Abstract
Contrast-induced encephalopathy (CIE) is a well-known but rare complication following contrast media administration. Its nonspecific clinical manifestations hinder diagnosis, particularly in the pediatric population. The majority of cases are reversible, with clinical improvement and resolution of signs noted on diagnostic imaging. Here, we report the case of a 2-month-old patient with a history of complex cardiovascular disease who presented with a single episode of seizure after undergoing cardiac catheterization with nonionic iodinated contrast media. CIE is diagnosed based on the signs and symptoms exhibited by the patient and the findings on plain head computed tomography (CT) scan. Subsequently, the absence of neurological symptoms and disappearance of the imaging alterations on a control CT are documented.
Keywords: Encephalopathy, Neurotoxicity, Contrast medium, Blood–brain barrier
Introduction
Contrast-induced encephalopathy (CIE) is a complication that occurs in patients who exhibit de novo neurological disorders after undergoing medical imaging procedures using iodinated contrast, particularly cardiac catheterization [1]. It is a complication that generally has a good prognosis; however, it should be carefully considered by the medical staff, and parents should be informed about it before the procedure.
The pathophysiology is unclear; however, it is believed to be related to chemical and osmotic factors of the contrast medium that can damage the blood–brain barrier [1,2]. Secondary osmotic alterations explain the appearance of cerebral edema. The presence of the contrast agent within the brain parenchyma causes an excitatory activity, which is eliminated within the subsequent 96 h depending on the renal function of the patient [1]. After this period, complete resolution of the neurological symptoms and computed tomography (CT) abnormalities occurs [1,2].
Patients usually experience new-onset focal epilepsy with tomographic findings indicating the retention of the contrast agents in the interstitium; however, this can confuse the physician if the possibility of CIE is not considered.
CIE accounts for up to 1% of cases undergoing these procedures [1]; however, its incidence could be higher, even more so considering that amnesia and cortical blindness are the most frequent manifestations in the adult population, both being difficult to detect in neonates and lactating patients. The most common neurological manifestations in pediatric patients are hemiplegia and focal or generalized clonic–tonic seizures, which are more frequent in children with a history of epilepsy or intracranial injury [1].
Case report
A 2-month-old female patient, born at full-term, was admitted at the pediatric cardiology department of our institution because of occasional cyanosis reported by her mother. She had a history of complex congenital heart disease with a single ventricle defect and double outlet right ventricle with D-transposition of the great arteries associated with ostium secundum‐type atrial septal defect involving the left vena cava. The patient had good general condition on physical examination, her weight was 5 kg, with a saturation level of 80% and no signs of respiratory distress. She was on furosemide and spironolactone treatment, both not known to be associated with seizures.
The interdisciplinary team decided to perform pulmonary artery banding. During the surgical procedure, the patient showed hemodynamic instability and cardiorespiratory arrest, requiring cardiac massage, which prevented the completion of the procedure. To supplement studies, catheterization was performed through the left femoral artery (catheter tip located into the single ventricle), with serial doses of 1-1.5 mL/kg over 30-minute period (total dose of 3 mL/kg (15 mL)) of nonionic iodinated contrast media (Iopromide).
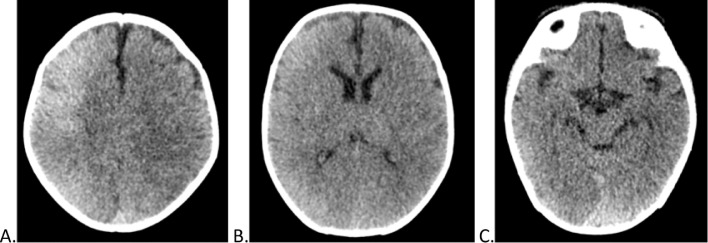
Approximately 12 hours after the procedure, the patient exhibited clonic movements of the left upper limb and persistent sucking movements. A plain head CT was performed (Fig. 1) after a single seizure episode, demonstrating a slight diffuse increase in the cortical density in the right frontal, parietal, and occipital lobes, and to a lesser extent in the left parietal and occipital lobes. There were no mass effect, bleeding, or other alterations.
Fig. 1.
Initial noncontrast head tomography, after cardiac catheterization. (A-C) Axial cuts from cranial to caudal. Slight diffuse increase in the density of predominantly frontal, parietal and occipital cortices in the right cerebral hemisphere, and to a lesser extent in the left parietal and occipital lobe. No expansive lesions or signs suggestive of a mass effect are observed.
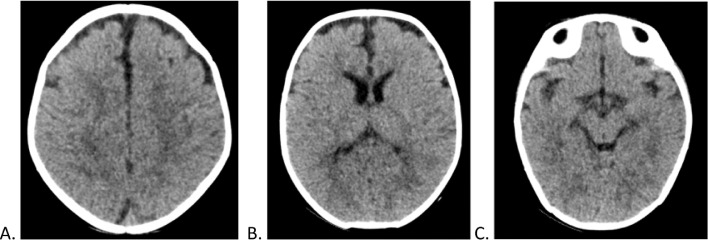
Considering the observed imaging findings and the single de novo seizure episode after contrast agent administration, CIE was diagnosed. The patient did not present new seizure episodes or neurological deterioration, and 11 days after catheterization, resolution of the CIE was observed on the control plain head CT scan (Fig. 2). Owing to factors related to her primary condition, she required follow-up and in-hospital management.
Fig. 2.
Control noncontrast head tomography, 11 days after cardiac catheterization. (A-C) Axial cuts from cranial to caudal. Absence of diffuse hyperdensity evidenced in the previous skull tomography
Discussion
CIE occurs when the contrast agent passes through the blood–brain barrier to the brain [2]. This barrier is related to the special features of the brain microcirculation that prevent the passage of molecules, ions, and cells from the blood to the brain [3].
The main components of the barrier are [4] as follows:
-
•
The tight junctions located between endothelial cells have various mechanisms (claudins, efflux pumps, electrical charges, etc.) that limit the entry of different substances
-
•
Endothelial cells lack the fenestrations present in other tissues
-
•
Absence of endocytic vesicles at the endothelial cell level
-
•
Presence of pericytes and glial cells acting as additional barriers
To date, the blood–brain barrier was believed to be immature in the fetus, embryo, and neonate, a situation that is currently known to be untrue [5]. This hypothesis suggested that patients in the neonatal stage are more prone to suffer pathologies such as CIE [5]. Currently, it is known that there are factors that may increase the barrier's permeability, particularly those related to increased cerebral blood flow (hypoxemia and hypercapnia) or systemic inflammatory states (neonatal sepsis and hyperbilirubinemia) [6].
Regarding contrast agents, osmotic and biochemical mechanisms have been postulated as they cause temporary opening of the tight junctions that allow the passage of contrast media to the cerebral cortex [2,6]. Although the ionic iodinated CIE is more frequent, there are multiple cases reported with nonionic media [2]. Regarding the injection of the contrast agent, the risk increases when using the intra-arterial route [2] because encephalopathy has been more frequently observed after angiography similar to that with cardiac catheterization.
Carboxyl ends, sodium ions, and benzene rings have been postulated as the cause of neurotoxicity in these patients because they act as excitatory substances at the neuronal level [2]. Patients with underlying epilepsy, intracranial lesions, predisposing factors to hyperflow, such as hypoxemia and hypercapnia, and neonates with inflammatory states such as sepsis and hyperbilirubinemia are more likely to experience encephalopathy.
The onset of neurological symptoms occurs between the first 2-12 hours and lasts up to 24-96 hours after injection of the contrast agent [1,2]. Transient neurological symptoms along with diagnostic imaging allow the diagnosis of CIE and exclude other complications that may arise in angiographic studies with contrast media, such as ischemic cerebrovascular accident and hemorrhage. The diagnosis is confirmed when the symptoms and the alterations in the images disappear, which can occur 25 hours following the administration of the contrast agent [7].
In plain head CT scan, the characteristic findings of CIE can range from diffuse cortical and subcortical hyperdensity to hyperdense lesions, enhancement of the sulci, cerebral edema, and enhancement of the subarachnoid spaces [8]. To distinguish CIE from other complications, several aspects should be carefully considered. In plain CT scan, the contrast medium has an approximate density of 100-300 Hounsfield Units (HU), whereas the density of blood ranges between 40 and 60 (HU). In addition to the above, the involvement of more than one vascular territory makes an ischemic or hemorrhagic event less likely to occur [7]. Finally, in magnetic resonance imaging (MRI), the areas affected by the CIE will be hyperintense in the T2, fluid-attenuated inversion-recovery, and diffusion-weighted imaging sequences. Unlike in ischemic events, the MRI apparent diffusion coefficient for the CIE will be normal [8].
In conclusion, CIE is a relevant differential diagnosis in patients presenting with de novo neurological alterations after undergoing procedures with iodinated contrast agents, particularly seizures and hemiplegia, while considering that amnesia and cortical blindness have also been described in adults. Head CT scan will be mandatory in these patients to exclude other causes of neurological symptoms and to identify whether the initial findings in the control images have been resolved.
Patient consent
Patient consent has been obtained.
References
- 1.Frye R, Newburger J, Nugent A, Sahin M. Focal seizure and cerebral contrast retention after cardiac catheterization. Pediatr Neurol. 2005;32:213–216. doi: 10.1016/j.pediatrneurol.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Torvik A, Walday P. Neurotoxicity of water -soluble contrast media. Acta Radiológica. 1995;36:221–229. doi: 10.1177/0284185195036s39927. [DOI] [PubMed] [Google Scholar]
- 3.Saunders N, Dziegielewska K, Kjeld Mollgard K, Habgood M. Physiology and molecular biology of barrier mechanisms in the fetal and neonatal brain. Physiol. 2018:5723–5756. doi: 10.1113/JP275376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominguez Ortega F, González Azpeitia G, Cidras Pidre M, Calvo Rosales J. Apertura reversible de la barrera hematoencefálica inducida por hipercapnia en hiperbilirrubinemia experimental. An Esp Pediatr. 1997;46:374–377. [PubMed] [Google Scholar]
- 5.Haussen DC, Modir R, Yavagal DR. Unilateral contrast neurotoxicity as a stroke mimic after cerebral angiogram. J Neuroimaging. 2011;XX:1–3. doi: 10.1111/j.1552-6569.2011.00655.x. [DOI] [PubMed] [Google Scholar]
- 6.Rajagopal R, Raju SN, Pandey NN, Sharma S. Contrast-related neurotoxicity: an entity not to be forgotten. J Clin Interv Radiol ISVIR. 2019;3:139–141. [Google Scholar]
- 7.Spina R, Simon N, Markus R. Contrast-induced encephalopathy following cardiac catheterization. Catheter Cardiovasc Interv. 2017;90:257–268. doi: 10.1002/ccd.26871. [DOI] [PubMed] [Google Scholar]
- 8.Bhargavi D, Trottier SA. Seizure and hemiplegia following contrast exposure: understanding contrast-induced encephalopathy. Case Rep Med. 2018 doi: 10.1155/2018/9278526. Article ID 9278526. [DOI] [PMC free article] [PubMed] [Google Scholar]