Abstract
The synthetic utility of aryl radicals has been established in the last century, however, their broad applications were hampered by ineffective generation methods. It was in the last decade, that a rapid development of various redox systems took place, thus trigerring a renaissance of ary radical chemistry. This tutorial review focuses on the start-of-the-art methods for generation of aryl radicals. Primarily, various light-induced systems, including photoredox catalysis, visible light transition metal catalysis,and chemistry of electron donor-acceptor complexes, are reviewed. The main current precursors of aryl radicals are evaluated together with the selected examples of their modern applications.
1. Introduction
Since the first introduction of Meerwein reaction1 (arylation of an alkene using diazonium salts), aryl radicals were proven to be versatile intermediates in organic synthesis, particularly in functional group interconversion and C–C bond-forming reactions (Fig. 1).2 Classically, generation of aryl radical was based on the use of stoichiometric reagents, such as AIBN/n-Bu3SnH for aryl halides3 or stoichiometric transition metal reductants for diazonium salts4. These methods suffer from vast undesired by-product formation, as well as low efficiency, which substantially limit their employment in organic synthesis. However, with the development of novel redox systems, primarily light-induced methodologies5, an interest in generation and employment of aryl radicals has been reignited. Novel catalytic protocols were developed, which made known chemistry much more practical and efficient. Moreover, new aryl radical precursors were recognized, expanding known transformations onto new classes of molecules. As a result, synthetic strategies relying on aryl radicals gained major recognition within the synthetic community.
Fig. 1.
Generation and transformations of aryl radicals.
Furthermore, it was demonstrated, that aryl radicals could be used not only for functionalization of arenes, but also to perform more challenging transformations, such as C–H functionalization reactions.6 The basis for this reactivity lies in the ability of aryl radical to perform efficient hydrogen atom transfer (HAT) step. This process is highly thermodynamically favoured based on the comparison of the bond-dissociation energy (BDE) of aromatic C(sp2)–H bond versus that of alkyl C(sp3)–H bond (Fig. 2).7 Thus, from thermodynamic standpoint, aryl radicals should be able to abstract hydrogen atom from strong unactivated C(sp3)-H bonds.8 Another important feature of aryl radicals is their relative ambiphilicity, which makes them efficient for abstracting hydrogens from both electron-rich and electro-poor C–H bonds. As described below, these properties found their applications in the newly developed synthetic methods.
Figure 2.
C–H bond-dissociation energy comparison.
It is apparent that many solutions developed for generation of aryl radical will have an impact on the field of redox chemistry in a broader sense, as these approaches may be used for generation of other types of radicals.
The aim of this tutorial review is to highlight major strategies for generation of aryl radicals developed in the last decade. A full spectrum of current aryl radical precursors is reviewed. The comparison between different precursors, as well as their advantages and disadvantages, are discussed. Major emphasis is placed on the mechanistic aspects of different methods and on how these aspects enable transformation in question.
2. Diazonium Salts
Diazonium salts are one among the oldest aryl radical sources known. Since publication of Meerwein arylation,1 many iterations of this transformation using variety of different reducing agents, primarily transition metals, have been developed.4 Diazonium salts are viewed as good aryl radical source for two reasons: first, availability of different aniline precursors for their synthesis; and second – its high reactivity due to low reduction potential (−0.16V vs SCE).9 Thus, it is not surprising that upon development of new redox systems, diazonium salts were among the first reagents to use.
2.1. Photoredox catalysis
Even though photo-catalyzed Pschorr cyclization has been known since 1984,10 only upon rapid development of photoredox catalysis in a past decade organic chemists have brought their attention towards development of more general photo-catalytic reactions of diazonium salts. Among first attempts is work by Sanford and co-workers published in 2011, where they have demonstrated a combination of photoredox and palladium catalysis for directed C–H arylation reaction (Scheme 1).11
Scheme 1.
Sanford’s directed arylation using diazonium salts.
This method relied on the generation of aryl radical via a single electron transfer from photo-excited ruthenium complex to diazonium salt – same as in the photo-catalyzed Pschorr cyclization mentioned before. Aryl radical addition to the palladium(II) complex 4 results in Pd(III) species 5. A single electron oxidation of 5 by ruthenium complex or another molecule of diazonium salt generates palladium(IV) complex 6, which, in turn, undergoes reductive elimination resulting in the formation of the product 3. This approach outlines ability of the photoredox chemistry to generate aryl radical under very mild conditions and compatibility with other known catalytic systems.
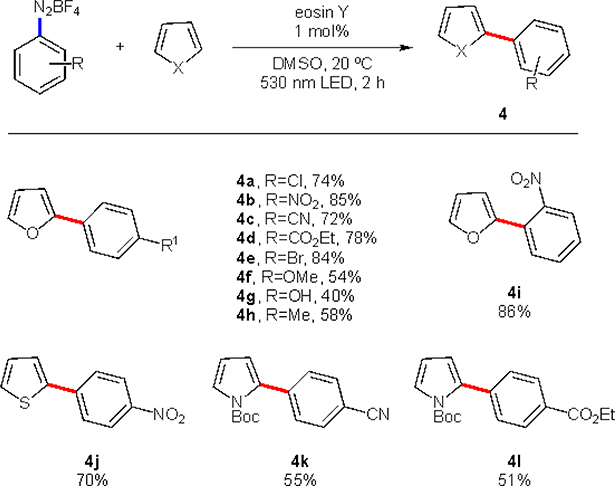
In 2012, König and co-workers reported eosin Y-catalyzed arylation of heteroarenes under green light irradiation.12 This method allows for C-H arylation of furans, pyrroles, and thiophenes with broad range of diazonium salts using just 1 molar percent of the photocatalyst (Scheme 2). Diazonium salts bearing electron-withdrawing groups typically perform best in radical chemistry. The same trend is observed in this work, where the salts bearing halo (4a, 4e), nitro (4b), cyano (4c), and ester (4d) groups in para position gave good-to-excellent yields in the reaction with furan. On the other hand, the reactions of more electron-rich salts, possessing methoxy (4f), hydroxy (4g), and alkyl (4h) substituents resulted in moderate outcomes. Mechanistically, this transformation is an example of oxidative quenching (Scheme 3). Upon photoexcitation of the catalyst under 530 nm irradiation, eosin Y engages in an SET with the diazonium salt 2, thus leading to aryl radical upon extrusion of dinitrogen. Since aryl radical possess enough electrophilicity,8 it is capable to add to electron rich systems, such as 5-membered heteroaromatics. After the C–C bond forming event, the formed radical 7 is oxidized by giving an electron to either eosin Y radical-cation or another molecule of diazonium salt. If latter occurs, that would be an example of a chain propagation event, one of the common routes in the photoredox chemistry. A subsequent loss of proton restores aromaticity, thus completing the product formation. This protocol showcases the ability of photo-redox systems to promote highly attractive C–C bond forming reactions. Naturally, such potential attracted chemists to develop more transformations based on this or similar systems.
Scheme 2.
König’s C–H arylation of heteroarenes.
Scheme 3.
Proposed mechanism for König’s C–H arylation of heteroarenes.
2.2. Reduction by nucleophilic bases
Generation of aryl radicals from diazonium salts using stoichiometric amounts of nucleophilic organic or inorganic bases has been known for almost hundred years. In 1924, Gomberg and Beckmann reported biaryl synthesis reaction of diazonium salts and benzene derivatives mediated by simple Brønsted base – hydroxide anion.13 Despite the use of stoichiometric amounts of the reductant, this approach is still relevant today. First of all, inorganic and organic bases are abundant and inexpensive. In addition, a great variety of such compounds is available. Thus, for particular conditions, an appropriate base can be identified to suit the system.
One of the modern applications of this strategy was reported by Gevorgyan and co-workers in 2019, which highlights a transition metal-free approach towards directed amination of alkyl C–H bonds of alcohols (Scheme 4, (a)).14 The authors used silicon based auxiliary-containing iodomethylene unit for this radical relay reaction. Upon reaction between diazonium salt and lithium formate, aryl radical is formed (Scheme 4, (b)), which then abstracts iodine atom from the auxiliary, thus resulting in a formation of an aryl iodide and methylene radical 10 adjacent to silicon. Such iodine atom transfer is guided by the same principles as HAT discussed in the introduction. Indeed, the BDE for C–I bond in iodobenze is 67 kcal/mol, while that C–I bond for primary alkyl iodide is around 57 kcal/mol only.7 The formed silylmethyl radical is primary and electrophilic. Consequently, it can engage in 1,n-HAT process effectively generating tertiary or secondary alkyl radicals at the aliphatic carbon chain of the alcohol. As in the case of iodine atom transfer, BDEs have essential role in the process, as BDE of the primary C–H bond is 2–3 kcal/mol higher than these for the secondary C–H and 4–5 kcal/mol for tertiary, respectively. In contrast to the silylmethyl radical 10, the formed transposed radical 11 is nucleophilic and, thus, is easily trapped by another equivalent of the diazonium salt.15 Upon completion of the process, alkyl aryl diazene product is formed. This protocol enabled synthesis of β- (9a-9i, 9l-m), γ- (9j) and δ- (9k) amino alcohols in moderate to good yields. Moreover, it was shown that this protocol can be used to transform molecules containing broad range of functional groups (9a-9g), thus demonstrating the potential for application in a more complex setting.
Scheme 4.
(a) Gevorgyan’s C–H amination reaction (b) Proposed mechanism.
It must be noted that the mechanism of reduction of diazonium salts with nucleophilic bases is not completely understood. It is generally believed that due to the high electrophilicity of the diazonium salts (2), a nucleophile could add to it to form diazene 12 (Scheme 5). This intermediate could either directly fragment into the aryl radical, dinitrogen gas and an oxygen centered radical (path a). Alternatively, aryl radical would form after a nucleophilic substitution and fragmentation of the formed diazo anhydride 13 (path b).16
Scheme 5.
Hypothetical patways towards aryl radicals from diazonium salts.
One of the new directions for this approach could be towards discovery of new single electron donors that, in addition to reduction, could participate in further steps. A great example of such avenue is oxyarylation of alkenes developed by Studer and co-workers in 2012 (Scheme 6).17 This transformation is build around SET process between TEMPONa reagent and a diazonium salt, which produces the desired aryl radical species, as well as a persistent TEMPO radical. The aryl radical readily adds across electron-rich double bond to produce a nucleophilic radical. The latter recombines with TEMPO, thus furnishing the carbooxygenation product 14. Selectivity and efficiency of this transformation is reinforced by the persistent radical effect. A broad range of diazonium salts can be used in this methodology (2a-i). With respect to the alkenes, styrenes turned to be the most effective radical acceptors (14g-r, 14u), which may be attributed to the formation of highly stabilized benzylic radicals upon addition process. Notably, good diastereoselectivity was obtained in the reaction of β-methylstyrene (14u).
Scheme 6.
(a) Studer’s oxyarylation of alkenes (b) Proposed mechanism.
2.3. Electrochemistry
Electrochemistry is one of the most powerful tools for performing electron transfers available to chemists.18 Naturally, there is an interest to apply electrochemistry for the development of robust diazonium salt transformations. In 2018, Mo and co-workers pursuing this goal reported an electrochemical version of the Sandmeyer reaction (Scheme 7 (a)).19
Scheme 7.
(a) Mo’s electrochemical Sandmeyer reaction (b) Upscale synthesis.
It was shown that tremendous range of aryl halides could be synthesized using platinum material for both cathode and anode. Efficiency of the process heavily varies depending on a substitution at the salt ranging from excellent (e.g. 15g) to poor (e.g. 15j). It is important to remember that for a particular target an optimal method could be adjusted accordingly. In addition, a gram-scale reaction was successfully performed pointing to the possibility for development of large, sustainable synthesis (Scheme 7 (b)). While electrochemistry is not widely applied to the transformations of diaznoium salt at this point, it may find broad application in future, particularly for the most challenging transformations.
2.4. Ball milling
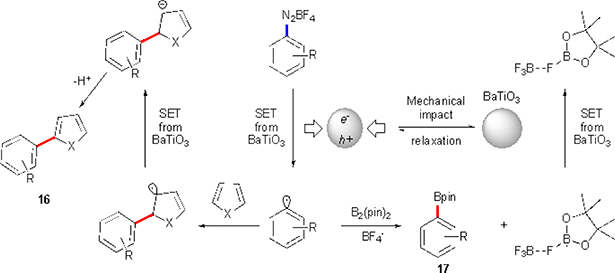
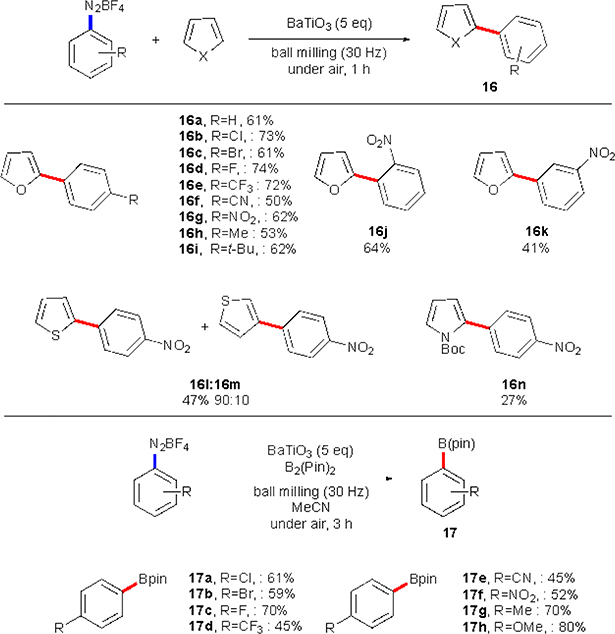
In 2019 Kubota, Ito and co-workers applied piezoelectric materials as an electron donors for the diazonium salts.20 This unprecedented methodology utilized a ball milling reaction setup in a combination with BaTiO3 nanoparticles to achieve C–H arylation of electron-rich heteroaromatics, as well as synthesis of aryl boronate esters. Xu and co-workers in 2010 studied water splitting mediated by BaTiO3 microfiber.21 In this work, nanoparticles were agitated by an ultrasound resulting in a polarization (piezoelectric effect) to induce a potential, which initiated water splitting. Ito, Kubota and co- workers postulated that mechanical impacts during the course of the milling could give similar polarization effect. Since water splitting is a very energy-demanding process (1.28V vs NHE), a potential generated should also be sufficient to reduce diazonium salts (Scheme 8). If successful, upon an electron transfer the dinitrogen extrusion would take place. The formed aryl radical would then add to a heterocycle or a bis(pinacolato)diborane in the same manner as seen in the previous works.
Scheme 8.
Proposed mechanism for Kubota’s and Ito’s C–H arylation and borylation reactions.
Indeed, it was demonstrated that a number of arylated heterocycles could be synthesized via this approach (Scheme 9). The scope of the diazonium salts successfully used in this transformation mostly matches that for alternative methodologies discussed previously. Borylation of the aryl diazonium salts was also achieved. Though, synthesis of mostly para-substituted compounds was demonstrated (17a-h). Perhaps the major shortcoming of the protocol is the use of a large excess of BaTiO3 nanoparticles (5 equivalents) despite its catalytic role in this transformation. However, the great strength of the protocol is the ease of almost complete recovery of the material. Moreover, the recovered nanoparticles could be reused multiple times without any additional reactivation procedures. Overall, this pioneering approach may significantly broaden utility of the mechanochemistry in organic synthesis, as now it can be viewed as a way of transforming mechanical energy into a redox potential for organic transformations. At this point, no other known approaches can achieve such energy conversion type.
Scheme 9.
Scope of Kubota’s and Ito’s C–H arylation and borylation reactions.
3. Aryl Halides
Although, as discussed above, diazonium salts are very potent sources for aryl radicals, they suffer several major drawbacks. These include their chemical instability and a limited scope of the synthesizable compounds available. The former issue makes some of these compounds difficult to handle, and thus reduces the practicality. Whereas the latter heavily limits their utility in the synthesis. Although a range of electron-poor to neutral aryl diazonium salts is routinely available, use of their electro-rich counterparts poses a great challenge. Functional group tolerance is also an issue, since diazonium moiety by itself is a highly reactive functionality. In this light, aryl halides pose as one of the possible alternatives due to a vast amount of compounds available, ease in synthesis, as well as higher stability compared to that of the diazonium salts. However, expectedly, the higher stability comes with the price of higher reduction potentials required for activation of these substrates.
3.1. Light Induced Homolysis
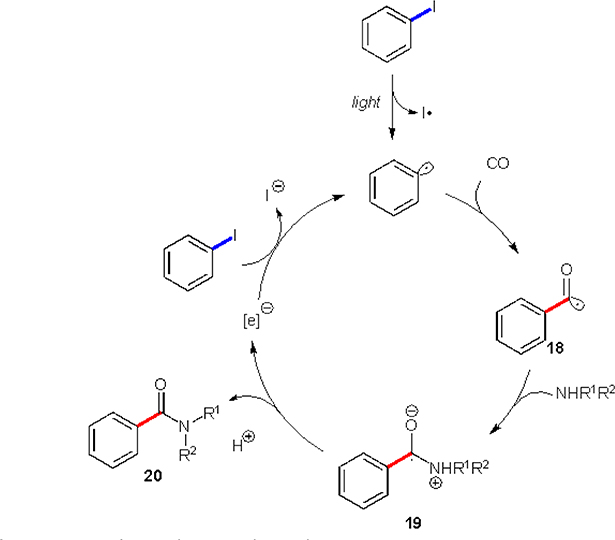
In 2015, Ryu and co-workers disclosed the possibility of aryl radical generation from corresponding iodides without any stoichiometric reductants or catalysts.22 Their proposal was based on the ability of UV light to trigger homolysis of the C–I bond in certain aryl iodides (Scheme 10). The authors hypothesized, that formed aryl radical would add to carbon monoxide, forming acyl radical 18, followed by a nucleophilic attack by amine resulting in a zwitterionic radical intermediate 19. These two steps have been previously reported to proceed under different conditions. In this work, the authors explored a radical chain process, where intermediate 19 acts as an electron donor to another molecule of aryl iodide, thus closing the cycle.
Scheme 10.
Ryu’s mechanistic hypothesis.
This approach allowed for efficient aminocarbonylation for a range of aryl iodides under high pressure of carbon monoxide (Scheme 11). Although this method is rather limited from synthetic standpoint, this work sends an important message that in certain systems a direct high energy input (such as photons in this case) is sufficient to trigger an overall highly valuable transformation without any additional chemical reagents.
Scheme 11.
Ryu’s scope of photoinduced aminocarbonylation.
3.2. Photoredox catalysis
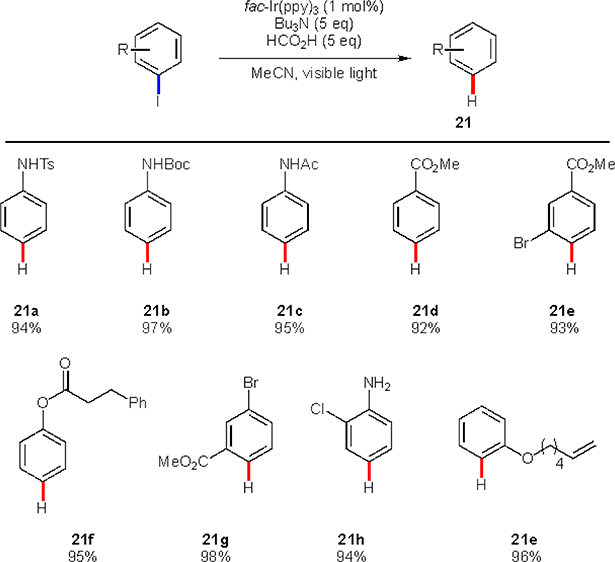
In 2012, Stephenson and co-workers demonstrated a photocatalyzed reduction of alkyl, vinyl and aryl iodides.23 This work exposed a potential of photoredox catalysis for cleavage of unactivated C-I bonds resulting in the formation of carbon-centered radicals. In order to make such transformation possible, the authors had to identify a photocatalyst that would be reducing enough to engage into an SET process with targeted substrates. Iridium based photocatalyst fac-Ir(ppy)3 was identified as a potential candidate due to a highly negative reduction potential (E Ir(IV)/Ir(III)=−1.73 V vs SCE). Indeed, this compound was able to catalyze the reduction of a number of N-protected iodoanilines (21a-c) and iodobenzenes, containing ester moiety (21d-g) (Scheme 12). This system exhibits high chemoselectivity, as other halogens were untouched in the course of the reaction (21e, 21g-h). However, it must be highlighted, that catalyst used in the reaction is among most reducing photocatalysts known. Hence, in order to develop reactions of aryl bromides or chlorides using direct photoredox conditions, a stronger reducing photocatalyst needs to be developed.
Scheme 12.
Stephenson’s reduction of aryl iodides.
The mechanism of this reduction follows an oxidative quenching redox cycle path (Scheme 13). First, the iridium catalyst, upon a photoexcitation engages in an SET with aryl iodide to produce iodide anion and aryl radical. An intermolecular HAT process between the formed radical and tributylamine occurs, furnishing the reduction product. As in the case with an iodine atom transfer seen earlier, the HAT here is guided by difference in BDE’s: 113 kcal/mol for benzene C–H vs 91 kcal/mol for C–H of methylene next to the nitrogen.7 In order to complete the catalytic cycle, the oxidized photocatalyst must be reduced. The role of reductant is fulfilled by either tributylamine, its post-HAT radical version, or formic acid present in the reaction mixture.
Scheme 13.
Proposed mechanism to Stephenson’s reduction.
In 2014, König and co-workers reported generation of aryl radicals from aryl bromides and -chlorides in the presence of perylene diimide (PDI) photocatalyst (Scheme 14).24 PDI undergoes photoexcitation with visible light, and upon subsequent reductive quench with triethylamine is converted to radical anion. The latter, which is stable in the absence of external oxidants, can harvest a second photon to form extremely strong reductant reducing entity. This method operating via two-photon excitation enabled reduction of aryl iodides (22a-d, 22e, 22g), bromides (22f, 22i), and even chlorides (22h, 22i-m) bearing primarily electron-withdrawing functional groups, such as acetyl, formyl, cyano, ester, and trifluoromethyl (Scheme 15). Notably, even with such capable reducing system, the arylhalides with OMe or NMe2 functionalities characterized by much higher reduction potentials, remained outside of the reach for generation of aryl radicals. Recently, however, the mechanism of this reaction has been revisited.25
Scheme 14.
König’s proposed mechanism of reduction.
Scheme 15.
König’s aryl halide reduction.
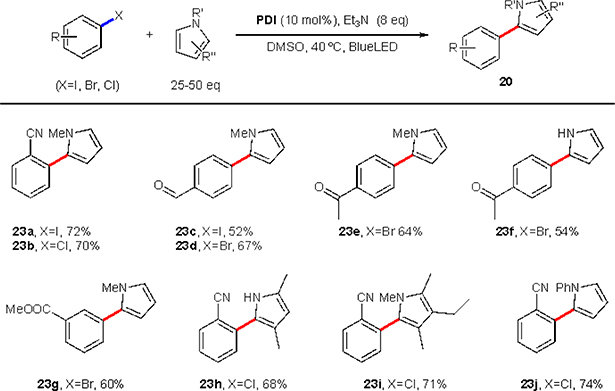
In addition to the reduction, the formed aryl radical could be trapped with large excess of electron-rich heteroaromatics, such as pyrroles (Scheme 16). Although, the scope of functionalities is limited to the electron-withdrawing groups only, this protocol is noteworthy, as it represents the first example of such C–C bond forming reactions using aryl bromides and even chlorides via photoredox catalysis.
Scheme 16.
König’s arylation of pyrroles.
3.3. Electrochemistry and photochemistry combination
A modified solution to the problem of generation of aryl radicals from bromides and chlorides was presented by the groups of Wickens26 and Lambert, Lin27. To the contrast of the previously discussed method, where a photocatalyst upon first excitation engages in the reductive quenching to form a radical anion, the authors utilized electrochemistry to achieve the initial electron transfer (Scheme 17). First, a ground state catalyst (PC) undergoes a single electron reduction at the surface of the cathode. Then a newly formed radical anion gets excited by a visible light to form a highly reducing entity, which in turn donates electron to the aryl halide. In contrast to König’s work, such approach eliminates necessity for the stochiometric reductant in the initial step. As a result, there is no additional byproducts forming within reaction medium and, hence, no problems associated with potential side reactions. In addition, the range of potential catalysts is greatly expanded. Not many compounds have the ability to absorb visible light and act as photocatalysts in neutral state, however, their radical anions are more capable of these processes.26–27
Scheme 17.
General mechanistic concept for combined electrochemistry-photochemistry activation mode of aryl halides.
Using this combination of electro- and photochemistry the authors succeeded in a range of different functionalization reactions. Thus, Lambert, Lin and co-workers developed borylation and stannylation reactions in the presence of 9,10-dicyanoanthracene (DCA) (Scheme 18).27 Both aryl bromides and -chlorides were successfully employed in these transformations. The spectrum of functional groups was extended in comparison to the previous methods, as aryl halides bearing electron-donating groups including methoxy (24b) and dimethylamino (24i) could now be engaged in reductive transformations. According to the calculations, the system could generate aryl radicals from chloride having reduction potential as negative as −2.94 V vs SCE (24g). It was estimated that the reduction potential of the DCA radical anion is about −3.2 V vs SCE, which is nearly as strongly reducing as lithium metal (−3.3 V)!
Scheme 18.
Lambert’s and Lin’s borylation and stannylation scope.
Wickens and co-workers, successfully applied similar combined electrochemical/photochemical approach for phosphorylation of aryl chlorides.26 As in work discussed above (Scheme 18)27, this approach allowed for a dramatic expansion of the functional group scope (Scheme 19). Naphtalene imide (NpMI) (analogue of PDI from König’s work) enabled reduction of substrates with potential as negative as −3.4 V vs SCE (25d). Moreover, both works showed that arylation of pyrroles could be achieved under presented conditions. Again, the previously inaccessible aryl halides containing electron-donating groups were successfully employed. In conclusion, these two works pushed the limits of photoredox catalysis beyond what was seemed possible. The achieved redox potentials and their synthetic application are truly impressive.
Scheme 19.
Scope of Wickens’ phosphorylation and arylation reactions.
3.4. Light induced transition metal catalysis
In the photoredox catalysis, transition metal-based photosensitisers serve as an electron-, energy-, or atom transfer agents, and do not directly participate in the bond forming events. In contrast, in photoexcited transition metal chemistry, the transition metal plays double duty by absorbing light and then by performing catalytic transformation.28 In 2016, Gevorgyan and co-workers demonstrated formation of aryl radical from aryl iodide in the presence of Pd(0) catalysts, thus presenting an alternative to the classical two electron oxidative addition between Pd(0) complex and aryl halides.29 Under blue light irradiation, the reaction between palladium complex and aryl iodide 25 presumably via SET results in formation of the new palladium hybrid radical intermediate 24 containing aryl radical and Pd(I) species (Scheme 20). Aryl radical generated in this process was shown to undergo a facile 1,5-HAT process to produce a transposed alkyl radical 26. This process is highly thermodynamically favourable, as the C–H bonds adjacent to heteroatoms are substantially weaker than the aliphatic alkyl C–H bonds. A subsequent β-hydrogen elimination, typical for the palladium chemistry results in desaturation product, silylenol ether 28.
Scheme 20.
Proposed mechanistic patway for Gevorgyans desaturation reaction.
Engagement of this hybrid Pd-radical reactivity, achieved in the absence of exogeneous photosensitizers or oxidants, enabled desaturation of a diverse range of silyl alkyl ethers (Scheme 21). Thus, a number of 1-arylethanol derivatives (28a-e), various alkyl substrates (28f-h, l), cyclic molecules (28i-k) as well as more complex structures (28m) could be dehydrogenated under these mild reaction conditions.
Scheme 21.
Scope of Gevorgyan’s desaturation protocol.
Involvement of radical intermediates in this transformation was unambiguously confirmed by the radical clock experiment (Scheme 22). Thus, subjecting substrate 29 having cyclopropyl moiety to the reaction conditions resulted in a formation of linear diene 33. This outcome is in a full agreement with the radical ring fragmentation of intermediate 31 resulting in the most stable benzylic radical 32. Radical 31, in turn, is formed via the HAT process from aryl radical 30. The product of the potential β-carbon elimination,30 the branched product 34, was not observed in the reaction mixture, thus ruling out involvement of traditional two-electron Pd chemistry in this transformation.
Scheme 22.
Radical clock experiment.
It is believed that this work not only opened a new chapter in the palladium chemistry, but also offered a new mild and general way for generation of aryl radicals. Recently, Arndsten and co-workers applied palladium/visible light combination to overcome long-standing limitations of carbonylation reaction (Scheme 23).31 In this impressive work, the authors succeeded to efficiently synthesize aroyl chlorides 36 (Nu=Cl) from aryl iodides 35 in the presence of Pd(0) catalyst! In addition, a range of nucleophiles, which typically are not efficient in the classical two-electron palladium carbonylation chemistry (such as bulky amines), could be employed in this method. The reaction proceeds via intermediacy of aryl radical.
Scheme 23.
Arndsten’s carbonylation protocol.
In addition, there are a few other reports, describing formation of aryl radicals under photoinduced transition metal catalysis.28
3.5. Electron Donor-Acceptor Complexes
In addition to the photoredox catalysis and light induced transition metal catalysis already discussed, electron donor-acceptor (EDA) approach has gained popularity in recent years for enabling efficient SET processes.32 It is based on a direct electronic interaction between donor (D) and acceptor (A) molecules via a reversible interaction between HOMO of D with LUMO of A resulting in a formation of new molecular aggregate, a visible light absorbing complex A---D (Fig. 3). Such aggregate exhibits absorption band not characteristic for either donor or accepter. Thus, in this scenario, two compounds that separately show no absorption in the visible light region form a complex that does exhibit such property. Upon light irradiation, SET takes place from the donor to acceptor yielding radical cation and radical anion pair. These two radical ions are engaged in the further transformations. It must be noted, that in order for this approach to work, the orbital energies of donor and acceptor must match for a desired interaction to take place.32
Fig. 3.
Generalized EDA complex formation.
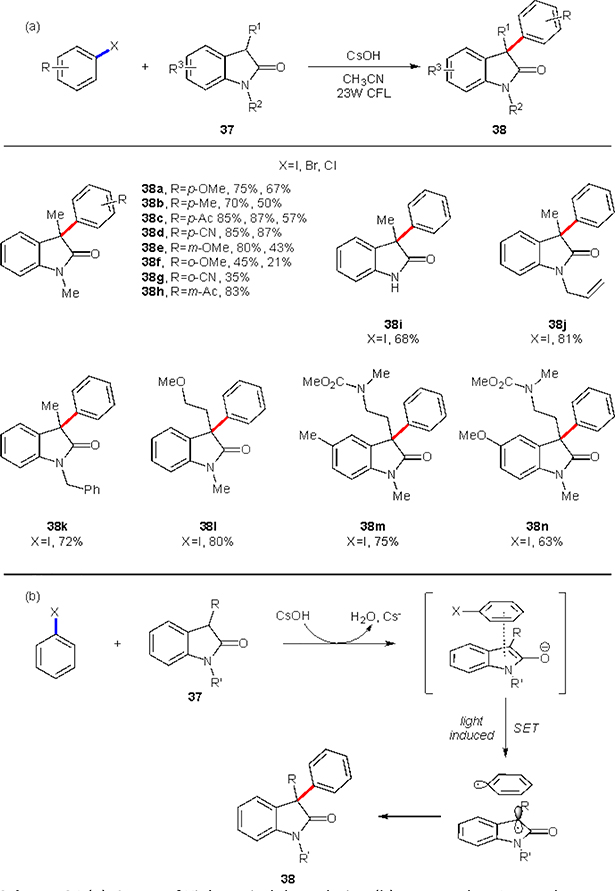
Most broadly EDA complexes are used for functionalization of aromatics, as conjugated pi-systems readily participate in such non-covalent interactions. In addition, orbital energies are readily adjustable by installing appropriate functionalities. Expectedly, the generation of aryl radicals was also attempted using this approach. One of more recent examples, in 2019, Xia and co-workers described the α-arylation of oxyindoles 37 utilizing visible light induced charge transfer pathway (Scheme 24(a)).33 This highly valuable protocol relied on the formation of an EDA complex between deprotonated oxyindole and an aryl halide (Scheme 24(b)). The formation of such EDA complex could be observed with a naked eye upon mixing the starting material in the presence of base, which results in a colored solution. Upon light induced electron transfer, two radical form, recombination of which results in the formation of product 38. Employing such a simple system, a variety of 3-aryloxyindoles was efficiently synthesized. Since this reaction is within the EDA paradigm, the aryl halides bearing electron-withdrawing functional groups re the most efficient. Nevertheless, aryl halides with methoxy group (38a,e,f) in different positions were shown to be prominent, as well. Oxyindole substituents were varied at nitrogen (38i-k), C-3 (38l, m) or C-5 (38m, n) positions. Functional groups in these positions have donating character, as expected.
Scheme 24.
(a) Scope of Xia’s oxyindole arylation (b) Proposed EDA complex.
4. Other Aryl Radical Precursors
Diazonium salts and aryl halides are two major sources of aryl radicals to day. However, there is an abundance of aromatic compounds available with other functionalities, such as phenols, carboxylic acids, boronic acids, etc. As usefulness of aryl radical became more and more apparent, synthetic chemists got interested in finding ways of generating aryl radicals with these materials. However, a number of synthetic problem for each particular functionality must have been solved in order to implement these compounds in reactions discussed in previous chapters.
4.1. Aryl Triflates
Generation of aryl radical from phenols is a highly attractive possibility due to the large abundance of the starting materials available. In the cases of diazonium salts and aryl halides, radical generation methods has been known for a long time. In a sense, we saw a surge of works exploiting known principles with much more effective modern systems. To the contrast, until very recently, it was not known how to activate the C–O bond of phenols toward formation of an aryl radical. A solution for this long standing problem was reported by Li and co-workers in 2017.34 The authors found that iodide anion under UV-C irradiation (254 nm), could perform SET to aryl triflate thus leading to the desired C–O bond cleavage (Scheme 25(a)). Notably, prior to Li’s work, any attempts of SET processes from various reductants to triflates invariably led to the cleavage of the S–O- rather than the C–O bond.35 It is not surprising, since the sulfur atom is the most electron-deficient site in the molecule, it hence is characterized by the highest electron affinity. The success of the protocol developed by the authors is built on the softness of the iodide anion as an electron donor. As discussed quite a few times already, bis(pinacolato)boronate ester is one of the best trapping reagents for the aryl radical. Thus, this new protocol allowed for a general and efficient synthesis of aryl boronate esters bearing both electron-donating (39a-d, h) and electron-withdrawing (39e-g) groups (Scheme 25(b)).
Scheme 25.
(a) Li’s proposed SET path for formation of aryl radical from aryl triflate.(b) Scope of borylation reaction.
In addition to that, the authors succeeded to convert aryl triflates into aryl iodides under slightly modified conditions. Such functional group interconversion is particularly interesting as it is very rarely seen. Despite an overall similarity to the borylation, there is an inherent problem with the stability of the aryl iodides under the conditions used. Aryl iodides are much more photo-labile than aryl triflates and, thus, under the UV-C conditions may undergo further undesired transformations. Nevertheless, the authors achieved synthesis of aryl iodides with a very broad functional group spectrum (Scheme 26). This work highlights the importance of not only the reduction potentials of the reagent, but also its hardness and softness matching with these of the substrate. A proper matching may enable previously not expected chemical transformations as it was elegantly demonstrated in this case.34
Scheme 26.
Scope of Li’s iodination of aryl triflates.
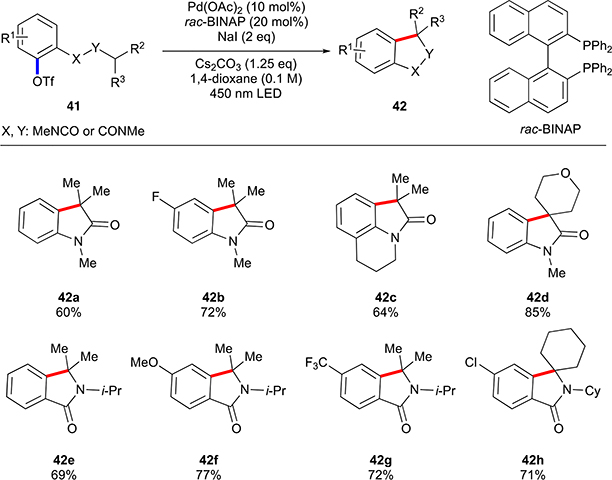
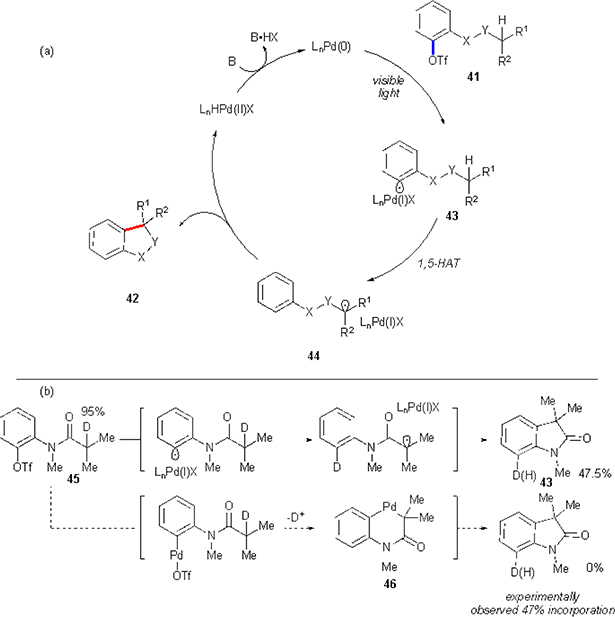
More recently, Gevorgyan and co-workers have showcased generation of aryl radical from triflates under visible light irradiation catalysed by palladium.36 Authors demonstrated that synthesis of certain oxyindoles and indolines could be achieved from respective aryl triflates via an intramolecular cyclization (Scheme 27).
Scheme 27.
Scope of Gevorgyan’s cyclization of aryl triflates.
This work is conceptually similar to the previous work by the same group discussed in aryl halide part (Scheme 19).29 In this case, palladium(0) complex reacts with an aryl triflate 41 resulting in the formation of aryl radical 43 (Scheme 28(a)). The latter engages in 1,5-HAT process, followed by a back cyclization, rearomatization sequence to furnish the reaction product 42. It must be noted, that the hydrogen translocation step (43→44) was shown to be equally efficient with both types of C–H bonds (electron rich in indolines, and -poor in oxyindoles). It is attributed to the ambiphilicity of the aryl radical discussed in the introduction. This, in addition to the large favourable differences between the BDEs of aromatic vs aliphatic C–H bonds, makes intramolecular HAT with aryl radicals a very facile and general process. While, at this point, the exact mechanistic pathway for the generation of the aryl radical is not clear, its intermediacy has been established by a series of experiments. In particular, the reaction of D-labelled substrate 45 revealed transfer of half of the initial deuterium to the product, which is consistent with the radical mechanism (Scheme 28(b)). Alternatively, if reaction would have proceeded via palladacycle 46, a complete deuterium loss should have occurred during the deprotonation step.
Scheme 28.
(a) Gevorgyan’s mechanistic proposal for cyclization of aryl triflates. (b) Labelling studies.
4.2. Aryl Carboxylic Acids
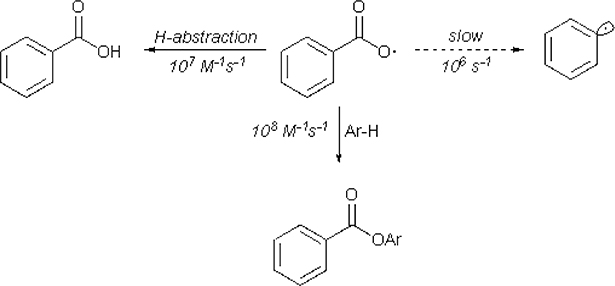
A direct generation of aryl radicals from carboxylic acids remains a particularly challenging task. The most obvious path of generation lies in the formation of a carboxyl radical, however due to the relatively low reaction rate of decarboxylation for benzoyl radicals (k=1.4•106 s−1) in comparison to its competitive reactions, such as intermolecular HAT (k=1.2•107 M−1 s−1) or addition to arenes (k=2.2•108 M−1 s−1) (Scheme 29), such path requires high energy input most of the time.37
Scheme 29.
Competetive reaction rates for reactions of benzoyl radical.
As an example, in 2012 Greaney and co-workers demonstrated radical reductive decarboxylation of aryl carboxylic acids under silver catalysis (Scheme 30).38 Their method relies on the established single electron oxidation of carboxylate by silver(II). Highly thermal conditions, as well as large excess of terminal oxidant K2S2O8, enabled complete conversion of carboxyl radical into aryl radical, which sequentially underwent hydrogen atom transfer with molecule of solvent.
Scheme 30.
Greaney’s radical protodecarboxylation.
In 2017, Glorious and co-workers proposed a solution to the slow decarboxylation event by redirecting reaction to an alternative pathway (Scheme 31).39 It was hypothesized, that if generation of aryl radical from carboxyl radical is not kinetically accessible, perhaps a different intermediate is necessary. Carboxyl radicals have been known to react rapidly with iodination reagents, forming benzoyl hipoiodites.40 It was envisioned that if a single electron transfer to such bromo-analogue, compound 48, would occur, a direct decarboxylative fragmentation would take place, thus resulting in a formation of a halogen anion and an aryl radical. Indeed, this approach has proven to be successful. By identifying a proper brominating reagent, as well as iridium-based photocatalyst, the authors achieved the formation of the carboxyl hipobromite in situ, followed by an SET from the reduced photocatalyst to bromite.
Scheme 31.
Glorious’ mechanistic hypothesis for formation of aryl radicals from benzoic acids.
Aryl radicals, formed in this manner were used in the synthesis of biaryls (Scheme 32). Notably, aryl radical generated in this manner could contain halogen functionalities in different positions (49a-g), as well as range of neutral-to-donating functional groups (49i-m, o). Perhaps the selectivity issue in the reaction with non-symmetrical arenes is the biggest drawback of this method, which reflects the low-selective nature of the radical addition (49p-r). Nonetheless, this work provides a novel avenue for the aryl radical generation from benzoic acids.
Scheme 32.
Scope of Glorious’ decarboxylative arylation.
4.3. Boronic Acids
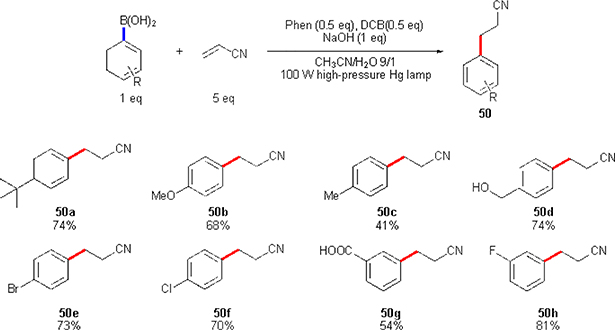
Aryl boronic acids and its various derivatives are widely used in organic synthesis. Thus not surprisingly, there is an interest for using these abundant materials for other purposes, such as generation of aryl radicals. However, to date, this field remains relatively undeveloped, as the methodologies developed rely on the employment of stoichiometric oxidants.41 Only recently, Yoshimi group developed a sub-stoichiometric process: light-induced Meerwein type arylation of Michael acceptors using aryl boronic acids.42 The authors used combination of phenanthrene (Phen) and 1,4-dicyanobenze (DCB) for their redox system (Scheme 33). Under these conditions, different aryl boronic acids bearing electron donating (24a-d), as well as withdrawing (24e-h) groups, were coupled with acrylonitrile with moderate-to-good efficiencies.
Scheme 33.
Yoshimi’s Meerwein arylation of Michael acceptors.
Mechanistically, this is a rare example of aryl radical generation via a single electron oxidation, rather than reduction process (Scheme 34). A photoexcited Phen participates in an SET with DCB. The formed Phen radical cation acts as a single electron oxidant in reaction with (trihydroxy)aryl borate 51, to produce free boric acid and aryl radical upon fragmentation. Sequentially, an aryl radical adds to the Michael acceptor, resulting in an electrophilic radical 52. A single electron reduction by DCB radical anion of the latter, followed by protonation completes the product formation.
Scheme 34.
Proposed mechanism for Yoshimi’s arylation of Michael acceptors.
4.4. Hypervalent Iodine
Iodine(III) reagents are widely applied in the radical chemistry.43 Naturally, aryl radical chemistry is well present within that field. For example, in 2013 Xiao and co-workers reported visible light-induced arylation of arenes and heteroarenes using diaryliodonium salts.44 The authors demonstrated that in presence of moderately reducing [Ru(bpy)3]Cl2 photocatalyst under blue light irradiation, arylation of various electron rich heteroaromatics by unsymmetrical diiodonium salts 53 is plausible (Scheme 35). In this manner, arenes with different mainly electron withdrawing groups were installed. Overall, reactivity of iodonium salts in the reaction is reminiscent with that of diazonium salts discussed above.
Scheme 35.
Scope of Xiao’s C–H arylation reaction.
Likewise, mechanistically this transformation follows the logic similar to that with diazonium salts (Scheme 36). The choice of aryl radical precursor and photocatalyst are the major differences between the methods. Apparently, in the case of unsymmetrical iodonium salts 53, a selectivity issue toward different aryl radicals may exist. The selectivity here is primarily guided by the steric effect; where an aryl iodide with bulkier ortho substituents is released preferentially, thus resulting in the formation of the less hindered aryl radical.
Scheme 36.
Proposed mechanism for Xiao’s C–H arylation reaction.
In 2016, Studer and co-workers demonstrated that generation of aryl radical from the same type of iodonium salts could be mediated by TEMPONa, a reagent discussed above in this review.45 Although, oxyarylation of styrene could be performed in this manner (Scheme 37(a)), the efficiency of method is quite low. In the same work, the authors highlighted employment of another reagent, iodine(III) compound, iodoanylidene malonates 56, which could be engaged in the similar type of process (Scheme 37(b)). This aryl radical precursor performed much better in the oxyarylation process, as not only the efficiency of the process was high (57a-g), but it also allowed for employment of diversely substituted iodoanylidines (57h-k).
Scheme 37.
Studer’s oxyarylation of alkenes using I(III) reagents.
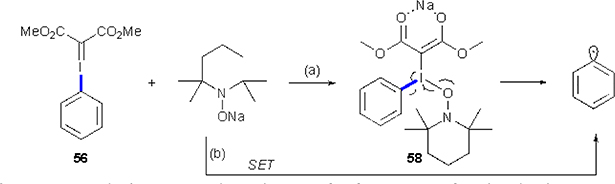
The authors proposed two pathways for the mechanism of this transformation (Scheme 38). According to the first scenario, TEMPONa attack on iodine atom resulting in the formation of an adduct 58, which undergoes a radical fragmentation to produce aryl radical, TEMPO radical, and dimethyl iodomalonate. Alternatively, a direct SET from the TEMPONa to iodoanylidene occurs, providing upon fragmentation the same overall outcome. While the first scenario may seem less probable due to the bulkiness of TEMPONa, the performed DFT calculation supported the feasibility of this pathway.
Scheme 38.
Studer’s proposed mechanism for formation of aryl radical.
4.5. Sulfur based sources
There are two major classes of sulfur based aryl radical precursors.46 Particularly widely used are sulfonium salts. In 2019, Ritter and co-workers developed a late stage fluorination protocol relying on employment of aryl sulfonium salts.47 This work is a great representative example of the chemistry in question. The methodology is based on photocatalytic activation of aryl thianthrenium salts (TT, TFT) in the combination with copper catalysis (Scheme 39). The photoexcited iridium photocatalyst first engages in a reductive quenching, most likely, by the copper(I) co-catalyst present in the medium. The formed IrII species then transfers an electron to sulfonium salt 59. The reduction potential of aryl thianthrenium salt’s is around −1.5 V vs SCE, which on average is higher than that for aryl bromides, but much lower than that of the diazonium salts, discussed previously. The formed aryl radical attacks the copper(II) catalyst, which possesses a fluoride ligand acquired from the medium. The reductive elimination from resulting Cu(III) species completes the product 60 formation and releases the Cu(I) co-catalyst. The Stern-Volmer studies revealed that TT, released in the course of reaction, is an excellent quencher for the excited IrIII, which makes it a potential electron shuffler between iridium and copper catalysts.
Scheme 39.
Mechanism of Ritter’s fluorination reaction.
The methodology demonstrated in this work enables fluorination of broad spectrum of arenes (Scheme 40). The major highlight of the work is ability to fluorinate densely functionalized complex molecules. Impressively, the reaction is selective at thiothrenium site even if molecule contains an iodide atom (60f).
Scheme 40.
Scope of Ritter’s fluorination reaction.
Sulfonyl chlorides is another class of sulfur-based radical sources used for generation of aryl radicals.48 A significant example of this was reported by Li and co-workers in 2013.49 It demonstrates the reaction between various aryl sulfonyl chlorides with 1,6-enynes under photoredox conditions resulting in a diverse range of 10a,11-dihydro-10H-benzo[b]fluorenes (Scheme 41).
Scheme 41.
Scope Li’s tandem cyclization products.
The oxidative quenching pathway was proposed for this cascade annulation reaction (Scheme 42). Upon SET, the sulfonyl chloride radical inion fragments releasing sulfur dioxide, chloride anion and an aryl radical. The latter attacks alkyne 61, triggering a series of radical cyclizations to produce A, which upon oxidation by the photocatalyst and a subsequent proton loss produces the polycyclic product 62. It is worth noting that aryl sulfonyl chloride was both aryl radical source initiating cyclizations as well as a final radical acceptor. This work is a great example of the employment of radical chemistry for a quick building of molecular complexity.
Scheme 42.
Proposed mechanism for Li’s tandem cyclization reaction.
Conclusions
In the past decade, the abundance of methods enabling generation of aryl radical has greatly increased. Primarily, it happened due to the rapid development of the photoredox catalysis. While older methods relied on various stoichiometric reagents, such as n-Bu3SnH or transition metal salts, the modern techniques are primarily catalytic, hence generating much less unnecessary and toxic by products. The scope of the aryl radical precursors has also been expanded. In addition to classical diazonium salts and halides, quite few other aryl radical precursor classes have been developed. Overall, aryl radical now is a versatile intermediate that could be accessed from a variety of starting materials by a range of methods. Due to the mildness of the newly developed protocols, the applicability of aryl radicals in modern chemistry has been emerging.
Key learing points.
The readers of this review will learn about:
Modern strategies towards generation of aryl radical.
Recently introduced aryl radical precursors.
Development of different redox systems towards generation of aryl radicals from more challenging substrates.
Important aryl radical applications in the synthesis.
Acknowledgements
We thank the National Institute of Health (GM120281), National Science Foundation (CHE-1955663), and Welch Foundation (Chair, AT-0041) for financial support.
Biography

Nikita Kvasovs
Nikita Kvasovs was born in Riga, Latvia. He received his BSc in chemistry from University of Latvia. In 2017, he joined prof. Gevorgyan’s group at University of Illinois at Chicago as a PhD student and later moved to University of Texas at Dallas. His research interest is focused around novel visible light induced transition metal catalyzed transformations.

Vladimir Gevorgyan
Vladimir Gevorgyan received his PhD from the Latvian Institute of Organic Synthesis. After two years of postdoctoral research (1992–1994, JSPS- and Ciba-Geigy International Postdoctoral Fellowships) at Tohoku University, Japan, and a visiting professorship (1995) at CNR, Bologna, Italy, he joined the faculty at Tohoku University (Assistant Professor, 1996; Associate Professor, 1997–1999). In 1999, Vladimir Gevorgyan moved to USA to join UIC (Associate Professor, 1999; Professor, 2003; LAS Distinguished Professor, 2012). In 2019, he joined the University of Texas at Dallas to become a Robert. A. Welch Distinguished Chair in Chemistry. Vladimir also holds a Professor position at the University of Texas Southwestern Medical Center. His group is interested in the development of novel synthetic methodology, particularly toward biologically relevant molecules.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Notes and references
- 1.Meerwein H, Büchner E and van Emster K, J. Prakt. Chem, 1939, 152, 237–266. [Google Scholar]
- 2.Pratsch G and Heinrich MR, in Radicals in Synthesis III, eds. Heinrich M and Gansäuer A, Springer Berlin Heidelberg, Berlin, Heidelberg, 2012, pp. 33–59. [Google Scholar]
- 3.(a) Kuivila HG, Acc. Chem. Res, 1968, 1, 299–305; [Google Scholar]; (b) Rosa AM, Lobo AM, Branco PS and Sundaresan P, Tetrahedron, 1997, 53, 285–298. [Google Scholar]
- 4.Galli C, Chem. Rev, 1988, 88, 765–792. [Google Scholar]
- 5.(a) Romero NA and Nicewicz DA, Chem. Rev, 2016, 116, 10075–10166; [DOI] [PubMed] [Google Scholar]; (b) Shaw MH, Twilton J and MacMillan DWC, J. Org. Chem, 2016, 81, 6898–6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Voica A-F, Mendoza A, Gutekunst WR, Fraga JO and Baran PS, Nat. Chem, 2012, 4, 629–635; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hollister KA, Conner ES, Spell ML, Deveaux K, Maneval L, Beal MW and Ragains JR, Angew. Chem. Int. Ed, 2015, 54, 7837–7841; [DOI] [PubMed] [Google Scholar]; (c) Friese FW, Mück-Lichtenfeld C and Studer A, Nat. Commun, 2018, 9, 2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo Y-R, Comprehensive Handbook of Chemical Bond Energies, CRC Press, Boca Raton, 1st edn., 2007. [Google Scholar]
- 8.De Vleeschouwer F, Geerlings P and De Proft F, Theor. Chem. Acc, 2012, 131, 1245. [Google Scholar]
- 9.Andrieux CP and Pinson J, J. Am. Chem. Soc, 2003, 125, 14801–14806. [DOI] [PubMed] [Google Scholar]
- 10.Cano-Yelo H and Deronzier A, J. Chem. Soc., Perkin Trans 2, 1984, 1093–1098. [Google Scholar]
- 11.Kalyani D, McMurtrey KB, Neufeldt SR and Sanford MS, J. Am. Chem. Soc, 2011, 133, 18566–18569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hari DP, Schroll P and König B, J. Am. Chem. Soc, 2012, 134, 2958–2961. [DOI] [PubMed] [Google Scholar]
- 13.Gomberg M and Bachmann WE, J. Am. Chem. Soc, 1924, 46, 2339–2343. [Google Scholar]
- 14.Kurandina D, Yadagiri D, Rivas M, Kavun A, Chuentragool P, Hayama K and Gevorgyan V, J. Am. Chem. Soc, 2019, 141, 8104–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Citterio A and Minisci F, J. Org. Chem, 1982, 47, 1759–1761. [Google Scholar]
- 16.Rüchardt BFC, Merz E, Spec. Publ. Chem. Soc, 1965, 19, 154. [Google Scholar]
- 17.Hartmann M, Li Y and Studer A, J. Am. Chem. Soc, 2012, 134, 16516–16519. [DOI] [PubMed] [Google Scholar]
- 18.Yan M, Kawamata Y and Baran PS, Chem. Rev, 2017, 117, 13230–13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Q, Sun B, Liu Z, Kao Y, Dong B-W, Jiang S-D, Li F, Liu G, Yang Y and Mo F, Chem. Sci, 2018, 9, 8731–8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubota K, Pang Y, Miura A and Ito H, Science, 2019, 366, 1500. [DOI] [PubMed] [Google Scholar]
- 21.Hong K-S, Xu H, Konishi H and Li X, J. Phys. Chem. Lett, 2010, 1, 997–1002. [Google Scholar]
- 22.Kawamoto T, Sato A and Ryu I, Chem. Eur. J, 2015, 21, 14764–14767. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen JD, D’Amato EM, Narayanam JMR and Stephenson CRJ, Nat. Chem, 2012, 4, 854–859. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh I, Ghosh T, Bardagi JI and König B, Science, 2014, 346, 725. [DOI] [PubMed] [Google Scholar]
- 25.Marchini M, Gualandi A, Mengozzi L, Franchi P, Lucarini M, Cozzi PG, Balzani V and Ceroni P, Phys. Chem. Chem. Phys, 2018, 20, 8071–8076. [DOI] [PubMed] [Google Scholar]
- 26.Cowper NGW, Chernowsky CP, Williams OP and Wickens ZK, J. Am. Chem. Soc, 2020, 142, 2093–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H, Kim H, Lambert TH and Lin S, J. Am. Chem. Soc, 2020, 142, 2087–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parasram M and Gevorgyan V, Chem. Soc. Rev, 2017, 46, 6227–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parasram M, Chuentragool P, Sarkar D and Gevorgyan V, J. Am. Chem. Soc, 2016, 138, 6340–6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miura M and Satoh T, in Palladium in Organic Synthesis: −/−, ed. Tsuji J, Springer Berlin Heidelberg, Berlin, Heidelberg, 2005, pp. 1–20. [Google Scholar]
- 31.Torres GM, Liu Y and Arndtsen BA, Science, 2020, 368, 318. [DOI] [PubMed] [Google Scholar]
- 32.Crisenza GEM, Mazzarella D and Melchiorre P, J. Am. Chem. Soc, 2020, 142, 5461–5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang K, Li N, Zhang Y, Li T and Xia C, Chem. Sci, 2019, 10, 3049–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, Yang X, Gao Y and Li C-J, J. Am. Chem. Soc, 2017, 139, 8621–8627. [DOI] [PubMed] [Google Scholar]
- 35.Jolly PI, Fleary-Roberts N, O’Sullivan S, Doni E, Zhou S and Murphy JA, Org. Biomol. Chem, 2012, 10, 5807–5810. [DOI] [PubMed] [Google Scholar]
- 36.Ratushnyy M, Kvasovs N, Sarkar S and Gevorgyan V, Angew. Chem. Int. Ed, 2020, 59, 10316–10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chateauneuf J, Lusztyk J and Ingold KU, J. Am. Chem. Soc, 1988, 110, 2886–2893. [Google Scholar]
- 38.Seo S, Taylor JB and Greaney MF, Chem. Commun, 2012, 48, 8270–8272. [DOI] [PubMed] [Google Scholar]
- 39.Candish L, Freitag M, Gensch T and Glorius F, Chem. Sci, 2017, 8, 3618–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.da Silva Corrêa CMM and Oliveira MABCS, J. Chem. Soc., Perkin Trans 2, 1983, 711–715. [Google Scholar]
- 41.Yan G, Yang M and Wu X, Org. Biomol. Chem, 2013, 11, 7999–8008. [DOI] [PubMed] [Google Scholar]
- 42.Iwata Y, Tanaka Y, Kubosaki S, Morita T and Yoshimi Y, Chem. Commun, 2018, 54, 1257–1260. [DOI] [PubMed] [Google Scholar]
- 43.Wang X and Studer A, Acc. Chem. Res, 2017, 50, 1712–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y-X, Xue D, Wang J-D, Zhao C-J, Zou Q-Z, Wang C and Xiao J, Synlett, 2013, 24, 507–513. [Google Scholar]
- 45.Hartmann M, Li Y, Mück-Lichtenfeld C and Studer A, Chem. Eur. J, 2016, 22, 3485–3490. [DOI] [PubMed] [Google Scholar]
- 46.Péter Á, Perry GJP and Procter DJ, Adv. Synth. Catal, 2020, 362, 2135–2142. [Google Scholar]
- 47.Li J, Chen J, Sang R, Ham W-S, Plutschack MB, Berger F, Chabbra S, Schnegg A, Genicot C and Ritter T, Nat. Chem, 2020, 12, 56–62. [DOI] [PubMed] [Google Scholar]
- 48.Chaudhary R and Natarajan P, ChemistrySelect, 2017, 2, 6458–6479. [Google Scholar]
- 49.Deng G-B, Wang Z-Q, Xia J-D, Qian P-C, Song R-J, Hu M, Gong L-B and Li J-H, Angew. Chem. Int. Ed, 2013, 52, 1535–1538. [DOI] [PubMed] [Google Scholar]