Abstract
Poultry production currently relies on the use of soybean as the main protein and energy source. Reducing its proportion in poultry diets and partly replacing it with local feedstuffs would improve sustainability by reducing dependence on importations and the environmental impact of production. In this study, we evaluated the impact of replacing soybean by sunflower meal, fava bean, canola meal, and dried distillers grains with solubles on the performance of rapid and slow growing chickens. Animals were reared in groups and on the floor. Individual BW and feed intake data were collected throughout each animal's life, thanks to an electronic feed station. At 5 wk (for broilers) and 12 wk (for slow growing chickens), the birds were slaughtered to obtain carcass composition and meat quality data. Adaptation to the alternative diet was studied separately for each genotype. Firstly, we performed ANOVA with diet effect on daily data of individual BW, feed intake, and feed conversion ratio. Secondly, the variability of performances within the group was studied by ANOVA with effects of diet, period, and their interaction. Finally, correlations between daily performances and final performances at slaughter were calculated to understand the construction of final phenotypes and to identify early indicators of final performances.
The results showed that the animals adapted well to the alternative diet, mean daily and final performances being mostly similar between the 2 diets for both genotypes (<3% on final BW). However, daily observations highlighted the critical importance of periods around dietary transitions by showing impacted performances for both genotypes. For example, feed conversion ratio of Label Rouge-alternative diet was 12 to 14% lower during the 3 d after transitions than during the 3 d before. It underlined the fact that adapting management of the batch to the alternative diet would be necessary. Correlations between daily and final performances showed that the slaughter performances of rapid growing chickens were mostly determined by BW whereas the main criterion was cumulative feed conversion for slow growing chickens. These correlations also suggested that reserves might be modified with the alternative diet, with rapid growing chickens giving rise to more glycogen reserves and less fat reserves.
Key words: alternative feedstuff, radio frequency identification device, kinetics, feed efficiency, feed intake
Introduction
Nutrition represents 50 to 70% of the production costs in poultry production (van Horne, 2018). A large part of these costs comes from the reliance on soybean and cereals to feed animals, which often compete with human nutrition (Leinonen and Kyriazakis, 2016). In Europe, soybean is mostly imported from America (European Commission, 2019). Moreover, Lathuillière et al. (2017) reported that soybean is a major cause of deforestation in Brazil and that maize culture requires a large amount of water. There is thus a motivation to reduce the need of these 2 feedstuffs in poultry diets in order to ensure the sustainability of poultry production in the context of growing world demand. Sunflower and rapeseed meals, by-products of the oil industry, and dried distillers grains with solubles (DDGS), by-product of bioethanol production, can be used as alternative sources of proteins. Moreover, their protein content varies according to the method of production (Laudadio et al., 2013). In order to compensate a potential lack of protein, other sources can be added to the diet, such as fava bean, a legume rich in protein with a sustainable worldwide production (Jensen et al., 2010). However, their incorporation is limited due to these beans richness in protease inhibitors, lectins, phenolic compounds, saponins, and non-starch polysaccharides that can affect the feed efficiency of the animals by impacting transit time, nutrient degradation, or anatomy of the digestive tract for example (Diaz et al., 2006). It has been shown that replacing soybean by a unique feedstuff had negative consequences on performances. For example, replacing soybean by fava bean led to low digestibility in methionine and cysteine (Koivunen et al., 2016). Regarding performances, replacing soybean by fava bean led to a decrease of 3 to 9% in BW with an increase of 5.7 to 8.0% in feed conversion ratio (FCR) in standard (STD) and Label Rouge (LR) chickens (Diaz et al., 2006; Bosco et al., 2013). Replacing it by DDGS improved BW by 2.1 to 3% without modifying FCR (Foltyn et al., 2013). Finally, replacement by canola meal increased FCR by 1% due to a 7.1% decrease of BW and daily feed intake (DFI) (Toghyani et al., 2017).Taking into account these results, one nutritional strategy could be using a mixture of these alternative feedstuffs (sunflower and canola meals, DDGS, fava beans) instead of a unique feedstuff, assuming that the complementarity between feedstuffs and the limitation of the proportion of each antinutritional factor would favor bird adaptation.
We thus evaluated the adaptation ability of 2 genotypes with different levels of growth rates and nutritional requirements, that is rapid growing STD chickens and slow growing LR chickens. We compared the kinetics of mean BW, feed intake (FI), and feed efficiency, as well as the variability of these traits between the alternative diet (AD) and the control diet (CD) from hatch to slaughter. Finally, analysis of the profiles of correlations between daily data and carcass and meat quality traits measured at slaughter was used to decipher how final phenotypes were constructed in both genotypes and diets and to find early predictors, other than morphological traits such as chicks or chickens' length and weight (Mendes and Akkartal, 2007; Moleenar et al., 2009). Measuring these traits in animals reared in individual cages induces a bias as it modifies animal feeding behavior and physical activity. Collective performances collected from birds reared in floor pens do not have this bias, but require a large number of animals for a rather poor statistical power (Gopinger et al., 2014; Alagawany et al., 2017). In order to be representative of production conditions (i.e., with animals reared on the floor and in groups), we thus collected individual FI and BW data with an automaton developed in our laboratory.
Materials and methods
The present study was performed in agreement with the French National Regulations for humane care and use of animals for research purposes with the authorization number 2018062715076382.V2-15695. Animals were reared at the PEAT INRAE poultry experimental facility (2018, https://doi.org/10.15454/1.5572326250887292E12), registered by the French Ministry of Agriculture under license number C-37-175-1 for animal experimentation (INRAE, Centre Val de Loire, Nouzilly, France).
Birds and Housing
Two batches of animals were reared successively for this experiment. In the first batch, 80 male Sasso naked neck chickens, a slow growing genotype dedicated to LR production, were reared from 1 to 82 d, between September and December 2018. In the second batch, 80 Cobb500 male chickens (STD) were reared from 1 to 35 d, between January and February 2019. Lighting and temperature schedules for both genotypes have been provided in Supplementary Table 1. At 1 d of age, the animals were identified with a wing band and an electronic radio frequency identification device (RFID) chip, and then weighed and placed in a pen on a floor covered with wooden chips. The RFID chip was placed at the base of the neck and secured with a plastic string passing under the skin. The pen was divided into 2 parts by a mesh bulkhead and the animals were placed in 1 of 2 groups, with an equal starting weight for both groups. In the first group, the animals were fed with a classic corn-soybean diet (CD) as is used in usual commercial conditions. In the second group, the animals were fed with an AD including less soybean meal and a higher proportion of alternative feedstuffs such as sunflower, rapeseed, and fava bean. The composition of the diets is shown in Table 1. Within a genotype, the diets were isoproteic and isoenergetic. The diets differed between the 2 genotypes in order to fulfill the needs of slow or fast growing broilers. A starter diet was given from hatch to 7 d for STD birds (2,850 kcal kg−1 DM; 21.5% CP) and upto 28 d for LR birds (2,750 kcal kg−1 DM; 20.0% CP). A grower diet was given from 8 to 22 d for STD chickens (2,900 kcal kg−1 DM; 20.0% CP) and from 29 to 63 d for LR chickens (2,850 kcal kg−1 DM; 18.0% CP). A finisher diet was given from 23 to 35 d for STD chickens (2,950 kcal kg−1 DM; 18.5% CP) and from 69 to 82 d for LR chickens (2,900 kcal kg−1 DM; 16.5% CP).
Table 1.
Composition and age of distribution of CD and AD for STD and LR genotypes.
| Ingredient (%) | STD |
LR |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD |
AD |
CD |
AD |
|||||||||
| 1–7 d | 8–22 d | 23–35 d | 1–7 d | 8–22 d | 23–35 d | 1–28 d | 29–63 d | 64–82 d | 1–28 d | 29–63 d | 64–82 d | |
| Corn | 30.650 | 35.970 | 39.800 | 20.420 | 18.890 | 23.500 | 29.620 | 42.920 | 46.620 | 18.250 | 16.950 | |
| Wheat | 30.100 | 30.100 | 30.100 | 30.100 | 30.100 | 30.100 | 38.550 | 30.100 | 30.100 | 40.100 | 57.950 | 45.100 |
| Fava bean | 12.000 | 13.000 | 10.000 | 13.000 | 12.000 | |||||||
| Soybean meal | 32.860 | 28.520 | 25.150 | 24.220 | 11.610 | 7.130 | 28.080 | 23.160 | 19.840 | 18.540 | 6.730 | 5.200 |
| Rapeseed meal | 5.000 | 5.000 | 8.000 | 5.000 | 5.000 | 5.000 | ||||||
| Wheat DDGS | 3.000 | 5.000 | 5.000 | 3.000 | 5.000 | |||||||
| High fiber sunflower meal | 8.120 | 7.730 | 5.190 | 5.020 | 8.000 | |||||||
| Soybean oil | 2.210 | 1.900 | 1.990 | 5.000 | 5.000 | 5.000 | 0.360 | 0.570 | 1.420 | 3.820 | 3.800 | |
| Corn gluten | 1.100 | |||||||||||
| Calcium carbonate | 0.710 | 0.169 | 0.002 | 0.655 | 0.142 | 0.600 | 0.274 | 0.300 | 0.590 | 0.390 | 0.350 | |
| Bicalcic phosphate | 2.160 | 1.850 | 1.540 | 2.050 | 1.730 | 1.400 | 1.970 | 1.870 | 1.560 | 1.880 | 1.540 | 1.350 |
| Salt | 0.236 | 0.207 | 0.211 | 0.192 | 0.150 | 0.158 | 0.270 | 0.246 | 0.280 | 0.254 | 0.180 | 0.230 |
| Vitamins and minerals | 0.400 | 0.400 | 0.400 | 0.400 | 0.400 | 0.400 | 0.400 | 0.400 | 0.400 | 0.400 | 0.400 | 0.400 |
| Sodium carbonate | 0.135 | 0.173 | 0.175 | 0.196 | 0.262 | 0.250 | 0.100 | 0.129 | 0.081 | 0.114 | 0.227 | 0.160 |
| DL-Met | 0.269 | 0.275 | 0.231 | 0.234 | 0.285 | 0.234 | 0.204 | 0.211 | 0.114 | 0.207 | 0.230 | 0.114 |
| HCL Lys | 0.176 | 0.264 | 0.250 | 0.287 | 0.414 | 0.392 | 0.154 | 0.243 | 0.125 | 0.183 | 0.373 | 0.214 |
| Thr | 0.076 | 0.111 | 0.094 | 0.088 | 0.157 | 0.135 | 0.052 | 0.087 | 0.010 | 0.062 | 0.140 | 0.032 |
| Val | 0.021 | 0.061 | 0.041 | 0.038 | 0.130 | 0.106 | ||||||
| Trp | 0.005 | |||||||||||
| Calculated composition | ||||||||||||
| AMEn, kcal/kg | 2,850 | 2,900 | 2,950 | 2,850 | 2,900 | 2,950 | 2,750 | 2,850 | 2,900 | 2,750 | 2,850 | 2,890 |
| CP, g/kg | 215.0 | 200.4 | 187.1 | 215.0 | 194.3 | 181.3 | 200.0 | 179.8 | 165.0 | 200.0 | 179.0 | 164.9 |
| Lys, g/kg | 11.200 | 10.900 | 10.000 | 11.200 | 10.900 | 10.000 | 10.000 | 9.500 | 7.800 | 10.000 | 9.500 | 7.810 |
| Met + Cys, g/kg | 8.400 | 8.170 | 7.500 | 8.400 | 8.170 | 7.500 | 7.500 | 7.200 | 6.000 | 7.500 | 7.200 | 6.000 |
| Trp, g/kg | 2.280 | 2.060 | 1.890 | 2.280 | 1.840 | 1.700 | 2.100 | 1.790 | 1.620 | 1.990 | 1.730 | 1.490 |
Abbreviations: AD, alternative diet; CD, control diet; DDGS, dried distillers grains with solubles; LR, Label Rouge; STD, standard.
Feed Station
BW and FI were individually and continuously recorded throughout the experiment, thanks to an electronic feed station (https://www.feed-a-gene.eu/media/bird-e-automate-de-consommation-alimentaire-pour-volailles). The feeder had a circular shape and consisted of 8 independent accesses to feed, without corridors, so that the chickens can express their natural feeding behavior. Each access included 1 feed tube, 1 feed trough, 1 antenna placed on the top of the feed trough to detect the animal's RFID chip, 1 scale for feed weight, and 1 scale to record animal weight placed under the tray on which the animal would climb to eat. The feed troughs and the trays could be changed according to the size of the animals. Raw data obtained from the station were 1) feed weight by access every second, 2) identity of animal, time, and access number every time an antenna detected a chip, and 3) mean animal weight during each visit. A visit was defined by consecutive readings of the same chip at the same access with less than 10 s between consecutive detections of the chip. All scales and antennas were connected to a central system of data acquisition. Because of electronic problems, data were acquired from 12 d on for the LR chickens. Reliable data could be obtained from day 3 onward for the STD chickens.
Meal Definition and Calculation of Feed Intake per Meal
Consecutive visits were grouped into meals as follows. A meal started each time a new chip was detected and ended when another one was read or when the chip was no longer detected during an interval of 2 min. This limit was defined using preliminary experiments during which we compared the behavior of animals obtained by video recording and data received from the station (unpublished data). Occasionally, the chip was not detected by the antenna immediately after an animal's arrival or the signal was lost before an animal's departure. In order to correct for this bias, we calculated the variance of feed weight data by intervals of 10 s before the start and after the end of the meal. Video analyses showed that a large variance of feed weight in the station (>0.1 g) is associated with pecking movement in the feed trough, and thus, that an animal is eating. Meal length was extended to include these periods of large variance.
For meal n starting at second Sn and ending at second En, FI (FIn) was calculated as the difference of mean feed weight recorded every second between meals n−1 and n and between meals n and n + 1. Outlier values of feed weights in these intervals were removed using the Cook's distance with a threshold of 1/k, where k is the number of values in the interval. FI of the meal was obtained as:
where FIn is the FI for meal n, FWi the feed weight at second i, Sn and Sn+1 the times at which meals n and n+1 start, En−1 and En the times at which meals n−1 and n end, Ci a coefficient equal to 0 if the FI value at second i was an outlier and 1 if not, NOV1 and NOV2 the number of outlier values removed between meals n−1 and n and between means n and n+1, respectively.
When less than 10 s separated 2 successive meals M1 and M2 of respective durations D1 and D2, we did not obtain enough stable feed weight values to calculate a reliable mean feed weight between M1 and M2. Total FI (FIM1M2) was calculated as the difference between mean feed weight before the start of M1 and after the end of M2. The FI of each meal (FIM1, FIM2) was then calculated according to the respective duration of each meal as:
In order to check the reliability of FI measured by the station, each time the feed tubes were refilled, the added quantity of feed was weighed and compared with the data obtained from the feed station after refilling.
The DFI was calculated as the sum of the FI of all meals eaten during a 24-hour period.
BW and Daily Gain Calculation
Before calculating individual BW on the different days, abnormal data were removed (weights below 25 g and above 3 times the mean BW of the previous day). Data outside the interval deviating from the mean by more than 3 SD were then removed. BW was then calculated as the mean of all available weight data during a day for each animal.
In order to check the reliability of animal weight data from the station, animals were weighed manually, weekly for STD chickens and every 2 wk for LR chickens.
ADG and FCR Model
In order to smoothen the daily variations of FCR, a moving average was used to calculate the daily feed conversion ratio, as already done in pigs earlier (Huynh-Tran et al., 2017). Among the different possibilities tested, a moving ADG over 5 d led to the lowest number of null or negative FCR values and the lowest daily coefficient of variation (CV) of FCR among individuals. Daily ADG and FCR of animal i on day j (ADGij, DFCRij) were thus calculated as:
with BWij being the mean weight of animal i on day j and DFIij the DFI of animal i on day j.
Cumulative Feed Conversion Ratio
The daily cumulative feed conversion ratio for animal i on day j (DCFCRij) was calculated as the ratio of cumulative FI between the first day of data collection and day j to the weight gain over the same period:
with DFIik being the DFI of animal i for day k and BWij the BW of animal i on day j.
Carcass Composition and Meat Quality
At 35 d for STD chickens and 82 d for LR chickens, the animals were weighed after 8 h of feed withdrawal and transferred to the slaughterhouse of the PEAT INRAE poultry experimental facility (2018, https://doi.org/10.15454/1.5572326250887292E12).
After 24 h of chilling, body composition was characterized by measuring breast meat yield (BMY), pectoralis major yield, pectoralis minor yield, abdominal fat yield (AFY), and thigh yield (TY) in relation to BW. Except for the abdominal fat which was considered entirely, to determine the yields only the right part of the animals was considered and the weight of the different parts was doubled to obtain those yields. Meat quality was evaluated on the pectoralis major muscle by measuring lightness (L∗), yellowness (b∗), and redness (a∗) of the meat with a Miniscan Spectrocolorimeter (HunterLab, Reston, VA) and ultimate pH (pHu) with a portable pH meter (model 506, Crison Instruments SA, Alella, Barcelona, Spain).
Analysis of Variance
Analyses were performed separately for each genotype, since the experiments had been conducted independently. The effect of the diet was first estimated separately for each day by applying the PROC ANOVA procedure of SAS 9.4 (2013; SAS Institute Inc., Cary) with diet as the single fixed effect to data calculated for each day: BW, ADG, DFI, daily feed conversion ratio (DFCR), and daily cumulative feed conversion ratio (DCFCR). In a second step, 3 rearing phases were defined according to the feeding period: starter, grower, and finisher phases when the animals were fed with the starter, grower, and finisher diets, respectively. The birds' response to the diet depending on the feeding period was then analyzed with the following ANOVA model:
with yijk being the trait for animal k with diet i and period j, Di the fixed effect of diet i, Pj the fixed effect of phase j (i.e., starter diet, grower diet, and finisher diet), DPij the interaction between diet i and phase j, and eijk the residual for animal k. Both individual daily phenotypes and their coefficients of variation (calculated within-day) were analyzed, in order to consider the birds' response in terms of mean and variability.
Diet effect on slaughter traits was estimated by one-way ANOVA within each genotype, with diet being the only fixed effect of the model.
Correlations With Daily Data
Correlations between the daily data (BW, ADG, DFI, and DFCR) and the data measured at slaughter (final BW and final cumulative feed conversion ratio [CFCRf], BMY, pectoralis major yield, pectoralis minor yield, AFY, TY, L∗, a∗, b∗, and pHu) were calculated by using the rcorr function of the package Hmisc of the R software (R Core team, 2013).
Results
Validation of Growth and Feed Intake Data
On average, the absolute value of the difference between manual and automatically recorded data of BW was low (2.2%). Similarly, the difference between the feed weight displayed by the feed station and the real feed weight at each refilling was low (0.3%).
Diet Effect on Growth Parameters
Effect on the Mean
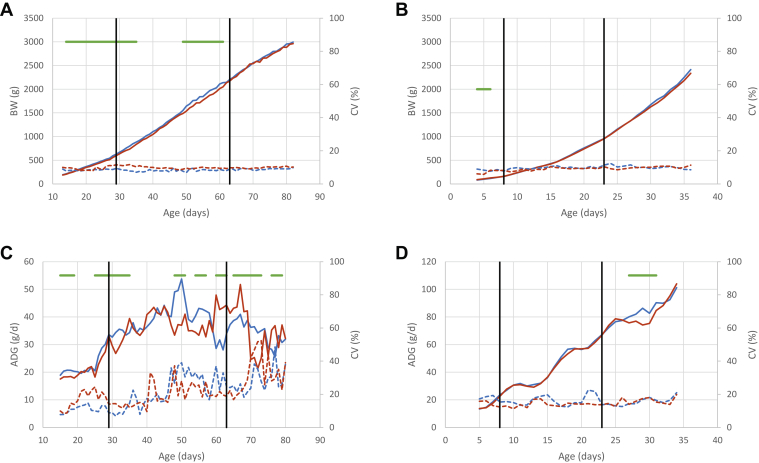
The ADG of LR chickens showed the same trends of kinetics in both diets, with a first phase of increase, followed by a plateau, and a final phase of decrease (Figure 1C). As the length of the plateau lasted 10 d with the AD diet and 20 d with the CD diet, ADG decreased earlier with AD (after 55 d) than with CD (after 70 d). The ADG of animals fed with the AD diet was 8 to 28% higher between 15 and 33 d (starter phase and start of grower phase), and 8 to 45% higher between 48 and 57 d (grower phase). In contrast, from 60 to 68 d (finisher phase), animals fed with AD showed a 10 to 40% lower ADG than those with the CD diet (Supplementary Table 2). This result was consistent with a slight advantage of BW for birds fed AD from 14 to 40 d and from 49 to 61 d (4.3–8.5%) and the absence of difference after this age (Figure 1A, Supplementary Table 3). Unlike the LR chickens, ADG increased until the end of the experiment for both diets in STD chickens (Figure 1D), which were slaughtered at a much younger age than LR chickens. Diet had a much smaller impact on STD than on LR chickens, as shown by the global analysis by feeding period in which the diet effect on ADG and BW was significant in STD chickens, but not in LR chickens (Table 2). Only during a 5-day period between 27 and 31 d, ADG was 5 to 15% higher with AD than with CD (Supplementary Table 2). Consistent with the absence of difference in ADG between diets, the growth curves of the STD birds were similar between the 2 diets (Figure 1B).
Figure 1.
Kinetics of the mean (solid line) and of the coefficient of variation (dotted line) for BW: (A) LR, (B) STD; and for ADG: (C) LR, (D) STD for chickens fed with classical diet (in red) or alternative diet (in blue). Black vertical lines indicate diet changes. Green horizontal lines indicate the periods of significance of the diet effect. Abbreviations: CV, coefficient of variation; LR, Label Rouge; STD, standard.
Table 2.
Diet and period effects on BW, ADG, DFI, DFCR, and DCFCR.
| Statistics | Effect | Level of effect | LR chickens |
STD chickens |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW (g) | ADG (gd−1) | DFI (gd−1) | DFCR | DCFCR | BW (g) | ADG (gd−1) | DFI (gd−1) | DFCR | DCFCR | |||
| LS means2 | Diet | AD | 1,472a | 31.7a | 98.3 | 3.09b | 1.95b | 724 | 47.8 | 93.6b | 1.79b | 1.44 |
| CD | 1,431b | 31b | 98.1 | 3.20a | 2.05a | 727 | 46.8 | 90.2a | 1.86a | 1.46 | ||
| Period1 | S | 370c | 21.3c | 46.5c | 2.32c | 1.70c | 121c | 17.3c | 27.9c | 1.67c | 1.25b | |
| G | 1,366b | 37.9b | 104.2b | 2.82b | 1.94b | 498b | 42.8b | 77.7b | 1.85b | 1.53a | ||
| F | 2,620a | 35.0a | 143.8a | 4.28a | 2.36a | 1,558a | 81.9a | 170.1a | 1.96a | 1.57a | ||
| Diet × period | AD × S | 381 | 22.1d | 45.9 | 2.20 | 1.64e | 117 | 17.5 | 28.1 | 1.54d | 1.26 | |
| AD × G | 1,398 | 38.6a | 105.8 | 2.80 | 1.86c | 493 | 43.1 | 76.6 | 1.86b,c | 1.51 | ||
| AD × F | 2,639 | 34.5c | 143.1 | 4.26 | 2.35a | 1,562 | 82.9 | 174.2 | 1.97a | 1.55 | ||
| CD × S | 359 | 20.5d | 47.1 | 2.44 | 1.77d | 125 | 17.1 | 27.7 | 1.79c | 1.24 | ||
| CD × G | 1,334 | 37.1b | 102.6 | 2.84 | 2.02b | 503 | 42.4 | 76.8 | 1.85c | 1.56 | ||
| CD × F | 2,601 | 35.5c | 144.5 | 4.30 | 2.38a | 1,554 | 80.9 | 166.0 | 1.95a,b | 1.57 | ||
| P-value | Diet | 0.003 | 0.020 | 0.870 | 0.010 | 0.001 | 0.811 | 0.090 | 0.050 | 0.030 | 0.506 | |
| Period | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | ||
| Diet × period | 0.277 | 0.001 | 0.107 | 0.100 | 0.001 | 0.842 | 0.432 | 0.070 | 0.001 | 0.523 | ||
Abbreviations: AD, alternative diet; CD, control diet; DCFCR, cumulative feed efficiency (daily cumulative feed conversion ratio); DFCR, feed efficiency (daily feed conversion ratio); DFI, daily feed intake; LR, Label Rouge; LS, least squares; STD, standard.
S: starter diet (d 1 to d 7 for STD, d 1 to d 28 for LR); G: grower diet (d 8 to d 22 for STD, d 29 to d 63 for LR); F: finisher diet (d 23 to d 35 for STD, d 69 to d 82 for LR).
Within effect, trait, and genotype, LS means values with different superscripts are significantly different (P < 0.05).
Effect on the Variability
The CV for ADG in STD chickens and for BW in both genotypes was stable and low at all ages, usually lower than 20% (Figures 1A–1D). In contrast, the CV for ADG in LR chickens varied with age for both diets, being stable until 35 d and increasing from 35 to 82 d up to values as high as 50% (Figure 1C). Despite similar trends, the kinetics of the CV of ADG during the 3 periods differs between the 2 diets. For the AD diet, CV increased from the starter to the grower diets while the increase occurred between the grower and finisher phases for the CD diet (Table 3). A significant interaction between diet and phase was also observed in BW variability in LR chickens. Indeed, LR animals fed with AD were 14.3% less variable than those fed with CD, only during the grower phase, whereas STD chickens fed with AD showed a 27.1% higher variability than those fed with CD over the whole period (Table 3).
Table 3.
Diet and period effects on the coefficient of variation of BW, ADG, DFI, DFCR, and DCFCR for each chicken genotype.
| Statistics | Effect | Level of effect | LR chickens |
STD chickens |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW (%) | ADG (%) | DFI (%) | DFCR (%) | DCFCR (%) | BW (%) | ADG (%) | DFI (%) | DFCR (%) | DCFCR (%) | |||
| LS means2 | Diet | AD | 8.7b | 20.3 | 21.6a | 28.0a | 18.0b | 10.8a | 13.0 | 20.5b | 23.9b | 33.1b |
| CD | 9.5a | 22.6 | 27.4b | 31.0b | 24.0a | 8.5b | 12.9 | 25.0a | 34.3a | 43.0a | ||
| Period1 | S | 8.8b | 14.1c | 15.9a | 19.3a | 16.1c | 8.5b | 13.4 | 26.9a | 42.3a | 52.4a | |
| G | 9.1a,b | 20.5b | 25.8b | 32.0b | 22.4b | 10.0a | 13.0 | 22.0b | 23.4b | 36.1b | ||
| F | 9.3a | 29.8a | 31.8c | 37.0c | 34.5a | 10.4a | 12.5 | 19.4c | 21.5c | 25.6c | ||
| Diet × period | AD × S | 8.7c | 11.1d | 16.1d | 16.2c | 15.2e | 9.8 | 13.6 | 26.6a | 37.6 | 58.4a | |
| AD × G | 8.4c | 21.7b,c | 23.9c | 32.4b | 19.9c | 11.5 | 13.5 | 17.7c | 16.7 | 26.1c | ||
| AD × F | 9.0b,c | 28.1a,b | 24.9b,c | 35.2a,b | 18.8c,d | 11.2 | 11.9 | 17.2b,c | 17.2 | 14.7d | ||
| CD × S | 8.9c | 17.1c,d | 15.7d | 22.5c | 17.0d,e | 7.3 | 13.2 | 27.2a | 47.0 | 46.3a,b | ||
| CD × G | 9.8a | 19.3c | 27.7b | 31.4b | 24.8b | 8.6 | 12.3 | 26.4a | 30.0 | 46.1a | ||
| CD × F | 9.7a,b | 31.6a | 38.7a | 38.8a | 30.1a | 9.6 | 13.3 | 21.5a,b | 25.8 | 36.4b | ||
| P-value | Diet | 0.001 | 0.130 | 0.001 | 0.020 | 0.001 | 0.001 | 0.940 | 0.001 | 0.001 | 0.001 | |
| Period | 0.010 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.615 | 0.001 | 0.001 | 0.001 | ||
| Diet × period | 0.002 | 0.046 | 0.001 | 0.036 | 0.001 | 0.192 | 0.105 | 0.020 | 0.276 | 0.001 | ||
Abbreviations: AD, alternative diet; CD, control diet; DCFCR, cumulative feed efficiency (daily cumulative feed conversion ratio); DFCR, feed efficiency (daily feed conversion ratio); DFI, daily feed intake; LR, Label Rouge; LS, least squares; STD, standard.
S: starter diet (day 1–day 7 for STD, day 1–day 28 for LR); G: grower diet (day 8–day 22 for STD, day 29–day 63 for LR); F: finisher diet (day 23–day 35 for STD, d 69 to d 82 for LR).
Within effect, trait, and genotype, LS means values with different superscripts are significantly different (P < 0.05).
Diet Effect on Feed Intake and Efficiency Traits
Effect on the Mean
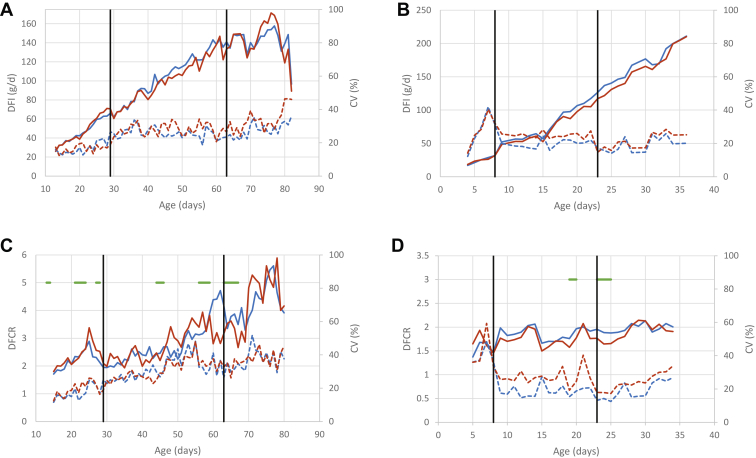
For both diets and genotypes, as expected, DFI increased with age (Figure 2). No difference was observed between the diets in LR chickens, except at 20, 26, 28, and 42 d, with no clear advantage for CD or AD (Figure 2A, Supplementary Table 4). In contrast, in STD chickens, DFI was continuously higher with AD than with CD, but the difference was significant only during the fourth week, before the last diet change (Figure 2B, Supplementary Table 4). During this period, DFI was 7.4 to 12.4% higher with AD than with CD. Summarizing the information by feeding period, we observed a diet effect in STD chickens, with DFI being 3.8% higher for chickens fed with AD than with CD (Table 2).
Figure 2.
Kinetics of the mean (solid line) and of the coefficient of variation (dotted line) for DFI: (A) LR, (B) STD; and for DFCR: (C) LR, (D) STD for chickens fed with classical diet (in red) or alternative diet (in blue). Black vertical lines indicate diet changes. Green horizontal lines indicate the periods of significance of the diet effect. Abbreviations: DFCR, daily feed conversion ratio; DFI, daily feed intake; LR, Label Rouge; STD, standard.
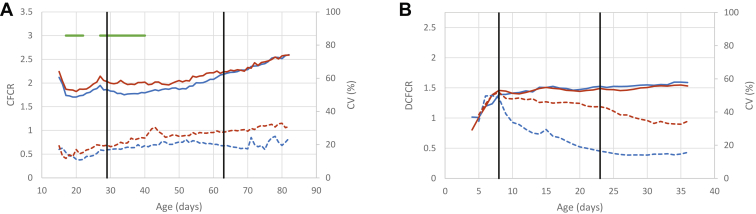
DFCR was highly variable between consecutive days, especially in LR chickens (Figures 2C and 2D, Supplementary Table 5), while the curves for DCFCR were smoothened (Figures 3A and 3B). Thus, in LR chickens DFCR was significantly better with AD for several days around the first diet change (17–32 d), but better for CD for several days around the second diet change (60–68 d), whereas a continuous difference was observed for DCFCR between 17 and 40 d, AD birds being 6.8 to 13% more efficient than CD birds during this period (Supplementary Table 6). Consistent with the other findings, when summarized by nutrition periods, diet effect was seen only during the starter phase for DFCR, while it was observed for both the starter and grower phases for DCFCR.
Figure 3.
Kinetics of the mean (solid line) and of the coefficient of variation (dotted line) for DCFCR (A) LR, (B) STD for chickens fed with classical diet (in red) or alternative diet (in blue). Black vertical lines indicate diet changes. Green horizontal lines indicate the periods of significance of the diet effect. Abbreviations: DCFCR, daily cumulative feed conversion ratio; LR, Label Rouge; STD, standard
Like the LR chickens, differences of DFCR between the diets in STD chickens were sporadic and limited to 5 d between 9 and 25 d (Figure 2D, Supplementary Table 5). During these 5 d, DFCR was 10.7 to 14.7% lower for CD birds. This was confirmed by the analysis of DFCR by period (Table 2), for which a diet by period interaction was significant, due to a positive effect of the AD diet, but only during the starter phase. When considering DCFCR, the diet effect was no longer significant (Figure 3B, Table 2).
Effect on the Variability
Change with age of DFI, DFCR, and DCFCR coefficients of variation differed between traits and genotypes, although similar trends were found between diets. The general trend was an increase in the CV of the 3 traits with age in LR chickens (Figures 2A, 2C and 3A) and a decrease in STD chickens (Figures 2B, 2D and 3B). Within each genotype, the CV of DFI and DFCR of LR increased with time, with a steeper slope in the starter phase than in the grower and finisher phases. The CV of DCFCR of LR-CD animals increased continuously whereas it remained stable after the first change of diet for LR-AD. In STD chickens, after a peak with high CV values during the starter phase, the CV decreased and stabilized during the grower and finisher phases for DFI and DFCR. A similar profile was observed for DCFCR, although the decrease in CV was more pronounced with AD than with CD.
Differences of variability between diets for DFI, DFCR, and DCFCR were strong in STD (Figures 2 and 3; Table 3). Alternative diet led to a decrease in the variability of those performances during the grower (DFI: −49%, DFCR: −30.4%, DCFCR: −44%) and finisher phases (DFI: −20%, DFCR: −30.4%, DCFCR: −58.4%) in STD chickens. In the case of LR chickens, the CV differed between diets during these phases for DFI and DCFCR traits. When significant, performances were less variable with the AD than with the CD diet.
Diet Effect on Carcass Composition and Meat Quality
Body composition and meat quality traits were not affected by diet in LR chickens, except for TY, which was slightly higher with the AD than with the CD diet (Table 4). In STD chickens, the abdominal fat percentage was significantly lower with AD compared to CD (−14%, P < 0.001). When fed with the CD diet, STD chickens had more acidic (lower pHu) and yellower (higher b∗ value) meat than those fed with AD. No diet effect was observed on the variability of the studied traits regardless of the genotype (data not shown).
Table 4.
Body composition and meat characteristics of LR and Cobb500 (STD) genotypes fed with either AD or CD.
| Trait1 | Genotype | LS means |
P-value |
|
|---|---|---|---|---|
| Diet |
Of diet effect |
|||
| AD | CD | |||
| Slaughter weight (g) | LR | 3,010 | 2,951 | 0.371 |
| STD | 2,334 | 2,355 | 0.720 | |
| AFY (%) | LR | 3.53 | 3.95 | 0.080 |
| STD | 1.57 | 1.83 | 0.001 | |
| BMY (%) | LR | 14.56 | 14.40 | 0.550 |
| STD | 20.44 | 20.40 | 0.970 | |
| TY (%) | LR | 25.64 | 25.16 | 0.030 |
| STD | 22.58 | 22.94 | 0.100 | |
| L∗ | LR | 48.76 | 49.14 | 0.520 |
| STD | 47.99 | 47.38 | 0.250 | |
| a∗ | LR | −1.06 | −1.09 | 0.860 |
| STD | −0.51 | −0.72 | 0.100 | |
| b∗ | LR | 9.82 | 9.48 | 0.230 |
| STD | 8.02 | 8.89 | 0.001 | |
| pHu | LR | 5.74 | 5.72 | 0.350 |
| STD | 5.89 | 5.79 | 0.001 | |
Abbreviations: AD, alternative diet; AFY, abdominal fat yield; BMY, breast muscle yield; CD, control diet; LR, Label Rouge; STD, standard; TY, thigh yield.
L∗: breast meat luminance, a∗: breast meat redness, b∗: breast meat yellowness, pHu: breast meat pH 24 h after slaughter.
Correlations Between Daily Traits and Cumulative Feed Conversion Ratio or Slaughter Traits
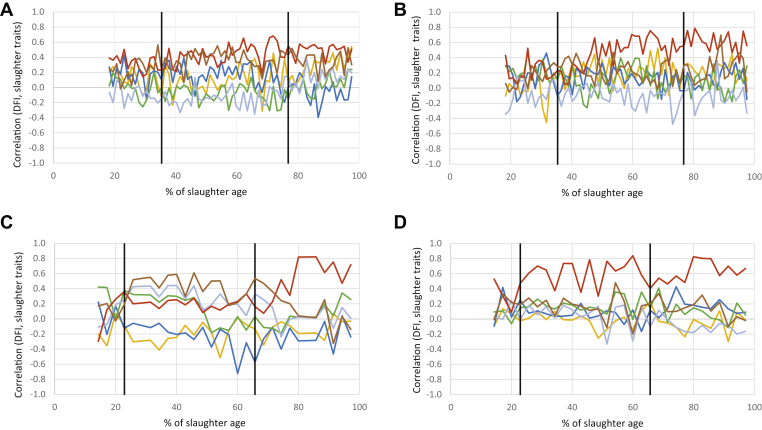
Feed Intake
On the whole, DFI was positively correlated with the CFCRf (Figure 4). In LR chickens, the correlation was lower during the starter phase (0.32 with AD, 0.22 with CD), increased during the grower phase (0.44 with AD, 0.47 with CD), and remained stable during the finisher phase (0.50 with AD, 0.61 with CD). In STD chickens, DFI and CFCRf were poorly correlated during the starter phase (on average 0.23 with AD and 0.40 with CD). During the grower phase, the correlation became stable and reached a higher level with CD (0.62 on average) than with AD (0.21 on average). During the finisher phase, a high correlation between DFI and CFCRf was maintained for STD chickens fed with CD diet (0.61), whereas it increased for those fed with the AD diet (0.53)(Supplementary Table 7).
Figure 4.
Profiles of correlations for LR: (A) AD, (B) CD; and for STD: (C) AD, (D) CD chickens between DFI and traits measured at slaughter (pHu in yellow, thigh yield in dark blue, AFP in green, BMY in light blue, CFCRf in red, BW at slaughter in brown). Black lines indicate diet changes. Abbreviations: AD, alternative diet; AFP, abdominal fat percentage; BMY, breast muscle yield; CD, control diet; CFCRf, final cumulative feed conversion ratio; DFI, daily feed intake; LR, Label Rouge; pHu, ultimate pH; STD, standard.
On the other hand, a moderate correlation with slaughter weight was observed for LR chickens starting at the first change of diet, stronger for those fed with AD (0.36) than with CD (0.24). We also observed a moderate positive correlation between DFI and breast final pH for these animals, particularly during the finisher phase (0.23 with CD, 0.32 with AD), whereas this correlation was low and negative in STD chickens (−0.04 with CD, −0.13 with AD). In STD chickens fed AD, the correlation between DFI and pHu was strongest at the beginning of the grower phase (−0.30 between 25.7 and 40% of the age at slaughter). During the same period, DFI was positively correlated with slaughter weight, as well as breast yield and AFYs (0.50, 0.40, and 0.30, respectively), whereas these correlations became weak during the finisher phase.
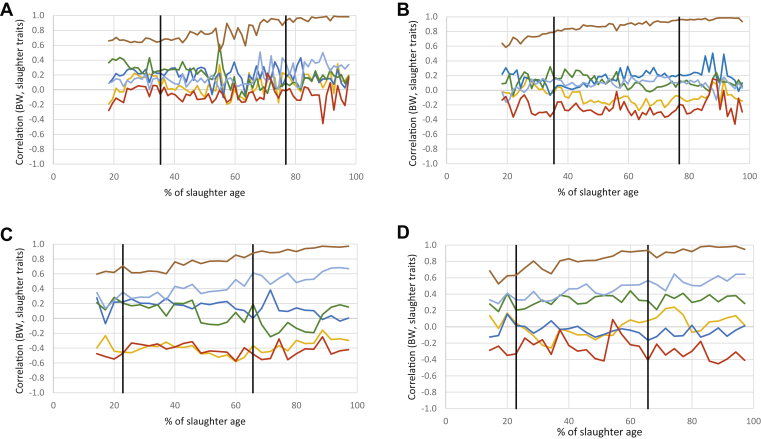
BW
As expected, the correlation between daily BW and slaughter weight increased with time to reach 1 on the last day (Figure 5). Even at the youngest ages, this correlation was found to be higher than 0.50, independent of the treatment. Correlations between BW and other slaughter traits were weak and rather stable with age in LR chickens. We only observed moderate positive correlations in LR-AD birds with fatness during the starter phase (0.32) and breast yield during the finisher phase (0.31). During this period, a moderate, positive correlation was also found with TY in LR-CD chickens (0.23). In contrast, corresponding correlations varied with age or diet in STD chickens. Thus, correlations with meat pHu or CFCRf were stable across ages, but more pronounced with the AD than with the CD diet (−0.39 and 0.01 for pHu and −0.43 and −0.27 for CFCRf, respectively). While weak correlations were found between daily BW and TY for both diets, different profiles were found for fat yield, the correlation being stable and moderate (0.32) for STD-CD chickens, but low for STD-AD chickens. Finally, correlations between daily BW and breast yield increased with age and reached quite significant values during the finisher phase in STD chickens (0.58 with AD, 0.56 with CD).
Figure 5.
Profiles of correlations for LR: (A) AD, (B) CD; and for STD: (C) AD, (D) CD chickens between BW and traits measured at slaughter (pHu in yellow, thigh yield in dark blue, AFP in green, BMY in light blue, CFCRf in red, BW at slaughter in brown). Black lines indicate diet changes. Abbreviations: AD, alternative diet; AFP, abdominal fat percentage; BMY, breast muscle yield; CD, control diet; CFCRf, final cumulative feed conversion ratio; LR, Label Rouge; pHu, ultimate pH; STD, standard.
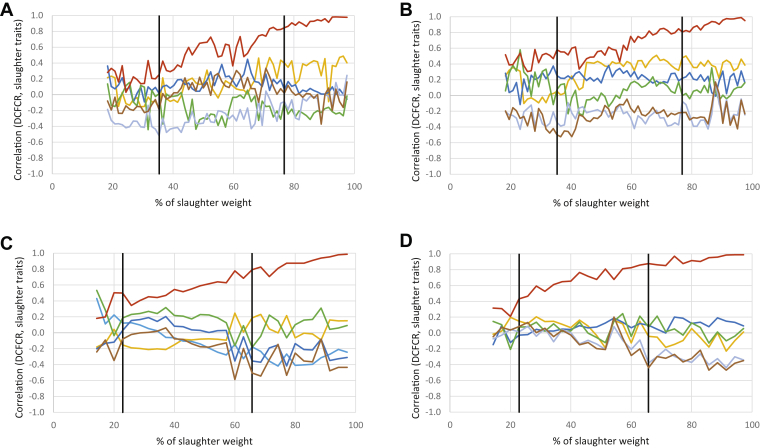
Cumulative Feed Efficiency
As expected, the correlation between DCFCR and CFCRf increased with age to reach 1 at slaughter (Figure 6). For LR chickens, better efficiency was associated with a higher breast yield and weight at slaughter, especially with CD (−0.29 and −0.47, respectively). Similar trends were observed for STD chickens during the finisher phase (−0.27 and −0.36 with AD, −0.33 and −0.34 with CD). Abdominal fat percentage and TY were poorly correlated with DCFCR. Finally, a lower breast meat pH and thus more acidic meat was associated with a lower DCFCR, at least for LR-CD chickens during the grower and finisher phases (0.40). This trend was not observed in STD chickens.
Figure 6.
Profiles of correlations for LR: (A) AD, (B) CD; and for STD: (C) AD, (D) CD chickens between DCFCR and traits measured at slaughter (pHu in yellow, thigh yield in dark blue, AFP in green, BMY in light blue, CFCRf in red, BW at slaughter in brown). Black lines indicate diet changes. Abbreviations: AD, alternative diet; AFP, abdominal fat percentage; BMY, breast muscle yield; CD, control diet; CFCRf, final cumulative feed conversion ratio; DCFCR, daily cumulative feed conversion ratio; LR, Label Rouge; pHu, ultimate pH; STD, standard.
Discussion
The aim of our study was to determine the capacity of adaptation of slow and fast growing chickens to a diet containing a mixture of alternative feedstuffs, in real conditions of production, that is on floor and in groups. Previous studies showed that FI recorded in cages differed from FI recorded on the floor. However, since many factors such as sex, diet composition, and cage or litter material influenced FI, BW, and FCR, the results of these studies were inconsistent (Akpobome and Fanguy, 1992; Plavnik et al., 2002; Santos et al., 2008; Simsek et al., 2014; Zhao et al., 2015). Automatons have already been developed to record FI on the floor. However, none are capable of simultaneously measuring FI and BW throughout the whole life of animals and without limiting the expression of natural behaviors due to the presence of systems of isolation of animals (Bley and Bessei, 2008; Howie et al., 2009; Tu et al., 2011; Basso et al., 2014; Yan et al., 2019). Thus, only synthetic FCR could be obtained with those automatons while ours was able to measure the kinetics of these types of traits.
The current study showed that differences between the 2 diets are moderate in terms of final performances in both genotypes, indicating that chickens are able to adapt to a diet composed of a mixture of alternative feedstuffs, with a higher proportion of wheat than corn and a partial replacement of soybean by DDGS, rapeseed, fava bean, and sunflower meals. Literature on the adaptation of chickens to a partial substitution of soybean by these feedstuffs showed contrasting results in both slow and fast growing chickens. Depending on the study, AD led to better, similar, or worse FCR (Diaz et al., 2006; Bosco et al., 2013; Foltyn et al., 2013; Méda et al., 2015; Koivunen et al., 2016; Alagawany et al., 2017; Toghyani et al., 2017). An absence of effect on FCR does not necessarily mean that there is no effect on performances that contribute to FCR. For example, for the LR chickens in this study as well as for the STD chickens in Diaz et al. (2006), the absence of an effect of the AD on FCR was due to a joint increase in FI and BW rate with AD. This discrepancy between studies could be due to many factors such as the animals (genotype, age, and sex) and the feedstuffs (quality, fiber percentage, and transformation process). The most striking difference in the adaptability of chickens to the AD was found in the variability of performances. Animals fed with AD had more homogeneous performances for FI and daily and cumulative FCR, especially in STD chickens.
Another interesting aspect of the daily data is that the data highlighted the importance of transition periods around diet changes. Modifications of performances around the time of the diet change could indicate a difficulty in adapting to the new diet if it appears after the transition or a necessity to change the diet earlier if it appears before the transition. These modifications are genotype and diet dependent and could be linked to several factors. For example, some diets have been shown to modify development of the digestive tract and thus its capacity of absorption (Nassiri Moghaddam et al., 2012). A difference of palatability between successive diets can be a cause of variations occurring after transitions. The drop we observed in weight gain despite the continuous increase in FI before the second diet change for the LR-AD chickens suggest that the animals' needs are not fulfilled anymore and that the diet change should have been done 3 to 4 d earlier, whereas this is not the case with the classic diet or with the STD chickens. Similarly, the strong increase in the CV of FI in STD chickens before the first diet change may indicate that this diet change occurs too late for some of the birds. This daily information could also help us to identify animals that are resilient to disturbances in their environment, especially around times of dietary transitions.
Finally, the correlation profiles between daily measurements and phenotypes measured at slaughter are useful to understand early indicators of final phenotypes. These indicators differ between genotypes and diets, which also highlights the fact that the final phenotype construction differs between genotypes and diets. For example, DFI is a good indicator of final FCR in STD chickens fed with CD, as the correlation between both traits is high as early as the first diet change. In contrast, when fed with AD, the correlation between both traits increased later, after the second diet change. The correlations between BW, AFY, BMY, and breast meat pHu in STD chickens also show that animals do not respond to CD and AD in the same way. For instance, although increased BW at early ages appears to be an indicator of increased BMY at slaughter for both diets, it also seems to be associated with higher muscle glycogen reserves which are the cause of lower pHu (Le Bihan-Duval et al., 2008) for birds fed the AD, and of higher abdominal fatness for birds fed the CD. This is maybe why the correlation between BW at an early age and CFCRf seems a little bit lower with CD than with AD, the energy cost of glycogen deposition in breast muscle being lower than the energy cost of abdominal fat deposition. In the current study, we also found indications showing that better FCR at early ages could be a predictor of higher breast development at slaughter in LR chickens, and could be of interest to limit the production costs of this alternative production and to satisfy the needs of the growing market of cuts and further processed products.
To conclude, both genotypes showed a good ability to adapt to ADs. Taking into account the costs of feedstuffs and mean FI, using these ADs would increase feed cost by 1.5% for LR chickens and by 3.4% for the STD chickens, close to the 0.5 to 4% of increase already found in the literature (Nguyen et al., 2012). This represents an increase of, respectively, 0.9 and 2% of the total production costs (Chenut, 2016). However, it has been shown that replacing soybean by local feedstuffs can decrease greenhouse gas emission by up to 41% depending on the percentage of replacement and the genotype (Méda et al., 2015). This element is important to evaluate the environmental impact of both diets, which has to be taken into account in the perspective of making poultry meat production more sustainable.
Acknowledgments
This study was supported by Feed-a-Gene, a project that has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement no. 633531.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2021.01.032.
Disclosures
The authors declare that they have no conflict of interest.
Supplementary data
References
- Akpobome G.O., Fanguy R.C. Evaluation of cage floor systems for production of commercial broilers. Poult. Sci. 1992;71:274–280. doi: 10.3382/ps.0710274. [DOI] [PubMed] [Google Scholar]
- Alagawany M., Attia A.I., Ibrahim Z.A., Mahmoud R.A., El-Sayed S.A. The effectiveness of dietary sunflower meal and exogenous enzyme on growth, digestive enzymes, carcass traits, and blood chemistry of broilers. Env. Sci. Poll. Res. Int. 2017;24:12319–12327. doi: 10.1007/s11356-017-8934-4. [DOI] [PubMed] [Google Scholar]
- Basso B., Lagüe M., Guy G., Ricard E., Marie-Etancelin C. Detailed analysis of the individual feeding behavior of male and female mule ducks. J. Anim. Sci. 2014;92:1639–1646. doi: 10.2527/jas.2013-7110. [DOI] [PubMed] [Google Scholar]
- Bley T.A.G., Bessei W. Recording of individual feed intake and feeding behavior of Pekin ducks kept in groups. Poult. Sci. 2008;87:215–221. doi: 10.3382/ps.2006-00446. [DOI] [PubMed] [Google Scholar]
- Bosco A.D., Ruggeri S., Mattioli S., Mugnai C., Sirri F., Castellini C. Effect of faba bean (vicia faba var. minor) inclusion in starter and growing diet on performance, carcass and meat characteristics of organic slow-growing chickens. It. J. Anim. Sci. 2013;12:e76. [Google Scholar]
- Chenut R. ITAVI; Paris, France: 2016. Performances techniques et coûts de production - Résultats 2015. [Google Scholar]
- Diaz D., Morlacchini M., Masoero F., Moschini M., Fusconi G., Piva G. Pea seeds (Pisum sativum), faba beans (Vicia faba var. minor) and lupin seeds (Lupinus albus var. multitalia) as protein sources in broiler diets: effect of extrusion on growth performance. It. J. Anim. Sci. 2006;5:43–53. [Google Scholar]
- European Commission United States is Europe’s main soya beans supplier with imports up by 112%. Eur. Comm. - Eur. Comm. 2019. https://ec.europa.eu/commission/presscorner/detail/en/IP_19_161
- Foltyn M., Rada V., Lichovnikova M., Dračková E. Effect of corn DDGS on broilers performance and meat quality. Acta Univ. Agric. Silvic. Mendelianae Brun. 2013;61:59–64. [Google Scholar]
- Gopinger E., Xavier E.G., Elias M.C., Catalan A.A.S., Castro M.L.S., Nunes A.P., Roll V.F.B. The effect of different dietary levels of canola meal on growth performance, nutrient digestibility, and gut morphology of broiler chickens. Poult. Sci. 2014;93:1130–1136. doi: 10.3382/ps.2013-03426. [DOI] [PubMed] [Google Scholar]
- Howie J.A., Tolkamp B.J., Avendano S., Kyriazakis I. The structure of feeding behavior in commercial broiler lines selected for different growth rates. Poult. Sci. 2009;88:1143–1150. doi: 10.3382/ps.2008-00441. [DOI] [PubMed] [Google Scholar]
- Huynh-Tran V.H., Gilbert H., David I. Genetic structured antedependence and random regression models applied to the longitudinal feed conversion ratio in growing Large White pigs. J. Anim. Sci. 2017;95:4752–4763. doi: 10.2527/jas2017.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E.S., Peoples M.B., Hauggaard-Nielsen H. Faba bean in cropping systems. Field Crops Res. 2010;115:203–216. [Google Scholar]
- Koivunen E., Partanen K., Perttila S., Palander S., Tuunainen P., Valaja J. Digestibility and energy value of pea (Pisum sativum L.), faba bean (Vicia faba L.) and blue lupin (narrow-leaf) (Lupinus angustifolius) seeds in broilers. Anim. Feed Sci. Technol. 2016;218:120–127. [Google Scholar]
- Lathuillière M.J., Miranda E.J., Bulle C., Couto E.G., Johnson M.S. Land occupation and transformation impacts of soybean production in Southern Amazonia, Brazil. J. Clean. Prod. 2017;149:680–689. [Google Scholar]
- Laudadio V., Bastoni E., Introna M., Tufarelli V. Production of low-fiber sunflower (Helianthus annuus L.) meal by micronization and air classification processes. Cyta – J. Food. 2013;11:398–403. [Google Scholar]
- Le Bihan-Duval E., Debut M., Berri C.M., Sellier N., Santé-Lhoutellier V., Jégo Y., Beaumont C. Chicken meat quality: genetic variability and relationship with growth and muscle characteristics. BMC Genet. 2008;10:53. doi: 10.1186/1471-2156-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen I., Kyriazakis I. How can we improve the environmental sustainability of poultry production? Proc. Nutr. Soc. 2016;75:265–273. doi: 10.1017/S0029665116000094. [DOI] [PubMed] [Google Scholar]
- Méda B., Lessire M., Dusart L., Bouvarel I., Berri C. 2015. Replacing soybean meal by alternative protein sources: multicriteria assessment of medium or slowgrowing chicken production systems. Proc. 20th Eur. Symp. Poult. Nut. Prague, Publisher, Tribun EU, Brno (Czech Republic) [Google Scholar]
- Mendes M., Akkartal E. Canonical correlation analysis for studying the relationships between pre- and post-slaughter traits of Ross 308 broiler chickens. Arch. Geflugelkd. 2007;71:267–271. [Google Scholar]
- Molenaar R., Reijrink I.A.M., Meijerhof R., van den Brand H. Correlation between chick length and chick weight at hatch and slaughter weight and breast yield in broilers. In: Hocking P.M., editor. Biology of Breeding Poultry. Cabi Publishing-C a B Int.; Wallingford, UK: 2009. p. 446. [Google Scholar]
- Moghaddam N.,H., Salari S., Arshami J., Golian A., Maleki M. Evaluation of the nutritional value of sunflower meal and its effect on performance, digestive enzyme activity, organ weight, and histological alterations of the intestinal villi of broiler chickens. J. Appl. Poult. Res. 2012;21:293–304. [Google Scholar]
- Nguyen T.T.H., Bouvarel I., Ponchant P., van der Werf H.M.G. Using environmental constraints to formulate low-impact poultry feeds. J. Clean. Prod. 2012;28:215–224. [Google Scholar]
- Plavnik I., Macovsky B., Sklan D. Effect of feeding whole wheat on performance of broiler chickens. Anim. Feed Sci. Technol. 2002;96:229–236. [Google Scholar]
- Santos F.B.O., Sheldon B.W., Santos A.A., Ferket P.R. Influence of housing system, grain type, and particle size on salmonella colonization and shedding of broilers fed triticale or corn-soybean meal diets. Poult. Sci. 2008;87:405–420. doi: 10.3382/ps.2006-00417. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc . SAS Institute Inc; Cary, NC: 2013. SAS/STAT® 13.1 User’s Guide. [Google Scholar]
- Simsek U.G., Erisir M., Ciftci M., Tatli Seven P. Effects of cage and floor housing systems on fattening performance, oxidative stress and carcass defects in broiler chicken. Kafkas Univ. Vet. Fak. Derg. 2014;20:727–733. [Google Scholar]
- R Core Team . 2013. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Toghyani M., Wu S.B., Pérez-Maldonado R.A., Iji P.A., Swick R.A. Performance, nutrient utilization, and energy partitioning in broiler chickens offered high canola meal diets supplemented with multicomponent carbohydrase and mono-component protease. Poult. Sci. 2017;96:3960–3972. doi: 10.3382/ps/pex212. [DOI] [PubMed] [Google Scholar]
- Tu X., Du S., Tang L., Xin H., Wood B. A real-time automated system for monitoring individual feed intake and body weight of group housed turkeys. Comp. Electron. Agric. 2011;75:313–320. [Google Scholar]
- Van Horne P.L.M. Wageningen Economic Research; Wageningen, The Netherlands: 2018. Page 40 in Competitiveness of the EU Poultry Meat Sector, Base Year 2017; International Comparison of Production Costs. Report 2018-116. [Google Scholar]
- Yan W., Sun C., Wen C., Ji C., Zhang D., Yang N. Relationships between feeding behaviors and performance traits in slow-growing yellow broilers. Poult. Sci. 2019;98:548–555. doi: 10.3382/ps/pey424. [DOI] [PubMed] [Google Scholar]
- Zhao X., Ren W., Siegel P.B., Li J., Yin H., Liu Y., Wang Y., Zhang Y., Honaker C.F., Zhu Q. Housing systems interacting with sex and genetic line affect broiler growth and carcass traits. Poult. Sci. 2015;94:1711–1717. doi: 10.3382/ps/pev128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.