Highlights
-
•
Septic arthritis of the shoulder is rare but potentially devastating. Bilateral shoulder septic arthritis is even more rare of a diagnosis.
-
•
Patients can often present with unimpressive physical examination findings, so it is of utmost importance to keep septic arthritis as a differential diagnosis.
-
•
It is paramount that patients be promptly treated with surgical intervention and the appropriate antibiotic therapy for preservation of life and limb, regardless of the chronicity or severity of the septic joint.
Keywords: Bilateral, Septic, Arthritis, Infection, Shoulder, Endocarditis
Abstract
Introduction
Septic arthritis is an orthopedic emergency that requires rapid diagnosis and treatment. It is typically caused by occult bacteremia which allows bacteria to seed the joint or local invasion of a soft tissue infection. Most cases of septic arthritis are caused by gram-positive bacteria, with the most common culprit being Staphylococcus Aureus. The reason septic arthritis is an orthopedic emergency is because of rapid destruction to cartilage. The mechanism of injury to cartilage is two-fold: bacterial enzymes are directly toxic to joint cartilage, and buildup of exudate can tamponade blood flow and cause anoxic injury. Typically, the knee is the most commonly involved joint. This is followed by the hip, ankle, elbow, wrist, and shoulder in descending order of occurrence. Polyarticular disease makes up a small percentage of these cases and if present, it is usually asymmetric and will involve at least one knee joint.
Presentation of case
Bilateral joint septic arthritis is relatively rare. We present an uncommon case of atraumatic bilateral septic shoulders in an elderly man with a history of heart disease and insidious bilateral shoulder pain after golfing 18 holes. This presentation is unique not only in its rarity but also in its impact on our understanding of septic arthritis in the setting of medical comorbidities and a relatively unimpressive presentation. With a recent golfing day just prior to presentation, differential diagnoses other than septic arthritis included deltoid/rotator cuff muscle strain, acute on chronic rotator cuff tendinosis, acute on chronic rotator cuff tearing, acute flare up of osteoarthritis, rheumatoid arthritis, or crystalline arthropathy. With elevated inflammatory markers and an equivocal physical examination, our patient underwent advanced imaging via MRI and subsequent bilateral glenohumeral joint diagnostic aspirations that were consistent with septic arthritis due to his complaining of contralateral shoulder pain shortly after his admission. Immediately after said diagnosis was made, the patient was taken back for emergent bilateral open irrigation and debridement, as septic arthritis is an orthopedic emergency, and went on to recover appropriately on culture-directed intravenous antibiotic therapy.
Discussion/conclusion
This case report is impactful with regard to clinical practice for multiple reasons. First and foremost it is a cautionary tale for all clinicians with regard to the level of suspicion one must have for polyarticular septic arthritis in the setting of the multiply painful patient. Second, it demonstrates the utility of advanced imaging in the equivocal patient. Lastly, it underscores the importance of prompt diagnosis and treatment, validating the existing algorithm for septic arthritis.
1. Introduction
1.1. Background
Septic arthritis is an orthopedic emergency. Its consequences are potentially devastating to both life and limb; thus, prompt diagnosis and treatment is of utmost importance. Patients usually present with pain/stiffness in the affected joint and fevers, however fever may be in only 40–60% of patients [1]. On physical examination, patients may have erythema and an effusion on inspection, warmth and tenderness to palpation, and an inability to bear weight or accept even minimal passive range of motion. Most recent literature suggests a mortality rate of 11% for monoarticular involvement with as high as 50% in polyarticular involvement [2]. Higher mortality rate is often correlated with comorbid conditions [3,4]. Risk factors for the development of septic joint include age, immunocompromised state (ie diabetes, rheumatoid arthritis, cirrhosis, human immunodeficiency virus), history of crystalline arthropathy, endocarditis or recent bacteremia, intravenous drug abuse, or recent joint injection [5].
The incidence of septic arthritis is relatively low, around 2–6/100,000 [6,7], with polyarticular being approximately 15% of said cases [3]. The knee is the most commonly involved joint with compromising approximately 50 percent of cases. This is followed by the hip, shoulder, elbow, ankle, and sternoclavicular joints [6].
The glenohumeral joint of the shoulder in particular is involved in approximately 3% of patients with monoarticular septic arthritis and 1% in polyarticular, with 88% of said individuals having a comorbid predisposing risk factor [2,6].
1.2. Rationale
Due to the rarity of polyarticular septic arthritis, let alone polyarticular glenohumeral septic arthritis in an afebrile patient, diagnosis and treatment can be delayed leading to potential catastrophic outcomes. As such, there is a concomitant paucity of literature regarding the work-up, management, and treatment of this condition.
In this report, we present a case of bilateral glenohumeral septic joint arthritis in an elderly male with a history of coronary artery disease, highlighting the sometimes insidious nature of this orthopedic emergency. This case report is impactful with regard to clinical practice for multiple reasons. First and foremost it is a cautionary tale for all clinicians with regard to the level of suspicion one must have for polyarticular septic arthritis in the setting of the multiply painful patient. Second, it demonstrates the utility of advanced imaging in the equivocal patient. Lastly, it underscores the importance of prompt diagnosis and treatment, validating the existing algorithm for the management of polyarticular septic arthritis. Of note, this case presentation has been designed in-line with the Surgical Case Report (SCARE) 2020 Guidelines [15].
2. Case presentation
2.1. Patient information
Our patient is a 76 year old male with a past medical history of coronary artery disease status post remote coronary artery bypass, mitral valve regurgitation, left ventricular hypertrophy, and atrial fibrillation/flutter who self-presented to the emergency department with insidious atraumatic right shoulder pain that began on the day of presentation at approximately 0300. Associated symptoms included body aches and chills, but remained afebrile throughout the shoulder pain episode. The initial pain resolved without intervention, but then returned with worsening pain, which prompted him to come to the emergency department. He stated that he played 18 holes of golf the day before his pain began, but denied any other inciting event.
Past Medical History: hyperlipidemia, hypertension, left ventricular hypertrophy, coronary artery disease, mitral valve regurgitation, polyneuropathy, paroxysmal atrial fibrillation, benign prostatic hyperplasia, and copper deficiency
Past Surgical History: coronary artery bypass grafting 20 years prior (2000), cardiac ablation 7 years prior (2013), repair of retinal detachment 7 years prior (2013), L5-L6 laminectomy and fusion 6 years prior (2014), and bilateral inguinal hernia repair 5 years prior (2015)
Medications: Aspirin 81 mg daily, Flomax0.4 mg daily, Voltaren 1% topical gel to apply QID PRN low back pain, Norvasc 10 mg daily, Norco 10/325 mg 0.5–1 tablet Q6h PRN low back pain.
Allergies: Cephalexin and latex, both causing a skin rash
Family History: Unremarkable
Social History: patient was a former half pack-per-day smoker from the age of 15 to the age of 45. He denied alcohol or recreational drug use.
Review of Systems: chronic low back pain but otherwise negative except for the history as described above
2.2. Clinical findings
During his initial emergency department presentation, he complained of severe right shoulder pain similar to the previous episode. Vital signs were within normal limits and the patient was afebrile. On physical examination, the patient had minimal tenderness to palpation over the right glenohumeral joint. On inspection there was no erythema, swelling, fluctuance, or induration. In fact, he had no noticeable wounds or lesions throughout his entire body. Active range of motion of the right shoulder was limited secondary to pain. Passive range of motion was 100 degrees of flexion, 100 degrees of abduction, 45 degrees of internal and external rotation with pain at the extremes of motion. Pain with axial loading of the right shoulder was also noted on the exam.
3. Diagnostic assessment and interpretation
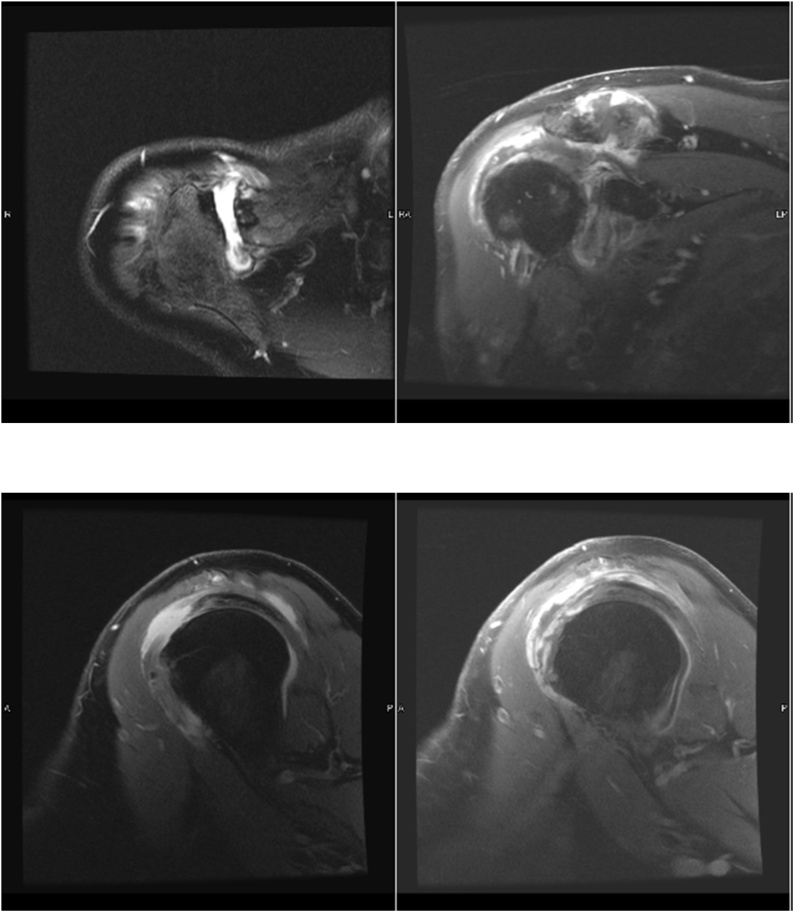
Radiographs of the right shoulder (Fig. 1) were found to be unremarkable and laboratory analysis including white blood cells (WBC) and C-reactive protein (CRP) were elevated at 33,100c/uL (normal range 4800-10,800 c/uL) and 11 mg/dL (normal range: 0–0.8 mg/dL), respectively. Due to a relatively unimpressive physical examination and elevated inflammatory markers, advanced imaging was pursued. The right shoulder MRI demonstrated supraspinatus tearing with intraarticular effusion consistent with septic arthritis (Fig. 2). Prompt aspiration was subsequently performed yielding 10cc of purulent appearing fluid. This was sent to the lab for analysis. Early the next morning, the patient also started reporting left shoulder pain. Examination of the left shoulder was consistent with his previous exam of the right shoulder with severely limited range of motion and pain with axial load; therefore, aspiration was also performed for the left shoulder for diagnostic testing without advanced imaging. Cell count for the shoulders was 197,500 c/um and 99,000 c/um for the right and left shoulders respectively - well above the diagnostic threshold of 50,000 c/um.
Fig. 1.
AP Radiograph of the right shoulder revealing AC joint degenerative changes but no acute osseous abnormality nor any soft tissue compromise. Sternotomy wires are evident.
Fig. 2.
Two Axial T2 weighted MRI cuts and two sagittal T2 weighted MRI cuts revealing a large acromioclavicular joint effusion with irregularly thickened capsule as well as a moderate glenohumeral effusion with a thickened capsule c/w infectious or inflammatory septic arthritis.
With a recent golfing day just prior to presentation, differential diagnoses other than septic arthritis included deltoid/rotator cuff muscle strain, acute on chronic rotator cuff tendinosis, acute on chronic rotator cuff tearing, acute flare up of osteoarthritis, rheumatoid arthritis, or crystalline arthropathy. However given the effusion found on MRI, the positive diagnostic aspirations for cell counts greater than 50,000 c/um, and no crystals being seen under microscopy, we were lead to a diagnosis of septic arthritis.Two positive blood cultures growing Streptococcus Agalactiae (Group B Streptococcus) that resulted one day after patient’s admission also reinforced our diagnosis for septic arthritis.
3.1. Diagnostic imaging
4. Intervention
After a period of being nothing-by-mouth (NPO), fluid resuscitation, empiric therapy with IV ceftriaxone and vancomycin, and medical optimization by the internal medicine team, the patient was then emergently taken for bilateral irrigation and debridement procedures via a deltopectoral approach approximately 24 h after presentation. Open as opposed to arthroscopic approaches were used due to the bilateral nature of the patient’s diagnosis as well as the intention of minimizing reoperations. This operation was performed with an experienced attending orthopedic surgeon with a fellowship in sports medicine and the assistance of a third-year orthopedic surgery resident in a community hospital setting.
4.1. Description of surgical procedure
The operative site was marked. He received IV antibiotics prior to the surgery which were continued, namely Ceftriaxone and Vancomycin. He was brought to the operating room table. General anesthesia was successfully obtained. Patient was placed in the semi beach chair position. All bony prominences were well padded and protected. A time-out was performed confirming the correct operative sides. Bilateral upper extremities were prepped and draped in normal sterile fashion.
A standard deltopectoral approach was utilized to access bilateral shoulders. Deltoid was retracted laterally. The pectoralis tendon was retracted medially.The conjoined tendon was identified and retracted medially. The biceps tendon was identified and used to follow into the rotator interval. The rotator interval was released to allow access to the glenohumeral joint. During the dissection of the left glenohumeral joint, immediate purulent material was encountered following rotator interval release. Sterile cultures were taken of the purulent material. The right shoulder subacromial space had evidence of purulent material and cultures were taken. The right glenohumeral space was also found to have purulent material. Next following adequate release of the rotator interval and biceps pulley debridement we irrigated 9 L of NS through each joint. Attention was made to release subdeltoid, subcoracoid and subacromial space. The above mentioned spaces were free of adhesions and loculations.
Next, a hemovac drain was placed into the bilateral shoulders deep to the deltopectoral interval. The drains were sutured in place. The subcutaneous layer was also closed using 3−0 absorbable monofilament suture. Hemostasis was obtained. Skin was closed using 3−0 nylon suture. The patient tolerated the procedure well without any complications. Sterile dressings were placed. Bilateral UltraSling shoulder immobilizers were placed with the arms in neutral rotation. The patient tolerated the procedure well without any complications and was taken to the recovery room in stable condition.
4.2. Postoperative course
Resulting blood and fluid cultures that were obtained upon initial presentation grew Streptococcus Agalactiae (Group B Streptococcus) and the patient was continued on intravenous (IV) ceftriaxone and vancomycin per recommendations from the infectious disease team. A transthoracic echocardiogram (TTE) performed on postoperative day 1 did not show evidence of obvious cardiac valve vegetations, however endocarditis could not be ruled out without a transesophageal echocardiogram (TEE) that was unfortunately never ordered. Over the next several days, we continued to trend CRP which hovered around 20 mg/dL for one week after his irrigation and debridement, therefore we considered repeating a washout in the operating room. However, on his eighth day post-operatively, the patient had a significant decrease in CRP to 3.80 mg/dL and the decision was made to not repeat another irrigation and debridement. Bilateral hemovacs drained were pulled due to decreased output 5 days after surgery. Due to a high suspicion of bacterial endocarditis by the internal medicine and infectious disease teams, a peripherally inserted central catheter (PICC) line was placed prior to discharge for a continued 6 weeks of Ceftriaxone therapy.
5. Follow-up and outcomes
Patient recovered over the next several days in the hospital with a slowly downtrending CRP. He was discharged home approximately two weeks after admission. With regard to follow-up, the patient was to see his primary care doctor and infectious disease team 1 week after discharge. He was to follow up with orthopedic surgery 10 days after discharge. In addition, he was to undergo intravenous ceftriaxone therapy daily for 6 weeks at an infusion center with weekly complete blood counts and complete metabolic panels. Patient was compliant and adhered to all of the above instructions and orders as prescribed.
The patient was last seen early approximately 3 months postoperatively and is doing well with no signs or symptoms of infection. His only complaint is weakness due to rotator cuff pathology. This was in-line with the expected clinical outcome.
There were no complications or adverse outcomes during the patient’s surgical procedure or during his two week stay.
6. Discussion
Septic arthritis is most commonly the result of microorganisms seeding a joint from underlying bacteremia, direct inoculation or contiguous spread. The infection leads to irreversible destruction to the involved joint as a result of proteolytic enzymes released from inflammatory cells [[5], [6], [7], [8]]. In addition, the built up exudative fluid puts pressure on the adjacent vessels and causes an ischemic environment. This produces a doubly devastating effect on local cartilage due to its dependence on oxygen diffusion through the synovium. Cartilage is avascular in nature, therefore it is dependent on synovial blood flow for oxygen delivery to the tissue. Built up exudative fluid can cause decreased blood flow to the synovium, stifling oxygen delivery to the cartilage, thus resulting in multiple mechanisms of injury [6]. Mortality from septic arthritis is approximately 11%, however severe complications such as limb loss have been reported in the literature [9].
Most cases of septic arthritis are caused by gram-positive bacterial organisms, likely due to their inherent ability to bind to connective tissue. Traditionally, Staphylococcus aureus has been known to be the most likely culprit, however a recent retrospective study found Group B Streptococcus emerging as the most frequently cultured pathogen [5,10]. In general, patients with septic arthritis present with a local erythematous area overlying a painful joint that has limited active and passive range of motion. Patients who develop septic arthritis secondary to Group B Streptococcus are more likely to have polyarticular involvement with involvement of less common sites such as those in the upper extremity [9,10]. A study by Leslie et al. showed that up to 75% of patients with glenohumeral septic arthritis initially presented afebrile with only mildly elevated inflammatory markers [14]. In addition, diagnosis of patients with glenohumeral arthritis is often delayed due to an atypical location and presentation. Similarly to the literature to date, our patient presented with polyarticular involvement with culture positive Group B Streptococcus. Furthermore, he did not present with erythematous regions over his painful shoulders; however, his diagnosis was not delayed.
The diagnosis of glenohumeral septic arthritis must take into account clinical and laboratory findings. Classically, a cell count of 50,000 c/um has been used as a marker for infection, however a cell count less than this has frequently been seen in patients with infections, especially those that are immunocompromised [6,8,11]. Criteria was outlined by Newman in 1976 when he suggested a diagnosis of septic arthritis was present if bacterial cultures can be isolated from joint fluid, isolation of bacteria from another source in the body, radiologic evidence, or turbid fluid from the affected joint [12]. Diagnosis is still predominantly clinical which can be confirmed with isolating bacteria in joint fluid aspirate. Imaging such as magnetic resonance imaging can be helpful, however it cannot differentiate septic causes versus inflammatory causes of arthritis [7]. In our patient, an elevated serum white blood cell count of 33,100 c/uL and c-reactive protein of 11 mg/dL provided us with a hint regarding a potentially inflammatory process. These laboratory values, in concert with a suspicious MRI, led to a diagnostic aspiration, ultimately revealing our causative diagnosis.
Septic arthritis of a native joint is an orthopedic emergency and rapid identification and treatment with joint irrigation and debridement as well as intravenous antibiotics is recommended [7]. Due to the high prevalence of methicillin-resistant staphylococcus aureus causing septic arthritis, targeted empiric antibiotic therapy such as vancomycin should be considered. In addition, gram-negative bacteria have been now shown to be increasing in prevalence and therefore cefepime or an antipseudomonal agent may also be given if the patient is immunocompromised, or has a history of intravenous drug use. Patients experiencing septic arthritis from an animal or human bite wound are at high risk of infection by oral flora and treatment should include penicillin to combat these organisms. Once the causative organism has been identified with fluid cultures, therapy can be narrowed based on sensitivities of the bacteria. Generally, patients will require antibiotic treatment for at least 6 weeks and may require up to 8 weeks of treatment depending on infection location and cause [1]. In addition to antibiotic treatment, multiple rounds of open or arthroscopic joint irrigation and debridement may be necessary. In fact, up to 30–40% of patients with glenohumeral arthritis may require multiple washouts for eradication of the infection [13]. Even then, 16.4% of patients may have recurrence at 40 months following initial eradication [4]. Thankfully, our patient did not require multiple irrigation and debridements but did complete his course of antibiotics.
A case series done by Leslie et al. demonstrated poor long term outcomes for patients with a delayed diagnosis of glenohumeral arthritis. All of the patients with a delay in diagnosis of 3 days ultimately progressed to a limited range of motion, which highlights the importance of early diagnosis and treatment [11,14]. Clearly, septic arthritis is not only dangerous due to its impact on the rest of the body, but also for its devastating consequences on quality of life.
Regarding strengths, this case report underscores the importance of prompt diagnosis and management of septic arthritis even in patients who do not present with grossly infected appearing extremities. It also validates an algorithm used to diagnose and treat polyarticular septic arthritis, namely, open surgical intervention and an extended period of intravenous antibiotics,
Regarding weaknesses, this case report may have benefitted from longer term follow up and evaluation; however, this patient did not continue to follow up after 3 months postoperatively.
7. Conclusion
Although our patient’s diagnosis of bilateral glenohumeral joint septic arthritis was prompt, his history of preceding physical activity was a red herring; his play of 18 holes of golf prior to presentation could lead many clinicians to a diagnosis of muscle strain, soft tissue sprain, or adhesive capsulitis. However, his physical examination, which ultimately led to further diagnostic labs and imaging, was paramount in the decision-making algorithm. Indeed, if there is any lesson to be drawn from this case report, it is that a diagnosis of septic arthritis should always be on a list of differential diagnoses, especially in those with chronic medical conditions and/or immunocompromised states such as our patient. This will lead to earlier diagnosis and treatment, helping prevent morbidity and mortality.
Declaration of Competing Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Sources of funding
The only source of funding utilized was the Community Memorial Health System: Graduate Medical Education program. The funding was utilized to submit this manuscript. There was otherwise not outside funding nor sponsorship used.
Ethical approval
The Community Memorial Health System IRB exempted our study due to its nature of being a case report with the understanding that patient would provide written consent to the study. If there are any questions or concerns regarding the ethics of this patient, please contact Dr. Graal Diaz at gdiaz.con@cmhshealth.org.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
All authors contributed equally to the composition of this manuscript.
Registration of research studies
Not Applicable.
Guarantor
Ammer Dbeis, DO.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Hotonu S.A., Khan S., Jeavons R. Bilateral shoulder septic arthritis in a fit and well 47-year-old man. BMJ Case Rep. 2015;2015(November (201)) doi: 10.1136/bcr-2015-211406. pp. bcr2015211406–bcr2015211406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clements J., Dinneen A., Heilpern G. Polyarticular septic arthritis in an immunocompetent patient. Ann. R. Coll. Surg. Engl. 2013;95(March (2)):e10–e11. doi: 10.1308/003588413X13511609955292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirchhoff C., Braunstein V., Buhmann (Kirchhoff) S., Oedekoven T., Mutschler W., Biberthaler P. Stage-dependant management of septic arthritis of the shoulder in adults. Int. Orthop. 2009;33(August (4)):1015–1024. doi: 10.1007/s00264-008-0598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweet M.C., Sheena G.J., Liu S., Fisk F.E., Lynch J.R., Muh S.J. Clinical characteristics and long-term outcomes after septic arthritis of the native glenohumeral joint: a 20-year retrospective review. Orthopedics. 2019;42(January (1)):e118–e123. doi: 10.3928/01477447-20181227-01. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg D.L., Sexton D.J. UpToDate; 1985. Septic Arthritis in Adults. p. 20. [Google Scholar]
- 6.Ross J.J. Septic arthritis of native joints. Infect. Dis. Clin. North Am. 2017;31(June (2)):203–218. doi: 10.1016/j.idc.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Mathews C.J., Weston V.C., Jones A., Field M., Coakley G. Bacterial septic arthritis in adults. Lancet. 2010;375(March (9717)):846–855. doi: 10.1016/S0140-6736(09)61595-6. [DOI] [PubMed] [Google Scholar]
- 8.Mehta P., Schnall S.B., Zalavras C.G. Septic arthritis of the shoulder, elbow, and wrist. Clin. Orthop. 2006;451(October):42–45. doi: 10.1097/01.blo.0000229322.30169.29. [DOI] [PubMed] [Google Scholar]
- 9.Wang V. A comparison of Streptococcus agalactiae septic arthritis and non-Streptococcus agalactiae septic arthritis. Singapore Med. J. 2018;59(October (10)):528–533. doi: 10.11622/smedj.2018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruksasakul R., Narongroeknawin P., Assavatanabodee P., Chaiamnuay S. Group B streptococcus is the most common pathogen for septic arthritis with unique clinical characteristics: data from 12 years retrospective cohort study. BMC Rheumatol. 2019;3(December (1)):38. doi: 10.1186/s41927-019-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen L. A retrospective review of native septic arthritis in patients: can we diagnose based on laboratory values? Cureus. 2020;(June) doi: 10.7759/cureus.8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman J.H. Review of septic arthritis throughout the antibiotic era. Ann. Rheum. Dis. 1976;35(June (3)):198–205. doi: 10.1136/ard.35.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel M.P., Perry K.I., Morrey M.E., Steinmann S.P., Sperling J.W., Cass J.R. Arthroscopic management of native shoulder septic arthritis. J. Shoulder Elbow Surg. 2013;22(March (3)):418–421. doi: 10.1016/j.jse.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 14.Leslie B.M., Harris J.M. Driscoll DSeptic arthritis of the shoulder in adults. J. Bone Jt. Surg. 1989;71(10):1516–1522. [PubMed] [Google Scholar]
- 15.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 Guideline: Updating Consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;(84):226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]