Abstract
The water extract of Centella asiatica (CAW) can improve cognitive and mitochondrial function and activate the nuclear factor erythroid 2-related factor 2 (NRF2) regulated antioxidant response pathway in aged mice. Here we investigate whether NRF2 activation is required for the cognitive and mitochondrial effects of prolonged CAW exposure during aging. Five-month-old NRF2 knockout (NRF2KO) and wild-type mice were treated with CAW for 1, 7, or 13 months. Each cohort was subjected to cognitive testing and hippocampal mitochondrial analyses. Age-related cognitive decline was accelerated in NRF2KO mice and while CAW treatment improved cognitive performance in wild-type mice, it had no effect on NRF2KO animals. Hippocampal mitochondrial function also declined further with age in NRF2KO mice and greater hippocampal mitochondrial dysfunction was associated with poorer cognitive performance in both genotypes. Long-term CAW treatment did not affect mitochondrial endpoints in animals of either genotype. These data indicate that loss of NRF2 results in accelerated age-related cognitive decline and worsened mitochondrial deficits. NRF2 also appears to be required for the cognitive enhancing effects of CAW during aging.
Keywords: Aging, Cognitive impairment, Mitochondrial dysfunction
1. Introduction
The elderly population in the United States has risen rapidly in recent years and is predicted to continue to rise with the number Americans ages 65 and older estimated to almost double by 2060 (Mather et al., 2015). This increase necessitates a parallel increase in identifying molecular targets and potential therapeutic agents that promote healthy aging and maintenance of cognitive function. The majority of elderly individuals experience some form of memory loss that affects their activities of daily life (Cansino, 2009; Johnson, 2016; Park, 2002) as well as impairments in executive function tasks, including attention, planning, inhibitory control, and cognitive flexibility (Buckner, 2004; Methqal, 2017; Park, 2002). Although the precise physiological underpinnings of this age-related cognitive decline are unknown, increased oxidative stress and mitochondrial dysfunction are thought to be contributing factors (Grimm and Eckert, 2017; Mattson and Arumugam, 2018).
The transcription factor NRF2 (nuclear factor erythroid 2-related factor 2; also called NFE2L2) regulates the endogenous antioxidant response pathway. It is ubiquitously expressed in the mammalian nervous system and protects cells from oxidative damage via increased transcription of cytoprotective genes through binding to antioxidant response elements in the promoters of antioxidant genes (Itoh, 1997; Ma and He, 2012; Motohashi, 2004). NRF2 activation has also been implicated in the regulation of mitochondrial function as well as biogenesis (Dinkova-Kostova, 2015; Vomhof- Dekrey and Picklo, 2012). We and others have shown that various drugs which can activate NRF2 enhance cognitive and mitochondrial function in animal models of healthy as well as pathological aging (Gray et al., 2018b,c; Majkutewicz, 2016; Majkutewicz et al., 2018; Senger et al., 2016; Zheng et al., 2017). Yet, it remains unclear whether NRF2 activation is necessary for these effects on cognitive and mitochondrial function.
The plant Centella asiatica (L.) Urban (Apiaceae), known in the United States as Gotu Kola, has been used for centuries in traditional Chinese and Ayurvedic medicinal practices to enhance cognition, mood, and memory (Shinomol et al., 2011). The water extract of Centella asiatica (CAW) is known to contain many NRF2 activating compounds (Brinkhaus et al., 2000; Fan et al., 2018; Gray et al., 2018a, 2014; Jiang et al., 2017; Liu et al., 2019; Yang et al., 2016) and has mainly demonstrated neuroprotective effects in both in vitro and in vivo models of aging and neurodegenerative disease (Kumar, 2002; Ponnusamy, 2008; Prakash, 2013; Shinomol, 2008a,b; Shinomol et al., 2011). Our own lab has previously shown that 2 weeks of oral CAW treatment can improve cognitive performance and increase hippocampal and cortical expression of mitochondrial genes as well as NRF2 and its antioxidant target genes in both healthy aged mice as well as Aβ overexpressing mice (Gray et al., 2016, 2018b, 2018c). However, we have not previously investigated whether these effects are related to events or independent consequences of CAW treatment. This study aims to address this question as well as investigate the effects of long-term CAW treatment during the normal aging process by comparing the cognitive and mitochondrial response of NRF2 knockout (NRF2KO) mice that express the transcription factor.
2. Methods
2.1. CAW
Dried C. asiatica stems and leaves (Lot number 170300206) were purchased through Oregon’s Wild Harvest, Redmond, OR. CAW was prepared as previously described (Gray et al., 2016, 2018b, 2018c). Briefly, 1200 g of the raw plant material was refluxed with 15 L of water for approximately 1.5 hours, in multiple small quantities. The extract was filtered to remove excess plant matter and lyophilized to a powder. Voucher samples of the raw C. asiatica are deposited at the Oregon State University Herbarium (OSC-V-258629) and our laboratory, and a representative CAW sample is stored in our laboratory at −20 °C.
2.2. Animals
Homozygous NRF2KO mice bred on a C57BL6 background and C57BL6 wild-type (WT) controls were obtained from The Jackson Laboratory. Litters were kept in a climate-controlled environment with a 12-hour light/12-hour dark cycle. Water and diet were supplied ad libitum, except during Odor Discrimination Reversal Learning testing when food was restricted at night and resupplied in the afternoon following testing. All methods were performed in correspondence with the NIH guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Portland VA Medical Center.
At 5 months of age, male and female NRF2KO and WT mice were exposed to CAW in the drinking water at 2 g/L. CAW water intake was monitored for each cage of animals and found to be the same between NRF2KO and WT animals (Supplementary Table 1). The CAW exposure continued through behavioral testing which was initiated after 1, 7, or 13 months of CAW treatment so that at each time point the cohorts of animals were 6, 12, or 18 months of age. Following 2 weeks of behavioral testing animals were sacrificed and tissue was harvested for mitochondrial and gene expression analysis (Fig. 1). The number of animals used in each behavioral test and biological assay can be found in Supplementary Tables 2A–C.
Fig. 1.

Timeline of treatment with CAW and behavioral experiments. Three cohorts of NRF2KO and WT mice were treated with CAW (2 g/L) at 5 months of age; the first group was cognitively tested at 6 months, the second at 12, and the third at 18. Following behavioral tests, mice were euthanized, and tissue was collected. Abbreviations: CAW, water extract of Centella asiatica; NRF2KO, nuclear factor erythroid 2-related factor 2 knockout; WT, wild type.
2.3. Behavioral testing
2.3.1. Learning acquisition test
This test consists of 2 separate stages: shaping and acquisition. In the shaping stage mice were introduced to the testing chamber and trained to dig for a food reward in lavender scented bedding. Mice were presented with one bowl containing the food reward that had been successively filled with bedding in 5 different levels: 0%, 25%, 50%, 75%, and 100%. The mouse proceeds to the acquisition step when it has successfully retrieved the reward 5 times in succession.
The acquisition stage follows the completion of the shaping stage. Mice were presented with 2 cups, one that contains dried beans and one with string. In every trial one digging material was scented with a mint odor and the other with vanilla. The odor and material pairings were randomly alternated between trials but balanced over the entirety of the acquisition phase so that each mouse was exposed to roughly equal combinations of each odor and digging material. The position of the baited cup, whether it was presented on the right or left side of the apparatus, was also balanced throughout testing. During the acquisition stage the bowl with the mint odor was always baited, regardless of the digging material. Example trials are outlined in Table 1. The criteria for completing the acquisition phase was 8 correct digs in any bout of 10 trials. The number of trials for each mouse to reach criteria was recorded. Mice were food restricted the night before each phase of the test in order to motivate the animals.
Table 1.
Example of test pairings for odor discrimination reversal learning test
| Right position | Left position | |
|---|---|---|
| Acquisition phase | D1 + O1 | D2 + O2 |
| D1 + O2 | D2 + O1 | |
| D2 + O1 | D1 + O2 | |
| D2 + O2 | D1 + O1 | |
| Shift phase | D1 + O1 | D2 + O2 |
| D1 + O2 | D2 + O1 | |
| D2 + O1 | D1 + O2 | |
| D2 + O2 | D1 + O1 |
Representative combinations of odor and digging material pairings are given during each phase of the ODRL Italicized indicates correct trial.
Key: D1, dried bean; D2, string; O1, vanilla; O2, mint; ODRL, odor discrimination reversal learning.
2.3.2. Object location memory
This test also has 3 stages: habituation, training, and testing. In the habituation phase, each mouse was introduced to an empty square arena (38 × 38 × 64 cm, built of white acrylonitrile butadiene styrene) for 2 10-minute sessions on 2 consecutive days. During the subsequent training stage animals are exposed to 2 identical objects in fixed locations for 10 minutes, once an hour in a 3-hour period. The testing phase occurred 2 hours after the final training phase (Fig. 2). In the testing phase, one of the identical objects was repositioned to a novel spatial location and the mouse was given the chance to examine the environment for 5 minutes. The time spent exploring the fixed and repositioned objects was evaluated via a camera placed above the arena, interfaced with ANYmaze video tracking system (Stoelting Co, Wood Dale, IL, USA). The percent preference was calculated by subtracting the percentage of time spent exploring the object in the familiar location from the percentage of time spent exploring the object in the novel location.
Fig. 2.
Set-up of OLM experiment. The mouse is exposed to 2 identical objects in fixed locations throughout training. Then after a time delay testing begins where one is moved to novel location and the amount of time the mouse spends exploring each object is measured. Abbreviations: OLM, object location memory.
2.4. Isolation of hippocampal mitochondria
Hippocampal mitochondria were isolated as previously described (Iuso et al., 2017) with slight alterations. Isolated hippo-campi were briefly submerged in cold isolation buffer which comprised of 70 mM sucrose, 2 mM HEPES, 1 mM EGTA, 220 mM mannitol, 5 mM KH2PO4, 5 mM MgCl2, and 0.5% BSA (fatty acid free) and homogenized using an Arrow Engineering JR4000 homogenizer. The homogenate was centrifuged at 500g for 5 minutes at 4 ° C. The supernatant fraction was then isolated and centrifuged at 14,000g for 10 minutes at 4 °C. Finally, the pellet fraction was resuspended in 12% Percoll and precisely layered on top of 24% Percoll and centrifuged at 16,000g for 20 minutes at 4 °C. Protein concentration was calculated by Bradford Assay.
2.5. Analysis of mitochondrial function
Mitochondrial function was evaluated using the Seahorse Bioscience XF24 Extracellular Flux Analyzer. Isolated hippocampal mitochondria were first plated on Seahorse XF culture plates (Seahorse Bioscience) (2 μg/well) with 3–4 replicate wells per animal. The plate was then centrifuged for 15 minutes at 2000g and oxygen consumption rates (OCRs) were quantified under different conditions using the MitoStress Kit as previously described (Iuso et al., 2017). Following 3 baseline measurements of OCR (reflecting basal respiration), a saturating concentration of ADP (2 mM) was added to achieve maximum state III respiration and 3 successive measurements were taken (denoted as ADP-stimulated respiration). The ATP synthase inhibitor oligomycin (2 μm) was then added to induce state IV respiration and 3 more measurements were made. Next the electron transport chain (ETC) accelerator carbonyl cyanide p-(trifluoromethoxy)phenyl-hydrazone (FCCP at 4 μm) was added to induce maximal uncoupled (state IIIu) respiration and after 3 measurements, the mitochondrial inhibitors rotenone (1 μm) and antimycin (1 μm) were added, and 3 final measurements were made. The respiratory control ratio (RCR) between state III and state IV respiration was also calculated.
2.6. ATP quantification
The ATP content of isolated hippocampal mitochondria was determined using the ATP determination kit (Life Technologies), as per the manufacturer’s instructions. Values were normalized to total protein content as determined by Bradford assay.
2.7. Gene expression
Hippocampal tissue was homogenized and RNA was extracted using Tri-Reagent (Molecular Research Center). RNA was then reverse transcribed using the Superscript III First Strand Synthesis kit (Invitrogen) to generate cDNA as per manufacturer’s instructions. Relative gene expression was determined using TaqMan Gene Expression Master Mix (Invitrogen) and commercially available TaqMan primers (Invitrogen) for mitochondrially encoded NADH dehydrogenase 1 (Mt-ND1), mitochondrially encoded cytochrome c oxidase 1 (Mt-CO1), mitochondrially encoded ATP synthase membrane subunit 6 (Mt-ATP6), and glyceraldehyde-3-phosphate dehydrogenase. Quantitative polymerase chain reaction was performed on a StepOne Plus Machine (Applied Biosystems) and analyzed using the delta-delta Ct method normalizing to glyceraldehyde-3-phosphate dehydrogenase expression.
2.8. Statistics
Using the general linear model (GLM), we analyzed behavioral outcomes with a three-way analysis of variance to test for the main effects of age (6, 12, 18 months), genotype (WT vs. NRF2KO), treatment group (CAW supplementation), and their interactions on cognition, hippocampal mitochondrial function, ATP content, and mitochondrial gene expression. For ease of interpretability, figures show results with 4 groups: WT, WT-CAW, NRF2KO, and NRF2KO- CAW. Based on our previous work showing a variable magnitude of response to CAW in male and female mice (Gray et al., 2016, 2018b; Matthews et al., 2019) and reports of differing levels of NRF2 expression between male and female animals (Rohrer et al., 2014; Xu et al., 2018), each sex was analyzed separately. These results can be found in the supplementary material. However, because in this study responses were similar for both males and females, results presented in the figures are both sexes combined together.
All outcome variables were assessed for normality. Learning acquisition, object location memory (OLM) % preference, and RCR were all normally distributed. Mt-ND1, Mt-CO1, and Mt-ATP6 were not normally distributed; a log transformation was performed and normality was established. Mt-ND1, Mt-CO1, and Mt-ATP6 analyses are on the log transformation. All behavior and mitochondrial gene expression variables were analyzed using the GLM. However, several mitochondrial function variables including ATP content as well as basal, and ADP-stimulated OCRs, violated normality and did not become normal with a log transformation. Therefore, these were analyzed using the nonparametric Wilcoxon rank-sum test. The predictors for each model were group status, age, and the interaction between group and age. Initial models included all possible interaction terms. However, only the interaction between age and genotype was statistically significant in most models. Since the other interaction terms can be considered irrelevant variables, including irrelevant variables in a model with bias parameter estimates, final models were run with only the main effects and the interaction between age and genotype. The parameter estimates reported in Supplementary Tables 3–8 reflect these revised parsimonious models for the combined model as well as the models with each sex analyzed separately. These statistical analyses were preformed using SAS 9.4.
All bar graphs have error bars reflecting standard error of the mean. Statistical significance for bar graphs was determined by analysis of variances with Tukey HSD pairwise comparisons. Significance was defined as p ≤ 0.05. These analyses were performed using GraphPad Prism 6.
3. Results
3.1. Loss of NRF2 accelerates age-related decline in learning and abolishes response to CAW
We have previously demonstrated that 2 weeks of CAW treatment improves learning in aged but not young WT mice (Gray et al., 2016). To determine the effects of long-term CAW exposure and whether NRF2 is required to observe them, we tested NRF2KO and WT mice at 6,12, and 18 months of age following treatment with CAW for 1, 7, or 13 months respectively. Mice were evaluated using the learning acquisition test in which the animal is tasked with learning to associate a reward with a specific cue (odor).
GLM analysis revealed that performance in the acquisition phase deteriorated with age (F(4,139) = 60.15, p < 0.0001; Fig. 3A). There was a statistically significant main effect for age (β = 0.89, p < 0.0001), older cohorts taking more trials to reach criteria than younger cohorts. There was a significant effect of treatment (β = 0.13, p < 0.05). Additionally, there was a significant interaction between age and genotype (β = −0.54, p < 0.0001), with NRF2KO performance deteriorating at a greater rate than WT. Specifically, WT CAW-treated animals had a slower rate of deterioration when compared to other groups. When looking at the 18-month cohort, it is apparent that control-treated NRF2KO mice took significantly more trials to reach criteria than control-treated WT animals (Fig. 3B) and that WT mice that were treated with CAW required significantly fewer trials to reach criteria than control-treated WT mice. This CAW-induced improvement was not observed in 18- month-old NRF2KO mice. Analysis of male and female response separately can be found in Supplementary Table 3.
Fig. 3.

NRF2KO accelerates age-related decline in learning and abolishes response to CAW: performance in the acquisition learning test worsened with age in mice of both genotypes (A). There was a significant interaction between age and genotype. A full description of the statistical analysis can be found in Supplementary Table 3. (B) At 18 months, a significant improvement in performance was evident in CAW-treated WT mice but CAW has no effect on NRF2KO animals.*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; n = 8–15 per group. Abbreviations: CAW, water extract of Centella asiatica; Ctrl, control; NRF2KO, nuclear factor erythroid 2-related factor 2 knockout; WT, wild type.
3.2. CAW treatment preserves spatial memory in aged WT but not NRF2KO mice
We have also previously reported that 2 weeks of CAW treatment improves spatial memory in healthy aging mice (Gray et al., 2016, 2018b). To validate these findings following long-term CAW treatment and to determine the requirement of NRF2, we used the OLM test. The OLM is a test of spatial memory wherein the mouse is exposed to 2 identical objects in fixed locations throughout training but during testing one object is moved to novel location (Fig. 2). If the mouse remembers the training location it should spend a greater amount of time with the object in the new location, quantified as the preference for the new location (time spent with the new location–time spent with the old location). In our experiment, all mice in each cohort spent comparable time exploring both objects during the training phase (data not shown). Again, this model was statistically significant (F (5, 132) = 17.22, p < 0.0001). There was again a significant effect of age with the older animals having a decreased preference for the novel location as compared to the younger animals (β = −0.73, p < 0.0001). There were 2 statistically significant interactions. The interaction between genotype and treatment (β = −0.24, p < 0.05) was statistically significant as well as the interaction between age and genotype (β = 0.42, p < 0.01) (Fig. 4A). At 18 months, the WT CAW-treated mice had a significantly stronger preference for the novel location than NRF2KO animals (Fig. 4B). A breakdown of the response of each sex separately can be found in Supplementary Table 4.
Fig. 4.
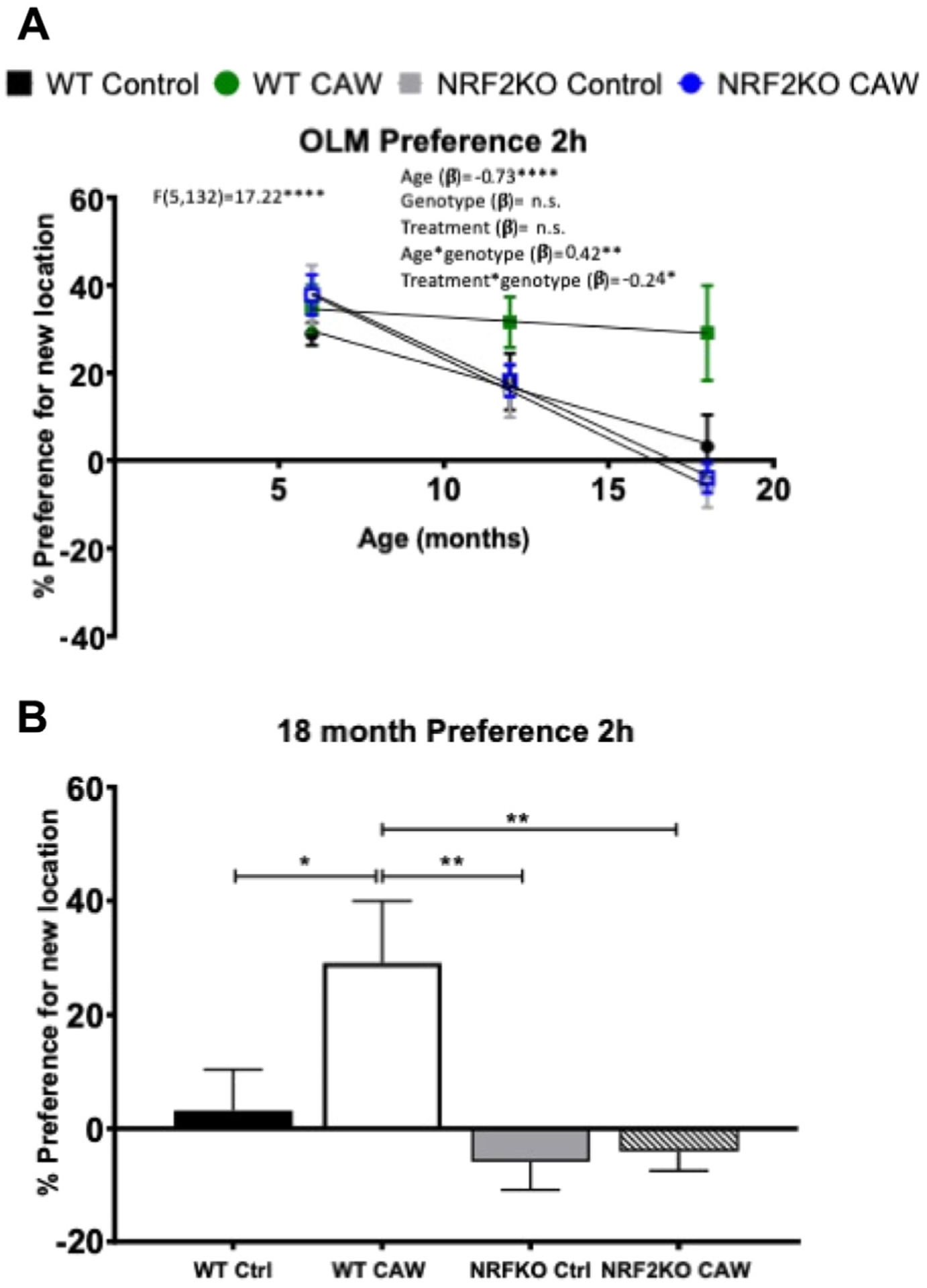
CAW treatment preserves spatial memory in aged WT but not NRF2KO mice: performance in OLM (A) declined significantly with age in mice of both genotypes. A significant interaction was detected between genotype and age and between treatment and genotype. A full description of the statistical analysis can be found in Supplementary Table 4. (B) CAW treatment increased preference for the novel location in 18-month-old WT mice but not NRFKO mice. Eighteen-month-old WT mice treated with CAW displayed a significantly greater preference for the novel location than NRF2KO mice. *p < 0.05, **p < 0.01, ****p < 0.0001; n = 8–15 per group. Abbreviations: CAW, water extract of Centella asiatica; Ctrl, control; NRF2KO, nuclear factor erythroid 2-related factor 2 knockout; OLM, object location memory; WT, wild type.
3.3. Aged NRF2KO mice have a greater decline in mitochondrial gene expression than WT mice
The expression of 3 different genes encoding enzymes in the ETC was measured from hippocampal tissue after euthanasia for each age and treatment group. There was a significant main effect of age for Mt-ND1 and Mt-CO1 (Fig. 5A and B) with expression declining in older animals (Mt-ND1: β = −0.57, p < 0.0001; Mt-CO1: β = −0.37, p < 0.001). A statistically significant interaction between age and genotype was also observed in Mt-ND1 (β = 0.33, p < 0.01) and Mt-CO1 expression (β = 0.26, p < 0.05). The details of the male and female mice separately can be found in Supplementary Tables 5 and 6. Although there were no significant effects of age, treatment, or genotype in the expression of Mt-ATP6 in all mice combined (Fig. 5C), for male mice there was both a significant main effect of age (β = −0.56, p < 0.0001) and a significant interaction between age and genotype (β = 0.47, p < 0.01) for Mt-ATP6 expression (Supplementary Table 7).
Fig. 5.

Diminished hippocampal mitochondrial gene expression is evident in aged NRF2KO mice: expression of (A) Mt-ND1 and (B) Mt-CO1 with age in all mice. A similar trend was observed in Mt-ATP6. There was a significant interaction between age and genotype for both Mt-ND1 and Mt-CO1. A full description of the statistical analysis can be found in Supplementary Tables 5–7. In the 18-month-old animals, both control and CAW-treated WT mice had significantly higher expression of Mt-ND1 than NRF2KO mice. CAW-treated WT mice also had significantly higher expression of Mt-CO1 than NRF2KO mice. A similar trend was observed for Mt-ATP6 as well. *p < 0.05; n = 8–15 per group. Abbreviations: CAW, water extract of Centella asiatica; Mt-ATP6, mitochondrially encoded ATP synthase membrane subunit 6; Mt-CO1,mitochondrially encoded cytochrome c oxidase 1; Mt-ND1, mitochondrially encoded NADH dehydrogenase 1NRF2KO, nuclear factor erythroid 2-related factor 2 knockout; WT, wild type.
Although no effects of CAW treatment were observed in either genotype with all mice combined at 18 months, there was a significant reduction in the expression of Mt-ND1 and Mt-CO1 in both control and CAW-treated NRF2KO female mice as compared to CAW-treated WT mice (Fig. 5D). The same trend was seen in the expression of Mt-ATP6 in 18-month-old mice.
3.4. Loss ofNRF2 exacerbates age-related mitochondrial dysfunction
Aged mice of both genotypes displayed impaired mitochondrial bioenergetic profiles relative to younger cohorts (Supplementary Fig. 1). Not only was overall oxygen consumption reduced with age but the response to accelerating inhibitory compounds was blunted in the older cohorts as compared to the younger ones. The RCR, a measure of how tightly coupled respiration is to phosphorylation and an indicator of overall mitochondrial health, was significantly reduced with age (F (4, 143) = 25.91, p < 0.0001; β = −0.67, p < 0.0001) but no interactions were found between age and genotype (Fig. 6A). However, at 18 months there was a significant reduction in RCR in both NRF2KO mice relative to CAW-treated WT animals (Fig. 6B). Additionally, although long-term CAW treatment did not significantly affect RCR in WT mice when analyzed together, there was a slight but statistically significant improvement in RCR in 18-month-old male WT mice (Supplementary Fig. 2). CAW treatment did not affect NRF2KO mice of either sex. Basal and ADP were not normally distributed, so we used a Wilcoxon rank-sum test to look at group difference at each age. Interactions cannot be assessed with this nonparametric test. There were no significant group differences at 6 or 12 months of age for either basal or ADP-stimulated respiration; however, statistically significant group differences were observed at 18 months for both basal (χ2 = 19.51, p < 0.01; Supplementary Fig. 3A) and ADP- stimulated respiration (χ2 = 11.50, p < 0.01; Supplementary Fig. 3B). Results for each sex separately can be found in Supplementary Fig. 4.
Fig. 6.

Aged NRF2KO mice have significantly diminished RCRs: (A) RCR declined significantly with age in all mice and there was no interaction between age and genotype. (B) In the 18-month-old cohort however, it was evident that NRF2KO had reduced RCRs relative to CAW-treated WT animals. **p < 0.01; n = 8–15. Abbreviations: CAW, water extract of Centella asiatica; Ctrl, control; NRF2KO, nuclear factor erythroid 2-related factor 2 knockout; RCR, respiratory control ratio; WT, wild type.
3.5. ATP content of mitochondria declines with age
Hippocampal ATP content was quantified by luminescent kit using mitochondria isolated from the hippocampus of treated animals. Overall, ATP content declined with age (Fig. 7A). ATP was not normally distributed, so we used a Wilcoxon rank-sum test to look at group difference at each age. Interactions cannot be assessed with this nonparametric test. At 18 months, there were significant group differences detected (χ2 = 16.91, p < 0.001; Fig. 7B). There were also significant group differences apparent at 12 months of age (Supplementary Fig. 5).
Fig. 7.

Hippocampal ATP content is lower in aged NRF2KO mice than WT nice: (A) hippocampal ATP content declined with age in all mice but because the data were not normally distributed the statistical significance of longitudinal effects between groups could not be determined. (B) Nonparametric analysis of the 18-month cohort revealed a significant group difference between Wilcoxon rank sums as determined by a χ2 test (χ2 = 16.91, p < 0.001). n = 8–15 per group. Abbreviations: CAW, water extract of Centella asiatica; Ctrl, control; NRF2KO, nuclear factor erythroid 2-related factor 2 knockout; WT, wild type.
3.6. Greater declines in hippocampal mitochondrial function are associated with poorer cognitive performance
In order to determine whether mitochondrial function was correlated with cognitive performance, we conducted Pearson correlations for normally distributed variables or Spearman correlations when variables were not normally distributed. Looking at all animals of both genotypes together both hippocampal ATP content and RCR were significantly correlated with cognitive performance. ATP and RCR were negatively correlated with the number of trials to reach criteria in the learning acquisition test (Fig. 8A and B) and positively correlated with 2-hour percent preference for the novel location in the OLM test (Fig. 8C and D). Additional correlations among behavioral tests and among mitochondrial variables are presented in Supplementary Figs. 6 and 7.
Fig. 8.
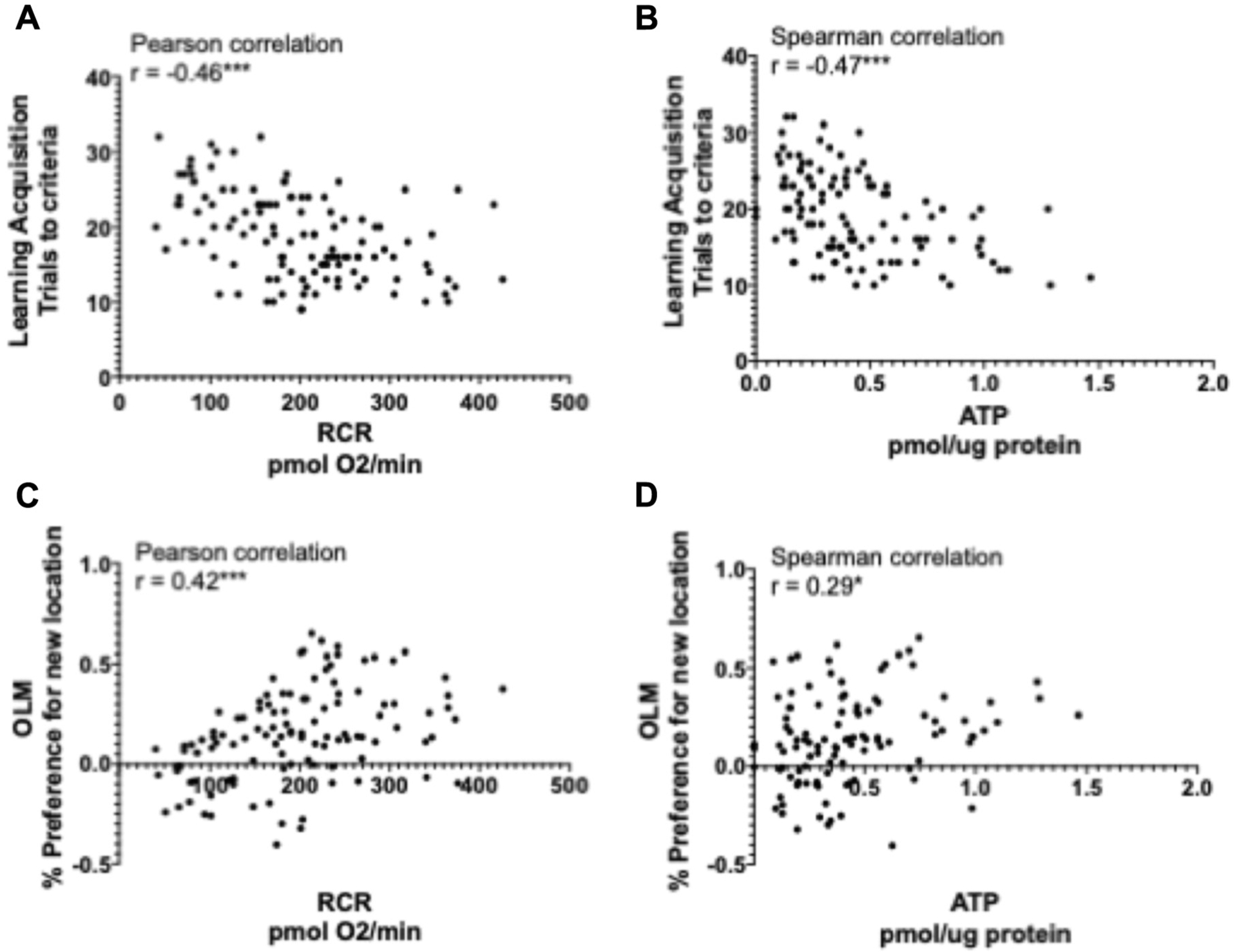
Hippocampal mitochondrial function is correlated with cognitive performance. Correlations between variables were determined by Pearson correlation for the normally distributed RCR values (A, C) and Spearman correlation as a nonparametric alternative for ATP (B, D) *p < 0.05, ***p < 0.001; n = 144. Abbreviations: OLM, object location memory; RCR, respiratory control ratio.
Interestingly when age was controlled for in the correlations and WT mice and KO mice were analyzed separately, these associations were altered. In WT animals (Table 2) RCR was still significantly correlated to trials to reach criteria in the learning test (r = −0.35, p < 0.01) and there was a similar trend toward a correlation with the 2-hour preference (r = 0.24, p > 0.05); however, RCR was not significantly correlated with cognitive outcome in NRF2KO mice (Table 3). When controlling for age, ATP content was not significantly correlated with cognitive outcome in WT or NRF2KO mice.
Table 2.
WT animal correlations adjusted for age
| WT mice | ||
|---|---|---|
| Learning acquisition | OLM 2 h % preference | |
| RCR | −0.35a | 0.24 |
| ATP | −0.10 | 0.03 |
Partial correlations adjusting for age between variables were determined by Pearson correlation for the normally distributed RCR values and Spearman correlation as a nonparametric alternative for ATP. Correlation coefficients (r) are given.
Key: OLM, object location memory; RCR, respiratory control ratio; WT, wild type.
p < 0.01; n = 51–55.
Table 3.
NRF2KO animal correlations adjusted for age
| NRF2KO mice | ||
|---|---|---|
| Learning acquisition | OLM 2 h % preference | |
| RCR | −0.07 | 0.09 |
| ATP | −0.10 | 0.010 |
Partial correlations adjusting for age between variables were determined by Pearson correlation for the normally distributed RCR values and Spearman correlation as a nonparametric alternative for ATP. Correlation coefficients (r) are given. n = 56–57.
Key: OLM, object location memory; NRF2KO, nuclear factor erythroid 2-related factor 2 knockout; RCR, respiratory control ratio.
4. Discussion
In the aging brain increased oxidative stress, resulting from a combination of free radical accumulation and diminished endogenous antioxidant defenses, is believed to contribute to bioenergetics and synaptic dysfunction as well as cognitive impairment (Franceschi and Campisi, 2014; Raz, 2018). This has led to a growing interest in therapies that target the NRF2-regulated antioxidant response pathway (Schmidlin et al., 2019). Here we evaluate one such potential therapy, CAW, exploring the cognitive and mitochondrial consequences of long-term treatment during aging as well as the requirement of NRF2 for those effects.
We found that age-related declines in spatial memory and learning were attenuated with exposure to water containing CAW 2 g/L in 18-month-old WT mice but not in age-matched NRF2KO mice. Although differences in water intake per cage of mice were not different between genotypes, it is important to note that the exact amount of CAW consumed by each individual animal was not measured in this study. This could account for some of the variabilities of responses seen within the CAW-treated groups. Future studies utilizing more stringent dosing methods to ensure more equal consumption, confirmed by monitoring of CAW compounds in the blood, would be able to address this issue. CAW treatment did not significantly affect cognitive performance in either of the younger (6 or 12 months) cohorts. This is in line with our previous work showing that 2 weeks of CAW treatment improves spatial memory in 20 months but not 2-month-old mice and that in aged mice CAW also enhances learning (Gray etal., 2016, 2018b). Notably, the amount of cognitive improvement observed in those studies was quite similar to what was seen here following 13 months of treatment. This would suggest that while prolonged CAW exposure does not appear to enhance the cognitive response relative to short-term treatment, it did not result in a diminished response that could have indicated a tolerance to the extract.
The cognitive enhancing effects of CAW observed at 18 months are likewise in line with previous reports of cognitive enhancing effects of constituent compounds from within the extract. CAW contains a complex mixture of compounds, including triterpenes, caffeoylquinic acids (CQAs), and other polyphenols, many of which have demonstrated cognitive enhancing properties. Asiatic acid is one of the signature triterpene compounds found in C. asiatica and CAW (Gray et al., 2014, 2018a; Siddiqui, 2007) and has repeatedly been shown to improve cognitive function in models of healthy aging as well as chemically induced cognitive impairment (Chaisawang et al., 2017; Loganathan and Thayumanavan, 2018; Nasiretal., 2011; Sirichoat et al., 2015; Umka Welbatetal., 2016; Xu et al., 2012). Polyphenols have likewise been reported to result in improvements in OLM performance (Carey et al., 2014; Matsui et al., 2009) and a recent study demonstrated that CQA treatment improves learning and memory deficits in a mouse model of accelerated brain aging (Sasaki et al., 2019).
In this study, we found that spatial learning declined with age in both genotypes but CAW-treated WT mice showed significant improvements in OLM performance relative to NRF2KO mice. We likewise saw a decline in learning with age in both genotypes, and by 18 months NRF2KO mice displayed poorer performance in learning acquisition test than their age-matched WT counterparts. This finding is in keeping with several recent studies implicating a role for NRF2 in aging and neurodegenerative disease. NRF2 localization and activity has been reported to be aberrant in both the Alzheimer’s and Parkinson’s disease brain which is believed to be associated with neurodegeneration observed (Ramsey et al., 2007). In the context of aging, ShRNA-mediated knockdown of NRF2 in the SAMP8 mouse model of accelerated aging exacerbated impairments in spatial and recognition memory (Ren et al., 2018). Similarly, NRF2 deletion has been shown to exacerbate cellular senescence and results in impaired memory performance in aged mice (Fulop et al., 2018; Gergues et al., 2018). In fact our own lab recently reported that 20-month-old NRF2KO mice have greater deficits in spatial memory and learning than aged-matched WT mice (Zweig et al., 2019). The current study builds on that previous work looking at a single time point by investigating the rate of cognitive decline in NRF2KO mice.
Declines in mitochondrial function as well as number are also known to occur in the aging brain (Sun et al., 2016). These mitochondrial changes in the brain are believed to precede and perhaps even induce cognitive decline in mouse models of aging and neurodegenerative disease (Forster, 1996; Yao J, 2009). In Alzheimer’s disease changes in mitochondrial dynamics have been shown to be early events, along with synaptic degeneration (Reddy et al., 2012). Additionally, it has been reported that β-amyloid localizes to mitochondrial membranes disrupting ETC activity, increasing ROS production and resulting in neuronal damage that leads to cognitive decline in that disease (Reddy and Beal, 2008). The correlation data from this study support a link between mitochondrial and cognitive function in the context of healthy aging. We observed that hippocampal ATP content and RCR were each significantly correlated with spatial memory and learning. However, with the exception of the correlation between RCR and learning acquisition in WT mice, these associations disappeared when adjusting for age, further underscoring that the relationship is primarily driven by aging.
In addition to observing a decline in mitochondrial function with age, we saw a parallel decrease in mitochondrial gene expression. The hippocampal expression of genes encoding enzymes in the ETC as well as the RCR, a metric of overall mitochondrial health, in isolated hippocampal mitochondria both diminished with age in all mice. There was no effect of CAW treatment on mitochondrial gene expression at any age, regardless of genotype. These results are in contradistinction to our previous reports of the effects of short-term CAW treatment in aged animals where we have consistently observed that 2 weeks of CAW treatment significantly increased hippocampal ETC gene expression in both male and female mice (Gray et al., 2016, 2018b). However, it is necessary to be cautious about making broader interpretations of these results as the present study only evaluated a small number of ETC genes. The ones selected (Mt-ND1, Mt-CO1, and Mt-ATP6) were chosen because they encode distinct enzymes in the ETC (complexes I, IV, and V respectively). The fact that we see similar effects of age, treatment, and the NRF2KO on the expression of each of these genes suggests that these effects are not restricted to one specific ETC complex. However, future work including analysis of a larger number of mitochondrial genes would be necessary to confirm this hypothesis and provide a more complete picture of the mitochondrial gene expression changes.
There was also no discernable effect of CAW on RCR at any age in either NRF2KO or WT mice. However by 18 months CAW-treated WT animals did have a significantly higher RCR than either control or CAW-treated NRF2KO animals. There was a similar, but nonsignificant, trend toward higher RCR in the control-treated WT animals relative to the NRF2KO animals. This trend is in line with our recent study in 20-month-old NRF2KO mice in which we found a similar impairment in hippocampal mitochondrial function tive to age-matched WT mice (Zweig et al., 2019) and with a recent study which found that mitochondrial respiration was decreased in the muscle of aged NRF2KO mice (Kitaoka et al., 2019). Conversely, NRF2 activating compounds have been shown to enhance mitochondrial function. Long-term treatment with highly purified olive oil improved spatial working memory and increased brain ATP levels (Reutzel et al., 2018). NRF2 was not specifically implicated in this study but monounsaturated fatty acids, which are abundant in olive oil, are well-established NRF2 activators (Cui et al., 2016; Wang et al., 2013). Asiatic acid and CQAs have also been shown to activate NRF2 (Fan et al., 2018; Gray et al., 2014, 2015; Meng et al., 2019) and treatment with asiatic acid and a CQA-rich sweet potato extract have each similarly been shown to affect brain energy metabolism, although again the role of NRF2 was not investigated (Nataraj et al., 2017; Sasaki et al., 2019). There are many mechanisms by which NRF2 can be activated including but not limited to induction of nuclear translocation by direct disruption with its cellular chaperone KEAP1 or phosphorylation of NRF2 at specific sites, or transcriptional alteration of either NRF2 or KEAP1 expression (Silva-Islas and Maldonado, 2018). It is likely that many different NRF2 activating compounds within CAW act by a variety of mechanisms. Studies are underway in our lab to explore the effects of individual compounds from CAW on NRF2 activation as well as their mode of activation and also their effects on mitochondrial activity and cognitive function.
The relatively subtle effect of CAW treatment on WT brain mitochondrial function was somewhat surprising. This is our first report of the effect of CAW on brain mitochondrial function in aged animals, so it is unknown whether the relatively subtle effects of long-term CAW treatment on in vivo mitochondrial bioenergetics observed here reflect a diminished response to prolonged exposure, or if CAW exposure for any duration does not have much of an effect on hippocampal mitochondrial bioenergetics. However, our previous study in the brains of β-amyloid overexpressing mice suggests that 2 weeks of CAW treatment does improve mitochondrial function at least in that pathological context (Gray et al., 2018c) which is in line with a recent report that likewise shows that NRF2 activation can improve metabolic deficits resulting from β-amyloid exposure (Sotolongo et al., 2020).
We also saw a pattern of decreased expression of the mitochondrial genes Mt-ND1 and Mt-CO1 with age in both genotypes. Although the effects of CAW treatment on WT animals were not apparent in the younger animals, at 18 months there was a significant reduction in the expression of these genes in both groups of NRF2KO mice compared to CAW-treated WT. To our knowledge, this is the first report of how loss of NRF2 affects hippocampal mitochondrial endpoints during aging and the exact link between NRF2 and mitochondrial endpoints is not well understood. This is particularly the case with regard to the link between NRF2 expression and mitochondrial gene expression. NRF2KO animals have been described as having lower levels of mt-DNA globally (Abdullah et al., 2012) and there does appear to be a link between declining NRF2 expression during aging and damage to mt-DNA which could lead to decreased expression (Li et al., 2018) which may be exacerbated in the complete absence of NRF2. It is also possible that the greater decrease in mitochondrial gene expression is due to the participation of NRF2 in the regulation of mitochondrial biogenesis. NRF2 activation has been shown to modulate the expression of ETC components as well as the expression of other regulators of biogenesis including PGC1a, PPARy, and Sirt1 (Abdullah et al., 2012; Cho et al., 2010; Huang et al., 2017; Lai et al., 2014) and so it is possible that without the input from NRF2 there was a greater decline in expression. It has been reported that NRF2 deficiency impairs skeletal muscle biogenesis and dynamics and this phenotype was most apparent in aged mice (Huang et al., 2019). However, further work is necessary to definitively identify the mechanistic link.
5. Conclusions
This is the first study to explore the cognitive and mitochondrial consequences of prolonged CAW treatment during aging and the role of the antioxidant regulatory transcription factor NRF2 in these effects. We found that while improvements in cognitive function were evident in WT mice that had been treated with the extract for 13 months, it was not of a greater magnitude than what we have reported previously following 2 weeks of CAW treatment. These cognitive enhancing effects were lost in mice that did not express NRF2. There were no robust effects of long-term CAW treatment on mitochondrial function in either genotype. Interestingly, NRF2KO mice displayed accelerated age-related cognitive deficits and exacerbated mitochondrial impairments at 18 months of age. However, due to the relatively small size of some of the groups it will be important to include these conditions in future experiment to replicate the results. Yet taken together the data presented here do suggest that NRF2 plays an important role in maintaining brain bioenergetics and cognitive function during aging and may be a useful target for anti-aging interventions. Furthermore, because cognitive impairment also accompanies oxidative stress and mitochondrial dysfunction in many neurological conditions, NRF2 activation, by CAW or other compounds, could have therapeutic relevance beyond healthy aging.
Supplementary Material
Acknowledgements
This work was funded by NIH-NCCIH grant R00AT008831 (NEG), NIH-NCCIH grant R01AT008099 (AS), NIH-NCCIH 5T32AT002688 on which KMW was a trainee, and a Department of Veterans Affairs Merit Review grant awarded to JFQ.
Footnotes
Disclosure statement
The authors report no conflicts of interest.
Declarations of interest: none.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neurobiolaging.2020.11.019.
References
- Abdullah A, Kitteringham NR, Jenkins RE, Goldring C, Higgins L, Yamamoto M, Hayes J, Park BK, 2012. Analysis of the role of Nrf2 in the expression of liver proteins in mice using two-dimensional gel-based prote-omics. Pharmacol. Rep 64, 680–697. [DOI] [PubMed] [Google Scholar]
- Brinkhaus B, Schuppan D, Hahn EG, 2000. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica. Phytomedicine 7, 427–448. [DOI] [PubMed] [Google Scholar]
- Buckner R, 2004. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron 44, 195–208. [DOI] [PubMed] [Google Scholar]
- Cansino S, 2009. Episodic memory decay along the adult lifespan: a review of behavioral and neurophysiological evidence. Int. J. Psychophysiol 71, 64–69. [DOI] [PubMed] [Google Scholar]
- Carey AN, Gomes SM, Shukitt-Hale B, 2014. Blueberry supplementation improves memory in middle-aged mice fed a high-fat diet. J. Agric. Food Chem 62, 3972–3978. [DOI] [PubMed] [Google Scholar]
- Chaisawang P, Sirichoat A, Chaijaroonkhanarak W, Pannangrong W, Sripanidkulchai B, Wigmore P, Welbat JU, 2017. Asiatic acid protects against cognitive deficits and reductions in cell proliferation and survival in the rat hippocampus caused by 5-fluorouracil chemotherapy. PLoS One 12, e0180650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Gladwell W, Wang X, Chorley B, Bell D, Reddy SP, Kleeberger SR, 2010. Nrf2-regulated PPAR{gamma} expression is critical to protection against acute lung injury in mice. Am. J. Respir. Crit. Care Med 182,170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Wang Q, Yi X, Zhang X, 2016. Effects of fatty acids on CYP2A5 and Nrf2 expression in mouse primary hepatocytes. Biochem. Genet 54, 29–40. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova A, Abramov AY, 2015. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med 88 (PtB), 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Chen Q, Wei L, Zhou X, Wang R, Zhang H, 2018. Asiatic acid ameliorates CCl4-induced liver fibrosis in rats: involvement of Nrf2/ARE, NF-kappaB/IkappaBalpha, and JAK1/STAT3 signaling pathways. Drug Des. Devel Ther 12, 3595–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster M, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS, 1996. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc. Natl. Acad. Sci. U S A 93, 4765–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Campisi J, 2014. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A. Biol. Sci. Med. Sci 69 (Suppl 1), S4–S9. [DOI] [PubMed] [Google Scholar]
- Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A, 2018. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience 40, 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergues MM, Moiseyenko A, Saad SZ, Kong AN, Wagner GC, 2018. Nrf2 deletion results in impaired performance in memory tasks and hyperactivity in mature and aged mice. Brain Res 1701,103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NE, Alcazar Magana A, Lak P, Wright KM, Quinn J, Stevens JF, Maier CS, Soumyanath A, 2018a. Centella asiatica - phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem. Rev 17,161–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NE, Harris CJ, Quinn JF, Soumyanath A, 2016. Centella asiatica modulates antioxidant and mitochondrial pathways and improves cognitive function in mice. J. Ethnopharmacol 180, 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NE, Morre J, Kelley J, Maier CS, Stevens JF, Quinn JF, Soumyanath A, 2014. Caffeoylquinic acids in Centella asiatica protect against amyloid-beta toxicity. J. Alzheimers Dis 40, 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NE, Sampath H, Zweig JA, Quinn JF, Soumyanath A, 2015. Centella asiatica attenuates amyloid-beta-induced oxidative stress and mitochondrial dysfunction. J. Alzheimers Dis 45, 933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NE, Zweig JA, Caruso M, Martin MD, Zhu JY, Quinn JF, Soumyanath A, 2018b. Centella asiatica increases hippocampal synaptic density and improves memory and executive function in aged mice. Brain Behav 8, e01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NE, Zweig JA, Caruso M, Zhu JY, Wright KM, Quinn JF, Soumyanath A, 2018c. Centella asiatica attenuates hippocampal mitochondrial dysfunction and improves memory and executive function in beta-amyloid overexpressing mice. Mol. Cell Neurosci 93, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm A, Eckert A, 2017. Brain aging and neurodegeneration: from a mitochondrial point of view. J. Neurochem 143, 418–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DD, Fan SD, Chen XY, Yan XL, Zhang XZ, Ma BW, Yu DY, Xiao WY, Zhuang CL, Yu Z, 2019. Nrf2 deficiency exacerbates frailty and sarcopenia by impairing skeletal muscle mitochondrial biogenesis and dynamics in an age-dependent manner. Exp. Gerontol 119, 61–73. [DOI] [PubMed] [Google Scholar]
- Huang K, Gao X, Wei W, 2017. The crosstalk between Sirt1 and Keap1/Nrf2/ARE anti-oxidative pathway forms a positive feedback loop to inhibit FN and TGF-beta1 expressions in rat glomerular mesangial cells. Exp. Cell Res 361, 63–72. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y, 1997. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun 236, 313–322. [DOI] [PubMed] [Google Scholar]
- Iuso A, Repp B, Biagosch C, Terrile C, Prokisch H, 2017. Assessing mitochondrial bioenergetics in isolated mitochondria from various mouse tissues using Seahorse XF96 analyzer. Methods Mol. Biol 1567, 217–230. [DOI] [PubMed] [Google Scholar]
- Jiang JZ, Ye J, Jin GY, Piao HM, Cui H, Zheng MY, Yang JS, Che N, Choi YH, Li LC, Yan GH, 2017. Asiaticoside mitigates the allergic inflammation by abrogating the degranulation of mast cells. J. Agric. Food Chem 65, 8128–8135. [DOI] [PubMed] [Google Scholar]
- Johnson S, Sacks PK, Turner SM, Gaynor LS, Ormerod BK, Maurer AP, Bizon JL, Burke SN, 2016. Discrimination performance in aging is vulnerable to interference and dissociable from spatial memory. Learn Mem 23, 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaoka Y, Tamura Y, Takahashi K, Takeda K, Takemasa T, Hatta H, 2019. Effects of Nrf2 deficiency on mitochondrial oxidative stress in aged skeletal muscle. Physiol. Rep 7, e13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MHV, 2002. Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. J. Ethnopharmacol 79, 253–260. [DOI] [PubMed] [Google Scholar]
- Lai L, Wang M, Martin OJ, Leone TC, Vega RB, Han X, Kelly DP, 2014. A role for peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1) in the regulation of cardiac mitochondrial phospholipid biosynthesis. J. Biol. Chem 289, 2250–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhao X, Hu Y, Sun H, He Z, Yuan J, Cai H, Sun Y, Huang X, Kong W, Kong W, 2018. Age-associated decline in Nrf2 signaling and associated mtDNA damage may be involved in the degeneration of the auditory cortex: implications for central presbycusis. Int. J. Mol. Med 42, 3371–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Li G, Tang H, Pan R, Wang H, Jin F, Yan X, Xing Y, Chen G, Fu Y, Dong J, 2019. Madecassoside ameliorates lipopolysaccharide-induced neurotoxicity in rats by activating the Nrf2-HO-1 pathway. Neurosci. Lett 709, 134386. [DOI] [PubMed] [Google Scholar]
- Loganathan C, Thayumanavan P, 2018. Asiatic acid prevents the quinolinic acid-induced oxidative stress and cognitive impairment. Metab. Brain Dis 33, 151–159. [DOI] [PubMed] [Google Scholar]
- Ma Q, He X, 2012. Molecular basis of electrophilic and oxidative defense: promises and perils of Nrf2. Pharmacol. Rev 64, 1055–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkutewicz I, Kurowska E, Podlacha M, Myslinska D, Grembecka B, Rucinski J, Pierzynowska K, Wrona D, 2018. Age-dependent effects of dimethyl fumarate on cognitive and neuropathological features in the streptozotocin-induced rat model of Alzheimer’s disease. Brain Res 1686,19–33. [DOI] [PubMed] [Google Scholar]
- Majkutewicz I, Kurowska E, Podlacha M, Myślińska D, Grembecka B, Ruciński J, Plucińska K, Jerzemowska G, Wrona D, 2016. Dimethyl fumarate attenuates intracerebroventricular streptozotocin-induced spatial memory impairment and hippocampal neurodegeneration in rats. Behav. Brain Res 308, 24–37. [DOI] [PubMed] [Google Scholar]
- Mather M, Jacobsen LA, Pollard KM, 2015. Aging in the United States Population Reference Bureau. [Google Scholar]
- Matsui N, Takahashi K, Takeichi M, Kuroshita T, Noguchi K, Yamazaki K, Tagashira H, Tsutsui K, Okada H, Kido Y, Yasui Y, Fukuishi N, Fukuyama Y, Akagi M, 2009. Magnolol and honokiol prevent learning and memory impairment and cholinergic deficit in SAMP8 mice. Brain Res 1305, 108–117. [DOI] [PubMed] [Google Scholar]
- Matthews DG, Caruso M, Murchison CF, Zhu JY, Wright KM, Harris CJ, Gray NE, Quinn JF, Soumyanath A, 2019. Centella asiatica improves memory and promotes antioxidative signaling in 5XFAD mice. Antioxidants (Basel) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Arumugam TV, 2018. Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metab 27,1176–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Li HY, Si CY, Liu YZ, Teng S, 2019. Asiatic acid inhibits cardiac fibrosis throughNrf2/HO-1 and TGF-beta1/Smads signaling pathways in spontaneous hypertension rats. Int. Immunopharmacol 74, 105712. [DOI] [PubMed] [Google Scholar]
- Methqal I, Provost JS, Wilson MA, Monchi O, Amiri M, Pinsard B, Ansado J, Joanette Y, 2017. Age-related shift in neuro-activation during a word-matching task. Front Aging Neurosci 9, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M, 2004. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med 10, 549–557. [DOI] [PubMed] [Google Scholar]
- Nasir MN, Habsah M, Zamzuri I, Rammes G, Hasnan J, Abdullah J, 2011. Effects of Asiatic acid on passive and active avoidance task in male Sprague-Dawley rats. J. Ethnopharmacol 134, 203–209. [DOI] [PubMed] [Google Scholar]
- Nataraj J, Manivasagam T, Justin Thenmozhi A, Essa MM, 2017. Neurotrophic effect of Asiatic acid, a triterpene of Centella asiatica against chronic 1-methyl 4- phenyl 1, 2, 3, 6-tetrahydropyridine hydrochloride/probenecid mouse model of Parkinson’s disease: the role of MAPK, PI3K-Akt-GSK3beta and mTOR signalling pathways. Neurochem. Res 42, 1354–1365. [DOI] [PubMed] [Google Scholar]
- Park D, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK, 2002. Models of visuospatial and verbal memory across the adult life span. Psychol. Aging 17, 299–320. [PubMed] [Google Scholar]
- Ponnusamy K, Mohan M, Nagaraja HS, 2008. Protective antioxidant effect of Centella asiatica bioflavonoids on lead acetate induced neurotoxicity. Med. J. Malaysia 63 (Suppl A), 102. [PubMed] [Google Scholar]
- Prakash A, 2013. Mitoprotective effect of Centella asiatica against aluminum- induced neurotoxicity in rats: possible relevance to its anti-oxidant and anti-apoptosis mechanism. Neurol. Sci 34, 1403–1409. [DOI] [PubMed] [Google Scholar]
- Ramsey CP, Glass CA, Montgomery MB, Lindl KA, Ritson GP, Chia LA, Hamilton RL, Chu CT, Jordan-Sciutto KL, 2007. Expression of Nrf2 in neurodegenerative diseases. J. Neuropathol. Exp. Neurol 66, 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Daugherty AM, 2018. Pathways to brain aging and their modifiers: free-radical-induced energetic and neural decline in senescence (friends) model - a mini-review. Gerontology 64, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Beal MF, 2008. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med 14, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, Shirendeb UP, Calkins MJ, Reddy AP, Mao P, Manczak M, 2012. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: implications to mitochondria-targeted antioxidant therapeutics. Biochim. Biophys. Acta 1822, 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren HL, Lv CN, Xing Y, Geng Y, Zhang F, Bu W, Wang MW, 2018. Down-regulated nuclear factor E2-related factor 2 (Nrf2) aggravates cognitive impairments via neuroinflammation and synaptic plasticity in the senescence-accelerated mouse prone 8 (SAMP8) mouse: a model of accelerated senescence. Med. Sci. Monit 24, 1132–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutzel M, Grewal R, Silaidos C, Zotzel J, Marx S, Tretzel J, Eckert GP, 2018. Effects of long-term treatment with a blend of highly purified olive secoiridoids on cognition and brain ATP levels in aged NMRI mice. Oxid Med. Cell Longev 2018, 4070935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer PR, Rudraiah S, Goedken MJ, Manautou JE, 2014. Is nuclear factor erythroid 2-related factor 2 responsible for sex differences in susceptibility to acetaminophen-induced hepatotoxicity in mice? Drug Metab. Dispos 42, 1663–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Davies J, Doldan NG, Arao S, Ferdousi F, Szele FG, Isoda H, 2019. 3,4,5-Tricaffeoylquinic acid induces adult neurogenesis and improves deficit of learning and memory in aging model senescence-accelerated prone 8 mice. Aging (Albany NY) 11, 401–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidlin CJ, Dodson MB, Madhavan L, Zhang DD, 2019. Redox regulation by NRF2 in aging and disease. Free Radic. Biol. Med 134, 702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger DR, Li D, Jaminet SC, Cao S, 2016. Activation of the Nrf2 cell defense pathway by ancient foods: disease prevention by important molecules and microbes lost from the modern western diet. PLoS One 11, e0148042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomol GK, 2008a. Effect of Centella asiatica leaf powder on oxidative markers in brain regions of prepubertal mice in vivo and its in vitro efficacy to ameliorate 3-NPA-induced oxidative stress in mitochondria. Phytomedicine 15, 971–984. [DOI] [PubMed] [Google Scholar]
- Shinomol GK, 2008b. Prophylactic neuroprotective property of Centella asiatica against 3-nitropropionic acid induced oxidative stress and mitochondrial dysfunctions in brain regions of prepubertal mice. Neurotoxicology 29, 948–957. [DOI] [PubMed] [Google Scholar]
- Shinomol GK, Muralidhara, Bharath MM, 2011. Exploring the role of “Brahmi” (Bacopa monnieri and Centella asiatica) in brain function and therapy. Recent Pat Endocr. Metab. Immune Drug Discov 5, 33–49. [DOI] [PubMed] [Google Scholar]
- Siddiqui BS, Aslam H, Ali ST, Khan S, Begum S, 2007. Chemical constituents of Centella asiatica. J. Asian Nat. Prod. Res 9, 407–414. [DOI] [PubMed] [Google Scholar]
- Silva-Islas CA, Maldonado PD, 2018. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res 134, 92–99. [DOI] [PubMed] [Google Scholar]
- Sirichoat A, Chaijaroonkhanarak W, Prachaney P, Pannangrong W, Leksomboon R, Chaichun A, Wigmore P, Welbat JU, 2015. Effects of Asiatic acid on spatial working memory and cell proliferation in the adult rat hippocampus. Nutrients 7, 8413–8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotolongo K, Ghiso J, Rostagno A, 2020. Nrf2 activation through the PI3K/GSK-3 axis protects neuronal cells from Abeta-mediated oxidative and metabolic damage. Alzheimers Res. Ther 12,13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Youle RJ, Finkel T, 2016. The mitochondrial basis of aging. Mol. Cell 61, 654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umka Welbat J, Sirichoat A, Chaijaroonkhanarak W, Prachaney P, Pannangrong W, Pakdeechote P, Sripanidkulchai B, Wigmore P, 2016. Asiatic acid prevents the deleterious effects of valproic acid on cognition and hippocampal cell proliferation and survival. Nutrients 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vomhof-Dekrey EE, Picklo MJ Sr., 2012. The Nrf2-antioxidant response element pathway: a target for regulating energy metabolism. J. Nutr. Biochem 23, 1201–1206. [DOI] [PubMed] [Google Scholar]
- Wang R, Paul VJ, Luesch H, 2013. Seaweed extracts and unsaturated fatty acid constituents from the green alga Ulva lactuca as activators of the cytoprotective Nrf2-ARE pathway. Free Radic. Biol. Med 57, 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SF, Ji LL, Wu Q, Li J, Liu J, 2018. Ontogeny and aging of Nrf2 pathway genes in livers of rats. Life Sci 203, 99–104. [DOI] [PubMed] [Google Scholar]
- Xu MF, Xiong YY, Liu JK, Qian JJ, Zhu L, Gao J, 2012. Asiatic acid, a pentacyclic triterpene in Centella asiatica, attenuates glutamate-induced cognitive deficits in mice and apoptosis in SH-SY5Y cells. Acta Pharmacol. Sin 33, 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Xu Y, Hu Y, Luo Y, Lu X, Tsui CK, Lu L, Liang X, 2016. Madecassic acid protects against hypoxia-induced oxidative stress in retinal microvascular endothelial cells via ROS-mediated endoplasmic reticulum stress. Biomed. Pharmacother 84, 845–852. [DOI] [PubMed] [Google Scholar]
- Yao J, I. R, Zhao L, Nilsen J, Hamilton RT, Brinton RD, 2009. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci 106, 14670–14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng KDX, Xiao N, Wu X, Wei Z, Fang W, Zhu Y, Zhang J, Chen X, 2017. Curcumin ameliorates memory decline via inhibiting BACE1 expression and beta-amyloid pathology in 5× FAD transgenic mice. Mol. Neurobiol 54, 1967–1977. [DOI] [PubMed] [Google Scholar]
- Zweig JA, Caruso M, Brandes MS, Gray NE, 2019. Loss of NRF2 leads to impaired mitochondrial function, decreased synaptic density and exacerbated age-related cognitive deficits. Exp. Gerontol [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


