Abstract
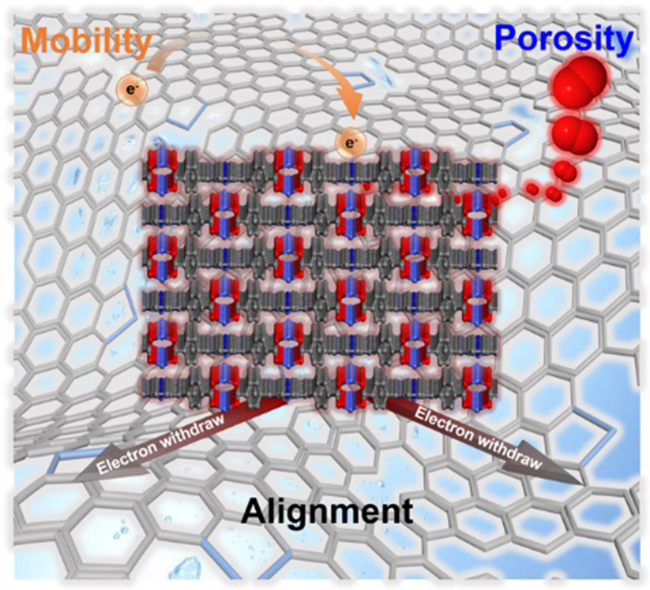
Tailoring the reaction kinetics is the central theme of designer electrocatalysts, which enables the selective conversion of abundant and inert atmospheric species into useful products. Here we show a supporting effect in tuning the electrocatalytic kinetics of oxygen reduction reaction (ORR) from four-electron to two-electron mechanism by docking metalloporphyrin-based metal-organic frameworks (MOFs) crystals on graphene support, leading to highly selective peroxide production with faradaic efficiency as high as 93.4%. A magic angle of 38.1° tilting for the co-facial alignment was uncovered by electron diffraction tomography, which is attributed to the maximization of π-π interaction for mitigating the lattice and symmetry mismatch between MOF and graphene. The facilitated electron migration and oxygen chemisorption could be ascribed to the supportive effect of graphene that disperses of the electron state of the active center, and ultimately regulates rate-determining step.
Electronic Supplementary Material
Supplementary material (synthesis protocols for control samples, morphological and structural characterizations, porosity, electrochemical properties and activities including SEM, TEM, XPS, Raman, AFM investigations) is available in the online version of this article at 10.1007/s12274-021-3382-3.
Keywords: metal-organic frameworks, nanocomposites, support effect, oxygen reduction reaction, peroxide selectivity
Electronic Supplementary Material
Docking MOF crystals on graphene support for highly selective electrocatalytic peroxide production
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Nos. 21522105 and 51861145313) and the Science & Technology Commission of Shanghai Municipality (17JC1404000). We acknowledge the support from the ShanghaiTech-SARI Joint Laboratory of Low-Carbon Energy Science, the Centre for High-resolution Electron Microscopy (CħEM, contract No. EM02161943), and the Analytical Instrumentation Center (Contract no. SPST-AIC10112914), SPST, ShanghaiTech University. The synchrotron X-ray portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource, a Directorate of SLAC National Accelerator Laboratory and an Office of Science User Facility operated for the US Department of Energy Office of Science by Stanford University. L. M. and F. L. acknowledge Department of Chemistry Startup fund at Virginia Tech; A. M. acknowledge the support of National Natural Science Foundation of China (Nos. 21850410448 and 21835002). We thank the STEM support from Dr. Weiyan Liu in CħEM. We also thank Prof. O. Terasaki, Prof. Z. Liu, Prof. Y. Ma, and Mr. T. Sun for their guidance in electron diffraction and XPS analyses. We thank and will remember Prof. Frank Tsung for his kind suggestion and encouragement, who left us forever on January 5, 2021 due to COVID-19.
References
- [1].Campos-Martin J M, Blanco-Brieva G, Fierro J L G. Hydrogen peroxide synthesis: An outlook beyond the anthraquinone process. Angew. Chem., Int. Ed. 2006;45:6962–6984. doi: 10.1002/anie.200503779. [DOI] [PubMed] [Google Scholar]
- [2].Wang K, Huang J H, Chen H X, Wang Y, Song S Q. Recent advances in electrochemical 2e oxygen reduction reaction for on-site hydrogen peroxide production and beyond. Chem. Commun. 2020;56:12109–12121. doi: 10.1039/D0CC05156J. [DOI] [PubMed] [Google Scholar]
- [3].Xia C, Xia Y, Zhu P, Fan L, Wang H T. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science. 2019;366:226–231. doi: 10.1126/science.aay1844. [DOI] [PubMed] [Google Scholar]
- [4].Perry S C, Pangotra D, Vieira L, Csepei L I, Sieber V, Wang L, de León C P, Walsh F C. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 2019;3:442–458. doi: 10.1038/s41570-019-0110-6. [DOI] [Google Scholar]
- [5].Jiang K, Zhao J J, Wang H T. Catalyst design for electrochemical oxygen reduction toward hydrogen peroxide. Adv. Funct. Mater. 2020;30:2003321. doi: 10.1002/adfm.202003321. [DOI] [Google Scholar]
- [6].Siahrostami S, Villegas S J, Mostaghimi A H B, Back S, Farimani A B, Wang H T, Persson K A, Montoya J. A review on challenges and successes in atomic-scale design of catalysts for electrochemical synthesis of hydrogen peroxide. ACS Catal. 2020;10:7495–7511. doi: 10.1021/acscatal.0c01641. [DOI] [Google Scholar]
- [7].Stamenkovic V R, Strmcnik D, Lopes P P, Markovic N M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2017;16:57–69. doi: 10.1038/nmat4738. [DOI] [PubMed] [Google Scholar]
- [8].Seh Z W, Kibsgaard J, Dickens C F, Chorkendorff I, Nørskov J K, Jaramillo T F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science. 2017;355:eaad4998. doi: 10.1126/science.aad4998. [DOI] [PubMed] [Google Scholar]
- [9].Luo M C, Guo S J. Strain-controlled electrocatalysis on multimetallic nanomaterials. Nat. Rev. Mater. 2017;2:17059. doi: 10.1038/natrevmats.2017.59. [DOI] [Google Scholar]
- [10].Escudero-Escribano M, Malacrida P, Hansen M H, Vej-Hansen U G, Velázquez-Palenzuela A, Tripkovic V, Schiøtz J, Rossmeisl J, Stephens I E L, Chorkendorff I. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. Science. 2016;352:73–76. doi: 10.1126/science.aad8892. [DOI] [PubMed] [Google Scholar]
- [11].Gong K P, Du F, Xia Z H, Durstock M, Dai L M. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science. 2009;323:760–764. doi: 10.1126/science.1168049. [DOI] [PubMed] [Google Scholar]
- [12].Chen G X, Xu C F, Huang X Q, Ye J Y, Gu L, Li G, Tang Z C, Wu B H, Yang H Y, Zhao Z P, et al. Interfacial electronic effects control the reaction selectivity of platinum catalysts. Nat. Mater. 2016;15:564–569. doi: 10.1038/nmat4555. [DOI] [PubMed] [Google Scholar]
- [13].Zheng Y, Zheng S S, Xue H G, Pang H. Metal-organic frameworks/graphene-based materials: Preparations and applications. Adv. Funct. Mater. 2018;28:1804950. doi: 10.1002/adfm.201804950. [DOI] [Google Scholar]
- [14].Sangwan V K, Beck M E, Henning A, Luo J J, Bergeron H, Kang J M, Balla I, Inbar H, Lauhon L J, Hersam M C. Self-aligned van der Waals heterojunction diodes and transistors. Nano Lett. 2018;18:1421–1427. doi: 10.1021/acs.nanolett.7b05177. [DOI] [PubMed] [Google Scholar]
- [15].Zhu X Y, Monahan N R, Gong Z Z, Zhu H M, Williams K W, Nelson C A. Charge transfer excitons at van der Waals interfaces. J. Am. Chem. Soc. 2015;137:8313–8320. doi: 10.1021/jacs.5b03141. [DOI] [PubMed] [Google Scholar]
- [16].Cheng R, Li D H, Zhou H L, Wang C, Wang A X, Jiang S, Liu Y, Chen Y, Huang Y, Duan X F. Electroluminescence and photocurrent generation from atomically sharp WSe2/MoS2 heterojunction p-n diodes. Nano Lett. 2014;14:5590–5597. doi: 10.1021/nl502075n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Su J, Li G D, Li X H, Chen J S. 2D/2D heterojunctions for catalysis. Adv. Sci. 2019;6:1801702. doi: 10.1002/advs.201801702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Popp T M O, Yaghi O M. Sequence-dependent materials. Acc. Chem. Res. 2017;50:532–534. doi: 10.1021/acs.accounts.6b00529. [DOI] [PubMed] [Google Scholar]
- [19].Pomerantseva E, Gogotsi Y. Two-dimensional heterostructures for energy storage. Nat. Energy. 2017;2:17089. doi: 10.1038/nenergy.2017.89. [DOI] [Google Scholar]
- [20].Furukawa H, Cordova K E, O’Keeffe M, Yaghi O M. The chemistry and applications of metal-organic frameworks. Science. 2013;341:1230444. doi: 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- [21].Islamoglu T, Goswami S, Li Z Y, Howarth A J, Farha O K, Hupp J T. Postsynthetic tuning of metal-organic frameworks for targeted applications. Acc. Chem. Res. 2017;50:805–813. doi: 10.1021/acs.accounts.6b00577. [DOI] [PubMed] [Google Scholar]
- [22].Ding Y J, Chen Y P, Zhang X L, Chen L, Dong Z H, Jiang H L, Xu H X, Zhou H C. Controlled intercalation and chemical exfoliation of layered metal-organic frameworks using a chemically labile intercalating agent. J. Am. Chem. Soc. 2017;139:9136–9139. doi: 10.1021/jacs.7b04829. [DOI] [PubMed] [Google Scholar]
- [23].Jiang H Q, Liu X C, Wu Y S, Shu Y F, Gong X, Ke F S, Deng H X. Metal-organic frameworks for high charge-discharge rates in lithium-sulfur batteries. Angew. Chem., Int. Ed. 2018;57:3916–3921. doi: 10.1002/anie.201712872. [DOI] [PubMed] [Google Scholar]
- [24].Dai H M, Sun J, Zhou Y, Zhou Z R, Luo W, Wei G F, Deng H X. Reticulation of 2D semiconductors by metal-organic approach for efficient hydrogen evolution. ACS Sustainable Chem. Eng. 2020;8:8102–8110. doi: 10.1021/acssuschemeng.0c02805. [DOI] [Google Scholar]
- [25].Lu X F, Liao P Q, Wang J W, Wu J X, Chen X W, He C T, Zhang J P, Li G R, Chen X M. An alkaline-stable, metal hydroxide mimicking metal-organic framework for efficient electrocatalytic oxygen evolution. J. Am. Chem. Soc. 2016;138:8336–8339. doi: 10.1021/jacs.6b03125. [DOI] [PubMed] [Google Scholar]
- [26].Lu X F, Xia B Y, Zang S Q, Lou X W. Metal-organic frameworks based electrocatalysts for the oxygen reduction reaction. Angew. Chem., Int. Ed. 2020;59:4634–4650. doi: 10.1002/anie.201910309. [DOI] [PubMed] [Google Scholar]
- [27].Shekhah O, Liu J, Fischer R A, Wöll C. MOF thin films: Existing and future applications. Chem. Soc. Rev. 2011;40:1081–1106. doi: 10.1039/c0cs00147c. [DOI] [PubMed] [Google Scholar]
- [28].Shekhah O, Wang H, Paradinas M, Ocal C, Schüpbach B, Terfort A, Zacher D, Fischer R A, Wöll C. Controlling interpenetration in metal-organic frameworks by liquid-phase epitaxy. Nat. Mater. 2009;8:481–484. doi: 10.1038/nmat2445. [DOI] [PubMed] [Google Scholar]
- [29].Hu P, Zhuang J, Chou L Y, Lee H K, Ling X Y, Chuang Y C, Tsung C K. Surfactant-directed atomic to mesoscale alignment: Metal nanocrystals encased individually in single-crystalline porous nanostructures. J. Am. Chem. Soc. 2014;136:10561–10564. doi: 10.1021/ja5048522. [DOI] [PubMed] [Google Scholar]
- [30].Zhao Y B, Kornienko N, Liu Z, Zhu C H, Asahina S, Kuo T R, Bao W, Xie C L, Hexemer A, Terasaki O, et al. Mesoscopic constructs of ordered and oriented metal-organic frameworks on plasmonic silver nanocrystals. J. Am. Chem. Soc. 2015;137:2199–2202. doi: 10.1021/ja512951e. [DOI] [PubMed] [Google Scholar]
- [31].Falcaro P, Okada K, Hara T, Ikigaki K, Tokudome Y, Thornton A W, Hill A J, Williams T, Doonan C, Takahashi M. Centimetrescale micropore alignment in oriented polycrystalline metal-organic framework films via heteroepitaxial growth. Nat. Mater. 2017;16:342–348. doi: 10.1038/nmat4815. [DOI] [PubMed] [Google Scholar]
- [32].Wu J X, Hou S Z, Zhang X D, Xu M, Yang H F, Cao P S, Gu Z Y. Cathodized copper porphyrin metal-organic framework nanosheets for selective formate and acetate production from CO2 electroreduction. Chem. Sci. 2019;10:2199–2205. doi: 10.1039/C8SC04344B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhao M T, Huang Y, Peng Y W, Huang Z Q, Ma Q L, Zhang H. Two-dimensional metal-organic framework nanosheets: Synthesis and applications. Chem. Soc. Rev. 2018;47:6267–6295. doi: 10.1039/C8CS00268A. [DOI] [PubMed] [Google Scholar]
- [34].Zagal J H, Koper M T M. Reactivity descriptors for the activity of molecular MN4 catalysts for the oxygen reduction reaction. Angew. Chem., Int. Ed. 2016;55:14510–14521. doi: 10.1002/anie.201604311. [DOI] [PubMed] [Google Scholar]
- [35].Lu Z Y, Chen G X, Siahrostami S, Chen Z H, Liu K, Xie J, Liao L, Wu T, Lin D C, Liu Y Y, et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 2018;1:156–162. doi: 10.1038/s41929-017-0017-x. [DOI] [Google Scholar]
- [36].Han L, Sun Y Y, Li S, Cheng C, Halbig C E, Feicht P, Hübner J L, Strasser P, Eigler S. In-plane carbon lattice-defect regulating electrochemical oxygen reduction to hydrogen peroxide production over nitrogen-doped graphene. ACS Catal. 2019;9:1283–1288. doi: 10.1021/acscatal.8b03734. [DOI] [Google Scholar]
- [37].Chen S C, Chen Z H, Siahrostami S, Higgins D, Nordlund D, Dimosthenis S, Kim T R, Liu Y Z, Yan X Z, Nilsson E, et al. Designing boron nitride islands in carbon materials for efficient electrochemical synthesis of hydrogen peroxide. J. Am. Chem. Soc. 2018;140:7851–7859. doi: 10.1021/jacs.8b02798. [DOI] [PubMed] [Google Scholar]
- [38].Tan Y M, Xu C F, Chen G X, Fang X L, Zheng N F, Xie Q J. Facile synthesis of manganese-oxide-containing mesoporous nitrogen-doped carbon for efficient oxygen reduction. Adv. Funct. Mater. 2012;22:4584–4591. doi: 10.1002/adfm.201201244. [DOI] [Google Scholar]
- [39].Valarselvan S, Manisankar P. Electrocatalytic reduction of oxygen at glassy carbon electrode modified by polypyrrole/anthraquinones composite film in various pH media. Electrochim. Acta. 2011;56:6945–6953. doi: 10.1016/j.electacta.2011.06.018. [DOI] [Google Scholar]
- [40].Ohnishi R, Katayama M, Cha D, Takanabe K, Kubota J, Domen K. Titanium nitride nanoparticle electrocatalysts for oxygen reduction reaction in alkaline solution. J. Electrochem. Soc. 2013;160:F501. doi: 10.1149/2.053306jes. [DOI] [Google Scholar]
- [41].Yasuda S, Yu L, Kim J, Murakoshi K. Selective nitrogen doping in graphene for oxygen reduction reactions. Chem. Commun. 2013;49:9627–9629. doi: 10.1039/c3cc45641b. [DOI] [PubMed] [Google Scholar]
- [42].Aijaz A, Fujiwara N, Xu Q. From metal-organic framework to nitrogen-decorated nanoporous carbons: High CO2 uptake and efficient catalytic oxygen reduction. J. Am. Chem. Soc. 2014;136:6790–6793. doi: 10.1021/ja5003907. [DOI] [PubMed] [Google Scholar]
- [43].Choi C H, Kwon H C, Yook S, Shin H, Kim H, Choi M. Hydrogen peroxide synthesis via enhanced two-electron oxygen reduction pathway on carbon-coated Pt Surface. J. Phys. Chem. C. 2014;118:30063–30070. doi: 10.1021/jp5113894. [DOI] [Google Scholar]
- [44].Park J, Nabae Y, Hayakawa T, Kakimoto M A. Highly selective two-electron oxygen reduction catalyzed by mesoporous nitrogen-doped carbon. ACS Catal. 2014;4:3749–3754. doi: 10.1021/cs5008206. [DOI] [Google Scholar]
- [45].Vikkisk M, Kruusenberg I, Joost U, Shulga E, Kink I, Tammeveski K. Electrocatalytic oxygen reduction on nitrogen-doped graphene in alkaline media. Appl. Catal. B: Environ. 2014;147:369–376. doi: 10.1016/j.apcatb.2013.09.011. [DOI] [Google Scholar]
- [46].Wang H, Yin F X, Li G R, Chen B H, Wang Z Q. Preparation, characterization and bifunctional catalytic properties of MOF(Fe/Co) catalyst for oxygen reduction/evolution reactions in alkaline electrolyte. Int. J. Hydrogen Energy. 2014;39:16179–16186. doi: 10.1016/j.ijhydene.2013.12.120. [DOI] [Google Scholar]
- [47].Favaro M, Ferrighi L, Fazio G, Colazzo L, Di Valentin C, Durante C, Sedona F, Gennaro A, Agnoli S, Granozzi G. Single and multiple doping in graphene quantum dots: Unraveling the origin of selectivity in the oxygen reduction reaction. ACS Catal. 2015;5:129–144. doi: 10.1021/cs501211h. [DOI] [Google Scholar]
- [48].Rameshkumar P, Praveen R, Ramaraj R. Electroanalysis of oxygen reduction and formic acid oxidation using reduced graphene oxide/gold nanostructures modified electrode. J. Electroanal. Chem. 2015;754:118–124. doi: 10.1016/j.jelechem.2015.07.011. [DOI] [Google Scholar]
- [49].Rigsby M L, Wasylenko D J, Pegis M L, Mayer J M. Medium effects are as important as catalyst design for selectivity in electrocatalytic oxygen reduction by iron-porphyrin complexes. J. Am. Chem. Soc. 2015;137:4296–4299. doi: 10.1021/jacs.5b00359. [DOI] [PubMed] [Google Scholar]
- [50].Wu J J, Zhang D, Niwa H, Harada Y, Oshima M, Ofuchi H, Nabae Y, Okajima T, Ohsaka T. Enhancement in kinetics of the oxygen reduction reaction on a nitrogen-doped carbon catalyst by introduction of iron via electrochemical methods. Langmuir. 2015;31:5529–5536. doi: 10.1021/acs.langmuir.5b00310. [DOI] [PubMed] [Google Scholar]
- [51].Fellinger T P, Hasché F, Strasser P, Antonietti M. Mesoporous Nitrogen-doped carbon for the electrocatalytic synthesis of hydrogen peroxide. J. Am. Chem. Soc. 2012;134:4072–4075. doi: 10.1021/ja300038p. [DOI] [PubMed] [Google Scholar]
- [52].Li B Q, Zhao C X, Liu J N, Zhang Q. Electrosynthesis of hydrogen peroxide synergistically catalyzed by atomic Co-Nx-C sites and oxygen functional groups in noble-metal-free electrocatalysts. Adv. Mater. 2019;31:1808173. doi: 10.1002/adma.201808173. [DOI] [PubMed] [Google Scholar]
- [53].Jung E, Shin H, Lee B H, Efremov V, Lee S, Lee H S, Kim J, Antink W H, Park S, Lee K S, et al. Atomic-level tuning of Co-N-C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 2020;19:436–442. doi: 10.1038/s41563-019-0571-5. [DOI] [PubMed] [Google Scholar]
- [54].Sun Y Y, Silvioli L, Sahraie N R, Ju W, Li J K, Zitolo A, Li S, Bagger A, Arnarson L, Wang X L, et al. Activity-selectivity trends in the electrochemical production of hydrogen peroxide over single-site metal-nitrogen-carbon catalysts. J. Am. Chem. Soc. 2019;141:12372–12381. doi: 10.1021/jacs.9b05576. [DOI] [PubMed] [Google Scholar]
- [55].Wang M J, Zhang N, Feng Y G, Hu Z W, Shao Q, Huang X Q. Partially pyrolyzed binary metal-organic framework nanosheets for efficient electrochemical hydrogen peroxide synthesis. Angew. Chem., Int. Ed. 2020;59:14373–14377. doi: 10.1002/anie.202006422. [DOI] [PubMed] [Google Scholar]
- [56].Byeon A, Cho J, Kim J M, Chae K H, Park H Y, Hong S W, Ham H C, Lee S W, Yoon K R, Kim J Y. High-yield electrochemical hydrogen peroxide production from an enhanced two-electron oxygen reduction pathway by mesoporous nitrogen-doped carbon and manganese hybrid electrocatalysts. Nanoscale Horiz. 2020;5:832–838. doi: 10.1039/C9NH00783K. [DOI] [PubMed] [Google Scholar]
- [57].Sun Y Y, Sinev I, Ju W, Bergmann A, Dresp S, Kühl S, Spöri C, Schmies H, Wang H, Bernsmeier D, et al. Efficient electrochemical hydrogen peroxide production from molecular oxygen on nitrogen-doped mesoporous carbon catalysts. ACS Catal. 2018;8:2844–2856. doi: 10.1021/acscatal.7b03464. [DOI] [Google Scholar]
- [58].Sun Y Y, Li S, Jovanov Z P, Bernsmeier D, Wang H, Paul B, Wang X L, Kühl S, Strasser P. Structure, activity, and faradaic efficiency of nitrogen-doped porous carbon catalysts for direct electrochemical hydrogen peroxide production. ChemSusChem. 2018;11:3388–3395. doi: 10.1002/cssc.201801583. [DOI] [PubMed] [Google Scholar]
- [59].Liu M H, Zhang H, Li Y L, Su H, Zhou W L, Zhao X, Cheng W R, Liu Q H. Crystallinity dependence for high-selectivity electrochemical oxygen reduction to hydrogen peroxide. Chem. Commun. 2020;56:5299–5302. doi: 10.1039/D0CC00139B. [DOI] [PubMed] [Google Scholar]
- [60].Li L Q, Tang C, Zheng Y, Xia B Q, Zhou X L, Xu H L, Qiao S Z. Tailoring selectivity of electrochemical hydrogen peroxide generation by tunable pyrrolic-nitrogen-carbon. Adv. Energy Mater. 2020;10:2000789. doi: 10.1002/aenm.202000789. [DOI] [Google Scholar]
- [61].Ko Y J, Choi K, Yang B, Lee W H, Kim J Y, Choi J W, Chae K H, Lee J H, Hwang Y J, Min B K, et al. A catalyst design for selective electrochemical reactions: Direct production of hydrogen peroxide in advanced electrochemical oxidation. J. Mater. Chem. A. 2020;8:9859–9870. doi: 10.1039/D0TA01869D. [DOI] [Google Scholar]
- [62].Dong Y Q, Su J X, Zhou S Q, Wang M, Huang S P, Lu C H, Yang H B, Fu F F. Carbon-based dots for the electrochemical production of hydrogen peroxide. Chem. Commun. 2020;56:7609–7612. doi: 10.1039/C9CC09987E. [DOI] [PubMed] [Google Scholar]
- [63].Zhao X, Wang Y, Da Y L, Wang X X, Wang T T, Xu M Q, He X Y, Zhou W, Li Y F, Coleman J N, et al. Selective electrochemical production of hydrogen peroxide at zigzag edges of exfoliated molybdenum telluride nanoflakes. Natl. Sci. Rev. 2020;7:1360–1366. doi: 10.1093/nsr/nwaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kim H W, Ross M B, Kornienko N, Zhang L, Guo J H, Yang P D, McCloskey B D. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts. Nat. Catal. 2018;1:282–290. doi: 10.1038/s41929-018-0044-2. [DOI] [Google Scholar]
- [65].Sa Y J, Kim J H, Joo S H. Active edge-site-rich carbon nanocatalysts with enhanced electron transfer for efficient electrochemical hydrogen peroxide production. Angew. Chem., Int. Ed. 2019;58:1100–1105. doi: 10.1002/anie.201812435. [DOI] [PubMed] [Google Scholar]
- [66].Zhao K, Su Y, Quan X, Liu Y M, Chen S, Yu H T. Enhanced H2O2 production by selective electrochemical reduction of O2 on fluorine-doped hierarchically porous carbon. J. Catal. 2018;357:118–126. doi: 10.1016/j.jcat.2017.11.008. [DOI] [Google Scholar]
- [67].Iglesias D, Giuliani A, Melchionna M, Marchesan S, Criado A, Nasi L, Bevilacqua M, Tavagnacco C, Vizza F, Prato M, et al. N-doped graphitized carbon nanohorns as a forefront electrocatalyst in highly selective O2 reduction to H2O2. Chem. 2018;4:106–123. doi: 10.1016/j.chempr.2017.10.013. [DOI] [Google Scholar]
- [68].Pham-Truong T N, Petenzi T, Ranjan C, Randriamahazaka H, Ghilane J. Microwave assisted synthesis of carbon dots in ionic liquid as metal free catalyst for highly selective production of hydrogen peroxide. Carbon. 2018;130:544–552. doi: 10.1016/j.carbon.2018.01.070. [DOI] [Google Scholar]
- [69].Lim B, Jiang M J, Camargo P H C, Cho E C, Tao J, Lu X M, Zhu Y M, Xia Y N. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science. 2009;324:1302–1305. doi: 10.1126/science.1170377. [DOI] [PubMed] [Google Scholar]
- [70].Wang D L, Xin H L, Hovden R, Wang H, Yu Y C, Muller D A, DiSalvo F J, Abruña H D. Structurally ordered intermetallic platinum-cobalt core-shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 2013;12:81–87. doi: 10.1038/nmat3458. [DOI] [PubMed] [Google Scholar]
- [71].Xie S F, Choi S I, Lu N, Roling L T, Herron J A, Zhang L, Park J, Wang J G, Kim M J, Xie Z X, et al. Atomic layer-by-layer deposition of Pt on Pd nanocubes for catalysts with enhanced activity and durability toward oxygen reduction. Nano Lett. 2014;14:3570–3576. doi: 10.1021/nl501205j. [DOI] [PubMed] [Google Scholar]
- [72].Yang J H, Yang J, Ying J Y. Morphology and lateral strain control of Pt nanoparticles via core-shell construction using alloy AgPd Core toward oxygen reduction reaction. ACS Nano. 2012;6:9373–9382. doi: 10.1021/nn303298s. [DOI] [PubMed] [Google Scholar]
- [73].Laokroekkiat S, Hara M, Nagano S, Nagao Y. Metal-organic coordination network thin film by surface-induced assembly. Langmuir. 2016;32:6648–6655. doi: 10.1021/acs.langmuir.6b01251. [DOI] [PubMed] [Google Scholar]
- [74].Lukasczyk T, Flechtner K, Merte L R, Jux N, Maier F, Gottfried J M, Steinrück H P. Interaction of cobalt(II) tetraarylporphyrins with a Ag(111) surface studied with photoelectron spectroscopy. J. Phys. Chem. C. 2007;111:3090–3098. doi: 10.1021/jp0652345. [DOI] [Google Scholar]
- [75].Kumar P, Kumar A, Sreedhar B, Sain B, Ray S S, Jain S L. Cobalt phthalocyanine immobilized on graphene oxide: An efficient visible-active catalyst for the photoreduction of carbon dioxide. Chem.-Eur. J. 2014;20:6154–6161. doi: 10.1002/chem.201304189. [DOI] [PubMed] [Google Scholar]
- [76].Malig J, Jux N, Kiessling D, Cid J J, Vazquez P, Torres T, Guldi M D. Towards tunable graphene/phthalocyanine-PPV hybrid systems. Angew. Chem., Int. Ed. 2011;50:3561–3565. doi: 10.1002/anie.201003364. [DOI] [PubMed] [Google Scholar]
- [77].Pham V D, Lagoute J, Mouhoub O, Joucken F, Repain V, Chacon C, Bellec A, Girard Y, Rousset S. Electronic interaction between nitrogen-doped graphene and porphyrin molecules. ACS Nano. 2014;8:9403–9409. doi: 10.1021/nn503753e. [DOI] [PubMed] [Google Scholar]
- [78].Wojcik A, Kamat P V. Reduced graphene oxide and porphyrin. An interactive affair in 2-D. ACS Nano. 2010;4:6697–6706. doi: 10.1021/nn102185q. [DOI] [PubMed] [Google Scholar]
- [79].Sharoyan E G, Sharoyan V E, Ovsyannikov M. EXAFS studies of organic molecular ferromagnets based on cobalt phthalocyanine: Na2.8CoPc. J. Porphyr. Phthalocyanines. 1998;2:237–241. doi: 10.1002/(SICI)1099-1409(199805/06)2:3<237::AID-JPP70>3.0.CO;2-T. [DOI] [Google Scholar]
- [80].Sarangi R, Cho J, Nam W, Solomon E I. XAS and DFT investigation of mononuclear cobalt(III) peroxo complexes: Electronic control of the geometric structure in CoO2 versus NiO2 systems. Inorg. Chem. 2011;50:614–620. doi: 10.1021/ic101730r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Zhang Q R, Tan X, Bedford N M, Han Z J, Thomsen L, Smith S, Amal R, Lu X Y. Direct insights into the role of epoxy groups on cobalt sites for acidic H2O2 production. Nat. Commun. 2020;11:4181. doi: 10.1038/s41467-020-17782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wang, Y. H.; Wang, X. T.; Ze, H. J.; Zhang, X. G.; Radjenovic, P. M.; Zhang, Y. J.; Dong, J. C.; Tian, Z. Q.; Li, J. F. Spectroscopic verification of adsorbed hydroxy intermediates in the bifunctional mechanism of the hydrogen oxidation reaction. Angew. Chem., Int. Ed., in press, DOI: 10.1002/anie.202015571. [DOI] [PubMed]
- [83].Hu A Q, Pang Q Q, Tang C, Bao J X, Liu H Q, Ba K, Xie S H, Chen J, Chen J H, Yue Y W, et al. Epitaxial growth and integration of insulating metal-organic frameworks in electrochemistry. J. Am. Chem. Soc. 2019;141:11322–11327. doi: 10.1021/jacs.9b05869. [DOI] [PubMed] [Google Scholar]
- [84].Wu J, Chen J H, Wang C, Zhou Y, Ba K, Xu H, Bao W Z, Xu X H, Carlsson A, Lazar S, et al. Metal-organic framework for transparent electronics. Adv. Sci. 2020;7:1903003. doi: 10.1002/advs.201903003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kumar A, Banerjee K, Foster A S, Liljeroth P. Two-dimensional band structure in honeycomb metal-organic frameworks. Nano Lett. 2018;18:5596–5602. doi: 10.1021/acs.nanolett.8b02062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lai L F, Potts J R, Zhan D, Wang L, Poh C K, Tang C H, Gong H, Shen Z X, Lin J Y, Ruoff R S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012;5:7936–7942. doi: 10.1039/c2ee21802j. [DOI] [Google Scholar]
- [87].Xing T, Zheng Y, Li L H, Cowie B C C, Gunzelmann D, Qiao S Z, Huang S M, Chen Y. Observation of active sites for oxygen reduction reaction on nitrogen-doped multilayer graphene. ACS Nano. 2014;8:6856–6862. doi: 10.1021/nn501506p. [DOI] [PubMed] [Google Scholar]
- [88].Parvez K, Wu Z S, Li R J, Liu X J, Graf R, Feng X L, Müllen K. Exfoliation of graphite into graphene in aqueous solutions of inorganic salts. J. Am. Chem. Soc. 2014;136:6083–6091. doi: 10.1021/ja5017156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Docking MOF crystals on graphene support for highly selective electrocatalytic peroxide production