Abstract
Extrapolating patterns from individuals to populations informs climate vulnerability models, yet biological responses to warming are uncertain at both levels. Here we contrast data on the heating tolerances of fishes from laboratory experiments with abundance patterns of wild populations. We find that heating tolerances in terms of individual physiologies in the lab and abundance in the wild decline with increasing temperature at the same rate. However, at a given acclimation temperature or optimum temperature, tropical individuals and populations have broader heating tolerances than temperate ones. These congruent relationships implicate a tight coupling between physiological and demographic processes underpinning macroecological patterns, and identify vulnerability in both temperate and tropical species.
Subject terms: Ecophysiology, Macroecology, Ichthyology
Nicholas Payne et al. use physiological and population-level abundance data from 823 fish species to examine how heating tolerance scales at both the individual and population level. This study shows that heating tolerance declines in the lab and the wild at the same rate, and for a given temperature, individuals and populations from tropical areas have broader heating tolerances than temperate species.
Introduction
Research into the relationships between physiological limitations of species and their distributions has a rich history in ecology, formalized by the concept of fundamental versus realized niches1. Renewed interest is represented by the field of ‘macrophysiology’2, which explores large scale physiological variation and its ecological implications. Anthropogenic climate change has re-invigorated interest in thermal tolerances3,4.
Comparing whether physiological functions of organisms and the performance of populations share responses to heating helps build a mechanistic understanding of heating responses across scales of biological organisation. However, relatively few generalizations can be made about patterns in the thermal tolerances of ectothermic animals5,6; less still about how tolerance patterns of individual organisms defined from laboratory experiments translate into those of populations in the wild7,8. Several comparative studies over the past decade have contrasted physiological thermal limits of species to the thermal limits of their geographical ranges, finding a number of interesting albeit complex patterns. For example, aquatic ectotherms appear to live closer to their physiological limits than do terrestrial species9, whereas accounting for body temperature suggests terrestrial animals regularly exceed physiological thermal limits, necessitating behavioural thermoregulation10. Ectotherms from warmer climates tend to have higher physiological thermal tolerances (e.g. CTmax), but such limits are relatively conserved across lineages, possibly due to fixed physiological boundaries11 or the non-linear increase in metabolism with temperature12.
Since metrics of heating tolerance at different biological scales measure very different properties13,14, the usefulness of laboratory-derived tolerance data for predicting climate change impacts is regularly questioned8,15–17. Nevertheless, understanding physiological variation via controlled experimentation could ultimately improve mechanistic understanding of ecological processes and therefore improve forecasts of species’ responses to climate change18,19. One potential means of enhancing the applicability of physiological information for ecological predictions is to show that physiological patterns translate into ecological ones. The metabolic theory of ecology20 is an influential example of macrophysiological patterns providing quantitative linkages between organismal, demographic and ecosystem processes, but similar frameworks are scarce for understanding how much heating organisms tolerate above temperatures to which they are acclimated or adapted.
We compared the temperature-dependence of physiological function in the laboratory with abundance in the wild by analysing data from two published datasets that together comprise more than 800 fish species. Our general aim was to explore whether there are similar patterns in fish thermal tolerance at the levels of individual physiology and abundance of natural populations. For physiological function of individual fish, we defined heating tolerance as the difference between the temperature a fish is acclimated to (Ta) and its upper critical thermal maximum in the laboratory (CTmax; measured as lethal temperatures or those coinciding with the loss of a critical physiological function; Fig. 1a). We called this ‘physiological heating tolerance’. For wild abundance of fish populations, ‘population heating tolerance’ was defined as the difference between the temperature at which abundance is greatest (Topt) and the temperature at the warm distribution limit of the species in its wild range (Tlim; Fig. 1b).
Fig. 1. Conceptual illustration of fish heating tolerances at different biological scales.
a At the individual level, Ta represents the temperature an individual is acclimated to in a laboratory experiment, and CTmax is the temperature coinciding with death or loss of critical functions. b At the population level, Topt is the temperature of highest abundance in the wild, and Tlim is the 95th percentile of maximum temperatures encountered by that species in its natural range.
We found striking similarities between patterns in physiological heating tolerances in the lab and population heating tolerances in the wild, with both declining with increasing temperature at similar rates. We also found that, at a given acclimation temperature or optimum temperature, tropical individuals and populations have broader heating tolerances than temperate ones. These shared patterns at different biological scales suggest a potentially tight link between physiological and demographic processes, and highlight vulnerabilities in fishes from both temperate and tropical regions.
Results
Physiological heating tolerances calculated from the laboratory estimates of critical performance are approximately two to three times higher than population heating tolerances calculated for abundance of wild fishes (Fig. 2a–b). This is not surprising given ectotherms are unlikely to live on the edge of their CTmax, and that heating tolerance decreases with slower rates of warming21,22. Indeed, individuals throughout a species’ wild range tend to be exposed to changes in environmental temperatures over much longer time scales (months and years) than are those that undergo laboratory experimentation (days)21. It could perhaps be expected that the larger temperature fluctuations seen in the wild would elevate heating tolerances relative to those of fish held at relatively stable temperature regime in laboratory settings, but such an effect may simply buffer the larger difference between the highest temperature at which they can be competitive in nature (Tlim) and their physiological limit (CTmax). The difference between physiological and population heating tolerance is consistent with expectations that thermal range decreases for higher levels of biological complexity14.
Fig. 2. Fish heating tolerances at different biological scales.
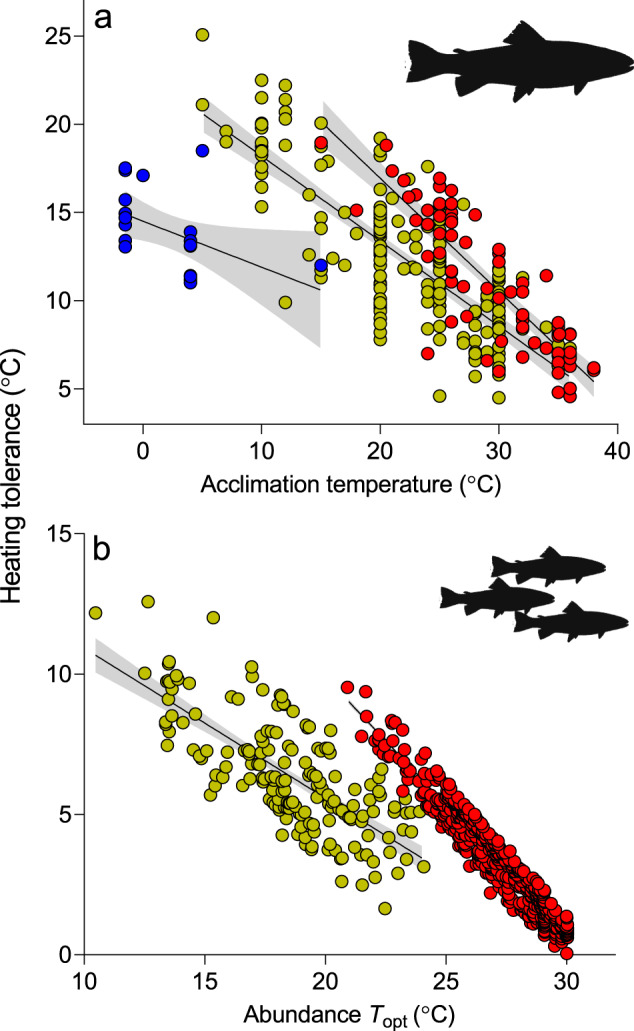
a At the individual level, physiological heating tolerance is CTmax − Ta, and b at the population level, population heating tolerance is Tlim − Topt (as per Fig. 1). Tropical, temperate and polar species are indicated by red, yellow and blue, respectively. For panel a, each symbol represents a mean measurement (n = 269) from 121 different species, and for b, each symbol represents a different species (n = 702). Lines represent regression mean and 95% CIs.
Both physiological and population heating tolerances strongly declined as ambient temperature increased (Fig. 2, Supplementary Tables 1–4; PGLS P < 0.001). That is, fishes acclimated to higher temperatures in the lab and wild fishes that were recorded at their highest densities at higher temperatures had lower heating tolerances (Fig. 2a–b). Moreover, the rates of these declines in heating tolerance with temperature were similar for individual physiology and wild populations (Fig. 2, Supplementary Tables 1–4).
Despite these similar, marked declines in heating tolerance toward higher temperatures, mean heating tolerances are broadly similar between temperate and tropical fishes at both physiological (12 vs 11 °C for temperate and tropical) and population (7 vs 3 °C) levels. As a result, because they generally experience warmer temperatures, tropical fishes tend to have larger heating tolerances than temperate ones at any given Ta or Topt. For example, a tropical fish population with Topt of 23 °C has roughly double the heating tolerance of a temperate species with the same Topt (Fig. 2b). Slopes were not significantly different between tropical and temperate species for physiological heating tolerance (PGLS interaction term P = 0.196, Supplementary Table 2), and only slightly steeper for tropical population heating tolerance (PGLS interaction term P < 0.001, Supplementary Table 4).
The similar rates of decline in heating tolerance across fish physiology and demography, with distinctive patterns for both tropical and temperate species, implicate a mechanistic link between these two biological scales. Whatever the ultimate mechanism underpinning the decline in physiological heating tolerance at higher Ta (and for tropical species to have higher heating tolerance at Ta than temperate ones at the same Ta), it is possible that the physiological scaling patterns seen in Fig. 2a are regulating the range of temperatures that fish species encounter in the wild, in turn driving the abundance scaling patterns seen in Fig. 2b. The transition to higher heating tolerances in tropical species compared to temperate ones at a given Ta or Topt does not seem readily explained by phylogenetic similarity, with closely related species spread across both guilds (Supplementary Fig. S1 and S2).
One possible explanation for why heating tolerances decline for warmer acclimated or adapted fishes, within either the temperate or tropical clades, is thermodynamics: 1 °C of heating tolerance represents a greater metabolic cost at higher Ta or Topt12,23 because metabolism scales exponentially with temperature rather than linearly24. Alternatively, heating tolerance may decline toward firm upper limits of enzyme activity and/or environmental temperature limits available in the ocean (a particularly likely explanation for heating tolerances of the warmest-adapted tropical fish populations, which may also explain the slightly steeper slope in Fig. 2b)25. A more traditional explanation is that declining heating tolerances are an adaptive response to the more-stable environments toward warmer, tropical regions26. However, environmental temperature variability would not explain the transition to higher heating tolerances seen for the tropical guild of fish—‘tropical’ species ought to have lower heating tolerances than ‘temperate’ ones at the same Ta or Topt. Disentangling the influence of temperature and seasonality (i.e. latitude) is a classic biogeographer’s problem, and our datasets imply that both factors may influence heating tolerance because heating tolerance declines with increasing temperature (Ta and Topt), but along different trajectories for tropical and temperate species.
The temperature an animal is acclimated to, Ta, is not necessarily the same as that species’ “physiologically-optimal” temperature because physiological Topt can be defined in many different ways, and different processes are often maximised at different temperatures16,27. For example, Atlantic cod Gadus morhua have variously been measured as having Topt for oxygen supply (% venous PO2) at 5 °C28, Topt for aerobic scope being as low as 7 °C or higher than 14 °C29,30, and Topt for growth rates ranging from 6 to 13 °C31. The acclimation process may improve performance at a given Ta, but it should be assumed neither that Ta equates to Topt for all physiological processes, nor that physiological Topt approximates Topt for natural abundance. Our physiological and population heating tolerance datasets clearly measure different things, but their similar patterns are perhaps unsurprising if Topt for abundance is considered from the perspective of it being the temperature at which most individuals of a species are ‘acclimated to’ in the wild (i.e. the commonest Ta in a species’ range).
Discussion
The similar scaling of patterns from the laboratory and the wild provides support for the application of across-species physiological trends to forecast how temperature governs distribution patterns (the basis of macrophysiology). Moreover, the observation of smaller heating tolerances at higher temperatures supports claims that ectotherms in warmer climates will be less resilient to future temperature rises6. Yet the transition to higher heating tolerances seen for tropical fishes than temperate species at the same Ta or Topt (Fig. 2) suggests climate resilience assessments need to include more complexity than thermal regimes alone (at least for fishes). Studies like ours that combine physiological and ecological information could help explain how tropical species elevate their heating tolerance, a question of central importance for understanding future resilience.
Methods
Underlying data
Data were derived from two recently published papers, with full physiological data compilation details and inclusion protocols found in Morley et al.32, and wild abundance data and modelling approaches in Waldock et al.7 Briefly, physiological data were compiled by Morley et al.32 from a literature search of studies that tested the upper temperature limits (critical or lethal endpoints) of fish that were acclimated at more than one experimental temperature. The search terms “acclimate” or “acclimation” and “temperature” were used in Google, Google Scholar and Web of Knowledge, and latitude of specimen collection was used to delineate tropical, temperate and polar species (<30, 30–60, >60°, respectively). This search returned high and low Ta measurements from 121 species (269 measurements in total) of juvenile and adult stages, from marine and freshwater habitats, and under experimental protocols that varied in some aspects between studies (e.g. rate of temperature ramping). These factors undoubtedly contribute to unexplained variation in our data. Topt and Tlim were estimated by fitting quantile generalised additive models to species abundance data compiled by the global Reef Life Survey of shallow fish communities parameter estimates in ref. 7, raw abundance data presented in ref. 33. These models related variation in abundance to thermal gradients, while accounting for confounding sources of environmental variation see ref. 7 for details.
Tlim was estimated as the 97.5th quantile of minimum and maximum temperatures recorded at reef survey sites over a 2-year period7, and tropical species were defined as those having Tlim > 29 °C. This division was chosen as it represents the threshold delineating the clear natural clustering of guilds (Fig. 2b). Waldock et al.7 accounted for the effect of additional environmental variation such as O2, phosphate, nitrate, current velocity, productivity, reef area, human population density, site depth, protection status scores and sampling intensity, and as such is robust to the confounding effects of environmental variation on thermal parameters. This dataset represented 702 species. Both datasets can be found in supporting online material in Morley et al.32 and Waldock et al.7, and are reproduced into a single file in supporting material of this paper.
Statistics and reproducibility
Analysis of both datasets was undertaken using the phylogenetic generalised least squares method, PGLS34, with the ‘caper’ package35 in R (version 3.3.0 R Foundation for Statistical Computing). Analyses that do not include phylogenetic information about the species represented treat each species as independent from all others. In reality, species are related and the covariance of traits exhibited by a species may be the result of its relatedness to other species with similar traits (phylogenetic inertia) rather than an instance of evolutionary adaptation see ref. 36 for illustration of the usefulness of PGLS methods. The fish phylogeny was based on a tree built using the ‘rotl’ package37, which can be seen in Supplementary Fig. S1. A measure of phylogenetic correlation, λ (lambda), was estimated by fitting PGLS models with different λ values to uncover which value maximised the log likelihood. Lambda quantifies the degree to which trait evolution deviates from a ‘Brownian motion’ model (traits evolving by the accumulation of small, random changes over time)38, and is thus considered to be a measure of the degree of phylogenetic correlation in the data39. λ = 1 retains the Brownian motion model, indicating that the trait covariance between any two species is directly proportional to their amount of shared evolutionary history, while λ = 0 indicates phylogenetic independence (the trait values across species are entirely unrelated to the phylogeny of those species). For the physiological dataset, we included in our models Ta, thermal guild (i.e. whether a species was derived from tropical, temperate or polar regions), whether the Ta treatment was the higher or the lower for that species, and interactions between Ta and those other two factors. For the population data, model terms were Topt, thermal guild, and their interaction. We did not include the type of tolerance metric (e.g. critical or lethal endpoints) as a factor in our models because they are defined and measured in different ways, vary across taxa and the type of tolerance metric has been shown to vary neither by acclimation capacity32 nor thermal tolerance breadth9. Alpha was set at 0.05 for all tests.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank Andrew Jackson, Jacinta Kong and Craig White for thoughtful discussion of thermal tolerance. N.L.P. was supported by a Science Foundation Ireland Starting Investigator Research Grant (18/SIRG/5549).
Author contributions
N.L.P., A.E.B. and S.A.M. conceived the study. L.G.H., J.A.S. and N.L.P. analysed the data. N.L.P. wrote the manuscript with contribution from S.A.M., L.G.H., J.A.S., R.S.S., C.W. and A.E.B.
Data availability
Data underlying the study can be found as Supplementary Data, and are available from paynen@tcd.ie upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-021-01773-3.
References
- 1.Hutchinson GE. Cold spring harbor symposium on quantitative biology. Cold Spring Harb. Symp. Quant. Biol. 1957;22:415–427. doi: 10.1101/SQB.1957.022.01.039. [DOI] [Google Scholar]
- 2.Chown S, Gaston K, Robinson D. Macrophysiology: large‐scale patterns in physiological traits and their ecological implications. Funct. Ecol. 2004;18:159–167. doi: 10.1111/j.0269-8463.2004.00825.x. [DOI] [Google Scholar]
- 3.Spicer, J. I., Morley, S. A. & Bozinovic, F. Physiological diversity, biodiversity patterns and global climate change: testing key hypotheses involving temperature and oxygen. Philos. Trans. R. Soc. B374 (2019). [DOI] [PMC free article] [PubMed]
- 4.Chown SL, Gaston KJ. Macrophysiology—progress and prospects. Funct. Ecol. 2016;30:330–344. doi: 10.1111/1365-2435.12510. [DOI] [Google Scholar]
- 5.Sunday JM, Bates AE, Dulvy NK. Thermal tolerance and the global redistribution of animals. Nat. Clim. Change. 2012;2:686–690. doi: 10.1038/nclimate1539. [DOI] [Google Scholar]
- 6.Deutsch CA, et al. Impacts of climate warming on terrestrial ectotherms across latitude. PNAS. 2008;105:6668–6672. doi: 10.1073/pnas.0709472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waldock C, Stuart‐Smith RD, Edgar GJ, Bird TJ, Bates AE. The shape of abundance distributions across temperature gradients in reef fishes. Ecol. Lett. 2019;22:685–696. doi: 10.1111/ele.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Payne NL, et al. Temperature dependence of fish performance in the wild: links with species biogeography and physiological thermal tolerance. Funct. Ecol. 2016;30:903–912. doi: 10.1111/1365-2435.12618. [DOI] [Google Scholar]
- 9.Sunday JM, Bates AE, Dulvy NK. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B. 2011;278:1823–1830. doi: 10.1098/rspb.2010.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunday JM, et al. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. PNAS. 2014;111:5610–5615. doi: 10.1073/pnas.1316145111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araujo MB, et al. Heat freezes niche evolution. Ecol. Lett. 2013;16:1206–1219. doi: 10.1111/ele.12155. [DOI] [PubMed] [Google Scholar]
- 12.Payne NL, Smith JA. An alternative explanation for global trends in thermal tolerance. Ecol. Lett. 2017;20:70–77. doi: 10.1111/ele.12707. [DOI] [PubMed] [Google Scholar]
- 13.Sinclair BJ, et al. Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecol. Lett. 2016;19:1372–1385. doi: 10.1111/ele.12686. [DOI] [PubMed] [Google Scholar]
- 14.Rezende EL, Bozinovic F. Thermal performance across levels of biological organization. Philos. Trans. R. Soc. B. 2019;374:20180549. doi: 10.1098/rstb.2018.0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes DK, Peck LS, Morley SA. Ecological relevance of laboratory determined temperature limits: colonization potential, biogeography and resilience of Antarctic invertebrates to environmental change. Glob. Change Biol. 2010;16:3164–3169. doi: 10.1111/j.1365-2486.2010.02198.x. [DOI] [Google Scholar]
- 16.Clark TD, Sandblom E, Jutfelt F. Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J. Exp. Biol. 2013;216:2771–2782. doi: 10.1242/jeb.084251. [DOI] [PubMed] [Google Scholar]
- 17.Norin T, Malte H, Clark TD. Aerobic scope does not predict the performance of a tropical eurythermal fish at elevated temperatures. J. Exp. Biol. 2014;217:244–251. doi: 10.1242/jeb.089755. [DOI] [PubMed] [Google Scholar]
- 18.Kearney M, Porter W. Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 2009;12:334–350. doi: 10.1111/j.1461-0248.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 19.Buckley LB, et al. Can mechanism inform species’ distribution models? Ecol. Lett. 2010;13:1041–1054. doi: 10.1111/j.1461-0248.2010.01506.x. [DOI] [PubMed] [Google Scholar]
- 20.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. doi: 10.1890/03-9000. [DOI] [Google Scholar]
- 21.Peck LS, Clark MS, Morley SA, Massey A, Rossetti H. Animal temperature limits and ecological relevance: effects of size, activity and rates of change. Funct. Ecol. 2009;23:248–256. doi: 10.1111/j.1365-2435.2008.01537.x. [DOI] [Google Scholar]
- 22.Richard J, Morley SA, Thorne MA, Peck LS. Estimating long-term survival temperatures at the assemblage level in the marine environment: towards macrophysiology. PLoS ONE. 2012;7:e34655. doi: 10.1371/journal.pone.0034655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dillon ME, Wang G, Huey RB. Global metabolic impacts of recent climate warming. Nature. 2010;467:704–706. doi: 10.1038/nature09407. [DOI] [PubMed] [Google Scholar]
- 24.Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–2251. doi: 10.1126/science.1061967. [DOI] [PubMed] [Google Scholar]
- 25.Stuart-Smith RD, Edgar GJ, Bates AE. Thermal limits to the geographic distributions of shallow-water marine species. Nat. Ecol. Evol. 2017;1:1846–1852. doi: 10.1038/s41559-017-0353-x. [DOI] [PubMed] [Google Scholar]
- 26.Janzen DH. Why mountain passes are higher in the tropics. Am. Nat. 1967;101:233–249. doi: 10.1086/282487. [DOI] [Google Scholar]
- 27.Martin TL, Huey RB. Why “Suboptimal” is optimal: Jensen’s inequality and ectotherm thermal preferences. Am. Nat. 2008;171:E102–E118. doi: 10.1086/527502. [DOI] [PubMed] [Google Scholar]
- 28.Pörtner H-O, et al. Cod and climate in a latitudinal cline: physiological analyses of climate effects in marine fishes. Clim. Res. 2008;37:253–270. doi: 10.3354/cr00766. [DOI] [Google Scholar]
- 29.Sylvestre E-L, Lapointe D, Dutil J-D, Guderley H. Thermal sensitivity of metabolic rates and swimming performance in two latitudinally separated populations of cod, Gadus morhua L. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2007;177:447–460. doi: 10.1007/s00360-007-0143-x. [DOI] [PubMed] [Google Scholar]
- 30.Claireaux G, Webber D, Lagardère J-P, Kerr S. Influence of water temperature and oxygenation on the aerobic metabolic scope of Atlantic cod (Gadus morhua) J. Sea Res. 2000;44:257–265. doi: 10.1016/S1385-1101(00)00053-8. [DOI] [Google Scholar]
- 31.Björnsson B, Steinarsson A. The food-unlimited growth rate of Atlantic cod (Gadus morhua) Can. J. Fish. Aquat. Sci. 2002;59:494–502. doi: 10.1139/f02-028. [DOI] [Google Scholar]
- 32.Morley S, Peck L, Sunday J, Heiser S, Bates A. Physiological acclimation and persistence of ectothermic species under extreme heat events. Glob. Ecol. Biogeogr. 2019;28:1018–1037. doi: 10.1111/geb.12911. [DOI] [Google Scholar]
- 33.Edgar GJ, Stuart-Smith RD. Systematic global assessment of reef fish communities by the Reef Life Survey program. Sci. Data. 2014;1:140007. doi: 10.1038/sdata.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grafen A. The phylogenetic regression. Philos. Trans. R. Soc. Lond. Ser. B, Biol. Sci. 1989;326:119–157. doi: 10.1098/rstb.1989.0106. [DOI] [PubMed] [Google Scholar]
- 35.Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S. The caper package: comparative analysis of phylogenetics and evolution in R. R. Package Version. 2013;5:1–36. [Google Scholar]
- 36.Halsey LG, Butler PJ, Blackburn TM. A phylogenetic analysis of the allometry of diving. Am. Nat. 2006;167:276–287. doi: 10.1086/499439. [DOI] [PubMed] [Google Scholar]
- 37.Michonneau F, Brown JW, Winter DJ. rotl: an R package to interact with the Open Tree of Life data. Methods Ecol. Evol. 2016;7:1476–1481. doi: 10.1111/2041-210X.12593. [DOI] [Google Scholar]
- 38.Freckleton R. The seven deadly sins of comparative analysis. J. Evol. Biol. 2009;22:1367–1375. doi: 10.1111/j.1420-9101.2009.01757.x. [DOI] [PubMed] [Google Scholar]
- 39.Freckleton RP, Harvey PH, Pagel M. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 2002;160:712–726. doi: 10.1086/343873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Data underlying the study can be found as Supplementary Data, and are available from paynen@tcd.ie upon request.


