Abstract
Single-cell RNA-Sequencing (scRNA-Seq) has improved our understanding of individual cell types in the human placenta. However, placental scRNA-Seq data is not readily accessible when trying to understand how expression patterns in model systems correspond to those from first trimester human placenta. Therefore, we developed PlacentaCellEnrich, a tool that takes a gene set as input, and then reports if the input set is enriched for genes with placenta cell-specific expression patterns, based on human placenta scRNA-Seq data. The PlacentaCellEnrich tool is freely available at https://placentacellenrich.gdcb.iastate.edu/ for non-profit academic use under the MIT license.
Keywords: scRNA-Seq, Trophoblast, Gene enrichment, Decidua, Cell subtype identification
Introduction
The placenta is derived from extraembryonic tissue and undergoes changes in structure and function during pregnancy to cater to the needs of the developing fetus [1][2]. The multiple functions of the placenta are coupled with the development of different trophoblast (TB) cells [3][4]. Due to the heterogeneous nature of the placenta, bulk RNA-Sequencing (RNA-Seq) data is insufficient for understanding how the expression of different TB cells contribute to the establishment and maintenance of pregnancy. Single-cell RNA-Sequencing (scRNA-Seq) helps address this, by allowing quantification of gene expression levels in the individual cell types of the placenta [5][6][7][8][9].
Due to restrictions on human placental research, mammalian and cell culture models are often used to study placental development [4]. Despite the availability of scRNA-Seq data in human placenta, an understanding of how gene expression patterns from model systems correspond to specific human placenta cells is not always clear. This is due to the difficulty in mapping human placental scRNA-Seq data to other organisms. Therefore, we developed “PlacentaCellEnrich” (https://placentacellenrich.gdcb.iastate.edu/), a user-friendly, interactive web application that can be used to compare gene lists generated in placenta model systems to gene expression in human cells, by carrying out a gene enrichment analysis.
Methods
scRNA-Seq Datasets
We used scRNA-Seq data for placental and decidual cells from Suryawanshi et al. [5] and Vento-Tormo et al. [6] consisting of expression data for 22 and 32 cell types respectively. In the Suryawanshi et al. study, data was generated using the Drop-seq protocol from 10x genomics [5]. However, in the Vento-Tormo et al. study, scRNA-Seq data was generated using both the SmartSeq2 and Drop-seq protocols, and then integrated using Seurat [6][10]. The raw count data from each study was normalized into transcript per million (TPM) like values by dividing the raw reads with sequencing depth and multiplying by a scaling factor of 10,000 to compare expression across cell clusters. We used only protein-coding genes for cell-specific gene enrichment. The PlacentaCellEnrich tool uses one-to-one orthologous gene mappings from Ensembl [11][12] to carry out cell-specific gene enrichment of gene lists from mouse, rat, and rhesus macaque, using human scRNA-Seq datasets.
Defining genes with cell-specific expression
Genes with cell-specific expression are defined based on the algorithm used in the Human Protein Atlas (HPA), that has also been implemented by the TissueEnrich tool [13][14]. Briefly, the genes were divided into six groups. These groups are:
Not Expressed: Genes with an expression level less than an expression threshold specified by user across all the cells.
Cell Enriched: Genes with an expression level greater than or equal to the expression threshold that also have at least five-fold higher expression levels in a particular cell type compared to all other cells.
Group Enriched: Genes with an expression level greater than or equal to the expression threshold that also have at least five-fold higher expression levels in a group of 2–7 cell types compared to all other cells, and that are not considered Cell Enriched.
Cell Enhanced: Genes with an expression level greater than or equal to the expression threshold that also have at least five-fold higher expression levels in a particular cell type compared to the average levels in all other cells, and that are not considered Cell Enriched or Group Enriched.
Expressed in all: Genes with an expression level greater than or equal to the expression threshold across all of the cells that are not in any of the above 4 groups.
Mixed: Genes that are not assigned to any of the above 5 groups.
Genes from the “Cell Enriched”, “Group Enriched”, and “Cell Enhanced” groups are classified as cell-specific genes.
Cell-specific gene enrichment
We used the hypergeometric test to calculate the enrichment of cell-specific genes in the input gene set. The p-value is calculated as:
and the fold-change is calculated as:
N is the total number of genes, K is the total number of cell-specific genes for a cell type, n is the number of genes in the input gene set, and k is the number of cell-specific genes in the input gene set. Multiple hypothesis correction is done using the Benjamini & Hochberg correction.
Background Genes
We provide an optional feature of using background genes for enrichment analysis. It should be noted that the background genes must contain all the genes in the input gene set. The p-value for the enrichment is calculated as:
and the fold-change is calculated as:
Nb is the total number of background genes, Kb is the total number of cell-specific genes for a cell type in background genes, n is the number of genes in the input gene set, and k is the number of cell-specific genes in the input gene set.
Results
Carrying out placenta cell-specific gene enrichment analysis
We used two human scRNA-Seq data sets to identify genes with cell-specific expression, as described in the methods section [5][6]. We then developed a user-friendly web application that analyzes a gene list to calculate placenta cell-specific gene enrichment using R Shiny (Version 1.4.0) [15]. Given a list of genes (e.g. differentially expressed genes from bulk RNA-seq data generated in placenta or genes in a scRNA-Seq cell cluster based on data generated in placenta model systems), the tool can be used to determine if the genes have cell-type specific expression in the human scRNA-Seq data sets. Below is a step-by-step tutorial for carrying out placenta cell-specific enrichment analysis, that is also outlined in Figure 1. A guided tutorial is also available by clicking the “Placenta Cell Gene Enrichment” tab on the homepage.
Fig. 1.
Overview of the PlacentaCellEnrich website and options available for analysis.
After navigating to the homepage (https://placentacellenrich.gdcb.iastate.edu/), click on the “Placenta Cell Gene Enrichment” tab.
Select the type of Gene Identifier for the input gene set. Users can enter the input genes as Gene Symbols or Ensembl IDs.
Select the organism of the input gene set. Currently, PlacentaCellEnrich supports input data for commonly used organisms with a hemochorial placenta including humans, mouse, rat, and rhesus macaque.
Select the scRNA-Seq data to use for enrichment analysis. Users can choose either of the two available human placental single-cell studies. We used orthologous genes to carry out enrichment analysis for mouse, rat, and rhesus macaque using these datasets.
Select the type of cell-specific genes used for the enrichment analysis. The recommended setting is “All”. The description of cell-specific genes is described in methods section.
Enter the expression threshold to define whether the gene is expressed or not. By default, this is set to the recommended value of 1.
Select the type of histogram plot. Users can plot the enrichment analysis in terms of either adjusted p-values or the fold-change, calculated during the enrichment analysis.
Enter the background gene set. This is optional, and users can use this feature if they want to carry out enrichment analysis using a custom gene set instead of all the genes in the datasets. Users can either copy the gene list in the text area or upload it in a file using the browse button. The genes should be one per line for both cases. More information about background sets is available in the methods section.
Enter the input gene set. Users can either copy the gene list in the text area or upload it in a file using the browse button. The genes should be one per line for both cases. Users can also click the “Sample List” to test the tool. As described on the help page, the sample list is comprised of the 1,000 most highly expressed genes from EVTs described in Okae et al.
Click the submit button to carry out enrichment analysis.
Click the bar of any cell type in the histogram to see the expression of cell-specific genes in a heatmap.
Click the download button to download the enrichment values in tab-separated text values.
Visualizing expression patterns of genes across placenta cell types
PlacentaCellEnrich also enables users to check the expression of genes across various placental cell types in the two human scRNA-Seq datasets [5][6]. Below is a step-by-step tutorial on how to visualize the expression of a gene across placental cells (Figure 1).
After navigating to the homepage (https://placentacellenrich.gdcb.iastate.edu/), click on the “Placenta Cell-Specific Genes” tab.
Select the organism of the placental scRNA-Seq data to use. Currently, PlacentaCellEnrich only has human datasets, so the only option is Homo Sapiens (default).
Select the scRNA-Seq dataset. Users can choose either of the two human placental scRNA-Seq studies.
Enter the expression threshold to define whether the gene is expressed or not. By default, this is set to the recommended value of 1.
Enter the gene in the input search box. The search option suggests the genes that are present in the dataset while the user is typing.
Click the submit button to view the expression of the input gene across placenta cell types.
Example usage scenarios of PlacentaCellEnrich
Identifying genes with cell-type specific expression in a human TB cell culture system
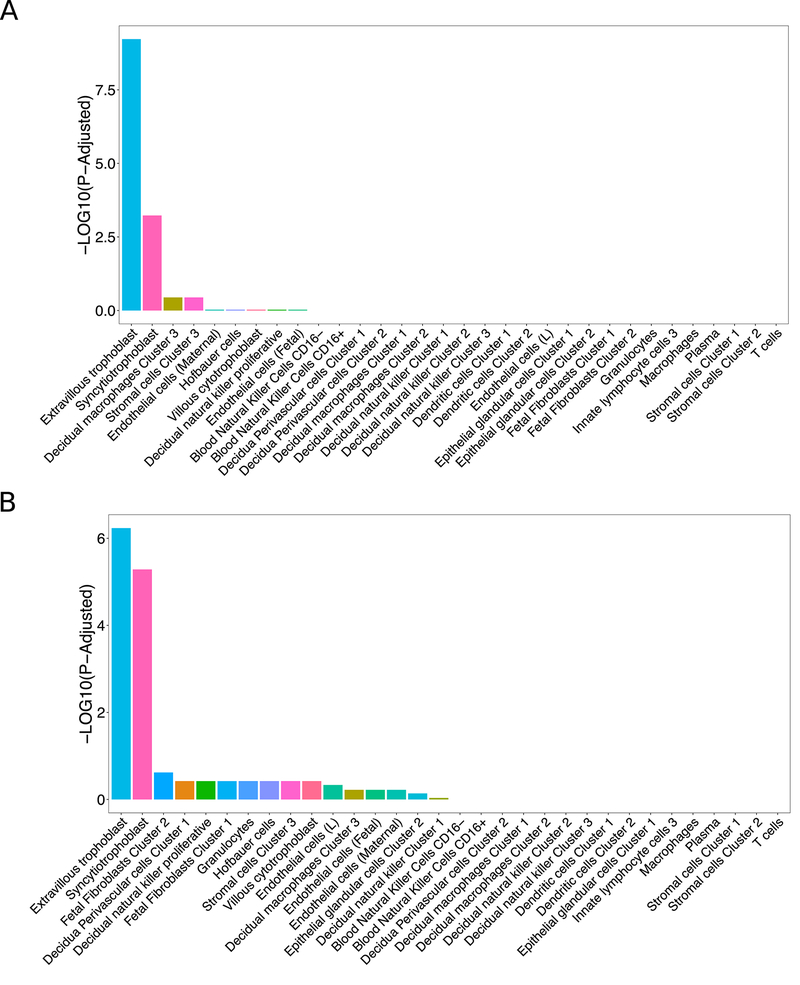
Okae H. et al. isolated TB stem cell lines from human blastocysts, cultured them, and differentiated them into syncytiotrophoblast (syncytioTB) and extravillous trophoblast (EVT) cells [16]. To demonstrate the use of our tool and the similarity of cells differentiated in Okae H. et al. to human placental cells, we used PlacentaCellEnrich on the 1,000 most highly expressed genes from syncytioTB and EVT cells differentiated in culture [16]. As expected, PlacentaCellEnrich shows that differentiated EVT and syncytioTB cells are enriched for genes specifically expressed in first trimester placenta EVTs and syncytioTB cells, respectively (Figure 2 and Supplementary Figure S1). Furthermore, PlacentaCellEnrich identified genes that are specifically expressed in first trimester EVT (or syncytioTB), and also expressed in the cells differentiated in culture (Supplementary Data). Many groups use cell culture models to recapitulate cell types from the first trimester placenta [17][18][19][20][21], and PlacentaCellEnrich can be used to determine the similarity between the gene expression profiles of the culture model cells to those from human placenta.
Fig. 2.
Bar chart showing placenta cell-specific enrichment of the 1000 most highly expressed genes in EVT (a) and syncytioTB (b) differentiated from TB stem cells. Data were analyzed in PlacentaCellEnrich the using Vento-Tormo et al. [6] dataset.
Comparing mouse and human placental gene expression profiles
In the mouse, TB giant cells proliferate rapidly around embryonic day (E) 7.5, prior to the establishment of blood flow, and by E9.5 blood flows between the mother and baby, transporting nutrients and oxygen [22]. To demonstrate how PlacentaCellEnrich can be used to compare the expression profiles in mouse placenta with human TB cells, we used RNA-Seq data generated from mouse fetal placental tissue at E7.5 and E9.5 [23]. We used the 1000 most highly expressed genes from these datasets. PlacentaCellEnrich shows the enrichment of both EVT and syncytioTB-specific genes at E7.5 and E9.5, with higher enrichment of syncytioTB-specific genes at E9.5 than E7.5 (Figure 3 and Supplementary Figure S2). This is expected, since syncytioTB help in nutrient transport, which is established by E9.5 [4]. PlacentaCellEnrich results also indicate that there is minimal decidual contamination in the microdissected mouse fetal placentas. Finally, using PlacentaCellEnrich, we identified EVT and syncytioTB specific genes that are expressed at each time point in mouse, and can be used to identify candidate genes for future studies (Supplementary Data).
Fig. 3.
Bar chart showing the placenta cell-specific enrichment of the 1000 most highly expressed genes in E7.5 (a), and E9.5 (b) mouse placenta. Data were analyzed in PlacentaCellEnrich using the Vento-Tormo et al. [6] dataset.
Comparing mouse and human decidual gene expression profiles
Decidualization of the endometrium is an essential process in the establishment of pregnancy. In mice, this process is first initiated at the antimesometrial (AM) side of the implantation site, and then spreads to the mesometrial (M) pole [24]. Zhao et al. investigated the molecular differences between these regions in the mouse decidua, and using RNA-seq found a total of 1,423 differentially expressed genes (DEGs) between the AM (811 upregulated genes) and M (612 upregulated genes) regions[25]. Using PlacentaCellEnrich, we found that the DEGs upregulated in the AM region are most highly enriched for 1st trimester human epithelial glandular cell-specific genes (Figure 4a and Supplementary Figure S3a), whereas the DEGs upregulated in the M region are most highly enriched with for Natural Killer cell-specific genes (Figure 4b and Supplementary Figure S3b). These results help link bulk RNA-Seq data from mouse decidua to specific human placental cell types based on gene expression information.
Fig. 4.
Bar chart showing the placenta cell-specific enrichment of genes upregulated in antimesometrial (a), and mesometrial (b) mouse decidua. Data were analyzed in PlacentaCellEnrich using the Vento-Tormo et al. [6] dataset.
Discussion
We developed PlacentaCellEnrich, a user-friendly web application that carries out placenta cell-specific gene enrichment using scRNA-Seq data from first-trimester human placenta cells. Identification of cell-specific genes in our tool is based on a method developed by the human protein atlas for identification of tissue-specific genes. Alternate methods could result in a different classification of genes, and future research could focus on statistical methodologies to define cell-specific genes. The enrichment analysis in PlacentaCellEnrich is carried out using the hypergeometric test, which has also been used in Gene Ontology (GO) enrichment analysis. In our analysis, however, we calculate the enrichment of cell-specific genes instead of the GO linked gene sets [26].
The two human scRNA-Seq data sets used in PlacentaCellEnrich were selected based on specific criteria. We used studies that made their processed data publicly available, and that also had at least ten cell clusters [5][6]. Due to the different processing pipelines, the two datasets we used were not merged, and it is recommended that users run analysis and view results using both datasets. It is also important to note that our tool uses cell cluster data as defined by the previous studies when performing enrichment analysis. It is possible that some cell populations were not fully characterized in those studies, and therefore would not be included in our tool. If future analysis identifies additional cell populations, our tool can be updated. Additionally, only protein-coding genes were used for calculation of placenta cell-specific genes. Although this filtering makes the results more focused, it may also lead to the exclusion of novel targets of interest, including miRNAs and other non-coding RNAs.
PlacentaCellEnrich allows users to input genes from model organisms, including mouse, rat, and rhesus macaque. We mapped the orthologous genes between humans and other model organisms using the Ensembl database. The Ensembl database annotates orthologous genes using BLAST [12]. To make the ortholog mapping more stringent, we only mapped the one-to-one orthologs. However, there are alternative ways to map orthologs between species, including using coding Sequence, Markov’s clustering, and other hybrid methods [27][28][29]. If a user prefers one of the alternate methods, they can first obtain the orthologous gene list using the method of their choice, and then use PlacentaCellEnrich.
We provided three usage scenarios that demonstrate how PlacentaCellEnrich can relate gene expression data from model cell culture systems or model organisms to gene expression data from first-trimester human placental cells. While we used the 1,000 most highly expressed genes for many analyses in this paper, important genes can also have lower expression. It is recommended that users select the gene sets for enrichment analysis depending on their research question, whether it be the N most highly expressed genes, sets of differentially expressed genes, or marker genes associated with scRNA-Seq cell clusters. PlacentaCellEnrich can be used to obtain gene expression patterns across placental cell types for individual genes of interest. The tool does not allow identification of enriched functional units across cell populations, such as potential ligand/receptor interactions. For this purpose, scRNA-Seq data can be analyzed with CellPhoneDB [30]. In summary, PlacentaCellEnrich is a valuable tool for the placenta research community, providing insights into single-cell expression profiles from first-trimester human placenta.
Supplementary Material
PlacentaCellEnrich is a webtool that carries out cell-specific gene enrichment analysis.
PlacentaCellEnrich identifies genes with placenta cell-specific expression.
PlacentaCellEnrich aids in comparing gene expression from human placenta to other model systems.
Acknowledgments
We thank Tuteja lab members (Haninder Kaur, Kelby Kies and Ha Vu) for testing the web application; and Levi Baber (LAS research IT director at Iowa State University) for setting up the web application server. Servers and IT support were provided by the Research IT group at Iowa State University http://researchit.las.iastate.edu.
Funding
This work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number RHD096083A to GT. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders/funding agencies.
Abbreviations1
- 1 TB
trophoblast
- RNA-Seq
RNA-Sequencing
- scRNA-Seq
Single-cell RNA-Sequencing
- TPM
Transcripts per million
- HPA
Human Protein Atlas
- syncytioTB
Syncytiotrophoblast
- EVT
Extravillous trophoblast
- E
Embryonic day
- M
Mesometrial
- AM
Antimesometrial
- GO
Gene Ontology
- DEGs
Differentially expressed genes
Footnotes
Conflict of interest statement
The authors have no conflicts of interests to declare.
Declarations of interest: None
Data Availability
The data used in the case studies is provided in the supplementary data file.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Burton GJ, Fowden AL, The placenta: A multifaceted, transient organ, Philos. Trans. R. Soc. B Biol. Sci 370 (2015). doi: 10.1098/rstb.2014.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Knöfler M, Haider S, Saleh L, Pollheimer J, Gamage TKJB, James J, Human placenta and trophoblast development: key molecular mechanisms and model systems, Cell. Mol. Life Sci. 76 (2019) 3479–3496. doi: 10.1007/s00018-019-03104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rossant J, Cross JC, Placental development: lessons from mouse mutants., Nat. Rev. Genet. 2 (2001) 538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- [4].Turco MY, Moffett A, Development of the human placenta, Development. 146 (2019) dev163428. doi: 10.1242/dev.163428. [DOI] [PubMed] [Google Scholar]
- [5].Suryawanshi H, Morozov P, Straus A, Sahasrabudhe N, Max KEA, Garzia A, Kustagi M, Tuschl T, Williams Z, A single-cell survey of the human first-trimester placenta and decidua, Sci. Adv. 4 (2018) eaau4788. doi: 10.1126/sciadv.aau4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, Park JE, Stephenson E, Polański K, Goncalves A, Gardner L, Holmqvist S, Henriksson J, Zou A, Sharkey AM, Millar B, Innes B, Wood L, Wilbrey-Clark A, Payne RP, Ivarsson MA, Lisgo S, Filby A, Rowitch DH, Bulmer JN, Wright GJ, Stubbington MJT, Haniffa M, Moffett A, Teichmann SA, Single-cell reconstruction of the early maternal–fetal interface in humans, Nature. 563 (2018) 347–353. doi: 10.1038/s41586-018-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu Y, Fan X, Wang R, Lu X, Dang YL, Wang H, Lin HY, Zhu C, Ge H, Cross JC, Wang H, Single-cell RNA-seq reveals the diversity of trophoblast subtypes and patterns of differentiation in the human placenta, Cell Res. 28 (2018) 819–832. doi: 10.1038/s41422-018-0066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tsang JCH, Vong JSL, Ji L, Poon LCY, Jiang P, Lui KO, Ni YB, To KF, Cheng YKY, Chiu RWK, Lo YMD, Integrative single-cell and cell-free plasma RNA transcriptomics elucidates placental cellular dynamics, Proc. Natl. Acad. Sci. U. S. A. 114 (2017) E7786–E7795. doi: 10.1073/pnas.1710470114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pique-Regi R, Romero R, Tarca AL, Sendler ED, Xu Y, Garcia-Flores V, Leng Y, Luca F, Hassan SS, Gomez-Lopez N, Single cell transcriptional signatures of the human placenta in term and preterm parturition, Elife. 8 (2019). doi: 10.7554/eLife.52004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Satija R, Farrell JA, Gennert D, Schier AF, Regev A, Spatial reconstruction of single-cell gene expression data, Nat. Biotechnol. 33 (2015) 495–502. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zerbino DR, Achuthan P, Akanni W, Amode MR, Barrell D, Bhai J, Billis K, Cummins C, Gall A, Girón CG, Gil L, Gordon L, Haggerty L, Haskell E, Hourlier T, Izuogu OG, Janacek SH, Juettemann T, To JK, Laird MR, Lavidas I, Liu Z, Loveland JE, Maurel T, McLaren W, Moore B, Mudge J, Murphy DN, Newman V, Nuhn M, Ogeh D, Ong CK, Parker A, Patricio M, Riat HS, Schuilenburg H, Sheppard D, Sparrow H, Taylor K, Thormann A, Vullo A, Walts B, Zadissa A, Frankish A, Hunt SE, Kostadima M, Langridge N, Martin FJ, Muffato M, Perry E, Ruffier M, Staines DM, Trevanion SJ, Aken BL, Cunningham F, Yates A, Flicek P, Ensembl 2018, Nucleic Acids Res. 46 (2018) D754–D761. doi: 10.1093/nar/gkx1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Aken BL, Ayling S, Barrell D, The Ensembl gene annotation system, Database. 2016 (2016). doi: 10.1093/database/baw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist P, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, Von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, Von Heijne G, Nielsen J, Pontén F, Tissue-based map of the human proteome, Science. 347 (2015) 1260419–1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- [14].Jain A, Tuteja G, TissueEnrich: Tissue-specific gene enrichment analysis, Bioinformatics. (2018). doi: 10.1093/bioinformatics/bty890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].W.C. and J.C. and J.A. and Y.X. and McPherson J, shiny: Web Application Framework for R, (2017). https://cran.r-project.org/package=shiny (accessed February 12, 2018).
- [16].Okae H, Toh H, Sato T, Hiura H, Takahashi S, Shirane K, Kabayama Y, Derivation of Human Trophoblast Stem Cells Article Derivation of Human Trophoblast Stem Cells, Stem Cell. (2018) 1–14. doi: 10.1016/j.stem.2017.11.004. [DOI] [PubMed] [Google Scholar]
- [17].Yabe S, Alexenko AP, Amita M, Yang Y, Schust DJ, Sadovsky Y, Ezashi T, Roberts RM, Comparison of syncytiotrophoblast generated from human embryonic stem cells and from term placentas., Proc. Natl. Acad. Sci. U. S. A. (2016) 1601630113-. doi: 10.1073/pnas.1601630113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jain A, Ezashi T, Roberts RM, Tuteja G, Deciphering transcriptional regulation in human embryonic stem cells specified towards a trophoblast fate, Sci. Rep. 7 (2017) 17257. doi: 10.1038/s41598-017-17614-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sheridan MA, Yang Y, Jain A, Lyons AS, Yang P, Brahmasani SR, Dai A, Tian Y, Ellersieck MR, Tuteja G, Schust DJ, Schulz LC, Ezashi T, Michael Roberts R, Early onset preeclampsia in a model for human placental trophoblast, Proc. Natl. Acad. Sci. U. S. A. 116 (2019). doi: 10.1073/pnas.1816150116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hadjantonakis AK, Siggia ED, Simunovic M, In vitro modeling of early mammalian embryogenesis, Curr. Opin. Biomed. Eng. 13 (2020) 134–143. doi: 10.1016/j.cobme.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Horii M, Li Y, Wakeland AK, Pizzo DP, Nelson KK, Sabatini K, Laurent LC, Liu Y, Parast MM, Human pluripotent stem cells as a model of trophoblast differentiation in both normal development and disease, Proc. Natl. Acad. Sci. U. S. A. 113 (2016) E3882–E3891. doi: 10.1073/pnas.1604747113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Woods L, Perez-Garcia V, Hemberger M, Regulation of Placental Development and Its Impact on Fetal Growth-New Insights From Mouse Models, Front. Endocrinol | Www.Frontiersin.Org. 9 (2018) 570. doi: 10.3389/fendo.2018.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tuteja G, Chung T, Bejerano G, Changes in the enhancer landscape during early placental development uncover a trophoblast invasion gene-enhancer network, Placenta. 37 (2016) 45–55. doi: 10.1016/j.placenta.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Das SK, Regional development of uterine decidualization: Molecular signaling by Hoxa-10, Mol. Reprod. Dev. 77 (2010) 387–396. doi: 10.1002/mrd.21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhao M, Zhang WQ, Liu JL, A study on regional differences in decidualization of the mouse uterus, Reproduction. 153 (2017) 645–653. doi: 10.1530/REP-16-0486. [DOI] [PubMed] [Google Scholar]
- [26].Hahne F, Huber W, Gentleman R, Falcon S, Falcon S, Gentleman R, Hypergeometric Testing Used for Gene Set Enrichment Analysis, in: Bioconductor Case Stud, Springer New York, 2008: pp. 207–220. doi: 10.1007/978-0-387-77240-0_14. [DOI] [Google Scholar]
- [27].Emms DM, Kelly S, OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy, Genome Biol. 16 (2015). doi: 10.1186/s13059-015-0721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li L, Stoeckert CJ, Roos DS, OrthoMCL: Identification of ortholog groups for eukaryotic genomes, Genome Res. 13 (2003) 2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Miller JB, Pickett BD, Ridge PG, JustOrthologs: a fast, accurate and user-friendly ortholog identification algorithm, Bioinformatics. 35 (2019) 546–552. doi: 10.1093/bioinformatics/bty669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Efremova M, Vento-Tormo M, Teichmann SA, Vento-Tormo R, CellPhoneDB: inferring cell–cell communication from combined expression of multi-subunit ligand–receptor complexes, Nat. Protoc. 15 (2020) 1484–1506. doi: 10.1038/s41596-020-0292-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.