitigate the virus infection. However, due to its close similarity with SARS and MERS viruses, several of the drugs that are in the pipeline for these viruses, as well as others like Ebola, have been used in clinical trials (summarized in Table 4, please note we have listed the drugs which are used alone or in combination). Data so far indicates that Remdesivir, a nucleotide analog (adenosine) that is incorporated into viral RNA and inhibit its replication, has promising results in patients that are treated with the drug at a very early stage of infection (2–3 days of infection). Interestingly, Remdesivir was originally developed to treat Ebola virus infection. Therefore, the repurposing of existing drugs is a way forward to find quick and timely treatment options. Also, the SARS-CoV-2 protein interactome analysis has identified several targets for which there are drugs available in the developmental stage, which could provide novel avenues to treat virus infection (Gordon et al., 2020). Likewise, high throughput quantitative mass spectrometry-based phospho-proteomics analysis of SARS-CoV-2-infected Vero E6 cells identified strong activation of p38 MAP kinases, casein kinase II (CK2), Ca++ and calmodulin-dependent kinases, PRKG1/2, and inhibition of cell cycle and cell growth kinases (Bouhaddou et al., 2020). Interestingly, inhibition of p38 MAP kinases, cyclin-dependent kinase (CDK), AXL, and PIKFYVE kinases inhibited virus replication in Vero and A549 cell lines (Bouhaddou et al., 2020), providing novel targets for antiviral drug development. In addition, monoclonal antibodies neutralizing virus are also being developed and being tested. Antiviral immunotherapy using INF-β as an aerosol in combination with lopinavir-ritonavir and ribavirin has also shown promising results in small trial. The triple therapy was effective in clearing the virus within 8 days from most of the patients (Hung et al., 2020). This may partly be explained by poor antiviral response by host and therefore INF-β might be very effective in activating antivirus response.
Table 4:
Clinical trials underway to treat SARS-CoV-2 infection (source: clinicaltrials.org and Clinical Trials Arena)
| Drugs | Target/mechanism |
|---|---|
| Remdesivir | Inhibits viral RNA synthesis |
| Danoprevir + Ritonavir | Protease inhibitor, Antiretroviral |
| Lopinavir + Ritonavir | Protease inhibitor |
| Hydroxychloroquine | Inhibits lysosomal activity (Discontinued) |
| Telmisartan | Angiotensin receptor blocker |
| Rintatolimod | Recombinant Interferon Alfa-2B |
| Meplazumab | Mab against CD147 membrane glycoprotein |
| Favipiravir | RNA dependent RNA polymerase |
| Galidesivir | Inhibits viral RNA synthesis |
| Nitazoxanide | Interfere with pyruvate:ferredoxin oxidoreductase |
| ACE inhibitors/ARB | Angiotensin-converting enzyme inhibitor, Angiotensin receptor blockers |
| Convalescent serum | Virus-neutralizing antibodies |
| Monoclonal antibodies to SARS-CoV-2 | Virus-neutralizing antibodies |
| Baricitinib | Janus Kinase inhibitor |
| Mesenchymal stem cells | Cell therapy |
| Famotidine | H2 blocker |
| Interferon β−1b | Antiviral immune response |
| Peginterferon lambda alfa-1a | Antiviral immune response |
| SARS-CoV-2-specific T-cells | Cytotoxic T-cells |
| NT-17 | Recombinant IL-17 |
| Isotretinoin | Papain-like protease inhibitor |
| MK-4482 | Antiviral |
| TXA127 | Angiotensin 1–7 |
| Clevudine | Pyrimidine analogue for HBV treatment |
| Opaganib | Sphingosine kinase-2 inhibitor |
Palliative/symptomatic treatments
An extensive literature review suggests that the majority of the patients that progress to severe form of the disease have sepsis-like symptoms with coagulopathy and multiple organ dysfunction (Wang et al., 2020; Zhou et al., 2020). Therefore, it is logical to think if palliative therapy used in sepsis could be used in COVID-19 patients. Interestingly, plasminogen inhalation therapy (that targets the clotting system) did show dramatic improvement of respiratory function in a small set of patients (Wang et al., 2020). Interestingly, inhibitors of blood clotting are also used to treat sepsis patients in clinics. Recent study using dexamethasone, a good old synthetic long acting corticosteroid (“Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report,” 2020) and reports of Tocilizumab (IL6 inhibitor) for treating COVID-19 complications, suggest a dysfunctional immune system being the cause of many complications. Likewise, given the fact that exosomes play a critical role in sepsis pathology (Essandoh et al., 2015; Raeven et al., 2018) and SARS-CoV-2 infection (Song et al., 2020), drugs targeting exosome pathways should be investigated in preclinical models. Interestingly, several drugs that target exosomes have been investigated for cancer and other diseases (reviewed in detail by Catalano & O’Driscoll (Catalano & O’Driscoll, 2020), summarized in Table 5), therefore should be investigated in pre-clinical studies to evaluate their efficacy as well as safety. In addition, mesenchymal stem cell-derived exosomes could also be used for therapeutic purposes in COVID-19 infection due to their immunomodulatory, anti-inflammatory, and regenerative properties (reviewed elsewhere in detail [(Akbari & Rezaie, 2020; Pinky et al., 2020)]. We also suggest the investigation of ceramide synthesis inhibitors in pre-clinical studies since exosome synthesis inhibitor targets this pathway (Essandoh et al., 2015). Also, ceramides have been known to activate inflammatory pathways in several metabolic and cardiovascular diseases (Bikman & Summers, 2011; Summers, 2018) that are known to have worse outcomes in COVID-19. Therefore, targeting this pathway might have a synergistic effect in controlling sepsis, inflammation, and virus dissemination through circulation. Interestingly, Opaganib, a sphingosine kinase-2 inhibitor is undergoing clinical trials for treating pneumonia caused by SARS-CoV-2 (NCT04467840).
Table 5:
Exosome inhibitors that are investigated for therapy in different diseases
| Pharmacological inhibitors | Target/mechanism of action | Effects |
|---|---|---|
| Calpeptin | Calpains/ Inhibition of MVs/EVs release | Increased anti-cancer drug susceptibility in cancer cell lines |
| Manumycin A | RAS GTPase/ Inhibition of EVs release | Anti-cancer activity, Increased wound healing |
| Y27632 | ROCK1 and ROCK2/ Inhibition of production and release of MVs | Endothelial cell dysfunction |
| Pantethine | Cholesterol synthesis/ inhibition of MVs formation and shedding | Anti-cancer effects, anti-sclerosis, decreased severity of cerebral malaria |
| Imipramine | Acid sphingomyelinase/ Inhibition of MVs and EVs generation | Inhibits osteoclast differentiation and bone loss, increased efficiency of cancer chemotherapy |
| GW4869 | Membrane neutral sphingomyelinase/ Inhibition of EVs production and release | Inhibited hypertrophic effect of cardiac fibroblasts, reduced drug-resistance in cancer cells, immune regulation |
| U0126 | MEK 1 and MEK 2/ Inhibition of MVs generation | Inhibits coagulant activity of monocytes and macrophages |
| NSC23766 | Rac1 GTPase/ Inhibition of MVs generation and release | Reduced MVs release from platelets in pre-clinical model of sepsis |
| Dimethyl amiloride (DMA) | Na+/Ca2+ channels/ Inhibition of EVs release | Increased efficiency of anti-tumor drugs |
| Sulfisoxazole | RABs and ESCRT pathway/ Inhibition of MVs release | Anti-bacterial and anti-cancer activity |
Acknowledgement:
This work is supported, in part, by the National Institutes of Health (NIH) grants HL138023 (to P.K. and J.Z.), and American Heart Association Transformational Project Award 19TPA34850100 (to P.K.) and T32 Training Grant T32EB023872 (to JH).
Abbreviations
- COVID-19
Coronavirus Disease 2019
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- SARS
Sever Acute Respiratory Syndrome
- ACE2
Angiotensin-converting Enzyme 2
- RNA
Ribonucleic Acid
- CDC
Center for Disease Control
- NIH
National Institute of Health
- MHC
Major Histocompatibility Complex
- HLA
Human Leucocyte Antigen
Footnotes
Competing Interests: None. All authors reported “Nothing to disclose”
References
- Akbari A, & Rezaie J (2020). Potential therapeutic application of mesenchymal stem cell-derived exosomes in SARS-CoV-2 pneumonia. Stem Cell Research & Therapy, 11(1), 356. 10.1186/s13287-020-01866-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenquer M, & Amorim MJ (2015). Exosome Biogenesis, Regulation, and Function in Viral Infection. Viruses, 7(9), 5066–5083. 10.3390/v7092862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen KG, Rambaut A, Lipkin WI, Holmes EC, & Garry RF (2020). The proximal origin of SARS-CoV-2. Nature Medicine, 26(4), 450–452. 10.1038/s41591-020-0820-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MR, Kashanchi F, & Jacobson S (2016). Exosomes in Viral Disease. Neurotherapeutics, 13(3), 535–546. 10.1007/s13311-016-0450-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenaccio C, Anticoli S, Manfredi F, Chiozzini C, Olivetta E, & Federico M (2015). Latent HIV-1 is activated by exosomes from cells infected with either replication-competent or defective HIV-1. Retrovirology, 12, 87–87. 10.1186/s12977-015-0216-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariza ME, Rivailler P, Glaser R, Chen M, & Williams MV (2013). Epstein-Barr virus encoded dUTPase containing exosomes modulate innate and adaptive immune responses in human dendritic cells and peripheral blood mononuclear cells. PloS One, 8(7), e69827–e69827. 10.1371/journal.pone.0069827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, Ibrahim H, Friedman GH, Thompson C, Alviar CL, Chadow HL, Fishman GI, Reynolds HR, Keller N, & Hochman JS (2020). ST-Segment Elevation in Patients with Covid-19—A Case Series. The New England Journal of Medicine. 10.1056/NEJMc2009020 [DOI] [PMC free article] [PubMed]
- Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, & Swaminathan S (2020). Acute Kidney Injury in COVID-19: Emerging Evidence of a Distinct Pathophysiology. Journal of the American Society of Nephrology, ASN.2020040419. 10.1681/ASN.2020040419 [DOI] [PMC free article] [PubMed]
- Bay M, Kirk V, Parner J, Hassager C, Nielsen H, Krogsgaard K, Trawinski J, Boesgaard S, & Aldershvile J (2003). NT-proBNP: A new diagnostic screening tool to differentiate between patients with normal and reduced left ventricular systolic function. Heart (British Cardiac Society), 89(2), 150–154. 10.1136/heart.89.2.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Morales R, Crespillo AJ, Fraile-Ramos A, Tabarés E, Alcina A, & López-Guerrero JA (2012). Role of the small GTPase Rab27a during herpes simplex virus infection of oligodendrocytic cells. BMC Microbiology, 12, 265–265. 10.1186/1471-2180-12-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikman BT, & Summers SA (2011). Ceramides as modulators of cellular and whole-body metabolism. The Journal of Clinical Investigation, 121(11), 4222–4230. 10.1172/JCI57144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, Møller R, Panis M, Sachs D, Albrecht RA, & tenOever BR (2020). SARS-CoV-2 launches a unique transcriptional signature from in vitro, ex vivo, and in vivo systems. BioRxiv, 2020.03.24.004655. 10.1101/2020.03.24.004655 [DOI]
- Bouhaddou M, Memon D, Meyer B, White KM, Rezelj VV, Correa Marrero M, Polacco BJ, Melnyk JE, Ulferts S, Kaake RM, Batra J, Richards AL, Stevenson E, Gordon DE, Rojc A, Obernier K, Fabius JM, Soucheray M, Miorin L, … Krogan NJ (2020). The Global Phosphorylation Landscape of SARS-CoV-2 Infection. Cell, 182(3), 685–712.e19. 10.1016/j.cell.2020.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Schäfer A, Lu S, Bilello JP, Desrosiers RC, Edwards R, Raab-Traub N, & Cullen BR (2006). Epstein-Barr virus microRNAs are evolutionarily conserved and differentially expressed. PLoS Pathogens, 2(3), e23–e23. 10.1371/journal.ppat.0020023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano M, & O’Driscoll L (2020). Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. Journal of Extracellular Vesicles, 9(1), 1703244. 10.1080/20013078.2019.1703244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, Xing F, Liu J, Yip CC-Y, Poon RW-S, Tsoi H-W, Lo SK-F, Chan K-H, Poon VK-M, Chan W-M, Ip JD, Cai J-P, Cheng VC-C, Chen H, … Yuen K-Y (2020). A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. The Lancet, 395(10223), 514–523. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R, & Perlman S (2017). Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Seminars in Immunopathology, 39(5), 529–539. 10.1007/s00281-017-0629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Li X, Chen M, Feng Y, & Xiong C (2020). The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovascular Research, 116(6), 1097–1100. 10.1093/cvr/cvaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MP, Papenburg J, Desjardins M, Kanjilal S, Quach C, Libman M, Dittrich S, & Yansouni CP (2020). Diagnostic Testing for Severe Acute Respiratory Syndrome–Related Coronavirus-2: A Narrative Review. Annals of Internal Medicine. 10.7326/M20-1301 [DOI] [PMC free article] [PubMed]
- Chugh PE, Sin S-H, Ozgur S, Henry DH, Menezes P, Griffith J, Eron JJ, Damania B, & Dittmer DP (2013). Systemically circulating viral and tumor-derived microRNAs in KSHV-associated malignancies. PLoS Pathogens, 9(7), e1003484–e1003484. 10.1371/journal.ppat.1003484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SL, Liu G, Abrao M, Smart N, & Heniford T (2020). Perspectives on Surgery in the Time of COVID-19: Safety First. Journal of Minimally Invasive Gynecology, 27(4), 792–793. 10.1016/j.jmig.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang C-CH, Newman A, Loehr L, Folsom AR, Elkind MS, Lyles MF, Kronmal RA, & Yende S (2015). Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA, 313(3), 264–274. 10.1001/jama.2014.18229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, & Decroly E (2020). The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Research, 176, 104742. 10.1016/j.antiviral.2020.104742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Li F, & Shi Z-L (2019). Origin and evolution of pathogenic coronaviruses. Nature Reviews Microbiology, 17(3), 181–192. 10.1038/s41579-018-0118-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme-Axford E, Donker RB, Mouillet J-F, Chu T, Bayer A, Ouyang Y, Wang T, Stolz DB, Sarkar SN, Morelli AE, Sadovsky Y, & Coyne CB (2013). Human placental trophoblasts confer viral resistance to recipient cells. Proceedings of the National Academy of Sciences, 110(29), 12048. 10.1073/pnas.1304718110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. (2020). New England Journal of Medicine. 10.1056/NEJMoa2021436 [DOI]
- Dijkman R, Jebbink MF, Deijs M, Milewska A, Pyrc K, Buelow E, van der Bijl A, & van der Hoek L (2012). Replication-dependent downregulation of cellular angiotensin-converting enzyme 2 protein expression by human coronavirus NL63. Journal of General Virology, 93(9), 1924–1929. 10.1099/vir.0.043919-0 [DOI] [PubMed] [Google Scholar]
- Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, Wang X, Hu C, Ping R, Hu P, Li T, Cao F, Chang C, Hu Q, Jin Y, & Xu G (2020). Clinical Features of 85 Fatal Cases of COVID-19 from Wuhan: A Retrospective Observational Study. American Journal of Respiratory and Critical Care Medicine. 10.1164/rccm.202003-0543OC [DOI] [PMC free article] [PubMed]
- Dukers DF, Meij P, Vervoort MBHJ, Vos W, Scheper RJ, Meijer CJLM, Bloemena E, & Middeldorp JM (2000). Direct Immunosuppressive Effects of EBV-Encoded Latent Membrane Protein 1. The Journal of Immunology, 165(2), 663. 10.4049/jimmunol.165.2.663 [DOI] [PubMed] [Google Scholar]
- Dykes IM (2017). Exosomes in Cardiovascular Medicine. Cardiology and Therapy, 6(2), 225–237. 10.1007/s40119-017-0091-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essandoh K, Yang L, Wang X, Huang W, Qin D, Hao J, Wang Y, Zingarelli B, Peng T, & Fan G-C (2015). Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochimica et Biophysica Acta, 1852(11), 2362–2371. 10.1016/j.bbadis.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Karakiulakis G, & Roth M (2020). Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? The Lancet Respiratory Medicine, 8(4), e21. 10.1016/S2213-2600(20)30116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, & Vander Heide RS (2020). Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. The Lancet. Respiratory Medicine, 8(7), 681–686. 10.1016/S2213-2600(20)30243-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraile-Ramos A, Cepeda V, Elstak E, & van der Sluijs P (2010). Rab27a is required for human cytomegalovirus assembly. PloS One, 5(12), e15318–e15318. 10.1371/journal.pone.0015318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Jiang D, Wen X-S, Cheng X-C, Sun M, He B, You L-N, Lei P, Tan X-W, Qin S, Cai G-Q, & Zhang D-Y (2020). Prognostic value of NT-proBNP in patients with severe COVID-19. Respiratory Research, 21(1), 83. 10.1186/s12931-020-01352-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardinassi LG, Souza COS, Sales-Campos H, & Fonseca SG (2020). Immune and Metabolic Signatures of COVID-19 Revealed by Transcriptomics Data Reuse. Frontiers in Immunology, 11, 1636. 10.3389/fimmu.2020.01636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber PP, Cabrini M, Jancic C, Paoletti L, Banchio C, von Bilderling C, Sigaut L, Pietrasanta LI, Duette G, Freed EO, Basile G. de S., Moita CF, Moita LF, Amigorena S, Benaroch P, Geffner J, & Ostrowski M (2015). Rab27a controls HIV-1 assembly by regulating plasma membrane levels of phosphatidylinositol 4,5-bisphosphate. The Journal of Cell Biology, 209(3), 435–452. 10.1083/jcb.201409082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudicessi JR, Roden DM, Wilde AAM, & Ackerman MJ (2020). Genetic susceptibility for COVID-19-associated sudden cardiac death in African Americans. Heart Rhythm, S1547–5271(20)30419-7. 10.1016/j.hrthm.2020.04.045 [DOI] [PMC free article] [PubMed]
- Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO, Simmons G, Hofmann H, Kuri T, Weber F, Eichler J, Drosten C, & Pöhlmann S (2010). Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. Journal of Virology, 84(2), 1198–1205. 10.1128/JVI.01248-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, O’Meara MJ, Guo JZ, Swaney DL, Tummino TA, Huettenhain R, Kaake RM, Richards AL, Tutuncuoglu B, Foussard H, Batra J, Haas K, Modak M, Kim M, Haas P, … Krogan NJ (2020). A SARS-CoV-2-Human Protein-Protein Interaction Map Reveals Drug Targets and Potential Drug-Repurposing. BioRxiv, 2020.03.22.002386. 10.1101/2020.03.22.002386 [DOI] [PMC free article] [PubMed]
- Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT, Rajan M, Reshetnyak E, Hupert N, Horn EM, Martinez FJ, … Safford MM (2020). Clinical Characteristics of Covid-19 in New York City. The New England Journal of Medicine. 10.1056/NEJMc2010419 [DOI] [PMC free article] [PubMed]
- Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L, Jadi RS, Marrama D, de Silva AM, Frazier A, Carlin AF, Greenbaum JA, Peters B, Krammer F, Smith DM, Crotty S, & Sette A (2020). Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell, 181(7), 1489–1501.e15. 10.1016/j.cell.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorov B, Attuil-Audenis V, Perugi F, Nedelec M, Watson S, Pique C, Darlix J-L, Conjeaud H, & Muriaux D (2009). A role for CD81 on the late steps of HIV-1 replication in a chronically infected T cell line. Retrovirology, 6, 28–28. 10.1186/1742-4690-6-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junyi Guo, Zheng Huang, Li Lin, & Jiagao Lv. (2020). Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Journal of the American Heart Association, 9(7), e016219. 10.1161/JAHA.120.016219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, & Lu Z (2020). Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiology. 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed]
- Hatswell AJ (2020). Learnings for Health Economics from the Early Stages of the COVID-19 Pandemic. PharmacoEconomics - Open, 1–3. 10.1007/s41669-020-00216-9 [DOI] [PMC free article] [PubMed]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, Drosten C, & Pöhlmann S (2020). SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell, 181(2), 271–280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Ma F, Wei X, & Fang Y (2020). Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. European Heart Journal. 10.1093/eurheartj/ehaa190 [DOI] [PMC free article] [PubMed]
- Hua A, O’Gallagher K, Sado D, & Byrne J (2020). Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. European Heart Journal. 10.1093/eurheartj/ehaa253 [DOI] [PMC free article] [PubMed]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, … Cao B (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England), 395(10223), 497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H-I, Lin J-Y, Chiang H-C, Huang P-N, Lin Q-D, & Shih S-R (2020). Exosomes Facilitate Transmission of Enterovirus A71 From Human Intestinal Epithelial Cells. The Journal of Infectious Diseases, 222(3), 456–469. 10.1093/infdis/jiaa174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung IF-N, Lung K-C, Tso EY-K, Liu R, Chung TW-H, Chu M-Y, Ng Y-Y, Lo J, Chan J, Tam AR, Shum H-P, Chan V, Wu AK-L, Sin K-M, Leung W-S, Law W-L, Lung DC, Sin S, Yeung P, … Yuen K-Y (2020). Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. The Lancet, 395(10238), 1695–1704. 10.1016/S0140-6736(20)31042-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PJ, Richards AM, Nicholls MG, Yandle TG, Doughty RN, & Espiner EA (1997). Immunoreactive amino-terminal pro-brain natriuretic peptide (NT-PROBNP): A new marker of cardiac impairment. Clinical Endocrinology, 47(3), 287–296. 10.1046/j.1365-2265.1997.2361058.x [DOI] [PubMed] [Google Scholar]
- Hussain M, Jabeen N, Raza F, Shabbir S, Baig AA, Amanullah A, & Aziz B (2020). Structural variations in human ACE2 may influence its binding with SARS-CoV-2 spike protein. Journal of Medical Virology, n/a(n/a). 10.1002/jmv.25832 [DOI] [PMC free article] [PubMed]
- Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, & Metra M (2020). Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiology. 10.1001/jamacardio.2020.1096 [DOI] [PMC free article] [PubMed]
- Izquierdo-Useros N, Naranjo-Gómez M, Erkizia I, Puertas MC, Borràs FE, Blanco J, & Martinez-Picado J (2010). HIV and mature dendritic cells: Trojan exosomes riding the Trojan horse? PLoS Pathogens, 6(3), e1000740. 10.1371/journal.ppat.1000740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski E, Narayanan A, Van Duyne R, Shabbeer-Meyering S, Iordanskiy S, Saifuddin M, Das R, Afonso PV, Sampey GC, Chung M, Popratiloff A, Shrestha B, Sehgal M, Jain P, Vertes A, Mahieux R, & Kashanchi F (2014). Human T-lymphotropic virus type 1-infected cells secrete exosomes that contain Tax protein. The Journal of Biological Chemistry, 289(32), 22284–22305. 10.1074/jbc.M114.549659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai H, & Kai M (2020). Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19. Hypertension Research: Official Journal of the Japanese Society of Hypertension, 1–7. 10.1038/s41440-020-0455-8 [DOI] [PMC free article] [PubMed]
- Zamaneh Kassiri, Jiuchang Zhong, Danny Guo, Ratnadeep Basu, Xiuhua Wang, Liu Peter P, Scholey James W, Penninger Josef M, & Oudit Gavin Y (2009). Loss of Angiotensin-Converting Enzyme 2 Accelerates Maladaptive Left Ventricular Remodeling in Response to Myocardial Infarction. Circulation: Heart Failure, 2(5), 446–455. 10.1161/CIRCHEARTFAILURE.108.840124 [DOI] [PubMed] [Google Scholar]
- Kita S, Maeda N, & Shimomura I (2019). Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. Journal of Clinical Investigation, 129(10), 4041–4049. 10.1172/JCI129193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, & Endeman H (2020). Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Research, 191, 145–147. 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, … Penninger JM (2005). A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nature Medicine, 11(8), 875–879. 10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Faiq MA, Pareek V, Raza K, Narayan RK, Prasoon P, Kumar P, Kulandhasamy M, Kumari C, Kant K, Singh HN, Qadri R, Pandey SN, & Kumar S (2020). Relevance of enriched expression of SARS-CoV-2 binding receptor ACE2 in gastrointestinal tissue with pathogenesis of digestive symptoms, diabetes-associated mortality, and disease recurrence in COVID-19 patients. BioRxiv, 2020.04.14.040204. 10.1101/2020.04.14.040204 [DOI] [PMC free article] [PubMed]
- Kwon Y, Nukala SB, Srivastava S, Miyamoto H, Ismail NI, Ong S-B, Lee WH, & Ong S-G (2020). Exosomes Facilitate Transmission of SARS-CoV-2 Genome into Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. BioRxiv, 2020.05.14.093583. 10.1101/2020.05.14.093583 [DOI]
- Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, & Wang X (2020). Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 10.1038/s41586-020-2180-5 [DOI] [PubMed]
- Lenassi M, Cagney G, Liao M, Vaupotic T, Bartholomeeusen K, Cheng Y, Krogan NJ, Plemenitas A, & Peterlin BM (2010). HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic (Copenhagen, Denmark), 11(1), 110–122. 10.1111/j.1600-0854.2009.01006.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure F-X, Bouadma L, Nguyen D, Parisey M, Wicky P-H, Behillil S, Gaymard A, Bouscambert-Duchamp M, Donati F, Le Hingrat Q, Enouf V, Houhou-Fidouh N, Valette M, Mailles A, Lucet J-C, Mentre F, Duval X, Descamps D, Malvy D, … Yazdanpanah Y (2020). Clinical and virological data of the first cases of COVID-19 in Europe: A case series. The Lancet Infectious Diseases, 20(6), 697–706. 10.1016/S1473-3099(20)30200-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, Su X, & Cao B (2020). SARS-CoV-2 and viral sepsis: Observations and hypotheses. The Lancet, 395(10235), 1517–1520. 10.1016/S0140-6736(20)30920-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P (2020). The Heart in COVID19: Primary Target or Secondary Bystander? JACC: Basic to Translational Science. 10.1016/j.jacbts.2020.04.001 [DOI] [PMC free article] [PubMed]
- Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss H-P, Blankenberg S, Püschel K, & Westermann D (2020). Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiology. 10.1001/jamacardio.2020.3551 [DOI] [PMC free article] [PubMed]
- Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, Zhang M, Tan J, Xu Y, Song R, Song M, Wang L, Zhang W, Han B, Yang L, Wang X, Zhou G, Zhang T, Li B, … Wang X (2020). Neutrophil-to-Lymphocyte Ratio Predicts Severe Illness Patients with 2019 Novel Coronavirus in the Early Stage. MedRxiv, 2020.02.10.20021584. 10.1101/2020.02.10.20021584 [DOI] [PMC free article] [PubMed]
- Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt J-D, Sacco C, Alexia B, Sandri MT, Barco S, & Humanitas COVID-19 Task Force. (2020). Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thrombosis Research, 191, 9–14. 10.1016/j.thromres.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C-M, Wang N, Yang X-L, Liu H-Z, Zhang W, Li B, Hu B, Peng C, Geng Q-B, Zhu G-J, Li F, & Shi Z-L (2018). Discovery of Novel Bat Coronaviruses in South China That Use the Same Receptor as Middle East Respiratory Syndrome Coronavirus. Journal of Virology, 92(13), e00116–18. 10.1128/JVI.00116-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maemura T, Fukuyama S, & Kawaoka Y (2020). High Levels of miR-483–3p Are Present in Serum Exosomes Upon Infection of Mice With Highly Pathogenic Avian Influenza Virus. Frontiers in Microbiology, 11, 144. 10.3389/fmicb.2020.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Rojas PP, Quiroz-García E, Monroy-Martínez V, Agredano-Moreno LT, Jiménez-García LF, & Ruiz-Ordaz BH (2020). Participation of Extracellular Vesicles from Zika-Virus-Infected Mosquito Cells in the Modification of Naïve Cells’ Behavior by Mediating Cell-to-Cell Transmission of Viral Elements. Cells, 9(1), 123. 10.3390/cells9010123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle D, Plein S, O’Donnell JS, Sharif K, & Bridgewood C (2020). Increased cardiovascular mortality in African Americans with COVID-19. The Lancet. Respiratory Medicine, 8(7), 649–651. 10.1016/S2213-2600(20)30244-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckes DG Jr, Gunawardena HP, Dekroon RM, Heaton PR, Edwards RH, Ozgur S, Griffith JD, Damania B, & Raab-Traub N (2013). Modulation of B-cell exosome proteins by gamma herpesvirus infection. Proceedings of the National Academy of Sciences of the United States of America, 110(31), E2925–E2933. 10.1073/pnas.1303906110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, & Manson JJ (2020). COVID-19: Consider cytokine storm syndromes and immunosuppression. The Lancet, 395(10229), 1033–1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menachery VD, Yount BL, Debbink K, Agnihothram S, Gralinski LE, Plante JA, Graham RL, Scobey T, Ge X-Y, Donaldson EF, Randell SH, Lanzavecchia A, Marasco WA, Shi Z-L, & Baric RS (2015). A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nature Medicine, 21(12), 1508–1513. 10.1038/nm.3985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno GK, Braun KM, Riemersma KK, Martin MA, Halfmann PJ, Crooks CM, Prall T, Baker D, Baczenas JJ, Heffron AS, Ramuta M, Khubbar M, Weiler AM, Accola MA, Rehrauer WM, O’Connor SL, Safdar N, Pepperell CS, Dasu T, … Friedrich TC (2020). Distinct patterns of SARS-CoV-2 transmission in two nearby communities in Wisconsin, USA. MedRxiv: The Preprint Server for Health Sciences, 2020.07.09.20149104. 10.1101/2020.07.09.20149104 [DOI]
- Nolte-’t Hoen E, Cremer T, Gallo RC, & Margolis LB (2016). Extracellular vesicles and viruses: Are they close relatives? Proceedings of the National Academy of Sciences, 113(33), 9155. 10.1073/pnas.1605146113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G, Rezza G, & Brusaferro S (2020). Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 10.1001/jama.2020.4683 [DOI] [PubMed]
- Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, & Butany J (2009). SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. European Journal of Clinical Investigation, 39(7), 618–625. 10.1111/j.1365-2362.2009.02153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SK, Velkoska E, Freeman M, Wai B, Lancefield TF, & Burrell LM (2014). From gene to protein-experimental and clinical studies of ACE2 in blood pressure control and arterial hypertension. Frontiers in Physiology, 5, 227–227. 10.3389/fphys.2014.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VB, Zhong J-C, Grant MB, & Oudit GY (2016). Role of the ACE2/Angiotensin 1–7 Axis of the Renin-Angiotensin System in Heart Failure. Circulation Research, 118(8), 1313–1326. 10.1161/CIRCRESAHA.116.307708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinky, Gupta S, Krishnakumar V, Sharma Y, Dinda AK, & Mohanty S (2020). Mesenchymal Stem Cell Derived Exosomes: A Nano Platform for Therapeutics and Drug Delivery in Combating COVID-19. Stem Cell Reviews and Reports. 10.1007/s12015-020-10002-z [DOI] [PMC free article] [PubMed]
- Premkumar L, Segovia-Chumbez B, Jadi R, Martinez DR, Raut R, Markmann A, Cornaby C, Bartelt L, Weiss S, Park Y, Edwards CE, Weimer E, Scherer EM, Roupael N, Edupuganti S, Weiskopf D, Tse LV, Hou YJ, Margolis D, … de Silva AM (2020). The RBD Of The Spike Protein Of SARS-Group Coronaviruses Is A Highly Specific Target Of SARS-CoV-2 Antibodies But Not Other Pathogenic Human and Animal Coronavirus Antibodies. MedRxiv: The Preprint Server for Health Sciences, 2020.05.06.20093377. 10.1101/2020.05.06.20093377 [DOI]
- Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, & Nagel E (2020). Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiology. 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed]
- Raeven P, Zipperle J, & Drechsler S (2018). Extracellular Vesicles as Markers and Mediators in Sepsis. Theranostics, 8(12), 3348–3365. 10.7150/thno.23453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnaiah V, Thumann C, Fofana I, Habersetzer F, Pan Q, de Ruiter PE, Willemsen R, Demmers JAA, Stalin Raj V, Jenster G, Kwekkeboom J, Tilanus HW, Haagmans BL, Baumert TF, & van der Laan LJW (2013a). Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proceedings of the National Academy of Sciences of the United States of America, 110(32), 13109–13113. 10.1073/pnas.1221899110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnaiah V, Thumann C, Fofana I, Habersetzer F, Pan Q, de Ruiter PE, Willemsen R, Demmers JAA, Stalin Raj V, Jenster G, Kwekkeboom J, Tilanus HW, Haagmans BL, Baumert TF, & van der Laan LJW (2013b). Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proceedings of the National Academy of Sciences of the United States of America, 110(32), 13109–13113. 10.1073/pnas.1221899110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranney ML, Griffeth V, & Jha AK (2020). Critical Supply Shortages—The Need for Ventilators and Personal Protective Equipment during the Covid-19 Pandemic. New England Journal of Medicine, 382(18), e41. 10.1056/NEJMp2006141 [DOI] [PubMed] [Google Scholar]
- Susmita Sahoo, & Losordo Douglas W (2014). Exosomes and Cardiac Repair After Myocardial Infarction. Circulation Research, 114(2), 333–344. 10.1161/CIRCRESAHA.114.300639 [DOI] [PubMed] [Google Scholar]
- Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, De Cobelli F, Tresoldi M, Cappelletti AM, Basso C, Godino C, & Esposito A (2020). Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. European Heart Journal. 10.1093/eurheartj/ehaa286 [DOI] [PMC free article] [PubMed]
- Sanche S, Lin YT, Xu C, Romero-Severson E, Hengartner N, & Ke R (2020). High Contagiousness and Rapid Spread of Severe Acute Respiratory Syndrome Coronavirus 2. Emerging Infectious Diseases, 26(7), 1470–1477. 10.3201/eid2607.200282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Recalde Á, Solano-López J, Miguelena-Hycka J, Martín-Pinacho JJ, Sanmartín M, & Zamorano JL (2020). COVID-19 and cardiogenic shock. Different cardiovascular presentations with high mortality. Revista Espanola de Cardiologia (English Ed.), 73(8), 669–672. 10.1016/j.rec.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos RAS, Simoes e Silva AC, Maric C, Silva DMR, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SVB, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss H-P, Speth R, & Walther T (2003). Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proceedings of the National Academy of Sciences of the United States of America, 100(14), 8258–8263. 10.1073/pnas.1432869100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorey JS, Cheng Y, Singh PP, & Smith VL (2015). Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Reports, 16(1), 24–43. 10.15252/embr.201439363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A, & Crotty S (2020). Pre-existing immunity to SARS-CoV-2: The knowns and unknowns. Nature Reviews Immunology, 20(8), 457–458. 10.1038/s41577-020-0389-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen MA, Khan S, Kazmi A, Bashir N, & Siddique R (2020). COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. Journal of Advanced Research, 24, 91–98. 10.1016/j.jare.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, & Huang C (2020). Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiology. 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed]
- Sil S, Hu G, Liao K, Niu F, Callen S, Periyasamy P, Fox HS, & Buch S (2020). HIV-1 Tat-mediated astrocytic amyloidosis involves the HIF-1α/lncRNA BACE1-AS axis. PLOS Biology, 18(5), e3000660. 10.1371/journal.pbio.3000660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões e Silva AC, Silveira KD, Ferreira AJ, & Teixeira MM (2013). ACE2, angiotensin-(1–7) and Mas receptor axis in inflammation and fibrosis. British Journal of Pharmacology, 169(3), 477–492. 10.1111/bph.12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-W, Lam SM, Fan X, Cao W-J, Wang S-Y, Tian H, Chua GH, Zhang C, Meng F-P, Xu Z, Fu J-L, Huang L, Xia P, Yang T, Zhang S, Li B, Jiang T-J, Wang R, Wang Z, … Shui G (2020). Omics-Driven Systems Interrogation of Metabolic Dysregulation in COVID-19 Pathogenesis. Cell Metabolism. 10.1016/j.cmet.2020.06.016 [DOI] [PMC free article] [PubMed]
- Song Y, Liu P, Shi XL, Chu YL, Zhang J, Xia J, Gao XZ, Qu T, & Wang MY (2020). SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut, gutjnl-2020-320891. 10.1136/gutjnl-2020-320891 [DOI] [PubMed]
- South AM, Diz DI, & Chappell MC (2020). COVID-19, ACE2, and the cardiovascular consequences. American Journal of Physiology-Heart and Circulatory Physiology, 318(5), H1084–H1090. 10.1152/ajpheart.00217.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers SA (2018). Could Ceramides Become the New Cholesterol? Cell Metabolism, 27(2), 276–280. 10.1016/j.cmet.2017.12.003 [DOI] [PubMed] [Google Scholar]
- Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL, Banovich NE, Barbry P, Brazma A, Collin J, Desai TJ, Duong TE, … HCA Lung Biological Network. (2020). SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nature Medicine. 10.1038/s41591-020-0868-6 [DOI] [PMC free article] [PubMed]
- Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang Y-Q, Wang Q, & Miao H (2020). Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduction and Targeted Therapy, 5(1), 33. 10.1038/s41392-020-0148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, Li D, Wang X, & Sun Z (2020). Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis: JTH, 18(4), 844–847. 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, & Arbustini E (2020). Myocardial localization of coronavirus in COVID-19 cardiogenic shock. European Journal of Heart Failure. 10.1002/ejhf.1828 [DOI] [PMC free article] [PubMed]
- Temme S, Eis-Hübinger AM, McLellan AD, & Koch N (2010). The Herpes Simplex Virus-1 Encoded Glycoprotein B Diverts HLA-DR into the Exosome Pathway. The Journal of Immunology, 184(1), 236. 10.4049/jimmunol.0902192 [DOI] [PubMed] [Google Scholar]
- Thevarajan I, Nguyen THO, Koutsakos M, Druce J, Caly L, van de Sandt CE, Jia X, Nicholson S, Catton M, Cowie B, Tong SYC, Lewin SR, & Kedzierska K (2020). Breadth of concomitant immune responses prior to patient recovery: A case report of non-severe COVID-19. Nature Medicine, 26(4), 453–455. 10.1038/s41591-020-0819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, & Xiao S-Y (2020). Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc. 10.1038/s41379-020-0536-x [DOI] [PMC free article] [PubMed]
- Urbanelli L, Buratta S, Tancini B, Sagini K, Delo F, Porcellati S, & Emiliani C (2019). The Role of Extracellular Vesicles in Viral Infection and Transmission. Vaccines, 7(3), 102. 10.3390/vaccines7030102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, & Munster VJ (2020). Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. New England Journal of Medicine, 382(16), 1564–1567. 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, & Moch H (2020). Endothelial cell infection and endotheliitis in COVID-19. The Lancet, 395(10234), 1417–1418. 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdecchia P, Cavallini C, Spanevello A, & Angeli F (2020). The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. European Journal of Internal Medicine, S0953–6205(20)30151-5. 10.1016/j.ejim.2020.04.037 [DOI] [PMC free article] [PubMed]
- Wan Y, Shang J, Graham R, Baric RS, & Li F (2020). Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. Journal of Virology, 94(7), e00127–20. 10.1128/JVI.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, & Peng Z (2020). Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed]
- Wang Janice, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, Yaffe MB, Moore HB, & Barrett CD (2020). Tissue Plasminogen Activator (tPA) Treatment for COVID-19 Associated Acute Respiratory Distress Syndrome (ARDS): A Case Series. Journal of Thrombosis and Haemostasis, n/a(n/a). 10.1111/jth.14828 [DOI] [PMC free article] [PubMed]
- Wang Jingyu, Wu F, Liu C, Dai W, Teng Y, Su W, Kong W, Gao F, Cai L, Hou A, & Jiang C (2019). Exosomes Released from Rabies Virus-Infected Cells May be Involved in the Infection Process. Virologica Sinica, 34(1), 59–65. 10.1007/s12250-019-00087-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, Tang C, Zhang N, Zhong N, & Li S (2020). Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. The Lancet Haematology, 7(5), e362–e363. 10.1016/S2352-3026(20)30109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CK, Lam CWK, Wu AKL, Ip WK, Lee NLS, Chan IHS, Lit LCW, Hui DSC, Chan MHM, Chung SSC, & Sung JJY (2004). Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clinical and Experimental Immunology, 136(1), 95–103. 10.1111/j.1365-2249.2004.02415.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, Graham BS, & McLellan JS (2020). Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science, 367(6483), 1260. 10.1126/science.abb2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, … Song Y (2020). Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Internal Medicine. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed]
- Wu Q, Zhou L, Sun X, Yan Z, Hu C, Wu J, Xu L, Li X, Liu H, Yin P, Li K, Zhao J, Li Y, Wang X, Li Y, Zhang Q, Xu G, & Chen H (2017). Altered Lipid Metabolism in Recovered SARS Patients Twelve Years after Infection. Scientific Reports, 7(1), 9110. 10.1038/s41598-017-09536-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurdinger T, Gatson NN, Balaj L, Kaur B, Breakefield XO, & Pegtel DM (2012). Extracellular vesicles and their convergence with viral pathways. Advances in Virology, 2012, 767694–767694. 10.1155/2012/767694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, & Wang F-S (2020). Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine, 8(4), 420–422. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xydakis MS, Dehgani-Mobaraki P, Holbrook EH, Geisthoff UW, Bauer C, Hautefort C, Herman P, Manley GT, Lyon DM, & Hopkins C (2020). Smell and taste dysfunction in patients with COVID-19. The Lancet Infectious Diseases, 20(9), 1015–1016. 10.1016/S1473-3099(20)30293-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, Ogawa H, Okumura K, Mukoyama M, & Nakao K (1994). Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation, 90(1), 195–203. 10.1161/01.cir.90.1.195 [DOI] [PubMed] [Google Scholar]
- Zeng J-H, Liu Y-X, Yuan J, Wang F-X, Wu W-B, Li J-X, Wang L-F, Gao H, Wang Y, Dong C-F, Li Y-J, Xie X-J, Feng C, & Liu L (2020). First case of COVID-19 complicated with fulminant myocarditis: A case report and insights. Infection. 10.1007/s15010-020-01424-5 [DOI] [PMC free article] [PubMed]
- Zhang C, Shi L, & Wang F-S (2020). Liver injury in COVID-19: Management and challenges. The Lancet Gastroenterology & Hepatology, 5(5), 428–430. 10.1016/S2468-1253(20)30057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X-J, Qin J-J, Cheng X, Shen L, Zhao Y-C, Yuan Y, Lei F, Chen M-M, Yang H, Bai L, Song X, Lin L, Xia M, Zhou F, Zhou J, She Z-G, Zhu L, Ma X, Xu Q, … Li H (2020). In-Hospital Use of Statins Is Associated with a Reduced Risk of Mortality among Individuals with COVID-19. Cell Metabolism, 32(2), 176–187.e4. 10.1016/j.cmet.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, & Cao B (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet (London, England), 395(10229), 1054–1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Woodson M, Sherman MB, Neelakanta G, & Sultana H (2019). Exosomes mediate Zika virus transmission through SMPD3 neutral Sphingomyelinase in cortical neurons. Emerging Microbes & Infections, 8(1), 307–326. 10.1080/22221751.2019.1578188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Chen K, Zou J, Han P, Hao J, & Han Z (2020). Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Frontiers of Medicine. 10.1007/s11684-020-0754-0 [DOI] [PMC free article] [PubMed]
- Zou Z, Yan Y, Shu Y, Gao R, Sun Y, Li X, Ju X, Liang Z, Liu Q, Zhao Y, Guo F, Bai T, Han Z, Zhu J, Zhou H, Huang F, Li C, Lu H, Li N, … Jiang C (2014). Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nature Communications, 5, 3594–3594. 10.1038/ncomms4594 [DOI] [PMC free article] [PubMed] [Google Scholar]


