Abstract
Atherosclerosis (AS) is the main pathological cause of coronary heart disease (CHD). Current clinical interventions including statin drugs can effectively reduce acute myocardial infarction and stroke to some extent, but residual risk remains high. The current clinical treatment regimens are relatively effective for early atherosclerotic plaques and can even reverse their progression. However, the effectiveness of these treatments for advanced AS is not ideal, and advanced atherosclerotic plaques—the pathological basis of residual risk—can still cause a recurrence of acute cardiovascular and cerebrovascular events. Recently, nanomedicine-based treatment strategies have been extensively used in antitumor therapy, and also shown great potential in anti-AS therapy. There are many microstructures in late-stage atherosclerotic plaques, such as neovascularization, micro-calcification, and cholesterol crystals, and these have become important foci for targeted nanomedicine delivery. The use of targeted nanoparticles has become an important strategy for the treatment of advanced AS to further reduce the residual risk of cardiovascular events. Furthermore, the feasibility and safety of nanotechnology in clinical treatment have been preliminarily confirmed. In this review, we summarize the application of nanomedicine delivery in the treatment of advanced AS and the clinical value of several promising nanodrugs.
Keywords: coronary heart disease, advanced atherosclerosis, vulnerable plaque, nanoparticles, targeted therapy, drug delivery
Introduction
The incidence and mortality rates of arteriosclerotic cardiovascular disease (ASCVD) are increasing year by year. Currently, strategies for the prevention of cardiovascular events include lifestyle interventions, such as diet, exercise, and smoking cessation; control of the risk factors, including hypertension, serum cholesterol levels, and diabetes; and pharmacological interventions, including administering lipid-lowering drugs to prevent plaque growth and instability and the use of antiplatelet drugs in patients with chronic coronary syndromes, but the efficacy and safety of the antiplatelet therapy in patients require further assessment in view of a balance between the prevention of ischemic events and the increased risk of bleeding. We have used advanced imaging techniques, such as optical coherence tomography (OCT), intravascular ultrasound (IVUS), or near-infrared spectroscopy (NIRS), to demonstrate that current clinical coronary artery disease treatment programs are relatively effective for early atherosclerotic plaques and can even reverse their progression [1, 2]. However, the effectiveness of these treatments for advanced atherosclerosis (AS) is not ideal, and advanced atherosclerotic plaques—the pathological basis of residual risk—can still cause a recurrence of acute cardiovascular and cerebrovascular events after the current treatment regimens [3–5].
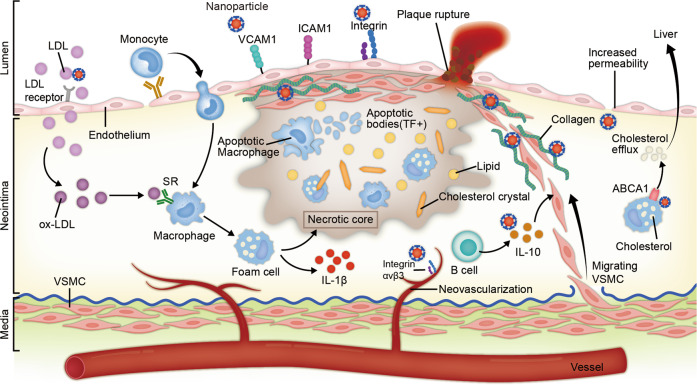
AS is a form of chronic inflammatory disease that leads to the deposition of lipids on the vascular wall. AS plaques begin to form in areas of impaired endothelial function in large- to medium-sized arteries, forming areas of frequent lesions. Recently, we and other researchers found that 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine, 25-hydroxycholesterol, and other types of lipid oxidation from oxidized low-density lipoprotein (oxLDL) can impair endothelial function significantly [6–8]. Impaired endothelial function may increase the penetration of macromolecules, such as lipoprotein, and increase the expression of chemotactic molecules, e.g., monocyte chemotactic protein 1 (MCP-1), and adhesion molecules, e.g., intercellular adhesion molecule (ICAM-1), vascular cell adhesion molecule (VCAM-1), E-selectin, and P-selectin. Moreover, the increased recruitment and accumulation of monocytes are crucial pathophysiological factors in AS [9, 10]. Monocytes differentiate into macrophages, which convert to foam cells by modifying apolipoprotein B (APO-B) containing LDL (oxidation and acetylation). Lipoprotein and immune cells in the vascular wall are subcutaneously deposited in the arteries, and the deposited LDL is transformed into oxLDL through the action of reactive oxygen species (ROS) secreted by the vascular cells. Macrophages are further induced to take up lipoprotein and form foam cells. These cycles constitute the first stages of AS. Lipoprotein and immune cells may decrease as inflammation subsides or lead to plaque progression, apoptosis, and neovascularization over subsequent years or decades.
The pathological features of advanced atherosclerotic lesions are a lipid or necrotic core composed of a large amount of lipid or necrotic cell deposits, increased numbers of inflammatory cells, a thin fibrous cap, and unstable neovascularization in the plaques. Immune cells release inflammation-degrading enzymes, such as matrix metalloproteinases (MMPs), which increase plaque instability. The clinical manifestations are erosion and rupture of the atherosclerotic plaques followed by thrombosis, which eventually leads to coronary lumen obstruction and adverse cardiovascular events. We and others have found that the formation of the atherosclerotic plaque is accompanied by thickening of the arterial intima. When the intima thickens to a certain extent, local hypoxia occurs, and the microvessels that originate from the outer membrane and nourish the blood vessels extend to the bottom of the plaque [11–13]. Subsequently, the process of neovascularization begins in plaques as a compensatory defense mechanism to restore nutrient supply to the vascular wall [14]. Neovascularization can promote cell transport and the recruitment of immune cells, which in turn promote inflammation and plaque progression and can even lead to plaque rupture. The lack of vascular wall cells and the poor connectivity of surface endothelial cells results in the high permeability and osmotic brittleness of the plaques; the vascular wall becomes prone to leakage and rupture, leading to internal bleeding, which increases the instability of the late atherosclerotic plaque.
Nanomedicine is defined as the application of nanotechnology in the monitoring, control, diagnosis, and treatment of biological systems [15]. Nanomedicine was originally developed in the field of anticancer therapy research, and recent studies have indicated that nanomedicine shows great potential for the diagnosis and treatment of AS [16]. The mechanisms behind the formation of late-stage atherosclerotic plaques are complex, and multiple molecular targets are known to play important roles. The systemic treatment strategies used in clinical practice reflect the limitations and substantial systemic side effects of current therapies. Therefore, strategies that specifically target multiple molecular sites or the late-stage atherosclerotic plaque microstructure could help to combat these limitations. Furthermore, nanomedicines can overcome the problem of rapid kidney excretion, thus maintaining the drugs in the blood circulation for longer periods. This characteristic is conducive to the extravasation of the vascular system so that the nanomaterials can accumulate and be adequately distributed throughout the required tissues or organs, achieving the maximum therapeutic effect at the minimum drug dose. In this paper, the use of nanomedicines in the treatment of advanced AS is systematically summarized (Table 1), and the potential clinical application and value of several promising nanomedicines, as well as the challenges faced in the development of nanomedicines, are discussed in depth.
Table 1.
Use of nano-drugs in the treatment of advanced AS.
| Therapeutic strategy | Drug | NPs | Target | Drug delivery | Animal model | In vivo fundings |
|---|---|---|---|---|---|---|
| Regulation of lipid metabolism | miR-33, miR-206, miR-223 [52] | chNPs: PEG and chitosan | Macrophage | Intrinsic targeting | C57BL/6 mice | In vivo, mice treated with miR-33-chNPs showed decreased reverse cholesterol transport (RCT) to the plasma, liver, and feces. |
| HDL [48] | rHDL: phospholipids, cholesterol and Apo-AI | ABCA1 transporter | Intrinsic targeting | Sprague-Dawley rats; ApoE−/− mice | In rats, release of inflammatory factors↓. In ApoE−/− mice, the aortic plaque area↓, lipid-rich plaques↓ | |
| PCSK9 siRNA [44] | Lipidoids: cholesterol, the phospholipid DSPC PEG2000-DMG and siRNA | Hepatocytes and immune cell | Intrinsic targeting | C57BL/6 mice; nonhuman primates | PCSK9 expression and plasma LDL-C levels↓ | |
| Na2SeO3 [53] | Selenium nanoparticles (SeNPs): GSH and BSA | Liver and blood vessel | Intrinsic targeting | ApoE−/− mice | Serum total cholesterol, triglyceride, LDL level↓, HDL level↑; the NO level and the activities of glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase in the serum and liver↑; foam cells and lipids in aortic plaques↓ | |
| Reduction of dead cell accumulation and plaque necrotic cores | IL-10 [55] | Col-IV IL-10 NP22 PEG and PLGA | Type IV collagen | Specific targeting | LDLR−/− mice | Efferocytosis in plaques in the aortic root↑; thickness of the fibrous-cap↑; size of necrotic cores↓ |
| Rapamycin [60] | RBC/RAP@PLGA: PLGA nanoparticles cloaked with the cell membrane of RBCs | Pathological lesion | Non-specific targeting | ApoE−/− mice | Atherosclerotic plaques area of aortic↓; lipid-rich plaques↓; size of necrotic cores↓; macrophage infiltration and MMP-9 expression in plaques↓ | |
| Rapamycin [61] | PNP: platelet membrane-coated nanoparticles | Pathological lesion | Non-specific targeting | ApoE−/− mice | Atherosclerotic plaques area of aortic↓; lipid-rich plaques↓; size of necrotic cores↓; macrophages in plaque↓; collagen fibers and smooth muscle cells in the plaque↑ | |
| Rapamycin [34] | ROS-sensitive material (Ox-bCD) and acid-labile material (Ac-bCD), both by functional modification of cyclodextrins | Pathological lesion | Non-specific targeting | ApoE−/− mice | Atherosclerotic plaques area of aortic↓; lipid-rich plaques↓; size of necrotic cores↓; macrophage infiltration and MMP-9 expression in plaques↓; collagen fibers in the plaque and thickness of the fibrous-cap↑ | |
| TPCD NP [33] | TPCD NP: conjugating a superoxide dismutase mimetic agent Tempol and a hydrogen peroxide-eliminating compound of phenylboronic acid pinacol ester onto a cyclic polysaccharide β-cyclodextrin | Macrophages and vascular smooth muscle cells | Non-specific targeting | ApoE−/− mice | Cholesterol crystals in plaques↓; size of necrotic cores↓; macrophage infiltration and MMP-9 expression in plaques↓; thickness of the fibrous-cap↑ | |
| Selective anti-inflammatory therapy | Rapamycin [71] | Leukosomes: integrates leukocyte-derived membrane proteins into the phospholipid bilayer. | Endothelium at the pathological lesion | Non-specific targeting | ApoE−/− mice | Proliferation of macrophages in aortic↓; the release of inflammatory factors and proinflammatory factor↓; atherosclerotic plaques area of aortic↓; MMP-9 activity↓ |
| Hyaluronan [70] | Hyaluronan Nanoparticles (HA-NPs) | CD44 cell surface receptor | Specific targeting | ApoE−/− mice | Atherosclerotic plaques area of aortic↓; macrophages in plaque↓; thickness of the fibrous-cap↑ | |
| Paclitaxel and methotrexate [67] | LDE: lipid core nanoparticles resemble the low-density lipoprotein structure | LDL receptor | Specific targeting | New Zealand white rabbits | Under LDE-PTX + LDE-MTX treatment, atherosclerotic plaques area of aortic and intima area↓; macrophage infiltration and MMP-9 expression in plaques↓ | |
| MnO2 [73] | EC-BSA-MnO2: dissociable MnO2 nanoparticle core shielded in the plasma membrane of endothelial cells (ECs). | Integrin β3 on EC surfaces | Specific targeting | ApoE−/− mice | Macrophage accumulation in plaques↓; atherosclerotic plaques area of aortic↓ | |
| Targeting neovascularization in plaques | Anti-miR-712 [76] | Cationic lipoparticles (CCLs) coated, decorated with peptide (VHPK) | Vascular cell adhesion molecule 1 (VCAM-1) | Specific targeting | ApoE−/− mice | Silencing of inflammatory endothelial cells↓; Plaque area and degree of left common carotid artery stenosis↓ |
| Fumagillin [79] | αvβ3-targeted paramagnetic nanoparticles | αvβ3-integrin | Specific targeting | Hyperlipidemic rabbits | Angiogenesis in plaque↓; combined with atorvastatin, the antiangiogenic effect was more significant and sustained |
Composition of nanocarriers for the treatment of atherosclerosis
Nanoparticles (NPs), which typically range from 1 to 100 nm, are similar in size to biological macromolecules, such as proteins and DNA. Unlike bulk materials, the intrinsic physical properties of nanomaterials vary. Nanocarriers may consist of organic materials (such as lipids, peptides, glycosylated compounds, hyaluronic acids, and even nucleic acids), metals, inorganic materials (such as iron oxide and gold), or different combinations of these materials. The NP surface can be modified with peptides, polymers, or antibodies that facilitate the selection of specific targets in cells or tissues, increasing drug availability and avoiding systemic side effects. The most commonly used are FDA-approved polymer nanocarriers, which are combined with standard drugs to improve absorption and bioavailability. Among them, lipid NPs, represented by liposomes, are the most widely used targeting strategies for atherosclerotic plaques. In the early stages of AS, liposomes targeting the endothelial dysfunction and lipid accumulation of macrophages are used, and in the later stages, a combination of liposomes and drugs targeting the ruptured edges of vulnerable plaques has been proven to be effective at low doses [17]. Liposomes are a type of traditional nanomedicine delivery carrier composed of a lipid bilayer. Depending on whether the interior of the liposome is lipophilic or hydrophilic, they can be used to carry lipophilic or water-soluble medicine, and cationic liposomes can also carry DNA, RNA, and other nucleic acids [18, 19]. The problem with exogenous nucleic acids, especially siRNA, miRNA, and other RNA molecules, is that they are quickly degraded during transport and cannot easily pass through the target cell membranes; however, packaging nucleic acids in liposomes helps to combat these barriers, allowing them to carry out functions inside the target cells [20].
An important mechanism that limits the use of nanocarrier liposomes to deliver traditional medicines is the human mononuclear phagocyte system (MPS), which recognizes NPs as foreign substances and engulfs them, altering the duration of drug retention in the blood and affecting drug distribution to tissues [21, 22]. The standard technique used to solve this problem is to modify the surface of NPs with polymers, such as polyethylene glycol (PEG), a process known as pegylation [23]. Because of the highly flexible and hydrophilic properties of PEG, the hydration layer formed by PEG can effectively reduce the adsorption of undesirable proteins on the surface, avoiding immune recognition and clearance, prolonging drug circulation, and enhancing targeted medicine delivery [24]. In response to the problem that pegylated drug-carrying NPs trigger an immune response and increase the risk of being cleared by the immune system [25], many biomimetic membranes, such as those of red blood cells, white blood cells, platelets, and endothelial cells, have been used to encapsulate NPs. The use of these membranes can avoid macrophage phagocytosis and improve the utilization rate of drugs, and many studies have shown that the therapeutic effect is better than that with pure PEG nanocarriers [26, 27]. In addition to liposomes, various micelles, such as chitosan and mannitol micelles; polymers, such as polyglycolic acid, poly lactic-co-glycolic acid (PLGA), and β-cyclodextrin; lipoproteins (natural and artificially modified); and other materials have been increasingly used in recent years for nanomedicine delivery. Nanocarriers of the same drug that act on different molecular targets, cells, or tissues can exert a variety of different effects in the body. Composite nanomaterials, which are used in multiple structures or material combinations for different drug applications, also provide additional strategies for the treatment of AS [28].
Targeting advanced atherosclerotic plaques with nanoparticles
The ongoing research and development of nanomaterials means that the approaches and mechanisms for targeted nanomedicine delivery to atherosclerotic plaques are continuously evolving (Fig. 1). However, the strategies can be summarized as two main types [29].
Fig. 1. Nanomedicine-based strategies for targeting advanced atherosclerotic plaques.
The nanoparticles (NPs) circulating in the blood enter plaques either through increased permeability (nonspecific targeting) or through specific receptors (e.g., vascular adhesion molecule 1 (VCAM-1), low-density lipoprotein (LDL) receptor and integrin) on the surface of vascular endothelial cells (specific targeting). After entering the plaque, some nanoparticles were targeted to the lesion through nonspecific targeting due to their own characteristics (such as the use of responsive nanomaterials to target the acidic or inflammatory microenvironments). Others specifically target certain cells (e.g., macrophages, endothelium and vascular smooth muscle cells) or the extracellular matrix, such as collagen, with targeting peptides on their surfaces.
The first is nonspecific targeting, also known as passive targeting. The tight connectivity of normal endothelial cells (<2 nm) limits the distribution of NPs, whereas the presence of large gaps in dysfunctional endothelial cells allows large molecules and NPs to seep out of local areas of plaque neovascularization. Arterial wall endodermal permeability increases in atherosclerotic lesions, allowing more lipoproteins and small particles, such as NPs, to migrate to the intimal layer [30]. Sites of neovascularization are vulnerable to leakage and to the enhanced permeability and retention effect (EPR effect), which leads to NP aggregation in the lesion [31]. The use of responsive nanomaterials to target the common microenvironments of different diseases is a nonspecific targeting method that has emerged in recent years and is achieving significant therapeutic effects in the fields of tumor [32] and cardiovascular treatment [33]. Dou et al. used β-cyclodextrin (β-CD)-based pH-reactive NP (Ac-bCD) and ROS-reactive NP (Ox-bCD) to encapsulate rapamycin (RAPA) to treat AS model mice; the therapeutic effect was found to be better than RAPA monotherapy and nonresponsive NP (PLGA)-wrapped RAPA [34].
The second is specific targeting, which is also known as active targeting. NPs bind to specific cells or molecules in the lesion through their surface ligands, and thus, the process of reaching the lesion site is considered active targeting. Scavenger receptors on the surface of macrophages (MSR-A, SR-BI, and CD-36), mannose receptors on the surface of macrophages (CD206), (VCAM-1) secreted by endothelial cells, a specific marker of neovascular endothelial cells in plaques known as integrin ανβ3, and type IV collagen exposed on the surface of advanced atherosclerotic plaques, as a result of endothelial connection disruption, can all be used as targets to direct nanomedicine into plaques, allowing it to play a local therapeutic role [35]. In addition, specific accumulation of therapeutic agents can also be achieved through the intrinsic targeting of natural NPs. Artificially modified lipoproteins retard the progression of AS by increasing serum APOA1 levels, reversing cholesterol transport, and regulating inflammation [36]. An siRNA for the PCSK9 gene encapsulated in a cationic liposome significantly reduced PCSK9 expression and plasma LDL-C levels [37].
Different strategies for stabilizing advanced atherosclerotic plaques
Regulation of lipid metabolism and reduction of lipid-rich plaques
As an independent risk factor for AS, we found that LDL deposition not only induces oxidative stress but also damages the endothelium [38], induces platelet activation and promotes the formation of vulnerable plaques, which is associated with the occurrence of adverse cardiovascular events, such as myocardial infarction (MI). In addition, NIRS was used to detect lipid-rich plaques associated with acute coronary syndrome and MI [3]. Our and many other studies have shown that cholesterol crystallization in plaques is also an important factor leading to plaque instability and local inflammation [39, 40]. Currently, commonly used lipid-lowering drugs, such as statins, can stabilize atherosclerotic plaques and reduce the incidence of MI. However, the residual risk associated with advanced AS plaques is still large, and the recurrence rate of cardiovascular events remains high [41]. Furthermore, side effects, such as liver damage, should not be ignored. The PCSK9-inhibitor evolocumab, a pro-protein converting enzyme, can significantly reduce circulating LDL cholesterol levels by reducing LDLR degradation and increasing LDL-C uptake; the enzyme has been approved for use in patients with familial hypercholesterolemia and cardiovascular disease who still require further LDL-C reduction at the maximum tolerated dose of statins [42]. In comparison, inclisiran, a novel PCSK9 inhibitor that silences the PCSK9 gene with siRNA, has a faster and more long-lasting therapeutic effect than evolocumab [43]. PCSK9 expression and plasma LDL-C levels were significantly reduced in high-risk cardiovascular patients in phase II clinical trials. The key to overcoming the difficulties with using siRNA, such as the impenetrability of cell membranes and the instability of exogenous RNA in the blood, is to coat the siRNA with lipidoids [44], as this facilitates their efficient delivery to the liver and inhibits the synthesis of PCSK9 in the liver.
Serum high-density lipoprotein (HDL) levels negatively correlate with the risk of coronary heart disease (CHD), mainly via reverse cholesterol transport (RCT), by preventing lipid accumulation by transporting excess cholesterol from surrounding cells and tissues to the liver for excretion and modulating inflammation to prevent cardiovascular disease. However, we found that normal anti-inflammatory HDL converts to proinflammatory HDL in AS, which not only loses the function of protecting blood vessels but also damages the vascular endothelium [16, 45, 46]. Therefore, cardiovascular events cannot be prevented by raising HDL cholesterol levels. The direct injection of recombinant HDL (rHDL) particles and boosting of APOA1 levels can have antiatherosclerosis effects. A recent work suggested the value of HDL-mimicking NPs for AS [47]. RHDL particles are self-assembled from phospholipids, cholesterol, and apo-AI, and those particles composed of different phospholipids have different inhibitory effects on the release of inflammatory factors, narrowing the aortic atherosclerotic plaque area, reducing the content of lipid-rich plaques, and delaying the progression of AS in ApoE−/− mice [48]. Several clinical studies also confirmed the efficacy of different types of rHDL for plaque regression and modification of the lipoprotein composition in patients [49, 50].
The cholesterol efflux of macrophages is the first and most important step of RCT and is related to the protective role of a unique form of selective autophagy called lipophagy against atherosclerosis in macrophages. Excess lipids in atherosclerotic plaques are carried to lysosomes and cleared by lipophagy [51]. Recently, researchers have used chitosan nanoparticles (chNPs) composed of PEG and chitosan polymers to transfer exogenous miR-33 into macrophages [52], reducing the expression of the target gene ABCA1 and the outflow of cholesterol from macrophages to apolipoprotein A1 (ApoA1). In contrast, chNPs coated with pro-exudative miRNAs, such as miR-206 and miR-223, had anti-AS effects in mice by upregulating ABCA1 expression and RCT. This confirms that miRNAs in NPs can be efficiently delivered to macrophages, where they can regulate ABCA1 expression and RCT. Recently, Guo et al. confirmed for the first time that selenium nanoparticles (SeNPs: synthesized from Na2SeO3 and other materials) regulate cholesterol metabolism through antioxidant selenium protein, reduce oxidative stress and therefore reduce hyperlipidemia and vascular damage in ApoE−/− mice on a high-cholesterol and high-fat diet [53]. SeNPs that focus on oxidative stress and treat AS through multiple pathways, such as metabolism and immunity, have promising potential applications for future AS treatment strategies.
Reduction in dead cell accumulation and plaque necrotic cores
The large necrotic core and thin-cap fibroatheromas (TCFAs) are key components of atherosclerotic vulnerable plaques. TCFAs with large necrotic cores are associated with poor prognosis in AS [9]. Reducing the size of the necrotic core or increasing the thickness of the fibrous cap undoubtedly increases the stability of advanced atherosclerotic plaques. The increased senescence and apoptosis of smooth muscle cells and the accumulation of oxLDL and other kinds of lipoproteins promoted the transformation of macrophages into foam cells, all of which increased the instability of late atherosclerotic plaques [54]. Macrophages with impaired function in advanced AS cannot clear a large number of dead cells that have engulfed lipids through efferocytosis, which results in the outflow of a large number of lipid necrotic cells and the infiltration of a large number of inflammatory cells. The anti-inflammatory cytokine interleukin-10 (IL-10) can block the accumulation of inflammatory cells and promote their excretion; remove pathogens, cell debris, and inflammatory cytokines; stimulate efferocytosis; and repair damaged tissues. Kamaly et al. developed a kind of sustained-release polymer NP (Col-IV IL-10 NP22) consisting of PEG and PLGA and containing IL-10. The NPs can enter atherosclerotic plaques through disturbed endothelial connections to bind with exposed type IV collagen (Col-IV) and release IL-10; they can be used to treat LDL−/− mice with advanced AS by increasing efferocytosis in plaques in the aortic root, increasing the thickness of the fibrous cap, and shrinking necrotic cores to reduce the formation of vulnerable plaques [55].
Autophagy plays a unique role in the delicate control of cell fate in the development of AS and neointimal hyperplasia in postpercutaneous coronary intervention restenosis [56]. In advanced AS, dysfunctional macroautophagy can make cells susceptible to apoptosis stimulation and impaired clearance of apoptotic cells, thus accelerating the progression of plaque [57]. RAPA, a targeted inhibitor of the mammalian target of RAPA (mTOR) pathway, induces autophagy to promote macrophage clearance, maintains plaque stability through autophagy-mediated cell death, reduces lipid accumulation, and reduces inflammation in plaques. Because of the side effects of the systemic administration of RAPA, such as hypertriglyceridemia, hypercholesterolemia, and interstitial lung disease, RAPA drug-eluting stents are currently used to reduce vascular inflammation and prevent restenosis after angioplasty by retarding vascular smooth muscle cell phenotype switching and hyperproliferation [58]. However, RAPA may cause systemic hyperlipidemia possibly because it suppresses LDL receptor expression in the liver. Furthermore, in advanced atherosclerosis plaques, excessive autophagy stimulation may present obstacles for the treatment of AS. Therefore, the optimal blood concentration of RAPA needs to be determined, and better delivery methods are needed to reduce systemic side effects. Studies have suggested that RAPA-containing liposomes have a high encapsulation rate of RAPA and a sustained-release effect of the release of RAPA [59]. Furthermore, numerous studies have demonstrated the antiatherogenic effectiveness of nanocarriers combined with RAPA in vivo; at the same time, there was no significant change in plasma lipoprotein and cholesterol and no significant adverse effects with long-term treatment [34, 60, 61].
Excess ROS can cause oxidative stress by acting as a signal to maintain physiological vascular homeostasis. Oxidative stress can promote the accumulation of dead cells in plaques by mediating the apoptosis of macrophage foam cells, thus causing secondary necrosis and increasing plaque instability [62]. Zhang et al. [33] modified β-cyclodextrin (β-CD) using a pinol hydroperoxide scavenging compound (a ROS response unit) of benzene borate and developed a broad-spectrum ROS scavenging nanomedicine system with antioxidant stress activity and a targeted effect, which was named TPCD NP. TPCD NP alleviated ROS-induced macrophage inflammation and apoptosis and effectively inhibited foam cell formation. Furthermore, TPCD NPs increased the stability of plaques in the aortic root of ApoE−/− mice fed a high-fat diet for 12 weeks, reduced cholesterol crystals in plaques, shrunk necrotic cores, reduced macrophage infiltration and MMP-9 expression in AS plaques, and increased the thickness of the fibrous cap.
Selective anti-inflammatory therapy
The inflammatory reaction is involved throughout the formation and development of AS. Macrophages and inflammatory factors play key roles in endothelial dysfunction, the lipid deposition of foam cells, and necrotic core formation. We found that reducing chronic inflammation and modulating immune system responses are likely to be the most promising therapeutic strategies, and they are currently the most popular targets for the treatment of AS [63, 64]. The Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) demonstrated that systemic anti-inflammatory therapy, i.e., interleukin-1β antibody canakinumab, reduced the incidence of cardiovascular disease in high-risk populations [65]. However, systemic targeted inflammation has the potential to inhibit innate immunity and disrupt host defenses against infection, and infections treated with anti-inflammatory therapy in the CANTOS trial had a higher mortality rate. Nanomedicine delivery can reduce systemic side effects by targeting anti-inflammatory agents locally. Methotrexate (MTX), a commonly used anti-inflammatory drug for autoimmune diseases, has been shown in recent years to reduce the risk of cardiovascular disease due to chronic inflammation. A previous clinical trial using a lipid nuclear nanoparticle (LDE) with a similar structure to LDL to carry paclitaxel (PTX) showed that these compounds reduce the toxicity of anticancer chemotherapeutic agents and have the potential to reduce the atherosclerotic lesion size in patients with cardiovascular disease [66]. Furthermore, Gomes et al. combined LDE-PTX treatment with methotrexate (MTX) associated with LDE (LDE-MTX) to treat atherosclerotic lesions in rabbits fed a high-fat diet and showed that the therapeutic effect of LDE-PTX was enhanced when combined with LDE-MTX [67]. However, the effectiveness of the treatment has yet to be confirmed in clinical trials. In addition to the delivery of anti-inflammatory drugs, NPs can be used to target tissues via their own anti-inflammatory properties. For AS, these properties can lead NPs to accumulate in the lesion to provide a therapeutic effect. Hyaluronic acid regulates cell adhesion, migration, and proliferation by binding to CD44 expressed on the surface of endothelial cells at the site of atherosclerotic inflammation. At sites of inflammation, the hyaluronic acid-rich microenvironment promotes tissue infiltration through the division of immune cells. Moreover, the biological characteristics of hyaluronic acid are largely determined by its polymerization, and highly polymerized hyaluronic acid has been proven to inhibit inflammation and have antiangiogenesis effects [68, 69]. Hyaluronic acid nanoparticles (HA-NPs) prepared with hyaluronic acid as the framework have been proven to have good plaque stabilization and anti-inflammatory effects in ApoE−/− mice [70]. Chronic inflammation leads to impaired endothelial function, and when overexpressed, activated adhesion molecules, such as VCAM-1 and ICAM-1, can bind to white blood cells and transfer directly to the vascular wall. Leukosomes, biomimetic NPs designed using this approach, can accumulate at the site of vascular injury by simulating the distribution of white blood cells in AS, deliver drugs to the lesion site and exert an anti-inflammatory effect on the vessel wall. For example, delivery of RAPA can treat AS by inhibiting the release of inflammatory factors, inhibiting the proliferation of macrophages, and narrowing the necrotic core of plaques [71]. This is one example of successful synergistic complementary treatment of a disease by combining the properties of NPs with the properties of drugs.
In recent years, our knowledge of plaque rupture mechanisms, including hemodynamic and physiological factors, such as shear stress and fluid dynamics, has increased. Endothelial shear stress caused by endothelial surface friction is closely related to the pathogenesis of AS, the formation of plaques, and the development of plaque vulnerability [40]. Laminar shear stress (LSS) induced by stable blood flow has anti-inflammatory and protective effects on AS. LSS rapidly stimulates the conformational activation of the shear force sensor integrin β3, inhibits downstream inflammatory signals, reduces mononuclear cell infiltration, and delays the occurrence of AS [72]. Gao et al. designed a type of MnO2 NP that accurately activates integrin β3. The biomimetic NPs form on the surface of endothelial cell membranes, preventing the removal of circulating NPs by the MPS; the biomimetic NPs, with reductive dissociation properties, have been shown to be effective high-efficiency integrin activators to inhibit downstream inflammatory signals and plaque formation [73].
Targeting neovascularization in plaques
Using OCT and IVUS to explore the characteristics of vulnerable plaques, we and other researchers have shown that patients with CHD with neovascularization in nonculprit plaques show more vulnerable OCT features [74, 75]. Moreover, plaques containing neovascularization are still at high risk even under statin therapy [74], and the multiple molecular targets of neovascularization in plaques highlight the need for nanomedicine therapy. VCAM-1, an adhesion molecule secreted by activated vascular endothelial cells at the site of atherosclerotic lesions, recruits inflammatory cells to the activated endothelial surface. Therefore, nanocarriers can target endothelial cells in AS plaques by carrying peptides specifically identified by VCAM-1 [76]. Kheirolomoom et al. modified the VHPK peptide on the surface of cationic liposomes containing anti-miR-712 molecules to construct a VHPK-CCL-anti-miR-712 NP that specifically targeted the aortic endothelial cells of mice. Furthermore, in vivo studies showed that selective and efficient silencing of inflammatory endothelial cells played an anti-AS role, and miRNA delivery was highly selective to the target organ [77].
Angiogenesis in atherosclerotic plaques is regulated by a variety of cytokines and platelet growth factors, one of which is vascular endothelial growth factor (VEGF). Anti-VEGF drugs and many other antiangiogenic drugs have been successfully used in a number of studies to combat tumor angiogenesis and extend the survival of patients with cancer [78]. In addition, integrin ανβ3 has long interested researchers as a target in atherosclerotic plaques, and the delivery of fumtrithromycin-NPs combined with atorvastatin to combat angiogenesis in plaques has the potential to substantially improve the stability of plaques [79]. However, whether the targeted inhibition of neovascularization in plaques in the early stages of AS can reduce the occurrence of adverse clinical events to improve the prognosis of atherosclerotic heart disease remains to be further studied and tested.
Future directions and challenges
The use of targeted NPs in the treatment of AS has become a hot topic and has provided many improved strategies and new research avenues for the treatment of patients with cardiovascular diseases. The precise therapeutic targets and the successful delivery of small molecules in vivo, even nucleic acids, provided by nanomedicine have created a potential therapeutic approach for AS that uses genetic information for accurate medicine and customized personalized therapy to improve the efficacy and reduce adverse reactions. For example, numerous genetic studies have demonstrated the role of target genes such as LDLR, APO-B, and PCSK9 and established the association between high LDL cholesterol levels and ASCVD [80]. In addition, a variety of miRNAs serve a vital cellular and molecular regulatory function in the formation and development of atherosclerotic plaques [81]. Using regulatory genes encapsulated in NPs to regulate the expression of target genes has been implemented in studies [37, 52]; perhaps we can modify NPs to carry out individualized treatment for different genetic backgrounds in the future. Therefore, nanomedicine-aided gene regulation is a potential therapeutic strategy in AS.
With the development of nanotechnology and the demand for effective treatments for cardiovascular diseases, a variety of new nanomaterials combined with different therapeutic drugs and new therapy targets are continuously being discovered and applied in animal models, many of which have shown potent and specific therapy effects, while some have been used in clinical trials for the treatment of AS [49, 50, 66]. Nanointervention with the delivery of silica–gold NPs has been proven to reduce total atheroma volume more in patients who have CAD with target lesions than in those who accepted stent implantation [82]. This study showed that the delivery of NPs with a bioengineered patch has a more pronounced effect and a more significant level of safety for AS, but the optimal technique of delivering NPs into the target tissue is still a key limitation. In addition, nanointervention is an invasive treatment. The feasibility and safety of NPs in clinical treatment have been preliminarily confirmed, but certain limitations remain in further clinical development with regard to their structural design, stability, targeting specificity, and toxicity. Therefore, to develop nanomedicine-based therapies as treatment methods that can be used in clinical practice, they need to be continuously optimized and subjected to medicine-based testing. Currently, nanotechnology has shown great potential for the diagnosis and treatment of cardiovascular diseases, and further research should focus on improving its stability and clinical translation effectiveness by increasing studies on the interaction between bionanomaterials and various components of plaques. As our knowledge increases and the therapeutic mechanisms involved are further explored and improved, nanomaterials have the potential to unlock an array of innovative research fields in the diagnosis and treatment of cardiovascular diseases.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (Grant Nos. 81971715 and 91739113 to JWT; 81770241, 81830013 and Distinguished Young Scholar Grant 81325001 to JSO), the International Cooperation project (2015DFA31070) from the Ministry of Science and Technology of China, the Guangdong Natural Science Fund Committee (Grant 2015A030312009), the Changjiang Scholars Program from the Ministry of Education of China, the Sun Yat-sen University Clinical Research 5010 Program, and the Program of National Key Clinical Specialties to JSO.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Le-chun Ou, Shan Zhong
Contributor Information
Jing-song Ou, Email: oujs@mail.sysu.edu.cn.
Jin-wei Tian, Email: tianjinweidr2009@163.com.
References
- 1.Dai J, Xing L, Hou J, Jia H, Hu S, Tian J, et al. Chronic kidney disease predicts coronary plaque vulnerability: an optical coherence tomography study. Coron Artery Dis. 2017;28:135–44. doi: 10.1097/MCA.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 2.Dong N, Xie Z, Dai J, Wang W, Sun R, Zhan Y, et al. Statin-induced improvements in vulnerable plaques are attenuated in poorly controlled diabetic patients with coronary atherosclerosis disease: a serial optical coherence tomography analysis. Acta Diabetol. 2016;53:999–1008. doi: 10.1007/s00592-016-0902-9. [DOI] [PubMed] [Google Scholar]
- 3.Waksman R, Di Mario C, Torguson R, Ali ZA, Singh V, Skinner WH, et al. Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: a prospective, cohort study. Lancet. 2019;394:1629–37. doi: 10.1016/S0140-6736(19)31794-5. [DOI] [PubMed] [Google Scholar]
- 4.Tian J, Dauerman H, Toma C, Samady H, Itoh T, Kuramitsu S, et al. Prevalence and characteristics of tcfa and degree of coronary artery stenosis: an OCT, IVUS, and angiographic study. J Am Coll Cardiol. 2014;64:672–80. doi: 10.1016/j.jacc.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 5.Tian J, Ren X, Vergallo R, Xing L, Yu H, Jia H, et al. Distinct morphological features of ruptured culprit plaque for acute coronary events compared to those with silent rupture and thin-cap fibroatheroma: a combined optical coherence tomography and intravascular ultrasound study. J Am Coll Cardiol. 2014;63:2209–16. doi: 10.1016/j.jacc.2014.01.061. [DOI] [PubMed] [Google Scholar]
- 6.Yan FX, Li HM, Li SX, He SH, Dai WP, Li Y, et al. The oxidized phospholipid povpc impairs endothelial function and vasodilation via uncoupling endothelial nitric oxide synthase. J Mol Cell Cardiol. 2017;112:40–8. doi: 10.1016/j.yjmcc.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Ou ZJ, Chen J, Dai WP, Liu X, Yang YK, Li Y, et al. 25-Hydroxycholesterol impairs endothelial function and vasodilation by uncoupling and inhibiting endothelial nitric oxide synthase. Am J Physiol Endocrinol Metab. 2016;311:E781–E790. doi: 10.1152/ajpendo.00218.2016. [DOI] [PubMed] [Google Scholar]
- 8.Que X, Hung MY, Yeang C, Gonen A, Prohaska TA, Sun X, et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature. 2018;558:301–6. doi: 10.1038/s41586-018-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arbab-Zadeh A, Fuster V. From detecting the vulnerable plaque to managing the vulnerable patient: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:1582–93. doi: 10.1016/j.jacc.2019.07.062. [DOI] [PubMed] [Google Scholar]
- 10.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 11.Ou J, Wang J, Xu H, Ou Z, Sorci-Thomas MG, Jones DW, et al. Effects of D-4F on vasodilation and vessel wall thickness in hypercholesterolemic LDL receptor-null and LDL receptor/apolipoprotein A-I double-knockout mice on western diet. Circ Res. 2005;97:1190–7. doi: 10.1161/01.RES.0000190634.60042.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian J, Hu S, Sun Y, Yu H, Han X, Cheng W, et al. Vasa vasorum and plaque progression, and responses to atorvastatin in a rabbit model of atherosclerosis: contrast-enhanced ultrasound imaging and intravascular ultrasound study. Heart. 2013;99:48–54. doi: 10.1136/heartjnl-2012-302775. [DOI] [PubMed] [Google Scholar]
- 13.Tian J, Hou J, Xing L, Kim SJ, Yonetsu T, Kato K, et al. Significance of intraplaque neovascularisation for vulnerability: optical coherence tomography study. Heart. 2012;98:1504–9. doi: 10.1136/heartjnl-2012-302445. [DOI] [PubMed] [Google Scholar]
- 14.Camare C, Pucelle M, Negre-Salvayre A, Salvayre R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017;12:18–34. doi: 10.1016/j.redox.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19:311–30. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 16.Chan CKW, Zhang L, Cheng CK, Yang H, Huang Y, Tian XY, et al. Recent advances in managing atherosclerosis via nanomedicine. Small. 2018;14:4. doi: 10.1002/smll.201702793. [DOI] [PubMed] [Google Scholar]
- 17.Kiaie N, Gorabi AM, Penson PE, Watts G, Johnston TP, Banach M, et al. A new approach to the diagnosis and treatment of atherosclerosis: the era of the liposome. Drug Disco Today. 2020;25:58–72. doi: 10.1016/j.drudis.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Xu P, Li SY, Li Q, Ren J, Van Kirk EA, Murdoch WJ, et al. Biodegradable cationic polyester as an efficient carrier for gene delivery to neonatal cardiomyocytes. Biotechnol Bioeng. 2006;95:893–903. doi: 10.1002/bit.21036. [DOI] [PubMed] [Google Scholar]
- 19.Schiener M, Hossann M, Viola JR, Ortega-Gomez A, Weber C, Lauber K, et al. Nanomedicine-based strategies for treatment of atherosclerosis. Trends Mol Med. 2014;20:271–81. doi: 10.1016/j.molmed.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Xu P, Li SY, Li Q, Van Kirk EA, Ren J, Murdoch WJ, et al. Virion-mimicking nanocapsules from ph-controlled hierarchical self-assembly for gene delivery. Angew Chem Int Ed Engl. 2008;47:1260–4. doi: 10.1002/anie.200703203. [DOI] [PubMed] [Google Scholar]
- 21.Sun W, Hu Q, Ji W, Wright G, Gu Z. Leveraging physiology for precision drug delivery. Physiol Rev. 2017;97:189–225. [Google Scholar]
- 22.Matsumoto Y, Nichols JW, Toh K, Nomoto T, Cabral H, Miura Y, et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat Nanotechnol. 2016;11:533–8. doi: 10.1038/nnano.2015.342. [DOI] [PubMed] [Google Scholar]
- 23.Xu P, Tang H, Li S, Ren J, Van Kirk E, Murdoch WJ, et al. Enhanced stability of core-surface cross-linked micelles fabricated from amphiphilic brush copolymers. Biomacromolecules. 2004;5:1736–44. doi: 10.1021/bm049874u. [DOI] [PubMed] [Google Scholar]
- 24.Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. Pegylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(Pt A):28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Q, Lai SK. Anti-peg immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:655–77. doi: 10.1002/wnan.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhen X, Cheng P, Pu K. Recent advances in cell membrane-camouflaged nanoparticles for cancer phototherapy. Small. 2019;15:e1804105. doi: 10.1002/smll.201804105. [DOI] [PubMed] [Google Scholar]
- 27.Ai X, Hu M, Wang Z, Zhang W, Li J, Yang H, et al. Recent advances of membrane-cloaked nanoplatforms for biomedical applications. Bioconjug Chem. 2018;29:838–51. doi: 10.1021/acs.bioconjchem.8b00103. [DOI] [PubMed] [Google Scholar]
- 28.Distasio N, Lehoux S, Khademhosseini A, Tabrizian M. The multifaceted uses and therapeutic advantages of nanoparticles for atherosclerosis research. Materials. 2018;11:E754. doi: 10.3390/ma11050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim KS, Song CG, Kang PM. Targeting oxidative stress using nanoparticles as a theranostic strategy for cardiovascular diseases. Antioxid Redox Signal. 2019;30:733–46. doi: 10.1089/ars.2017.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y, Lobatto ME, Kawahara T, Lee Chung B, Mieszawska AJ, Sanchez-Gaytan BL, et al. Probing nanoparticle translocation across the permeable endothelium in experimental atherosclerosis. Proc Natl Acad Sci USA. 2014;111:1078–83. doi: 10.1073/pnas.1322725111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lobatto ME, Calcagno C, Millon A, Senders ML, Fay F, Robson PM, et al. Atherosclerotic plaque targeting mechanism of long-circulating nanoparticles established by multimodal imaging. ACS Nano. 2015;9:1837–47. doi: 10.1021/nn506750r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu B, Chen H, Li X, Zhao C, Liu Y, Zhu L, et al. PH-responsive flower-like micelles constructed via oxime linkage for anticancer drug delivery. RSC Adv. 2014;4:48943–51. [Google Scholar]
- 33.Wang Y, Li L, Zhao W, Dou Y, An H, Tao H, et al. Targeted therapy of atherosclerosis by a broad-spectrum reactive oxygen species scavenging nanoparticle with intrinsic anti-inflammatory activity. ACS Nano. 2018;12:8943–60. doi: 10.1021/acsnano.8b02037. [DOI] [PubMed] [Google Scholar]
- 34.Dou Y, Chen Y, Zhang X, Xu X, Chen Y, Guo J, et al. Non-proinflammatory and responsive nanoplatforms for targeted treatment of atherosclerosis. Biomaterials. 2017;143:93–108. doi: 10.1016/j.biomaterials.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Koradia A, Kamato D, Popat A, Little PJ, Ta HT. Treatment of atherosclerotic plaque: Perspectives on theranostics. J Pharm Pharmacol. 2019;71:1029–43. doi: 10.1111/jphp.13092. [DOI] [PubMed] [Google Scholar]
- 36.Kornmueller K, Vidakovic I, Prassl R. Artificial high density lipoprotein nanoparticles in cardiovascular research. Molecules. 2019;24:E2829. doi: 10.3390/molecules24152829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray KK, Stoekenbroek RM, Kallend D, Leiter LA, Landmesser U, Wright RS, et al. Effect of an sirna therapeutic targeting PCSK9 on atherogenic lipoproteins: prespecified secondary end points in orion 1. Circulation. 2018;138:1304–16. doi: 10.1161/CIRCULATIONAHA.118.034710. [DOI] [PubMed] [Google Scholar]
- 38.Stepp DW, Ou J, Ackerman AW, Welak S, Klick D, Pritchard KA., Jr Native LDL and minimally oxidized LDL differentially regulate superoxide anion in vascular endothelium in situ. Am J Physiol Heart Circ Physiol. 2002;283:H750–759. doi: 10.1152/ajpheart.00029.2002. [DOI] [PubMed] [Google Scholar]
- 39.Dai J, Tian J, Hou J, Xing L, Liu S, Ma L, et al. Association between cholesterol crystals and culprit lesion vulnerability in patients with acute coronary syndrome: an optical coherence tomography study. Atherosclerosis. 2016;247:111–7. doi: 10.1016/j.atherosclerosis.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Lee KY, Chang K. Understanding vulnerable plaques: current status and future directions. Korean Circ J. 2019;49:1115–22. doi: 10.4070/kcj.2019.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nilsson J. Atherosclerotic plaque vulnerability in the statin era. Eur Heart J. 2017;38:1638–44. doi: 10.1093/eurheartj/ehx143. [DOI] [PubMed] [Google Scholar]
- 42.Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–9. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 43.Lin XL, Xiao LL, Tang ZH, Jiang ZS, Liu MH. Role of PCSK9 in lipid metabolism and atherosclerosis. Biomed Pharmacother. 2018;104:36–44. doi: 10.1016/j.biopha.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 44.Whitehead KA, Dorkin JR, Vegas AJ, Chang PH, Veiseh O, Matthews J, et al. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat Commun. 2014;5:4277. doi: 10.1038/ncomms5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ou J, Ou Z, Jones DW, Holzhauer S, Hatoum OA, Ackerman AW, et al. L-4f, an apolipoprotein A-1 mimetic, dramatically improves vasodilation in hypercholesterolemia and sickle cell disease. Circulation. 2003;107:2337–41. doi: 10.1161/01.CIR.0000070589.61860.A9. [DOI] [PubMed] [Google Scholar]
- 46.Ou ZJ, Li L, Liao XL, Wang YM, Hu XX, Zhang QL, et al. Apolipoprotein A-I mimetic peptide inhibits atherosclerosis by altering plasma metabolites in hypercholesterolemia. Am J Physiol Endocrinol Metab. 2012;303:E683–694. doi: 10.1152/ajpendo.00136.2012. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Zhang X, Millican R, Creutzmann JE, Martin S, Jun HW. High density lipoprotein mimicking nanoparticles for atherosclerosis. Nano Converg. 2020;7:6. doi: 10.1186/s40580-019-0214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwendeman A, Sviridov DO, Yuan W, Guo Y, Morin EE, Yuan Y, et al. The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti-inflammatory properties. J Lipid Res. 2015;56:1727–37. doi: 10.1194/jlr.M060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kempen HJ, Gomaraschi M, Simonelli S, Calabresi L, Moerland M, Otvos J, et al. Persistent changes in lipoprotein lipids after a single infusion of ascending doses of MDCO-216 (apoa-IMilano/POPC) in healthy volunteers and stable coronary artery disease patients. Atherosclerosis. 2016;255:17–24. doi: 10.1016/j.atherosclerosis.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 50.Bisgaier CL, Ackermann R, Rea T, Rodrigueza WV, Hartman D. ApoA-IMilano phospholipid complex (ETC-216) infusion in human volunteers. Insights into the phenotypic characteristics of ApoA-IMilano carriers. Pharmacol Res. 2016;111:86–99. doi: 10.1016/j.phrs.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Yang M, Zhang Y, Ren J. Autophagic regulation of lipid homeostasis in cardiometabolic syndrome. Front Cardiovasc Med. 2018;5:38. doi: 10.3389/fcvm.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen MA, Wyatt H, Susser L, Geoffrion M, Rasheed A, Duchez AC, et al. Delivery of microRNAs by chitosan nanoparticles to functionally alter macrophage cholesterol efflux in vitro and in vivo. ACS Nano. 2019;13:6491–505. doi: 10.1021/acsnano.8b09679. [DOI] [PubMed] [Google Scholar]
- 53.Guo L, Xiao J, Liu H, Liu H. Selenium nanoparticles alleviate hyperlipidemia and vascular injury in ApoE-deficient mice by regulating cholesterol metabolism and reducing oxidative stress. Metallomics. 2020;12:204–17. doi: 10.1039/c9mt00215d. [DOI] [PubMed] [Google Scholar]
- 54.Zhu H, Wang Z, Dong Z, Wang C, Cao Q, Fan F, et al. Aldehyde dehydrogenase 2 deficiency promotes atherosclerotic plaque instability through accelerating mitochondrial ROS-mediated vascular smooth muscle cell senescence. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1782–92. doi: 10.1016/j.bbadis.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 55.Kamaly N, Fredman G, Fojas JJ, Subramanian M, Choi WI, Zepeda K, et al. Targeted interleukin-10 nanotherapeutics developed with a microfluidic chip enhance resolution of inflammation in advanced atherosclerosis. ACS Nano. 2016;10:5280–92. doi: 10.1021/acsnano.6b01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu H, Zhang Y. Life and death partners in post-pci restenosis: apoptosis, autophagy, and the cross-talk between them. Curr Drug Targets. 2018;19:1003–8. doi: 10.2174/1389450117666160625072521. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Sowers JR, Ren J. Targeting autophagy in obesity: from pathophysiology to management. Nat Rev Endocrinol. 2018;14:356–76. doi: 10.1038/s41574-018-0009-1. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, Yang F, Zou S, Qu L. Rapamycin: A bacteria-derived immunosuppressant that has anti-atherosclerotic effects and its clinical application. Front Pharmacol. 2018;9:1520. doi: 10.3389/fphar.2018.01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miao ZL, Deng YJ, Du HY, Suo XB, Wang XY, Wang X, et al. Preparation of a liposomal delivery system and its in vitro release of rapamycin. Exp Ther Med. 2015;9:941–6. doi: 10.3892/etm.2015.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Zhang K, Qin X, Li T, Qiu J, Yin T, et al. Biomimetic nanotherapies: red blood cell based core-shell structured nanocomplexes for atherosclerosis management. Adv Sci. 2019;6:1900172. doi: 10.1002/advs.201900172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song Y, Huang Z, Liu X, Pang Z, Chen J, Yang H, et al. Platelet membrane-coated nanoparticle-mediated targeting delivery of rapamycin blocks atherosclerotic plaque development and stabilizes plaque in apolipoprotein E-deficient (Apoe−/−) mice. Nanomedicine. 2019;15:13–24. doi: 10.1016/j.nano.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 62.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–55. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liang SJ, Zeng DY, Mai XY, Shang JY, Wu QQ, Yuan JN, et al. Inhibition of orai1 store-operated calcium channel prevents foam cell formation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:618–28. doi: 10.1161/ATVBAHA.116.307344. [DOI] [PubMed] [Google Scholar]
- 64.Su Y, Yuan J, Zhang F, Lei Q, Zhang T, Li K, et al. MicroRNA-181a-5p and microRNA-181a-3p cooperatively restrict vascular inflammation and atherosclerosis. Cell Death Dis. 2019;10:365. doi: 10.1038/s41419-019-1599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thompson PL, Nidorf SM. Anti-inflammatory therapy with canakinumab for atherosclerotic disease: lessons from the CANTOS trial. J Thorac Dis. 2018;10:695–8. doi: 10.21037/jtd.2018.01.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shiozaki AA, Senra T, Morikawa AT, Deus DF, Paladino-Filho AT, Pinto IM, et al. Treatment of patients with aortic atherosclerotic disease with paclitaxel-associated lipid nanoparticles. Clinics. 2016;71:435–9. doi: 10.6061/clinics/2016(08)05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomes FLT, Maranhao RC, Tavares ER, Carvalho PO, Higuchi ML, Mattos FR, et al. Regression of atherosclerotic plaques of cholesterol-fed rabbits by combined chemotherapy with paclitaxel and methotrexate carried in lipid core nanoparticles. J Cardiovasc Pharmacol Ther. 2018;23:561–9. doi: 10.1177/1074248418778836. [DOI] [PubMed] [Google Scholar]
- 68.Ossipov DA. Hyaluronan-based delivery of therapeutic oligonucleotides for treatment of human diseases. Expert Opin Drug Deliv. 2019;16:621–37. doi: 10.1080/17425247.2019.1617693. [DOI] [PubMed] [Google Scholar]
- 69.Yao SY, Shen ML, Li SJ, Wu XD, Zhang MM, Ma LN, et al. Application of a mechanically responsive, inflammatory macrophage-targeted dual-sensitive hydrogel drug carrier for atherosclerosis. Colloids Surf B Biointerfaces. 2020;186:110718. doi: 10.1016/j.colsurfb.2019.110718. [DOI] [PubMed] [Google Scholar]
- 70.Beldman TJ, Senders ML, Alaarg A, Perez-Medina C, Tang J, Zhao Y, et al. Hyaluronan nanoparticles selectively target plaque-associated macrophages and improve plaque stability in atherosclerosis. ACS Nano. 2017;11:5785–99. doi: 10.1021/acsnano.7b01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boada C, Zinger A, Tsao C, Zhao P, Martinez JO, Hartman K, et al. Rapamycin-loaded biomimetic nanoparticles reverse vascular inflammation. Circ Res. 2020;126:25–37. doi: 10.1161/CIRCRESAHA.119.315185. [DOI] [PubMed] [Google Scholar]
- 72.Wang L, Luo JY, Li B, Tian XY, Chen LJ, Huang Y, et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016;540:579–82. doi: 10.1038/nature20602. [DOI] [PubMed] [Google Scholar]
- 73.Gao W, Yang H, Liu X, Liu Z, Tong L, Sun Y, et al. Reductively dissociable biomimetic nanoparticles for control of integrin-coupled inflammatory signaling to retard atherogenesis. Chem Commun. 2019;55:11535–8. doi: 10.1039/c9cc06039a. [DOI] [PubMed] [Google Scholar]
- 74.Liu X, Sun C, Gu X, Liu X, Wang X, Wang X, et al. Intraplaque neovascularization attenuated statin benefit on atherosclerotic plaque in cad patients: a follow-up study with combined imaging modalities. Atherosclerosis. 2019;287:134–9. doi: 10.1016/j.atherosclerosis.2019.06.912. [DOI] [PubMed] [Google Scholar]
- 75.Papaioannou TG, Kalantzis C, Katsianos E, Sanoudou D, Vavuranakis M, Tousoulis D. Personalized assessment of the coronary atherosclerotic arteries by intravascular ultrasound imaging: Hunting the vulnerable plaque. J Pers Med. 2019;9:E8. doi: 10.3390/jpm9010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bala G, Blykers A, Xavier C, Descamps B, Broisat A, Ghezzi C, et al. Targeting of vascular cell adhesion molecule-1 by 18F-labelled nanobodies for PET/CT imaging of inflamed atherosclerotic plaques. Eur Heart J Cardiovasc Imaging. 2016;17:1001–8. doi: 10.1093/ehjci/jev346. [DOI] [PubMed] [Google Scholar]
- 77.Kheirolomoom A, Kim CW, Seo JW, Kumar S, Son DJ, Gagnon MK, et al. Multifunctional nanoparticles facilitate molecular targeting and miRNA delivery to inhibit atherosclerosis in ApoE−/− mice. ACS Nano. 2015;9:8885–97. doi: 10.1021/acsnano.5b02611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen J, Sun X, Shao R, Xu Y, Gao J, Liang W. Vegf sirna delivered by polycation liposome-encapsulated calcium phosphate nanoparticles for tumor angiogenesis inhibition in breast cancer. Int J Nanomed. 2017;12:6075–88. doi: 10.2147/IJN.S142739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Winter PM, Caruthers SD, Zhang H, Williams TA, Wickline SA, Lanza GM. Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. JACC Cardiovasc Imaging. 2008;1:624–34. doi: 10.1016/j.jcmg.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lange LA, Hu Y, Zhang H, Xue C, Schmidt EM, Tang ZZ, et al. Whole-exome sequencing identifies rare and low-frequency coding variants associated with LDL cholesterol. Am J Hum Genet. 2014;94:233–45. doi: 10.1016/j.ajhg.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andreou I, Sun X, Stone PH, Edelman ER, Feinberg MW. MiRNAs in atherosclerotic plaque initiation, progression, and rupture. Trends Mol Med. 2015;21:307–18. doi: 10.1016/j.molmed.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kharlamov AN, Tyurnina AE, Veselova VS, Kovtun OP, Shur VY, Gabinsky JL. Silica-gold nanoparticles for atheroprotective management of plaques: results of the NANOM-FIM trial. Nanoscale. 2015;7:8003–15. doi: 10.1039/c5nr01050k. [DOI] [PubMed] [Google Scholar]