Abstract
Malaria is still a global health concern with more than 400,000 death annually. Personal protection using mosquitoes’ repellent is an effective prevention strategy, especially in endemic areas. The toxic effects of synthetics repellents and their adverse effects on fabricated goods have made the development of green repellent critical. In this study, ingredients of Zataria multiflora essential oil (ZMEO) were identified using GC–MS analysis. Solid-lipid nanoparticles containing ZMEO (1%) were prepared (SLN-ZMEO) using the high-pressure homogenizer method. The repellent activity of ZMEO and SLN-ZMEO was investigated using Klun and Debboun method and compared together. Besides, their cytotoxicity on a human skin normal cell line (HFFF2) was evaluated. Five major components of ZMEO were carvacrol (27.05%), thymol (26.452%), γ-terpinene (15.144%), o-cymene (13.584%), and α-pinene (9.483%). The SLN-ZMEO showed a spherical shape with a particle size of 134 ± 7 nm. Moreover, their polydispersity index (PDI), zeta potential and entrapment efficiency were determined as 0.24 ± 0.1, − 9.82 ± 0.95 mV and 64.6 ± 3.8%, respectively. Interestingly, the protection time of nanoformulation (93 ± 5 min) was three times longer than that of the non-formulated essential oil (29 ± 2 min). Interestingly, both samples did not show cytotoxicity on HFFF2. Therefore, the prepared nanoformulation can be used as a green and potent repellent.
Keywords: Solid lipid nanoparticle, Anopheles stephensi, Repellent activity, Protection time, Zataria multiflora
Introduction
Vector-borne diseases account for more than 17% of all infectious diseases and caused around 700.000 deaths annually (Achee et al. 2019; WHO 2019). The most important vector-borne diseases are transmitted by three genera of mosquitoes, including Aedes (Dengue and Yellow Fever), Culex (Japanese encephalitis and West Nile fever), and Anopheles (Lymphatic Filariasis, Malaria) (Rahimi et al. 2019; Seufi and Galal 2010). It has been estimated that around 228 million new malaria cases and 405,000 deaths occurred just in 2018 (Hanafi-Bojd et al. 2020; World Health Oraganization 2020). Furthermore, Anopheles stephensi is one of the main malaria vectors in three regions of the World Health Organization (WHO); African Region (351 million people), Eastern Mediterranean (317 million people), South-East Asia Region (1.61 billion people) (Vatandoost et al. 2019; WHO 2019).
Personal protection using repellents is an appropriate way to prevent human contact with the mosquitoes, especially in areas where there is a high environmental burden (MaryamTavassoli et al. 2015; Pirmohammadi et al. 2016). Repellents are introduced as substances that deterring arthropods from landing or biting on the skin of the human, animal, or a surface in general (Mozaffari et al. 2014; Paluch et al. 2010). Most of the commercial repellents are based on N, N-diethyl-m-methylbenzamide (DEET), which are effective against a wide range of insects (Gillij et al. 2008; Tavassoli et al. 2015). However, it shows a toxic effect on humans and harms plastic, synthetic fabric, and painted surfaces (Legeay et al. 2018; Syed and Leal 2008). Thus developing new repellants with potent activity and lower side effects are crucial.
Plant-derived essential oils (EO)s with a wide range of activities such as repellency effect are an attractive source for the development of green repellent (Nerio et al. 2010; Toolabi et al. 2018). However, because some components of EOs are volatile, their use is particularly challenging at low concentrations. Recently, the nanoformulating of EOs has been introduced as a promising strategy for improving the volatility of EOs. Nanoemulsion, nanogel, niosome, solid-lipid nanoparticle (SLN), and polymeric nanoparticles are the proper forms for this purpose (Osanloo et al. 2018a, b).
SLNs are a new generation of nano-size emulsions where an oil (cargo) has been substituted by a solid lipid; therefore, are good candidates for the loading of EOs. Besides, SLN offers unique properties such as large surface area, high drug loading, and the interaction of phases at the interfaces (del Pozo-Rodríguez et al. 2007; Mukherjee et al. 2009).
In this study, the ingredients of Zataria multiflora EO (ZMEO) were identified by GC–MS analysis. For the first time, a green nano-repellent for An. stephensi was developed by loading of ZMEO in SLNs (SLN-ZMEO). After that, the protection time of ZMEO (1%) and SLN-ZMEO (1%) was compared. Also, the samples' cytotoxicity on the human skin normal cell line (HFFF2) was investigated.
Materials and methods
Materials
Zardband Pharmaceutical Co. (Yasoj, Iran) generously provided the ZMEO. Stearic acid, tween 80, span 60, and absolute ethanol (99.8%) were purchased from Merck Co. (Germany). Deionized water was purified using a Milli-Q system (Milli-pore, Direct-Q). Pasteur Institute of Iran provided the HFFF2 cell line. Powder 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), and tablets of phosphate-buffered saline (PBS) were purchased from Sigma-Aldrich (USA). Dimethyl Sulfoxide (DMSO), penicillin–streptomycin, RPMI, and trypsin were bought from Shellmax (China). Fetal bovine serum (FBS) was bought from Gibco (USA).
GC–MS procedure
For identification components of ZMEO, GC–MS analysis was performed using a 7890A Network GC system coupled with a 5975C VL MSD with Triple-Axis Detector (Agilent Technologies, Santa Clara, CA, USA). The separation of the EO components was carried out on HP-5MS silica fused column (30 m length, 0.25 mm i.d, and 0.25 µM film thickness). The initial temperature of the column was set at 40 °C and fixed for 1 min. Then, it increased with 3 °C/min to the final heat of 250 °C and held for 20 min. Other instrument parameters were set as follows: split-flow 100 mL/min, septum purge 6 mL/min, and the column flow rate of 1 mL/min. Helium gas with a purity of 99.99% was used as the carrier gas. The ZMEO components were identified using the method described in our previous report (Osanloo et al. 2018b).
Preparation of SLN-ZMEO
The ZMEO loaded SLNs (SLN-ZMEO) were prepared using a high-pressure homogenizer method, as described previously with slight modification (Lai et al. 2006). The ZMEO (1% w/w) was dissolved in the mixture of melted solid lipid (stearic acid 4%—85 °C) and lipophilic surfactant (span 60—2%). The EO-loaded lipid dispersed in a hot aqueous surfactant solution (tween 80—4%). The obtained mixture was homogenized using a high-shear homogenizer (D-91126 Schwa Bach, Heidolph, Germany) for 1 min at 8000 rpm. The obtained pre-emulsion was then homogenized at high pressure (3 cycles, 500 bar) using an APV Micron Lab 40 (APV Systems, Unna, Germany) thermostat at 90 °C for reducing the size of the nanoparticles. The final sample (SLN-ZMEO) was used for further investigations, such as characterization and repellent bioassays.
Characterization of SLN-ZMEO
Size, morphology and zeta potential analyses
Malvern zetasizer (Malvern Instruments, UK) was used to determine the particle size, polydispersity index (PDI), and zeta-potential of the nanoparticles. In this method, the sample was measured at 25 °C with an angle detection of 90°. The samples' concentration for analysis on the ZetaSizer was 20–400 k counts per second (KCPS), and the intensity of diffraction was 100,000 counts per second.
The transmission electron microscopy (TEM, CM 30, and Phillips, Netherlands) was utilized to determine the shape of SNL-ZMEO. Briefly, the SLN samples were first diluted two times with distilled water. One drop of the diluted sample was placed on a 200-mesh carbon-coated copper grid, followed by stained with 2% phosphotungstic acid solution and dried at room temperature.
Entrapment efficiency
A serial dilution of ZMEO (150–350 µg/mL) was prepared using ethanol to determine the maximum absorption wavelength (λ max). Their absorbance was screened in a range of 200–400 nm. The wavelength with the highest absorption at all concentrations was selected as λ max. After that, the standard calibration curve for ZMEO at the mentioned concentrations was plotted; absorbance (at λ max) vs. concentration (in µg/mL). The obtained regression equation was used for determining the amount of ZMEO in the sample.
The entrapment efficiency of ZMEO in SLNs was calculated using the indirect approach, i.e., determining non-loaded ZMEO and deducting it from the initial amount, using Eq. 1. For this purpose, SLN-ZMEO was centrifuged using a HERMLE, Z36HK machine (Germany) for 20 min at 25,000 rpm. After that, filtration with a pore size of 0.22 μm (n = 3) was carried out. Finally, ZMEO content in the supernatant was determined; absorbance at λ max was placed in the regression equation.
| 1 |
where Wi and Wf are the initial drug amount and the drug present in supernatant, respectively.
Evaluating protection times of ZMEO and SLN-ZMEO
The repellent activity was investigated using Klun & Debboun (K&D) method with slight modification. K&D devises including four separate rectangular cells (4 × 3 × 5) with a sliding valve at the bottom of each (see Fig. 1). The device was fastened on hands of the volunteer, and the valves were opened; mosquitoes exposed with the skin. Protection time defined as the interval between (min) the use of the samples (ZMEO 1% and SLN-ZMEO (having 1% ZMEO) and the first mosquito bite.
Fig. 1.
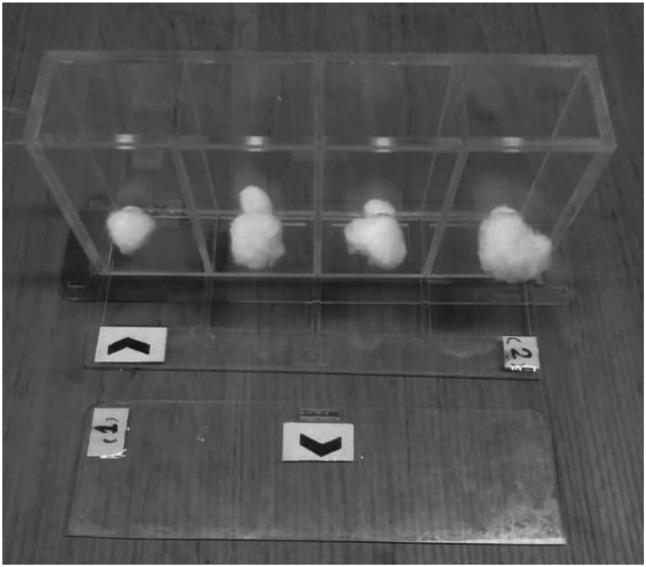
K&D devise
Before starting the test, the skin of the volunteer was disinfected using 70% alcohol. Then, four rectangles with a 12 cm area are marked on it (like to K&D device). The first rectangle was treated with ethanol as the control group. Three others stained with ZMEO or SLN-ZMEO separately. After that, 20 nulliparous female mosquitoes (7–9 day aged), starved for 12–24 h, were shed inside each of cell. The exposure was stoped; as soon as the first bite of the volunteer (Ghosh et al. 2014).
Evaluation cytotoxicity of SLN-ZMEO and ZMEO
MTT assay was used for investigating cytotoxicity of ZMEO and SLN-ZMEO against the HFFF2. The cell line was cultured in RPMI complete medium (containing FBS 12% and Penicillin–Streptomycin 1%) and was incubated at 37 °C, air (95%) and CO2 (5%). The cells were seeded in a 96-well plate and incubated for another 24 h for attaching the cells and reached confluence 70–80%. After that, the liquid content of wells was replaced with 50 µL of RPMI complete fresh medium and 50 µL of each of SLN-ZMEO and ZMEO (separately). The treated plate was then incubated for four hours (more than twice as much as observed protection time).
After incubation, the liquid content of the well was discarded, and 100 μL/well of MTT solution (dissolved in RPMI) was added and incubated for another 3 h. Then, 100 μL of DMSO was added to each well and mixed thoroughly to dissolve the dye crystals. Finally, the absorbance (A) of the wells was measured at 570 nm, using a plate reader (Synergy HTX Multi-Mode Reader, USA). The cell viability at each concentration was calculated by Eq. 2. Noted, in each plate control group (n = 3) were considered; no treatment was applied.
| 2 |
Statistical analyses
All the tests were repeated three times, and final values reported as mean ± standard deviations. Drawing of table and calculation of means and standard deviations were performed using Excell software (v 2010, Microsoft Office, USA). Protection time and cytotoxicity of ZMEO and SLN-ZMEO were compared using an independent sample t-test with a confidence interval of 95% using SPSS software (v.21, IBM, USA).
Results and discussion
Ingredients of ZMEO
Thirty-six identified compounds in ZMEO using GC–MS analysis are listed In Table 1. Its five major components were α-pinene (9.483%), o-cymene (13.584%), γ-terpinene (15.144%), thymol (26.452%), and carvacrol (27.05%).
Table 1.
Identified ingredients in ZMEO using GC–MS analysis
| No | Ingredients | Area | % | Retention time | Retention index |
|---|---|---|---|---|---|
| 1 | α-Thujene | 29,584,668 | 0.463 | 9.193 | 604 |
| 2 | α-Pinene | 230,827,972 | 3.616 | 9.483 | 613 |
| 3 | Camphene | 12,684,943 | 0.199 | 10.036 | 625 |
| 4 | β-Pinene | 37,479,693 | 0.587 | 11.248 | 646 |
| 5 | 1-Octen-3-ol | 5,959,451 | 0.093 | 11.569 | 704 |
| 6 | 3-Octanone | 19,800,469 | 0.310 | 11.827 | 710 |
| 7 | β-Myrcene | 75,429,639 | 1.182 | 11.991 | 715 |
| 8 | 3-Octanol | 6,953,388 | 0.109 | 12.274 | 722 |
| 9 | α-Phellandrene | 10,994,122 | 0.172 | 12.502 | 727 |
| 10 | 3-Carene | 3,721,087 | 0.058 | 12.757 | 734 |
| 11 | α-Terpinene | 92,129,256 | 1.443 | 13.083 | 742 |
| 12 | o-Cymene | 684,964,276 | 10.731 | 13.584 | 755 |
| 13 | Limonene | 58,427,992 | 0.915 | 13.688 | 757 |
| 14 | 1,8-Cineole | 159,004,914 | 2.491 | 13.804 | 760 |
| 15 | β-trans-ocimene | 2,749,288 | 0.043 | 14.607 | 780 |
| 16 | γ-Terpinene | 391,836,818 | 6.139 | 15.144 | 794 |
| 17 | cis-sabinenehydrate | 7,459,667 | 0.117 | 15.472 | 802 |
| 18 | α-terpinolene | 9,282,541 | 0.145 | 16.408 | 820 |
| 19 | Linalool | 126,174,339 | 1.977 | 17.12 | 835 |
| 20 | Borneol | 10,394,978 | 0.163 | 20.101 | 895 |
| 21 | 4-Terpineol | 59,753,322 | 0.936 | 20.617 | 905 |
| 22 | Fenchyl alcohol | 50,866,771 | 0.797 | 21.692 | 925 |
| 23 | 2-Isopropyl-5-methyl-1-methoxybenzene | 39,249,785 | 0.615 | 23.281 | 955 |
| 24 | Carvacrol methyl ether | 84,815,310 | 1.329 | 23.705 | 963 |
| 25 | l-Carvone | 7,071,401 | 0.111 | 24.462 | 977 |
| 26 | trans-anthole | 13,697,016 | 0.215 | 25.594 | 998 |
| 27 | Thymol | 1,608,717,376 | 25.202 | 26.452 | 1015 |
| 28 | Carvacrol | 1,930,172,282 | 30.238 | 27.050 | 1026 |
| 29 | Thymyl acetate | 75,349,373 | 1.180 | 28.69 | 1057 |
| 30 | Carvacryl acetate | 129,791,198 | 2.033 | 29.477 | 1072 |
| 31 | trans-caryophyllene | 140,081,621 | 2.195 | 31.291 | 1107 |
| 32 | γ-Selinene | 4,619,485 | 0.072 | 31.6 | 1113 |
| 33 | Aromadendrene | 76,499,808 | 1.332 | 32.066 | 1123 |
| 34 | Ledene | 38,088,110 | 0.597 | 34.35 | 1168 |
| 35 | ( +) spathulenol | 52,363,148 | 0.820 | 37.648 | 1234 |
| 36 | Caryophyllene oxide | 87,065,417 | 1.364 | 37.823 | 1238 |
Prepared SLN-ZMEO
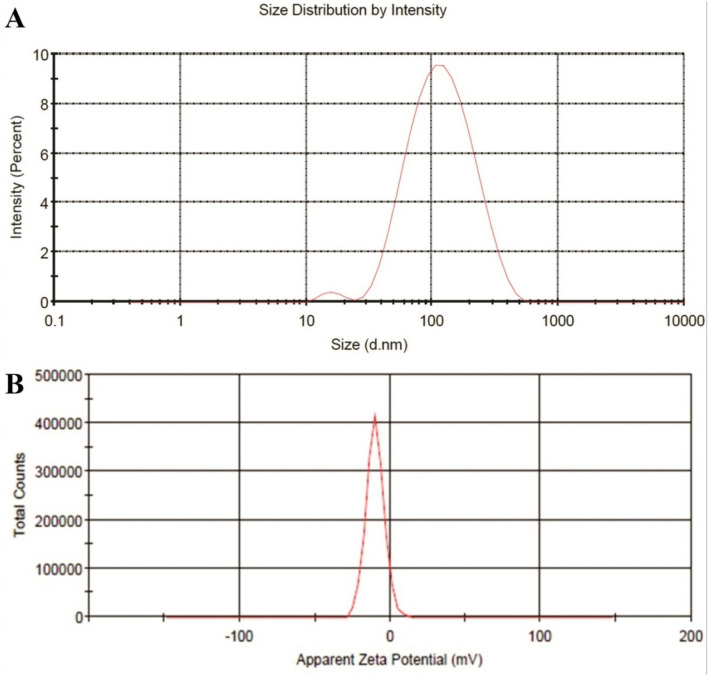
DLS analysis of SLN-ZMEO is depicted in Fig. 2a; the particle size and PDI of the SLN-ZMEO were 134 ± 7 nm and 0.24 ± 0.1, respectively. Also, its zeta potential was measured as −9.82 ± 0.95 mV (see Fig. 2b). SLN-ZMEO was spherical as TEM analysis demonstrated in Fig. 3. Some reports have been found on the preparation of SLN-ZMEO or SLN loaded with other herbal substances; however, the final formulations were not mosquitoes repellent. For instance, formulating and characterizing SLN-ZMEO with the average size of SLN was 650 nm had been described in a study (Moghimipour et al. 2013). In another one, SLN-ZMEO was prepared as antifungal agents with a particle size of 255.5 ± 3 nm, PDI 0.369 ± 0.05, and zeta potential 37.8 ± 0.8 mV (Nasseri et al. 2016). Furthermore, the z-average of carvacrol loaded SLN was reported in the range of 15–25 nm (He et al. 2019). Also, SLN containing Eugenia caryophyllata EO was prepared as antibacterial agents with a particle size of 397–786 nm (Fazly Bazzaz et al. 2018).
Fig. 2.
a DLS analysis of SLN nanoparticles containing ZMEO with the particle size of 134 ± 7 nm and PDI 0.24 ± 0.1, b Zeta potential of SLN nanoparticles containing ZMEO −9.82 ± 0.95
Fig. 3.
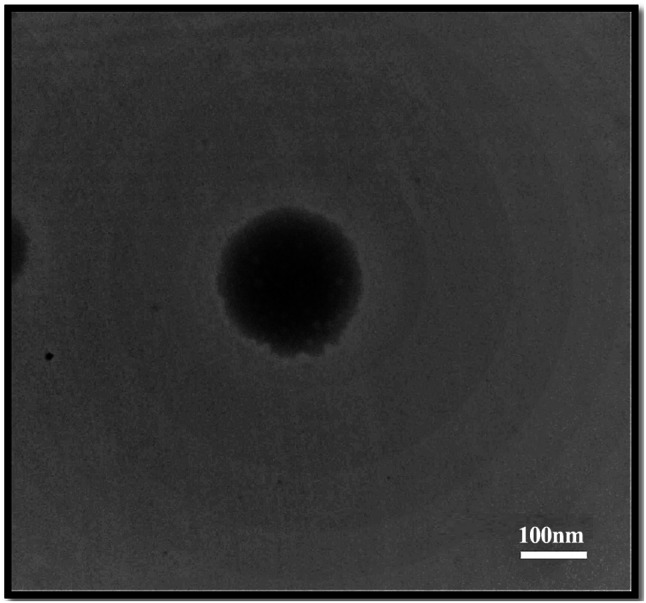
TEM image of SLN nanoparticles containing ZMEO with
The entrapment efficiency of SLN-ZMEO
The maximum absorption wavelength of ZMEO was obtained at 236 nm by spectrophotometer analysis. From Fig. 4, the linear regression equation for ZMEO was found, y = 0.0052x + 0.0014 (R2: 0.9997). The entrapment efficiency of ZMEO in SLNs was achieved at 64.6 ± 3.8%.
Fig. 4.

Used calibration curve for determining the amount of ZMEO in the supernatant of SLN-ZMEO
Comparison protection times of ZMEO and SLN-ZMEO
The protection times of the samples are shown in Fig. 5. After the topical application of SLN-ZMEO, protection time 93 ± 5 min was observed. This amount significantly better than non-formulated ZMEO with a protection time of 29 ± 2 (independent sample t-test, sig < 0.05).
Fig. 5.
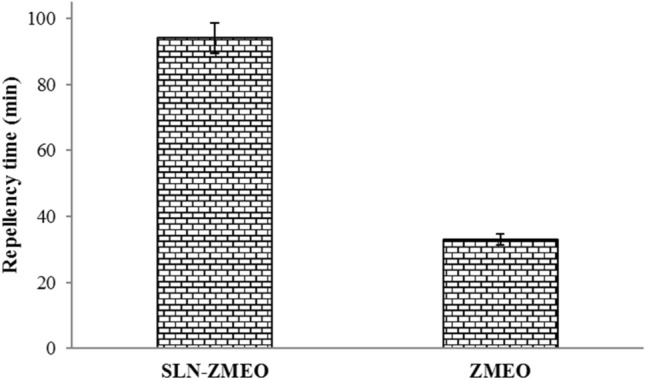
Protection time of SLN nanoparticles containing ZMEO 1% compared with non-formulated ZMEO (1%)
From the literature, no report was found on the repellent activity of nanoformulation against Anopheles spp. However, there are few studies in this field, i.e., the development of EO-based nanoformulation as a mosquito repellent.
Nanoemulsion of Eucalyptus globulus with a particle size of 17.1 nm was prepared. Protection time of the EO 15% lower than its nanoemulsion form against a mixture of Culex spp.; 59 < 170 min. Although this study provides useful information, the ingredients of the used EO were not reported, the active agent has not been known (Navayan et al. 2017). In another study, nanoemulsion of citronella oil (20%) with a particle size of 135 nm was prepared. It showed a protection time of 170 min, against Aedes aegypti (Sakulku et al. 2009). In these two studies, the concentration of the used EOs was high; their pungent odor makes it impossible for the consumer to use.
Furthermore, another report was found on the microencapsulation of lemongrass EO; it was related to preparing texture (polyester) with repellent activity (Anitha et al. 2011). In two other studies, synthetic insect repellent, including diethylphenylacetamide and permethrin, was encapsulated in polyethylene glycol and ethyl cellulose nanoparticles with particle sizes of 149 and 400 nm, respectively. They impregnated onto cotton fabrics as a repellent against C. quinquefasciatus and C. pipiens (Balaji et al. 2017; Türkoğlu et al. 2020).
Nanoemulsions are nano-size mixtures of oil and aqueous phases. Its preparation process is more straightforward than other nanoformulations. Therefore, in the above-mentioned EO-based formulation, nanoemulsion was prepared. In nanoemulsion, oil droplets (cargo) only surrounded and protected by surfactant molecules (Osanloo et al. 2017). Therefore, the protection of cargos is less than other nanoformulations. It seems a reason for used high amounts of EOs for the studies mentioned above.
In this study, ZMEO (1%) was loaded into the solid-lipid nanoparticles (SLN-ZMEO). In SLNs, oil droplets surrounded by slid-lipids such as stearic, oleic, linoleic acids (Rassouli and Al-Qushawi 2018). Besides, the stability of the system was supplied by using the surfactants (Garcês et al. 2018; Yadav et al. 2013). Therefore, the protection time of SLN-ZMEO contained ZMEO 1% was longer (~ 300%) than, and non-formulated ZMEO (1%). Improvement of protection times resulted from the control of the volatility of ZMEO as well as its slow release.
Cytotoxicity of ZMEO and SLN-ZMEO
Figure 6 shows that ZMEO and SLN-ZMEO did not show significant cytotoxicity compared to the control group (one-way ANOVA, sig > 0.05), unlike significant differences in the performance of the two samples, neither of them was toxic.
Fig. 6.

Cytotoxicity of ZMEO and SLN-ZMEO against HFFF2 cell line
We recently developed a green nanoformulation as a larvicide; it had no cytotoxicity on the HFFF2 cell line. In that research, EO of Artemisia dracunculus was encapsulated in chitosan nanoparticles. Besides, A. dracunculus EO at a concentration of 25% did not show cytotoxic effect (Osanloo et al. 2019). From the literature, ZM extract did not cause significant cellular toxicity up to the highest examined concentration of 200 µg/mL (Aghamohammadi et al. 2015). Another study reported that emu oil increased the growth of HFFF2 more than 100%; the anti-inflammatory effect of the EO was investigated (Vahedian et al. 2020).
Conclusion
In this study, ingredients of ZMEO were identified using GC–MS analysis. Then, solid-lipid nanoparticles containing ZMEO (1%) were prepared. For the first time, the protection time of an EO-based nanoformulation was investigated against An. stephensi. About a 300% increase in the protection time of nanoformulation was observed in comparison to non-formulated ZMEO. Interestingly both ZMEO and SLN-ZMEO had no cytotoxic effect on the human normal cell line, HFFF2.
Acknowledgments
This study was supported by the Iranian Ministry of Health grants for young researchers (No. 97367). It has also been ethically approved by the ethical committee at Fasa University of Medical Sciences, Fasa, IRAN, IR.FUMS.REC.1398.087. The process of mosquito-repellent tets was fully described to the candidates. They volunteered to participate in this research and were supported by the corresponding author during the test and one month later for any possible side effects; however, no side effects were observed.
Compliance with ethical standard
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Achee NL, et al. Alternative strategies for mosquito-borne arbovirus control. PLoS Negl Trop Dis. 2019;13(3):e0007275. doi: 10.1371/journal.pntd.0007275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghamohammadi A, Hosseinimehr SJ, Ghasemi A, Azadbakht M, Pourfallah TA. Radiosensitization effects of a Zataria multiflora extract on human glioblastoma cells. Asian Pac J Cancer Prev. 2015;16:7285–7290. doi: 10.7314/APJCP.2015.16.16.7285. [DOI] [PubMed] [Google Scholar]
- Anitha R, Ramachandran T, Rajendran R, Mahalakshmi M. Microencapsulation of lemon grass oil for mosquito repellent finishes in polyester textiles. Elixir Bio Phys. 2011;40:5196–5200. [Google Scholar]
- Balaji APB, Ashu A, Manigandan S, Sastry TP, Mukherjee A, Chandrasekaran N. Polymeric nanoencapsulation of insect repellent: evaluation of its bioefficacy on Culex quinquefasciatus mosquito population and effective impregnation onto cotton fabrics for insect repellent clothing. J King Saud Univ Sci. 2017;29:517–527. doi: 10.1016/j.jksus.2016.12.005. [DOI] [Google Scholar]
- del Pozo-Rodríguez A, Delgado D, Solinís MA, Gascón AR, Pedraz JL. Solid lipid nanoparticles: formulation factors affecting cell transfection capacity. Int J Pharm. 2007;339:261–268. doi: 10.1016/j.ijpharm.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Fazly Bazzaz B, Khameneh B, Namazi N, Iranshahi M, Davoodi D, Golmohammadzadeh S. Solid lipid nanoparticles carrying Eugenia caryophyllata essential oil: the novel nanoparticulate systems with broad-spectrum antimicrobial activity. Lett Appl Microbiol. 2018;66:506–513. doi: 10.1111/lam.12886. [DOI] [PubMed] [Google Scholar]
- Garcês A, Amaral M, Lobo JS, Silva A. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: a review. Eur J Pharm Sci. 2018;112:159–167. doi: 10.1016/j.ejps.2017.11.023. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Ozek T, Tabanca N, Ali A, ur Rehman J, Khan IA, Rangan L. Chemical composition and bioactivity studies of Alpinia nigra essential oils. Ind Crops Prod. 2014;53:111–119. doi: 10.1016/j.indcrop.2013.12.026. [DOI] [Google Scholar]
- Gillij YG, Gleiser RM, Zygadlo JA. Mosquito repellent activity of essential oils of aromatic plants growing in Argentina. Bioresour Technol. 2008;99:2507–2515. doi: 10.1016/j.biortech.2007.04.066. [DOI] [PubMed] [Google Scholar]
- Hanafi-Bojd AA, Vatandoost H, Yaghoobi-Ershadi MR. Climate change and the risk of malaria transmission in Iran. J Med Entomol. 2020;57:50–64. doi: 10.1093/jme/tjz131. [DOI] [PubMed] [Google Scholar]
- He J, Huang S, Sun X, Han L, Chang C, Zhang W, Zhong Q. Carvacrol loaded solid lipid nanoparticles of propylene glycol monopalmitate and glyceryl monostearate: preparation, characterization, and synergistic antimicrobial activity. Nanomater. 2019;9:1162. doi: 10.3390/nano9081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Wissing SA, Müller RH, Fadda AM. Artemisia arborescens L. essential oil-loaded solid lipid nanoparticles for potential agricultural application: preparation and characterization. AAPS Pharm Sci Tech. 2006;7:E10. doi: 10.1208/pt070102. [DOI] [PubMed] [Google Scholar]
- Legeay S, Clere N, Apaire-Marchais V, Faure S, Lapied B. Unusual modes of action of the repellent DEET in insects highlight some human side effects. Eur J Pharmacol. 2018;825:92–98. doi: 10.1016/j.ejphar.2018.02.033. [DOI] [PubMed] [Google Scholar]
- MaryamTavassoli MS, Hassan Vatandoost M, Abai R, Khoobdel M, Bakhshi H, Rafi F. Repellency effects of picaridin and DEET against Anopheles stephensi on human volunteers. J Entomol Zool Stud. 2015;3:343–347. [Google Scholar]
- Moghimipour E, Ramezani Z, Handali S. Solid lipid nanoparticles as a delivery system for Zataria multiflora essential oil: formulation and characterization. Curr Drug Deliv. 2013;10:151–157. doi: 10.2174/1567201811310020001. [DOI] [PubMed] [Google Scholar]
- Mozaffari E, et al. Chemical composition, larvicidal and repellency properties of Cionura erecta (L.) Griseb against malaria vector, Anopheles stephensi Liston (Diptera: Culicidae) J Arthropod Borne Dis. 2014;8(2):147–155. [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009;71:349–358. doi: 10.4103/0250-474X.57282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasseri M, Golmohammadzadeh S, Arouiee H, Jaafari MR, Neamati H. Antifungal activity of Zataria multiflora essential oil-loaded solid lipid nanoparticles in-vitro condition. Iran J Basic Med Sci. 2016;19:1231. [PMC free article] [PubMed] [Google Scholar]
- Navayan A, Moghimipour E, Khodayar MJ, Vazirianzadeh B, Siahpoosh A, Valizadeh M, Mansourzadeh Z. Evaluation of the mosquito repellent activity of nano-sized microemulsion of Eucalyptus globulus essential oil against culicinae. Jundishapur J Nat Pharm Prod. 2017;12:e55626. [Google Scholar]
- Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils: a review. Bioresour Technol. 2010;101:372–378. doi: 10.1016/j.biortech.2009.07.048. [DOI] [PubMed] [Google Scholar]
- Osanloo M, Amani A, Sereshti H, Abai MR, Esmaeili F, Sedaghat MM. Preparation and optimization nanoemulsion of Tarragon (Artemisia dracunculus) essential oil as effective herbal larvicide against Anopheles stephensi. Ind Crops Prod. 2017;109:214–219. doi: 10.1016/j.indcrop.2017.08.037. [DOI] [Google Scholar]
- Osanloo M, Assadpour S, Mehravaran A, Abastabar M, Akhtari J. Niosome-loaded antifungal drugs as an effective nanocarrier system: a mini review. Curr Med Mycol. 2018;4:31. doi: 10.18502/cmm.4.4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanloo M, Sereshti H, Sedaghat MM, Amani A. Nanoemulsion of Dill essential oil as a green and potent larvicide against Anopheles stephensi. Environ Sci Pollut. 2018;25:6466–6473. doi: 10.1007/s11356-017-0822-4. [DOI] [PubMed] [Google Scholar]
- Osanloo M, Sedaghat MM, Sereshti H, Rahmani M, Saeedi Landi F, Amani A. Chitosan nanocapsules of tarragon essential oil with low cytotoxicity and long-lasting activity as a green nano-larvicide. J Nanostruct. 2019;9:723–735. [Google Scholar]
- Paluch G, Bartholomay L, Coats J. Mosquito repellents: a review of chemical structure diversity and olfaction. Pest Manag Sci. 2010;66:925–935. doi: 10.1002/ps.1974. [DOI] [PubMed] [Google Scholar]
- Pirmohammadi M, et al. Chemical composition and repellent activity of Achillea vermiculata and Satureja hortensis against Anopheles stephensi. J Arthropod Borne Dis. 2016;10:201. [PMC free article] [PubMed] [Google Scholar]
- Rahimi S, Vatandoost H, Abai MR, Raeisi A, Hanafi-Bojd AA. Status of resistant and knockdown of west Nile vector, culex pipiens complex to different pesticides in Iran. J Arthropod Borne Dis. 2019;13:284. [PMC free article] [PubMed] [Google Scholar]
- Rassouli A, Al-Qushawi A. Lipid-based nanoparticles as novel drug delivery systems for antimicrobial agents. Iran J Vet Sci Technol. 2018;10(2):1–16. [Google Scholar]
- Sakulku U, Nuchuchua O, Uawongyart N, Puttipipatkhachorn S, Soottitantawat A, Ruktanonchai U. Characterization and mosquito repellent activity of citronella oil nanoemulsion. Int J Pharm. 2009;372:105–111. doi: 10.1016/j.ijpharm.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Seufi AM, Galal FH. Role of Culex and Anopheles mosquito species as potential vectors of rift valley fever virus in Sudan outbreak, 2007. BMC Infect Dis. 2010;10:65–65. doi: 10.1186/1471-2334-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Z, Leal WS. Mosquitoes smell and avoid the insect repellent DEET. Proc Natl Acad Sci. 2008;105:13598–13603. doi: 10.1073/pnas.0805312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli M, et al. Repellency effects of picaridin and DEET against Anopheles stephensi on human volunteers. J Entomol Zool Stud. 2015;3:343–347. [Google Scholar]
- Toolabi R, Abai MR, Sedaghat MM, Vatandoost H, Shayeghi M, Tavakoli S, Aghdam MS. Larviciding activity of Acroptilon repens extract against Anopheles stephensi, Culex pipiens and Culex quinquefaciatus under laboratory conditions. Pharmacogn J. 2018;10(3):453–456. doi: 10.5530/pj.2018.3.74. [DOI] [Google Scholar]
- Türkoğlu GC, Sarıışık AM, Erkan G, Yıkılmaz MS, Kontart O. Micro- and nano-encapsulation of limonene and permethrin for mosquito repellent finishing of cotton textiles. Iran Polym J. 2020;29:321–329. doi: 10.1007/s13726-020-00799-4. [DOI] [Google Scholar]
- Vahedian V, et al. Anti-inflammatory activity of emu oil-based nanofibrous scaffold through downregulation of IL-1, IL-6, and TNF-α pro-inflammatory cytokines. Horm Mol Biol Clin Investig. 2020;41(2):2019–2052. doi: 10.1515/hmbci-2019-0052. [DOI] [PubMed] [Google Scholar]
- Vatandoost H, et al. Efficacy of extractions of Iranian native plants against main malaria vector, Anopheles stephensi in Iran for making appropriate formulation for disease control. J Arthropod Borne Dis. 2019;13(4):344–352. [PMC free article] [PubMed] [Google Scholar]
- WHO (2019) World malaria report 2019
- World Health Oraganization (2020) Vector Control. https://www.who.int/vector-control/en/ Accessed 2020
- Yadav N, Khatak S, Sara US. Solid lipid nanoparticles—a review. Int J Appl Pharm. 2013;5:8–18. [Google Scholar]