Abstract
Joubert Syndrome is a rare autosomal recessive genetic disorder characterized by a distinctive midbrain-hindbrain malformation that gives the appearance of “the molar tooth sign” on axial magnetic resonance imaging (MRI). Mutations in the implicated genes, affect proteins integral to cellular structures like the primary cilium, basal bodies and centromeres, categorizing Joubert syndrome as a ciliopathy. The most common clinical manifestations include moderate to severe hypotonia in early infancy with ataxia developing later in life, abnormal breathing patterns (tachypnea, apnea), atypical eye movements, development delay and intellectual disabilities. Differential diagnosis between different ciliopathies is challenging due to the overlapping clinical features. French neurologist Marie Joubert was the first to describe the clinical findings in 1969 and later the disorder was named after her.
In this report, we present the case of a newborn female patient who was admitted to the neonatal intensive care unit 12 hours after birth, presenting with dyspnea, cyanosis, signs of respiratory distress and seizures. During the course of her hospitalization elevated levels of urea and creatinine were detected and after an abdominal ultrasound and CT evaluation bilateral renal hyperplasia and polycystic kidney disease were discovered. An MRI of the head and neck revealed the presence of inferior vermis agenesis, with a medial crack in cerebellum, a partial dysgenesis of corpus callosum, an underlying and thicker cerebral peduncle, as well as the molar tooth sign suggesting a diagnosis of Joubert syndrome. The diagnosis was ultimately confirmed through molecular genetic testing. Through this case report, we hope to draw attention to this rare and elusive group of disorders and emphasize the value of a prompt diagnosis and a proactive and multidisciplinary approach in the management of these patients.
Keywords: Joubert syndrome, ciliopathy, CEP290, molar tooth sign, MRI
Introduction
Joubert syndrome (JS) (OMIM 213300) is a rare and clinically heterogeneous group of disorders defined by the presence of a midbrain-hindbrain malformation known as the molar tooth sign (MTS), a pathognomonic neuroradiologic finding characterized by cerebellar vermis hypoplasia, abnormally deep interpeduncular fossa at the level of the isthmus and upper pons, horizontalized, thickened and elongated superior cerebellar peduncles, that represents the hallmark of this diverse group of disorders [1]. Prevalence is reported to be around 1:80,000 to 1:100,000 but is believed to be underestimated [1,2]. In the past, the term Joubert syndrome and related disorders (JSRD) has been used to describe conditions that share the presence of the molar tooth sign, clinical features of JS and multiple organ involvement, but in current literature Joubert syndrome has been adopted as the accepted term to describe all types of JS [2].
Joubert syndrome is a genetically heterogeneous disorder with numerous implicated genes, mutations are by and large inherited in an autosomal recessive pattern, with the exception of a few rare cases that are inherited in an X-linked recessive pattern [2], [3], [4].
Classic Joubert manifests with hypotonia in early infancy and ataxia later in life, developmental delay and the presence of the molar tooth sign. Nonetheless, the majority of patients present with multiple organ involvement including ocular, renal and hepatic involvement [1], [2], [3],5]. Based on the phenotypic presentation, Joubert syndrome is classified in 6 different subgroups: pure JS, JS with ocular defect, JS with renal defect, JS with oculo-renal defects, JS with hepatic defect, JS with oro-facio-digital defects [1].
The most common neurological signs include abnormal breathing patterns, tachypnea, sleep apnea, hypotonia, abnormal eye and tongue movements, intellectual disability and ataxia [1], [2], [3],5]. Associated eye defects include abnormal development of the retina, coloboma, nystagmus, strabismus, and ptosis [1], [2], [3],5]. Renal involvement usually encompasses nephronophthisis alone, both nephronophthisis and cystic dysplasia as well as cases resembling autosomal recessive polycystic kidney disease [1], [2], [3]. Distinctive facial features may also be present such as a broad forehead, arched eyebrows, ptosis, widely spaced eyes, low-set ears and a triangle-shaped mouth [1,2]. Less common clinical features include polydactyly, cleft lip or palate and seizures [1,2].
Diagnosis is usually suspected based on the clinical manifestations. There is a consensus among authors that the presence of the molar tooth sign, evident on axial MRI is mandatory [1], [2], [3]. Ultimately diagnosis is established through molecular genetic testing, either a multigene panel or comprehensive genomic sequencing [2]. Genetic counseling, carrier testing for at-risk family members, preimplementation testing and prenatal diagnosis for future pregnancies are strongly recommended [2,3,5].
A multidisciplinary approach is fundamental in the management of Joubert syndrome. Neurological manifestations in early infancy like hypotonia and the associated breathing anomalies may require respiratory assistance (supplemental oxygen, CPAP, noninvasive or invasive ventilation and in rare cases tracheostomy) and stimulatory medications [1,3]. Nutritional support is crucial, due to possible feeding problems arising from the moderate to severe hypotonia during infancy [1], [2], [3]. Patients should undergo a thorough evaluation to assess for multiorgan involvement, particularly ocular, renal and hepatic problems. Patients should be followed-up closely with yearly pediatric, neuropsychological and developmental assessment, ophthalmological evaluation, urinalysis, abdominal ultrasound and a comprehensive metabolic panel, to ensure a timely diagnosis and management of possible complications [3].
A global cognitive and behavioral assessment is paramount to providing adequate neuropsychological support and rehabilitation tailored to each patient's needs which includes speech therapy, physical and occupational therapy as well as educational support. Prognosis varies greatly depending on the extent of organ involvement and developmental delay.
Case report
Herein, we present the case of a newborn female patient, the third child of nonconsanguineous parents, born at term (39 weeks) via an elective C-section following a normal pregnancy. At birth, the patient presented with dyspnea, cyanosis and signs of respiratory distress. She was admitted to the neonatal intensive care unit (NICU) where continuous positive airway pressure (CPAP) with supplemental oxygen (FiO2 30%) was administered. Parenteral nutrition and antibiotic therapy were initiated as well. On admission, the patient also presented with seizures for which she received a dose of phenobarbital and continued receiving a maintenance dose during her hospitalization. During her stay in the NICU the patient experienced episodes of elevated blood pressure that were managed using nifedipine and received packed red blood cells (PRBC) transfusions to treat the underlying anemia. No recurrence of seizures was reported during her stay.
Physical examination revealed a normal skull configuration with an open anterior fontanelle, pupils were equal and reactive to light. The patient's weight on admission was 3460 kg, her height was 50 cm and her head circumference 34,5 cm. Cardiac and pulmonary auscultation were unremarkable, the abdomen soft and palpable. Skin turgor was normal, peripheral pulses were present and symmetrical bilaterally. Spontaneous motor activity was present. Notably, severe axial hypotonia and an incomplete Moro reflex were evident during examination.
A comprehensive workup was ordered including blood and urine cultures that identified the presence of Klebsiella pneumoniae. A lumbar puncture for a CSF culture was ordered to rule out central nervous system infections. The patient was initially started on a combination of meropenem and cloxacillin for 8 days, then monotherapy with meropenem was continued until discharge for a total duration of 11 days.
Cardiac ultrasound showed absence of congenital heart defects, open fetal structures were present, normal heart chambers, an intact interventricular septum and an ejection fraction (EF) of 66%. Abdominal ultrasound evidenced enlarged and hyperechogenic kidneys with loss of corticomedullary differentiation. Visualization of the pyelocaliceal system was normal, with no signs of obstruction. A CT of the abdomen was thereafter requested that showed bilateral renal hyperplasia and evidence of polycystic kidney disease. During hospitalization, the metabolic panel reported elevated levels of urea (11.79 mmol/L) and creatinine (243 mcmol/L). Before being discharged from NICU the values of urea were (4.1 mmol/I) and creatinine (140 mol/I). Both the fundoscopic examination and the hearing screening test were normal.
An ENT consult raised doubts about a possible mild laryngomalacia that was subsequently confirmed through a fiberoptic bronchoscopy examination that also excluded synchronous bronchial anomalies. The patient was seen by a pediatric neurologist and a head and neck MRI was requested. Initially a diagnosis of Dandy Walker malformation with no clear signs of obstruction of the foramen magnum was suspected, however upon closer reevaluation of the MRI the presence of inferior vermis agenesis with a medial crack in the cerebellum, a partial dysgenesis of corpus calosum, an underlying and thicker cerebral peduncle as well the molar tooth sign, all pointed to Joubert syndrome as a potential diagnosis.
The definitive diagnosis was established through molecular genetic testing. Genome sequencing revealed a mutation involving the CEP290 gene that ultimately confirmed the diagnosis of Joubert syndrome.
Discussion
Joubert syndrome represents a rare group of clinically and genetically heterogeneous disorders with the molar tooth sign (MTS), the radiological evidence of cerebellar vermis hypoplasia, abnormally deep interpeduncular fossa at the level of the isthmus and upper pons, horizontalized, thickened and elongated superior cerebellar peduncles, as the hallmark of the syndrome [1,6]. From a histopathological perspective, the molar tooth sign is characterized by a severe hypoplasia of the cerebellar vermis, fragmentation of cerebellar nuclei, dysplasia of pontine and medullary structures and a lack of decussation of the superior cerebellar peduncles and of the corticospinal tracts [1].
Due to its predominantly autosomal recessive inheritance pattern, couples with an affected child carry a 25% chance of producing an affected offspring. There are about 33 different genes implicated in the pathogenesis of Joubert syndrome [2]. Mutations in the AHI1 gene are responsible for about 11% of the affected population, mutations in the NPHP1 gene are believed to give rise to approximately 1%-2% of the cases and mutations in the CEP290 gene are believed to account for 4%-10% of all the cases [7]. The proteins encoded by these genes are part of cellular structures called primary cilium and/or basal body and centromere [5]. They are believed to play an important structural and functional role in these organelles.
Seeing as the culprit mutation in our patient was found in the CEP290 gene, for the purpose of this case report we have focused on the proposed role CEP290 gene plays in the pathogenesis of Joubert syndrome. CEP290 gene encodes a centrosomal protein termed nephrocystin-6 that modulates the activity of a transcription factor (ATF4) which is believed to be instrumental in renal cyst formation [2,5,8]. This would explain the frequent association between CEP290 mutations and Joubert syndrome with renal involvement, as well as other syndromes such as Senior-Loken, Meckel, Bardet-Biedl with overlapping clinical features [2,3]. Recently, it has been proposed that CEP290 gene product may also play a role in the microtubule-based ciliary transport, in the vesicle transport, the development and maintenance of the cilium [5,9,10,11].
Most frequently reported mutations include small deletions, insertions, splice-site, a few missense mutations resulting in frameshift mutations that ultimately produce truncated versions of this centrosomal protein [2,3]. These loss-of-function mutations are believed to cause disease, but the underlying pathogenetic mechanisms remain to be elucidated [12]. Owing to the fundamental role the centrosome plays in chromosome segregation and cell regulation, as well as the fact that they are present during the cerebellar murine embryogenesis, it has been hypothesized that loss-of-function mutations of CEP290 gene are responsible for the severe clinical picture of Joubert syndrome and other pathologies [2,9], thus underscoring the importance of primary cilium, basal bodies and centrosomes in normal cellular function.
Through this case report, we hope to draw attention to this rare and elusive group of disorders and emphasize the value of a prompt diagnosis and a proactive and multidisciplinary approach in the management of these patients.
Conclusion
It is our hope, this case report helps give insight into the diverse and heterogenous clinical picture of ciliopathies, in particular Joubert syndrome. At the same time, we aim to highlight the importance of a timely diagnosis in adopting a proactive approach in the management of these disorders, tailored to the individual needs of each patient, in hopes of influencing and improving their overall prognosis (Fig. 1, Fig. 2).
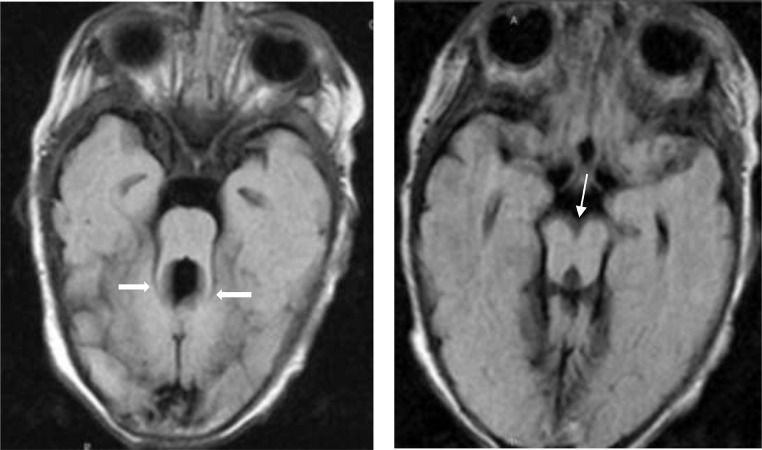
Fig. 1.
FLAIR ax sequence (A and B). There is an elongation of the superior cerebellar peduncles including thickened, elongated, parallel, and horizontally orientated superior cerebellar peduncle(thick white arrows)that gives the shape of the molar tooth (molar tooth sign), the absence of vermis and in B (thin white arrow) the deepening of the interpeduncular fossa.
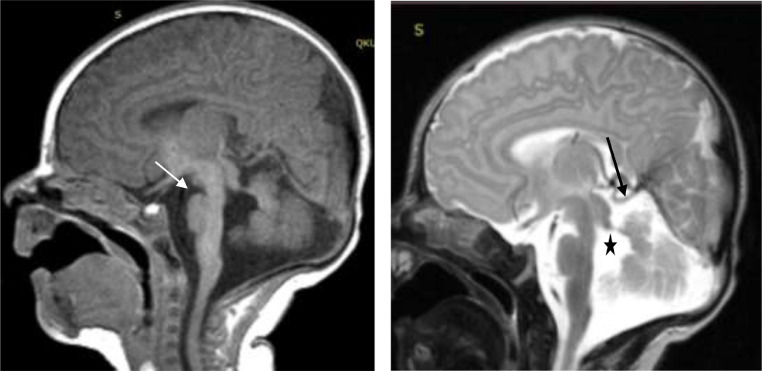
Fig. 2.
Sequence T1 sag (A) a deepened IF (white arrow) and T2 sag. (B) absence/hypoplasia of the vermis is observed(long black arrow), an enlarged fourth ventricle (black asterisk).
Patient consent
Patient consent has been obtained.
References
- 1.Brancati Francesco, Dallapiccola, Bruno, Maria Valente Enza. Joubert Syndrome and related disorders. Orphanet J Rare Dis. 2010;5:20. doi: 10.1186/1750-1172-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parisi M, Glass I. Joubert Syndrome. 2003 Jul 9 [Updated 2017 Jun 29] In: Adam MP, Ardinger HH, Pagon RA, editors. GeneReviews. University of Washington; Seattle (WA): 2020. https://www.ncbi.nlm.nih.gov/books/NBK1325/ Seattle1993-Available from: [PubMed] [Google Scholar]
- 3.Parisi M., Doherty D., Chance P., Glass Ian A. Joubert syndrome (and related disorders) (OMIM 213300) Eur J Hum Genet. 2007;15:511–521. doi: 10.1038/sj.ejhg.5201648. [DOI] [PubMed] [Google Scholar]
- 4.Coene KLM, Roepman Ronald, Doherty Dan, Afroze Bushra, Kroes Hester, Letteboer Stef.J.F. OFD1 is mutated in X-linked Joubert syndrome and interacts with LCA5-encoded lebercilin. Am J Hum Genet. 2009;85(4):465–481. doi: 10.1016/j.ajhg.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doherty D. Joubert syndrome: insights into brain development, cilium biology, and complex disease. Semin Pediatr Neurol. 2009;16(3):143–154. doi: 10.1016/j.spen.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maria BL, Quisling RG, Rosainz LC, Yachnis AT, Gitten J, Dede D. Molar tooth sign in Joubert syndrome: clinical radiologic, and pathologic significance. J Child Neurol. 1999;14:368–376. doi: 10.1177/088307389901400605. [DOI] [PubMed] [Google Scholar]
- 7.“Joubert syndrome”. National Organization for Rare Disorders (NORD).Retrieved 8 November 2020
- 8.Sayer John, Otto Edgar A, O’Toole John F, Nurnberg Gudrun, Michael Kennedy, Becker Christian. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006;38:674–681. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 9.Chang Bo, Khanna Hemant, Hawes Norman, Jimeno David, He Shirley, Lillo Concepcion. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006;15:1847–1857. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J, Krishnaswami SR, Gleeson JG. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet. 2008;17:3796–3805. doi: 10.1093/hmg/ddn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsang WY, Bossard Carine, Khanna Hemant, Peranen Johan, Swaroop Anand, Malhotra Vivek. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev Cell. 2008;15:187–197. doi: 10.1016/j.devcel.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valente Enza Maria, Silhavy Jennifer L, Brancati Francesco, Barrano Giuseppe, Krishnaswami Suguna Rani, Castori Marco. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet. 2006;38(6):623–625. doi: 10.1038/ng1805. [DOI] [PubMed] [Google Scholar]