Half of heart failure patients have a preserved ejection fraction (HFpEF), a highly morbid syndrome with very few effective treatments1. HFpEF is historically associated with hypertension and left ventricular hypertrophy, with myocytes displaying fibrosis-independent passive stiffening yet preserved force development at systolic calcium levels2. Over the past few decades the HFpEF phenotype has become increasingly dominated by marked obesity, coinciding with a global pandemic, particularly prominent in the United States. Importantly, obesity worsens HFpEF prognosis and is associated with right ventricular dysfunction3. Whether and how such clinical changes impact RV myocyte function remains unknown.
Here, we measured passive stiffness and calcium-activated force in skinned cardiomyocytes obtained from RV septal endomyocardial biopsies in patients with HFpEF. The diagnostic criteria and features of the JHMI HFpEF cohort, including extensive demographics, phenomics, biopsy protocol, and control tissue features, have all been recently reported4. The protocol was approved by the JHU Institutional Review Board and all subjects provided informed consent. Starting with a group of 111 HFpEF patients in our biopsy database, quartiles of systolic blood pressure, sex-adjusted LV mass index (LVMi)4 and body mass index (BMI) were determined. Each patient was scored 0-3 for each; diabetes mellitus was +1 if present, 0 if not. A hypertension/hypertrophy score: Ht/Hp = SBP+LVMi and obesity/diabetes score: Ob/Dm = BMI+DM and their ratio were determined. Ht/Hp patients had a value ≥3 and ratio in the top quintile (≥2); Ob/Dm had a score ≥3 and ratio in the lowest quintile (<0.667), and Mixed matched both with a ratio 0.667-2.0. Major differentiating features are shown in Figure-A. Ht/Hp patients had a mean BMI of 30 kg/m2, systolic pressure of 160 mmHg, and LV hypertrophy, very similar to patients in prior HFpEF myofilament studies2. Mean BMI was 41 kg/m2 in Ob/Dm, systolic pressure 130 mmHg, and less LV hypertrophy, while Mixed matched the BMI of Ob/Dm and SBP/LVMi of Ht/Hp. NT-proBNP was highest in Ht/Hp consistent with hemodynamic load and less obesity. Right heart load (pulmonary artery systolic and wedge pressures were near identical among the groups. There were no significant differences in sex (67% female), ethnicity (67% African-American), New-York Heart Association Function Class (67% III-IV), diabetes (69%), atrial fibrillation (24%), or chronic medications (50% beta-blockers, 43% calcium-channel blockers, 62% ACE/ARB, 90% loop diuretics) between the groups. Flash frozen biopsies stored in optimal cutting temperature compound at −140 °C were randomly selected (n=13-15) for each HFpEF group, and RV septal control tissue (n=10) obtained from non-obese 27±6 kg/m2 donors without LV hypertrophy 82.8±8.6 gm/m1.7 (mean±SD)4.
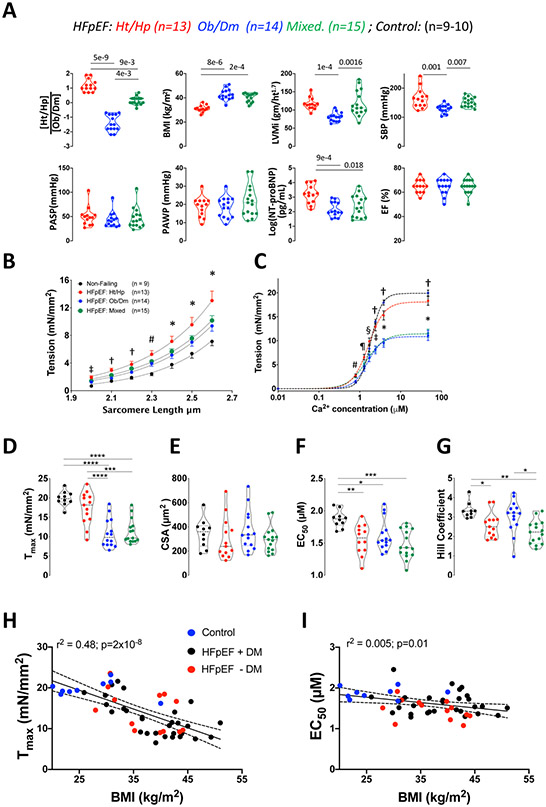
Figure:
A) Clinical characterization of three HFpEF subgroups: hypertension/hypertrophy (Ht/Hp), obesity/diabetes (Ob/Dm), and Mixed. Violin plots show median and 25/75 percentiles for each variable. (Ht/Hp)/(Ob/Dm) index ratio, body mass index (BMI), sex-adjusted left ventricular mass index (LVMi), systolic blood pressure (SBP), pulmonary arterial systolic pressure (PASP), pulmonary arterial wedge pressure (PAWP), log(NTproBNP), and ejection fraction (EF). Kruskal Wallis test followed by Dunn’s multiple comparisons, p-values displayed in each panel. B) Passive myocyte tension-sarcomere length (SL) dependence for control and 3 HFpEF groups. Relations are fit to a mono-exponential. Group effect on Tension-SL relation p=7x10−6 (2-way repeated measures analysis of variance, 2W-RMANOVA). Symbols show Tukey multiple comparisons test at each SL: * p<0.01 CON vs Ht/Hp; p<0.05 CON vs Mixed; # p<0.001 CON vs Ht/Hp and Mixed, p<0.02 vs Ob/Dm; † p<0.005 CON vs Ht/Hp and Mixed; ‡ p<0.001 CON vs Ht/Hp and Mixed, p=0.007 CON vs Ob/Dm. C) Tension-calcium relations for control and HFpEF. Curves are fit to the Hill equation, analyzed by 2W-RMANOVA (overall group effect p=10−6), symbols for Tukey multiple comparisons test: * p<0.001, † p<0.0001, ‡ p<0.002 versus both Ob/Dm and Mixed; § p<0.03 Ht/Hp versus Ob/Dm and Mixed; ¶ p<0.05 Ht/Hp versus CON and Ob/Dm; # p<0.01 CON vs Ht/Hp and Mixed; p=0.025 Ob/Dm vs Ht/Hp and p=0.05 vs Mixed. D) Violin plots for Tmax, E) myocyte cross sectional area (CSA) F) EC50 G) Hill coefficient. 1-way ANOVA, Tukey multiple comparisons: **** p<10−6; *** p<3x10−4; ** <0.01; * p<0.05. H) Negative correlation between BMI and Tmax. Data are color coded to show HFpEF patients with or without diabetes mellitus (DM), and Controls. Linear regression and 95% confidence bands (employing all the data) are shown. Regression equation: Tmax = −0.462 x BMI + 31. I) Linear regression analysis for the relation between EC50 and BMI using same approach as in Panel H. Regression equation EC50 = −0.01315*X + 2.102.
Active and passive sarcomere function was determined from single myocytes isolated in skinning solution from the biopsy pieces as previously described5. Tension (force/cross sectional area)-calcium (Ca2+) relations were measured at a constant sarcomere length (SL=2.1 μM) from 0-46.8 μM Ca2+, and fit to the Hill equation: T = Tmax × Cah/(EC50h + Cah), yielding maximal tension (Tmax, mN/mm2), calcium sensitivity (EC50, Ca2+ at 50% peak force), and cooperativity (h). Passive stiffness in Ca2+-free buffer was measured from 2.0 to 2.6 μm SL. Several cells (2-4) were studied from each biopsy, individually fit to the Hill equation and the results averaged prior to entry into group analyses.
Passive force-SL curves for Ht/Hp cells had higher tension at all SL (stiffer) versus controls (p=1x10−9) and were also stiffer than Ob/Dm cells (p=6x10−4; RMANCOVA; Figure, B). Ob/Dm and Mixed curves were nearly superimposable and both stiffer than controls. Thus, RV myocyte stiffness is greater with Ht/Hp phenotypes but less so when marked obesity predominates.
Active force-SL curves revealed striking reduction of Tmax in Ob/Dm and Mixed HFpEF versus Ht/Hp and CON (Tmax =11±3.7, 11±3.1, 17.3±4.2, 20±1.9, mN/mm2, respectively, Figure, C, D). RV myocyte cross-sectional area was similar among the groups (Figure, E) consistent with similar pulmonary loading. At lower Ca2+, the relations were left-shifted quantified by reduced EC50 in each HFpEF group versus CON (Figure, F). This might contribute to diastolic stiffening and/or resting contractility, but this impacted each group similarly. Both Ht/Hp and Mixed myocytes also displayed lower Hill coefficient (reduced cooperativity Figure, G), suggesting this feature of contraction was more influenced by hemodynamic load than by obesity.
Combining all groups, Tmax negatively correlated with BMI (Tmax, r=−0.69, p=2x10−8, Figure, H), with controls, and HFpEF patients +/− DM falling along the same relation. Multiple regression including BMI, DM, sex adjusted LV mass index, log(NT-proBNP), RV systolic and right atrial/pulmonary artery wedge pressure ratio, yielded only BMI correlating with Tmax (p=0.0006). BMI slightly but significantly negatively correlated with EC50 (Figure I), but not with the Hill coefficient (not shown, p=0.97).
Thus, HFpEF patients with Class II or greater obesity exhibit substantially depressed RV systolic sarcomere function but less passive myocyte stiffening when compared to myocytes from patients with a primarily Ht/Hp phenotype. These findings may help explain worse outcomes in obese HFpEF, and pose a potentially new approach to therapy involving sarcomere stimulators.
Acknowledgments
Sources of Funding:
Supported by American Heart Association Go-Red-For-Women Network of Excellence Grants: 16SFRN28620000 (DAK), 16SFRN27870000 (KS), National Heart Lung Blood Institute: R35:HL-135827 (DAK), T32-HL007227 (VSH, MIA), and K23-HL146889-02 (SH).
Footnotes
Disclosures:
The authors have no conflicts to disclose.
REFERENCES
- 1.Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2020;17:559–573. doi: 10.1038/s41569-020-0363-2 [DOI] [PubMed] [Google Scholar]
- 2.Borbely A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, Stienen GJ and Paulus WJ. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–781. doi: 10.1161/01.CIR.0000155257.33485.6D [DOI] [PubMed] [Google Scholar]
- 3.Prenner SB and Mather PJ. Obesity and heart failure with preserved ejection fraction: A growing problem. Trends Cardiovasc Med. 2018;28:322–327. doi: 10.1016/j.tcm.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 4.Hahn VS, Knutsdottir H, Luo X, Bedi K, Margulies KB, Haldar SM, Stolina M, Yin J, Khakoo AY, Vaishnav J, et al. Myocardial Gene Expression Signatures in Human Heart Failure with Preserved Ejection Fraction. Circulation. 2020. doi: 10.1161/CIRCULATIONAHA.120.050498 [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu S, Kokkonen-Simon KM, Kirk JA, Kolb TM, Damico RL, Mathai SC, Mukherjee M, Shah AA, Wigley FM, Margulies KB, et al. Right Ventricular Myofilament Functional Differences in Humans With Systemic Sclerosis-Associated Versus Idiopathic Pulmonary Arterial Hypertension. Circulation. 2018;137:2360–2370. doi: 10.1161/CIRCULATIONAHA.117.033147 [DOI] [PMC free article] [PubMed] [Google Scholar]