Abstract
Background
A research priority in finding a cure for HIV is to establish methods to accurately locate and quantify where and how HIV persists in people living with HIV (PLWH) receiving suppressive antiretroviral therapy (ART). Infusing copper-64 (64Cu) radiolabelled broadly neutralising antibodies targeting HIV envelope (Env) with CT scan and positron emission tomography (PET) identified HIV Env in tissues in SIV infected non-human primates . We aimed to determine if a similar approach was effective in people living with HIV (PLWH).
Methods
Unmodified 3BNC117 was compared with 3BNC117 bound to the chelator MeCOSar and 64Cu (64Cu-3BNC117) in vitro to assess binding and neutralization. In a clinical trial 64Cu-3BNC117 was infused into HIV uninfected (Group 1), HIV infected and viremic (viral load, VL >1000 c/mL; Group 2) and HIV infected aviremic (VL <20 c/mL; Group 3) participants using two dosing strategies: high protein (3mg/kg unlabeled 3BNC117 combined with <5mg 64Cu-3BNC117) and trace (<5mg 64Cu-3BNC117 only). All participants were screened for 3BNC117 sensitivity from virus obtained from viral outgrowth. Magnetic resonance imaging (MRI)/PET and pharmacokinetic assessments (ELISA for serum 3BNC117 concentrations and gamma counting for 64Cu) were performed 1, 24- and 48-hours post dosing. The trial (clincialtrials.gov NCT03063788) primary endpoint was comparison of PET standard uptake values (SUVs) in regions of interest (e.g lymph node groups and gastrointestinal tract).
Findings
Comparison of unmodified and modified 3BNC117 in vitro demonstrated no difference in HIV binding or neutralisation. 17 individuals were enrolled of which 12 were dosed including Group 1 (n=4, 2 high protein, 2 trace dose), Group 2 (n=6, 2 high protein, 4 trace) and Group 3 (n=2, trace only). HIV+ participants had a mean CD4 of 574 cells/microL and mean age 43 years. There were no drug related adverse effects and no differences in tissue uptake in regions of interest (e.g lymph node gut, pharynx) between the 3 groups. In the high protein dosing group, serum concentrations of 3BNC117 and gamma counts were highly correlated demonstrating that 64Cu-3BNC117 remained intact in vivo.
Interpretation
In PLWH on or off ART, the intervention of infusing 64Cu-3BNC117 and MRI/PET imaging over 48 hours, was unable to detect HIV-1 env expression in vivo. Future studies should investigate alternative radiolabels such as zirconium which have a longer half-life in vivo.
Funding
Funded by the Alfred Foundation, The Australian Centre for HIV and Hepatitis Virology Research with additional support from the Division of AIDS, National Institute of Allergy and Infectious Disease, US National Institutes of Health (U19AI096109). JHM and SRL are supported by the Australian National Health and Medical Research Council.
Keywords: HIV, Imaging, Clinical trial, PET scan, Broadly neutralising antibodies
Research in context.
Evidence before this study
Prior to this trial there was no reports in humans of radiolabeling anti-HIV broadly neutralising antibodies for the purpose of infusing them and performing subsequent positron emission tomography (PET) based imaging to quantify and locate tissue sites of HIV. This was performed in a macaque trial with a copper-64 radiolabeled anti-SIV broadly neutralising antibody in macaques on and off antiretroviral therapy (ART) and was able to detect SIV in tissue sites. If a safe non-invasive method could be developed in people to locate and quantify HIV in tissue this would be a critical tool to understand HIV persistence on ART.
Added value of this study
This trial did not detect HIV in tissue sites despite extensive pre-clinical work demonstrating that the modified version of the broadly neutralising antibody 3BNC117 was able to bind and neutralize HIV, investigating different dosing strategies of radiolabeled 3BNC117 in the clinical trial and also including HIV uninfected control participants in the trial for direct comparison of images with HIV-infected participants. This trial that demonstrates the safe deployment of non-invasive PET-based imaging methods with radiolabeled broadly neutralising antibodies to image HIV to drive this research field in new directions.
Implications of all the available evidence
In contrast to the macaque trial this study argues that copper-64 radiolabeled anti-HIV broadly neutralized antibodies are not suitable for PET-based imaging to locate and quantify HIV. Future studies should consider alternate strategies such as using a radionuclide with a longer radioactive half-life that would allow imaging for several days post infusion and allow temporal changes in uptake and improved blood pool clearance to be assessed.
Alt-text: Unlabelled box
1. Introduction
Antiretroviral therapy (ART) has dramatically reduced HIV-related morbidity and mortality for people living with HIV (PLWH), however treatment is needed lifelong. The major barrier to a cure for HIV is long lived and proliferating latently infected CD4+ T-cells [1,2], which are highly enriched in lymph node (LN) tissue and the gastrointestinal (GI) tract [3]. A major challenge to understanding where and how HIV persists on ART in tissues, is the difficulty in using tissue biopsies which are invasive, expensive and only assess a tiny fraction of an organ [3].
The number of latently infected cells is almost ten times higher in tissues such as the GI tract or LN where drug concentrations of antiretrovirals have been shown to be lower than in blood [3,4]. Furthermore, detection of constitutive and inducible expression of viral protein in PLWH on ART, not just viral RNA or DNA [5], has significant implications for the efficacy of therapeutic interventions such as broadly neutralising antibodies (bNAb), which can target and potentially eliminate infected cells that express viral protein. Hence bNAbs and other therapeutic vaccines are being actively pursued as cure strategies [6]. Developing a safe non-invasive method to locate and quantify HIV protein in tissue will be a critical tool to understand and eventually eliminate HIV persistence on ART.
Diagnostic imaging using Positron Emission Tomography (PET) with antibodies radiolabelled with a positron-emitting radionuclide can be used to quantitate biodistribution and localisation of antibodies. This approach is now extensively used in oncology and isotopes for various elements have been used, including iodine, indium, zirconium and copper. The high molecular weight of IgG antibodies (150 kDa) and their interaction with the Fc-receptor means that they may take days to clear from the blood-pool and accumulate in target tissues. The positron-emitting radionuclide copper 64 (64Cu) has a positron emission energy that is similar to fluorine-18 but has a longer radioactive half-life of 12.7 hours which allows for PET imaging up to 48 hours after injection. Copper-64 has also been safely applied in humans to quantify the distribution of various cancers [7,8] and allows for low effective doses of radiation which are required to study healthy volunteers.
PET imaging has also been used to detect simian immunodeficiency virus (SIV) in non-human primates (NHP) before and after suppressive ART [9]. In that study, a broadly neutralising antibody against the SIV envelope protein 7D3 was labelled with copper-64, and then PET/CT imaging was performed to detect SIV envelope protein in tissue. In NHP off ART, there was increased uptake of the radiolabelled antibody in the GI tract, genital tract and axillary and inguinal LNs. In animals on ART with an undetectable viral load in plasma, there was uptake of the antibody in tissue including colon, spleen, the male genital tract and LNs. Uptake of radiolabelled antibody was clearly different from uninfected NHP and the distribution was consistent with other markers of virus persistence, including measurement of SIV DNA and RNA. Whether a similar approach can be used in PLWH remains unknown.
3BNC117 is a potent bNAb that targets the CD4-binding site and has been demonstrated to neutralise 195 of 237 HIV strains across 6 clades [10]. In clinical trials it has been administered to PLWH both on and off ART with a single infusion of 3BNC117 resulted in a 2 log10 reduction in plasma HIV RNA in viremic individuals [11]. In aviremic individuals on ART, infusions of 3BNC117 alone resulted in prolonged time to viral rebound after ceasing ART consistent with neutralisation of free virus and potential depletion of infected cells [12,13].
Here we report the results of a clinical trial that infused 64Cu-3BNC117 into HIV-uninfected participants and HIV-infected participants on and off ART with serial PET/MRI scans. The objectives of this trial were to determine (i) if modifying 3BNC117 would impact its ability to bind and neutralize HIV, (ii) the safety of 64Cu-3BNC117, and (iii) the in vivo distribution of 64Cu-3BNC117 in PLWH as compared to uninfected controls. We also compared dosing with labelled antibody alone or a combination of labelled and unlabelled antibody, to permit evaluation of all tissue types and whether they could impact the assessment of the distribution of HIV infected cells.
2. Methods
2.1. Study design
This was an open-label phase I clinical trial to assess 64Cu-3BNC117 for its safety and potential to detect tissue sites of HIV in HIV-uninfected individuals and HIV-infected individuals on and off ART.
Study participants were enrolled in three cohorts, HIV-uninfected controls, HIV-infected viremic participants off ART and HIV-infected aviremic participants receiving ART. Participants undertook five visits over a 14-day period plus a screening visit. Eligible subjects received an infusion of 3mg/kg 3BNC117 bound to no more than 80 MBq of 64Cu which combines the small amount of labelled antibody (< 5 mg 64Cu-3BNC117) with the remainder of the unlabeled antibody in a single infusion at the baseline visit (Day 0). The labelled and unlabelled antibody were combined in a 250 mL bag of normal saline (0.9% sodium chloride) which was infused intravenously over 60 minutes via an intravenous line containing a 0.22 micron filter. During the course of the trial the design was amended, and subsequent participants received only the labelled antibody dosed as; up to 5 mg of 3BNC117 bound to 120-130 MBq of 64Cu. This trace dosing was prepared in a 5 mL syringe and infused via slow push over 5 minutes. The amended dose of 64Cu was increased after detailed dosimetry on the first participants demonstrated that 130 MBq of 64Cu could be administered and stay under the approved effective dose of 4.7 mSv. Planned recruitment was for 2 HIV uninfected individuals and 4 HIV infected individuals per group. The trial was registered on clincialtrials.gov (NCT03063788)
2.2. Preparation of MeCOSar-3BNC117 and radiolabeling with 64Cu
NHS-MeCOSar was dissolved in DMSO, mixed with 3BNC117 in PBS at 4 different molar ratios (5x, 10x, 15x, 20x NHS-MeCOSar to GMP-3BNC117) and incubated at room temperature for 1 hour. The reaction was purified via spin column (10kDa MWCO), washed twice with 0.1M ammonium acetate in saline (pH 5.8), sterile filtered and aliquoted at a concentration of 13.3 mg/mL. SEC was used to monitor the stability of GMP-3BNC117 and the different MeCOSar-3BNC117 constructs. LC-MS was used to assess changes in molecular weight associated with addition of MeCOSar.
Radiolabeling was achieved by incubating copper-64 (370 MBq) with MeCOSar-3BNC117 (4 mg) at room temperature for 15 minutes. The crude reaction mixture was purified via centrifugal filtration (50kDa MWCO), washed twice with saline, reconstituted in saline containing 5% human serum albumin (HAS) and sterile filtered using a 0.22 micron filter. Radiochemical purity was determined by instant thin-layer chromatography (iTLC) using 10 mM EDTA in 0.1M phosphate buffer (pH 7) as a mobile phase. Protein integrity of the radiolabeled construct was monitored via size-exclusion high performance liquid chromatography (SEC-HPLC). Stability of [64Cu]Cu-MeCOSar-3BNC117 was assessed in human serum at 37⁰C over 48 hours.
2.3. Binding and neutralisation assays for modified 3BNC117
Three assays were developed to assess binding and neutralisation of 3BNC117 compared with MeCOSar-3BNC117 and 64Cu-MeCOSar-3BNC117. The assay for the fully radiolabeled construct was also developed so it could be used as a validation assay when study drug radiolabeling was performed during the clinical trialr
1) Enzyme-linked immunosorbent assay (ELISA) assay. 50 ng HIV AD8 gp140 antigen combined with 10x coating buffer (200mM TrisHCl and 1M NaCl in Milli-Q [MQ] water) was coated on Immuno Maxisorp 96 well microplates. Each well was washed with phosphate buffered saline supplemented with Tween 20 (PBST) then blocked with 5% bovine serum albumin (BSA). Plates were then sealed and incubated at room temperature for 2 hours, shaking at 300 rpm. Serial dilutions of GMP-3BNC117, MeCOSar-3BNC117, 64Cu-MeCOSar-3BNC117, or isotype control from 1ug/mL to 7.8 ng/mL were added to microplates with 5% BSA used as a negative control with plates incubated at room temperature for 2 hours, shaking at 300 rpm. 100uL of a secondary horse radish peroxidase (HRP)-conjugated anti-Fc antibody used at a dilution of 1:1000 was added to each well then incubated for 1 hour, shaking at 300 rpm. Wells were washed 6x in 200uL PBST, then dried. 100uL of chromogenic TMB substrate was added to each well, incubated for 15-20 minutes then the reaction was stopped with 1M Sulfuric Acid in MQ water. Absorbance at 450 nm was assessed by a light reader
2) Cell binding assay. Binding of 3BNC117 to cell surface expressed Env was performed as previously described [14]. Briefly, human embryonic kidney cells (HEK 293T) cells were transfected with Env expression plasmids for HIV strains AD8 and NL4.3 using lipofectamine 2000. 24h post transfection the cells were incubated with 10µg/mL 3BNC117, MeCOSar-3BNC117 or serum from a HIV+ viremic individual (HIVIG), followed by incubation with a FITC-conjugated rabbit anti-human F(ab’)2 Ig (Chemicon). FITC expression was measured by fluorescence-activated cell sorting (FACS) which corresponds to the level of 3BNC117 binding to Env transfected cells and the relative fluorescence was calculated as previously described [14].
3) Neutralisation assay. Env pseudotyped luciferase reporter viruses were produced and titrated in JC53 cells as previously described [15]. Reporter viruses pseudotyped with subtype B Env strains with high (NL4.3 and AD8) and moderate sensitivity (TRO.11) to neutralising antibodies were produced [14]. These reporter viruses were incubated in increasing concentrations of 3BNC117 and MeCOSar-3BNC117 (0.0032µg/mL to 10µg/mL) for 1 hour at 37°C. The virus and antibody mixture were then added to JC53 cells and incubated at 37°C for a total of 72 hours. The level of reporter virus entry was measured by luciferase activity in cell lysates (Promega) according to the manufacturer's instructions. Luminescence was measured using a FLUOStar microplate reader (BMG Labtech). Virus entry in wells containing antibody was normalized to that of wells incubated with PBS. Inhibition curves were generated with a non-linear function and least squares analysis in Prism (Graphpad). The IC50 was determined from these curves.
2.4. Study drug
3BNC117 is a recombinant, fully human IgG1- kappa monoclonal antibody recognizing the CD4 binding site on the HIV-1 envelope [10]. The antibody was originally cloned from an HIV-1-infected viremic controller enrolled in the International HIV Controller Study [10], expressed in Chinese hamster ovary cells (clone 5D5-5C10), and purified using standard methods. The 3BNC117 drug substance was produced at Celldex Therapeutics Fall River (Massachusetts, USA) GMP facility, and the drug product fill-finished at Gallus BioPharmaceuticals (New Jersey, USA).
The positron emitting isotope 64Cu has a half life of 12.8 hours allowing for effective doses of radiation under 5 milliSieverts, a dose considered acceptable for healthy volunteers. 64Cu was produced in the cyclotron at the Department of Molecular Imaging and Therapy at Austin Health, Melbourne using standardised methods as previously described [16].
Radiolabelling was performed on pre-conjugated MeCOSar-3BNC117 within a Class II Biohazard cabinet at a sterile workstation as described above. Radioconjugate formulated in saline containing 5% HSA was drawn into a syringe and a sterile 0.22 micron filter attached. The syringe containing up to 130 MBq of 64Cu-3BNC117, as measured in a radioactive dose calibrator, was transferred to the imaging facility for participant intravenous infusion.
2.5. PET/MRI
Subjects were injected with between 60 to 130 MBq of 64Cu-3BNC117. Following injection, whole-body MR-PET scans were undertaken on a Siemens Biograph mMR scanner at Monash Biomedical Imaging at three sessions with nominal time delays of 1.5, 24 and 48 hrs. Scans were 90-120 minutes in duration with scans on days 1 and 2 not imaging below the knees to maximise recording of PET data in regions where HIV reservoirs were most expected and to help minimise the overall scan time. A series of modified Dixon sequences were used to generate the MR attenuation correction information.
2.6. Pharmacokinetics
Two methods were used to establish PK over time with samples taken at 5 minutes, 1, 4, 24 and 48 hours post infusion.
The radioactivity measure was recorded as counts per minute (cpm) with serum obtained from participants aliquoted and counted in a gamma scintillation counter. Duplicate standards prepared from the injected material were counted at each time point with serum samples to enable calculations to be corrected for the isotope physical decay. The results were expressed as % injected dose per litre (%ID/L) and µg/mL.
Quantitative determination of 3BNC117 in human serum was performed in triplicate using a validated sandwich ELISA assay that uses an anti-idiotypic antibody specifically recognizing 3BNC117 and results expressed as µg/mL11 ELISA was performed on sera from the 3 mg/kg dose level only due to the assay's limits of quantitation for 3BNC117 at the trace dose level.
For PK analysis a two compartment IV bolus model with macro-parameters, no lag time and first order elimination was fitted to first infusion 64Cu-3BNC117 (µg/mL) and ELISA 3BNC117 (µg/mL) measurements for each patient using un-weighted non-linear, least squares with Phoenix WinNonlin version 8 (Certara, Princeton, NJ). Estimates were determined for the pharmacokinetic parameters: T ½ a and T ½ b, the initial and terminal elimination half-lives; VSS, volume at steady state; Cmax (maximum serum concentration); AUC (area under the serum concentration curve extrapolated to infinite time); and CL (total serum clearance). Statistical comparison of individual patient data was not possible due to the small sample size. Comparison of cpm and ELISA PK parameters was performed by Student's t-test with P < 0.05 indicating significant differences. We chose a parametric test given that simulation studies have shown that the t-test is robust to non-normality and small samples sizes [17] and because statistical significance can only be reached using a Wilcoxon test if a minimum of 6 paired data shift in the same direction.
2.7. Study subjects
The study was conducted at either the Department of Infectious Diseases, Alfred Hospital, Melbourne or the Department of Infectious Diseases, Monash Health, Melbourne between October 2018 and December 2019 where 17 individuals were enrolled. PET/MRI scanning and infusions were conducted at Monash Biomedical Imaging, Monash University, Melbourne. (ClinicalTrials.gov number NCT03063788). Inclusion criteria for all participants was age 18 to 65 years and able to provide written informed consent. Inclusion criteria for HIV-uninfected participants also required a negative HIV Ag/Ab test at screening and being amenable to HIV risk reduction counselling and to maintain behaviour consistent with low risk of HIV exposure during the course of the trial. Inclusion criteria HIV-infected participants was a CD4+ T-cell counts >300 cells/μL at screening and with HIV that was sensitive to 3BNC117 by either a viral outgrowth assay or the PhenoSense mAb assay from Monogram Biosciences. One group of HIV-infected participants was off ART for at least 8 weeks with a viral load at screening > 1000 copies/mL and a second group of HIV infected participants had to be receiving ART for at least 12 months with a viral load < 50 copies/mL over that time and a viral load < 20 copies/mL at screening. Individuals with Hepatitis B or C infection and abnormal laboratory examinations were exclusion criteria. Women of childbearing potential were required to have a negative pregnancy test and adhere to contraceptive measures until 8 weeks after receiving the study drug.
2.8. Ethics
Participants were only enrolled after obtaining informed consent and the trial was approved by the Alfred Health Ethics Committee (Approval HREC/16/Alfred/184)
2.9. Screening participants for sensitivity to 3BNC117
Individuals were screened for trial inclusion by two methods (viral outgrowth assay (VOA) and the PhenoSense mAb assay) that assessed capacity of participant virus to neutralize 3BNC1117.
One of two VOAs were performed on peripheral blood mononuclear cells (PBMCs) were isolated from 100-150mL blood from each participant. CD4+ T-cells were isolated from PBMCs by negative selection using the CD4+ T-Cell Isolation Kit, Human (Miltenyi Biotec). In general, the viremic individuals were screened using a CD8-depleted lymphoblast VOA, as described previously [18]. Briefly, participant CD4+ T-cells were stimulated for 24 hrs with PHA (1ug/mL, Thermo Fisher Scientific) in a minimum of six replicate wells of 1.5-3 million cells, followed by co-culture with CD8-depleted lymphoblasts (lymphoblasts were CD8-depleted using the CD8 MicroBeads, Human kit, Miltenyi Biotec). The CD8-depleted lymphoblasts were added in a 1:1 ratio with the CD4+ T-cells. A half media change was performed every 3-4 days and a half culture change was performed every 7th day with the CD8-depleted lymphoblasts being replenished. Aviremic individuals were screened using a MOLT-4-CCR5 cell VOA, as previously described [19]. Briefly, participant CD4+ T-cells were stimulated for 48 hrs with anti-CD3/CD28 microbeads (1 bead per cell, Life Technologies) followed by co-culture with MOLT4-CCR5 cells at a 5:1 ration of T-cells to MOLT4-CCR5 cells. A half culture split was performed every 3-4 days and the supernatants were stored for analysis. Neutralisation was assessed using a TZM-bl cell line that contains a tat-responsive luciferase reporter gene as previously described [20]. This assay assesses the ability of 3BNC117 to prevent infection of TZM-bl cells by virus obtained from supernatant. The supernatant harvested from each replicate VOA well was assessed for neutralization in triplicate, three wells containing 10ug/mL 3BNC117 and three wells without 3BNC117. Luciferase activity was measured 48 hrs after addition of supernatant to the TZM-bl cells using the BriteLite Plus Reporter Gene Assay System (Perkin Elmer). Supernatant from mock cultured cells was used as a negative control. Virus was considered sensitive to 3BNC117 if the luciferase signal in the absence of 3BNC117 was above three times the standard deviation of the negative control wells while the luciferase signal was within three times the standard deviation of the negative control wells in the presence of 3BNC117. Virus was also considered sensitive to 3BNC117 if there was a greater than 10-fold reduction in luciferase signal between the wells containing 3BNC117 versus the wells without 3BNC117. Virus was considered insensitive if there was no difference in luciferase signal between the wells containing versus not containing 3BNC117 and they were both above three times the standard deviation of the negative control wells.
The PhenoSense mAb Assay platform generates pseudovirions that express envelope proteins representing the quasispecies of plasma HIV RNA envelope sequences from viremic individuals or cell-associated HIV DNA envelope sequences from aviremic individuals. These virus particles containing participant derived envelope and a luciferase reporter were assessed by determining their capacity to be neutralised at different concentrations of 3BNC117 and therefore prevent infection of the target cell line. Results are reported as the concentration of 3BNC117 required to inhibit viral replication by 50% (IC50) [21].
2.10. PET image analysis
Qualitative analysis of the biodistribution of 64Cu-3BNC117 was performed by visual inspection of PET images comparing uninfected control subjects with HIV infected viremic and aviremic participants across the three scan timepoints by two experienced Nuclear Medicine physicians.
Quantitative assessment of image datasets for dosimetry analysis was also performed. Using the MIM [22] viewer environment, the reconstructed PET images were fused with a selected Dixon sequence MR image with well-defined organ delineation. The MRI was used to assist in whole organ markup of selected organs for the determination of the cumulated activity per organ where the selected organs were: Liver, Bladder, Red Marrow, Kidney and Heart. The whole-body cumulated activity was also measured from which the remainder-body term was calculated. In the case of the red-marrow term, an average activity concentration was determined which was then scaled by the standard red-marrow mass term provided in the ICRP133 publication [23].
Cumulative activities for each organ were determined from area under the curve (AUC) analysis of the 3-timepoint PET activity curves with a single exponential fit. These cumulated activities were then input to the OLINDA environment which generates a table of radiation dose received by a standard list of organs using the ICRP60 [24] based tissue weightings. A standard 70 kg adult male phantom model was adopted for the dosimetric evaluation.
2.11. Statistical methods
Continuous variables were summarized using mean and confidence intervals (CI) or median and inter quartile ranges (IQR), as appropriate. Numbers and percentage distribution were quantified for categorical variables.
Adverse events were summarized according to severity assessment showing the number of events, and the causality assessment.
The biodistribution of 64Cu-3BNC117 was determined qualitatively from PET images, and descriptive data reported. Dosimetry data and pharmacokinetic parameters were assessed by one-way ANOVA across the three trial groups: HIV uninfected, HIV infected viremic and aviremic (SigmaStat 4.0, Systat Software Inc.).
Differences between the ELISA and gamma counting methods to compare pharmacokinetic parameters in the same participants were assessed by paired T test, with P <0.05 indicating significant differences (SigmaStat 4.0, Systat Software Inc.).
2.12. Study monitoring
An independent data safety monitoring committee established a study progress and safety monitoring plan. They reviewed safety data for all participants at a minimum of 6-monthly intervals and approved the submission to alter the dosing strategy during the trial.
2.13. Role of funding source
Funders had no role in study design, data collection, data analyses, interpretation, or writing of the manuscript
3. Results
3.1. Radiolabeling 3BNC117 with NHS-MeCOSar and 64Cu
Cage amine ligands of the sarcophagine type (3,6,10,13,16,19-hexaazabicyclo[6.6.6]- icosane = Sar) form stable complexes with copper(II) and are well suited for diagnostic imaging with copper-64 [25]. In this work we conjugated 1-CH3,8-NH2CO(CH2)3CO2H-3,6,10,13,16,19-hexaazabicyclo[6.6.6]- icosane, MeCOSar to 3BNC117. Reaction of the N-hydroxysuccinimydyl (NHS) ester of MeCOSar with 3BNC117 followed by purification allowed isolation of MeCOSar-3BNC117. The conjugate was characterized by size-exclusion chromatography and electrospray mass spectrometry which identified between 1 and 3 MeCOSar chelators were attached the antibody with an average of 1.6 (Figure S1a, S1b).
Radiolabeling of MeCOSar-3BNC117 with copper-64 was achieved in 72 % ± 8 % (n=9) isolated radiochemical yield after incubation at room temperature for 15 minutes at pH 5-6. The radiochemical purity and specific activity at the end of synthesis were 97 MegaBecquerels (MBq) ± 12 MBq (n=9) and 99.5% ± 0.3% (n=9), respectively. SEC-high-performance liquid chromatography (HPLC) of 64Cu-MeCOSar-3BNC117 showed negligible aggregation after radiolabeling (Figure S1c). Stability in human serum at 37⁰C was excellent with 96 % ± 1 % (n=3) of 64Cu-MeCOSar-3BNC117 intact after 48 hours.
3.2. Modified antibody does not affect binding or neutralisation in vitro
We then confirmed that modifying 3BNC117 to MeCOSar-3BNC117 and 64Cu-MeCOSar-3BNC117 did not affect binding and neutralisation to HIV Env. We assessed binding to gp140 coated plates by enzyme-linked immunosorbent assay (ELISA) to determine if 3BNC117 retained binding to Env after radiolabeling. Similar levels of binding were observed between 3BNC117, MeCOSar-3BNC117 and 64Cu-MeCOSar-3BNC117 suggesting that the addition of chelator and copper-64 did not significantly affect the binding activity of 3BNC117 (Figure S2a).
We next assessed binding of the 3BNC117 and MeCOSar-3BNC117 antibodies to Env in the context of cell surface expression. In brief, we stably expressed HIV envelope protein from a CCR5 and CXCR4 using HIV infectious clone (AD8 and NL4.3, respectively) on the surface of human embryonic kidney cells (HEK 293T). We then assessed cell binding of 3BNC117 and MeCOSar-3BNC117 by addition of a FITC labeled anti-human IgG and flow cytometry (Figure S2b and S2c). We found no differences in fluorescence intensity for 3BNC117 or MeCOSar-3BNC117 when bound to cells expressing either HIV strain.
Finally, we assessed the neutralisation activity of 3BNC117 and MeCOSar-3BNC117 antibodies. In brief reporter viruses pseudotyped with different strains of HIV envelope (AD8, NK4.3, TRO.11) were used to infect JC53 cells in the presence of increasing concentrations of 3BNC117 or MeCOSar-3BNC117 and assessed for their ability to neutralise virus and inhibit infection. We observed minimal difference in the dose inhibition curves between 3BNC117 and MeCOSar-3BNC117 (Figure S2d). We observed no difference in the inhibitory concentration 50% (IC50), between 3BNC117 and MeCOSar-3BNC117 for the three different reporter viruses (Figure S2e). Overall, we demonstrated that addition of the MeCOSar chelator and copper-64 had no appreciable effect on 3BNC117 function.
3.3. Study participants
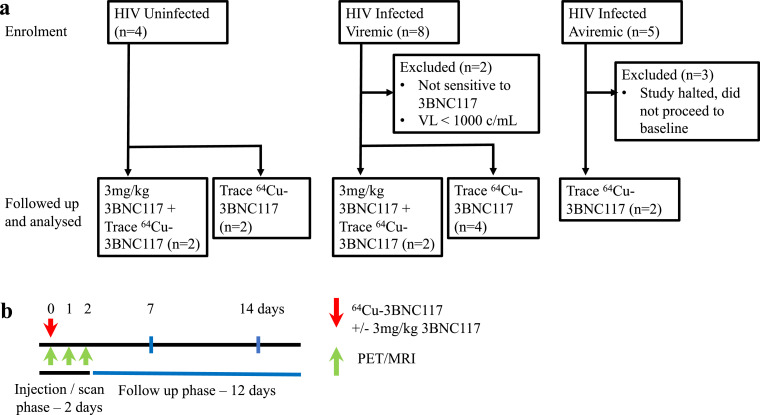
Between October 2018 and December 2019, a total of 17 individuals were enrolled across two study sites in Melbourne, Australia. These comprised four uninfected participants as controls, eight HIV-infected viremic participants and five HIV-infected aviremic participants receiving ART. (Table 1) All HIV-infected participants were screened for infection with virus sensitive to 3BNC117. We stimulated peripheral blood mononuclear cells (PBMC) with phytohaemagglutinin (PHA) + IL-2 and performed a viral outgrowth assay and used virus from the supernatant to infect TZM-bl cells to determine sensitivity to neutralization with 3BNC117. In addition, we used the PhenoSense mAb assay that assessed 3BNC117 neutralisation of pseudovirions that included HIV envelope from each participant. All viremic individuals were off ART with HIV RNA in plasma above 1000 copies/mL and CD4+ T-cell counts above 300 cells/µL, and aviremic individuals had received ART for at least 12 months with HIV RNA < 20 copies/mL. Four individuals (two uninfected, two viremic) completed the trial at the originally approved dosing schedule of 3 mg/kg 3BNC117 with up to 80MBq of 64Cu-3BNC117 and the subsequent six participants completed the trial with an amended dosing protocol that only gave a trace dose of 64Cu-3BNC117 (less than 5 mg), and up to 130 MBq of 64Cu. Detailed dosimetry of the first four participants allowed for this change in 64Cu dose while still adhering to an effective radiation dose of 4.7 mSv, required by regulators in Australia [26]. Two viremic participants failed screening because of a screening viral load below 1000 copies/mL in one participant and virus that was not sensitive to 3BNC117 in another participant. Three aviremic participants that were screened did not proceed to infusion and scanning after an interim analysis demonstrated no clear difference in images from HIV uninfected and infected participants (Fig. 1). All HIV infected participants were male, except for one female participant in the viremic group who received trace dosing.
Table 1.
Baseline characteristics of study participants.
| Group | Study ID | Age | Gender | Dose | CD4 cells / microL | Screening Viral load (c/mL) | Baseline Viral Load (c/mL) | Screening Viral Outgrowth Assay (VOA)a | Screening PhenoSense mAb IC50 (μg/mL)b |
|---|---|---|---|---|---|---|---|---|---|
| HIV uninfected | 101 | 43 | Male | 3 mg/kg + trace | NA | NA | NA | NA | NA |
| 102 | 31 | Female | 3 mg/kg + trace | NA | NA | NA | NA | NA | |
| 103 | 56 | Female | Trace | NA | NA | NA | NA | NA | |
| 104 | 58 | Male | Trace | NA | NA | NA | NA | NA | |
| HIV Infected viremic mg/kg + trace' does the number 3 have its own line? this would be better if '3 mg/kg' was on one line of the cell and the next line of the cell was '+ trace'"?> | 201 | 49 | Male | NA | 600 | 65977 | NA | 0/4 (0%) | > 50 |
| 202 | 49 | Female | Trace | 377 | 33200 | 59052 | 7/10 (70%) | 0.20679 | |
| 203 | 42 | Male | 3 mg/kg + trace | 495 | 130187 | 76486 | 12/12 (100%) | 0.03503 | |
| 204 | 28 | Male | 3 mg/kg + trace | 1012 | 1239 | 331 | 59/62 (95%) | 0.03233 | |
| 205 | 57 | Male | NA | 557 | 79 | NA | NA | NA | |
| 206 | 61 | Male | Trace | 560 | 5649 | 6631 | 5/27 (19%)c | 0.70811 | |
| 207 | 41 | Male | Trace | 391 | 1656 | 651 | 8/8 (100%)d | 0.04288 | |
| 208 | 28 | Male | Trace | 840 | 27209 | 395598 | 13/35 (37%)d,e | 0.07172 | |
| Median (IQR) | 45.5 (37.8-51) | 559 (469 - 660) | 16429 (1552 - 41394) | 32841 (2146-72127) | |||||
| HIV infected aviremicf | 301 | 36 | Male | Trace | 468 | < 20 | < 20 | 4/5 (80%) | 0.04288 |
| 302 | 60 | Male | Trace | 451 | < 20 | < 20 | 10/10 (100%) | 0.02806 |
NOTES:
Reported as the number of wells sensitive to 3BNC117 as a proportion of the number of wells positive for virus
Concentration of antibody required to inhibit viral replication by 50%
Had additional wells considered partially sensitive to 3BNC117. 22/27 wells positive if these wells included
Using Aviremic VOA
including wells that were partially sensitive to 3BNC117, means that 27/35 wells were positive
3 individuals screened but not included for baseline analysis due to study termination and therefore were not included; NA, not applicable; IQR, interquartile range.
Fig. 1.
Trial design. (a) Consolidated Standards of Reporting Trials (CONSORT) flow diagram for 17 enrolled participants. (b) Schematics of study design. Red arrow indicates time of 64Cu-3BNC117 administration with or without additional unlabelled 3mg/kg 3BNC117. Green arrows indicate time of PET/MRI scans.
3.4. No drug related adverse events
No serious adverse events and no adverse events related to study drug were reported. Two individuals experienced back ache after being in the scanner that improved after coming out of the scanner and then worsened again in the scanner on the subsequent study days. This was Grade 1 in severity and related to study procedures. All other adverse events were unrelated to study procedures. There was a single Grade 2 adverse event which was headache that started on day 13 of the study protocol (Table 2).
Table 2.
Adverse events.
| Adverse eventsa | HIV uninfected | HIV infected viremic | HIV infected aviremic | Total |
|---|---|---|---|---|
| Related to study procedures | ||||
| Back acheb | 1 | 1 | 2 | |
| Unrelated to study procedures | ||||
| Vomiting | 1 | 1 | ||
| Diarrhoea | 1 | 1 | ||
| Urinary Frequency | 1 | 1 | ||
| Headache | 1 | 1 | ||
| Conjunctivitis | 1 | 1 | ||
| Nasal congestion | 1 | 1 |
NOTES:
All events Grade 1 except for the headache event which was Grade 2 There were no laboratory adverse events
Symptoms worsened after being in scanner and then improved and then worsened again after being in scanner on subsequent imaging days, both thought related to lying in scanner and not study drug.
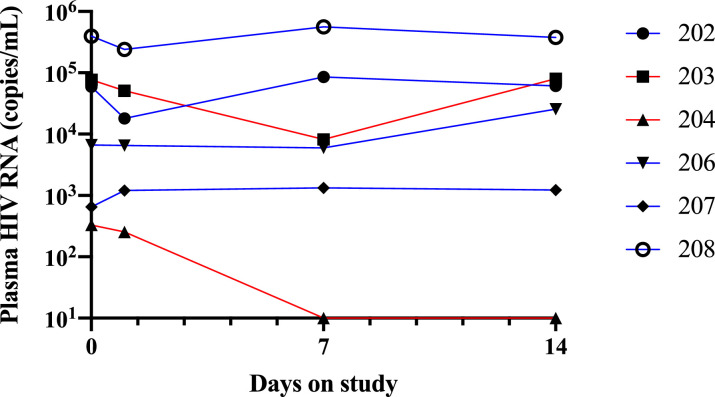
3.5. Reduction in plasma HIV RNA in viremic individuals receiving the 3mg/kg dose
Consistent with previously reported findings [11] individuals receiving 3 mg/kg 3BNC117 experienced a > 1 log10 drop in plasma HIV RNA over the first week after infusion. Viremic participants who received the trace dosing protocol had no change in viral load over the first week (Fig. 2).
Fig. 2.
HIV RNA in viremic participants. Red line received 3 mg/kg 3BNC117 + trace dosing. Blue line received trace dosing only.
3.6. Pharmacokinetics
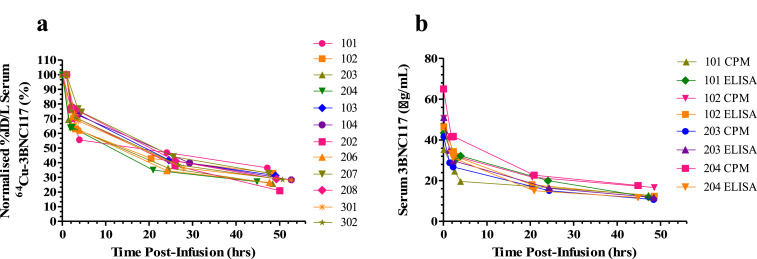
Pharmacokinetic (PK) results for 64Cu-3BNC117 (Table S1) demonstrated a terminal (β) half-life of 2 to 3 days by both methods of measurement, with total mean (± SD) serum clearance ranging from 61.7 (± 4.9) to 157.6 (± 32) mL/hr, but no statistically significant differences in clearance rates between uninfected and infected participants (ANOVA P=0.095) or between dose levels. We compared serum antibody concentrations by an anti-idiotype ELISA, and measured radioactivity in blood. For participants receiving the 3 mg/kg dosing there was comparable PK clearance for ELISA and gamma counting results (Fig. 3).
Fig. 3.
Pharmacokinetic analysis 3BNC117 serum clearance a) Normalised 64Cu-3BNC117 serum clearance for all patients, b) 3mg/kg dose cohort determined by ELISA and gamma counting (CPM).
The comparable PK findings from ELISA and gamma counting (Table S2) at the 3mg/kg dose level (where accuracy of ELISA was consistent with limits of detection of the assay) were consistent with 64Cu and 3BNC117 remaining coupled in-vivo.
3.7. Imaging and dosimetry
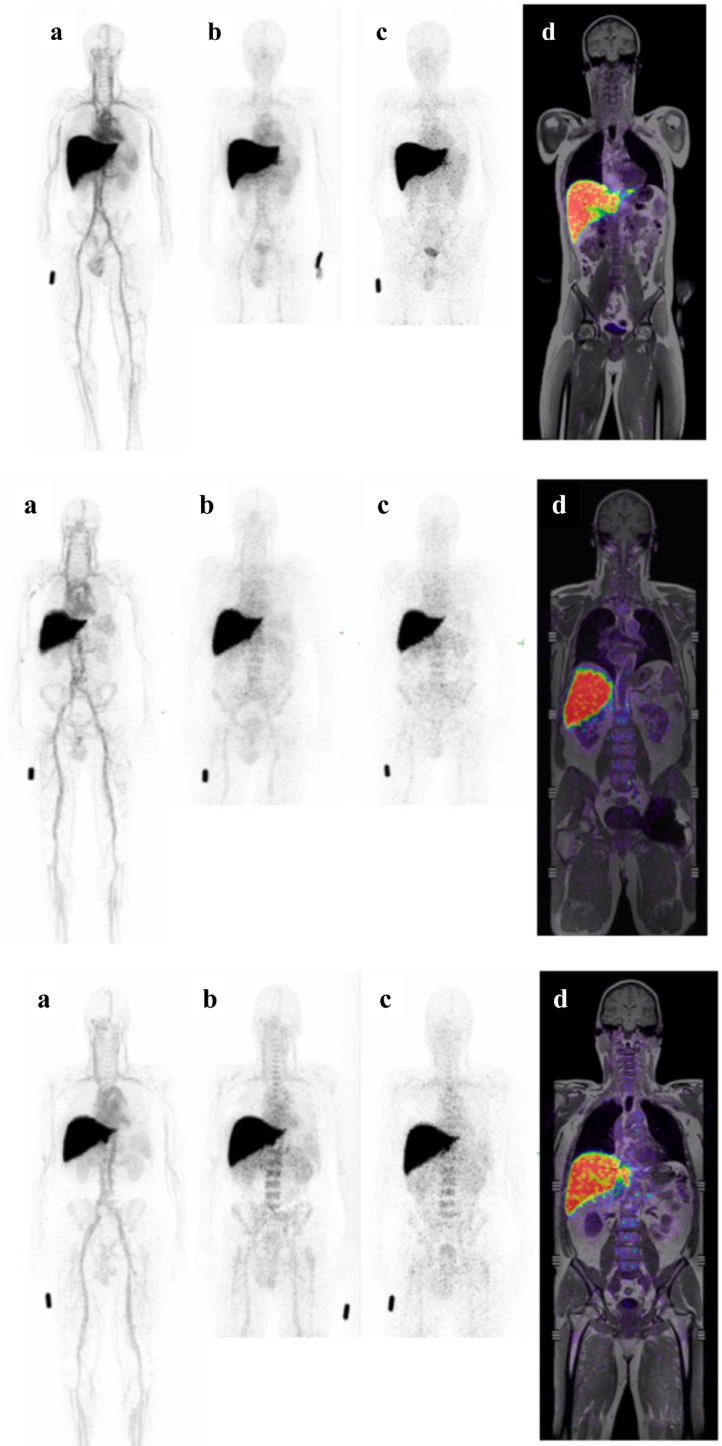
The PET/MRI images for all subjects at all time points were assessed qualitatively and showed consistent patterns of gradual blood pool clearance of 64Cu-3BNC117, and uptake in liver and in bone marrow (Fig. 4). The biodistribution showed no change between dose levels (ie. trace dose vs trace dose + 3mg/kg 3BNC117), and no differences in biodistribution pattern was seen between cohorts (control, HIV +ve aviremic, HIV +ve viremic). Liver and bone marrow uptake were consistent with expected catabolism of the 64Cu-3BNC117 construct. There was no uptake of 64Cu-3BNC117 seen in other organs (eg. lymph nodes, GI tract, lymphatic tissue) in any subject.
Fig. 4.
. 64Cu-3BNC117 Biodistribution from 3 representative participants that receiving trace dosing who were uninfected (top panel [IPH-104]), viremic (middle panel [IPH-208]) and aviremic (bottom panel [IPH-302]). Within each panel there are Positron Emission Tomography (PET) Maximum Intensity Projection (MIP) images at (a) 1, (b) 24 and (c) 48 hours after dosing and a (d) slice of fused coronal Positron Emission Tomography Magnetic Resonance Imaging (MRI) 48 hours after dosing.
The radiation dosimetry for 64Cu-3BNC117 for source organs of Liver, Bladder, Red Marrow, Kidney and Heart, showed a whole-body Effective Dose (HWB) of 37.1 +/- 6.4 µSv/MBq (Table S3). No difference in whole body dose was evident between cohorts (control, HIV +ve aviremic, HIV +ve viremic) (Table S3). Individual organ dosimetry is shown in Table S4.
4. Discussion
We report the findings from the first PET/MRI imaging clinical trial of radiolabelled antibodies administered to healthy controls and PLWH. Injection of 64Cu-3BNC117 followed by serial imaging over 48 hours in HIV-infected and uninfected participants was safe, and no drug related adverse events were observed. We demonstrated pre-clinical data that modified 3BNC117 was able to bind and neutralize HIV envelope with similar efficiency to 3BNC117 and that radiolabeling of MeCOSar-3BNC117 with copper-64 was possible at room temperature in just 15 minutes to give 64Cu-3BNC117 in very high radiochemical yields. 64Cu-3BNC117 showed similar biodistribution and pharmacokinetics between controls, viremic and aviremic HIV-infected participants, when administered at a tracer dose (< 5 mg injected) and when co-administered with supplemental naked 3BNC117 at 3mg/kg dose levels. No uptake of 64Cu-3BNC117 in previously reported HIV reservoirs such as lymphoid tissue or GI tract were seen.
There were several key and important differences between this human clinical trial and the prior published non-human primate study [9]. Firstly, the anti-SIV broadly neutralising antibody 7D3 was a murine IgG while 3BNC117 is a human IgG. In humans and macaques, murine IgG is immunogenic with the production of anti-murine antibodies and has a shorter half-life reported down to one day in humans [27]. Secondly, the NHP study used a pegylated antibody to prolong its half-life and decrease the potential adverse immune reactions to the murine antibodies. The differences in pharmacokinetics and target of 7D3 compared to 3BNC117 may have impacted on the timing of visualization of HIV envelope in tissues by imaging, which could explain the lack of uptake of 64Cu-3BNC117 in our study.
We addressed the possibility of differences in protein dose impacting on biodistribution of 64Cu-3BNC117 by utilizing two different dosing strategies, tracer dosing as in the prior NHP trial, and tracer dosing combined with unlabelled antibody (3mg/kg) which would address any possible impact of a peripheral tissue "sink" and to facilitate binding to HIV targets [28]. The lack of difference in biodistribution between the two dose levels indicates that the temporal course of uptake of 64Cu-3BNC117 may be a factor in assessing HIV envelope expression in tissue, and imaging at longer time points may be beneficial. We also observed a shorter metabolic half-life of 3BNC117 compared to the prior phase I study [11], however we only sampled blood for 48 hours post infusion which will underestimate the terminal half-life clearance, and our Cmax levels and initial clearance curves are highly comparable to the prior study [11].
The expression of HIV envelope in vivo, even in viremic individuals is likely lower than in SIV-infected NHP or when imaging tumor antigens in oncology. In HIV infection, expression of HIV env has been reported at approximately 10 envelope trimers expressed per HIV virion. In contrast, in SIV infection, expression of SIV env per SIV virion is up to ten times higher [29,30].
This relative lack of antigen target in HIV compared to SIV infection and also in oncology studies where typically 103-105 membrane associated protein targets exist per cell [31] may explain why we were unable to detect HIV env expression in vivo in this study. This difference needs to be an important consideration when considering future technologies to image HIV. It should be noted that this imaging technique aims to detect HIV envelope on infected cells and not cell free virus. Other contributing factors include the fact that SIV infection typically generates higher viral loads and increased doses of radioactivity can be given in a NHP study. For example the five viremic macaques imaged by Santangelo et al had a mean weight and viral load of 7.6kg and 570860 copies/mL, respectively and received 81 MBq of copper-64 [9]. This compares to our viremic participants who received tracer dosing in this trial who had a mean weight and viral load of 89.8 kg and 115483 copies/mL, respectively and received 129 MBq of 64Cu-3BNC117. Infusion of a moderately increased dose of 64Cu-3BNC117 could have been offset by the fact that the macaques were over 10 times smaller with higher viral loads compared to the HIV-infected participants imaged in this study.
Our study had several limitations. First, we enrolled a relatively small sample size as this was an early phase trial that may have meant a very low specific signal could have been missed. However, given we examined two different dosing strategies in six participants with clear viremia, it is unlikely that a positive imaging signal was missed. We also only employed the trace dosing strategy in the aviremic group based on our findings from uninfected and viremic cohorts, so cannot draw any conclusions about imaging with 3mg/kg dosing for aviremic individuals. We used a copper-64 radioisotope with a 12-hour half-life, to allow for imaging over the first 48 hours of dosing and an effective radiation dose below 5 mSv, which is required when recruiting uninfected controls in the Australian regulatory environment. The half-life of 3BNC117 is approximately nine days in viremic individuals and 17 days in uninfected individuals [11]. Therefore the 48-hour imaging window in this trial allowed for accurate assessment of the initial distribution and equilibrium phase of the antibody. However, if data beyond this timepoint is required to image the uptake of antibody into tissues this was not able to be captured using 64Cu-3BNC117. It is possible that we didn't detect HIV envelope due to competition from endogenous antibody with the labeled antibody potentially blocking binding to HIV envelope on infected cells. This could be evaluated using pseudotyped virus to infect cells in the presence and absence of labeled 3BNC117 and patient sera and quantification of binding of the antibody using flow cytometry, as previously described [32]. An experiment such as this could identify if endogenous antibodies were able interfere with binding of radiolabeled 3BNC117 to infected cells in vivo. However, we think that this possible explanation is unlikely given the clear binding of labeled anti-env antibody observed in prior studies of SHIV-infected non-human primates.
Although we were unable to detect HIV envelope expression in this study, alternative imaging strategies should be evaluated. One approach could be studies with alternate non-human primate models using chimeric simian-human immunodeficiency viruses (SHIVs) that carry envelope derived from HIV and an appropriate labelled antibody targeting this Env. Other clinical trials could include radiolabeling with a positron-emitting radionuclide with a longer radioactive half-life (e.g. zirconium-89, t1/2 = 79 h) that would allow PET images to be acquired several days post infusion and may allow temporal changes in uptake and improved blood pool clearance to be assessed. Other potential strategies include increasing the available target by combining more than one broadly neutralising antibody that target different sites on envelope, for example combining an antibody such as 3BNC117 that targets the CD4 binding site with an antibody targeting the V3 loop allowing for close to 100% coverage of HIV quasispecies within an individual. Alternatively, use of a latency reversal agent that could increase expression of HIV proteins or performing the imaging procedures soon after ART interruption are possible strategies that may optimise this technique to image the distribution of HIV.
Although the administration of 64Cu-3BNC117 did not demonstrate a difference between uninfected and infected participants, the radiolabeled antibody was safe and, further clinical trials of imaging techniques are warranted.
Data sharing
Publicly available datasets are not available for this trial but requests can be made to the corresponding author.
Declaration of Competing Interest
PD holds intellectual property in, this area of research, which has been licensed from the University of Melbourne to Clarity Pharmaceuticals and has a financial interest in Clarity Pharmaceuticals. PD serves on the Scientific Advisory Board of Clarity Pharmaceuticals. MCN has a patent for 3BNC117 licensed to Gilead and is a Member of the Scientific Advisory Board of Celldex Pharmaceuticals and Frontier Bioscience. SRL reports grants from National Health and Medical Research Council of Australia (NHMRC), grants from National Institutes of Health (NIH), during the conduct of the study; grants from American Foundation for AIDS Research (amfAR), grants from Gilead Sciences, grants from Merck, grants from ViiV, grants from Leidos, grants from Wellcome Trust, grants from Australian Centre for HIV and Hepatitis Virology Research (ACH2), grants from Melbourne HIV Cure Consortium, grants from Victorian Department of Health and Human Services (DHHS), grants from Medical Research Future Fund (MRFF), Other authors have nothing to disclose.
Acknowledgments
Acknowledgements
We would like to acknowledge the contribution of all the trial participants which made the study possible, Monogram Biosciences for assistance performing screening assays for the trial, Clarity Pharmaceuticals for providing the copper chelator and Socheata Chea for assistance with laboratory procedures.
Funding
Funded by the Alfred Foundation, The Australian Centre for HIV and Hepatitis Virology Research with additional support from the Division of AIDS, National Institute of Allergy and Infectious Disease, US National Institutes of Health (U19AI096109). JHM and SRL are supported by the Australian National Health and Medical Research Council.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103252.
Appendix. Supplementary materials
References
- 1.Chun TW, Carruth L, Finzi D. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 2.Finzi D, Hermankova M, Pierson T. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 3.Estes JD, Kityo C, Ssali F. Defining total-body AIDS-virus burden with implications for curative strategies. Nat Med. 2017;23:1271–1276. doi: 10.1038/nm.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher CV, Staskus K, Wietgrefe SW. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A. 2014;111:2307–2312. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardons M, Baxter AE, Massanella M. Single-cell characterization and quantification of translation-competent viral reservoirs in treated and untreated HIV infection. PLoS pathogens. 2019;15 doi: 10.1371/journal.ppat.1007619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caskey M, Klein F, Nussenzweig MC. Broadly neutralizing anti-HIV-1 monoclonal antibodies in the clinic. Nat Med. 2019;25:547–553. doi: 10.1038/s41591-019-0412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mortimer JE, Bading JR, Colcher DM. Functional imaging of human epidermal growth factor receptor 2-positive metastatic breast cancer using (64)Cu-DOTA-trastuzumab PET. J Nucl Med. 2014;55:23–29. doi: 10.2967/jnumed.113.122630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sevcenco S, Klingler HC, Eredics K. Application of Cu-64 NODAGA-PSMA PET in Prostate Cancer. Adv Ther. 2018;35:779–784. doi: 10.1007/s12325-018-0711-3. [DOI] [PubMed] [Google Scholar]
- 9.Santangelo PJ, Rogers KA, Zurla C. Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nat Methods. 2015;12:427–432. doi: 10.1038/nmeth.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheid JF, Mouquet H, Ueberheide B. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caskey M, Klein F, Lorenzi JC. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheid JF, Horwitz JA, Bar-On Y. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016;535:556–560. doi: 10.1038/nature18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu CL, Murakowski DK, Bournazos S. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science. 2016;352:1001–1004. doi: 10.1126/science.aaf1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterjovski J, Churchill MJ, Ellett A. Asn 362 in gp120 contributes to enhanced fusogenicity by CCR5-restricted HIV-1 envelope glycoprotein variants from patients with AIDS. Retrovirology. 2007;4:89. doi: 10.1186/1742-4690-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roche M, Tumpach C, Symons J. CXCR4-using HIV strains predominate in naive and central memory CD4(+) T cells in people living with HIV on antiretroviral therapy: implications for how latency is established and maintained. J Virol. 2020;94 doi: 10.1128/JVI.01736-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponigera SS, Tochon-Danguya HJ, Panopoulosa HP. Proceedings of the 4th International Workshop on Targetry and Target Chemistry AIP Conf Proc. Vol. 1509. 2012. Automated production of 124 I and 64 Cu using IBA Terimo and Pinctada metal electroplating and processing modules; pp. 114–119. [Google Scholar]
- 17.de Winter JC. Using the Student's t-test with extremely small sample sizes. Pract Assess Res Eval. 2013;18:1–12. [Google Scholar]
- 18.Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol. 2005;304:3–15. doi: 10.1385/1-59259-907-9:003. [DOI] [PubMed] [Google Scholar]
- 19.Massanella M, Yek C, Lada SM. Improved assays to measure and characterize the inducible HIV reservoir. EBioMedicine. 2018;36:113–121. doi: 10.1016/j.ebiom.2018.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Gao F, Mascola JR. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeves J, Zheng Y, Olefsky M, et al. Susceptibility to anti-HIV bnAbs is concordant in pre-ART plasma and on-ART PBMC samples: ACTG NWCS413. Conference for Retroviruses and Opportunistic Infections. Seattle, WA, USA2019.
- 22.MIM Software Inc. https://www.mimsoftware.com 11 March 2020.
- 23.Bolch WE, Jokisch D, Zankl M. ICRP Publication 133: The ICRP computational framework for internal dose assessment for reference adults: specific absorbed fractions. Ann ICRP. 2016;45:5–73. doi: 10.1177/0146645316661077. [DOI] [PubMed] [Google Scholar]
- 24.ICRP 1990 recommendations of the international commission on radiological protection. ICRP Publication 60. Ann ICRP. 1991;21 [PubMed] [Google Scholar]
- 25.Alt K, Paterson BM, Ardipradja K. Single-chain antibody conjugated to a cage amine chelator and labeled with positron-emitting copper-64 for diagnostic imaging of activated platelets. Mol Pharm. 2014;11:2855–2863. doi: 10.1021/mp500209a. [DOI] [PubMed] [Google Scholar]
- 26.ARPANSA. Code of practice for exposure of humans to ionizing radiation for research purposes. 2015:1-36.
- 27.Ghetie V, Ward ES, Vitetta ES. Handbook of anticancer pharmacokinetics and pharmacodynamics. Humana Press; Totowa, NJ: 2004. Pharmacokinetics of Antibodies and Immunotoxins in Mice and Humans. [Google Scholar]
- 28.Burvenich IJG, Parakh S, Parslow AC, Lee ST, Gan HK, Scott AM. Receptor occupancy imaging studies in oncology drug development. AAPS J. 2018;20:43. doi: 10.1208/s12248-018-0203-z. [DOI] [PubMed] [Google Scholar]
- 29.Zhu P, Chertova E, Bess J., Jr. Electron tomography analysis of envelope glycoprotein trimers on HIV and simian immunodeficiency virus virions. Proc Natl Acad Sci U S A. 2003;100:15812–15817. doi: 10.1073/pnas.2634931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chertova E, Bess JW, Jr., Crise BJ. Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), Is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 2002;76:5315–5325. doi: 10.1128/JVI.76.11.5315-5325.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boonstra MC, de Geus SW, Prevoo HA. Selecting targets for tumor imaging: an overview of cancer-associated membrane proteins. Biomark Cancer. 2016;8:119–133. doi: 10.4137/BIC.S38542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren Y, Korom M, Truong R. Susceptibility to neutralization by broadly neutralizing antibodies generally correlates with infected cell binding for a panel of clade B HIV reactivated from latent reservoirs. J Virol. 2018;92 doi: 10.1128/JVI.00895-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.