Abstract
In this study, we aimed to record, for the first time, parasitic infestation by the isopod Probopyrus pandalicola on the prawn Macrobrachium acanthurus, as well as to register some ecological interactions. We hypothesized that the parasitic infection is able to negatively affect the prawn’s nutritional condition and that this interaction can modify growth relationships in male individuals. We collected both parasitized (n = 25) and parasite-free (n = 25) individuals in several locations of the Contas River, state of Bahia, Brazil, which had their morphometric characteristics determined, including of the parasites. Relative growth models were constructed for both groups in order to compare slopes and intercepts and determine if the growth patterns are modified by the parasite. We also determined the body condition of the prawns, which was also compared between the two groups. Our results clearly demonstrated that the parasitic infection is able to induce modifications in relative growth patterns in male individuals and that this isopod is capable of reducing the nutritional condition of the prawns. This study indicates that this parasite can induce deleterious effects in the prawn, but individually. Further studies should be conducted to assess the relevance of our findings in conservation and management.
Keywords: Crustacea, Condition factor, Animal health, Infestation
Introduction
The genus Macrobrachium (Bate, 1868) includes species commonly found in lentic and lotic environments (Coelho and Ramos-Porto 1984) and are also widely exploited in both aquaculture and artisanal fisheries, being an important source of economic development in many latin american regions (Thompson 1980; Maciel and Valenti 2009; García-Guerrero et al. 2015). The species Macrobrachium acanthurus (Wiegmann, 1836) is a prawn that usually inhabits estuarine and freshwater environments across Latin America, where they can be found underneath rocks and among aquatic vegetation (Coelho 1963). Because of its large body size (females can attain around 108 mm and males 138 mm of body size) (Valenti et al. 1986), this prawn is very commonly exploited by artisanal fisheries around many regions in Latin America (New et al. 2000).
Parasitic interactions among isopods and prawns are common and reasonably well known in the scientific literature, mainly given that many parasitic isopods are crustacean specific and feed on their hemolymph (Walker 1977; Boxshall et al. 2005). More specifically, ectoparasitic isopods from the family Bopyridae usually infect prawns as their definitive hosts and remain attached to their branchial chambers until it dies (Beck 1980b; Chaplin-Ebanks and Curran 2007), mainly because of the capacity of the female isopod to avoid being released along with the old exoskeleton by instantly latching onto the new structure, allowing the parasite to grow along with its host (Cash and Bauer 1993). This happens in many species of the genus Macrobrachium (Collart 1990; Román-Contreras 1996; Oliveira et al. 2000; Conner and Bauer 2010; Vargas-Ceballos et al. 2016). Impacts from these infections can vary from castration, feminization in males (specifically consisting of impairment of claw growth and modification of growth patterns), behavioural and metabolic disturbances (Neves et al. 2000; Lester 2005; Chaplin-Ebanks and Curran 2007; Calado et al. 2008; Dumbauld et al. 2011). However, the intensity or even the presence of these effects can vary from specie to specie, and thus it is important to investigate the species-specific responses, as this may be important information for ecological studies, conservation, and aquaculture management (Ninawe and Selvin 2009). To date, there is no report of parasitic infection by bopyrid isopods in the freshwater prawn Macrobrachium acanthurus.
The species Probopyrus pandalicola (Packard, 1879) is a highly distributed parasitic isopod that is known to infect several species within the family Palaemonidae (Beck 1980b; Conner and Bauer 2010; Sherman and Curran 2015). Although some research demonstrated that the infection by this isopod does no visible harm in the respective hosts (Morris 1948; Calado et al. 2006), other studies showed that it is capable of inducing harmful effects in the vitality of juvenile hosts (Anderson 1990), sexual sterilization (Sherman and Curran 2015), impairment of growth and reproductive traits (Van Wyk 1982) and induce feeding behaviour disturbances (Bass and Weis 1999). This highlights even more the necessity of assessing the specific response of each species to this parasite, given the highly diverse nature and the conjoint of factors that are able to influence the interactions between hosts and parasites (Williams and Boiko 2012).
In this study, we aimed to record, for the first time, infection by a bopyrid isopod in the species Macrobrachium acanthurus and assessed some ecological outcomes of the host-parasite interaction. More precisely, we tested the hypothesis that the parasitic infection is able to reduce nutritional condition (H1) and modify patterns of growth in male individuals (feminization) (H2). Both hypotheses are based on the fact that parasitic infections, since they rely on host exploitation for growth and reproduction, can limit the host’s available energy reserves by a conjoint of factors (Sánchez et al. 2018).
Materials and methods
During fieldwork (a total of six samples realized each two months in the year 2017), we manually collected specimens of Macrobrachium acanthurus by passing sieves in the marginal vegetation in several locations in the Contas River, located in the north-eastern state of Bahia, Brazil (Fig. 1). It is the most important river in its respective basin and one of the largest in the state of Bahia, allowing the presence of a diverse range of economic activities such as agriculture and fishing because of its large water flow and habitat diverisity (SRH 2007; de Barros et al. 2020) In the laboratory, the prawns were dissected towards removing the parasitic isopods and identified regarding sex, measured their total length (tip of the telson to the proximal end of the rostrum) with a 0.01 mm precision calliper and obtained their weight (precision of 0.01 g) (the shrimps were weighed after removing the isopod). We then used the key provided by Markham (1985) to properly identify the species Probopyrus pandalicola, which also had their length measured. All examined material is currently stored in the Natural History Museum of the Federal University of Alagoas (UFAL), Maceió, AL, Brazil (Coleção de Carcinologia, register number CB001).
Fig. 1.
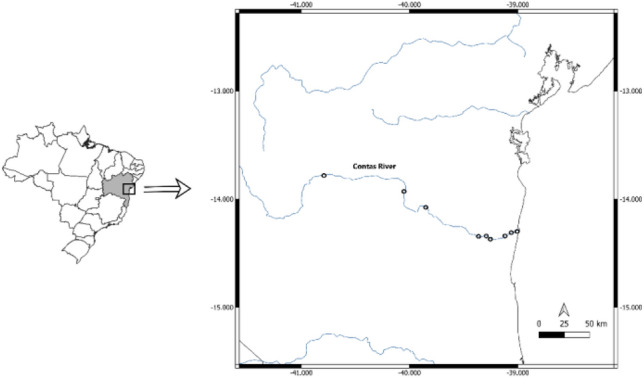
Location of the sampling sites (black circles) in the Contas River, state of Bahia, Brazil
We utilized the Fulton’s condition factor, defined by the formula K = (W/L3)0.105 (Froese 2006) to assess the shrimp’s body condition, where “W” is the weight in grams and “L” is the body length (TL) in mm. The multiplication by 105 intended to bring K close to unity. Basically, this formula states that, in a given length, the more mass the individual has, better the nutritional condition is (Froese 2006). As suggested by Sánchez et al. (2018), condition indexes are a reliable approach to measuring infection-fitness relationships, whereas it can also be utilized towards investigating outcomes of anthropogenic impacts in populations (de Barros et al. 2020). Then, to test the first hypothesis (H1) we utilized a t test to verify if there is any difference in the nutritional condition between parasitized and parasite-free individuals. We also analysed the relationship between parasite body size (female length) and host body size (total length) using a Pearson correlation test. To test the second hypothesis (H2), we constructed log–log relative growth models of the relationship CC x TL for both parasitized and parasite-free males. Then, we applied an analysis of covariance (ANCOVA) to test for parasite induced differences in relative growth by comparing slopes (Andrade and Estévez-Pérez 2014). The data were previously tested for normality and homogeneity of variances by the Shapiro–Wilk and Levene’s test, respectively, before any other statistical analysis. Null hypothesis were rejected when p < 0.05 (Zar 2010).
Results
We analysed a total of 50 male individuals, from which 25 had the isopod parasite in their branchial chambers (Fig. 2) (TL ranging from 18.6 to 35.6 mm) and also 25 had no infestation (TL ranging from 25.6 to 70.4 mm) (Table 1). We had no record of bilateral infestation and the ratio between infestation in the right and left branchial chambers was very close to 1:1. Host size was strongly correlated with parasite size (Fig. 3).
Fig. 2.
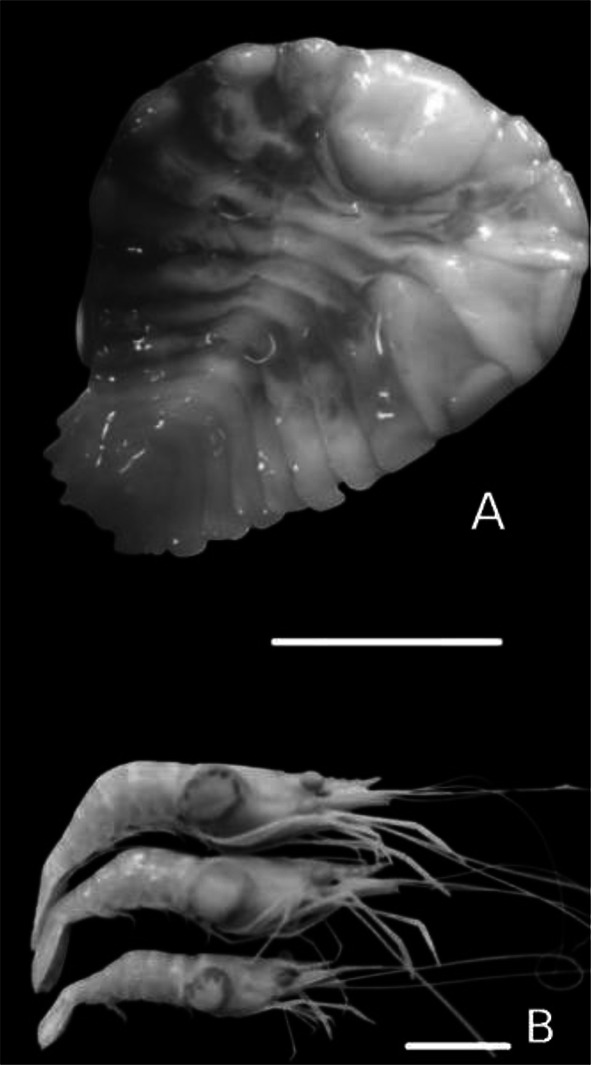
Ventral view of a female of Probopyrus pandalicola (a) and infested shrimps (b). Above scale is equal to 5 mm, while below scale is equal to 12 mm
Table 1.
Means and standard deviations of Total Length and Weight for both parasitized and parasite free individuals, as well as of the isopod
| Host | Parasite | ||
|---|---|---|---|
| Parameter | Parasitized (n = 25) | Parasite-free (n = 25) | |
| Total length (mm) | 26.9 ± 4.39 | 39.91 ± 10.46 | 7.5 ± 1.5 |
| Weight (g) | 0.34 ± 0.22 | 1.59 ± 1.48 | 0.02 ± 0.01 |
Fig. 3.
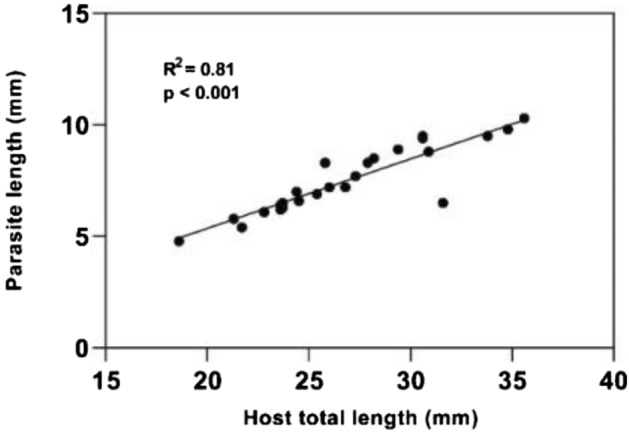
Correlation between host total length and parasite total length
We found that the Fulton’s Condition Factor was significantly smaller in the infested prawns (Fig. 4), hence confirming our first hypothesis (H1). Similarly, the analysis of covariance (ANCOVA) demonstrated that patterns of growth in male infested individuals were different than those of parasite-free prawns (F = 24.48, p < 0.001) (Fig. 5). This confirmed the second hypothesis (H2).
Fig. 4.
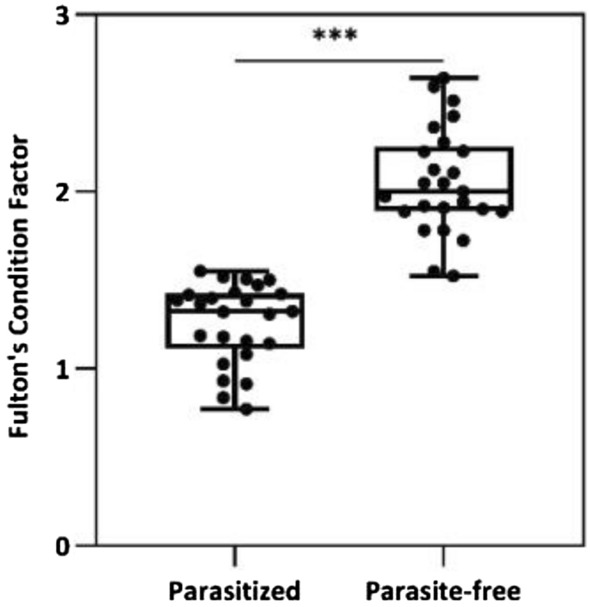
Comparison of the Fulton’s Condition Index between infested and non-infested individuals
Fig. 5.
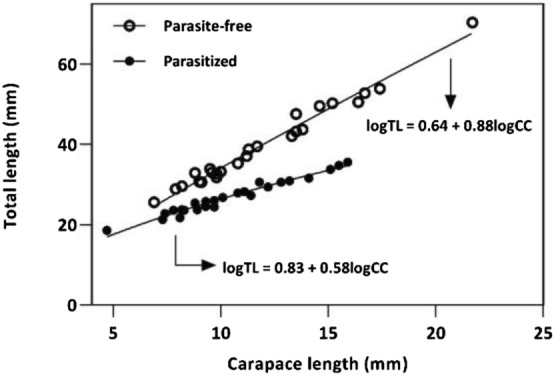
Growth regression lines and equations of male parasitized and parasite-free individuals
Discussion
This is the first study to record and briefly analyse the relationship between the palaemonid prawn Macrobrachium acanthurus and the parasitic isopod Probopyrus pandalicola. We observed that this parasite is able to induce strong modifications in the growth patterns in male prawns. More specifically, our results suggested that parasitized individuals grew much slower because they had the relationship between carapace length (CL) and total length (TL) altered, as represented by the equation parameters in the Fig. 5. This can possibly be a result of the haemolymph loss caused by the feeding of the parasite (Walker 1977). The subsequent loss of nutrients caused by it can disturb and inhibit the synthesis of hormonal compounds that are thought to stimulate maturation processes (Fingerman 1997; Subramoniam 2011; Swetha et al. 2011). Finally, it is likely that the parasitized individual becomes sexually sterile (Sherman and Curran 2015). Although some decapod crustaceans have evolved to be adapted to nutrient loss and even starvation for considerable periods of time (Vinagre and Chung 2016), this can in fact also cause modification of growth patterns, altered survival and body composition in some other species that are not (Wu et al. 2004). Veritably, parasitic infections are known to produce a significant negative effect in reproductive aspects of many marine species (Hua et al. 2017), including in infestations by isopods on crustaceans (Beck 1980a; Abu-Hakima 1984; Sherman and Curran 2015). Likewise, the work conducted by Dumbauld et al. (2011) suggested that the infection by a bopyrid isopod in the mud shrimp Upogebia pugettensis limited their maximum size and weight, besides causing a considerable decrease in population size. Similar work conducted by Smith et al. (2008) also revealed impairment of mass gain in the same species. In Palaemonidae prawns, specifically, little is known about the effects of bopyrid isopods. Research by Anderson (1990) suggested lethal effects of this parasite in species of Palaemonetes, which had higher mortality rates when compared to non-infected prawns. The study by Sherman and Curran (2015) demonstrated a clear sexual sterilization in the daggerblade grass shrimp Palaemonetes pugio (Holthuis, 1949), an effect that can potentially affect recruitment processes depending on the magnitude of the infection. For the same species, there are reports of decreased survival time, although it appears to also interact with temperature (Sherman and Curran, 2013).
We also observed that the parasitic infestation can generate a remarkable decrease in the body condition of the prawns, represented in this study by the Fulton’s Condition Factor (K). Indeed, the negative relationship between parasitic infestations and body/nutritional condition indexes is well established in the literature, with a mix of factors being able to influence it (Sánchez et al. 2018). It is mainly acknowledged on the limitation of the host’s movements (e.g. foraging/looking for resources, directly linked to energy gains) (Ghai et al. 2015), energetic outcomes of fighting the parasites back (even though that evidence in the scientific literature for immunity expenses are so far blended) (Bonneaud et al. 2003; Eraud et al. 2005; Nilsson et al. 2007), environmental factors that can lead to increased probability of transmission such as resource scarcity (Ostfeld et al. 2005; Becker et al. 2015) and fluid loss (Walker 1977; Smith et al. 2008). Particularly, in the studied shrimp, it is more likely that the relative contribution of fluid loss and limitation of movements are far more important in explaining the worsened nutritional condition, since the loss of haemolymph in crustaceans are commonly linked to nutrient loss and limitation of movements can modify feeding behaviours and also provide an important diminution of nutrient intake (Felten and Guerold 2001; Lester 2005).
Anderson (1990) stated that Probopyrus pandalicola reaches its infective stage at estuaries, which are also home to the prawn Macrobrachium acanthurus. This means that the infection usually occurs in early life stages, since estuarine environmental conditions are essential for the larvae to develop into a juvenile (Choudhury 1971; Gamba 1982; Bertini et al. 2014). The limitations of our study consisted, specifically, in the lack of any temporal record, excluding the possibility to assess the dynamics of the relationship over a timescale. Similarly, we were not able to record the parasite prevalence in the general prawn population. Because of it, more studies should be conducted in order to acquire data on this and determine the ecological relationships at a greater extent, as it should help to draw better strategies towards fishing and aquaculture management, as well as conservation. Although our data clearly demonstrated that patterns of growth in males of Macrobrachium acanthurus were modified, other minor limitation consisted in the lack of male cheliped growth data, which is thought to be a better indicator of feminization in shrimps (Choi et al. 2004; Smith et al. 2008).
We therefore conclude that our data demonstrated clear impacts by the bopyrid parasitic isopod Probopyrus pandalicola on the host prawn Macrobrachium acanthurus, which consisted in impairment of growth in male individuals, possibly pointing out to feminization, and worsened nutritional condition, probably as a result of nutrient loss and impaired foraging/feeding. Although our data remains conclusive about some ecological impacts of the host-parasite relationships, more studies should be conducted regarding temporal variation and prevalence of this parasite in the host population, in order to assess the extent of those outcomes.
Funding
This research was not supported by funding.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abu-Hakima R. Preliminary observations on the effects of Epipenaeon elegans Chopra (Isopoda; Bopyridae) on reproduction of Penaeus semisulcatus de Haan (Decapoda; Penaeidae) Int J Invertebr Reprod Dev. 1984;7(1):51–62. [Google Scholar]
- Anderson G. Postinfection mortality of Palaemonetes spp. (Decapoda: Palaemonidae) following experimental exposure to the bopyrid isopod Probopyrus pandalicola (Packard)(Isopoda: Epicaridea) J Crustacean Biol. 1990;10(2):284–292. [Google Scholar]
- Andrade JM, Estévez-Pérez MG. Statistical comparison of the slopes of two regression lines: a tutorial. Anal Chim Acta. 2014;838:1–12. doi: 10.1016/j.aca.2014.04.057. [DOI] [PubMed] [Google Scholar]
- Bass CS, Weis JS. Behavioral changes in the grass shrimp, Palaemonetes pugio (Holthuis), induced by the parasitic isopod, Probopyrus pandalicola (Packard) J Exp Mar Biol Ecol. 1999;241(2):223–233. [Google Scholar]
- Beck JT. The effects of an isopod castrator, Probopyrus pandalicola, on the sex characters of one of its caridean shrimp hosts, Palaemonetes paludosus. Biol Bull. 1980;158(1):1–15. [Google Scholar]
- Beck JT. Larval and adult habitats of a branchial bopyrid Probopyrus pandalicola on one of its freshwater shrimp hosts Palaemonetes paludosus. Crustaceana. 1980;38(3):265–270. [Google Scholar]
- Becker DJ, Streicker DG, Altizer S. Linking anthropogenic resources to wildlife–pathogen dynamics: a review and meta-analysis. Ecol Lett. 2015;18(5):483–495. doi: 10.1111/ele.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertini G, Baeza JA, Perez E. A test of large-scale reproductive migration in females of the amphidromous shrimp Macrobrachium acanthurus (Caridea: Palaemonidae) from south-eastern Brazil. Mar Freshw Res. 2014;65(1):81–93. [Google Scholar]
- Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G. Assessing the Cost of Mounting an Immune Response. Am Nat. 2003;161(3):367–379. doi: 10.1086/346134. [DOI] [PubMed] [Google Scholar]
- Boxshall G, Lester R, Grygier MJ, Hoeg JT, Glenner H, Shields JD, Lützen J (2005) Crustacean parasites. Rohde K.
- Calado R, Vitorino A, Dinis MT. Bopyrid isopods do not castrate the simultaneously hermaphroditic shrimp Lysmata amboinensis (Decapoda: Hippolytidae) Dis Aquat Org. 2006;73(1):73–76. doi: 10.3354/dao073073. [DOI] [PubMed] [Google Scholar]
- Calado R, Bartilotti C, Goy JW, Dinis MT. Parasitic castration of the stenopodid shrimp Stenopus hispidus (Decapoda: Stenopodidae) induced by the bopyrid isopod Argeiopsis inhacae (Isopoda: Bopyridae) J Mar Biol Assoc U K. 2008;88(2):307–309. [Google Scholar]
- Cash CE, Bauer RT. Adaptations of the branchial ectoparasite Probopyrus pandalicola (Isopoda: Bopyridae) for survival and reproduction related to ecdysis of the host, Palaemonetes pugio (Caridea: Palaemonidae) J Crustacean Biol. 1993;13(1):111–124. [Google Scholar]
- Chaplin-Ebanks SA, Curran MC. Prevalence of the bopyrid isopod Probopyrus pandalicola in the grass shrimp, Palaemonetes pugio, in four tidal creeks on the South Carolina-Georgia coast. J of Parasitol. 2007;93(1):73–77. doi: 10.1645/GE-3537.1. [DOI] [PubMed] [Google Scholar]
- Choi JH, Jamieson G, Han KH, Hung SY. Parapenaeon consolidatum (Isopoda: Bopyridae) and the relative growth and reproduction of Metapenaeopsis dalei (Decapoda: Penaeidae) in South Korea. J Shellfish Res. 2004;23(1):237–243. [Google Scholar]
- Choudhury PC. Complete larval development of the palaemonid shrimp Macrobrachium carcinus (L.), reared in the laboratory (Decapoda, Palaemonidae) Crustaceana. 1971;20(1):51–69. [Google Scholar]
- Coelho PA. Observações preliminares sobre a biologia e a pesca dos camarões do gênero Macrobrachium Bate, 1868 (Decapoda Palaemonidae) no Estado de Pernambuco. Brasil. Trabs. IO Univ. Recife. 1963;3:75–81. [Google Scholar]
- Coelho PA, Ramos-Porto M. Camarões de água doce do Brasil: distribuição geográfica. Revista brasileira de Zoologia. 1984;2(6):405–410. [Google Scholar]
- Collart OO. Interactions Entre Le Parasite Probopyrus Bithynis (Isopoda, Bopyridae) Et L’Un De Ses Hôtes, La Crevette Macrobrachium Amazonicum (Decapoda, Palaemonidae) Crustaceana. 1990;58(3):258–269. [Google Scholar]
- Conner SL, Bauer RT. Infection of adult migratory river shrimps, Macrobrachium ohione, by the branchial bopyrid isopod Probopyrus pandalicola. Invertebr Biol. 2010;129(4):344–352. [Google Scholar]
- de Barros MSF, dos Santos Calado TC. Plastic ingestion lead to reduced body condition and modified diet patterns in the rocky shore crab Pachygrapsus transversus (Gibbes, 1850)(Brachyura: Grapsidae) Mar Pollut Bull. 2020;156:111249. doi: 10.1016/j.marpolbul.2020.111249. [DOI] [PubMed] [Google Scholar]
- de Barros MSF, dos Santos Calado TC, dos Santos EV, Silva AS, de Andrade Albuquerque LG. Population biology and sexual dimorphism in the freshwater prawn Atya scabra (Decapoda: Atyidae) in the De Contas River, Bahia. Brazil. Revista de Biología Tropical. 2020;68(3):743–751. [Google Scholar]
- Dumbauld BR, Chapman JW, Torchin ME, Kuris AM. Is the collapse of mud shrimp (Upogebia pugettensis) populations along the Pacific coast of North America caused by outbreaks of a previously unknown bopyrid isopod parasite (Orthione griffenis)? Estuar Coasts. 2011;34(2):336–350. [Google Scholar]
- Eraud C, Duriez O, Chastel O, Faivre B. The energetic cost of humoral immunity in the Collared Dove, Streptopelia decaocto: is the magnitude sufficient to force energy-based trade-offs? Funct Ecol. 2005;19(1):110–118. [Google Scholar]
- Felten V, Guerold F. Hyperventilation and loss of hemolymph Na+ and Cl-in the freshwater amphipod Gammarus fossarum exposed to acid stress: a preliminary study. Dis Aquat Organ. 2001;45(1):77–80. doi: 10.3354/dao045077. [DOI] [PubMed] [Google Scholar]
- Fingerman M. Roles of neurotransmitters in regulating reproductive hormone release and gonadal maturation in decapod crustaceans. Invertebr Reprod Dev. 1997;31(1–3):47–54. [Google Scholar]
- Froese R. Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J Appl Ichthyol. 2006;22(4):241–253. [Google Scholar]
- Gamba AL. Macrobrachium: its presence in estuaries of the northern Venezuelan coast (Decapoda, Palaemonidae) Caribb J Sci. 1982;18(1–4):23–28. [Google Scholar]
- García-Guerrero M, de los Santos Romero R, Vega-Villasante F, Cortes-Jacinto E. Conservation and aquaculture of native freshwater prawns: the case of the cauque river prawn Macrobrachium americanum (Bate, 1868) Lat Am J Aquat Res. 2015;43(5):819–827. [Google Scholar]
- Ghai RR, Fugere V, Chapman CA, Goldberg TL, Davies TJ. Sickness behaviour associated with non-lethal infections in wild primates. Proc R Soc B Biol Sci. 2015;282(1814):20151436. doi: 10.1098/rspb.2015.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Wuerthner VP, Jones DK, Mattes B, Cothran RD, Relyea RA, Hoverman JT. Evolved pesticide tolerance influences susceptibility to parasites in amphibians. Evol Appl. 2017;10(8):802–812. doi: 10.1111/eva.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RJG. Crustacean parasites. Marine parasitology. Sidney: University of New England; 2005. pp. 123–169. [Google Scholar]
- Maciel CR, Valenti WC. Biology, fisheries, and aquaculture of the Amazon River prawn Macrobrachium amazonicum: a review. Nauplius. 2009;17(2):61–79. [Google Scholar]
- Markham JC. A review of the bopyrid isopods infesting caridean shrimps in the northwestern Atlantic Ocean, with special reference to those collected during the Hourglass Cruises in the Gulf of Mexico. Bureau Of Marine Research, St. Petersburg, Florida: Florida Department of Natural Resources; 1985. [Google Scholar]
- Morris JA (1948) Studies on the Host-parasite Relationship of Probopyrus Pandalicola (Packard). (No. 8). Catholic University of America Press
- Neves CA, Santos EA, Bainy ACD. Reduced superoxide dismutase activity in Palaemonetes argentinus (Decapoda, Palemonidae) infected by Probopyrus ringueleti (Isopoda, Bopyridae) Dis Aquat Org. 2000;39(2):155–158. doi: 10.3354/dao039155. [DOI] [PubMed] [Google Scholar]
- New MB, D’Abramo LR, Valenti WC, Singholka S. Sustainability of freshwater prawn culture. Freshwater Prawn Culture: The Farming of Macrobrachium rosenbergii. Oxford: Blackwell; 2000. pp. 429–434. [Google Scholar]
- Nilsson J, Granbom M, Råberg L. Does the strength of an immune response reflect its energetic cost? J Avian Biol. 2007;38(4):488–494. [Google Scholar]
- Ninawe AS, Selvin J. Probiotics in shrimp aquaculture: avenues and challenges. Crit Rev Microbiol. 2009;35(1):43–66. doi: 10.1080/10408410802667202. [DOI] [PubMed] [Google Scholar]
- Oliveira E, da Silva Castagini A, Masunari S. The population structure of Probopyrus floridensis (Isopoda, Bopyridae), a parasite of Macrobrachium potiuna (Decapoda, Palaemonidae) from the Perequê River, Paranaguá Basin, southern Brazil. Crustaceana. 2000;73(9):1095–1108. [Google Scholar]
- Ostfeld RS, Glass GE, Keesing F. Spatial epidemiology: an emerging (or re-emerging) discipline. Trends Ecol Evol. 2005;20(6):328–336. doi: 10.1016/j.tree.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Román-Contreras R. A new species of Probopyrus (Isopoda, Bopyridae), parasite of Macrobrachium americanum Bate, 1868 (Decapoda, Palaemonidae) Crustaceana. 1996;69(2):204–210. [Google Scholar]
- Sánchez CA, Becker DJ, Teitelbaum CS, Barriga P, Brown LM, Majewska AA, et al. On the relationship between body condition and parasite infection in wildlife: a review and meta-analysis. Ecol Lett. 2018;21(12):1869–1884. doi: 10.1111/ele.13160. [DOI] [PubMed] [Google Scholar]
- Sherman MB, Curran MC. The effect of the bopyrid isopod Probopyrus pandalicola (Packard, 1879)(Isopoda, Bopyridae) on the survival time of the daggerblade grass shrimp Palaemonetes pugio Holthuis, 1949 (Decapoda, Palaemonidae) during starvation at two different temperatures. Crustaceana. 2013;86(11):1328–1342. [Google Scholar]
- Sherman MB, Curran MC. Sexual sterilization of the daggerblade grass shrimp Palaemonetes pugio (Decapoda: Palaemonidae) by the bopyrid isopod Probopyrus pandalicola (Isopoda: Bopyridae) J Parasitol. 2015;101(1):1–5. doi: 10.1645/14-596.1. [DOI] [PubMed] [Google Scholar]
- Smith AE, Chapman JW, Dumbauld BR. Population structure and energetics of the bopyrid isopod parasite Orthione griffenis in mud shrimp Upogebia pugettensis. J Crustacean Biol. 2008;28(2):228–233. [Google Scholar]
- SRH – Secretaria de Recursos Hídricos (2007) Resolução No 20, de 23 de Agosto de 2007. http://www.semarh.ba.gov.br/Legislacao/RESOLUCAOCONERH/CONERHn20prorrogaprazoContas.pdf. Accessed 27 Sep 2020
- Subramoniam T. Mechanisms and control of vitellogenesis in crustaceans. Fish Sci. 2011;77(1):1–21. [Google Scholar]
- Swetha CH, Sainath SB, Reddy PR, Reddy PS. Reproductive endocrinology of female crustaceans: perspective and prospective. J Mar Sci Res Dev S. 2011;3:1–13. [Google Scholar]
- Thompson RK (1980) Aquaculture of Macrobrachium rosenbergii in Mauritius commercial production of juveniles. In: Giant Prawn 1980: an international conference on Macrobrachium (Freshwater Prawn) Farming, Bangkok (Thailand), 15–21 Jun 1980
- Valenti WC, Mello JD, Lobão VL. Dinâmica da reprodução de Macrobrachium acanthurus (Wiegmann, 1836) e Macrobrachium carcinus (Linnaeus, 1758) do Rio Ribeira de Iguape (Crustacea, Decapoda, Palaemonidae) Ciência e Cultura. 1986;38(7):1256–1262. [Google Scholar]
- Van Wyk PM. Inhibition of the growth and reproduction of the porcellanid crab Pachycheles rudis by the bopyrid isopod Aporobopyrus muguensis. Parasitol. 1982;85(3):459–473. [Google Scholar]
- Vargas-Ceballos MA, López-Uriarte E, García-Guerrero MU, Vega-Villasante F, Román-Contreras R, Akintola SL, et al. Infestation of Probopyrus pacificensis (Isopoda: Bopyridae) in Macrobrachium tenellum (Caridea: Palaemonidae) in the Ameca River, Jalisco, Mexico: prevalence and effects on growth. Pan-Am J Aquat Sci. 2016;11(1):39–46. [Google Scholar]
- Vinagre AS, Chung JS. Effects of starvation on energy metabolism and crustacean hyperglycemic hormone (CHH) of the Atlantic ghost crab Ocypode quadrata (Fabricius, 1787) Mar Biol. 2016;163(1):3. [Google Scholar]
- Walker SP. Probopyrus pandalicola: discontinuous ingestion of shrimp hemolymph. Exp Parasitol. 1977;41(1):198–205. doi: 10.1016/0014-4894(77)90145-x. [DOI] [PubMed] [Google Scholar]
- Wu L, Dong S, Jiang Z. Research advances in ecophysiological effects of starvation on crustacean. J Appl Ecol. 2004;15(4):723. [PubMed] [Google Scholar]
- Williams JD, Boyko CB. The global diversity of parasitic isopods associated with crustacean hosts (Isopoda: Bopyroidea and Cryptoniscoidea) PLoS One. 2012;4:e35350. doi: 10.1371/journal.pone.0035350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. Upper Saddle River, NJ: Pearson Prentice-Hall; 2010. [Google Scholar]


