Abstract
Tumor cells form immune escape and subsequently obtain unlimited proliferation ability due to the abnormal immune surveillance mediated by immune checkpoints. Among this class of immune checkpoints, PD-1/PD-L1 was recognized as an anticancer drug target for many years, and so far, several monoclonal antibodies have achieved encouraging outcome in cancer treatment by targeting the PD-1/PD-L1 signaling pathway. Due to the inherent limitations of antibodies, the development of small molecule inhibitors based on PD-1/PD-L1 signaling pathway is gradually reviving in decades. In this review, we summarized a number of small molecule inhibitors based on three different therapeutic approaches interfering PD-1/PD-L1 signaling pathway: (1) blocking direct interaction between PD-1 and PD-L1; (2) inhibiting transcription and translation of PD-L1; and (3) promoting degradation of PD-L1 protein. The development of these small molecule inhibitors opens a new avenue for tumor immunotherapy based on PD-1/PD-L1 signaling pathway.
Keywords: immune checkpoints, PD-1/PD-L1, small molecule inhibitors, cancer immunotherapy
Introduction
Immune checkpoint molecules work as protective factors for the body's immune system, for instance, cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), cluster of differentiation 28 (CD28), T cell immunoglobulin and mucin domain 3 (TIM-3), galectin-9 (Gal-9), programmed cell death-1 (PD-1), and programmed cell death-ligand 1 (PD-L1) can regulate the immune system to an appropriate intensity to avoid autoimmune responses caused by excessive activated immune cells [1]. However, when immune checkpoint molecules are overexpressed or overactivated, immune function is inhibited. By taking advantage of this phenomenon, tumor cells that excessively activate immune checkpoint molecules prevent local immune cells from escaping surveillance and clearance, thus accelerating tumor growth.
The PD-1/PD-L1 signaling pathway was discovered relatively early. In the tumor microenvironment, activated T cells express high levels of PD-1, which induce the expression of PD-L1 in local tissues by causing the release of cytokines, such as interferon gamma (IFN-γ), interleukins (ILs), and tumor necrosis factor-α (TNF-α) [2]. Upregulated PD-L1 prevents excessive activation of T cells, maintains the immune system’s tolerance to self-antigens, and reduces the immune response against the surrounding normal tissue after combining with PD-1 on T cells via protein–protein interactions. Therefore, blocking the interaction of PD-1 and PD-L1 can reverse immunosuppressive conditions and improve the killing of tumor cells by the body’s immune cells. In 2014, nivolumab, the first PD-1 monoclonal antibody drug, was approved to treat melanoma [3, 4]. In recent years, anti-PD-L1 monoclonal antibodies, such as atezolizumab [5], avelumab [6, 7], and durvalumab [7, 8], have also shown positive responses in clinical trials for a variety of malignancies, including melanoma, metastatic non-small-cell lung cancer, bladder cancer, and skin Merkel cell carcinoma (Table 1).
Table 1.
Inhibitors of PD-1/PD-L1 signaling pathway
| Name | Classification | Target | Research stage | Indications |
|---|---|---|---|---|
| Nivolumab [2, 3] | Antibody drugs | PD-1 | On the market | NSCLC |
| Atezolizumab [4] | PD-L1 | On the market | Bladder cancer | |
| Avelumab [5, 6] | PD-L1 | On the market | Skin merkel cell carcinoma | |
| Durvalumab [6, 7] | PD-L1 | On the market | NSCLC | |
| AUNP-12 [10] | Small molecule drugs | The binding of PD-1 to PD-L1 | In preclinical research | Melanoma |
| DPPA-1 [37] | In preclinical research | Colon cancer | ||
| TPP-1 [38] | In preclinical research | LCLC | ||
| BMS-202 [11, 12] | In preclinical research | |||
| CA-170 [44] | In clinical research | NSCLC | ||
| JQ1 [52] | Small molecule drugs | The expression of PD-L1 | In clinical research | Lymphoma |
| eFT508 [57] | In clinical research | Liver cancer | ||
| Osimertinib [61] | In preclinical research | NSCLC | ||
| PlatycodinD [62] | In preclinical research | NSCLC | ||
| PD-LYLSO [71] | Small molecule drugs | The degradation of PD-L1 | In preclinical research | – |
| Curcumin [72] | In preclinical research | Breast cancer | ||
| Metformin [73–75] | In preclinical research | Breast cancer |
However, antibody drugs are associated with several disadvantages, such as immunogenicity issues and the poor permeability of tumor tissues, which lead to the overall low response rate of PD-1/PD-L1 antibody drugs [9]. Tumor cells continually activate the PD-1/PD-L1 signaling pathway by overexpressing PD-L1 to trigger multiple immune suppression mechanisms. From this perspective, this binding can also be interrupted by inhibiting the expression of PD-L1 or promoting its degradation. Increasing research is devoted to intervening in the PD-1/PD-L1 signaling pathway by applying small molecule compounds such as peptides and peptidomimetics to address this problem. The first multipeptide human PD-1 (hPD-1) inhibitor, AUNP-12, reported in 2014, was jointly developed by Aurigene and Pierre Fabre Laboratories in India [10]. Bristol-Myers squibb (BMS) and Curis Pharmaceuticals have developed BMS-202 and CA-170, which interrupt the interaction of PD-1 and PD-L1 [11, 12]. Currently, these small molecular compounds are in the preclinical research stage. In this review, we first describe the formation of tumor escape mechanisms based on immune checkpoints and briefly summarize the efficacy and limitations of anti-PD-1/PD-L1 monoclonal antibodies and the necessity to develop small molecule drugs. Finally, we introduce small molecule drugs based on three different therapeutic methods of targeting the PD-1/PD-L1 signaling pathway.
Immune escape mechanisms based on the PD-1/PD-L1 signaling pathway
Under normal conditions, the body's immune system has an immune surveillance function. When malignant cells appear, the immune system can specifically recognize and remove these “nonself” cells to prevent tumor growth. However, in some cases, malignant cells prohibit immune responses against tumors by upregulating immunosuppressive molecules or downregulating immune-activated molecules, thereby achieving immune escape and immortalization [13]. PD-1/PD-L1 has been the most studied negative regulatory immune checkpoint-related axis in recent years and plays a prominent role in tumor immune escape (Fig. 1). PD-1, consisting of 288 amino acid residues, also known as PDCD1 and CD279, is a type I transmembrane protein and belongs to the B7/CD28 receptor superfamily [14, 15]. PD-L1 (CD274) and PD-L2 (CD273) are two ligands of PD-1 consisting of 290 and 270 amino acid residues, respectively, which are also type I transmembrane proteins and belong to the B7/CD28 family, and PD-L1 and PD-L2 have 37% sequence homology. PD-L1 has a wider range of expression than PD-L2, resulting in PD-L1 playing a major part in tumor cell immune escape [16, 17].
Fig. 1.
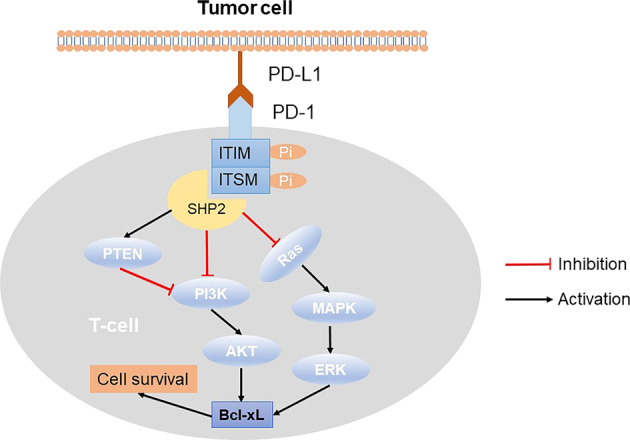
Immune checkpoint PD-1/PD-L1 signaling pathway. Binding of PD-1 and PD-L1 causes phosphorylation of the ITIMs and ITSMs in the intracellular domain of PD-1, which recruits tyrosine acid phosphatase SHP-2 to suppress PI3K/AKT, Ras/MAPK/ERK signaling pathway, ultimately leading to death of T cell
The structure of PD-1 consists of four parts: an immunoglobulin variable region (IgV), a transmembrane region, immunoreceptor tyrosine-based inhibitory motifs (ITIMs), and immunoreceptor tyrosine-based switch motifs (ITSMs) [14, 18]. The interaction of PD-1 and PD-L1 induces ITIMs and ITSMs to be phosphorylated in the intracellular domain of PD-1, which recruits tyrosine acid phosphatase Src homology phosphatase 1 (SHP-1) and Src homology phosphatase 2 (SHP-2) [18, 19]. These phosphatases dephosphorylates several key proteins in the T cell antigen receptor (TCR) signaling pathway and repress signaling pathways downstream of the TCR, such as phosphoinositide 3-kinase (PI3K), protein kinase B (PKB/AKT), mammalian target of rapamycin (mTOR), rat sarcoma (RAS), mitogen-activated protein kinase (MAPK/MEK), extracellular regulated protein kinase (ERK), etc. In turn, it inhibits the transcription of related genes, hinders the progression of T cell cycle and the expression of related proteins, and ultimately blunts the production of cytokines and the proliferation and differentiation of T cells, causing their immune function to be lost [20–22].
From antibodies to small molecule inhibitors
Since 2014, a variety of anti-PD-1/PD-L1 monoclonal antibody drugs have been approved by the FDA, and these monoclonal antibody drugs have made much progress in the clinical treatment of various tumors. The survival of many cancer patients is dramatically prolonged, and some patients even have complete remission. However, some studies have indicated that it is impossible for macromolecular antibody drugs to effectively penetrate tumor tissues and reach all regions of the tumor to accumulate at a sufficient concentration [23]. Furthermore, the immunogenicity of antibody drugs will induce the body to produce anti-antibodies and lead to the loss of efficacy [9]. In the meantime, the destruction of immune system function leads to an imbalance in immune tolerance that subsequently results in unsuppressed immune responses, which may be clinically manifested as autoimmune side effects that cause collateral damage to normal organ systems and tissues, including liver, gastrointestinal tract, lung, skin, and endocrine system [24]. In the dermatological safety analysis of melanoma patients, skin toxicity was observed in 34% of patients treated with nivolumab and 39% of patients treated with pembrolizumab [25, 26]. In addition, clinical follow-up data show that not all patients are sensitive to monoclonal antibody therapy. In melanoma treatment, PD-1 antibodies exhibited a 50% response rate, but the overall response rate for other solid tumors was generally low, ~15%–20% [27]. Monoclonal antibodies are difficult to produce, expensive and inconvenient to store and transport, which might limit the clinical application of PD-1/PD-L1 antibody drugs.
In contrast, small molecule inhibitors have significant advantages in dealing with these problems compared to monoclonal antibodies. Small molecule inhibitors are more suitable for oral administration and can reduce the target occupancy time by regulating the half-life of the drug, thereby avoiding the occurrence of serious immune-related adverse events. Moreover, easy transportation and storage, stability, superior membrane permeability, and other features make small molecule inhibitors favorable for clinical treatments in the future [28]. Current preclinical studies reveal that small molecule compounds have a better capability than antibodies to repress tumor growth and migration, and their biosafety is favorable [29]. Although the development of small molecule inhibitors lags behind that of antibody drugs, the controllable pharmacokinetic characteristics and mature development pipeline associated with small molecule inhibitors may overcome the existing problems of antibody drugs and allow them to replace monoclonal antibodies or serve as complementary therapies. Therefore, it is urgent to look for small molecule inhibitors targeting the PD-1/PD-L1-signaling pathway to improve tumor immunotherapy.
Small molecule inhibitors based on the PD-1/PD-L1 signaling pathway
Blocking the binding of PD-1 and PD-L1
PD-L1 is mainly made up of three parts: a short cytoplasmic tail region, a transmembrane region, and an IgV and IgC-like extracellular domain. In 2015, the crystal structural of the PD-1/PD-L1 binding interaction in humans was deciphered, and the molecules were found to bind at a 1:1 ratio [30]. The interaction pattern is very similar to that of the T cell receptor and the IgV-type region of an antibody, which is mediated by the GFCC′ beta sheet positivity in the protein–protein interaction region [31]. When PD-1 binds to PD-L1, its CC′ loop at Met70-Asp77 will be rotated 90° from the “open” state, beyond the binding site, and converted to a “closed” state. This change gives rise to four pairs of hydrogen bonds between PD-1 and the PD-L1 heterodimer (Gln75 of PD-1 forms three pairs of hydrogen bonds with Asp26 and Arg125 of PD-L1, while Thr76 of PD-1 and Tyr123 of PD-L1 form a pair). These bonds provide a structural biological foundation for the discovery of small molecule inhibitors aiming to block the link between PD-1 and PD-L1. It has also been pointed out that there are three key sites on the surface of the human PD-L1 protein [31]. The hydrophobic pocket formed by Ile134 and Ile126 can be combined with the six-membered aromatic ring and the aliphatic hydrocarbon branch, respectively, which are considered to be the binding sites of small molecule drugs. However, the research also shows that after PD-1 and PD-L1 form a dimer, hydrophobic and polar interactions exist at the interface between them, and some residues continue to form hydrogen bonds due to the transformation of the PD-1 spatial conformation, which increases the design difficulty of small molecule inhibitors for blocking this interaction.
Peptide-based small molecule inhibitors
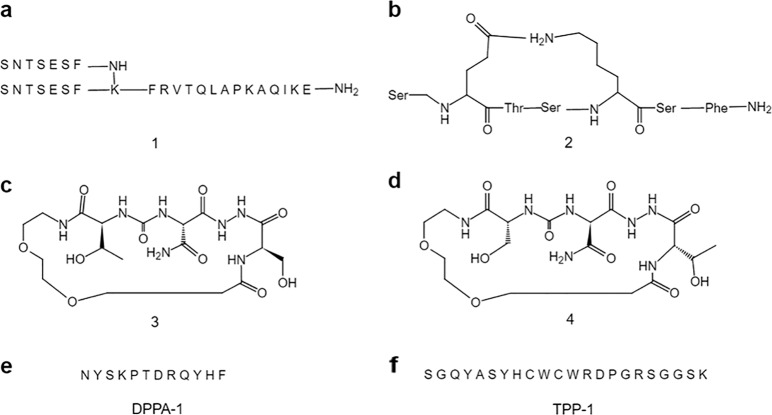
AUNP-12 is a branched 29 amino acid peptide that can be engineered to contain some sequences of the extracellular PD-1-binding domain of the human PD-1 protein [10]. This inhibitor is expected to bind PD-1 and inhibit the interaction between PD-1 and PD-L1. It is said that it can inhibit the growth and metastasis of primary tumors and maintain antitumor immune activity for at least 24 h with minimal toxicity. AUNP-12 is able to effectively control the occurrence of immunotherapy-related adverse events (irAEs) due to its metabolic half-life, which is shorter than that of one monoclonal antibody reported in 2014. In human PD-L2 (hPD-L2)-expressing HEK293 cells, a binding assay showed that AUNP-12 was able to interrupt the binding of PD-1 to PD-L2, and the EC50 reached 0.72 nmol/L. Furthermore, the EC50 was 0.41 nmol/L in an experiment to rescue peripheral blood mononuclear cell proliferation [10]. Animal experiments show that AUNP-12 has promising anti-PD-L1 activity and can effectively inhibit the growth and metastasis of tumor cells. AUNP-12 has promising biosafety and does not show significant adverse reactions at any dose. The specific structure of the compound has not been disclosed, and the possible structure of AUNP-12 is speculated to be similar to that of compound 1 according to the related patent disclosed by the company (Fig. 2a). On the basis of the findings with AUNP-12, researchers have generated additional compounds. In 2015, Aurigene developed a class of cyclic peptide compounds (Fig. 2). In the mouse spleen cell fluorescent dye proliferation assay, compound 2 could induce the proliferation of mouse spleen cells. Moreover, compound 2 significantly inhibited tumor metastasis and reduced the incidence of tumor metastasis by 54% in the high-metastatic B16F10 mouse melanoma subcutaneous xenograft model [32, 33]. In addition, in the presence of recombinant mouse PD-L1, when a test compound with a concentration of 100 nmol/L is added to mouse spleen cells stimulated by anti-CD3/CD28 antibody (1 μg/mL), the rescue capability of the compound can be evaluated, and the rescue rates of compounds 3 and 4 in mouse spleen cells reached 95% and 94%, respectively [34, 35].
Fig. 2.
Peptide-based small molecule inhibitors targeting PD-1 and PD-L1
The research team led by Liu Lei used phage display technology to screen the D-form peptide DPPA-1, which is composed of 12 amino acids (Fig. 2e). The amino acid sequence was found to be NYSKPTDRQYHF, and DPPA-1 has specific affinity for PD-L1. In a binding test with PD-L1, the Kd reached 0.51 μmol/L. Later, in the CT26 xenograft mouse model, the peptide notably inhibited tumor growth [36]. The polypeptide TPP-1 was reported in 2017 to target PD-L1 (Fig. 2f). TPP-1 is a polypeptide with an amino acid sequence of SGQYASYHCWCWRDPGRSGGSK (22 amino acids), and the Kd of TPP-1 binding to PD-L1 reached 74 nmol/L. In a xenograft mouse model using the large cell lung cancer cell line H460, TPP-1 significantly increased IFN-γ secretion and granzyme B expression and reduced tumor volume [37].
Nonpeptide-based small molecule inhibitors
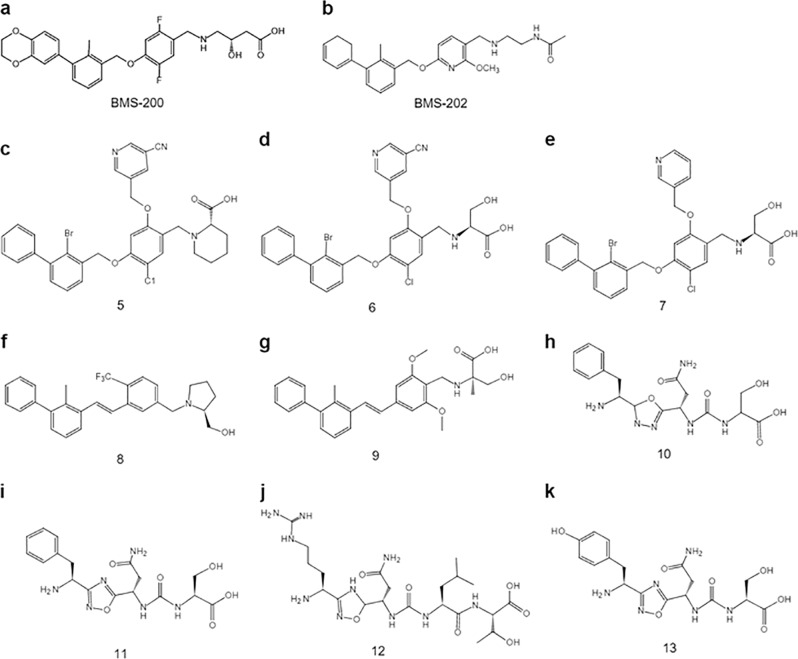
The BMS company has performed an in-depth exploration of small molecule inhibitors based on the PD-1/PD-L1 signaling pathway. This company disclosed the first patent on biphenyl-type immunomodulators in 2015. These small molecule inhibitors are aimed at overcoming immunological antagonistic diseases caused by activation of PD-1 and PD-L1, such as tumors and HCV, by blocking PD-1/PD-L1 binding [38]. The structures of two groups of small molecule inhibitors that include a biphenyl core structure have been revealed: one structure is the 3-(2,3-dihydro-1,4-benzodioxan-6-yl)-2-methylphenyl methanol scaffold (such as found in BMS-200), and the other is based on the 2-methyl-3-biphenyl methanol scaffold (such as found in BMS-202) [11, 12] (Fig. 3a, b). The IC50 values of these small molecule inhibitors were assessed through homogeneous time-resolved fluorescence (HTRF)-binding assays and ranged from 0.92 nM to 14.25 μM [11, 12]. Later, Zak used BMS-202 as a model compound to clarify the action mechanism of such small molecule compounds [30]. The research indicated that such small molecule compounds work on the surface of the PD-L1 protein, leading to dimerization of PD-L1, while BMS-202 is bound to two dimerized PD-L1 cylinders in the shape of a hydrophobic cavity. The surface of the PD-L1/PD-L1 interaction after dimerization is highly similar to that of the PD-1/PD-L1 interaction, resulting in the inability of PD-1 and PD-L1 to undergo normal interactions, which ultimately blocks the signaling pathway (Fig. 4).
Fig. 3.
Nonpeptide-based small molecule inhibitors targeting PD-1/PD-L1
Fig. 4.
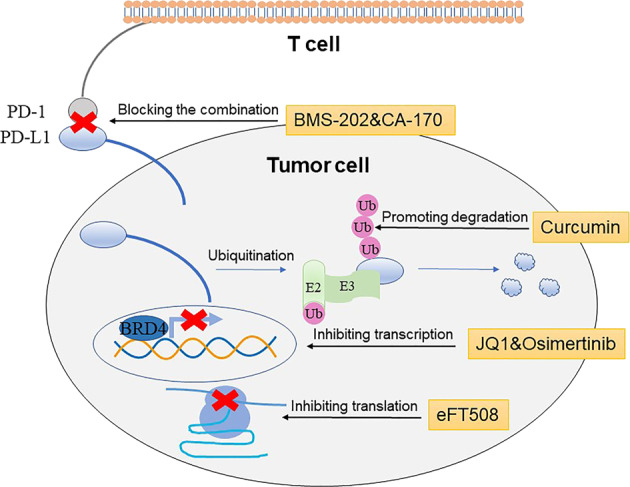
Methods for inhibiting PD-1/PD-L1 signaling pathway. BMS-202 and CA-107 interrupt the combination of PD-1 and PD-L1; JQ1 prevents BRD4 from combining with the PD-L1 promoter region to inhibit transcription; eFT508 reduces phosphorylation level of eIF4E to suppress PD-L1 protein translation; Curcumin inhibits CSN5 activity to promote the ubiquitination degradation of PD-L1
A series of patents from Feng's research group were published that disclosed a class of bromobenzyl ether derivatives that can significantly block the interaction of PD-1 and PD-L1. The structure of this kind of compound is similar to that of the compounds disclosed by BMS, but the difference is that the methyl group in the biphenyl structure is substituted with bromine. In the HTRF test, the activity of most compounds reached the nmol level, and the IC50 values of some compounds were <1 × 10−13 mol/L: the IC50 values of compounds 5 and 6 were 8.0 × 10−14 and 4.5 × 10−13 mol/L, respectively (Fig. 3c, d). In experiments with extracted human monocytes, PD-L1 was added to inhibit T lymphocytes that had been activated via an anti-CD3/CD28 antibody, and the ability of the test compounds to inactivate PD-L1 was examined. The experimental results showed that these compounds could partially inhibit the inhibitory function of PD-L1 on IFN-γ secretion at 10 nmol/L. In the mouse B16F10 subcutaneous xenograft model, the sodium salt form of compound 7 at a dose of 15 mg/kg significantly inhibited the growth of subcutaneous tumors in terms of both tumor volume and weight, and its inhibitory rate on tumor weight reached 64.11% [39–41] (Fig. 3e).
A class of aromatic acetylene and aromatic vinyl compounds was disclosed in 2018, including compounds 8 and 9 (Fig. 3f, g). In HTRF tests, compounds 8 and 9 had IC50 values of 18 and 48 nmol/L, respectively, and the PD-1/PD-L1 interaction was significantly inhibited [42].
CA-170, developed jointly by Curis Pharmaceuticals of the United States and Aurigene Pharmaceuticals of India, is a small molecule dual inhibitor of oral anti-V-domain immunoglobulin suppressor of T cell activation (VISTA) and anti-PD-1 [43]. It is also the only small molecule inhibitor of PD-1/PD-L1 that has entered clinical research whose structure has not been disclosed. However, it is speculated that it may be a class of 1,2,4-oxadiazole or 1,3,4-oxadiazole, and the substituted side chain in such compounds is generally an amino acid residue base (Fig. 3h–k). In the presence of recombinant mouse PD-L1 protein, the activity of the compound was characterized by determining the proliferation rate of spleen cells rescued by the compound, and the rescue rate of compound 11 reached 99% at a dose of 100 nmol/L [44]. Preclinical data in vivo indicated that CA-170 could significantly eliminate the inhibition of T cells by PD-L1, promote T cell differentiation and proliferation, and induce production of IFN-γ [45]. In a variety of tumor models in vivo, CA-170 displays similar antitumor activity to PD-1 monoclonal antibodies. From the published clinical data, CA-170 has the best effect on non-small-cell lung cancer (NSCLC) and Hodgkin's lymphoma, and its overall clinical benefit rate (CBR) is 70% and 77.8%, respectively, in these diseases. It is surprising that the clinical benefit rate of the low-dose group (400 mg/d) is higher than that of the high-dose group (800 mg/d), regardless of the overall tumor type [46]. A similar phenomenon was found in the in vitro IFN-γ secretion test and the peripheral monocyte rescue experiment. At low doses, the immunosuppressive effect increased with increasing drug concentration, but CA-170 lost its immunosuppressive ability with increasing drug concentration [47]. It is worth mentioning that CA-170 is significantly better than monoclonal antibodies in terms of biosafety because there are no obvious adverse reactions at doses up to 1200 mg/d. Therefore, CA-170 has great research and application prospects as a small molecule inhibitor targeting PD-1/PD-L1 (Fig. 4).
Suppressing the expression level of PD-L1
PD-L1 is expressed in a great diversity of tumor types. The expression of PD-L1 is monitored not only through the cancer-promoting pathway in tumor cells but also by various factors in the tumor microenvironment (TME) [48]. Signaling pathways such as MAPK and PI3K/Akt, which play an essential role in the growth, proliferation, and migration of tumor cells, can upregulate the expression of PD-L1 to repress immune clearance by T cells. Therefore, prohibiting these signaling pathways by applying related inhibitors can reduce PD-L1 expression and exert a promising antitumor effect. In addition, various transcriptional regulatory factors in cells, including myelocytomatosis viral oncogene (MYC), cyclin-dependent kinase 5 (CDK5), bromodomain-containing protein 4 (BRD4), and signal transducer and activator of transcription 3 (STAT3), have been found to play a direct or indirect role in the transcription of PD-L1, and inhibitors of these factors can interfere with the transcription of PD-L1 to achieve a goal of inhibiting its expression. Gene transcription is only a part of protein expression, and the expression of the target protein also needs to be realized through translation, posttranslational modification, and other mechanisms. Similarly, inhibiting the translation of PD-L1 is also capable of reducing PD-L1 levels to play an antitumor role. Immune checkpoint inhibitors allow the immune system to reidentify and attack tumor cells by inhibiting the action of immune checkpoint proteins, but this method is not universal and uniform. For example, Tecentriq plays a role in NSCLC by blocking the PD-1/PD-L1-signaling pathway [49]. However, when lung cancer is transferred to other organs, its therapeutic effect is reduced. This phenomenon inspires a further question: instead of repressing the function of these proteins after synthesis, why not directly inhibit the production of these proteins? It makes sense that inhibiting the production of PD-L1 from the source via blocking PD-L1 transcription, translation, and other methods may have more unexpected effects than inhibiting its function after production (Fig. 4).
BRD4 is a member of the BET family and contains two bromodomains that recognize acetylated lysine residues, which are necessary for chromatin remodeling and transcription activation [50]. It has been found that BRD4 can positively regulate the expression of PD-L1 [51]. Analysis of the TCGA database indicates that BRD4 gene amplification is present in many types of tumors, and BRD4 is significantly positively related to the expression of PD-L1 [52]. In lymphoma cells and epithelial ovarian cancer cells, both BRD4 knockdown and the BET bromodomain inhibitor JQ1 remarkably downregulated PD-L1 mRNA levels and protein expression, and JQ1 reduced PD-L1 expression in a time-dependent and dose-dependent manner [50, 51]. ChIP experiments found that BRD4 combines with the PD-L1 promoter, and JQ1 could block the binding between BRD4 and the PD-L1 promoter. Similarly, IFN-γ enhances the binding of BRD4 to the PD-L1 promoter, which can be inhibited by JQ1 [51] (Fig. 4). STAT3 can upregulate the transcription of PD-L1 by directly combining with the PD-L1 promoter. PD-L1 expression is elevated after oncogene anaplastic lymphoma kinase (ALK) mutation, while knockdown of STAT3 restrains this effect [53]. Latent membrane protein 1 (LMP1) positively regulates PD-L1 by promoting phosphorylation of STAT3, whereas inhibiting phosphorylation of STAT3 can reduce the PD-L1 expression induced by LMP1, which is achieved via the Janus kinase 3 (JAK3) inhibitor CP-690550 [52].
Recently, researchers began to recognize that oncogenes affect the translation process. Different oncogenes influence the translation process in distinct ways, thereby regulating the expression of related proteins to induce an imbalance of immune checkpoints. Eukaryotic translation initiation factor 4E (eIF4E) is a translation initiation factor that specifically combines with the 5′ end of mRNA and plays a significant regulatory role in the initial stage of protein synthesis. eIF4E is closely connected with tumor occurrence, infiltration and transfer and is highly expressed in various human malignant tumors. Phosphorylation of eIF4E at serine 209 positively regulates its self-carcinogenic activity and stimulates specific mRNA translation [54, 55]. A major study by the University of California, San Francisco (UCSF), showed that the new drug eFT508 (tomivosertib) inhibits the synthesis of PD-L1 protein, which not only delays tumor growth but also influences the ability of tumors to evade immune responses successfully in a mouse model with liver cancer [56]. To better understand the translational changes in the tumor, the researchers established two mouse models of liver cancer. One model had mutated forms of common liver cancer oncogenes, MYC and Kirsten rat sarcoma (KRAS), and the other only carried a mutated KRAS oncogene. Although both groups of mice carried liver tumors, tumors developed earlier and grew and spread faster to other organs in MYC–KRAS mice than in KRAS only mice. Next, researchers examined transcription and translation in tumors from two mouse models. The study found that the mutated MYC–KRAS gene led to higher levels of translated mRNA than the mutated KRAS gene. The most important finding was that the gene for the immune checkpoint protein PD-L1 was more active in MYC–KRAS tumors, which had approximately five times more PD-L1 protein than KRAS tumors. Dr. Ruggero found that higher levels of PD-L1 translation enabled MYC–KRAS tumors to escape the immune system and spread much more rapidly. When they used eFT508 in mice that carried MYC–KRAS tumors to inhibit PD-L1 translation by blocking its chemical modification and activation, PD-L1 decreased eIF4E levels, and the number of cancer cells was evidently reduced. This treatment also slowed tumor growth, prevented tumor spreading, and increased the survival rate of mice. However, eFT508 did not affect the survival of KRAS tumor rice. This study demonstrated that eFT508 inhibited ribosome binding to RNA, reduced PD-L1 protein levels in tumor cells, and remarkably increased the survival rate of mice bearing liver cancer (Fig. 4).
Osimertinib (AZD9291), the third type of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), is approved by the FDA for the treatment of non-small-cell lung cancer with a T790M resistance mutation in EGFR. Osimertinib is an irreversible inhibitor of EGFR that can form covalent bonds with molecular targets to produce a more sustained response and reduce the possibility of drug resistance. Clinical studies show that PD-L1 expression may be higher in EGFR mutant NSCLC patients than in NSCLC patients without EGFR mutations [57–59]. Dr. Lu has proven that the inhibitory effect on EGFR induced by osimertinib can reduce the protein level of PD-L1 by suppressing PD-L1 mRNA expression and inducing PD-L1 degradation via the proteasome pathway in EGFR-driven NSCLC cells, which may enhance T cell immune activity and the killing of tumor cells [60] (Fig. 4). Moreover, in further studies, Dr. Lu’s research team discovered that platycodin D, which is a natural product separated from an edible and medicinal plant (the bellflower), is able to reduce the protein level of PD-L1 in lung cancer cells by inducing its release into extracellular media, which provides new possibilities for the application of natural products in cancer immunotherapy [61].
Promoting degradation of PD-L1
Existing antibody drugs are able to connect to and block PD-L1 on the surface of tumor cells, but recent studies have pointed out that PD-L1 produced by tumor cells is also present in circulating endosomes, the Golgi apparatus, and vesicles in cells. PD-L1 in cancer cells has a cancer-promoting function and supplies and renews inactivated PD-L1 on the cell surface, which may be one of the causes of antibody drug failure. Therefore, the complete removal of PD-L1 inside the cell and on the cell surface may be a more effective blocking strategy. There are three main pathways of degradation for intracellular proteins: the lysosomal pathway, the ubiquitination pathway, and the caspase pathway. The lysosomal pathway is mainly triggered by the recognition of the protein or the endocytosis of the protein into the lysosome, and degradation is carried out by the corresponding enzyme in the acidic environment of the lysosome; the contents are then transported to the cytosol through a carrier protein on the lysosomal membrane to supplement the metabolic contents of the cytosol [62]. The ubiquitin–proteasome pathway is an important pathway for the specific degradation of proteins. It is involved in various metabolic activities of the body, mainly degrading cyclin, spindle-related proteins, transcription factors such as nuclear factor κB (NF-κB), cell surface receptors such as EGFR, tumor suppressors such as P53, and oncogene products. The ubiquitin–proteasome pathway mechanism is dependent on ATP and is finished within two steps. First, ubiquitin is transferred to a target protein with the help of a series of ubiquitin ligases to achieve polyubiquitination. Then, the polyubiquitinated protein is recognized and hydrolyzed by the 26S proteolytic enzyme [63]. Last, the caspase pathway is the protein degradation pathway related to apoptosis. Caspases exist in the form of zymogens under normal conditions and are activated after apoptosis is initiated. The proteins that are degraded in apoptosis include DNA damage repair enzymes, U1 small nuclear ribonucleoprotein components, lamin, actin and lining proteins [64]. The degradation of these enzymes and proteins leads to apoptosis of cells, whose debris is eventually engulfed and digested by phagocytic cells. Therefore, scientists can design inducers to guide PD-L1 in tumor cells towards the degradation pathway, reduce the level of PD-L1 in tumor cells and suppress the ability of tumor cells to escape immunity to achieve antitumor effects.
Dr. Xu discovered a significant association between Huntingtin-interacting protein 1-related (HIP1R) and PD-L1: according to the OncoBinder multigroup prediction model, there is often copy number loss of the HIP1R gene in tumor cells with PD-L1 gene amplification [65]. This suggests that the HIP1R gene may be detrimental to high expression of PD-L1, and tumor cells might selectively delete the HIP1R gene during development. The researchers conducted a series of validations and studies. First, they found that HIP1R could directly interact with PD-L1 and deliver PD-L1 for lysosomal degradation via the lysosomal-targeted signaling pathway. Consumption of HIP1R in tumor cells leads to the accumulation of PD-L1 and ultimately inhibits T cell-mediated cytotoxicity and promotes tumor immune escape [66, 67]. Further, by mutating the HIP1R gene, they found a key sequence that bound directly to PD-L1 and transported it to the lysosome, which was later confirmed to be a lysosomal sorting sequence located at the carboxy terminus of HIP1R. This lysosomal sorting sequence with a “dileucine” pattern is in charge of the targeted transport of PD-L1 into the lysosome. HIP1R transports PD-L1 to the surface of the lysosome by binding to the adaptor protein (AP) complex and relies on the endosomal sorting complexes required for transport (ESCRT) complex to allow PD-L1 to enter the multivesicular body (MVB) and lysosomes [68, 69]. Finally, the researchers were inspired by this mechanism to fuse two peptides derived from HIP1R (which are bound to PD-L1 and transported to lysosomes) and designed a new molecule, PD-LYLSO, that could target PD-L1 for degradation [70]. Functional experiments showed that PD-LYSO can guide more PD-L1 to lysosomes for degradation and activate tumor cell killing by T cells. The research team applied for a patent for the invention of the PD-LYSO peptide and is currently conducting preclinical research.
COP9 signalosome complex 5 (CSN5) is an important ubiquitin ligase that modulates PD-L1 ubiquitination and affects tumor immunity by regulating the level of PD-L1 ubiquitination. It has been found in breast cancer models that TNF-α secreted by macrophages can positively regulate PD-L1 protein expression during posttranslational modifications without affecting PD-L1 transcription [71]. TNF-α activates the transcription of CSN5 and promotes the expression of CSN5 by activating the P65 subunit of NF-κB. Highly expressed CSN5 combines with PD-L1 and deubiquitinates PD-L1 to enhance the stability of PD-L1. In contrast, inhibitors of CSN5 will ubiquitinate PD-L1 and cause it to be shuttled to the degradation pathway; for instance, the CSN5 inhibitor curcumin can reduce tumor burden and improve the survival rate in mice, increase the proportion of CD8+ TILs and reduce PD-L1 expression in tumor cells [71] (Fig. 4). Since curcumin enhances the efficacy of anti-CTLA-4 antibody drugs, CSN5-targeted inhibitors in combination with PD-1/PD-L1 antibody drugs or CTLA-4 antibody drugs may serve as new approaches to tumor immunotherapy.
Metformin is a widely used oral hypoglycemic drug for the treatment of type 2 diabetes (T2D) and has been considered to be a clinically safe and well-tolerated drug for decades. In addition, metformin has an antitumor effect and preserves highly cytotoxic T lymphocyte (CTL) activity in tumor tissues [72, 73]. These findings suggest that the antitumor effect of metformin could have a part in the immune response to tumor progression. A recent study found that metformin can lead to the degradation of PD-L1 and prevent tumor cells from undergoing immune escape [74]. The researchers compared the antitumor effects of metformin between normal immune-active 4T1 breast cancer models and severe combined immunodeficiency (SCID) mice. Tumor tissue was significantly reduced only in immunocompetent mice after a week of metformin treatment, while there was no remarkable change in immunodeficient mice, indicating that the antitumor function of metformin is closely connected with the immune system. Further studies have discovered that metformin can activate adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK), phosphorylate the serine at position 195 (S195) of the PD-L1 protein and lead to dysfunction of PD-L1, which later cannot be recognized by the protein transport network and stays on the endoplasmic network. The S195-phosphorylated PD-L1 undergoes a conformational change and accumulates, which in turn induces degradation of PD-L1 after it binds to ER-associated protein degradation (ERAD) [75]. The researchers next attempted to treat mice with metformin and CTLA-4 inhibitors in three different models of breast cancer, melanoma, and colon cancer, and this combination therapy achieved good therapeutic effects: lowered tumor burden, increased survival rate, increased CTL activity, and prevented mouse weight loss. Therefore, these results suggest that scientists can develop new clinical effects from existing drugs, which may open new avenues for cancer immunotherapy.
Discussion
Obstructing the interaction of PD-1 and PD-L1 by using monoclonal antibody drugs has shown impressive effects in cancer immunotherapy. However, the disadvantages of the antibodies cannot be avoided within years, therefore, it is important to design and synthesize small molecule inhibitors that interrupt the interaction of PD-1 and PD-L1. Although the binding activity of small molecule inhibitors to targets is somewhat lower than that of antibody drugs, they have mature development pipelines and controllable pharmacokinetic properties, making it possible to bypass the faults of antibody drugs. However, the interaction between PD-1 to PD-L1 is a protein–protein interaction, and their contact interfaces are highly flat and hydrophobic. In addition, they have large interaction surfaces and few binding pockets for traditional small molecules, making it difficult to find a proper site that is suitable for a small molecule. Moreover, the structural regions of the PD-1/PD-L1 interaction induce orthogonal conformational changes of PD-1 and PD-L1, thus causing the binding ability of small molecule compounds to be challenged, which ultimately results in the inability of small molecule inhibitors to interfere with the binding of PD-1 and PD-L1, while monoclonal antibodies do not have this issue. To solve these difficulties, it is possible to employ the crystal structure of PD-1/PD-L1 to screen out candidate molecules with potential activity in the compound library through computer simulation and virtual screening. Homogeneous time-resolved fluorescence technology is used to carry out high-throughput screening of the candidate antagonistic molecules of PD-1/PD-L1 binding and to select compounds with better activity for in-depth research.
In response to existing problems based on studies of small molecule compounds that interrupt PD-1/PD-L1 binding, researchers have been exploring other effective intervention pathways to design small molecule drugs, and many scientists have focused on the regulatory mechanisms of PD-L1 protein expression. Many transcription factors have been found that enhance the transcription of PD-L1. Among them, MYC is widely expressed in many tumors and can directly initiate PD-L1 transcription [76]; STAT1 and STAT3 play important roles in PD-L1 transcription initiated by various pathways [77, 78]; while hypoxia inducible factor-1α (HIF-1α) mainly mediates hypoxic microenvironment-induced PD-L1 transcriptional expression [79]. However, the expression level of PD-L1 varies widely in different populations, and many patients have low expression or even no expression of PD-L1. Under such circumstances, it is futile to attempt to achieve therapeutic responses by directly reducing the expression of PD-L1. Alternatively, combined with PD-L1 protein level regulators, PD-1 immunotherapy can improve the therapeutic effect in tumors. CDK4 and CDK6 inhibitors promote Fizzy-related protein 1 (FZR1) to degrade speckle-type POZ protein (SPOP) and increase PD-L1 expression by blocking cyclin CDK4-mediated phosphorylation of SPOP [80]. Further studies showed that CDK4/6 inhibitors combined with PD-1 immunotherapy could significantly reduce tumor volume and improve the survival of tumor-bearing mice [80].
After translation, the stability of the protein is a key factor determining the abundance of its expression. Current studies have found that a variety of posttranslational modifications, including ubiquitination, glycosylation, phosphorylation, and palmitoylation, are critical for regulating the stability of the PD-L1 protein. For example, glycosylation of PD-L1 at the N192, N200, and N219 sites inhibits its degradation [81], thereby prolonging the half-life of PD-L1 and increasing protein stability; the T180 and S184 sites of PD-L1 can be phosphorylated by glycogen synthase kinase-3β (GSK3β), which enhances the binding to E3 ubiquitin ligase β-transducin repeat-containing protein (β-TrCP) and finally leads to ubiquitin proteasome pathway degradation [81]; palmitoyl-transferase zinc-finger DHHC domain-containing protein 9 (zDHHC9) can regulate the protein stability of PD-L1 by mediating palmitoylation of the C272 site of PD-L1 [82]. Many of the above studies have indicated that PD-L1 will eventually be degraded by the ubiquitin–proteasome pathway. Therefore, from this perspective, intervening in the degradation of PD-L1 is expected to become a new therapeutic strategy. However, the degradation of PD-L1 caused by small molecule modulators will cause off-target effects, compensatory feedback mechanisms, etc., which will have a negative impact on the therapeutic effect. To solve these problems, combined with proteolysis targeting chimera (PROTAC) technology, E3 ligases can bind to PD-L1 and label it with ubiquitin molecules, triggering the proteasome to degrade PD-L1, thereby achieving therapeutic purposes.
Studies have shown that some tumors, such as prostate cancer tumors, have low response rates to PD-L1 antibodies and may develop drug resistance [83]. The previously assumed answer to this low response rate was that in these patients, the PD-L1 protein level in tumor cells was low or even completely absent [84]. Dr. Robert and his team found that prostate cancer cells highly express PD-L1; more precisely, they secrete PD-L1 in exosomes instead of simply expressing them in the cell membrane [85]. These exosomes are produced by cancer cells and reach the lymph nodes through the lymphatic system or blood flow. In the lymph nodes, the PD-L1 protein acts as a molecular destroyer, remotely disarming immune cells and preventing them from locating the tumor to initiate an anticancer attack [86]. PD-L1 from the exosomes does not suppress the immune response on the surface of the tumor cells but exerts an inhibitory effect before the immune cells arrive. It is surprising that PD-L1 from exosomes has significant resistance to existing checkpoint inhibitors different from those found on the surface of tumor cells, but the specific cause is unclear. Therefore, preventing exosomes from releasing PD-L1 will become a new therapeutic approach, either alone or in association with current checkpoint inhibitors, which are expected to overcome the resistance of most patients to existing immunological checkpoint inhibitors. Although small molecule inhibitors have many advantages, their affinity for the target is hardly comparable to that of antibody drugs, and they are prone to off-target effects, which greatly reduces the therapeutic effect and may even bring unknown off-target toxicity. At the same time, the uncertainty of intracellular PD-L1 expression and the short half-life of small molecule drugs are additional reasons why it is difficult for small molecule inhibitors to achieve good in vivo activity.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81930102 to BY; No. 81872885 to JC), and the Talent Project of Zhejiang Association for Science and Technology (No. 2018YCGC002 to JC).
Competing interests
The authors declare no competing interests.
Contributor Information
Bo Yang, Email: yang924@zju.edu.cn.
Ji Cao, Email: caoji88@zju.edu.cn.
References
- 1.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–52. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Sullivan Coyne G, Madan RA, Gulley JL. Nivolumab: promising survival signal coupled with limited toxicity raises expectations. J Clin Oncol. 2014;32:986–8. doi: 10.1200/JCO.2013.54.5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkinson E. Nivolumab success in untreated metastatic melanoma. Lancet Oncol. 2015;16:e9. doi: 10.1016/S1470-2045(14)71129-5. [DOI] [PubMed] [Google Scholar]
- 5.Inman BA, et al. Atezolizumab: a PD-L1-blocking antibody for bladder cancer. Clin Cancer Res. 2017;23:1886–90. doi: 10.1158/1078-0432.CCR-16-1417. [DOI] [PubMed] [Google Scholar]
- 6.Sidaway P. Skin cancer: Avelumab effective against Merkel-cell carcinoma. Nat Rev Clin Oncol. 2016;13:652. doi: 10.1038/nrclinonc.2016.156. [DOI] [PubMed] [Google Scholar]
- 7.Shultz D. Three drugs approved for urothelial carcinoma by FDA. Cancer Discov 2017; 7:659–60. [DOI] [PubMed]
- 8.Powles T, O'Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma updated results from a phase 1/2 open-label study. JAMA Oncol 2017;3:e172411. [DOI] [PMC free article] [PubMed]
- 9.Naidoo J, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375–91. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasikumar PGN, Ramachandra M. Aurigene Discovery Technologies Limited, assignee. Immunosuppression modulating compounds. 2017.
- 11.Chupak LS, Zheng X. Preparation of compounds useful as immunomodulators. Bristol‐Myers Squibb Company: USA; 2018.
- 12.Chupak LS, Ding M, Martin SW. Preparation of substituted 2,4‐dihydroxybenzylamines as immunomodulators. Bristol‐Myers Squibb Company: USA; 2017.
- 13.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–61. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375:1767–78. doi: 10.1056/NEJMra1514296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan S, et al. An unexpected N-terminal loop in PD-1 dominates binding by nivolumab. Nat Commun. 2017;8:14369. doi: 10.1038/ncomms14369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–47. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 17.Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol. 2013;94:25–39. doi: 10.1189/jlb.1212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin DY, et al. The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc Natl Acad Sci U S A. 2008;105:3011–6. doi: 10.1073/pnas.0712278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazar-Molnar E, et al. Crystal structure of the complex between programmed death-1 (PD-1) and its ligand PD-L2. Proc Natl Acad Sci U S A. 2008;105:10483–8. doi: 10.1073/pnas.0804453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153–67. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 21.Hui E, et al. T cell costimulatory receptor CD28 is a primary target for PD-1–mediated inhibition. Science. 2017;355:1428–33. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patsoukis N, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez HL, et al. Antibody–drug conjugates: current status and future directions. Drug Discov Today. 2014;19:869–81. doi: 10.1016/j.drudis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book 2015;35:76–83. [DOI] [PubMed]
- 25.Weber JS. Safety profile of nivolumab (NIVO) in patients (pts) with advanced melanoma (MEL): a pooled analysis. J Clin Oncol 2015;33:785–92. [DOI] [PubMed]
- 26.Robert C, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 27.Hu W, Wei H, Li K, Li P, Lin J, Feng R. Downregulation of USP32 inhibits cell proliferation, migration and invasion in human small cell lung cancer. Cell Prolif 2017;50:e12343. [DOI] [PMC free article] [PubMed]
- 28.Chen SH, et al. Combination of 4-1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol Res. 2015;3:149–60. doi: 10.1158/2326-6066.CIR-14-0118. [DOI] [PubMed] [Google Scholar]
- 29.Zhan MM, et al. From monoclonal antibodies to small molecules: the development of inhibitors targeting the PD-1/PD-L1 pathway. Drug Discov Today. 2016;21:1027–36. doi: 10.1016/j.drudis.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Zak KM, et al. Structure of the complex of human programmed death 1, PD-1, and its ligand PD-L1. Structure. 2015;23:2341–8. doi: 10.1016/j.str.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zak KM, et al. Structural basis for small molecule targeting of the programmed death ligand 1 (PD-L1) Oncotarget. 2016;7:30323–35. doi: 10.18632/oncotarget.8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasikumar PGN, Ramachandra M. Immunomodulating and antitumor cyclic compounds from the BC loop of human programmed cell death 1 protein. Aurigene Discovery Technologies Limited; 2016.
- 33.Sasikumar PGN, Ramachandra M. Therapeutic immunomodulating compounds as programmed cell death 1 signaling pathway inhibitors for treating cancers and infections. Aurigene Discovery Technologies Limited; 2015.
- 34.Sasikumar PGN, Ramchandra M. Preparation of immunomodulator peptidomimetic compounds as programmed cell death 1 signaling pathway inhibitors for treating cancers and infections. Aurigene Discovery Technologies Limited; 2015.
- 35.Sasikumar PGN, Ramachandra M. Therapeutic cyclic compounds as immunomodulators. Aurigene Discovery Technologies Limited; 2016.
- 36.Chang HN, et al. Blocking of the PD-1/PD-L1 Interaction by a D-peptide antagonist for cancer immunotherapy. Angew Chem Int Ed Engl. 2015;54:11760–4. doi: 10.1002/anie.201506225. [DOI] [PubMed] [Google Scholar]
- 37.Li C, et al. Peptide blocking of PD-1/PD-L1 interaction for cancer immunotherapy. Cancer Immunol Res. 2018;6:178–88. doi: 10.1158/2326-6066.CIR-17-0035. [DOI] [PubMed] [Google Scholar]
- 38.Gillman KW. Macrocyclic peptides useful as immunomodulators; 2016.
- 39.Feng Z, Chen X, Yang Y. Benzyl phenyl ether derivative preparation method therefor, and pharmaceutical composition and uses thereof; 2017.
- 40.Feng Z, Chen X, Yang Y. Bromo benzyl phenyl ether derivative preparation method therefor, and pharmaceutical composition and uses thereof; 2017.
- 41.Feng Z, Chen X, Yang Y. Phenylate derivative preparation method therefor, and pharmaceutical composition and uses thereof; 2017.
- 42.Wang Y, Xu Y, Zhang T. Aromatic acetylene or aromatic ethylene compound, intermediate, preparation method, pharmaceutical composition and use thereof; 2018.
- 43.Sasikumar P, Sudarshan N, Gowda N. AUPM‐170: first‐in‐class, oral immune checkpoint inhibitor of PD‐L1/2 and VISTA. In: AACR annual meeting; 2015.
- 44.Sasikumar P, Ramachandra M. VISTA signaling pathway inhibitory compounds useful as immunomodulators. Aurigene Discovery Technologies Limited; 2018.
- 45.Sasikumar P, Sudarshan N, Gowda N. Oral immune checkpoint antagonists targeting PD‐L1/VISTA and PD‐L1/ TIM3 for cancer therapy. In: AACR annual meeting; 2016.
- 46.Carreterogonzalez A,LD, Ghanem I, et al. Analysis of response rate with ANTI PD-1/PD-L1 monoclonal antibodies in advanced solid tumors: a meta-analysis of randomized clinical trials. Oncotarget. 2018;9:8706–15. doi: 10.18632/oncotarget.24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Powderly J, Patel MR, Lee JJ, Brody J, Meric-Bernstam F, Hamilton E, et al. 1141PDCA-170, a first in class oral small molecule dual inhibitor of immune checkpoints PD-L1 and VISTA, demonstrates tumor growth inhibition in pre-clinical models and promotes T cell activation in Phase 1 study. Ann Oncol 2017;28:dx376.007.
- 48.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and T-regs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci Transl Med 2013;5:200ra116. [DOI] [PMC free article] [PubMed]
- 49.Tecentriq (package insert). South San Francisco, CA: Genentech Inc; 2018.
- 50.Hogg SJ, et al. BET-bromodomain inhibitors engage the host immune system and regulate expression of the immune checkpoint ligand PD-L1. Cell Rep. 2017;18:2162–74. doi: 10.1016/j.celrep.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu HR, et al. BET bromodomain inhibition promotes anti-tumor immunity by suppressing PD-L1 expression. Cell Rep. 2016;16:2829–37. doi: 10.1016/j.celrep.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fang W, et al. EBV-driven LMP1 and IFN-gamma up-regulate PD-L1 in nasopharyngeal carcinoma: implications for oncotargeted therapy. Oncotarget. 2014;5:12189–202. doi: 10.18632/oncotarget.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marzec M, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci U S A. 2008;105:20852–7. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Furic L, et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci U S A. 2010;107:14134–9. doi: 10.1073/pnas.1005320107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herdy B, et al. Translational control of the activation of transcription factor NF-kappaB and production of type I interferon by phosphorylation of the translation factor eIF4E. Nat Immunol. 2012;13:543–50. doi: 10.1038/ni.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu Y, et al. Translation control of the immune checkpoint in cancer and its therapeutic targeting. Nat Med. 2019;25:301–11. doi: 10.1038/s41591-018-0321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azuma K, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25:1935–40. doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- 58.D'Incecco A, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112:95–102. doi: 10.1038/bjc.2014.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang YN, et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget. 2015;6:14209–19. doi: 10.18632/oncotarget.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang XM, et al. Osimertinib (AZD9291) decreases programmed death ligand-1 in EGFR-mutated non-small cell lung cancer cells. Acta Pharmacol Sin. 2017;38:1512–20. doi: 10.1038/aps.2017.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang MY, et al. Platycodin D triggers the extracellular release of programed death Ligand-1 in lung cancer cells. Food Chem Toxicol. 2019;131:110537. doi: 10.1016/j.fct.2019.05.045. [DOI] [PubMed] [Google Scholar]
- 62.Wang CW, Klionsky DJ. The molecular mechanism of autophagy. Mol Med. 2003;9:65–76. [PMC free article] [PubMed] [Google Scholar]
- 63.Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17:1807–19. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- 64.Luo H, Wong J, Wong B. Protein degradation systems in viral myocarditis leading to dilated cardiomyopathy. Cardiovasc Res. 2010;85:347–56. doi: 10.1093/cvr/cvp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Coillie S, et al. OncoBinder facilitates interpretation of proteomic interaction data by capturing coactivation pairs in cancer. Oncotarget. 2016;7:17608–15. doi: 10.18632/oncotarget.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Juneja VR, et al. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. 2017;214:895–904. doi: 10.1084/jem.20160801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li CW, et al. Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1. Cancer Cell. 2018;33:187–201 e10. doi: 10.1016/j.ccell.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amorim NA, et al. Interaction of HIV-1 Nef protein with the host protein Alix promotes lysosomal targeting of CD4 receptor. J Biol Chem. 2014;289:27744–56. doi: 10.1074/jbc.M114.560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhai Q, et al. Identification and structural characterization of the ALIX-binding late domains of simian immunodeficiency virus SIVmac239 and SIVagmTan-1. J Virol. 2011;85:632–7. doi: 10.1128/JVI.01683-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang HB, et al. HIP1R targets PD-L1 to lysosomal degradation to alter T cell-mediated cytotoxicity. Nat Chem Biol. 2019;15:42–4. doi: 10.1038/s41589-018-0161-x. [DOI] [PubMed] [Google Scholar]
- 71.Lim SO, et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell. 2016;30:925–39. doi: 10.1016/j.ccell.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Viollet B, et al. Cellular and molecular mechanisms of metformin: an overview. Clin Sci. 2012;122:253–70. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eikawa S, et al. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A. 2015;112:1809–14. doi: 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cha JH, et al. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol Cell. 2018;71:606–20 e7. doi: 10.1016/j.molcel.2018.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zanetti G, et al. COPII and the regulation of protein sorting in mammals. Nat Cell Biol. 2012;14:20–28. doi: 10.1038/ncb2390. [DOI] [PubMed] [Google Scholar]
- 76.Casey SC. MYC regulates the antitumor immune response through CD47 and PD-L1 (vol 352, aaf7984, 2016) Science. 2016;353:229–229. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cerezo M, et al. Translational control of tumor immune escape via the eIF4F-STAT1-PD-L1 axis in melanoma. Nat Med. 2018;24:1877–87. doi: 10.1038/s41591-018-0217-1. [DOI] [PubMed] [Google Scholar]
- 78.Atsaves V, et al. PD-L1 is commonly expressed and transcriptionally regulated by STAT3 and MYC in ALK-negative anaplastic large-cell lymphoma. Leukemia. 2017;31:1633–7. doi: 10.1038/leu.2017.103. [DOI] [PubMed] [Google Scholar]
- 79.Noman MZ, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–90. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang JF, et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature. 2018;553:91–5. doi: 10.1038/nature25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li CW, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016;7:12632. doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang Y, et al. Palmitoylation stabilizes PD-L1 to promote breast tumor growth. Cell Res. 2019;29:83–6. doi: 10.1038/s41422-018-0124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goswami S, Aparicio A, Subudhi SK. Immune checkpoint therapies in prostate cancer. Cancer J. 2016;22:117–20. doi: 10.1097/PPO.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martin AM, et al. Paucity of PD-L1 expression in prostate cancer: innate and adaptive immune resistance. Prostate Cancer Prostatic Dis. 2015;18:325–32. doi: 10.1038/pcan.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ruivo CF, et al. The biology of cancer exosomes: insights and new perspectives. Cancer Res. 2017;77:6480–8. doi: 10.1158/0008-5472.CAN-17-0994. [DOI] [PubMed] [Google Scholar]
- 86.Poggio M, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177:414–27. doi: 10.1016/j.cell.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]