Abstract
The assessment and monitoring of the tissue perfusion is extremely important in critical conditions involving circulatory shock. There is a wide range of established methods for the assessment of cardiac output as a surrogate of oxygen delivery to the peripheral tissues. However, the evaluation of whether particular oxygen delivery is sufficient to ensure cellular metabolic demands is more challenging. In recent years, specific biochemical parameters have been described to indicate the status between tissue oxygen demands and supply. In this review, the authors summarize the application of some of these biochemical markers, including mixed venous oxygen saturation (SvO2), lactate, central venous–arterial carbon dioxide difference (PCO2 gap), and PCO2 gap/central arterial-to-venous oxygen difference (Ca–vO2) for hemodynamic assessment of tissue perfusion. The thorough monitoring of the adequacy of tissue perfusion and oxygen supply in critical conditions is essential for the selection of the most appropriate therapeutic strategy and it is associated with improved clinical outcomes.
Keywords: Microcirculation, Circulatory shock, Tissue perfusion, Hemodynamic monitoring, Oxygen saturation, Lactate
Introduction
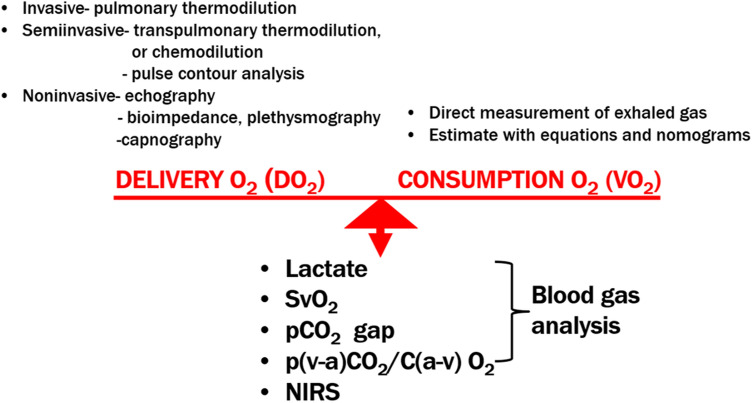
In individuals experiencing circulatory shock, it is essential to know whether cardiac output (CO) is sufficient to address tissue demands. Regardless of the type of shock, however, the ultimate consequences remain unchanged and have the same definition: a failure of oxygen (O2) utilization and cell metabolism caused by hypoperfusion resulting from circulatory failure—either the macrocirculation (heart and great vessels) or the microcirculation (capillaries, blood elements, cells) [1]. Hypoperfusion can be defined as a supply of O2 that does not adequately address the needs of cells [2, 3]. Failure of O2 use leads to anaerobic metabolism which is the source of several detectable products and byproducts. There is a broad spectrum of methods (from non-invasive to invasive) for measuring O2 supply for which CO is usually used as a surrogate in clinical practice [4] (Fig. 1).
Fig. 1.
Complexity of the evaluation of global circulatory status. DO2, oxygen delivery; VO2, oxygen consumption; NIRS, near-infrared spectroscopy oximetry; PCO2 gap, central venous–arterial carbon dioxide difference; SvO2, mixed venous oxygen saturation
However, it is difficult to measure O2 consumption because it can be estimated from nomograms or measured directly using exhaled gases; regardless, however, neither method is suitable for routine clinical use [5].
In treating any type of circulatory shock, sufficient CO must be ensured to fulfill tissue demands. CO is determined by heart rate (HR) and stroke volume (SV), according to the following equation:
SV depends on preload, afterload, and contractility. In clinical practice, two approaches are used to increase SV (and CO): adding volume (to increase preload based on the Frank–Starling law) and administering agents with a positive inotropic effect (i.e., inotropes) to increase cardiac contractility. However, it is well known from clinical trials that the administration of higher doses of both volume and/or inotropes is associated with worse outcomes [6–9]. There is no rigorous threshold of CO that should be reached in treating circulatory shock, and the goal is to increase CO only as much as needed to ensure adequate perfusion [1]. The adequacy of perfusion (or hypoperfusion) is difficult to assess in clinical practice. Instrumental methods examining the microcirculation (e.g., videomicroscopic techniques) are not well established for clinical use [10]. Clinical signs of hypoperfusion are not very sensitive and manifest only in the later stages of shock [11]. Currently, the easiest way to assess the adequacy of perfusion and relationship between O2 supply and demand(s) is, therefore, the measurement of biochemical markers related to O2 metabolism (Fig. 1). However, the interpretation of the measured values requires understanding of the complex physiological principles in the context of other hemodynamic findings. The aim of our review was, therefore, to summarize current possibilities of the assessment and monitoring of tissue perfusion adequacy and interpretation of the values in different critical circulatory situations. The most frequently used parameters in clinical practice include mixed venous oxygen saturation (SvO2), lactate levels, partial pressure of carbon dioxide (PCO2) gap, and surrogates of the respiratory quotient (RQ). Evidence supporting the use of these parameters in individuals who experience septic shock is quite robust; however, they are also applicable to those who experience cardiogenic shock [2, 3]. Although sex and age may affect the course of shock (e.g., different immune response), it seems that these factors do not influence the clinical use of the parameters of tissue perfusion adequacy [12, 13].
Global oxygen metabolism
Oxygen delivery (DO2) is expressed by the equation:
The major part of O2 in the blood is carried by hemoglobin (HGB). Only a clinically insignificant amount of O2 is physically dissolved and, therefore, is usually omitted:
where SaO2 is the saturation of HGB, PaO2 is the partial pressure of arterial oxygen, 1.38 represents the ml of oxygen bound to 1 g of HGB, and 0.0031 is the solubility coefficient of oxygen in plasma [14].
O2 consumption (VO2) can be calculated using the Fick principle (uptake of substance by an organ is proportional to the flow to the organ and arteriovenous concentration difference of the substance):
where CaO2 and CvO2 represent arterial and venous O2 concentrations, respectively, or
Under normal physiological conditions, O2 consumption depends only on the metabolic state (i.e., the higher metabolic rate the higher the O2 consumption) and is not influenced by DO2. This is based on the fact that DO2 greatly (up to five times) exceeds O2 consumption and serves as the delivery reserve for the body [15]. Therefore, under physiological conditions, VO2 is delivery independent and fluctuation in usual DO2 does not affect O2 consumption. There are two compensatory mechanisms for maintaining the equilibrium between VO2 and DO2. If O2 demands become higher, the compensatory increase in delivery will occur by increasing CO (first mechanism). If the increase in CO is not sufficient, then O2 extraction (EO2) from HGB (EO2 = SaO2 − SvO2) will rise (second mechanism). Increasing EO2 is associated with a decrease in SvO2. EO2 is approximately 25–30% in healthy resting conditions, and its possible increase provides a delivery reserve [16]:
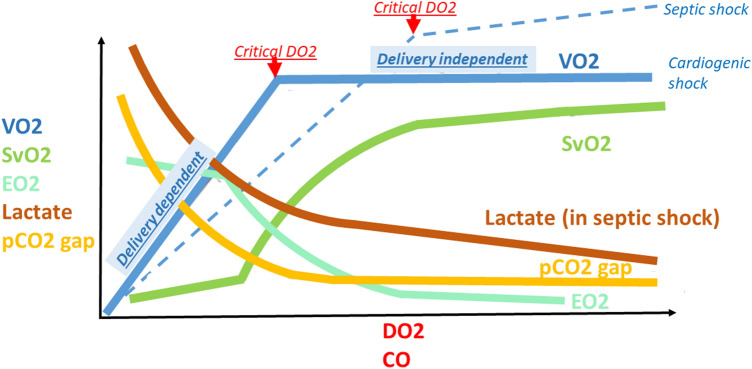
If the capacity of an organism to increase CO is diminished or compromised (e.g., heart failure), DO2 can be further raised only by an increase in EO2. If this mechanism is also depleted (SvO2 decline to 50%), the critical DO2 (the least CO necessary to fulfill tissue demands) is reached and switched to adverse anaerobic metabolism with lactate production [14]. If both compensatory mechanisms are exhausted, O2 consumption becomes entirely dependent on DO2, and is known as delivery-dependent VO2 [14] (Fig. 2).
Fig. 2.
Relationship between levels of the parameters of global oxygen (O2) metabolism and O2 delivery (DO2) or cardiac output (CO). pCO2 gap, central venous–arterial carbon dioxide difference; SvO2, mixed venous oxygen saturation; VO2, oxygen consumption; EO2, oxygen extraction
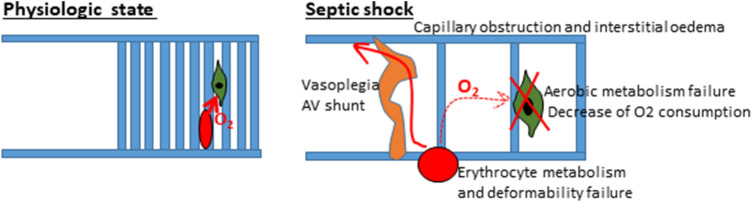
Primary failure of the macrocirculation (i.e., pump [heart] or great vessels [e.g., pulmonary embolism]) is known as cardiogenic shock and is a failure of DO2. Primary failure of the microcirculation and cell metabolism is known as distributive shock (e.g., septic shock) and is a failure of EO2 (Fig. 3). There is a higher level of critical DO2 in septic shock (dotted line in Fig. 2) due to failure of the microcirculation and cellular O2 use, which leads to malfunction of EO2 and a decrease in functional capillary density (heterogeneity of the capillary bed with good and poor perfusion). Therefore, some patients experiencing septic shock may benefit from increasing CO to higher values. However, this increase must be navigated by SvO2 and other parameters because routine increase to supranormal levels of CO may be associated with worse outcomes [7]. There can also be an uncoupling of the macro- and microcirculation when normalization of the macrocirculation (i.e., CO) does not improve microcirculation and cell metabolism [17–19].
Fig. 3.
Difference between oxygen (O2) delivery at the microcirculation level in physiological conditions and in septic shock. Thrombosis and edema of capillaries and interstitial edema (due to increased permeability) lead to reduction in microcirculation net and prolonging of diffusion distance for O2. Ateriovenous shunts bypass oxygenated blood directly in the veins. Malfunction of oxidative enzymes lead to decrease in O2 use. Collectively, this induces the failure of O2 extraction
Venous blood oxygen saturation (SvO2, ScvO2)
Saturation of HGB by O2 in the venous blood can be measured in mixed venous blood (i.e., SvO2) in the pulmonary artery using a pulmonary artery catheter (PAC) or in central venous blood (ScvO2) using a central venous catheter (CVC) placed in the internal jugular vein or subclavian vein [20].
Physiological principles
Generally, SvO2 values change with DO2 and EO2. SvO2 changes with DO2 (and CO) are non-linear (Fig. 2). There are only small changes in the independency zone from SvO2 > 70% (where compensation occurs through an increase in CO) and in dependency zone from SvO2 < 40% (where compensation through O2 extraction is depleted). The shape of the curve describing the relationship between SvO2 and DO2 is S-shaped and reflects the dissociation curve of oxy-HGB [20].
Normally, SvO2 values range from 65 to 80%. Generally, lower values imply low DO2 and a higher value means lower EO2 (Table 1). There are, however, more factors that lower SvO2 aside from low CO, including low HGB (anemia), low SaO2 (hypoxia), or high VO2 (i.e., O2 consumption). Increased consumption can be due to hyperthermia, shivering (thermogenesis), or cramps (epileptic seizure), increased consumption by breathing muscles while weaning from mechanical ventilation, or by psychomotoric agitation. If HGB concentration, arterial saturation, and consumption are optimized, then SvO2 depends solely on CO [21].
Table 1.
Relationship between mixed venous oxygen saturation (SvO2) and adequacy of oxygen (O2) delivery
| SvO2 | Adequacy of O2 delivery |
|---|---|
| > 80% | Low O2 extraction, low O2 cell metabolism |
| 65–80% | Normal O2 delivery → normal O2 extraction |
| 50–65% | Low O2 delivery → compensatory increased O2 extraction |
| 30–50% |
Critical O2 delivery → O2 extraction depleted Switch to anaerobic metabolism |
| < 25% | Cell death |
Higher SvO2 can be also caused by excessive DO2 or low consumption (e.g., sedation, myorelaxation, therapeutic hypothermia); however, it is rarely encountered and, therefore, high SvO2 is always an alert for EO2 or O2 metabolism failure. The possibility of cardiac disease with left-to-right shunt must also be excluded [14].
Difference between ScvO2 and SvO2
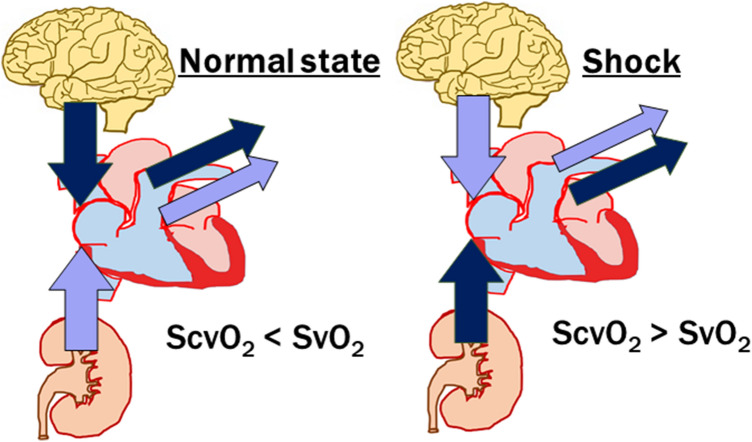
SvO2 represents the saturation of HGB by oxygen in the mixed venous blood drawn from the pulmonary artery using a PAC, and contains blood from the superior vena cava, inferior vena cava, and coronary sinus. SvO2 is, therefore, a marker of global EO2 in the entire body. However, the insertion of a PAC is an especially invasive procedure with many potential risks. ScvO2 represents the saturation of HGB by O2 from the CVC, usually from the subclavian or jugular vein, and indicates regional EO2 from the upper part of the body under physiological conditions higher (due to high O2 demands of the brain) than in the lower part because of the inflow of highly oxygenated venous blood from the kidneys (renal blood flow is as high as one-quarter of CO). In healthy conditions, ScvO2 is generally 2–7% lower than in the mixed venous blood SvO2 containing blood from the kidneys (i.e., ScvO2 < SvO2). However, during circulatory shock, the situation is much different. Centralization of the circulation leads to vasoconstriction in the visceral organs, with decreasing perfusion and conserving blood for the brain. For this reason, EO2 in the lower part of the body is higher than in the upper part, and ScvO2 is higher than SvO2, which contains deoxygenated splanchnic blood (ScvO2 > SvO2). The difference increases with the severity of shock and can reach 18% [22, 23] (Fig. 4). There are more variables, such as the position of the CVC (ScvO2 and SvO2 become similar when a CVC is placed more distally in the right atrium) or lowering demands of the brain by sedation [24]. Although the absolute values of ScvO2 and SvO2 may differ, their trends are the same, and ScvO2 can be used as surrogate for SvO2 to assess perfusion adequacy [15].
Fig. 4.
Explanation of the difference between saturation of hemoglobin by O2 in central venous blood (ScvO2) and mixed venous oxygen saturation (SvO2) under physiological conditions and in circulatory shock
Measurement
SvO2 can be measured either intermittently from blood samples or continually using special catheters (either a PAC or CVC) equipped with optic sensors (light emitted from the tip of the catheter and reflected light from erythrocytes is measured using spectrophotometry). Although these systems need to be calibrated, they provide comparable values [25, 26].
Clinical applications
Marker of tissue hypoxia (adequacy of CO)
The measurement of SvO2 is recommended by guidelines for CO adequacy monitoring [6, 27]. It provides information about hypoxia according to the amount of extracted O2. As mentioned above, the correlation between CO and SvO2 is worse in distributive shock (i.e., septic shock) due to extraction failure, and high SvO2 does not exclude low DO2. However, even in those states, if SvO2 is low, it means DO2 is low [1].
Prognostic markers and goal-directed therapy
Patients who experience septic shock have a worse prognosis when SvO2 is either low (< 65%) or high (> 80%) [6]. Until recently, SvO2 was recommended as a parameter for guiding the resuscitation of circulation in the early stage(s) of septic shock based on evidence of mortality reduction (guidelines from 2012 stated a goal of SvO2 > 65% and ScvO2 > 70%) [28]. The recommendations changed in 2016 after publication of three clinical trials that did not confirm the prognostic effect [29–31]. One reason is that baseline SvO2 may be high due to EO2 failure. However, there were other reasons for high baseline SvO2 in those trials than in previous trials; more specifically, patients were less sick and SvO2 was measured after initial volume treatment. Therefore, although the current recommendation for the use of SvO2 for goal-directed therapy (GDT) is not as strong as before, it is still suggested in patients with low SvO2 [1, 6].
Marker of incoming distributive shock (uncoupling)
A sudden unexplained elevation in SvO2 may imply the development of extraction (i.e., EO2) failure and microcirculation damage (e.g., systemic inflammatory response or septic shock) [14, 32].
Marker of catheter wedging
When using a PAC equipped with an optic sensor (described above), high SvO2 indicates wedging, either unintentionally when the catheter is placed too distally, or appropriate wedging during the measurement of pulmonary capillary wedge pressure [5].
Not a marker of local hypoxia
SvO2 is not a sensitive marker of local hypoxia (e.g., acute limb ischemia). In these situations, SvO2 will be normal due to the majority of blood with normal SvO2 originating from other organs [6].
Near-infrared spectroscopy oximetry
Near-infrared spectroscopy (NIRS) oximetry is a non-invasive method that uses self-adhesive patches equipped with sensors (light emitter and sensors of reflected light are spaced several centimeters from one another) placed on the skin. The light penetrates several centimeters into the tissue and, using several algorithms, provides information regarding the status of oxygenation of HGB in the microcirculation (i.e., mixture of arterioles, capillaries, and venules (peripheral saturation, rSO2) 3–4 cm under the skin. Because the majority of blood is pooled in the veins, the value is driven, in large part, by venous saturation. Therefore, NIRS oximetry behaves in a manner similar to SvO2 (i.e., reflects CO) and is falsely high in conditions with O2 extraction failure (e.g., sepsis). Hypoperfusion is obvious when rSO2 < 50% or if there is a drop > 20% from baseline. It has been shown that NIRS oximetry values correlate with CO in cardiogenic shock. Currently, this method is increasingly used for non-invasive hemodynamic monitoring [33–36].
Lactate
Physiological principles
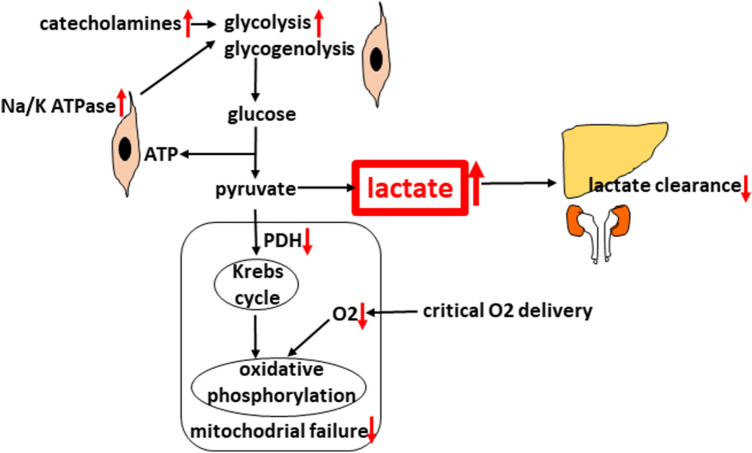
The glucose molecule is metabolized to pyruvate without the need for O2, generating 2 ATP molecules and known as anaerobic glycolysis. In the presence of O2, pyruvate enters the mitochondria, where pyruvate dehydrogenase (PDH) converts pyruvate into the acetylkoenzyme A, which enters the Krebs cycle followed by oxidative phosphorylation (1 glucose molecule generates 36 ATP molecules). When available O2 drops to critical levels, DO2 pyruvate is metabolized by lactate dehydrogenase into lactate. Therefore, lactate is considered to be a marker of anaerobic metabolism. Aside from the hypoxic explanation, however, there are also non-hypoxic pathways for lactate production not related to hypopefusion in shock that are either increased production or decreased clearance. Non-hypoxic production occurs in septic shock due to excessive ß-adrenergic stimulation of muscle cells by intrinsic mechanisms or by the administration of catecholamines [37, 38]. It leads to excessive glycogenolysis and glycolysis. Increased glycolysis produces an abundance of pyruvate that overwhelms the capacity of PDH, thus leading to lactate production. Another reason is malfunction of PDH and other mitochondrial enzymes of aerobic metabolism induced by septic toxins. The liver and kidneys are responsible for clearance of up to 90% of lactate (lactate is converted back to pyruvate by entering the Krebs cycle or is used for gluconeogenesis in the Cori cycle). In case of their hypoperfusion or enzyme failure, clearance is diminished [14, 39] (Fig. 5).
Fig. 5.
Glucose metabolism and the production of lactate
Measurement
Blood lactate level is routinely measured from blood samples (point-of-care test). Even during the first hours of shock or during decompensation, it is sufficient to measure lactate levels every 1–2 h due to its slower kinetics [6, 28]. There are even systems for continuous invasive monitoring of lactate levels [40].
Clinical applications
Marker of anaerobic metabolism (adequacy of CO)
Lactate informs about perfusion indirectly by reflecting anaerobic metabolism [6]. Because it requires switch of metabolism it is late marker of hypoperfusion not as sensitive in detecting early stages of hypoperfusion as SvO2, PCO2 gap or PCO2 gap/Ca–vO2. The cut-off value indicating hypoperfusion is > 2 mmol/l. Lactate exhibits a similar biphasic curvilinear shape of dependence on CO like other parameters, except for septic shock, where the normalization occurs slower (Fig. 2, lactate in septic shock, lactate in non-septic state—curve would be similar to PCO2 curve) [41, 42]. First, it is due to non-hypoxic reasons for lactate elevation and, second, to microvascular uncoupling. The correlation between lactate and CO is, therefore, weaker. Improving CO initially causes a rapid drop in lactate, followed by persistent only slowly decreasing lactate levels despite the already normalized perfusion. Therefore, trying to normalize lactate could lead to harmful over-resuscitation by fluid and inotropes [8]. Normalization of PCO2 gap and PCO2 gap/Ca–vO2 ratio (faster reacting markers of anaerobic metabolism) would suggest that perfusion is normalized and lactate level is elevated for other reasons. Lactate/pyruvate ratio was proposed to discriminate non-hypoxic lactate elevation (> 18 indicates anaerobic metabolism) but it is not widely used due to technical difficulties with measuring pyruvate [14].
Prognostic marker and GDT
Lactate is the only parameter to have clear evidence for GDT and is strongly recommended for navigation of treatment by guidelines [6, 28]. Both high value and slow clearance are associated with worse prognosis. Conversely, bringing lactate levels under 2 mmol/l or clearance > 20% every 2 h in the early stage(s) of septic shock (first 8 h) or > 50% in the first 6 h is associated with improved outcomes [1, 6, 43, 44].
Marker of distributive shock (uncoupling)
When CO and SvO2 are normal, increased lactate level can imply microvascular and cellular failure [1, 41].
Marker of local hypoxia
Lactate levels can be elevated also if local hypoxia occurs (e.g., acute limb ischemia). Global hypoxia can be ruled out based on other perfusion parameters that would be normal [14].
PCO2 gap (∆PCO2, Pv–aCO2)
PCO2 gap is the difference between venous and arterial partial pressures of CO2.
Physiological principles
Unlike O2, only 5% of CO2 is reversibly bound to proteins, mainly HGB (to the amino group creating carbamino HGB). On the other hand, CO2 is more physically dissolved in blood than O2 because it is 20 times more soluble, but still comprises only 5% of CO2 in the blood. The majority (90%) of CO2 in blood is in the form of bicarbonate: the CO2 originating from tissues combines with water (H2O) to form H2CO3. This takes place mainly in erythrocytes catalyzed by carbonic anhydrase (only a minority of CO2 is created slowly uncatalyzed in plasma). H2CO3 dissociates in erythrocytes into HCO3− and H+. HCO3− leaves the erythrocytes via a bicarbonate/chloride exchanger and is dissolved in blood flowing to the lungs, where the reverse reaction occurs (in erythrocytes and the lung endothelium), catalyzed by carbonic anhydrase and bringing H2O and CO2 [15]. CO2 is highly lipophilic and freely diffuses through membranes and is exhaled by the lungs. The CO2 dissociation curve (relation between PCO2 and content of CO2) is curvilinear (unlike O2, which is S-shaped); however, in the physiological range, it is near linear, which is why CO2 content can be substituted by PCO2 * k (dissociation coefficient).
As mentioned above, PCO2 gap is the difference between partial pressure of CO2 in arterial and venous blood. As described for SvO2, the Fick principle can also be applied: CO2 production (VCO2) is proportional to a flow through the tissues and arteriovenous concentration difference in CO2 [45, 46]:
where Ca and CvCO2 represent the arterial and venous concentrations of CO2, respectively.
Also mentioned above, concentration can by calculated from partial pressure (PCO2) as follows:
and
PCO2 gap is proportional to CO2 production (VCO2) in tissues and inversely related to CO (i.e., flow through the tissues (elimination from tissues) [47]. In normoxemia, aerobic production of CO2 occurs in the Krebs cycle. In hypoxemia, VCO2 remains relatively stable because, although aerobic production of CO2 decreases, it is partly counterbalanced by increased anaerobic production. In fact, VCO2 slightly decreases during hypoxia despite anaerobic CO2 generation; however, for clinical purposes, it can be considered constant. Anaerobic CO2 production comes from increased production of H+ buffered by HCO3– (further converted to CO2). The source of H+ is mainly from ATP hydrolysis, then lactate production (although lactate production does not generate H+ directly because one H+ is generated to make pyruvate from glucose, but one H+ is consumed to make lactate from pyruvate), and other enzymes producing H+. In normoxemic conditions, H+ is consumed in oxidative phosphorylation, which is not the case in anaerobic metabolism [14, 39] (Fig. 6).
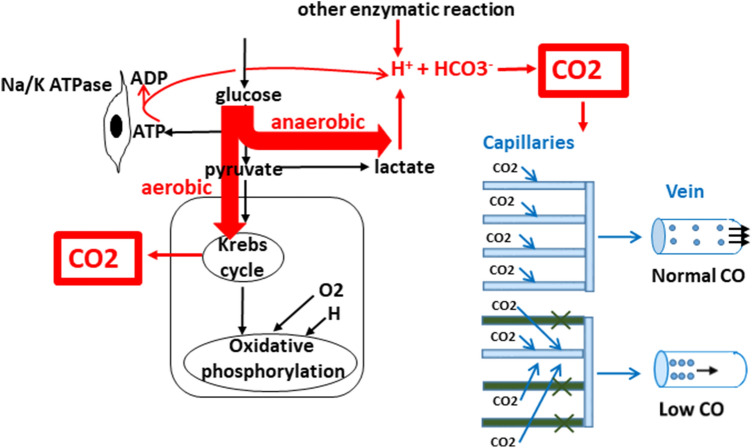
Fig. 6.
Production of carbon dioxide (CO2) and its relationship with cardiac output (CO). All produced CO2 easily diffuses and dissolves in blood. Transport from tissues to the venous blood does not restrict clearance from tissues. All produced CO2 always gets to the venous blood without accumulation in the tissues, and its concentration in the veins depends only on venous return. Higher CO leads to smaller venous CO2 concentration and smaller arteriovenous difference. Lower CO leads to slower flow through capillaries and the entire CO2 production is dissolved in smaller venous blood volume, known as the “stagnation phenomenon”. This is why there is higher amount of CO2 dissolved in venous blood and higher arteriovenous difference. The same applies to reduced capillary net (despite normal CO) when CO2 from areas with damaged net is drained by remaining capillaries leading inevitably to high CO2 concentration. HCO3−, bicarbonate
This explains why PCO2 gap cannot be used to detect hypoxia and anaerobic metabolism—normal PCO2 gap does not mean the absence of hypoxia. As mentioned above, because VCO2 is essentially constant in normoxia and hypoxia, and because the diffusibility through membranes and solubility in blood is very high (not restricting CO2 elimination from tissues), PCO2 gap is determined solely by capillary venous outflow (i.e., CO, that clears produced CO2 [45]:
When DO2 was lowered beyond critical value in a canine model, either by reducing blood flow using blood with normal SaO2, or by preserved blood flow but with low SaO2. The former lead to an increase in PCO2 gap, whereas the latter did not change PCO2 gap [48].
Difference between PcvCO2 and PvCO2
The values of PCO2 gap exhibit the same trends as SvO2 when using mixed venous blood (P(v–a)CO2) or central venous blood (∆PCO2). Again, similar to the case of SvO2, the use of central venous blood is widely accepted as a surrogate for calculation of PCO2 gap [46, 49].
Measurement of PCO2 gap
PCO2 gap is routinely measured from blood samples (point-of-care test); however, there are some limitations. The blood capacity for CO2 is increased (dissociation curve is not linear) with low HGB saturation with O2 (hypoxia, Haldane effect) and acidosis by carrying more CO2 by HGB. In severe hypoxia and acidosis, CO2 content can be increased by these factors at a given PCO2 and, therefore, calculation of CO2 gap from pCO2 can be imprecise [14].
Clinical applications
Marker of venous return (adequacy of CO)
Venous content of CO2 and PCO2 gap depends, in fact, only on microvasculatory venous return (Fig. 6) and PCO2 gap reflects venous return from the capillary bed and the adequacy of the microcirculation [14].
In the state of coupling macro- and microcirculation, it indirectly reflects CO and has similar biphasic curvilinear shape of dependence on CO similar to other parameters [50] (Fig. 2). It does not have as robust evidence as other parameters on GDT; however, guidelines have recommended the use of PCO2 gap to help assess the adequacy of CO as well as to guide therapy [1]. In normal conditions (normal CO and homogenous healthy capillary bed), all CO2 production is rapidly washed out, and venoarterial PCO2 gradient is minimal. PCO2 gap > 6 mmHg (0.8 kPa) is the cut-off value that implies inadequate CO; in that case, the therapeutic option could be to increase CO with the aim of normalizing PCO2 gap. On the other hand, in shock with persistent elevation of lactate levels (see below), normalized PCO2 gap will indicate the risk for potentially harmful over-resuscitation using fluid and inotropes. The variation of CO2 occurs faster than lactate changes; therefore, it is more sensitive marker to hemodynamic changes [14].
In contrast, in the uncoupling state (distributive shock), there is a weak correlation between PCO2 gap and CO (similar to SvO2) because of decreased functional capillary density, with areas with good and poor perfusion (Fig. 6); this can lead to elevation of venous CO2 content and PCO2 gap despite normal or high CO. In such situations, some patients may benefit from an increase in CO to supranormal value if signs of hypoperfusion persist [51].
Prognostic marker
Persistent elevation of PCO2 gap in patients with septic shock has been shown to be associated with worse prognosis [52].
Not a marker of hypoxia
As mentioned above, PCO2 gap does not indicate the metabolic impact of hypoperfusion—it does not reflect hypoxia [47].
Cv–aCO2/Ca–vO2 ratio
Physiological principles
This ratio is derived from the RQ, which reflects the ratio of moles of CO2 generated per mole of O2; it can be directly measured by calorimetry and expressed by the equation:
However, it can be also calculated based on the Fick principle:
Using the partial pressure of CO2, it can be obtained a surrogate of RQ:
In aerobic metabolism, one O2 molecule leads approximately to the production of one CO2 molecule, and RQ = 1. In hypoperfusion leading to anaerobic metabolism, there is a decline in both VO2 and VCO2; however, this decline is asymmetric. As mentioned above, VCO2 decreases only slightly due to counterbalancing of the aerobic production decrease by an increase in anaerobic production:
Therefore RQ > 1 implies a switch to anaerobic metabolism (Fig. 7).
Fig. 7.
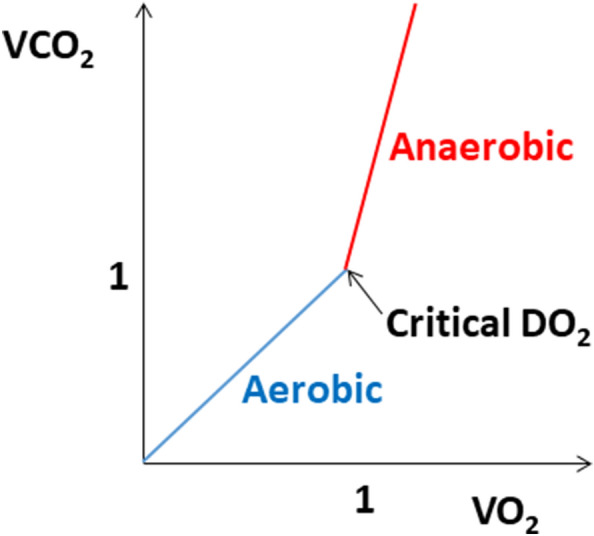
Relationship between carbon dioxide output (VCO2) and oxygen consumption (VO2) (the respiratory quotient) under aerobic and anaerobic metabolism. DO2, oxygen delivery
The Cv–aCO2/Ca–vO2 ratio appears to correspond with lactatemia and RQ measured by calorimetry; however, according to some trials, the surrogate PCO2 gap/Ca–vO2 ratio may be imprecise due to the Haldane effect [14, 45].
Clinical applications
Marker of anaerobic metabolism (adequacy of CO)
A PCO2 gap/Ca–vO2 > 1.4 implies anaerobic metabolism. Its advantage compared to lactate is an earlier reaction [45]. The elevation of both PCO2 gap/Ca–vO2 and lactate strongly indicate ongoing anaerobic metabolism. If PCO2 gap/Ca–vO2 is elevated but lactate levels are normal, it can suggest the onset of anaerobic metabolism (early stage[s] of shock). If PCO2 gap/Ca–vO2 is normal but elevated lactate levels persist, it suggests resolution of aerobic metabolism with persistent lactate elevation from non-hypoxic causes (see above), and over-resuscitation with fluids and inotropes is discouraged [14, 53].
Prognostic marker
It has been shown that patients with septic shock and increased PCO2 gap/Ca–vO2 have a worse prognosis [54]. In contrast to lactate levels, evidence supporting PCO2 gap/Ca–vO2 for GDT is lacking.
Marker of distributive shock (uncoupling)
When CO and SvO2 are high, increased lactate and PCO2 gap/Ca–vO2 can imply microvascular and cellular failure [55].
Algorithm for assessment and monitoring of microcirculation and tissue perfusion
The abovementioned biochemical parameters of global O2 metabolism are used as clinical markers of different aspects of the microcirculation and tissue perfusion status (Table 2). The precise analysis and accurate interpretation of the measured values enable the recognition of the specific cause of tissue hypoperfusion and optimize the therapeutic intervention. We propose an algorithm for the evaluation of tissue perfusion and microcirculation status and related therapeutic consequences that are based on the findings (Fig. 8).
Table 2.
Summary of the clinical use of selected biochemical parameters of global oxygen (O2) metabolism for the assessment of microcirculation and tissue perfusion
| Parameter | Clinical application as a marker | Cut-off value(s) |
|---|---|---|
| SvO2 |
Hypoxia Extraction O2 from hemoglobin Microcirculation and cell failure (high SvO2) |
< 65% > 80% |
| Lactate |
Hypoxia Anaerobic metabolism Strongest data for GDT Also detects local hypoxia |
> 2 mmol/l |
| PCO2 gap | Venous return—perfusion |
> 0.8 kPa (> 6 mmHg) |
| PCO2 gap/Ca–vO2 |
Hypoxia Anaerobic metabolism |
> 1.4 |
Ca–vO2, central venous-to-arterial CO2 difference; GDT, goal-directed therapy; PCO2 gap, central venous–arterial carbon dioxide difference; SvO2, mixed venous oxygen saturation
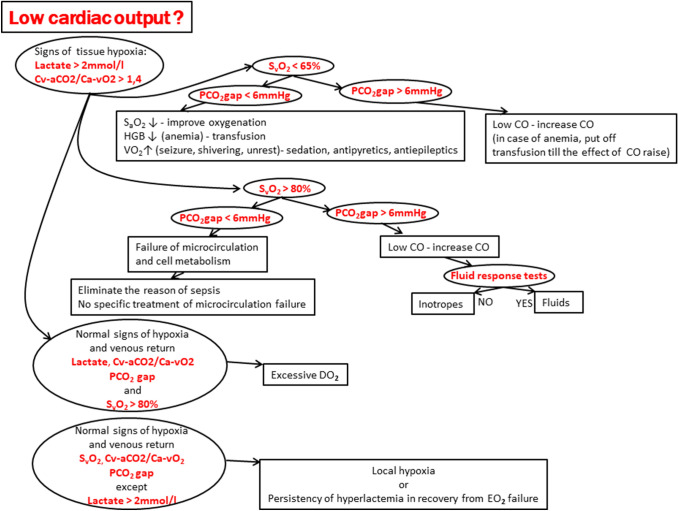
Fig. 8.
Algorithm for the use of parameters of global oxygen metabolism (
adapted from Mallat et al. [45] and Vallet et al. [56]). Ca–vO2, central venous-to-arterial oxygen difference; CO, cardiac output; DO2, oxygen delivery; HGB, hemoglobin; PCO2 gap, central venous–arterial carbon dioxide difference; SaO2, oxygen saturation; SvO2, mixed venous oxygen saturation; VO2, oxygen consumption; ↑, increase; ↓, decrease
Conclusion
The assessment and monitoring of the microcirculation and tissue perfusion is extremely important in conditions involving circulatory shock. Whereas parameters of the macrocirculation, such as blood pressure or CO, are relatively easily available and are amenable to simple interpretation, the situation at the microcirculatory level is significantly more complex. Moreover, there is often only very limited correlation between the findings at the macrocirculation and microcirculation levels, and therapies directed at simply normalizing the macrocirculation without the knowledge of the status of the microcirculation can be even harmful. Currently, the available methods for evaluating the microcirculation are very limited. In recent years, biochemical markers of global O2 metabolism have become routinely used in the assessment of tissue perfusion. However, the interpretation of these values must be based on knowledge of physiological principles and in the context of other findings. Nevertheless, there is mounting evidence that accurate assessment and monitoring of tissue perfusion using parameters of global O2 metabolism is essential for the selection of the most appropriate therapeutic strategy and may improve therapeutic outcomes in patients with critical circulatory conditions.
Acknowledgements
The work was supported by an Institutional grant MH CZ—DRO (Nemocnice Na Homolce—NNH, 00023884), IG150501.
Abbreviations
- ∆PCO2
Venous–arterial carbon dioxide difference from central venous blood
- CaO2
Arterial oxygen concentration
- Ca–vO2
Arterial–venous difference in oxygen concentration
- CO
Cardiac output
- CVC
Central venous catheter
- CvO2
Venous oxygen concentration
- DO2
Oxygen delivery
- EO2
Oxygen extraction
- GDT
Goal-directed therapy
- HGB
Hemoglobin
- HR
Heart rate
- NIRS
Near-infrared spectroscopy
- PAC
Pulmonary artery catheter
- PaCO2
Partial pressure of arterial carbon dioxide
- PaO2
Partial pressure of arterial oxygen
- PCO2 gap
Venous–arterial carbon dioxide difference
- PcvCO2
Partial pressure of central venous carbon dioxide
- PDH
Pyruvate dehydrogenase
- Pv–aCO2
Venous–arterial carbon dioxide partial pressure difference
- PvCO2
Partial pressure of mixed venous carbon dioxide
- RQ
Respiratory quotient
- rSO2
Peripheral oxygen saturation
- SaO2
Arterial oxygen saturation
- ScvO2
Central venous oxygen saturation
- SV
Stroke volume
- SvO2
Mixed venous oxygen saturation
- VCO2
Carbon dioxide production
- VO2
Oxygen consumption
Author contributions
MJ wrote the first draft of the manuscript. PO critically reviewed and revised the manuscript for important intellectual content. Both authors approved the final version for publication.
Data availability
Not applicable.
Code availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL, Vincent JL, Rhodes A. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1795–1815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O'Neill W, Ornato JP, Stelling K, Thiele H, van Diepen S, Naidu SS. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94:29–37. doi: 10.1002/ccd.28329. [DOI] [PubMed] [Google Scholar]
- 3.Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40:2671–2683. doi: 10.1093/eurheartj/ehz363. [DOI] [PubMed] [Google Scholar]
- 4.Saugel B, Vincent JL. Cardiac output monitoring: how to choose the optimal method for the individual patient. Curr Opin Crit Care. 2018;24:165–172. doi: 10.1097/MCC.0000000000000492. [DOI] [PubMed] [Google Scholar]
- 5.Ragosta M. Textbook of clinical hemodynamics. Philadelphia: Elsevier; 2018. [Google Scholar]
- 6.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 7.Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717–1722. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]
- 8.Vincent JL, Bihari DJ. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;331:1160–1161. doi: 10.1056/NEJM199410273311715. [DOI] [PubMed] [Google Scholar]
- 9.Elbers P, Rodrigus T, Nossent E, Malbrain ML, Vonk-Noordegraaf A. Fluid therapy in critically ill patients: perspectives from the right heart. Anaesthesiol Intensive Ther. 2015;47:s38–s43. doi: 10.5603/AIT.a2015.0080. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson S, Nilsson J, Sturesson C. Non-invasive imaging of microcirculation: a technology review. Med Devices (Auckl) 2014;7:445–452. doi: 10.2147/MDER.S51426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ait-Oufella H, Lemoinne S, Boelle PY, Galbois A, Baudel JL, Lemant J, Joffre J, Margetis D, Guidet B, Maury E, Offenstadt G. Mottling score predicts survival in septic shock. Intensive Care Med. 2011;37:801–807. doi: 10.1007/s00134-011-2163-y. [DOI] [PubMed] [Google Scholar]
- 12.Angele MK, Pratschke S, Hubbard WJ, Chaudry IH. Gender differences in sepsis: cardiovascular and immunological aspects. Virulence. 2014;5:12–19. doi: 10.4161/viru.26982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khirfan G, Almoushref A, Naal T, Abuhalimeh B, Dweik RA, Heresi GA, Tonelli AR. Mixed venous oxygen saturation is a better prognosticator than cardiac index in pulmonary arterial hypertension. Chest. 2020 doi: 10.1016/j.chest.2020.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinsky MR, Teboul JL, Vincent JL. Hemodynamic monitoring. New York: Springer; 2019. [Google Scholar]
- 15.Pearse R, Hodes A. Mixed and central venous oxygen saturation. In: Vincent JL, editor. Yearbook of intensive care and emergency medicine 2005. New York: Springer; 2005. [Google Scholar]
- 16.Bryan-Brown CW. Tissue blood flow and oxygen transport in critically ill patients. Crit Care Med. 1975;3:103–108. doi: 10.1097/00003246-197505000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Ince C, Sinaasappel M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med. 1999;27:1369–1377. doi: 10.1097/00003246-199907000-00031. [DOI] [PubMed] [Google Scholar]
- 18.Fink MP. Cytopathic hypoxia. Is oxygen use impaired in sepsis as a result of an acquired intrinsic derangement in cellular respiration? Crit Care Clin. 2002;18:165–175. doi: 10.1016/s0749-0704(03)00071-x. [DOI] [PubMed] [Google Scholar]
- 19.De Backer D, Creteur J, Dubois MJ, Sakr Y, Koch M, Verdant C, Vincent JL. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med. 2006;34:403–408. doi: 10.1097/01.ccm.0000198107.61493.5a. [DOI] [PubMed] [Google Scholar]
- 20.Lough M. Hemodynamic monitoring, evolving technologies and clinical practice. St. Louis: Elsevier; 2015. [Google Scholar]
- 21.Caille V, Squara P. Oxygen uptake-to-delivery relationship: a way to assess adequate flow. Crit Care. 2006;10(Suppl 3):S4. doi: 10.1186/cc4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinhart K, Rudolph T, Bredle DL, Hannemann L, Cain SM. Comparison of central-venous to mixed-venous oxygen saturation during changes in oxygen supply/demand. Chest. 1989;95:1216–1221. doi: 10.1378/chest.95.6.1216. [DOI] [PubMed] [Google Scholar]
- 23.Ramakrishna MN, Hegde DP, Kumaraswamy GN, Gupta R, Girish TN. Correlation of mixed venous and central venous oxygen saturation and its relation to cardiac index. Indian J Crit Care Med. 2006;10:230–234. doi: 10.4103/0972-5229.29841. [DOI] [Google Scholar]
- 24.Cavaliere F, Zamparelli R, Martinelli L, Scapigliati A, De Paulis S, Caricato A, Gargaruti R, Cina A. Blood from the right atrium may provide closer estimates of mixed venous saturation than blood from the superior vena cava. A pilot study. Minerva Anestesiol. 2014;80:11–18. [PubMed] [Google Scholar]
- 25.Li L, Subramaniaam B, Aguirre AD, Andrawes MN, Tearney GJ (2016) In-vivo continuous monitoring of mixed venous oxygen saturation by photoacoustic transesophageal echocardiography. Photonic therapeutics and diagnostics XII
- 26.Liakopoulos OJ, Ho JK, Yezbick A, Sanchez E, Naddell C, Buckberg GD, Crowley R, Mahajan A. An experimental and clinical evaluation of a novel central venous catheter with integrated oximetry for pediatric patients undergoing cardiac surgery. Anesth Analg. 2007;105:1598–1604. doi: 10.1213/01.ane.0000287657.08434.dc. [DOI] [PubMed] [Google Scholar]
- 27.Levy B, Bastien O, Karim B, Cariou A, Chouihed T, Combes A, Mebazaa A, Megarbane B, Plaisance P, Ouattara A, Spaulding C, Teboul JL, Vanhuyse F, Boulain T, Kuteifan K. Experts’ recommendations for the management of adult patients with cardiogenic shock. Ann Intensive Care. 2015;5:52. doi: 10.1186/s13613-015-0052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric S Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Investigators A, Group ACT. Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, Williams P. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 30.Pro CI, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, Coats TJ, Singer M, Young JD, Rowan KM, Pro MTI. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 32.Textoris J, Fouche L, Wiramus S, Antonini F, Tho S, Martin C, Leone M. High central venous oxygen saturation in the latter stages of septic shock is associated with increased mortality. Crit Care. 2011;15:R176. doi: 10.1186/cc10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheeren TW, Schober P, Schwarte LA. Monitoring tissue oxygenation by near infrared spectroscopy (NIRS): background and current applications. J Clin Monit Comput. 2012;26:279–287. doi: 10.1007/s10877-012-9348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103(Suppl 1):i3–i13. doi: 10.1093/bja/aep299. [DOI] [PubMed] [Google Scholar]
- 35.Mozina H, Podbegar M. Near-infrared spectroscopy for evaluation of global and skeletal muscle tissue oxygenation. World J Cardiol. 2011;3:377–382. doi: 10.4330/wjc.v3.i12.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostadal P, Kruger A, Vondrakova D, Janotka M, Psotova H, Neuzil P. Noninvasive assessment of hemodynamic variables using near-infrared spectroscopy in patients experiencing cardiogenic shock and individuals undergoing venoarterial extracorporeal membrane oxygenation. J Crit Care. 2014;29(690):e11–e15. doi: 10.1016/j.jcrc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 37.James JH, Fang CH, Schrantz SJ, Hasselgren PO, Paul RJ, Fischer JE. Linkage of aerobic glycolysis to sodium-potassium transport in rat skeletal muscle. Implications for increased muscle lactate production in sepsis. J Clin Investig. 1996;98:2388–2397. doi: 10.1172/JCI119052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy B, Desebbe O, Montemont C, Gibot S. Increased aerobic glycolysis through beta2 stimulation is a common mechanism involved in lactate formation during shock states. Shock. 2008;30:417–421. doi: 10.1097/SHK.0b013e318167378f. [DOI] [PubMed] [Google Scholar]
- 39.Suetrong B, Walley KR. Lactic acidosis in sepsis: it’s not all anaerobic: implications for diagnosis and management. Chest. 2016;149:252–261. doi: 10.1378/chest.15-1703. [DOI] [PubMed] [Google Scholar]
- 40.Chavez J, Glaser S, Krom Z. Continuous lactate measurement devices and implications for critical care: a literature review. Crit Care Nurs Q. 2020;43:269–273. doi: 10.1097/CNQ.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 41.Bakker J. Lactate levels and hemodynamic coherence in acute circulatory failure. Best Pract Res Clin Anaesthesiol. 2016;30:523–530. doi: 10.1016/j.bpa.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Hernandez G, Luengo C, Bruhn A, Kattan E, Friedman G, Ospina-Tascon GA, Fuentealba A, Castro R, Regueira T, Romero C, Ince C, Bakker J. When to stop septic shock resuscitation: clues from a dynamic perfusion monitoring. Ann Intensive Care. 2014;4:30. doi: 10.1186/s13613-014-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puskarich MA, Trzeciak S, Shapiro NI, Arnold RC, Heffner AC, Kline JA, Jones AE, Emergency Medicine Shock Research N Prognostic value and agreement of achieving lactate clearance or central venous oxygen saturation goals during early sepsis resuscitation. Acad Emerg Med. 2012;19:252–8. doi: 10.1111/j.1553-2712.2012.01292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernandez G, Ospina-Tascon GA, Damiani LP, Estenssoro E, Dubin A, Hurtado J, Friedman G, Castro R, Alegria L, Teboul JL, Cecconi M, Ferri G, Jibaja M, Pairumani R, Fernandez P, Barahona D, Granda-Luna V, Cavalcanti AB, Bakker J, The ASI, the Latin America Intensive Care N. Hernandez G, Ospina-Tascon G, Petri Damiani L, Estenssoro E, Dubin A, Hurtado J, Friedman G, Castro R, Alegria L, Teboul JL, Cecconi M, Cecconi M, Ferri G, Jibaja M, Pairumani R, Fernandez P, Barahona D, Cavalcanti AB, Bakker J, Hernandez G, Alegria L, Ferri G, Rodriguez N, Holger P, Soto N, Pozo M, Bakker J, Cook D, Vincent JL, Rhodes A, Kavanagh BP, Dellinger P, Rietdijk W, Carpio D, Pavez N, Henriquez E, Bravo S, Valenzuela ED, Vera M, Dreyse J, Oviedo V, Cid MA, Larroulet M, Petruska E, Sarabia C, Gallardo D, Sanchez JE, Gonzalez H, Arancibia JM, Munoz A, Ramirez G, Aravena F, Aquevedo A, Zambrano F, Bozinovic M, Valle F, Ramirez M, Rossel V, Munoz P, Ceballos C, Esveile C, Carmona C, Candia E, Mendoza D, Sanchez A, Ponce D, Ponce D, Lastra J, Nahuelpan B, Fasce F, Luengo C, Medel N, Cortes C, Campassi L, Rubatto P, Horna N, Furche M, Pendino JC, Bettini L, Lovesio C, Gonzalez MC, Rodruguez J, Canales H, Caminos F, Galletti C, Minoldo E, Aramburu MJ, Olmos D, Nin N, Tenzi J, Quiroga C, Lacuesta P, Gaudin A, Pais R, Silvestre A, Olivera G, Rieppi G, Berrutti D, Ochoa M, Cobos P, Vintimilla F, Ramirez V, Tobar M, Garcia F, Picoita F, Remache N, Granda V, Paredes F, Barzallo E, Garces P, Guerrero F, Salazar S, Torres G, Tana C, Calahorrano J, Solis F, Torres P, Herrera L, Ornes A, Perez V, Delgado G, Lopez A, Espinosa E, Moreira J, Salcedo B, Villacres I, Suing J, Lopez M, Gomez L, Toctaquiza G, Cadena Zapata M, Orazabal MA, Pardo Espejo R, Jimenez J, Calderon A, Paredes G, Barberan JL, Moya T, Atehortua H, Sabogal R, Ortiz G, Lara A, Sanchez F, Hernan Portilla A, Davila H, Mora JA, Calderon LE, Alvarez I, Escobar E, Bejarano A, Bustamante LA, Aldana JL. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA. 2019;321:654–664. doi: 10.1001/jama.2019.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mallat J, Lemyze M, Meddour M, Pepy F, Gasan G, Barrailler S, Durville E, Temime J, Vangrunderbeeck N, Tronchon L, Vallet B, Thevenin D. Ratios of central venous-to-arterial carbon dioxide content or tension to arteriovenous oxygen content are better markers of global anaerobic metabolism than lactate in septic shock patients. Ann Intensive Care. 2016;6:10. doi: 10.1186/s13613-016-0110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Beest PA, Lont MC, Holman ND, Loef B, Kuiper MA, Boerma EC. Central venous-arterial pCO(2) difference as a tool in resuscitation of septic patients. Intensive Care Med. 2013;39:1034–1039. doi: 10.1007/s00134-013-2888-x. [DOI] [PubMed] [Google Scholar]
- 47.Mallat J, Lemyze M, Tronchon L, Vallet B, Thevenin D. Use of venous-to-arterial carbon dioxide tension difference to guide resuscitation therapy in septic shock. World J Crit Care Med. 2016;5:47–56. doi: 10.5492/wjccm.v5.i1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vallet B, Teboul JL, Cain S, Curtis S. Venoarterial CO(2) difference during regional ischemic or hypoxic hypoxia. J Appl Physiol (1985) 2000;89:1317–1321. doi: 10.1152/jappl.2000.89.4.1317. [DOI] [PubMed] [Google Scholar]
- 49.Cuschieri J, Rivers EP, Donnino MW, Katilius M, Jacobsen G, Nguyen HB, Pamukov N, Horst HM. Central venous-arterial carbon dioxide difference as an indicator of cardiac index. Intensive Care Med. 2005;31:818–822. doi: 10.1007/s00134-005-2602-8. [DOI] [PubMed] [Google Scholar]
- 50.Creteur J, De Backer D, Sakr Y, Koch M, Vincent JL. Sublingual capnometry tracks microcirculatory changes in septic patients. Intensive Care Med. 2006;32:516–523. doi: 10.1007/s00134-006-0070-4. [DOI] [PubMed] [Google Scholar]
- 51.Shoemaker WC, Appel PL, Kram HB. Role of oxygen debt in the development of organ failure sepsis, and death in high-risk surgical patients. Chest. 1992;102:208–215. doi: 10.1378/chest.102.1.208. [DOI] [PubMed] [Google Scholar]
- 52.Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, Fumagalli R. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med. 1995;333:1025–1032. doi: 10.1056/NEJM199510193331601. [DOI] [PubMed] [Google Scholar]
- 53.He H, Long Y, Liu D, Wang X, Tang B. The Prognostic value of central venous-to-arterial CO2 difference/arterial-central venous O2 difference ratio in septic shock patients with central venous O2 saturation >/=80. Shock. 2017;48:551–557. doi: 10.1097/SHK.0000000000000893. [DOI] [PubMed] [Google Scholar]
- 54.He HW, Liu DW, Long Y, Wang XT. High central venous-to-arterial CO2 difference/arterial-central venous O2 difference ratio is associated with poor lactate clearance in septic patients after resuscitation. J Crit Care. 2016;31:76–81. doi: 10.1016/j.jcrc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 55.Monnet X, Julien F, Ait-Hamou N, Lequoy M, Gosset C, Jozwiak M, Persichini R, Anguel N, Richard C, Teboul JL. Lactate and venoarterial carbon dioxide difference/arterial-venous oxygen difference ratio, but not central venous oxygen saturation, predict increase in oxygen consumption in fluid responders. Crit Care Med. 2013;41:1412–1420. doi: 10.1097/CCM.0b013e318275cece. [DOI] [PubMed] [Google Scholar]
- 56.Vallet B, Pinsky MR, Cecconi M. Resuscitation of patients with septic shock: please “mind the gap”! Intensive Care Med. 2013;39:1653–1655. doi: 10.1007/s00134-013-2998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.