Abstract
Resolvins are a group of specialized proresolving lipid mediators (SPMs) enzymatically produced from omega-3 fatty acids during acute inflammation response to infections or tissue injury. Resolvin D1 (RvD1) is one of resolvins and is well studied in resolution of inflammation to treat inflammatory diseases. Resolution of inflammation includes the inhibition of polymorphonuclear leukocyte recruitment and reduced cytokine production. However, effective delivery of RvD1 to inflammatory tissues is challenging because of its lack of tissue targeting and poor physicochemical properties. Here, we proposed nanovesicles made from human neutrophil membrane which can specifically target inflamed tissues, and we loaded RvD1 on the surface of nanovesicles and antibiotic (ceftazidime, CEF) inside nanovesicles for improved treatment of bacterial infections. In a mouse model of bacterium-induced peritonitis, we demonstrated that human neutrophil cell membrane-formed vesicles (NMVs) enhanced inflammation resolution and bacterial killing after co-delivery of RvD1 and CEF. Our studies reveal that neutrophil nanovesicles may be critical for enhanced therapy to infectious diseases.
Introduction
Inflammation is the immune response to tissue injury or infections, and it is involved with the activation of leukocytes and their interactions with the blood vasculature [1, 2]. Polymorphonuclear leukocytes (neutrophils) play a central role in the inflammation response. For example, neutrophil adhesion to activated endothelial cells of blood vessels, and their subsequent transmigration are the essential events to inhibit bacterial invasion and their growth[3]. If neutrophils are out of control, excessive neutrophil infiltration may cause a wide range of inflammatory disorders, such as acute lung inflammation/injury [4], sepsis [5], vascular diseases (such as rheumatoid arthritis, inflammatory bowel disease, atherosclerosis ischemia-reperfusion injury) [6–9]. Targeting neutrophils by administration of antibodies [9, 10] to block neutrophil transmigration showed the reduction of inflammation response, but potentially leading to vulnerable infections. Similarly, anti-inflammation agents that target NF-κB pathways may cause the immunosuppression that may be susceptible to infections[11]. Recent studies have shown that inflammation can be actively resolved by endogenous regulators that can terminate inflammatory reactions, promoting tissue healing and repair, unlike anti-inflammatory therapies that only inhibit specific pathways [12, 13]. This new resolution pharmacology illustrates the next generation of anti-inflammatory therapies [14].
Resolvin D series are one of inflammation resolution agents that are enzymatically produced from ω−3 fatty acid, docosahexaenoic acid (DHA) [15]. Resolvins are potent molecules that control excessive inflammatory responses via binding of specific GPCRs with a high affinity and stereoselectivity. Resolvin D1 (RvD1) is one type of resolvins and is well studied [16]. RvD1 terminates neutrophil recruitment and downregulates cellular adhesion between neutrophils and endothelium through its binding to GPCRs (such as FPR2/ALX receptors). In addition, RvD1 can decrease the levels of several inflammatory factors, such as TNF-α, IL-1β and IL-6. This inflammation resolution of RvD1 demonstrates the organ-protection in several diseases related to the eyes, lungs and kidney, there is no observable immunosuppression [17–20]. Although resolvins are produced at inflammatory sites during acute inflammation, they are quickly and enzymatically degraded [13]. Therefore, treating inflammatory or infectious diseases requires the delivery of resolvins to inflammatory tissues [21], but resolvins do not possess the tissue selection and lack of stability in vivo. Here, we proposed cell membrane-derived nanovesicles that can specifically target inflammatory sites for delivering RvD1.
Cell membrane-derived drug delivery platforms have been intensively studied since they demonstrate several novel properties compared to synthetic nanoparticles, for example, endogenous tissue targeting, biocompatibility, long circulation and genetic engineering [21–25]. There are several platforms of cell membrane-derived nanovesicles [26–28], such as single cell-line derived vesicles, combinatory vesicles made from two cell lines, nanoparticle-loaded vesicles and artificial cell membrane vesicles. We recently employed a cell line of HL60 cells as a model of neutrophils to generate cell membrane nanovesicles that can specifically target activated endothelial cells during acute inflammation. Using these nanovesicles, we have delivered anti-inflammatory agents (TPCA-1 or piceatannol) to treat acute lung inflammation/injury and sepsis [22, 29]. HL60 cells are derived from leukemia cells, therefore this system lacks the translation. In this report, we proposed to generate cell membrane nanovesicles from human neutrophils and addressed whether the nanovesicles can specifically target inflammatory tissues. To test our hypothesis, we established a mouse model of bacterium-induced peritonitis. Accumulation of bacteria in peritoneal cavity causes acute inflammation responses. Neutrophils are activated and adhere to activated endothelium at infectious sites. We hypothesized that neutrophil membrane nanovesicles may bind to activated endothelial cells because the nanovesicles possess membrane adhesion molecules of their parent neutrophils. Thus, the nanovesicles would be novel drug carriers to deliver therapeutics to treat peritonitis.
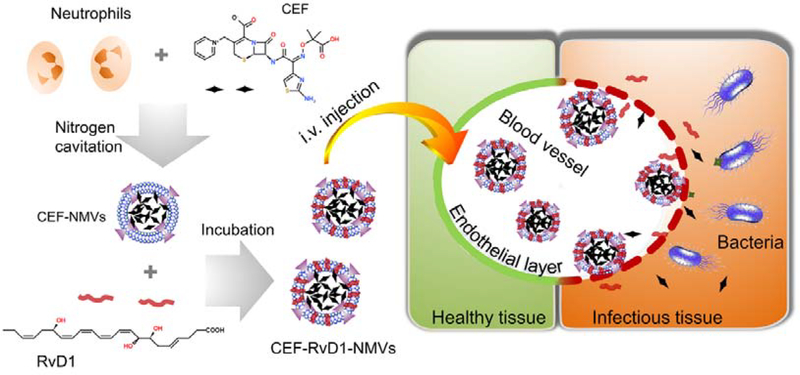
Figure 1 shows our hypothesis. To examine our concept, we have generated cell membrane-formed vesicles (NMVs) from human neutrophils using nitrogen cavitation [29] and established a mouse model of Pseudomonas aeruginosa (P. aeruginosa)-induced peritonitis. To treat bacterial infections, we designed the nanovesicles co-loaded with RvD1 on the surface of nanovesicles and antibiotic (ceftazidime, CEF) inside nanovesicles. The in vitro and in vivo experiments showed that neutrophil nanovesicles can target inflammatory tissues and decreased inflammation responses and cleared bacteria. Collectively, our studies reveal the potential of neutrophil membrane-formed vesicles as a novel drug delivery system to treat infectious diseases.
Figure 1. Schematic shows that human neutrophil membrane-derived nanovesicles can specifically bind to inflamed vasculature in bacterial infections.
Bacterial infections cause the inflammatory response. During inflammation response, activated neutrophils bind to endothelium via several intercellular adhesion molecules, such as ICAM-1 expressed on endothelium and integrin β2 expressed on neutrophils. We proposed that neutrophil membrane-derived nanovesicles may bind to activated endothelium like their parent neutrophils, thus delivering therapeutics to infectious tissues for improved anti-infection. In this study, we proposed to co-load RvD1 (a new inflammation resolution agent) and antibiotic (ceftazidime) in nanovesicles to treat a mouse model of bacterium-induced peritonitis.
Methods and Materials
Reagents
Histopaque 10771, Histopaque 11191, formaldehyde solution, and dimethylsulfoxide (DMSO, purity >99.5%), were purchased from Sigma-Aldrich (St. Louis, MO). Fluorescently-labeled antibodies, and antibodies including FITC-anti-CD11b and APC-anti-CD15 antibodies, monoclonal anti-ICAM-1isotype, and enzyme-linked immunosorbent assay (ELISA) kits for TNF-α, IL-1β and IL-6, were purchased from Biolegend (San Diego, CA). Lipid staining dye DiO (3,3′-dihexadecyloxacarbocyanine perchlorate), DiD (1,1-Dioctadecyl-3,3,3,3-tetramethylindodicarbocyanine), Penicillin-streptomycin (pen-strep) and glutamine (100×) were purchased from Life Technologies (Grand Island, NY). Anti-ICAM-1, anti-integrin β2, anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Pierce™ BCA protein assay kits and ECL Western blotting substrates were obtained from Thermo Fisher Scientific (Rockford, IL). RvD1 was purchased from Cayman Chemical (Ann arbor, MI). CEF was bought from TCI (Tokyo, Japan).
Cells, bacteria, and animals
Human umbilical vein endothelial cells (HUVECs), cell culture medium EBM supplemented with EGM Singlequot (for HUVECs) and RPMI-1640 were purchased from Lonza (Walkersville, MD). Trypsin 0.25% solution and HBSS buffer (without Ca2+, Mg2+ and phenol red) were obtained from Corning (Corning, NY). The bacterium strain P. aeruginosa was purchased from American Type Culture Collection Center (ATCC, Manassas, VA). CD-1 mice were purchased from Envigo (Indianapolis, IN) and they were acclimated in the animal facility for 1 week before they were enrolled in the studies. Mice were housed under a 12-h light-dark cycle at 23°C in a specific pathogen free (SPF) vivarium and had ad libitum access to food and water. All animal protocols were approved by the Institutional Animal Care and Use Committee at Washington State University.
Isolation of human neutrophils
All protocols of human subjects have been approved by the Institutional Review Board at Washington State University. The experiments were fully explained to participants before blood was drawn. The informed consent was signed by all participants and the documents were stored in the College of Pharmacy and Pharmaceutical Sciences at the Washington State University. Venous blood was collected by antecubital venipuncture from healthy volunteers and the blood was transferred to a test tube containing EDTA-K3. Human neutrophils were isolated by gradient density centrifugation method after the whole blood was transferred in a 15 ml polypropylene centrifuge tube containing Histopaque 10771 and 11191 reagents [30]. Briefly, 3 ml of Histopaque 10771 was carefully layered on top of 3 ml of Histopaque 11191 in a centrifuge tube. Subsequently, 6 ml of the collected blood was placed on this discontinuous density gradient. The tube was centrifuged at 890 g for 30 min at 20 °C. The layer containing neutrophils was collected and centrifuged to obtain neutrophils for future experiments. The neutrophil suspension was diluted by 2 times in PBS without Ca2+ and Mg2+. The neutrophil suspension was then centrifuged at 870 g for 5 min at 4 °C. The supernatant was removed, and the red blood cell (RBC) lysis buffer was added. The suspension was left on the bench for 30 min to allow red blood cells to completely lyse. Neutrophils were then collected and washed once with PBS. The neutrophil purity was determined by a fluorescence microscope (Eclipse Ts2, Nikon, Japan) after neutrophils were stained with DAPI. The neutrophil purity was also assessed by flow cytometry after neutrophils were stained with FITC-anti-CD11b and APC-anti-CD15 antibodies.
Preparation and characterization of NMVs
To activate neutrophils, they were resuspended with 10% human plasma-contained PBS and incubated at 37°C for 1 hour at a final concentration of 100 μg/ml LPS. The cells were then pelleted and resuspended in HBSS at 1–5×106/ml. The suspension was transferred to a nitrogen cavitation chamber to prepare cell membrane-formed vesicles as described previously [22, 29]. The sizes of NMVs were measured by dynamic light scattering (DLS) method (Nano ZS90 Malvern Zetasizer, Westborough, MA).
Cell surface markers characterized by western blot.
Cells or NMVs were diluted using a buffer and the suspension was loaded in a 12% sulfate-polyacrylamide gel in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Then, the bands in SDS-PAGE were transferred to a polyvinylidene fluoride (PVDF) membrane (Merck Millipore Corporation, Billerica, MA), followed by the incubation with primary antibodies (such as anti-integrin β2, anti-ICAM-1 and anti-GAPDH monoclonal antibody) for 2 h at the room temperature. Then, the blots were incubated with secondary goat anti-mouse IgG-HRP (1:5 000; Santa Cruz Biotechnology) for 2 h. Washed with Tris buffered saline and 0.05% Tween 20 (TBS-T), the blots were treated with the chemiluminescent HRP substrate (Thermo Scientific,) and chemiluminescence was detected using the Bio-Rad ChemiDoc XRS imager (Bio-Rad, Hercules, CA).
Uptake of NMVs by endothelial cells
To label NMVs, NMVs were incubated with DiD or DiO (0.25%) at 37°C for 30 min, and then free DiD or DiO was removed by centrifugation at 100 000 g for 30 min. HUVECs were seeded in a 12-well culture plate at a density of 5×104 cells/well for 24 h before the experiment. HUVECs were activated by being incubated with 25 ng/ml of TNF-α for 4 h.
For the confocal fluorescence imaging, HUVECs were cultured on a coverslip coated with gelatin. 5 μg of DiD-labeled NMVs (called DiD-NMVs) was added to the cells in 1 ml of EBM medium containing 2% FBS in the presence of a final concentration of 5 μg/ml of anti-ICAM-1 antibody or its isotype antibody, and HUVECs were incubated in a CO2 incubator for 30 min. The cells were stained with Hoechst 33342 and Cell Mask Green dye (Invitrogen, Eugene, OR) for 15 min. Thereafter, the cells were fixed with 4% formaldehyde for 15 min and mounted for imaging using a confocal microscope (A1R plus, Nikon, Japan).
For a flow cytometry assay, 5 μg of DiO-labeled NMVs (called DiO-NMVs) was added to the cells in 1 ml of EBM medium containing 2% FBS in the presence of a final concentration of anti-ICAM-1 antibody or its isotype antibody at 5 μg/ml, and HUVECs were incubated in a CO2 incubator for 30 min. After incubation, HUVECs were washed with PBS three times and harvested in PBS. The cellular uptake of vesicles was determined using a Gallios flow cytometer (Beckman Coulter, Brea, CA. Data were analyzed using Kaluza software 2.0 (Beckman Coulter, Brea, CA).
Loading of CEF and RvD1 in NMVs
Neutrophils were resuspended with 10% human plasma-contained PBS and incubated at 37 °C for 1 hour at a final concentration of 100 μg/ml of LPS. The cells were then pelleted and resuspended in HBSS at 1–5×106/ml. The cells were resuspended in HBSS containing 10, 20, or 30 mg/ml CEF, respectively, at 1–5×106/ml. The suspension was transferred to a nitrogen cavitation chamber to generate nanovesicles. The suspension was centrifugated at 2000 g for 20 min to remove cell nuclei. The supernatant was centrifugated once at 100 000 g for 30 min to remove free CEF and free cytosol proteins. The pelleted NMVs were resuspended in PBS and RvD1 was added at the final concentrations of 200, 600 or 1 000 ng/ml, respectively. The suspension was centrifugated at 100 000 g for 30 min to remove free RvD1. The vesicles passed through a membrane with the porous size of 200 nm to make uniform size of vesicles. The final loading of CEF and RvD1 in NMVs (CEF-RvD1-NMVs) was determined by high performance liquid chromatography (HPLC).
Drug release of CEF-RvD1-NMVs
CEF-RvD1-NMVs (about 1 ml) were placed in a dialysis tube with the cutoff molecular weight at 30 000 (Da) and were dialyzed in 20 ml PBS. The tubes were placed on a shaker and the shaking speed was set at 100 rpm. At different time points, 10 μl of CEF-RvD1-NMVs was sampled and CEF and RvD1 contents were measured by HPLC. For RvD1, the HPLC was set in a mobile phase of methanol:KH2PO4 (20 mM at pH 3.5) = 32:68 at 1ml/min. The column was Restek C18 250×4.6 mm. The RvD1 signal was monitored at 301 nm. The minimum detection concentration was 0.5 μg/ml. CEF was determined by HPLC in a mobile phase of methanol: KH2PO4 (20 mM at pH3.5) = 25:75 at 1 ml/min. The column was Restek C18 250×4.6 mm. The signal was monitored at 260 nm. The minimum detection concentration of CEF was 0.2 μg/ml.
Stability and toxicity assay
NMVs (15 μl/vial) were stored at −20 °C. At 0, 2 4 and 8 days, NMVs were thawed and 5 μl of the samples was taken to determine the size measurement using DLS. The rest samples were dissolved in 90 μl of methanol and the concentrations of RvD1 and CEF were determined by HPLC as described above. To measure the toxicity of RvD1-CEF-NMVs on HUVECs, HEK 293T and NHF cells, a 96-well plate at a density of 5 000 cells/well and cultured overnight. CEF-RvD1-NMVs at various concentrations were incubated with the cells for 20 h at 37°C. The MTS cell proliferation kit (Promega, Madison, WI) was used to assess cell viability according to the manufacturer guidance.
Therapeutic effects of CEF-RvD1-NMVs in a mouse model
CD-1 (24–26 g, male) mice were intraperitoneally (i.p.) inoculated with P. aeruginosa (105 colony forming unit, CFU). 2 h later, HBSS, RvD1, CEF, RvD1/CEF, CEF-RvD1-NMVs (1.6% CEF and 0.013% RvD1) were i.v. given to the mice (at 67 ng of RvD1/mouse and 10 μg of CEF/mouse). At 20 h after the bacterial inoculation, the blood and peritoneal exudates were collected. P. aeruginosa titers, the cell infiltration and cytokine levels were determined. Cytokines (TNF-α, IL-1β and IL-6) in peritoneal fluids were measured using commercial ELIISA kits according to the manufacturer’s instructions.
NMVs inhibited inflammation response in HUVECs
HUVECs were seeded in a 12-well plate at 105 /well one day prior to the experiment. The wells were replaced with a fresh medium containing TNF-α at 50 ng/ml. CEF (5 μg/ml), CEF-NMVs (at 5 μg/ml of CEF), RvD1(33 ng/ml), RvD1-NMVs (at 33 ng/ml of RvD1), CEF+RvD1(at 5 μg/ml of CEF and 33 ng/ml of RvD1), CEF-RvD1-NMVs (at 5 μg/ml of CEF and 33 ng/ml of RvD1) were added to the wells, respectively. 4 h later, the cells were washed once with PBS, and the cells were lysed with 80 μl of cell lysis buffer. The total proteins were quantified by BCA, and each sample at 20 μg of proteins was loaded on 15% SDS-PAGE for electrophoresis. Western blots were performed to determine ICAM-1 expression using anti-ICAM-1 antibodies. GAPDH was as an internal standard.
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
MIC is defined as the lowest concentration of an antimicrobial agent that inhibits the growth of a microorganism and MBC is to determine the lowest concentration at which an antimicrobial agent kills microorganisms. To determine the MIC, NMVs loaded with CEF or both CEF and RvD1 and free drugs were serially diluted and were added to a 96-well plate. 10 μl of bacteria (108 CFU/ml) was added to each well (200 μl/well), and the samples were incubated at 37°C for 24 h. The values of OD600 of the samples were measured and the MIC was defined by 95% inhibition of bacteria. To measure MBC, 106 CFU P. aeruginosa were incubated with CEF (5 μg/ml) or CEF-NMVs (at 5 μg/ml of CEF) in PBS for 4 h. After bacteria were washed, the bacteria were incubated in LB agar at 37°C for 24 h. Then the bacteria were accounted to determine the MBC. MBC is defined by 99.9% of bacterial colonies that were killed. All experiments were carried out in triplicates.
Statistics
Differences between two groups were evaluated using the Student’s t-test method, and differences among multiple groups were evaluated using the one-way ANOVA method. P < 0.05, P < 0.01, P<0.001 were considered statistically significant, very significant and extremely significant, respectively.
Results and Discussion:
NMVs made from human neutrophils using nitrogen cavitation.
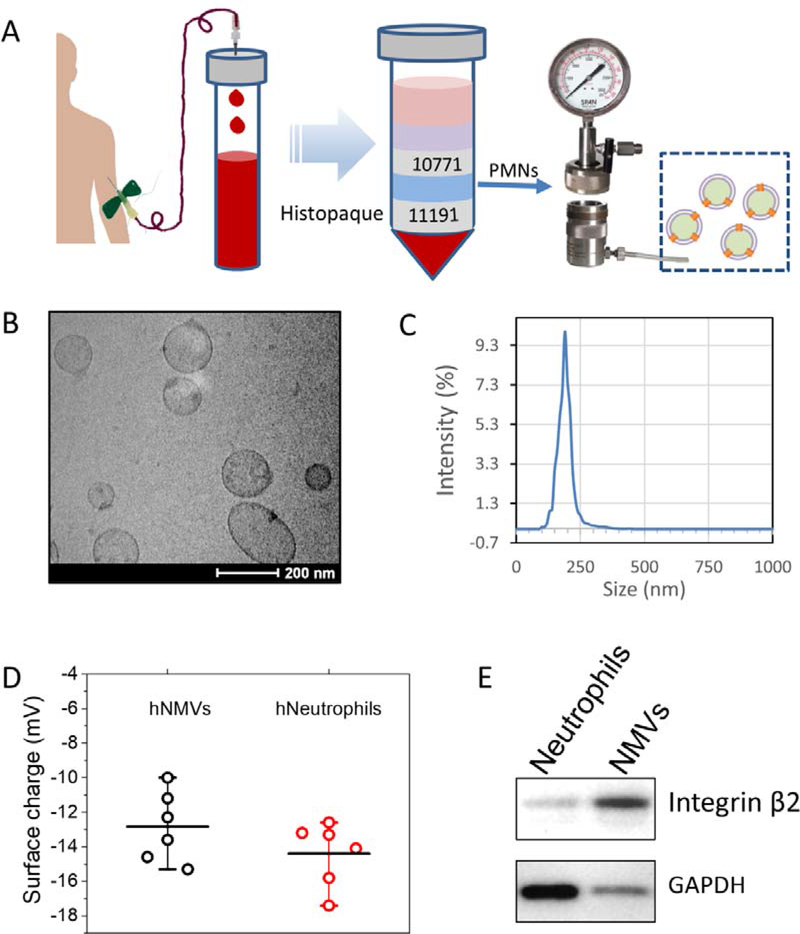
Our lab has established a method to generate cell membrane-derived nanovesicles from HL 60 cells using nitrogen cavitation [22, 29]. Nitrogen cavitation was a process in which cell suspension was placed in a chamber, and a high pressure of nitrogen gas was applied to the cells. When the nitrogen pressure was rapidly released, a cavitation force was formed inside the cells to disrupt the cell membrane. Cytosols and cell nuclei were released from the cells, and the cell membrane was sealed to form vesicles. We utilized serial centrifugations to remove nuclei and cytosols, and we finally obtained cell membrane-derived vesicles. We employed a similar protocol to generate NMVs from human neutrophils (Fig. 2A). The blood from either male or female healthy volunteers was collected. We isolated neutrophils using Histopaque reagents (Histopaque 10771 and 11191). Neutrophils were between Histopaque reagents and RBCs were in the bottom of a centrifuge tube. The neutrophils were collected, and their purity was more than 95% based on the analysis of flow cytometry using two human neutrophil markers (CD11b and CD15) (Fig. S1). Generally, we obtained 1–3×107 neutrophils from 60 ml of the whole blood.
Figure 2. Generation of NMVs and their physical and biological properties.
(A) Illustration of generation of human neutrophil membrane nanovesicles including human blood collection, isolation of neutrophils and generation of NMVs. (B) Cryo-TEM image of NMVs. (C) Size distribution of NMVs measured using dynamic light scattering (DLS). (D) Zeta potentials of NMVs and their parent neutrophils. (E) Western blots of NMVs and neutrophils at the same amount loading of proteins.
We generated NMVs from human neutrophils using the nitrogen cavitation technique. To study a morphology of NMVs, we used cryogenic transmission electron microscopy (cryo-TEM) because this microscopy allows to image natural morphologies of nanomaterials [22, 29]. The image showed that NMVs were liposome-like structures, indicating that NMVs were made of cell membrane (Fig. 2B). In addition, we measured the size of NMVs using dynamics light scattering and observed that the size of NMVs was 192.4 ±8.2 nm (n=6) (Fig. 2C) and the polydispersity index (PDI) was 0.21. This result is consistent with the cryo-TEM image (Fig. 2B). We also measured the surface zeta potential of NMVs, and the value was −12.8±2.0 mV (n=6), similar to that of their source cells of neutrophils (−14.4±1.84 mV, n=6) as shown in Fig. 2D. The result indicates that the surface properties of NMVs are similar to those of neutrophils, suggesting that NMVs were made from cell membrane. This supports our observation of NMVs using cryo-TEM. Integrin β2 is a major marker of activated neutrophils and is required for neutrophil adhesion to activated endothelial cells of blood vessels [31, 32]. Western blots (Fig. 2E) showed that NMVs contained integrin β2. Interestingly, at the same amount of protein loading for NMVs and neutrophil lysis, the level of integrin β2 in NMVs was higher than that in neutrophils. However, the level of GAPDH, a cytosol maker in NMVs was much lower than that in neutrophils. The results suggested that NMVs were made from cell membrane. Collectively, the results indicate that we successfully generated cell membrane-formed nanovesicles from human neutrophils.
NMVs specifically bind to endothelial cells
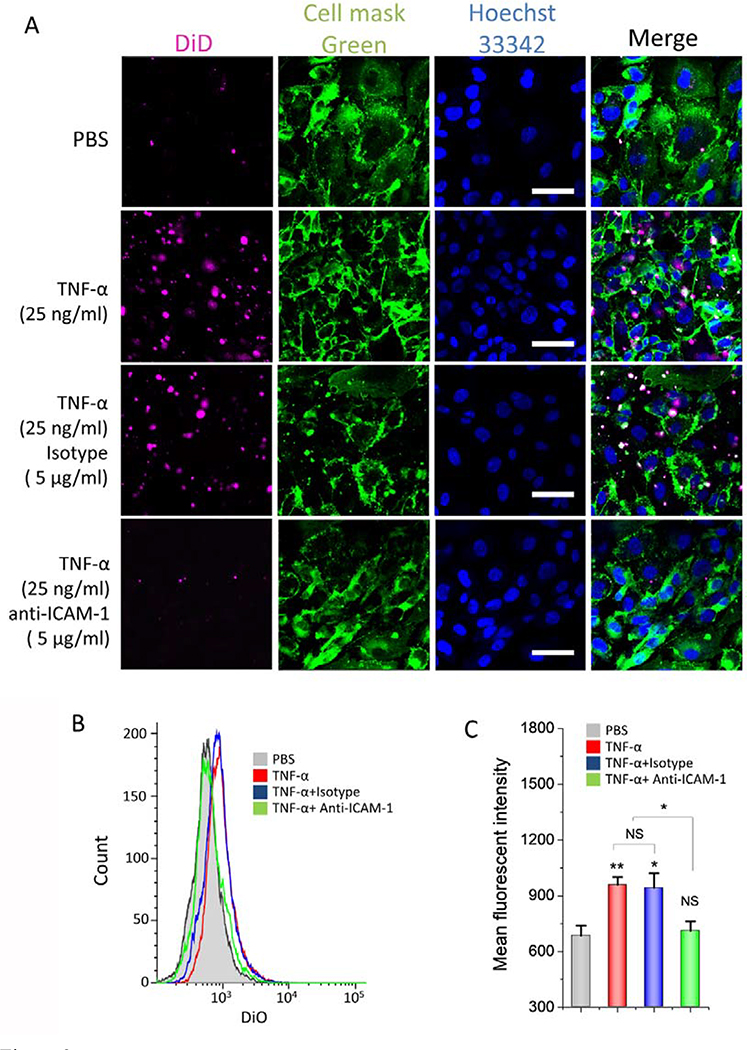
NMVs maintained intercellular adhesion molecules of neutrophils, thus they may interact with endothelial cells for tissue targeting. During acute inflammation response to lipopolysaccharide (LPS), neutrophils and endothelium are activated, and neutrophils bind to endothelium [1]. This process is strongly dependent on intercellular adhesion molecules, such as integrin β2 on neutrophils and ICAM-1 on endothelium [33]. We studied the expression of integrin β2 and ICAM-1 in vitro (Fig. S2). We found that their expression was increased after cells were treated with inflammatory factors, and it was time dependent. Next, we addressed whether NMVs interacted with endothelial cells (Fig. 3A). When we incubated NMVs with activated HUVECs, we observed the increased NMVs in endothelial cells compared to the case when HUVECs were treated with PBS, suggesting that activation of HUVECs was required for the uptake of NMVs. When we pretreated HUVEVs with ICAM-1 antibodies, we observed that the binding of NMVs to HUVECs dramatically decreased. As a control, the isotype antibodies of ICAM-1 did not affect the uptake of NMVs by HUVECs. All these results indicate that ICAM-1 is required for the binding of NMVs via integrin β2. Next, we performed the flow cytometry to quantitatively measure the interactions between NMVs and HUVECs (Fig. 3B and C). The results are consistent with the fluorescent confocal images.
Figure 3. NMVs bind to inflamed endothelial cells.
(A) In vitro binding of NMVs to activated endothelial cells characterized by confocal microscopy. HUVECs were treated with TNF-α at 25 ng/ml for 4 h or without TNF-α before the nanoparticle uptake experiments were performed. Then, DiD-NMVs (5 μg) were incubated with HUVECs at 37°C for 30 min. Anti-ICAM-1 antibody or its isotype antibody was used to block the binding of NMVs to endothelial cells. Scale bar, 50 μm. (B) Flow cytometry used to quantitatively measure the binding of DiD-NMVs to activated endothelial cells and (C) their quantification. Data are presented as the means ± SD, n=3. *P < 0.05; **P < 0.05.
Co-loading of RvD1 and CEF in NMVs.
Inflammatory diseases are associated with the failure of inflammation resolution after infections [34, 35]. To treat infectious diseases (bacterial infections) is needed to administrate antibiotics and inflammation resolution regents. RvD1 is enzymatically produced from DHA and is one of new inflammation resolution agents. RvD1 is a lipid molecule, so it can be loaded in the lipid membrane of NMVs [36]. Most antibiotics are water soluble so they can be trapped inside NMVs.
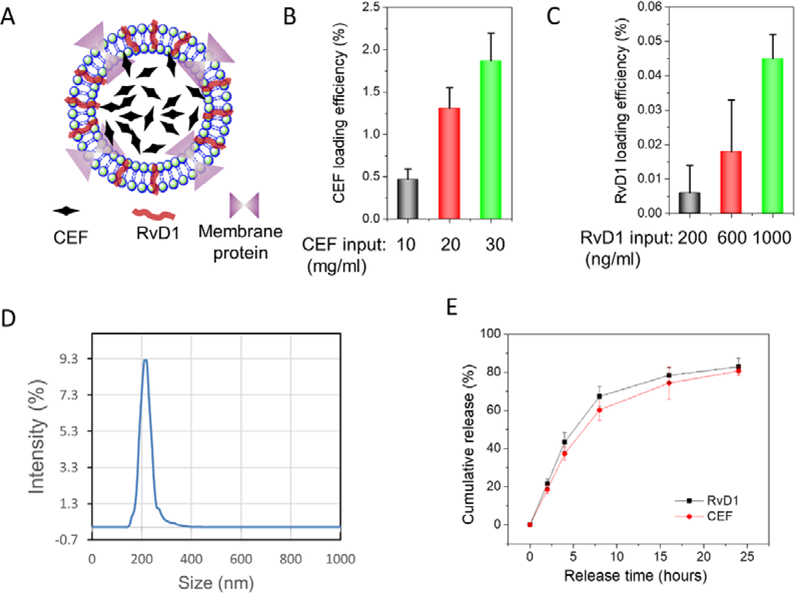
We co-loaded CEF, one of antibiotics and RvD1 in NMVs (Fig. 4A). To load CEF, we added CEF in a cell suspension buffer before using nitrogen cavitation to generate NMVs. When cells were disrupted and cell membrane was sealed to form NMVs, CEF was trapped inside NMVs. At 30 mg/ml CEF, the loading of CEF was 1.5% (Fig. 4B), and the loading efficiency was linearly increased with adding of CEF. After we loaded CEF inside NMVs, we loaded RvD1 to NMVs by incubating RvD1 with NMVs. We found that the loading of RvD1 was increased with amounts of RvD1 (Fig. 4C). We measured the sizes of NMVs after RvD1 and/or CEF were loaded (Fig. 4D and Fig. S3), finding that their sizes were similar to NMVs without the drug loading. The results indicated that the drug loading was unlikely to affect the structure of NMVs. The PDI of NMVs loaded with drugs was 0.22.
Figure 4. Co-loading of RvD1 and CEF in NMVs.
(A) Schematic shows the co-loading of CEF-RvD1-NMVs. CEF was trapped inside NMVs and RvD1 was incorporated to a lipid bilayer of NMVs. Loading efficiencies of CEF (B) and of RvD1 (C) in NMVs (n=3). (D) The size distribution of CEF-RvD1-NMVs determined by DLS. (E) Release profiles of CEF and RvD1 in CEF-RvD1-NMVs at 37°C (n=3).
Drug release of CEF and RvD1 was determined and both drugs had similar release profiles (Fig. 4E). The controlled drug release suggests that NMVs are good carriers to deliver therapeutics (Fig. 4E). We also studied the stability of NMVs. After CEF-RvD1-NMVs were stored at −20°C, we found that NMVs can maintain CEF for a week (Fig. S4A). RvD1 contents slightly decreased over the time (Fig. S4B), but NMVs increased the stability of RvD1 compared to free drug [37]. We also observed that the size of CEF-RvD1-NMVs did not change under our storage conditions (Fig. S4C). Furthermore, the toxicity studies of drug-loaded NMVs on HUVECs, HEK293T and NHF cells indicated that CEF-RvD1-NMVs were nontoxic to normal tissues (Fig. S5). Collectively, NMVs showed a novel drug delivery system that may deliver two types of drugs to target different cellular pathways.
CEF-RvD1-NMVs diminish inflammation response and increase bacterial killing in a mouse model of bacterium-induced peritonitis
We measured the MIC (minimum inhibitory concentration) of CEF to P. aeruginosa in vitro and found that the MIC was 3.7 μg/ml (Fig. S6). Interestingly, CEF-loaded NMVs showed the similar MIC compared to free CEF, suggesting that CEF can release from NMVs to kill bacteria. This is consistent with the results of drug release from NMVs (Fig. 4E). The results in Fig. S6 also showed that RvD1 did not change the MIC of ECF because RvD1 only targets the host. The previous studies showed that RvD1 had in vivo therapeutic effects below 100 ng/mouse [20]. Therefore, we proposed the doses of CEF at 10 μg/mouse and of RvD1 at 67 ng/mouse in the mouse peritonitis induced by P. aeruginosa because the chosen dose of CEF was close to its MIC and RvD1 was in the range of doses used in the mouse model.
Next, we addressed whether NMVs were good carriers to treat bacterium-induced peritonitis. We i.p. injected 105 CFUs of P. aeruginosa into a mouse to establish the bacterium-induced peritonitis model. CEF-RvD1-NMVs and other control formulations were i.v. administered 2 h after injection of bacteria. 20 h later, neutrophils, bacterial CFUs and cytokines were measured (Fig. 5A).
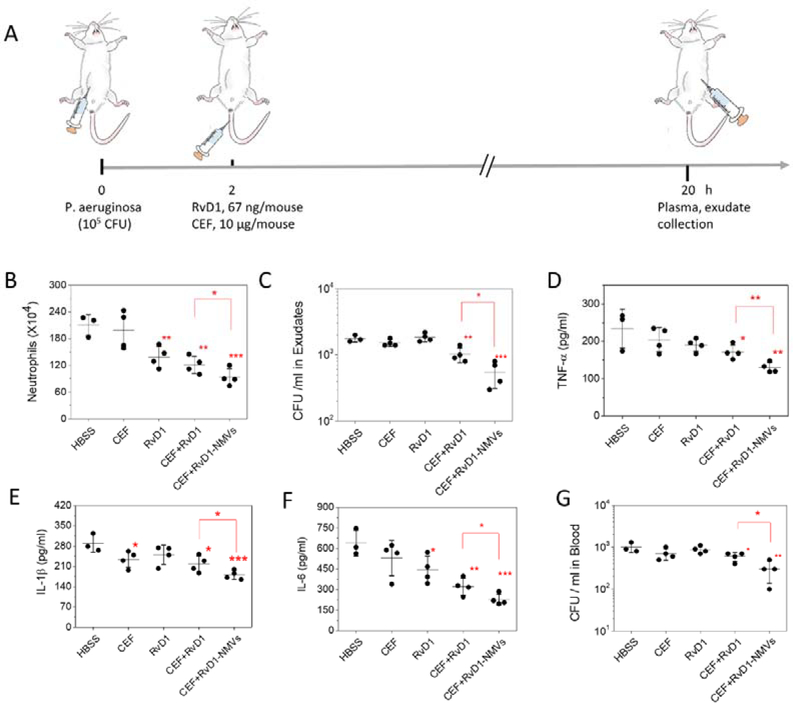
Figure 5. Co-delivery of CEF and RvD1 using NMVs enhances inflammation resolution and bacterial killing in a mouse model of bacterium-induced peritonitis.
(A) Animal protocol for bacterium-induced peritonitis. Neutrophil numbers (B) and CFU (C) in the peritoneal cavity. CFUs were measured using LB plates. (D-F) Cytokines of TNF-α, IL-1β and IL-6 in the peritoneal cavity. (G) CFUs of P. aeruginosa in blood. Data are presented as the mean ± SD, n=3, 4. The differences were assessed by one-way ANOVA and *P < 0.05, **P < 0.01, *** P < 0.001 compared to control (HBSS) unless specified otherwise. Copyright of the animal image was obtained from Encapsula NanoScience.
After we collected peritoneal fluids, we measured infiltrated neutrophils (Fig. 5B). We observed that NMVs dramatically decreased neutrophil infiltration to peritoneal cavity compared to free RvD1, CEF or their combination. Interestingly, CEF did not change the level of infiltrated neutrophils, but RvD1 indeed decreased neutrophil infiltration, suggesting that RvD1 showed the anti-inflammation effect. We also measured bacterial numbers in peritoneal cavity and found NMVs decreased the level of bacteria compared to the controls. Interestingly, only combination of CEF and RvD1 can inhibit bacterial growth compared to free drugs (Fig. 5C). Finally, we analyzed several cytokines including TNF-α, IL-1β and IL-6, and found the similar trend that NMVs alleviated inflammatory responses in contrast with free drugs and their combination (Fig. 5D–F). We also measured the bacterial level in blood and found that CFUs in blood were reduced when NMVs were used (Fig. 5G).
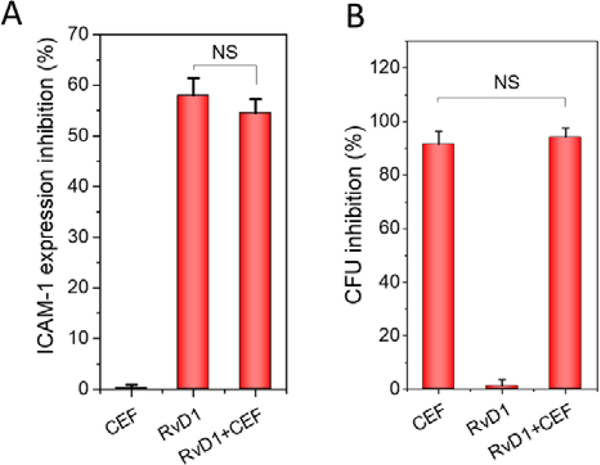
To address the molecular mechanism on inflammation resolution and killing of bacteria when we delivered RvD1 and CEF, we performed the in vitro experiments. Upregulation of ICAM-1 is a marker for inflammation response[2], so we addressed whether RvD1 and CEF affected ICAM-1 expression (Fig. 6A and Fig. S7). We observed that RvD1 inhibited the ICAM-1 expression, but CEF did not. The results indicated that RvD1 regulated inflammation pathways, but CEF did not. Similarly, we measured the bacterial killing when we treated with RvD1 and CEF (Fig. 6B and Fig. S8). The results showed that CEF prevented the bacterial growth, but RvD1 did not. The in vitro experiments may explain the molecular mechanism on resolution of inflammation and bacterial killing when we delivered RvD1 and CEF using NMVs. The enhanced therapeutic effect was observed when we treated mice using NMVs (Fig. 5) because NMVs can target inflamed peritoneal vasculature to increase the delivery efficiency of RvD1 and CEF. Infectious tissues include multiple cells that may contribute to inflammation resolution and bacterial killing, so the combinatory effects of RvD1 and CEF may be more complex.
Figure 6. RvD1 and CEF inhibits ICAM-1 expression of endothelial cells and bacterial growth, respectively.
(A) HUVECs were activated by TNF-α at 50 ng/ml in the presence of CEF (5 μg/ml), RvD1 (33ng/ml) and CEF+RvD1 (5 μg/ml and 33 ng/ml, respectively) for 4 h. The ICAM-1 expression was quantified based on western blots as shown in Fig. S7. (B) P aeruginosa was incubated with or without RvD1 (33 ng/ml), CEF (5 μg/ml) CEF+RvD1 (5 μg/ml and 33 ng/ml) for 24 h. The inhibition of bacterial growth was quantified based on the data in Fig. S8. The data were expressed as means ± SD, n=3. One-way ANOVA was used for statistical analysis. NS, not significance.
We also addressed whether NMVs impacted on antibiotic-resistance. We measured the MBCs (minimum bactericidal concentrations) of CEF and CEF-NMVs using P. aeruginosa, finding that their MBC was similar (Fig. S9). The results suggested that NMVs may not decrease antibiotic resistance. However, these in vitro experiments may not represent in vivo environments of infectious tissues because of multiple cells and blood vessel barriers in infectious tissues. We have shown that NMVs can target infectious sites, thus decreasing the dose of administering antibiotics. Therefore, it is possible that NMVs may decrease antibiotic resistance via the reduction of antibiotic dosage, but this concept is needed to investigate in the future.
Postoperative peritonitis (PP) involves the cytokine production and leukocyte infiltration in peritoneal exudates[38]. P. aeruginosa is an opportunistic pathogen in hospitals which may cause a peritoneal infection related peritonitis [39, 40]. CEF is a new ‘third generation’ of cephalosporin to treat a broad range of antimicrobial infections in clinic[41]. As seen in Fig. 5C, at a dose of 10 μg of CEF/mouse, CEF did not kill bacteria. However, co-delivery of RvD1 and CEF inhibited bacterial growth in peritoneal cavity, suggesting that targeting of both inflammation pathways and pathogens may benefit the therapy of peritonitis. Our results are consistent with enhanced resolution of inflammation in murine peritonitis by delivery of Resolvin D with neutrophil extracellular vesicles [42]. The recent studies show that RvD1 is the natural production of DHA to resolve inflammatory responses during acute inflammation. When RvD1 specifically binds G protein-coupled receptors (GPCRs) (such as ALX and GPR32), RvD1 displays potent and stereoselective anti-inflammatory actions, such as limiting neutrophil infiltration and pro-resolving actions [42]. Furthermore, RvD1 actions on human neutrophils decrease the actin polymerization and block LTB4-regulated adhesion molecules (including integrin β2) [43]. In the peritonitis mouse model (Fig. 5), we have demonstrated that RvD1 can diminish inflammation responses. This anti-inflammation effect may be associated with the inflammation resolution via RvD1 interacting with GPCRs to inhibit neutrophil infiltration and cytokine production.
Various nanoparticles (polymer NPs, dendrimers, solid lipid NPs and liposomes) have been investigated to deliver antibiotics to infectious lesions [21, 44], but they are needed to conjugate vascular targeting ligands for increased delivery of therapeutics at diseased sites. Our NMVs are made from human neutrophils and possess unique vascular targeting proteins expressed on neutrophils. The method of nitrogen cavitation can be scaled up for the production of NMVs and there is a rich resource of human neutrophils. Therefore, NMVs may have the impact on developing personalized nanomedicine. However, it is needed to study the pharmacokinetics of drugs and their biodistribution, thus we will mechanically address how NMVs specifically target inflamed vasculature in the peritonitis model.
Conclusion:
In summary, we have generated cell membrane-derived nanovesicles from human neutrophils and demonstrated that they can specifically target inflamed endothelium. Inflammation is the immune response to infections, so specifically delivering therapeutics to inflammatory sites is critical to treat infectious diseases. We have shown that using neutrophil nanovesicles we can co-deliver RvD1 and CEF to peritonitis tissues, thus improving the resolution of inflammation and bacterial killing. Our results reveal that neutrophil membrane-derived nanovesicles may be a novel drug delivery system to treat infectious diseases.
Supplementary Material
Acknowledgement
The work was supported by NIH grants R01EB027078 to Z. W.
Footnotes
Interest disclosure
The authors declare no interests.
References
- [1].Kolaczkowska E, Kubes P, Neutrophil recruitment and function in health and inflammation, Nat Rev Immunol 13(3) (2013) 159–75. [DOI] [PubMed] [Google Scholar]
- [2].Chu D, Dong X, Shi X, Zhang C, Wang Z, Neutrophil-Based Drug Delivery Systems, Adv Mater 30(22) (2018) e1706245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang CY, Gao J, Wang Z, Bioresponsive Nanoparticles Targeted to Infectious Microenvironments for Sepsis Management, Adv Mater 30(43) (2018) e1803618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang CY, Dong X, Gao J, Lin W, Liu Z, Wang Z, Nanoparticle-induced neutrophil apoptosis increases survival in sepsis and alleviates neurological damage in stroke, Sci Adv 5(11) (2019) eaax7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF, Neutrophils in development of multiple organ failure in sepsis, Lancet 368(9530) (2006) 157–69. [DOI] [PubMed] [Google Scholar]
- [6].Tabas I, Glass CK, Anti-Inflammatory Therapy in Chronic Disease: Challenges and Opportunities, Science 339(6116) (2013) 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Phillipson M, Kubes P, The neutrophil in vascular inflammation, Nat Med 17(11) (2011) 1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wagner DD, Frenette PS, The vessel wall and its interactions, Blood 111(11) (2008) 5271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang ZJ, Li J, Cho J, Malik AB, Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils, Nat Nanotechnol 9(3) (2014) 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lockwood CM, Elliott JD, Brettman L, Hale G, Rebello P, Frewin M, Ringler D, Merrill C, Waldmann H, Anti-adhesion molecule therapy as an interventional strategy for autoimmune inflammation, Clin Immunol 93(2) (1999) 93–106. [DOI] [PubMed] [Google Scholar]
- [11].Dinarello CA, Anti-inflammatory Agents: Present and Future, Cell 140(6) (2010) 935–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN, Resolvin D1 binds human phagocytes with evidence for proresolving receptors, Proceedings of the National Academy of Sciences of the United States of America 107(4) (2010) 1660–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN, Infection regulates pro-resolving mediators that lower antibiotic requirements, Nature 484(7395) (2012) 524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Perretti M, Leroy X, Bland EJ, Montero-Melendez T, Resolution Pharmacology: Opportunities for Therapeutic Innovation in Inflammation, Trends Pharmacol Sci 36(11) (2015) 737–755. [DOI] [PubMed] [Google Scholar]
- [15].Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL, Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals, The Journal of experimental medicine 196(8) (2002) 1025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bannenberg G, Serhan CN, Specialized pro-resolving lipid mediators in the inflammatory response: An update, Biochim Biophys Acta 1801(12) (2010) 1260–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tourdias T, Mori N, Dragonu I, Cassagno N, Boiziau C, Aussudre J, Brochet B, Moonen C, Petry KG, Dousset V, Differential aquaporin 4 expression during edema build-up and resolution phases of brain inflammation, Journal of neuroinflammation 8 (2011) 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Morris T, Stables M, Hobbs A, de Souza P, Colville-Nash P, Warner T, Newson J, Bellingan G, Gilroy DW, Effects of low-dose aspirin on acute inflammatory responses in humans, Journal of immunology 183(3) (2009) 2089–96. [DOI] [PubMed] [Google Scholar]
- [19].Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN, Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation, J Clin Invest 121(2) (2011) 569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN, Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis, Nature 461(7268) (2009) 1287–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dong XY, Zhang CY, Gao J, Wang ZJ, Targeting of Nanotherapeutics to Infection Sites for Antimicrobial Therapy, Adv Ther-Germany 2(11) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gao J, Wang SH, Wang ZJ, High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy, Biomaterials 135 (2017) 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hu CMJ, Fang RH, Copp J, Luk BT, Zhang LF, A biomimetic nanosponge that absorbs pore-forming toxins, Nat Nanotechnol 8(5) (2013) 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dehaini D, Wei XL, Fang RH, Masson S, Angsantikul P, Luk BT, Zhang Y, Ying M, Jiang Y, Kroll AV, Gao WW, Zhang LF, Erythrocyte-Platelet Hybrid Membrane Coating for Enhanced Nanoparticle Functionalization, Adv Mater 29(16) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fang RNH, Hu CMJ, Chen KNH, Luk BT, Carpenter CW, Gao WW, Li SL, Zhang DE, Lu WY, Zhang LF, Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles, Nanoscale 5(19) (2013) 8884–8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gao J, Dong X, Wang Z, Generation, purification and engineering of extracellular vesicles and their biomedical applications, Methods (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang S, Dong X, Gao J, Wang Z, Targeting Inflammatory Vasculature by Extracellular Vesicles, AAPS J 20(2) (2018) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang S, Gao J, Wang Z, Outer membrane vesicles for vaccination and targeted drug delivery, Wiley Interdiscip Rev Nanomed Nanobiotechnol 11(2) (2019) e1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gao J, Chu DF, Wang ZJ, Cell membrane-formed nanovesicles for disease-targeted delivery, J Control Release 224 (2016) 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Freitas M, Porto G, Lima JL, Fernandes E, Isolation and activation of human neutrophils in vitro. The importance of the anticoagulant used during blood collection, Clinical biochemistry 41(7–8) (2008) 570–5. [DOI] [PubMed] [Google Scholar]
- [31].Gorina R, Lyck R, Vestweber D, Engelhardt B, beta(2) Integrin-Mediated Crawling on Endothelial ICAM-1 and ICAM-2 Is a Prerequisite for Transcellular Neutrophil Diapedesis across the Inflamed Blood-Brain Barrier, J Immunol 192(1) (2014) 324–337. [DOI] [PubMed] [Google Scholar]
- [32].Lynam E, Sklar LA, Taylor AD, Neelamegham S, Edwards BS, Smith CW, Simon SI, beta(2)-integrins mediate stable adhesion in collisional interactions between neutrophils and ICAM-1-expressing cells, J Leukocyte Biol 64(5) (1998) 622–630. [DOI] [PubMed] [Google Scholar]
- [33].Nourshargh S, Alon R, Leukocyte Migration into Inflamed Tissues, Immunity 41(5) (2014) 694–707. [DOI] [PubMed] [Google Scholar]
- [34].Gilroy D, De Maeyer R, New insights into the resolution of inflammation, Semin Immunol 27(3) (2015) 161–168. [DOI] [PubMed] [Google Scholar]
- [35].Feehan KT, Gilroy DW, Is Resolution the End of Inflammation?, Trends Mol Med 25(3) (2019) 198–214. [DOI] [PubMed] [Google Scholar]
- [36].Gc JB, Szlenk CT, Gao J, Dong X, Wang Z, Natesan S, Molecular Dynamics Simulations Provide Insight into the Loading Efficiency of Proresolving Lipid Mediators Resolvin D1 and D2 in Cell Membrane-Derived Nanovesicles, Mol Pharm 17(6) (2020) 2155–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Maddipati KR, Zhou SL, Stability and analysis of eicosanoids and docosanoids in tissue culture media, Prostaglandins Other Lipid Mediat 94(1–2) (2011) 59–72. [DOI] [PubMed] [Google Scholar]
- [38].Augustin P, Tran-Dinh A, Valin N, Desmard M, Crevecoeur MA, Muller-Serieys C, Woerther PL, Marmuse JP, Bronchard R, Montravers P, Pseudomonas aeruginosa post-operative peritonitis: clinical features, risk factors, and prognosis, Surgical infections 14(3) (2013) 297–303. [DOI] [PubMed] [Google Scholar]
- [39].Dela Ahator S, Zhang L, Small Is Mighty-Chemical Communication Systems in Pseudomonas Aeruginosa, Annual review of microbiology (2019). [DOI] [PubMed] [Google Scholar]
- [40].Wang SH, Gao J, Li M, Wang LG, Wang ZJ, A facile approach for development of a vaccine made of bacterial double-layered membrane vesicles (DMVs), Biomaterials 187 (2018) 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Giri P, Patel H, Srinivas NR, Review of Clinical Pharmacokinetics of Avibactam, A Newly Approved non-beta lactam beta-lactamase Inhibitor Drug, In Combination Use With Ceftazidime, Drug Res 69(5) (2019) 245–255. [DOI] [PubMed] [Google Scholar]
- [42].Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN, Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing, J Immunol 186(10) (2011) 5543–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Serhan CN, Chiang N, Resolution phase lipid mediators of inflammation: agonists of resolution, Current opinion in pharmacology 13(4) (2013) 632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Abed N, Couvreur P, Nanocarriers for antibiotics: a promising solution to treat intracellular bacterial infections, Int J Antimicrob Agents 43(6) (2014) 485–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.