Abstract
Lifestyle factors may affect mental health and play a critical role in the development of neurodegenerative diseases including Alzheimer’s disease (AD). However, whether the temperatures of daily beverages have any impact on cognitive function and AD development has never been studied. In this study, we investigated the effects of daily drinking water temperatures on cognitive function and AD development and progression in mice and the underlying mechanisms. Cognitive function of mice was assessed using passive avoidance test, open field test, and Morris water maze. Wild-type Kunming mice receiving intragastric water (IW, 10 mL/kg, 2 times/day) at 0 °C for consecutive 15 days displayed significant cognitive defects accompanied by significant decrease in gain of body weight, gastric emptying rate, pepsin activity, and an increase in the energy charge in the cortex when compared with mice receiving the same amount of IW at 25 °C (a temperature mimicking most common drinking habits in human), suggesting the altered neuroenergetics may cause cognitive decline. Similarly, in the transgenic APPwse/PS1De9 familial AD mice and their age- and gender-matched wild-type C57BL/6 mice, receiving IW at 0 °C, but not at 25 °C, for 35 days caused a significant time-dependent decrease in body weight and cognitive function, accompanied by a decreased expression of PI3K, Akt, the glutamate/GABA ratio, as well as neuropathy with significant amyloid lesion in the cortex and hippocampus. All of these changes were significantly aggravated in the APPwse/PS1De9 mice than in the control C57BL/6 mice. These data demonstrate that daily beverage at 0 °C may alter brain insulin-mediated neuroenergetics, glutamate/GABA ratio, cause cognitive decline and neuropathy, and promote AD progression.
Keywords: Alzheimer’s disease, cognition, drinking water, temperature, glutamate, GABA, insulin signaling, APPwse/PS1De9 familial AD mice
Introduction
It is widely recognized that lifestyle factors may affect brain health and play a critical role in the development of neurodegenerative diseases such as dementia and Alzheimer’s disease (AD) [1, 2]. Water/beverage consumption is one of the most important lifestyle factors and plays a critical role in metabolism and brain health [3]. There is a wide range of individual differences in the amount of daily water intake as well as individual preferences for drink temperature. For example, most people in China prefer warm or hot drinks such as hot tea or boiled water in all seasons, but many people in the United States prefer cold or iced drinks even in the winter. Interestingly, the incidence of AD in China is less than 1/3 of that in the United States [4]; however, whether beverage temperature has impacts on cognitive function and AD development and thus causes the difference in the incidence of AD has never been investigated.
In this study, therefore, we first examined the effects of drinking water at temperatures that correspond to the most common habits of humans, specifically, ice water (0 °C), cold water (4 °C), and normal water (25 °C), on a daily basis on cognitive function in normal wild-type Kunming mice; we found that the intragastric admission of 0 °C water, but not 25 °C water, induced significant cognitive decline. We then investigated the effects of drinking 0 °C water and 25 °C water on a daily basis on cognitive function and neuropathy in transgenic APPswe/PS1De9 familial AD mice and age- and gender-matched wild-type C57BL/6 mice. We further studied the possible underlying mechanisms and their impacts on the development and progression of AD.
Materials and methods
Animals and experimental protocols
To examine the effects of beverage temperature on cognitive performance, normal drinking water (25 °C), cold water(4 °C), and ice water (0 °C) were used to mimic the most common drinking habits of humans. After an adaptive intragastric feeding period of 7 days, wild-type Kunming mice (male, 6 weeks old, 28 ± 2 g, Institute of Laboratory Animals of Sichuan Academy of Medical Sciences, Chengdu, China) were divided randomly into two groups and were intragastrically administered 25 °C, 4 °C, and 0 °C water(10 mL/kg, 2 times/day) for the following 15 consecutive days.
To examine the effects of beverage temperature on AD development and progression, after an adaptive intragastric feeding period of 7 days, the familial AD transgenic APPswe/PS1De9 (APP/PS1) (A) mice and age- and gender-matched C57BL/6 wild-type (W) mice (male, 3 months old, 28 ± 2 g, Beijing Huafukang Bioscience Co. Inc., Beijing, China) in the control groups (the A-25 °C mice and W-25 °C mice) were intragastrically administered normal temperature water (25 °C) (10 mL/kg, 2 times/day) for the following consecutive 35 days, while the mice in the test groups (the A-0 °C mice and W-0 °C mice) received the same amount (10 mL/kg, 2 times/day) of 0 °C ice water for the following consecutive 35 days.
All animals were provided free and unlimited access to normal chow and water, housed under suitable temperature (22 ± 2 °C) and humidity (65% ± 5%) conditions in an animal observation room, and treated according to ethical practices adhering to international regulations (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85–23, 1985, revised 1996) and animal protocols approved by the ethical committee of the Institute of Material Medica Integratio and Transformation for Brain Disorders.
Behavioral test
The passive avoidance test was performed with a PAT-8 video analysis system (TechMan Soft, Sichuan, China) and an apparatus consisting of eight lit rooms and paired dark rooms of equal size (15.5 cm × 14 cm × 21 cm). The test was performed daily for 2 consecutive days. Before the test, the mice were habituated to the experimental environment for 30 min and allowed to familiarize themselves with the apparatus for 5 min; the mice were individually placed in a lit room and allowed free access to the corresponding dark room when a trap door was opened. Subsequently, the mice were individually placed in a lit room, and the trap door was closed. The video analysis system began recording when the door was opened, and the escape latency (ET) and error frequency of the mice were determined over 5 min.
The OFT-100 open field experiment system consisting of a cuboid box (62.5 × 74 × 51 cm3) was used to perform the open field test. The bottom of the box was mapped by the system. The mice were habituated to the experimental environment for 30 min and were allowed to familiarize themselves with the apparatus for 5 min before the test. Afterwards, the mouse was placed in the central location, and the video analysis system started to record movement in 5 min intervals. The ratio of time spent inactive and moving, the total distance traveled, the distance traveled in the center zone, and velocity were calculated.
A Morris water maze (MWM) system (Noldus, Netherlands) consisting of a swimming pool (120 cm in diameter, 50 cm in height) and a platform (6.5 cm in diameter and 15 cm in height) was used to assess spatial learning and memory in mice before and after the treatment. The pool was filled with water (23 ± 2 °C) to a depth of 16 cm and divided into four quadrants: the southeast, northeast, southwest, and northwest quadrants. The escape platform was fixed in a permanent position 1 cm under the water surface in the southeast quadrant throughout the course of the MWM test. In the hidden platform experiment, a mouse was placed in the water in the northwest quadrant facing the pool wall and allowed to swim until it found the hidden platform within 60 s. If the mouse could not find the platform within 60 s, it was gently guided to the platform and kept there 10 s, and the ET was recorded as 60 s. Training trials were performed twice a day for 5 days. The spatial probe trial was performed on the next day at the end of the hidden platform experiment. The platform was removed, and a mouse was placed in water in the northwest and allowed to swim freely for 120 s. The ET in the hidden platform experiment on 5 successive days and the percentage of time spent in the quadrant containing the platform, the number of times the target quadrant was crossed, the percentage of distance traveled in the target quadrant, the number of platform crossings, the time spent in the quadrant containing the platform, swimming speed, and trajectory were recorded in the spatial probe trial.
Gastric emptying rate (GER)
All Kunming mice were fasted but allowed access to drinking water on day 14, and 24 h later, they were lavaged with 0.2 mL 0.1% methyl orange solution for 90 min. Twenty minutes later, the mice were sacrificed by cervical dislocation, and a suspension of the gastric contents was collected in 10 mL distilled water. The suspension (pH 6–6.5) was centrifuged at 2000 r/min for 10 min. The absorbance of the supernatant (A1) and benchmark (A2) was detected with an ultraviolet spectrophotometer at 420 nm. The GER was calculated according to the following formula [5]: GER = (A1 − A2)/A2 × 100%.
Pepsin activity assay
Glass capillaries (φ 1 mm × 10 cm) were filled with freshly prepared egg white and inserted into hot water (85 °C) to solidify the egg white in the capillary. The supernatant of the prepared succus gastricus (1 mL) was placed in a cuvette with plugs, and then 15 mL of 0.05 mol/L hydrochloric acid was added to the cuvette and mixed. The plugs were tightened after two prepared capillary tubes were inserted and then the cuvette was incubated in a thermostatic bath for 24 h at 37 °C. The length of the transparent part of the capillary was measured, and the mean and pepsin activities (U/ml) were calculated according to the following formula [5]: pepsin activity = mean2 × 16.
Hematoxylin and eosin (HE) staining
Paraffin-embedded right hemispheres were cut into 5 μm-thick sections and then stained successively with HE (Thermo Fisher, USA). The pathomorphological changes in the hippocampi and cortices of the mice were examined.
Determination of the energy charge (EC)
The EC in the hippocampus and cortex was measured with HPLC as described previously [6]. In brief, the samples were analyzed by an ODS HYPERSIL C18 chromatographic column (5 μm, 250 mm × 4.6 mm; Thermo Fisher) with 0.05 mol/L phosphate buffer salt (A; pH = 6.5) and methanol (B) as the mobile phase. The column temperature was 25 °C, and the sample chamber temperature was 10 °C. The injection volume was 10 µL, and the flow rate was 1.0 mL/min. The UV detection wavelength was 254 nm. The EC was calculated according to the following formula:
Real-time PCR
Total RNA was extracted from the hippocampus and cortex with a Thermo Scientific GeneJET RNA Purification Kit (Thermo Fisher, USA), and reverse transcription was performed with a Thermo Scientific RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher, USA) according to the manufacturer’s manual. Real-time PCR was performed with SYBR Premix DimerEraserTM (Perfect Real Time) (TaKaRa, Japan) on a Real-time PCR Detection System (Bio-Rad, USA). Each sample was analyzed in triplicate with the following primers: PI3K, forward: 5′-ACC AGC AGG ATC AAG TTG TCA-3′, reverse: 5′-GAA GTA CGG GTG TACT CCTCAT-3′; Akt, forward: 5′-ACTT CTC AGT GGC ACA ATG CCA-3′, reverse: 5′-CAT GGA AGG TGC GCT CAA TGAC-3′; GAPDH, forward: 5′-AGG TCG GTG TGA ACG GAT TTG-3′, reverse: 5′-TGT AGA CCA TGT AGT TGA GGTCA-3′. The relative mRNA expression of PI3K and Akt relative to that of GAPDH was calculated by 2−ΔΔCt as described previously [7].
Western blot analysis
Harvested hippocampi and cortices were homogenized with RIPA lysis buffer, and the total protein concentrations were detected by a BCA assay kit (Multi Sciences, China). The target proteins were separated by electrophoresis on 8% SDS-PAGE gels and transferred to PVDF membranes. The membranes were blocked with 5% BSA at 25 °C for 90 min and then incubated overnight at 4 °C with the following primary antibodies: anti-GAPDH (1:2000, Servicebio, China), anti-phospho-PI3K, anti-PI3K, anti-Akt, and anti-phospho-Akt (1:1000, Cell Signaling Technology, USA). The membranes were incubated with HRP-conjugated goat anti-rabbit IgG secondary antibodies (1:1000, Multi Sciences, China) at 25 °C for 90 min after the membranes were washed 3 times with TBST. Then, the blots were visualized by enhanced chemiluminescence with an Ultra ECL Kit (Multi Sciences, China). The grayscale value of each protein was assessed using Quantity One software (Bio-Rad, USA).
Immunohistochemistry
Immunohistochemistry of the right hemispheres was performed as previously described [8]. Citrate buffer (pH = 6.0) was used for antigen retrieval, and the sections were incubated with primary antibody (PI3K, 1:40; Akt, 1:200; Aβ, 1:300, Cell Signaling Technology, USA; NeuN, 1:200, Abcam, UK) overnight at 4 °C and then incubated with secondary antibody for 30 min at 37 °C. The sections were stained with hematoxylin after color development with a DAB kit. Images of Akt and PI3K staining at three identical locations (the hippocampus, the cortex, and between the cortex and hippocampus) were collected for each sample, and the average optical density of each sample was then calculated based on the average optical density of each image determined by Image-Pro Plus 6.0 (Media Cybernetics, USA). Images of Aβ staining were obtained, and senile plaques were counted to assess the changes in Aβ after the mice were administered ice water.
Enzyme-linked immunosorbent assay (ELISA)
Appropriate cortical tissues were homogenized with normal saline (1 mg:9 µL) containing cOmplete tablets (Roche, Basel, Switzerland) on ice for 10 min and then centrifuged at 3500 r/min for 10 min at 25 °C. The supernatant was then separated and used to determine the concentrations of insulin (Excell Bio, China), glutamate and γ-aminobutyric acid (GABA) (Nanjing Jiancheng, China) in the brain tissue with kits.
Statistical analysis
All data are shown as the mean ± SEM. The sample sizes (n) of both C57BL/6 and APP/PS1 mice required for the passive avoidance test were estimated by power analysis and calculated according to the following equation and based on previously published data: n = 2(μα + μβ)2σ2/δ2, where α = level of significance (type I error rate), β = probability of failing to detect an existing difference (type II error rate (1-β is referred to as the power of the study)), μα = mean value at α, μβ = mean value at β, σ = population standard deviation, δ = margin of error on estimate [9, 10]. The mean ET in the MWM was analyzed by two-way ANOVA followed by the least significant difference test, and the number of days acted as the repeated-measure factor between sessions [11]. All of the other data were analyzed by the Anderson–Darling test. Samples with a normal distribution were analyzed by one-way ANOVA. If the sample variance was not homogeneous, as determined by Fisher’s least significant difference test, the Kruskal–Wallis test was used. Nonparametric tests were used for data that were not normally distributed. Student’s t test was performed for comparisons between two groups. Differences were considered statistically significant when P < 0.05. All statistical tests were performed using SPSS software package 16.0 for Windows.
Results
Effects of the temperature of intragastrically administered water on the cognitive performance of KM mice
To examine the effects of beverage temperature on cognitive performance, we designed experiments to administer animals with intragastric water (IW) at three different temperatures that mimic the most common drinking habits of humans, namely, normal water (25 °C), cold water (4 °C), and ice water (0 °C). In addition to being given free access to normal chow and regular drinking water, the mice in each group were administered IW (10 mL/kg, 2 times/day) at a temperature of 25, 4, or 0 °C daily. ET and body weight were monitored for 15 days. The GER, gastric pepsin activity, and the EC in the cortex were measured at the end of the protocols on day 15.
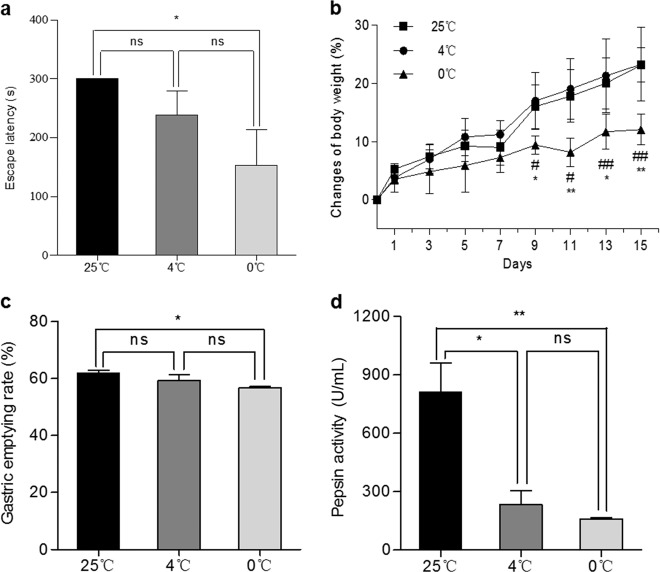
Figure 1a shows the ET of the mice after daily treatment with IW at a temperature of 25, 4, or 0 °C for 15 days. The ET of the mice in the 0 °C IW group was shorter than that of the 4 °C IW group (P > 0.05 vs. 0 °C, n = 6) and the 25 °C IW group(P < 0.05 vs. 0 °C, n = 6). While daily treatment with 4 °C IW caused a slight shortening of the ET, no statistical significance was observed (P > 0.05 vs. 25 °C, n = 6). These results indicated that compared with daily treatment with 25 °C IW, daily treatment with 0 °C IW caused a significant decline in cognitive function.
Fig. 1. Effects of drinking water temperature on the learning ability, memory, body weight, and gastric function of Kunming mice.
Equal amounts of water (10 mL/kg) at temperatures corresponding to the most common habits of humans, namely, normal temperature (25 °C) and ice water (0 °C) were given to the mice intragastrically 2 times/day for 15 consecutive days. a The escape latency in the passive avoidance test was calculated. Mean ± SEM; *P < 0.05, n = 6. b The body weight was measured and calculated every 2 days. Mean ± SEM; n = 6, *P < 0.05 and **P < 0.01 vs. 25 °C; #P < 0.05 and ##P < 0.01 vs. 4 °C. The gastric emptying rate (c n = 3) and pepsin activity (d n = 3) were measured and calculated. Mean ± SEM; *P < 0.05 and **P < 0.01.
As shown in Fig. 1b, compared with the mice treated with 25 °C IW and 4 °C IW, the mice treated with 0 °C IW had a great reduction in body weight gain from day 3 and significantly reduced body weight gain from day 9, suggesting that 0 °C IW may cause gastrointestinal and energy metabolism dysfunction. We therefore examined the GER and pepsin activity of the mice treated with IW at different temperatures. As shown in Fig. 1c, the GER of the mice treated with 0 °C IW was significantly lower than that of mice treated with warmer water (25 °C IW). Furthermore, 0 °C IW also significantly reduced pepsin activity (Fig. 1d). These results indicated that ice water can directly compromise gastrointestinal function, reducing the GER and pepsin activity.
Therefore, we further investigated whether 0 °C IW affects the EC in the brain. While no significant differences in the concentration of ATP (131.11 ± 9.88 nmol/L vs. 135.83 ± 22.67 nmol/L, n = 6, P = 0.6282), ADP (2.16 ± 0.72 nmol/L vs. 2.43 ± 0.78 nmol/L, n = 6, P = 0.2571), or AMP (3.77 ± 0.30 mol/L vs. 3.55 ± 0.66 mol/L, n = 6, P = 0.9230) in the cortex were observed between the 0 °C IW and 25 °C IW groups, the EC of mice in the 0 °C IW group was significantly higher than that in the 25 °C IW group (0.034 ± 0.003 vs. 0.041 ± 0.006, n = 6, P = 0.0443). In addition, we noticed that the ratio of ADP/ATP (0.014 ± 0.002 vs. 0.019 ± 0.004, n = 6, P = 0.0370) in the mice treated with 0 °C IW was significantly increased, while the ratio of AMP/ATP (27.59 ± 1.21 vs. 26.17 ± 3.18, n = 6, P = 0.4956) was not significantly different. These results suggested that 0 °C IW caused a significant increase in the EC and altered neuroenergetics, which may cause a decline in cognitive function [12–14].
Effects of daily administration of 0 °C IW on the development and progression of AD
Based on the experimental evidence that the daily administration of 0 °C IW significantly alters energy metabolism and causes a disturbance of neuroenergetics and a decline in cognitive function in KM mice, we hypothesized that beverage temperature may play a crucial role in the development and progression of AD. To test this hypothesis, we further investigated the effects of the daily administration of 0 °C IW and 25 °C IW on the development of cognitive decline in AD by using familial AD transgenic APPswe/PS1De9 (APP/PS1) mice and age- and gender-matched wild-type C57BL/6 mice.
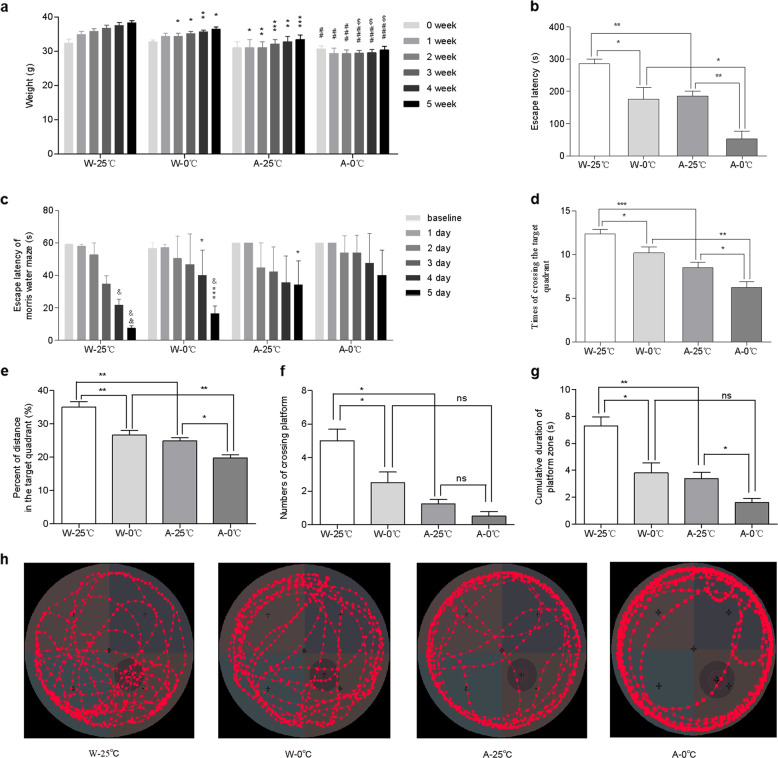
Similar to what was observed in the wild-type KM mice, the increase in the body weight of the wild-type C57BL/6 mice treated with 10 mL/kg 0 °C IW 2 times/day (W-0 °C) for 35 consecutive days was lower than that of the wild-type control mice treated with the same amount of 25 °C IW (W-25 °C), as shown in Fig. 2a. The ET of the W-0 °C mice was significantly shorter than that of the W-25 °C mice (Fig. 2b). These data are similar to what was observed in the KM mice and further confirmed that 0 °C IW may retard not only body weight increase but also cognitive function. Similarly, the increase in the body weight of the APP/PS1 model mice treated with 0 °C IW (10 ml/kg, 2 times/day) (A-0 °C) for 35 consecutive days was lower than that of the APP/PS1 control mice treated with the same amount of 25 °C IW (A-25 °C). The body weight of the mice in the A-0 °C group was significantly lower than that of the mice in the W-0 °C group (Fig. 2a). The ET of the A-0 °C mice was significantly shorter than that of the A-25 °C mice, the W-25 °C mice and the W-0 °C mice (Fig. 2b), strongly suggesting that the 0 °C ice water may cause cognitive decline and promote the development and progression of AD in APP/PS1 mice.
Fig. 2. Effects of the daily administration of 0 °C IW on the cognitive function and body weight of APP/PS1 mice.
a The body weight of wild-type C57BL/6 (W, n = 5) and APP/PS1 mice (AD model, A, n = 4) was monitored before and after the administration of intragastric water (IW, 10 mL/kg, 2 times/day) at a temperature of 0 °C (the W-0 °C and A-0 °C groups) or 25 °C (the W-25 °C and A-25 °C groups) for 35 consecutive days. Mean ± SEM; *P < 0.05, **P < 0.01, and ***P < 0.001 vs. the W-25 °C group; #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. the W-0 °C group; $P < 0.05 vs. the A-25 °C group. b The cognitive performance of APP/PS1 mice in the passive avoidance test, as determined by the escape latency. Mean ± SEM; *P < 0.05 and **P < 0.01. c Escape latency in the Morris water maze test. Mean ± SEM; *P < 0.05 and ***P < 0.001 vs. the W-25 °C group; &P < 0.05 and &&P < 0.01 vs. the baseline of each group. d The number of target quadrant crossings in the spatial probe test. Mean ± SEM; *P < 0.05, **P < 0.01, and ***P < 0.001. e The percentage of distance traveled in the target quadrant in the spatial probe test. Mean ± SEM; *P < 0.05 and **P < 0.01. f The number of platform quadrant crossings in the spatial probe test. Mean ± SEM; *P < 0.05. g The time spent in the quadrant containing platform in the spatial probe test. Mean ± SEM; *P < 0.05 and **P < 0.01. h Representative traces of each group.
Therefore, the MWM test was used to further examine the effects of 0 °C IW on spatial learning and memory in APP/PS1 and wild-type mice. As shown in Fig. 2c, a time-dependent shortening of the ET was observed in all groups of mice. Compared with baseline, the ET of mice in the W-25 °C group was significantly shorter on Day 4 (P < 0.05, n = 5) and Day 5 (P < 0.01, n = 5), while the ET of mice in the W-0 °C group was significantly shorter on Day 5 (P < 0.05, n = 5), indicating a decline in spatial learning and memory in the W-0 °C mice. When compared with that of the W-25 °C mice, the ET of the W-0 °C mice was significantly prolonged on Day 4 (P < 0.05) and Day 5 (P < 0.001), further confirming that 0 °C IW caused a decline in the cognitive function of these wild-type mice. In the APP/PS1 mice, however, although there was no significant difference in the ET compared with baseline among the mice in the A-25 °C and A-0 °C groups after Day 5, the relative shortening of the ET of the APP/PS1 mice was 0.64 times that of the C57BL/6 mice, indicating that the spatial learning ability and memory of the APP/PS1 mice were already compromised. Moreover, as shown in Fig. 2h, the APP/PS1 mice were mainly active at the edge of the pool, while the wild-type mice were more active outside the edge of the pool, which indicated that the spatial memory of the APP/PS1 mice was impaired. Meanwhile, the intensity of activity of the W-0 °C group in the quadrant containing the platform was lower than that of the W-25 °C group, and the frequency of activity of the A-0 °C group in the quadrant that contained the platform was higher than that of the A-25 °C group. Furthermore, in the spatial probe test, although there was no significant difference in the percentage of time spent in the platform quadrant observed among these groups, the number target quadrant crossings made by the W-25 °C and A-25 °C mice was significantly higher than that made by the W-0 °C and A-0 °C mice, respectively (Fig. 2d). The relative decrease in the number of target quadrant crosses made by the APP/PS1 mice was 1.02 times that made by the C57BL/6 mice. Meanwhile, the percentage of distance traveled in the target quadrant, the number of platform crossings, and the time spent in the quadrant containing the platform by the W-0 °C group were lower than those of the W-25 °C group, and those of the A-25 °C group were lower than those of the W-25 °C group (Fig. 2e–g). The percentage of distance traveled in the target quadrant and the time spent in the quadrant containing the platform by the A-0 °C group was lower than those by the A-25 °C group (Fig. 2e, g), and the percentage of distance traveled in the target quadrant by the A-0 °C group was also lower than that by the A-25 °C group. In addition, no significant difference in swimming velocity was observed among the groups (18.75 ± 0.61 and 19.67 ± 1.03 cm/s for the W-25 °C and W-0 °C groups, respectively, n = 5, P > 0.05; 16.83 ± 1.63 and 15.84 ± 1.42 cm/s for the A-25 °C and A-0 °C groups, respectively, n = 4, P > 0.05), indicating that the observed changes in spatial learning were not caused by individual differences in the swimming ability of the mice. The performance of the APP/PS1 mice in the spatial probe test were consistent with previous reports [15, 16]. The results suggested that long-term daily consumption of drinking water at a temperature of 0 °C exacerbated cognitive decline in the APP/PS1 mice.
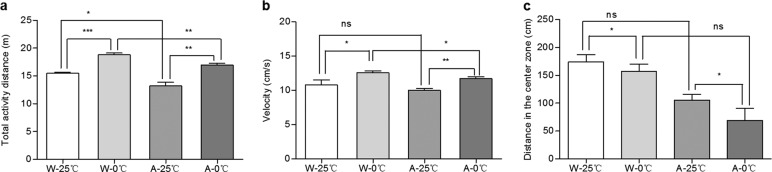
Figure 3 shows the neuropsychiatric changes in the mice observed in the open field test. The total distance traveled by the mice in the W-0 °C group was significantly longer than that traveled by the mice in the W-25 °C group (P < 0.001); similarly, the total distance traveled by the mice in the A-0 °C group was significantly longer than that traveled by the mice in the A-25 °C group (P < 0.01), indicating that 0 °C IW increased the total distance traveled (Fig. 3a). At the same time, compared with 25 °C IW, 0 °C IW also significantly increased the velocity of both wild-type and APP/PS1 mice (Fig. 3b). Moreover, the distance traveled in the center zone by the mice in W-0 °C and A-0 °C groups was markedly shorter than that traveled by the mice in W-25 °C and A-25 °C groups (Fig. 3c), indicating that 0 °C IW caused emotional changes and increased anxiety, as demonstrated by the increases in distance traveled and velocity and decreases in the distance traveled in the center zone. In addition, when compared to the wild-type control mice, the APP/PS1 mice treated with 25 °C IW or 0 °C IW traveled a significantly shorter distance. These results further indicated that 0 °C IW exacerbated neuropsychiatric changes and anxiety in the APP/PS1 AD mice.
Fig. 3. Effect of the daily administration of 0 °C IW on neuropsychiatric changes in APP/PS1 mice.
The open field test was used to observe the neuropsychiatric changes in wild-type C57BL/6 mice (W, n = 5) and APP/PS1 mice (AD model, A, n = 4) after they were administered intragastric water (IW, 10 mL/kg, 2 times/day) at a temperature of 0 °C (the W-0 °C and A-0 °C groups) or 25 °C (the W-25 °C and A-25 °C groups) for 35 consecutive days, and the total distance traveled (a), velocity (b), and distance traveled in the center zone (c) were recorded. Mean ± SEM; *P < 0.05, **P < 0.01, and ***P < 0.001.
Effects of the daily administration of 0 °C IW on the insulin signaling transduction pathway in brain tissues
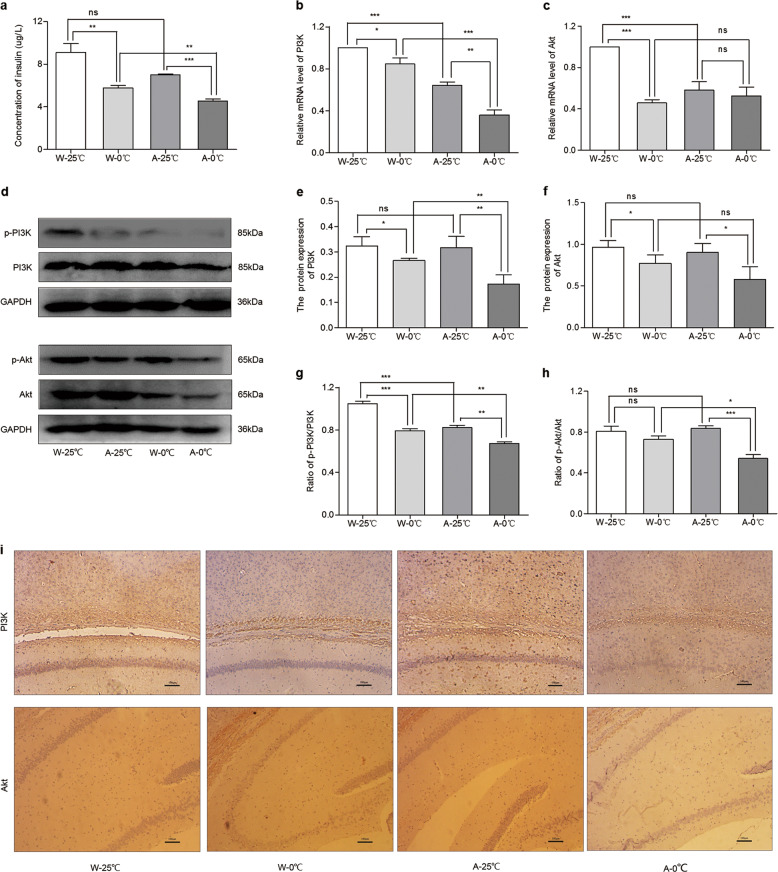
Since the daily administration of 0 °C IW caused a significant increase in the EC and since insulin plays a key role in the regulation of energy production and balance in the brain [17], we further examined whether insulin signaling in the brain is affected by ice water treatment. The concentration of insulin in the cortex of the mice was measured. As shown in Fig. 4a, the insulin concentration in the W-0 °C and A-0 °C mice was significantly lower than that in the W-25 °C (P < 0.01) and A-25 °C mice (P < 0.001), respectively. Therefore, the daily consumption of 0 °C drinking water may reduce the concentration of insulin in brain tissues.
Fig. 4. Effects of the daily administration of 0 °C IW on insulin signaling pathways.
After the wild-type C57BL/6 mice (W, n = 5) and APP/PS1 mice (AD model, A, n = 4) were administered intragastric water (IW, 10 mL/kg, 2 times/day) at either 0 °C (the W-0 °C and A-0 °C groups) or 25 °C (the W-25 °C and A-25 °C groups) for 35 consecutive days, the concentration of insulin (a) and mRNA levels of PI3K (b) and Akt (c) were measured. Then, Western blotting was used to further examine the protein expression and phosphorylation of PI3K and Akt (d–h). The protein expression of PI3K (upper row) and Akt (lower row) under the conditions in e and f was further examined by immunohistochemistry (i) to confirm the findings observed by Western blot analysis. Mean ± SEM; W-25 °C and W-0 °C, n = 5; A-25 °C and A-0 °C, n = 4; *P < 0.05, **P < 0.01, and ***P < 0.001.
PI3K and Akt are two key downstream signaling messengers of insulin-mediated energy regulation pathways [18]. Therefore, it is important to examine whether the expression of PI3K and Akt in brain tissues is affected by the daily consumption of 0 °C drinking water. The mRNA expression of PI3K (Fig. 4b) and Akt (Fig. 4c) in the W-0 °C and A-0 °C mice was significantly lower than that in the W-25 °C and A-25 °C mice, respectively. Then, we used immunohistochemistry to examine the protein expression of PI3K and Akt in brain tissues. As shown in Fig. 4d, the expression of PI3K and Akt in the W-0 °C mice (0.25 ± 0.01 and 0.20 ± 0.01, respectively, n = 5) was significantly lower than that in the W-25 °C mice (0.27 ± 0.01 and 0.22 ± 0.01, respectively, n = 5, P < 0.01). Similarly, the protein expression of PI3K and Akt in the A-0 °C mice (0.24 ± 0.01 and 0.20 ± 0.01, respectively) was also significantly lower than that in the A-25 °C mice (0.26 ± 0.01 and 0.24 ± 0.01, respectively, n = 4, P < 0.05). To further confirm the protein expression of PI3K and Akt determined by immunohistochemistry, we used western blotting to analyze protein expression (Fig. 4e). The results showed that the expression of PI3K in the W-0 °C mice (0.32 ± 0.03 vs. 0.27 ± 0.01 n = 5, P < 0.05) was obviously lower than that in the W-25 °C mice and that the expression of PI3K in the A-0 °C mice was also significantly lower than that in the A-25 °C mice (0.32 ± 0.04 vs. 0.17 ± 0.04 n = 4, P < 0.01). Meanwhile, the expression of Akt in the W-0 °C mice and A-0 °C mice was significantly lower than that in the W-25 °C mice (0.97 ± 0.07 vs. 0.77 ± 0.09 n = 5, P < 0.05) and A-25 °C mice (0.90 ± 0.10 vs. 0.58 ± 0.13 n = 4, P < 0.05), respectively.
Next, we detected the phosphorylation levels of PI3K (p-PI3K) and Akt (p-Akt) (Fig. 4e–g). The p-PI3K/PI3K ratio in the W-0 °C mice and A-0 °C mice was markedly lower than that in the W-25 °C mice and A-25 °C mice, respectively. The p-Akt/Akt ratio in the A-0 °C mice was evidently higher than that in the A-25 °C mice. Therefore, the daily consumption of 0 °C IW for 35 days caused a significant decrease in the expression of PI3K and Akt in brain tissues.
Effects of the daily administration of 0 °C IW on the balance of neurotransmitters
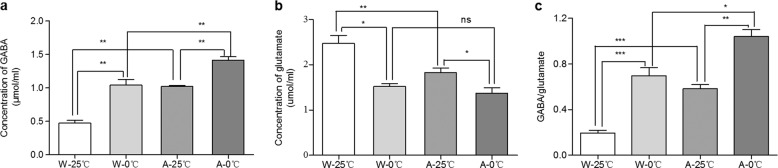
Glutamate and GABA are important neurotransmitters that maintain the physiological balance of nervous excitation and inhibition [19]. Thus, we next examined whether the temperature of IW affects the concentrations of glutamate and GABA in the cortices of mice. As shown in Fig. 5a, c, the glutamate concentration in the brains of the W-0 °C mice was distinctly lower than that in the brains of the W-25 °C mice. The glutamate concentration in the brains of the A-0 °C mice was significantly lower than that in the brains of the A-25 °C mice. The decrease in the concentration of glutamate in the APP/PS1 mice was 0.77 times that in wild-type C57BL/6 mice treated with 0 °C IW. In contrast, the GABA concentration in the brains of the W-0 °C mice was significantly higher than that in the brains of the W-25 °C mice, and the GABA concentration in the brains of the A-0 °C mice was significantly higher than that in the brains of the A-25 °C mice. The increase in the concentration of GABA in the APP/PS1 mice was 0.41 times that in the wild-type C57BL/6 mice. Therefore, the daily consumption of 0 °C drinking water caused a significant change in the balance between glutamate and GABA, which may have contributed to the changes in the behavioral performance of the mice in the corresponding groups.
Fig. 5. Effects of the daily administration of 0 °C IW on the balance of excitatory/inhibitory neurotransmitters.
After the wild-type C57BL/6 mice (W, n = 5) and APP/PS1 mice (AD model, A, n = 4) were administered intragastric water (IW, 10 mL/kg, 2 times/day) at either 0 °C (the W-0 °C or A-0 °C group) or 25 °C (the W-25 °C and A-25 °C group) for 35 consecutive days, the concentrations of GABA (a) and glutamate (b) in cortical tissues were measured by ELISA, and the GABA and glutamate ratio was calculated (c). Mean ± SEM; *P < 0.05, **P < 0.01, and ***P < 0.001.
Neuropathological effects of the daily administration of 0 °C IW
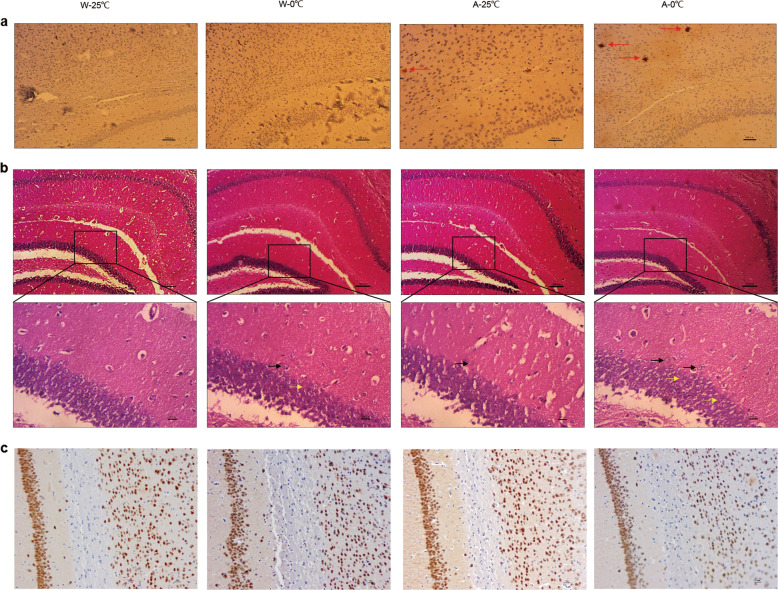
The above findings of a significant change in energy metabolism prompted us to further investigate whether the daily administration of 0 °C IW induces pathomorphological changes in brain tissues. As shown in Fig. 6a, while no significant Aβ plaques (red arrow) were observed in either the W-25 °C mice or the W-0 °C mice, the number of Aβ plaques in the A-0 °C mice (14.5 ± 2.5, n = 4) was significantly higher than that in the A-25 °C mice (7.0 ± 1.0, n = 4, P < 0.0085 vs. A-0 °C). Therefore, the daily administration of 0 °C IW promoted amyloid lesions in the cortices of APP/PS1 mice. In addition, it also induced partially scattered nerve cell arrangement (green arrow), a decrease in the number of neurons and an enlarged gap (blue arrow) in the CA3 region of the brain (Fig. 6b) in the APP/PS1 mice. We used NeuN staining to further examine whether the daily administration of 0 °C IW causes damage to hippocampal and cortical neurons. As shown in Fig. 6c, the number of NeuN-positive cells in W-25 °C and A-25 °C mice was higher than that in W-0 °C and A-0 °C mice, respectively. The density (IOD/area) of NeuN-positive cells in W-25 °C mice was significantly higher than that in W-0 °C mice (0.34 ± 0.01 vs. 0.42 ± 0.01, P < 0.0021). Similarly, the density (IOD/area) of NeuN-positive cells in A-25 °C mice was significantly higher than that in A-0 °C mice (0.31 ± 0.01 vs. 0.40 ± 0.01, P < 0.0005). Together, these results strongly suggested that the long-term daily consumption of 0 °C drinking water promoted the development of AD in APP/PS1 mice.
Fig. 6. Effect of the daily administration of 0 °C IW on amyloid lesions and neuropathy in the cortices and hippocampi of APP/PS1 mice.
After the wild-type C57BL/6 mice (W) and APP/PS1 mice (AD model, A) were administered intragastric water (IW, 10 mL/kg, 2 times/day) at either 0 °C (the W-0 °C and A-0 °C groups) or 25 °C (the W-25 °C and A-25 °C groups) for 35 consecutive days, amyloid lesions and neuropathy in the cortex and hippocampus were observed by immunohistochemistry and H&E staining, respectively. a Yellowish-brown or black-brown senile plaques (red arrow) in the cortex and hippocampus of each mouse were counted (×200). b Lesions in the cortex and hippocampus were observed, and lesions with scattered nerve cells (green arrow) and enlarged gaps (blue arrow) in the CA3 region of the hippocampus are shown (×100; magnification: ×400). c Representative NeuN staining of cells in each group. Brown or black-brown cells are NeuN-positive cells (×200).
Discussion
Many previous studies have shown that unhealthy lifestyles may affect brain health and play a critical role in the pathogenesis and development of neurodegenerative diseases such as cognitive decline, dementia, and AD [1, 2]. One of the most important lifestyle factors in metabolism and brain health is the daily consumption of beverages, including water [3, 20–22]. While most studies emphasize the importance of the quantity and quality of beverages, very little is currently known about the impact of beverage temperature on cognitive function and AD development. In this study, we provide the first compelling experimental evidence that the long-term daily consumption of 0 °C water not only impairs cognitive function but also promotes AD development and progression. We further explored the underlying mechanisms of the effects of ice water on cognitive function and found that the daily consumption of 0 °C water caused (1) a decrease in pepsin activity and an increase in the EC that resulted in impaired function of insulin signaling pathways and a significantly decrease in the protein expression of PI3K and Akt in the cortex and hippocampus; (2) an imbalance in the synthesis of neurotransmitters (glutamate vs GABA) in the brain; and (3) an increase in Aβ plaques and neuronal injury in the hippocampi and cortices of familial AD (APP/PS1) mice. These findings shed new light on the significant impact of lifestyle, specifically the temperature of drinking water or beverages, on the pathogenesis of cognitive dysfunction, and the development of AD. These findings also provide novel mechanistic insights into the effects of food/beverage temperature on cognitive function and dementia. These findings are important for the prevention and treatment of AD.
It is well known that water metabolism plays a significant role in both gastrointestinal function and cognitive function, which is closely related to gastrointestinal function through the gut–brain axis [22, 23]. To date, however, there has been no report on the role of drinking water temperature on cognitive function.
In this study, we provide the first evidence that the long-term daily consumption of iced water can aggravate cognitive decline. Although the number of mice subjected to the passive avoidance test was relatively small, the results are significant and compelling because the sample sizes were determined by power analysis, the 3R principle of animal use in experiments and previous studies [9, 10]. Our power analysis (n = 2 × (μα + μβ)2 × σ2/δ2) based on the ET data from the passive avoidance experiment indicated that our sample size (wild-type mice, n = 5; APP/PS1 mice, n = 4)was sufficient for statistical analysis of these data. Similar samples sizes for the passive avoidance and MWM tests have been used in other published papers [9, 10].
We next examined whether drinking water temperature affects gastric function, pepsin activity, and cognition and memory through the gut–brain axis. We found that the daily consumption of 0 °C drinking water for 14 consecutive days significantly decreased the GER and pepsin activity in wild-type KM mice and that these decreases were accompanied by decreases in body weight and ET (Fig. 1). The decline in gastrointestinal function led to insufficient energy supply to the brain and an increased EC in brain tissues. Similar results were observed in wild-type C57BL/6 mice and APP/PS1 AD mice treated with 0 °C IW for 35 consecutive days (Fig. 2). We also found that the insulin pathway, which is critical for the regulation of energy metabolism, was downregulated, as demonstrated by significant decreases in the concentrations of insulin and its two key downstream messengers in the brain. This may be the major mechanism responsible for the ice water-induced decline in cognitive and memory and exacerbation of AD progression.
It is well established that insulin binds to its receptor and leads to the automatic phosphorylation of insulin receptor substrate-1 [24]. It then activates PI3K/Akt to participate in glycometabolism. Numerous previous studies have shown that insulin signal disorder or insulin resistance contributes to the pathogenesis of AD [25–27]. This process may be related to the activation of glycogen synthase kinase 3β, which can induce Aβ accumulation and tau protein phosphorylation [28]. Recent evidence suggests however, that tau deletion aggravates insulin resistance in tau knockout mice [29]. In this study, we found that the daily consumption of 0 °C drinking water for 35 days significantly reduced the insulin concentration and the protein levels of phosphorylated PI3K and Akt in the brain tissues of APP/PS1 mice.
The decrease in PI3K/Akt phosphorylation was not consistent with the finding that the ATP concentration was elevated in the brain. We therefore further examined the possibility that insulin signaling may not be the only mechanism by which the regulation of energy metabolism is affected by drinking water temperature. Other pathways, such as AMPK signaling [30] and the Warburg effect [31], may take part in the production of energy. Further analysis revealed that the ADP/ATP ratio was increased significantly, indicating the activation of AMPK signaling.
Glutamate and GABA are the major contributors to the homeostatic balance of the excitatory and inhibitory amino acid neurotransmitter systems [32]. In addition to acetylcholine, other neurotransmitters, such as glutamate and GABA, also play an important role in AD [32, 33]. Accumulated evidence suggests that GABA type A receptors and transporter 3/4 are increased in AD patients and model mice [34, 35]. GABA levels are elevated in the brains and cerebrospinal fluid of AD patients [36, 37]. In this study, we found that the daily consumption of 0 °C drinking water caused a significant rise in the GABA level and a decrease in the glutamate content in the cortex and hippocampus. This suggests that the daily consumption of 0 °C drinking water causes an imbalance in excitatory and inhibitory amino acids that may boost the pathophysiology of depression [38], especially in elderly people and AD patients [38]. Our results from the open field test showed that the daily consumption of 0 °C drinking water may accelerate the formation of AD-like abnormal behavior induced through imbalances in the synthesis and activity of neurotransmitters.
A significant change in energy metabolism or neuroenergetics caused by daily 0 °C IW induced pathomorphological changes in brain tissues, as evidenced by the significantly increased number of Aβ plaques in the A-0 °C mice compared with the A-25 °C mice. Therefore, the daily administration of 0 °C IW promoted amyloid lesions in the cortices of AD mice. Amyloid protein deposition has been found in the brains of 12-week-old APP/PS1 mice, and thioflavin S staining, has shown that Aβ deposition in the brains of these mice increases by 179 times from 12 to 54 weeks [39]. Another study also showed that APP/PS1 mice have very low Aβ40 and Aβ42 levels at 2 months of age and that these levels begin to increase at 3–4 months of age [40]. Numerous other studies have also provided evidence that Aβ deposition is present in the brains of 3- to 4-month-old APP/PS1 mice [41–43]. The long-term daily administration of 0 °C IW also induced partially scattered nerve cell arrangement (green arrow), a decreased number of neurons, and an enlarged gap (blue arrow) in the CA3 region of the brain (Fig. 6b) in the APP/PS1 mice. In addition, NeuN staining further revealed that the daily administration of 0 °C IW caused damage to hippocampal and cortical neurons (Fig. 6c). Together, these results strongly suggested that the daily consumption of 0 °C drinking water promoted the development of AD in APP/PS1 mice.
In summary, this study provided experimental evidence (Fig. 7) that the long-term daily consumption of beverages at 0 °C may alter energy metabolism, central insulin-mediated neuroenergetics, and the glutamate/GABA ratio, cause cognitive decline and neuropathy and promote AD progression in mice.
Fig. 7. Schematic of the molecular mechanism underlying the cognitive decline induced by the long-term consumption of ice water in APP/PS1 mice.
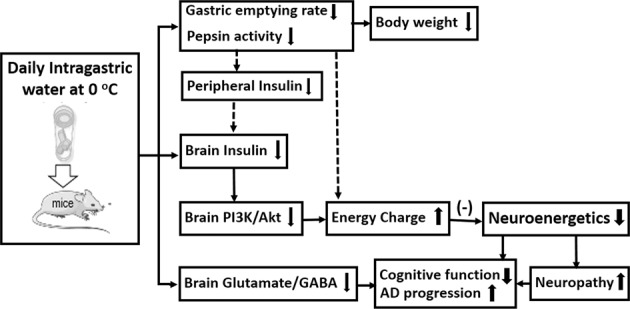
The long-term consumption of ice water induces neuropathy, such as abnormal nerve cell activity, aggravated senile plaque formation and neuronal injury, and cognitive decline that is related to (1) decreases in pepsin activity and the gastric emptying rate, which cause a decrease in calorie intake and an increase in the consumption of stored energy in the body, leading to a loss of body weight; (2) a decrease in calorie intake that induces insufficient peripheral insulin and causes less insulin to be transported into the brain, which inhibits PI3K/Akt signaling, leading to abnormal energy metabolism (changes in ATP, the EC and the ADP/ATP ratio); and (3) an imbalance in neurotransmitters (the glutamate/GABA ratio).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Key Program; No. 81430100 to SX) and by the Science and Technology Strategic Cooperation Programs of Luzhou Municipal People’s Government and Southwest Medical University (Nos. 2017LZXNYD-P01 and 2019LZXNYD-Duan Team to DDD).
Author contributions
JPW and SJX designed the study; WW, YD, and LXQ performed the research; JPW and WW contributed new reagents or analytic tools; YQW analyzed the data; JPW wrote the paper; and DDD critically reviewed, edited, and revised the paper.
Competing interests
The authors declare no competing interests.
Contributor Information
Dayue Darrel Duan, Email: dduan@swmu.edu.cn.
Shi-jun Xu, Email: xushijun@cdutcm.edu.cn.
References
- 1.Schulz JB, Deuschl G. Influence of lifestyle on neurodegenerative diseases. Nervenarzt. 2015;86:954–9. doi: 10.1007/s00115-014-4252-y. [DOI] [PubMed] [Google Scholar]
- 2.Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. 2016;5:934–43. doi: 10.1016/S1474-4422(16)30029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowman GL, Dayon L, Kirkland R, Wojcik J, Peyratout G, Severin IC, et al. Blood–brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers Dement. 2018;14:1640–50. doi: 10.1016/j.jalz.2018.06.2857. [DOI] [PubMed] [Google Scholar]
- 4.Alzheimer’s Association. 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:332–84. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Xiao E, Xiong H, Zhao Y, Deng X, Mei Z. Study on acute toxicity and animal gastrointestinal activity of crude and processed products of Entada phaseoloides. Zhong Yao Cai. 2010;33:1704–7. [PubMed] [Google Scholar]
- 6.Dai Y, Ma T, Ren X, Wei J, Fu W, Ma Y, et al. Tongluo Xingnao effervescent tablet preserves mitochondrial energy metabolism and attenuates cognition deficits in APPswe/PS1De9 mice. Neurosci Lett. 2016;630:101–8. doi: 10.1016/j.neulet.2016.07.044. [DOI] [PubMed] [Google Scholar]
- 7.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C T method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 8.Fu W, Dai Y, Ma T, Wei J, Chen H, Xu S. Tongluo Xingnao effervescent tablet reverses memory deficit and reduces plaque load in APPswe/PS1dE9 mice. Exp Ther Med. 2018;15:4005–13. doi: 10.3892/etm.2018.5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avgeropoulos N, Kelley B, Middaugh L, Arrigo S, Persidsky Y, Gendelman HE, et al. SCID mice with HIV encephalitis develop behavioral abnormalities. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:13–20. doi: 10.1097/00042560-199805010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Bustamante C, Bilbao P, Contreras W, Martínez M, Mendoza A, Reyes A, et al. Effects of prenatal stress and exercise on dentate granule cells maturation and spatial memory in adolescent mice. Int J Dev Neurosci. 2010;28:605–9. doi: 10.1016/j.ijdevneu.2010.07.229. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J, Chen X, Song Y, Zhang Y, Zhou L, Wan L. Deficit of RACK1 contributes to the spatial memory impairment via upregulating BECLIN1 to induce autophagy. Life Sci. 2016;151:115–21. doi: 10.1016/j.lfs.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Trevisiol A, Nave KA. Brain energy metabolism: conserved functions of glycolytic glial cells. Cell Metab. 2015;22:361–3. doi: 10.1016/j.cmet.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Tang BL. Brain activity-induced neuronal glucose uptake/glycolysis: is the lactate shuttle not required? Brain Res Bull. 2017;137:225–8. doi: 10.1016/j.brainresbull.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Jha MK, Morrison BM. Glia-neuron energy metabolism in health and diseases: New insights into the role of nervous system metabolic transporters. Exp Neurol. 2018;309:23–31. doi: 10.1016/j.expneurol.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Tang W, Chao FL, Zhou CN, Jiang L, Zhang Y, et al. Four-month treadmill exercise prevents the decline in spatial learning and memory abilities and the loss of spinophilin-immunoreactive puncta in the hippocampus of APP/PS1 transgenic mice. Neurobiol Dis. 2020;136:104723. doi: 10.1016/j.nbd.2019.104723. [DOI] [PubMed] [Google Scholar]
- 16.Qin C, Wu S, Chen B, Wu X, Qu K, Liu J, et al. Effect of ganoderma lucidum preparation on the behavior, biochemistry and autoimmune parameters of mouse models of APP/PS1 double transgenic Alzheimer’s disease. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2017;39:330–5. doi: 10.3881/j.issn.1000-503X.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Suckale J, Solimena M. The insulin secretory granule as a signaling hub. Trends Endocrinol Metab. 2010;21:599–609. doi: 10.1016/j.tem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Collins BC, Gillet LC, Rosenberger G, Röst HL, Vichalkovski A, Gstaiger M, et al. Quantifying protein interaction dynamics by SWATH mass spectrometry: application to the 14-3-3 system. Nat Methods. 2013;10:1246–53. doi: 10.1038/nmeth.2703. [DOI] [PubMed] [Google Scholar]
- 19.Ford TC, Nibbs R, Crewther DP. Glutamate/GABA+ ratio is associated with the psychosocial domain of autistic and schizotypal traits. PLoS One. 2017;12:e0181961.. doi: 10.1371/journal.pone.0181961.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarzinger M, Pollock BG, Osm H, Dufouil C, Rehm J, Group QDS. Contribution of alcohol use disorders to the burden of dementia in France 2008-13: a nationwide retrospective cohort study. Lancet Public Health. 2018;3:e124–32. doi: 10.1016/S2468-2667(18)30022-7. [DOI] [PubMed] [Google Scholar]
- 21.Spear LP. Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci. 2018;19:197–214. doi: 10.1038/nrn.2018.10. [DOI] [PubMed] [Google Scholar]
- 22.Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21:738–48. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Betiku OC, Yeoman CJ, Gaylord TG, Americus B, Olivo S, Duff GC, et al. Water system is a controlling variable modulating bacterial diversity of gastrointestinal tract and performance in rainbow trout. PLoS One. 2018;13:e0195967. doi: 10.1371/journal.pone.0195967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang H, Habte-Tsion H-M, Ge X, Ren M, Xie J, Miao L, et al. Dietary arginine affects the insulin signaling pathway, glucose metabolism and lipogenesis in juvenile blunt snout bream Megalobrama amblycephala. Sci Rep. 2017;7:7864. doi: 10.1038/s41598-017-06104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geert Jan B, Reagan LP. Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci. 2015;16:660–71. doi: 10.1038/nrn4019. [DOI] [PubMed] [Google Scholar]
- 26.Malkki H. Alzheimer disease: Insulin resistance could be linked to risk of AD via reduced glucose uptake. Nat Rev Neurol. 2015;11:485. doi: 10.1038/nrneurol.2015.147. [DOI] [PubMed] [Google Scholar]
- 27.Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14:168–81. doi: 10.1038/nrneurol.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avrahami L, Farfara D, Shaham-Kol M, Vassar R, Frenkel D, Eldar-Finkelman H. Inhibition of glycogen synthase kinase-3 ameliorates β-amyloid pathology and restores lysosomal acidification and mammalian target of rapamycin activity in the alzheimer disease mouse model in vivo and in vitro studies. J Biol Chem. 2013;288:1295–306. doi: 10.1074/jbc.M112.409250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marciniak E, Leboucher A, Caron E, Ahmed T, Tailleux A, Dumont J, et al. Tau deletion promotes brain insulin resistance. J Exp Med. 2017;214:2257–69. doi: 10.1084/jem.20161731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin S-C, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Cell Metab. 2017;27:299–313. doi: 10.1016/j.cmet.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17:113–24. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira AC, Mao X, Jiang CS, Kang G, Milrad S, McEwen BS, et al. Dorsolateral prefrontal cortex GABA deficit in older adults with sleep-disordered breathing. Proc Natl Acad Sci U S A. 2017;114:10250–55. doi: 10.1073/pnas.1700177114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992;326:1464–8. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- 34.Kwakowsky A, Calvo-Flores Guzmán B, Pandya M, Turner C, Waldvogel HJ, Faull RL. GABAA receptor subunit expression changes in the human Alzheimer’s disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. J Neurochem. 2018;145:374–92. doi: 10.1111/jnc.14325. [DOI] [PubMed] [Google Scholar]
- 35.Wu Z, Guo Z, Gearing M, Chen G. Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer’s disease model. Nat Commun. 2014;5:4159. doi: 10.1038/ncomms5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samakashvili S, Ibáñez C, Simó C, Gil-Bea FJ, Winblad B, Cedazo-Mínguez A, et al. Analysis of chiral amino acids in cerebrospinal fluid samples linked to different stages of Alzheimer disease. Electrophoresis. 2011;32:2757–64. doi: 10.1002/elps.201100139. [DOI] [PubMed] [Google Scholar]
- 37.Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH, et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat Med. 2014;20:886. doi: 10.1038/nm.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frisardi V, Panza F, Farooqui AA. Late-life depression and Alzheimer’s disease: the glutamatergic system inside of this mirror relationship. Brain Res Rev. 2011;67:344–55. doi: 10.1016/j.brainresrev.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Wengenack TM, Whelan S, Curran GL, Duff KE, Poduslo JF. Quantitative histological analysis of amyloid deposition in Alzheimer’s double transgenic mouse brain. Neurosci. 2000;101:939–44. doi: 10.1016/s0306-4522(00)00388-2. [DOI] [PubMed] [Google Scholar]
- 40.Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann Neurol. 2004;55:801–14. doi: 10.1002/ana.20101. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, He J, Zhang R, Zhu S, Wang J, Kong L, et al. Sensorimotor gating and memory deficits in an APP/PS1 double transgenic mouse model of Alzheimer’s disease. Behav Brain Res. 2012;233:237–43. doi: 10.1016/j.bbr.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 42.He J, Qiao JP, Zhu S, Xue M, Chen W, Wang X, et al. Serum β-amyloid peptide levels spike in the early stage of Alzheimer-like plaque pathology in an APP/PS1 double transgenic mouse model. Curr Alzheimer Res. 2013;10:979–86. doi: 10.2174/15672050113106660159. [DOI] [PubMed] [Google Scholar]
- 43.Collins JM, King AE, Woodhouse A, Kirkcaldie MTK, Vickers JC. Age moderates the effects of traumatic brain injury on beta-amyloid plaque load in APP/PS1 mice. J Neurotrauma. 2019;36:1876–89. doi: 10.1089/neu.2018.5982. [DOI] [PubMed] [Google Scholar]