Abstract
Background: Demonstration of exit block from the pulmonary vein (PV) to the left atrium after PV isolation (PVI) is not always possible after demonstration of entrance block. We examined factors associated with demonstrable exit block and the relationship between demonstrable exit block and subsequent PV reconnection.
Methods and Results: The subjects consisted of 227 patients (908 PV; mean patient age, 59.2±10.8 years; 72.2% male) who underwent radiofrequency PVI, 49 of whom proceeded to the second session after a mean duration of 563.4±456.3 days after the first session. In the first session, exit block was demonstrated in 73.1% of PV, and the predictors were superior PV, longitudinal diameter of the PV, and spontaneous activity in the PV. In the second session (n=49), exit block was demonstrated in 51.0% (33.1% in PV without reconnection vs. 79.7% in PV with reconnection, P<0.0001). Spontaneous activity (OR, 2.74; 95% CI: 1.12–7.03, P=0.0272) and use of a contact force-sensing catheter (OR, 0.42, 95% CI: 0.20–0.85, P=0.0151) were independent predictors of PV reconnection, but demonstrable exit block was not (OR, 1.58; 95% CI: 0.74–3.46, P=0.2377).
Conclusions: Inability to demonstrate exit block was not associated with increased risk of future PV reconnection.
Key Words: Atrial fibrillation, Catheter ablation, Exit block, Pulmonary vein isolation, Pulmonary vein reconnection
Pulmonary vein isolation (PVI) has been established as an essential and standard approach in catheter ablation (CA) for atrial fibrillation (AF). Despite recent improvement in CA technology, AF recurs at a significantly high rate after successful PVI.1,2 Spontaneous electrical activity originating from the myocardial sleeve at the ostium of the pulmonary vein (PV) and/or antrum of the left atrium (LA) is known to be a major trigger source of AF.3 Therefore, the primary goal of PVI is complete electrical isolation of the PV from the LA, given that electrical reconnection between them plays an important role in the recurrence of AF.1,4 Guidelines recommend confirmation of the presence of entrance block (LA to PV; class I indication) at least, and of exit block (PV to LA) if possible (class IIb) to ensure completion of electrical isolation when performing PVI,5 but it is not always possible to demonstrate exit block after PVI, and the question of whether non-demonstrable exit block is associated with an increased risk of PV reconnection after PVI has not been clarified. The purpose of this study was therefore to clarify the prevalence and significance of exit block in the context of PVI and its association with long-term PV reconnection, which is responsible for the recurrence of AF.
Methods
Subjects
This study was a single-center retrospective study conducted at Sapporo Medical University Hospital. The subjects consisted of 247 consecutive patients with drug-refractory and symptomatic AF who underwent first-time radiofrequency (RF) CA at the present institute between 2011 and 2016. One patient in whom PVI could not be successfully achieved and 19 patients who did not have 4 PV (e.g., a prominent left common PV and a previous history of surgery for lung cancer), were excluded from this study. Thus, 227 patients (908 PV) contributed to data analysis regarding exit block. Of the 227 patients, 49 had AF recurrence and proceeded to the second session, but 1 patient did not have evaluation of exit block. Paroxysmal AF (PAF) was diagnosed when AF terminated in ≤7 days and both AF and sinus rhythm (SR) had been documented on 12-lead electrocardiography (ECG) and/or Holter monitoring. Non-PAF was defined as continuous AF persisting for >7 days.5
Catheter Ablation
Anti-arrhythmic agents were discontinued on admission (i.e., 2 days before CA). 3-D reconstructed computed tomography (CT) of the heart and PV was performed 1 day before CA except for 4 patients with renal dysfunction. The longitudinal diameter of the PV ostium was measured on 3D-CT. A 20-multipole size-variable circular catheter (LASSO 2515, Biosense Webster, Diamond Bar, CA, USA) and an appropriate-sized 10- or 20-multipole circular catheter (EP star Libero 12.5–22.5 mm, Japan lifeline, Tokyo, Japan) were used for recording of the electrogram and pacing in the PV. CA was performed under deep sedation using continuous infusion of dexmedetomidine and propofol.
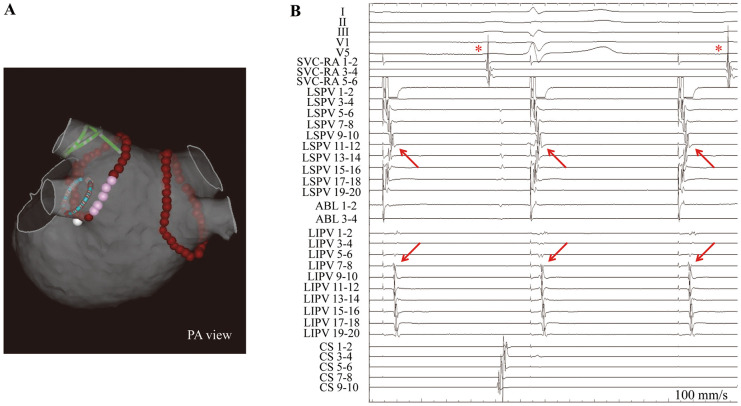
Two circular catheters were positioned at the ostium of the ipsilateral PV (double Lasso technique) and all of the patients underwent extensive encircling PVI (Figure 1A). RF ablation was performed under the guidance of a CARTO system (Biosense Webster) using a 3.5-mm-tip open-irrigated catheter; and a contact force (CF)-sensing catheter (NaviStar ThermoCool SmartTouch, Biosense Webster) was used after December 2014. Although the basic RF energy setting was 30 W and 20 s for each point, the power and duration were reduced to 20 W and 10–15 s, respectively, at the left-sided posterior wall or at a site close to the esophagus. When the PV potential was not eliminated by a single circular line, detailed mapping along the lesion line and additional touch-up ablation at a residual conduction gap were performed. Linear ablation at the carina site was required in some cases. If AF was ongoing even after PVI, SR was restored by intra-cardiac or external electrical cardioversion. Pacing from the LA appendage (LAA) or superior vena cava (SVC) was performed to distinguish PV potential from far-field potential, contributing to careful assessment of the entrance block.6 Sequential pacing from bipolar electrodes of the circular catheter in the PV was performed until PV sleeve musculature was captured to evaluate the presence of the exit block after completion of the entrance block (i.e., disappearance of PV potentials). PV pacing was performed during SR at 80–100 beats/min and a fixed output of 5.0 V/0.2 ms. Exit block was defined as local capture of the PV sleeve musculature without conduction to the LA (Figure 1B). Additional ablation was performed when unidirectional exit conduction was observed during PV pacing. If local capture of the PV sleeve was not obtained on PV pacing, the location and/or size of the circular catheter were adjusted again to optimize the contact between the PV and circular catheter electrodes. The definition of inability to demonstrate the exit block was loss of local capture of the PV sleeve musculature without conduction from PV to LA. Injection of adenosine triphosphate was performed at least 20 min after PVI, and additional ablation was repeated until dormant conduction was successfully eliminated. Spontaneous activity in the PV observed using the circular catheter was checked and marked on the record. Although additional ablation other than PVI was left to the discretion of individual operators, linear ablation of the cavotricuspid isthmus was performed in patients who had documented or induced atrial flutter. LA posterior wall isolation encircled by the roof line and bottom line and/or SVC isolation were performed in patients with non-PAF. PV reconnection was defined as an entrance conduction (i.e., the presence of PV potential) in the next session. Electrophysiological assessment and judgment were performed by more than 2 cardiac electrophysiologists.
Figure 1.
Representative (A) 3-D electroanatomic map and (B) intracardiac electrocardiogram (ECG). (A) Representative ablation line of pulmonary vein (PV) isolation and position of the circular catheters. Brown tags, sites of radiofrequency application; pink tags, sites close to the esophagus. (B) Exit block demonstrated by local capture of the PV sleeve musculature (arrows) without conduction to the left atrium. Asterisk, sinus rhythm. ABL, ablation catheter; CS, coronary sinus; LIPV, left inferior PV; LSPV, left superior PV; PA, posteroanterior; RA, right atrium; SVC, superior vena cava.
Follow-up
All of the patients received ECG at the present outpatient clinic or at clinics of physicians who referred the patients to the present institute at 3, 6, 9, 12, 18, and 24 months after CA or any time when symptomatic. Twenty-four-hour Holter monitoring was performed at 6 months and at 2 years after CA. Use of anti-arrhythmic agents for AF was discontinued at 3 months after CA. Recurrence of AF was defined as AF or atrial tachycardia (AT) documented on 12-lead ECG or 24-h Holter monitoring (cut-off duration >30 s in cases documented on Holter ECG) after a blanking period of 3 months after the prior CA.5
Statistical Analysis
Continuous data are expressed as mean±SD, and categorical variables are given as n (%). Differences in continuous variables between 2 groups were assessed using the Mann-Whitney U-test. Categorical variables were analyzed using the chi-squared test, and Fisher’s exact test was used when appropriate. To determine independent predictors, multivariate logistic regression analysis was performed. P<0.05 was considered statistically significant. Variables with P<0.05 on univariate analysis were entered into a multivariable model. Data analysis was performed using commercially available statistical analysis software (JMP version 11.0.0; SAS Institute, Cary, NC, USA).
Results
Baseline clinical subject characteristics are listed in Table 1. Mean patient age was 59.2±10.8 years, and 72.2% of the patients were male. PAF was present in 70.5% of the patients, and 15.4% of the patients had structural heart disease including hypertrophic cardiomyopathy (n=21), valvular heart disease (n=7), dilated cardiomyopathy (n=2), ischemic cardiomyopathy (n=2), congenital heart disease (n=4), and hemochromatosis (n=1). Ninety patients (39.6%) had hypertension. Mean CHADS2 score (1 point for congestive heart failure, hypertension, age ≥75 years, and diabetes mellitus, and 2 points for history of stroke or transient ischemic attack) was 0.82±0.93, and mean CHA2DS2-VASc score (1 point for congestive heart failure, hypertension, age 65–74, diabetes mellitus, female gender, vascular disease, and 2 points for age ≥75, and history of stroke or transient ischemic attack) was 1.49±1.30. The longitudinal diameter of the PV ostium was 18.1±3.3 mm, and the superior PV (SPV) was significantly larger than the inferior PV (IPV; 19.4±3.1 mm vs. 16.8±2.9 mm, P<0.001). On echocardiography, the LA diameter was 39.0±7.1 mm, LA volume index was 39.2±13.7 mL/m2, and left ventricular ejection fraction was 61.5±9.5%. A CF-sensing catheter was used in 38.8% of the patients in the first session and in 57.1% of the patients in the second session.
Table 1.
Baseline Clinical Characteristics (n=227)
| Age (years) | 59.2±10.8 |
| Male | 164 (72.2) |
| BMI (kg/m2) | 24.6±3.3 |
| PAF (vs. non-PAF) | 160 (70.5) |
| Structural heart disease | 35 (15.4) |
| Hypertension | 90 (39.6) |
| Diabetes mellitus | 26 (11.5) |
| CHADS2 score | 0.82±0.93 |
| CHA2DS2-VASc score | 1.49±1.30 |
| Anatomical data | |
| Longitudinal diameter of the PV ostium (mm) | 18.1±3.3 |
| Left superior PV (mm) | 19.6±3.3 |
| Left inferior PV (mm) | 16.3±2.4 |
| Right superior PV (mm) | 19.1±3.0 |
| Right inferior PV (mm) | 17.2±3.2 |
| LAD (mm) | 39.0±7.1 |
| LAVI (mL/m2) | 39.2±13.7 |
| LVEF (%) | 61.5±9.5 |
| Laboratory data | |
| eGFR (mL/min/1.73 m2) | 72.2±17.9 |
| BNP (pg/mL) | 124.3±198.9 |
| Procedural data | |
| CF-sensing catheter in 1 st session | 88 (38.8) |
| CF-sensing catheter in 2nd session | 28/49 (57.1) |
Data given as n (%) or mean±SD. BMI, body mass index; BNP, brain natriuretic peptide; CF, contact force; eGFR, estimated glomerular filtration rate; LAD, left atrial diameter; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; PAF, paroxysmal atrial fibrillation; PV, pulmonary vein.
Demonstrable Exit Block: Prevalence and Predictors
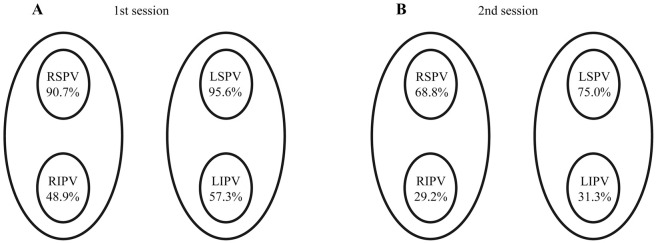
In the first session, entrance block was achieved in all PV, but exit block was demonstrated in only 73.1% (664/908) of the PV (i.e., 2.93±0.94 PV per patient; Table 2). Demonstrable exit block was noted in 95.6% of left SPV (LSPV), in 57.3% of left IPV (LIPV), in 90.7% of right SPV (RSPV), and in 48.9% of right IPV (RIPV; Figure 2A). There was no PV in which unidirectional exit conduction was observed after completion of the entrance block. The prevalence of demonstrable exit block was higher in the SPV than in the IPV (93.2% vs. 53.1%, P<0.0001) and also higher in the left-sided PV (LPV) than in the right-sided PV (RPV; 76.4% vs. 69.8%, P=0.0298). On univariate analysis, SPV, LPV, longitudinal diameter of the PV, spontaneous activity in the PV, and presence of dormant conduction were associated with demonstrable exit block in the first session. On multivariate analysis, SPV (OR, 8.44; 95% CI: 5.11–14.6, P<0.0001), longitudinal diameter of the PV (OR, 1.15 per 1 mm; 95% CI: 1.07–1.24, P=0.0002), and spontaneous activity in the PV (OR, 11.5; 95% CI: 3.41–72.0, P=0.0009) were independent predictors of demonstrable exit block in the first session.
Table 2.
Predictors of Demonstrable Exit Block in the First Session
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Exit block (−) n=244 PV |
Exit block (+) n=664 PV |
P-value | OR | 95% CI | P-value | |
| Anatomical data | ||||||
| PV location (LSPV/LIPV/RSPV/RIPV) | 10/97/21/116 | 217/130/206/111 | ||||
| Superior PV (vs. inferior PV) | 31 (12.7) | 423 (63.7) | <0.0001* | 8.44 | 5.11–14.6 | <0.0001* |
| Left-sided PV (vs. right-sided PV) | 107 (43.9) | 347 (52.3) | 0.0298* | 1.21 | 0.81–1.81 | 0.3653 |
| Longitudinal diameter of PV ostium (mm) | 16.5±3.0 | 18.6±3.2 | <0.0001* | 1.15 | 1.07–1.24 | 0.0002* |
| Procedural data (first session) | ||||||
| CF-sensing catheter | 84 (34.4) | 268 (40.4) | 0.1073 | |||
| Carina line | 17 (7.0) | 63 (9.5) | 0.2905 | |||
| Spontaneous PV activity | 3 (1.2) | 122 (18.4) | <0.0001* | 11.5 | 3.41–72.0 | 0.0009* |
| Dormant conduction | 13/176 (7.4) | 72/514 (14.0) | 0.0233* | 1.82 | 0.94–3.74 | 0.0885 |
Data given as n (%) or mean±SD. *P<0.05. CF, contact force; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; PV, pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Figure 2.
Demonstrable exit block in each pulmonary vein (PV) as a percentage of PV isolations in (A) the first session and (B) the second session. LIPV, left inferior PV; LSPV, left superior PV; RIPV, right inferior PV; RSPV, right superior PV.
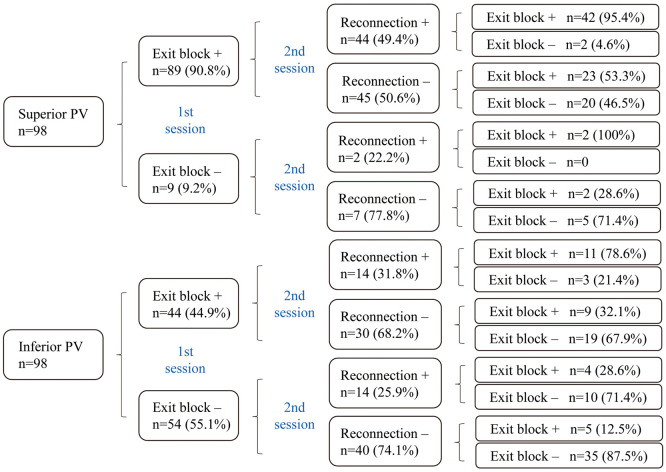
Forty-nine patients proceeded to the second session due to recurrence of AF after a mean interval of 563.4±456.3 days from the first session, and PV reconnection was observed in 37.8% (74/196) of all PV. Re-isolation of the PV was attempted and entrance block was achieved in all reconnected PV in the second session. Exit block was demonstrated in 51.0% (98/192) of all PV at the end of the second session (Table 3). The detailed clinical course of the 49 patients is shown in Figure 3. The prevalence of demonstrable exit block was 33.1% (39/118) in PV without reconnection and 79.7% (59/74) in PV with reconnection (P<0.0001). The prevalence of demonstrable exit block was lower in the second session than in the first session: 75.0% in the LSPV, 31.3% in the LIPV, 68.8% in the RSPV, and 29.2% in the RIPV (Figure 2B). The SPV had a significantly higher prevalence of demonstrable exit block than did the IPV (71.9% vs. 30.2%, P<0.0001). On univariate analysis, SPV, longitudinal diameter of the PV, spontaneous activity in the PV, exit block in the first session, and PV reconnection were associated with demonstrable exit block in the second session. On multivariate analysis, SPV (OR, 2.77; 95% CI: 1.13–6.99, P=0.0276), spontaneous activity in the PV (OR, 3.96; 95% CI: 1.29–14.9, P=0.0240), exit block in the first session (OR, 3.65; 95% CI: 1.53–9.13, P=0.0042), and PV reconnection (OR, 5.75; 95% CI: 2.55–13.7, P<0.0001) were independent predictors of demonstrable exit block at the end of the second session.
Table 3.
Predictors of Demonstrable Exit Block in the Second Session
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Exit block (−) n=94 PV |
Exit block (+) n=98 PV |
P-value | OR | 95% CI | P-value | |
| Anatomical data | ||||||
| PV location (LSPV/LIPV/RSPV/RIPV) | 12/33/15/34 | 36/15/33/14 | ||||
| Superior PV (vs. inferior PV) | 27 (28.7) | 69 (70.4) | <0.0001* | 2.77 | 1.13–6.99 | 0.0276* |
| Left-sided PV (vs. right-sided PV) | 45 (47.9) | 51 (52.0) | 0.6651 | |||
| Longitudinal diameter of PV ostium (mm) | 17.6±3.8 | 19.4±3.4 | 0.0004* | 1.01 | 0.90–1.14 | 0.8241 |
| Procedural data | ||||||
| CF-sensing catheter in the 1 st session | 28 (29.8) | 24 (24.5) | 0.4219 | |||
| CF-sensing catheter in the 2nd session | 58 (61.7) | 50 (51.0) | 0.1478 | |||
| Carina line in the 1 st or 2nd session | 12 (12.8) | 14 (14.3) | 0.8344 | |||
| Spontaneous PV activity in 1 st or 2nd session | 4 (4.3) | 42 (42.9) | <0.0001* | 3.96 | 1.29–14.9 | 0.0240* |
| Dormant conduction in the 2nd session | 0/45 (0) | 4/69 (5.8) | 0.1519 | |||
| Demonstrable exit block in the 1 st session | 44 (46.8) | 85 (86.7) | <0.0001* | 3.65 | 1.53–9.13 | 0.0042* |
| PV reconnection | 15 (16.0) | 59 (60.2) | <0.0001* | 5.75 | 2.55–13.7 | <0.0001* |
| Interval between 1 st and 2nd sessions (days) | 600.9±498.0 | 535.2±420.8 | 0.5500 | |||
Data given as n (%) or mean±SD. *P<0.05. Abbreviations as in Table 2.
Figure 3.
Detailed clinical course of the 49 patients who underwent second ablation. One patient (4 pulmonary veins [PV]) was not checked for exit block at the second session.
Subgroup analysis was performed in order to delineate the characteristics of PV likely to lose excitability (Table 4). Of the 129 PV with demonstrable exit block in the first session, exit block at the end of the second session could not be confirmed in 44 PV (34.1%). On univariate analysis, loss of excitability of the PV sleeve between the first and second sessions was associated with SPV, spontaneous automaticity in the PV, and PV reconnection. The interval between the first and second sessions was not associated with this phenomenon. On multivariate analysis, spontaneous activity in the PV (OR, 0.21; 95% CI: 0.04–0.72, P=0.0223) and PV reconnection (OR, 0.13; 95% CI: 0.04–0.36, P=0.002) were independent predictors of loss of excitability of the PV sleeve between the first and second sessions.
Table 4.
Predictors of PV Sleeve Loss of Excitability Between the First and Second Sessions
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| PV sleeve loss of excitability | P-value | OR | 95% CI | P-value | ||
| (−) n=85 PV | (+) n=44 PV | |||||
| Anatomical data | ||||||
| PV location (LSPV/LIPV/RSPV/RIPV) | 35/12/30/8 | 11/11/11/11 | ||||
| Superior PV (vs. inferior PV) | 65 (76.5) | 22 (50.0) | 0.0031* | 0.46 | 0.18–1.16 | 0.1035 |
| Left-sided PV (vs. right-sided PV) | 47 (55.3) | 22 (50.0) | 0.5822 | |||
| PV longitudinal diameter (mm) | 19.6±3.4 | 19.0±3.6 | 0.2160 | |||
| Procedural data | ||||||
| CF-sensing catheter in the 1 st session | 20 (23.5) | 14 (31.8) | 0.3993 | |||
| CF-sensing catheter in the 2nd session | 42 (49.4) | 28 (63.6) | 0.1394 | |||
| Carina line in the 1 st or 2nd session | 14 (16.5) | 6 (13.6) | 0.8001 | |||
| Spontaneous PV activity in 1 st or 2nd session | 41 (48.2) | 3 (6.8) | <0.0001* | 0.21 | 0.04–0.72 | 0.0223* |
| Dormant conduction in the 2nd session | 4 (6.4) | 0 (0) | 0.5678 | |||
| PV reconnection | 53 (62.4) | 5 (11.4) | <0.0001* | 0.13 | 0.04–0.36 | 0.0002* |
| Interval between 1 st and 2nd sessions (days) | 535.6±417.6 | 585.1±510.9 | 0.9485 | |||
Data given as n (%) or mean±SD. *P<0.05. Abbreviations as in Table 2.
Predictors of Long-Term PV Reconnection
Forty-nine patients underwent re-do ablation after a mean interval of 563.4±456.3 days from the first session. PV reconnection was observed in 83.7% of the patients (41/49) and in 37.8% of all PV (74/196), that is, 1.51±1.06 PV per patient (Table 5). The proportion of PV reconnections was 49.0% in the LSPV, 18.4% in the LIPV, 44.9% in the RSPV, and 38.8% in the RIPV. PV reconnection was more frequently observed in the SPV than in the IPV (46.9% vs. 28.6%, P=0.0120). On univariate analysis, SPV, longitudinal diameter of the PV, use of a CF-sensing catheter, spontaneous activity in the PV, and exit block in the first session were associated with PV reconnection in the second session. On multivariate analysis, spontaneous activity in the PV (OR, 2.74; 95% CI: 1.12–7.03, P=0.0272) and use of a CF-sensing catheter (OR, 0.42; 95% CI: 0.20–0.85, P=0.0151) were independent predictors of PV reconnection in the second session, although exit block in the first session was not identified as a significant predictor (OR, 1.58; 95% CI: 0.74–3.46, P=0.2377).
Table 5.
Predictors of Long-Term PV Reconnection
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| PV reconnection | P-value | OR | 95% CI | P-value | ||
| (−) n=122 PV | (+) n=74 PV | |||||
| Anatomical data | ||||||
| PV location (LSPV/LIPV/RSPV/RIPV) | 25/40/27/30 | 24/9/22/19 | ||||
| Superior PV (vs. inferior PV) | 52 (42.6) | 46 (62.2) | 0.0120* | 1.45 | 0.66–3.20 | 0.3537 |
| Left-sided PV (vs. right-sided PV) | 65 (53.3) | 33 (44.6) | 0.3023 | |||
| PV longitudinal diameter (mm) | 18.1±3.7 | 19.1±3.5 | 0.0387* | 1.002 | 0.91–1.11 | 0.9676 |
| Procedural data | ||||||
| CF-sensing catheter in the 1 st session | 42 (34.4) | 14 (18.9) | 0.0226* | 0.42 | 0.20–0.85 | 0.0151* |
| Carina line in the 1 st session | 11 (9.0) | 11 (14.9) | 0.2457 | |||
| Spontaneous PV activity in the 1 st session | 10 (8.2) | 18 (24.3) | 0.0028* | 2.74 | 1.12–7.03 | 0.0272* |
| Dormant conduction in the 1 st session | 9/83 (10.8) | 8/43 (18.6) | 0.2741 | |||
| Demonstrable exit block in the 1 st session | 75 (61.5) | 58 (78.4) | 0.0177* | 1.58 | 0.74–3.46 | 0.2377 |
| Interval between 1 st and 2nd sessions (days) | 542.8±448.7 | 597.4±469.6 | 0.3443 | |||
Data given as n (%) or mean±SD. *P<0.05. Abbreviations as in Table 2.
Discussion
In the present study, exit block (i.e., local capture of the PV sleeve musculature without conduction to the LA) after PVI was confirmed in 73.1% of all PV in the first session, and in 51.0% of all PV in the second session. The predictors of demonstrable exit block were SPV, longitudinal diameter of the PV, and spontaneous activity in the PV in the first session; and SPV, spontaneous activity in the PV, demonstrable exit block in a prior session, and PV reconnection in the second session. Non-use of a CF-sensing catheter and spontaneous activity in the PV were predictors of long-term PV reconnection. In contrast, inability to demonstrate exit block was not associated with increased risk of future PV reconnection.
Demonstrable Exit Block and Predictors of Exit Block
Squara et al reported that sleeve capture during PV pacing could be demonstrated in only 60.9% (81% in first-time procedures and 40% in redo) of the isolated PV, while it was uniformly demonstrated before ablation in all non-isolated PV.7 Gerstenfeld et al reported that exit block was present in only 58% of PV that were ablated in SR and in only 48% of PV that were ablated in AF.8 They concluded that a substantial proportion of PV lost their excitability during the process of PVI, and the present results are consistent with these previous reports. The percentage of the exit block was also reported to be affected by pacing output and pacing rate,9,10 although we performed PV pacing at a fixed output of 5.0 mV/0.2 ms and a pacing rate between 80 and 100 beats/min. In a study by Vijayaraman et al, capture of the PV sleeve without conduction to the LA could be demonstrated in 91% of all veins on high output pacing up to 20 mA at 2 ms.9 Although the percentage of demonstrable exit block might be augmented by high output pacing, the present results suggest that inability to demonstrate exit block is not associated with increased risk of future PV reconnection.
Although it is not clear why some PV sleeves lose their excitability during the process of PVI, a few electrophysiological mechanisms have been proposed. First, ablation of the PV antrum could modify the ganglionated plexi (GP) or axons extending from the GP to the PV myocardium,11 leading to a decrease in the excitability of the PV sleeve. Second, the electrophysiological property of the myocardium around the ablation site could be negatively altered (e.g., source-sink mismatch), and pacing threshold could be elevated.12–15 The present predictors of demonstrable exit block (i.e., SPV, longitudinal diameter of the PV, and spontaneous activity in the PV) were surrogates for a greater amount of myocardium or higher intensity of the connection of myocardial fibers at the PV sleeve. Approximately one-third of the PV with demonstrable exit block in the first session did not have demonstrable exit block at the end of the second session, and this loss of excitability of the PV sleeve between the first and second sessions was frequently observed in the PV without spontaneous activity and reconnection. Although a lower percentage of demonstrable exit block in the second session has already been reported,7 the present study has shown for the first time the relationship between long-term electrical isolation and the increased rate of non-demonstrable exit block in the same patients undergoing the first and second ablation procedures. A PV with a smaller amount of viable myocardium or fibers is likely to lose its excitability,7 and this process might be facilitated by regression of the PV sleeve musculature caused by long-term electrical isolation. However, further investigation is needed to clarify the mechanism of the loss of demonstrable exit block.
Demonstrable Exit Block and PV Reconnection
The rate of PV reconnection was 37.8% in the present study, which is similar to that in a previous report.16 Kim et al reported that PV reconnection was observed in 40.9% of PV (234/572) in a second ablation that was performed 23.7±19.9 months after the first procedure.16 PV are a major source of atrial triggers,3 and recovered PV conduction is a major cause of AF recurreence.1,4 Therefore, the presence of exit block is theoretically presumed to have a favorable effect on long-term outcome after PVI, and this issue has been discussed in several reports. During a 30-min observation, Chen et al showed that bidirectional block of the PV–LA junction could reduce acute PV reconnection.17 In contrast, Squara et al reported that PV with local sleeve capture after PVI were much more likely than non-captured PV to have reconnection using adenosine.7 In the present study, on univariate analysis non-demonstrable exit block was associated with long-term PV isolation, but this association was not seen on multivariate analysis (Table 5). It is difficult to reconcile earlier studies7,17 with the present study, but a possible explanation is that non-demonstrable exit block is due to the smaller mass of the PV sleeve musculature, where RF energy can easily ablate electrical conduction. In contrast with demonstrable exit block, the use of a CF-sensing catheter was a predictor of long-term PV isolation in the present study as in previous studies.18–21 Spontaneous PV activity was also an independent predictor of PV reconnection, possibly being attributable to a larger amount of PV sleeve musculature.
Although non-demonstrable exit block was not associated with increased risk of future PV reconnection, we cannot conclude as to whether assessment of exit block is an unnecessary maneuver or not. Duytschaever et al argued that entry block (i.e., complete elimination of PV potentials obtained on circular mapping catheter) was a sufficient endpoint for PVI because residual PV-LA exit conduction was rare (only 0.6% of PV) after proven LA-PV entry block.22 However, Kim et al reported that 9% of patients had exit conduction even after achievement of the entrance block,16 and that pacing in the PV may unmask a residual unidirectional conduction. This is one of the possible benefits of pacing in the PV, although it is rare in clinical practice.22–24 Although demonstration of exit block may be helpful for confirming existing unidirectional conduction from the PV to the LA at least in some cases, the relationship between demonstrable exit block and PV reconnection or recurrence of AF needs to be investigated further in a large prospective study.
Study Limitations
There are several limitations in this study. First, this study was a single-center retrospective study and the number of patients was relatively small. Second, assessment of the exit block by pacing in the PV was not performed before PVI. Squara et al, however, reported that all PV without entrance block demonstrated sleeve capture and that none of the PV had exit block prior to ablation,7 indicating that loss of excitability of the PV sleeve was exclusively caused by PVI. Third, we performed PV pacing using fixed output and cycle length. Thus, we cannot exclude the possibility that results might have been slightly different if a different protocol of PV pacing had been used. Fourth, we might have underestimated the percentage of demonstrable exit block, because we performed pacing only in the PV, not from the PV antrum. There is a possibility that pacing at the PV antrum close to the ablation line (e.g., using an ablation catheter), could have identified more exit blocks or even unidirectional exit conduction in PV without demonstrable exit block. Fifth, the presence or absence of spontaneous PV activity was based on sporadic monitoring with a circular catheter during the CA procedure. The duration of monitoring for spontaneous activity was different in each of the PV. Sixth, patients without AF recurrence were not included in the analysis of the relationship between exit block and PV reconnection, leading to possible bias. Sixth, the present RF ablation findings may not be applicable to cryoballoon (CB) ablation. Aryana et al reported that patients who received AF ablation using CB were less likely to have PV reconnection at the redo procedure than patients who received RF ablation with an open-irrigated and non-force-sensing catheter,25 suggesting that the importance of assessment of exit block after PVI differs between RF and CB ablations.
Conclusions
Exit block after PVI by RF ablation was not observed in 26.9% of the PV in the first session, and this percentage increased to 49.0% by the next session, after a mean interval of 563.4±456.3 days. Inability to demonstrate exit block, however, was not associated with increased risk of future PV reconnection.
Disclosures
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare no conflicts of interest.
IRB Information
This study was approved by the institutional ethics committee of Sapporo Medical University (reference number 312-73).
References
- 1. Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, et al.. Long-term outcomes of catheter ablation of atrial fibrillation: A systematic review and meta-analysis. J Am Heart Assoc 2013; 2: e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prystowsky EN, Padanilam BJ, Fogel RI.. Treatment of atrial fibrillation. JAMA 2015; 314: 278–288. [DOI] [PubMed] [Google Scholar]
- 3. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al.. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339: 659–666. [DOI] [PubMed] [Google Scholar]
- 4. Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FT, et al.. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: Lessons from double Lasso technique. Circulation 2005; 111: 127–135. [DOI] [PubMed] [Google Scholar]
- 5. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al.. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017; 14: e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ip JE, Markowitz SM, Cheung JW, Liu CF, Thomas G, Lessner SJ, et al.. Method for differentiating left superior pulmonary vein exit conduction from pseudo-exit conduction. Pacing Clin Electrophysiol 2013; 36: 299–308. [DOI] [PubMed] [Google Scholar]
- 7. Squara F, Liuba I, Chik W, Santangeli P, Zado ES, Callans DJ, et al.. Loss of local capture of the pulmonary vein myocardium after antral isolation: Prevalence and clinical significance. J Cardiovasc Electrophysiol 2015; 26: 242–250. [DOI] [PubMed] [Google Scholar]
- 8. Gerstenfeld EP, Dixit S, Callans D, Rho R, Rajawat Y, Zado E, et al.. Utility of exit block for identifying electrical isolation of the pulmonary veins. J Cardiovasc Electrophysiol 2002; 13: 971–979. [DOI] [PubMed] [Google Scholar]
- 9. Vijayaraman P, Dandamudi G, Naperkowski A, Oren J, Storm R, Ellenbogen KA.. Assessment of exit block following pulmonary vein isolation: Far-field capture masquerading as entrance without exit block. Heart Rhythm 2012; 9: 1653–1659. [DOI] [PubMed] [Google Scholar]
- 10. Yagishita A, Gimbel JR, Arruda M.. Rate-dependent exit conduction block from pulmonary vein to left atrium after entrance block: New implications of pacing rate to confirm bidirectional conduction block. Circ Arrhythm Electrophysiol 2016; 9: e003871. [DOI] [PubMed] [Google Scholar]
- 11. Nakagawa H, Scherlag BJ, Patterson E, Ikeda A, Lockwood D, Jackman WM.. Pathophysiologic basis of autonomic ganglionated plexus ablation in patients with atrial fibrillation. Heart Rhythm 2009; 6: S26–S34. [DOI] [PubMed] [Google Scholar]
- 12. Joyner RW, Wilders R, Wagner MB.. Propagation of pacemaker activity. Med Biol Eng Comput 2007; 45: 177–187. [DOI] [PubMed] [Google Scholar]
- 13. Andrade JG, Nattel S, Macle L.. Pulmonary vein exit block despite recovery of entry conduction during redo-ablation for atrial fibrillation. Europace 2015; 17: 752. [DOI] [PubMed] [Google Scholar]
- 14. Ge YZ, Shao PZ, Goldberger J, Kadish A.. Cellular electrophysiological changes induced in vitro by radiofrequency current: Comparison with electrical ablation. Pacing Clin Electrophysiol 1995; 18: 323–333. [DOI] [PubMed] [Google Scholar]
- 15. Spector P.. Principles of cardiac electric propagation and their implications for re-entrant arrhythmias. Circ Arrhythm Electrophysiol 2013; 6: 655–661. [DOI] [PubMed] [Google Scholar]
- 16. Kim JY, Kim SH, Song IG, Kim YR, Kim TS, Kim JH, et al.. Achievement of successful pulmonary vein isolation: Methods of adenosine testing and incremental benefit of exit block. J Interv Card Electrophysiol 2016; 46: 315–324. [DOI] [PubMed] [Google Scholar]
- 17. Chen S, Meng W, Sheng He D, Chen G, Zhang F, Yan Y, et al.. Blocking the pulmonary vein to left atrium conduction in addition to the entrance block enhances clinical efficacy in atrial fibrillation ablation. Pacing Clin Electrophysiol 2012; 35: 524–531. [DOI] [PubMed] [Google Scholar]
- 18. Neuzil P, Reddy VY, Kautzner J, Petru J, Wichterle D, Shah D, et al.. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: Results from the EFFICAS I study. Circ Arrhythm Electrophysiol 2013; 6: 327–333. [DOI] [PubMed] [Google Scholar]
- 19. Park CI, Lehrmann H, Keyl C, Weber R, Schiebeling J, Allgeier J, et al.. Mechanisms of pulmonary vein reconnection after radiofrequency ablation of atrial fibrillation: The deterministic role of contact force and interlesion distance. J Cardiovasc Electrophysiol 2014; 25: 701–708. [DOI] [PubMed] [Google Scholar]
- 20. Nakamura K, Naito S, Sasaki T, Minami K, Take Y, Shimizu S, et al.. Predictors of chronic pulmonary vein reconnections after contact force-guided ablation: Importance of completing electrical isolation with circumferential lines and creating sufficient ablation lesion densities. J Interv Card Electrophysiol 2016; 47: 321–331. [DOI] [PubMed] [Google Scholar]
- 21. El Haddad M, Taghji P, Phlips T, Wolf M, Demolder A, Choudhury R, et al.. Determinants of acute and late pulmonary vein reconnection in contact force-guided pulmonary vein isolation: Identifying the weakest link in the ablation chain. Circ Arrhythm Electrophysiol 2017; 10: e004867. [DOI] [PubMed] [Google Scholar]
- 22. Duytschaever M, De Meyer G, Acena M, El-Haddad M, De Greef Y, Van Heuverswyn F, et al.. Lessons from dissociated pulmonary vein potentials: Entry block implies exit block. Europace 2013; 15: 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim J, Desai S, Jadonath R, Beldner SJ.. The importance of bidirectional block during pulmonary vein isolation. Pacing Clin Electrophysiol 2013; 36: e143–e145. [DOI] [PubMed] [Google Scholar]
- 24. Nentwich K, Duytschaever M, Deneke T.. Pulmonary vein isolation: Does bidirectional conduction block matter? Herzschrittmacherther Elektrophysiol 2014; 25: 121–122. [DOI] [PubMed] [Google Scholar]
- 25. Aryana A, Singh SM, Mugnai G, de Asmundis C, Kowalski M, Pujara DK, et al.. Pulmonary vein reconnection following catheter ablation of atrial fibrillation using the second-generation cryoballoon versus open-irrigated radiofrequency: Results of a multicenter analysis. J Interv Card Electrophysiol 2016; 47: 341–348. [DOI] [PubMed] [Google Scholar]