Abstract
The first description of the medical use of licorice appeared in “Shennong Bencao Jing”, one of the well-known Chinese herbal medicine classic books dated back to 220–280 AD. As one of the most commonly prescribed Chinese herbal medicine, licorice is known as “Guo Lao”, meaning “a national treasure” in China. Modern pharmacological investigations have confirmed that licorice possesses a number of biological activities, such as antioxidation, anti-inflammatory, antiviral, immune regulation, and liver protection. 18β-glycyrrhetinic acid is one of the most extensively studied active integrants of licorice. Here, we provide an overview of the protective effects of 18β-glycyrrhetinic acid against various acute and chronic liver diseases observed in experimental models, and summarize its pharmacological effects and potential toxic/side effects at higher doses. We also make additional comments on the important areas that may warrant further research to support appropriate clinical applications of 18β-glycyrrhetinic acid and avoid potential risks.
Keywords: 18β-glycyrrhetic acid, licorice preparation, liver injury, glycyrrhetinic acid target
Introduction
18β-Glycyrrhetinic acid (18β-GA) is an in vivo metabolic component of glycyrrhizic acid (GL) formed after two sugar moieties are removed by the intestinal flora. 18β-GA is widely considered one of the main active substances of licorice [1]. The current research on the biological activities of 18β-GA mainly focuses on its adrenal cortical hormone-like properties and anti-inflammatory [2, 3], immunoregulatory [4], anti-tumor [5, 6], anti-injury [7], and antioxidative activities [8, 9]. Based on the liver protective properties of licorice, some scholars have suggested that 18β-GA may also possess a strong liver protective effect [10–12]. In this review, we will summarize the liver protective effects of 18β-GA and its mechanisms in different models of liver injury.
Biological activity of 18β-GA
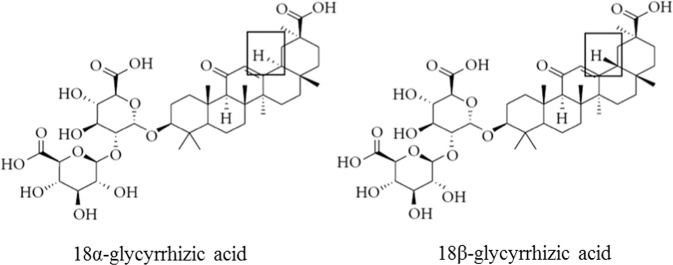
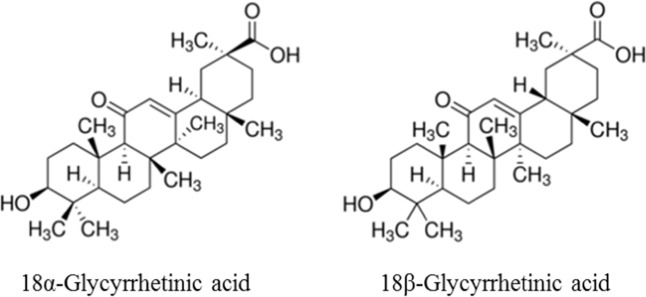
Licorice was initially described in “Shennong Bencao Jing”, an ancient Chinese book on agriculture and medicinal plants. Licorice remains the most commonly used traditional Chinese medicine today. It is also a commonly used ingredient in food, beverages, tobacco, and cosmetics. One of the most important known naturally occurring components of licorice is GL. This component has been explicitly recommended as a hepatoprotective drug by several guides [13, 14]. There are two epimers of GL, namely, 18α-GL and 18β-GL (Fig. 1) [9]. After oral administration, 18α-GL accumulates mainly in the liver [9] and has potent antifibrotic effects, which are significantly higher than those of 18β-GL [15]. 18α-GL is the main active ingredient of TianqingGanmei and Ganlixin, two anti-inflammatory and liver enzyme-lowering hepatoprotective drugs developed in China. Additionally, pharmacokinetic studies have shown that 18α- and 18β-GL can be converted to their respective metabolites, 18α- and 18β-GA, at a significantly higher rate. A preliminary study suggested that 5 min after mice were intraperitoneally administered 18α-GL and 18β-GL, the substances were distributed in all organs. Except for those in blood, the cumulative levels of these substances were the highest in the liver, and the blood concentration of 18α-GL was significantly higher than that of 18β-GL. As time passed, 18α-GL decreased rapidly, and by 3 h, the 18α-GL content was only one tenth of the 18β-GL content. This indicates that the metabolism rate of 18α-GL in mice is significantly faster than that of 18β-GL [16]. Furthermore, pharmacokinetics studies have indicated that the rate of conversion of 18α-GL to GA (including 18α-GA and 18β-GA) is significantly higher than that of 18β-GL. This may be the main reason why 18α-GL is more potent than 18β-GL. When GL is hydrolyzed, the content of 18β-GA is above 97% [17]. In addition, almost 100% of GL is irreversibly transformed into 18β-GA in the gastrointestinal tract by bacterial β-D-glucuronidase after it is orally administered [12, 18, 19]. Therefore, 18β-GA is the main metabolite of GL and the main active component of licorice root [20]. GA is a pentacyclic triterpenoid derivative of beta-amyrin and is the aglycone of GL [12], which has two major optical isomers, as shown in Fig. 2.
Fig. 1.
Chemical structure of glycyrrhizic acid (GL)
Fig. 2.
Chemical structure of glycyrrhetinic acid (GA)
18β-GA protects against different types of liver diseases
The liver is the main metabolic and defensive organ of the human body, and it performs multiple functions, such as biotransformation and detoxification [21]. When the liver is overloaded, liver dysfunction, massive hepatocyte necrosis, abnormal liver histopathology, and liver failure develop, resulting in various complications, such as jaundice, hepatic encephalopathy, hepatic nephropathy, and even death. According to statistics, the number of liver disease cases worldwide is as high as 1.3 billion, and approximately one-third of these cases occur in China [22]. Liver disease can be divided into chronic, subacute, and acute liver diseases based on the onset of liver injury [23]. Subacute liver injury refers to acute liver dysfunction that occurs in chronic liver disease. However, due to regional differences in the definition and diagnostic criteria of subacute liver injury both domestically and internationally and because there is no unified prognostic evaluation system, relatively few studies on subacute liver injury have been performed. Due to the different pathogeneses and clinical manifestations of various liver diseases and the mutual influence of pathogenic factors, the treatment of liver injuries remains a difficult challenge to healthcare providers.
Acute liver injury
Recent wide-ranging studies on acute liver injury have focused on massive hepatocyte death or the loss of liver function caused by various etiologies or pathogenic factors in the absence of chronic liver disease. The causes of acute liver injury are numerous and complex and include viruses, drugs, excessive alcohol intake, ischemia, and food poisoning. The incidence of liver damage remains high in China and around the world.
Drug-induced liver injury (DILI)
Toxic damage to the liver caused by various prescription or nonprescription drugs or their metabolites is called drug-induced liver injury (DILI) and is common. DILI is one of the most common and serious adverse drug reactions and can cause acute liver failure or even death [24]. The main reason for the susceptibility of the liver to adverse drug reactions is probably its central role in biotransformation, which involves cytochrome P450 (CYP; phase 1), conjugation (phase 2), and transportation (phase 3) [20, 25, 26]. Recent studies have shown that 18β-GA pretreatment can minimize cyclophosphamide-induced hepatotoxicity [27] and reduce methotrexate-induced oxidative stress and liver injury [28], both of which are closely related to the activation of the Nrf2 [29] and PPARγ pathways, which may be related to ERK [30] or Sirt1 [7]. A variety of Chinese herbal medicines contain hepatotoxic pyrrolizidine alkaloids (PAs), which may cause acute and chronic liver damage in humans [31]. 18β-GA pretreatment for three days significantly reverses PA-induced liver injury in SD rats, as indicated by significant reductions in serum GOT and GPT levels and various transaminase levels [32]. It was reported that 18β-GA significantly reduces APAP-induced hepatic inflammatory cell infiltration (which involves the JNK and NF-kB pathways [33]), decreases HMGB1-TLR4 signaling activation [29], downregulates CYP2E1 expression and significantly reduces ROS production [34]. In addition, 18β-GA can increase the expression level of Sirt 6, inhibit the translocation of the HMGB1 protein from the nucleus and reverse the lipopolysaccharide-induced extracellular accumulation of HMGB1 through the same mechanism [35]. 18β-GA pretreatment can significantly reduce liver ALT and AST levels and serum fatty acid and carnitine level and improve APAP-induced hepatotoxicity by reversing the metabolism of fatty acids [11]. Conversely, it has also been reported that GL can rapidly improve APAP-induced liver damage by directly inhibiting TNFα-induced hepatocyte apoptosis. However, 18β-GA does not have the potential to treat the effects of excess APAP [36]. 18β-GA promotes the production and accumulation of GSH, improves the viability of hepatocytes, and alleviates hepatotoxicity induced by isoniazid (INH) [37]. 18β-GA pretreatment significantly improves 2-AAF-induced lipid peroxidation and decreases ALT and AST levels, xanthine oxidase activity, and 2-phase detoxification enzyme activity. 18β-GA has a potential hepatoprotective effect by attenuating oxidative stress, inflammation, and hyperproliferation [38].
Cholestatic liver injury
Cholestasis is a complication of a variety of hepatobiliary diseases. The main manifestation of cholestasis is abnormal bile secretion or excretion. α-Naphthyl isothiocyanate (ANIT) is a toxin widely used to mimic human clinical cholestatic liver damage. It was found that 18β-GA can promote cholestasis by promoting bile flow and has significant effects against ANIT-induced liver injury in rats [39, 40]. Data have also demonstrated that 18β-GA can prevent ANIT-induced liver damage by regulating the expression of bile acid transporters and reversing bile acid metabolites [39]. Our laboratory also demonstrated that 18β-GA can promote bile acid efflux transporter expression, regulate bile acid balance, and improve ANIT-induced cholestatic liver injury by activating the Sirt1/FXR signaling pathway [7]. The 18β-GA derivative TY501 also reduces LCA-induced cholestatic liver injury by activating the FXR-mediated upregulation of efflux transporters such as Bsep, Mrp2, and Ntcp [41].
Fulminant liver failure
Fulminant hepatic failure (FHF) is caused by many factors, such as viral infection and APAP overdose. FHF is characterized by rapid hepatocyte death and a loss of liver function with temporal and regional differences as well as poor prognosis that may be related to high mortality [42]. Liver injury induced by D-galactosamine/lipopolysaccharide (D-GalN/LPS) is the most commonly used experimental model of fulminant hepatic failure. A mouse model of acute inflammation induced by Propionibacterium acnes/LPS has been used to study the potential mechanism of 18β-GA. 18β-GA can improve acute P. acnes-induced liver injury by downregulating MyD88 expression, inhibiting NF-κB activation, and decreasing MIP-1α expression in Kupffer cells [43]. 18β-GA pretreatment can inhibit IRAK-1, subsequently hinder the MAPK and NF-κB signaling pathways, inhibit TNF-α production, and reduce LPS/D-GalN-induced liver inflammation and liver failure by upregulating IRAK-M [44]. 18β-GA has a strong anti-inflammatory effect by inhibiting the expression of NO, PGE2 and ROS induced by LPS and the expression of proinflammatory genes by inhibiting NF-κB and PI3K activities [45–47]. 18β-GA significantly inhibits the expression of the dendritic cell (DC) surface molecules CD80, CD86, and major histocompatibility complex (MHC) class I and class II and the production of interleukin-12 in LPS-stimulated DCs and thus has the potential to treat DC-related acute and chronic liver diseases [48].
Chronic liver disease
Chronic liver injury is a long-term progressive disease caused by a variety of factors that can progress to liver fibrosis and cirrhosis [49]. Common experimental chronic liver injury models include alcoholic liver injury, nonalcoholic fatty liver disease, and CCl4-induced liver fibrosis.
Fatty liver disease
Fatty liver disease is the excessive deposition of lipids in the liver caused by excessive fat intake and decreased triglyceride secretion [50]. The incidence of fatty liver disease in China is reported to be as high as 10% [51] and is increasing each year. Thus, fatty liver disease is one of the most serious public health threats in China. It was demonstrated that 18β-GA inhibits fat production by inhibiting MAPK activation and significantly reduces the obesity index, lipid deposition and plasma lipid levels in HFD-induced animals [52]. 18β-GA can also inhibit the expression of cathepsin B and enzyme activity by stabilizing the integrity of lysosomes and mitochondria, significantly reducing FFA/HFD-induced hepatic lipotoxicity and protecting against fatty liver disease [53]. 18β-GA can significantly improve hepatic steatosis, inflammation and fibrosis induced by methionine- and choline-deficient diets by regulating the bile acid balance and inhibiting inflammatory damage [54].
Licorice has been shown to have a significant protective effect against alcohol-induced fatty liver disease due to its potent anti-inflammatory and antioxidant activities [55]. Glycyrrhizic acid, as one of the major active compounds in licorice, also has significant effects against alcoholic liver disease (ALD) by regulating oxidative stress and lipid metabolism [56]. Glycyrrhizic acid may also prevent alcoholic hepatitis (AH) through its anti-inflammatory effect [57]. It is well known that excessive drinking causes ALD. Its main features are fat accumulation, inflammation and scarring. The current standard drug treatments for alcoholic liver disease, such as corticosteroids and pentoxifylline, which are mainly used to reduce inflammation in patients with acute alcoholic hepatitis, target the inflammatory pathway. In addition, 18β-GA is an active in vivo metabolite of glycyrrhizic acid (GL), which has also been shown to have significant anti-inflammatory and lipid metabolism regulatory effects. To conclude, the aforementioned scientific data suggest that 18β-GA has a potent protective effect against alcohol-induced liver injury.
Hepatic fibrosis
Hepatic fibrosis is the abnormal proliferation of connective tissue in the liver caused by a variety of factors, including viral hepatitis, alcohol, autoimmune diseases, drug induction and biliary obstruction [58]. Further development of liver cirrhosis leads to hepatic fibrosis. Long-term subcutaneous injection of CCl4 is widely used to model liver fibrosis in animals, as CCl4 can cause lipid peroxidation and destroy the membrane structure of hepatocytes [59, 60]. 18β-GA can exert strong effects against chronic liver fibrosis by activating the nuclear translocation of Nrf2 to reverse CCl4-induced liver oxidative stress in mice [61]. 18β-GA inhibits CCl4-induced hepatocyte apoptosis via the p53-dependent mitochondrial pathway, thereby delaying the progression of hepatic fibrosis in rats [62]. 18β-GA can prevent alcohol- and CCl4-induced liver fibrosis in rats by inhibiting the proliferation and activation of HSCs to reduce the production of collagen [63]. 18β-GA significantly inhibits liver fibrosis in mice by inhibiting the nuclear accumulation of Smad3 and Smad3-dependent type 1 collagen synthesis and the transdifferentiation of resting HSCs into activated HSCs [64].
Bile duct ligation (BDL) is another animal model that is widely used to simulate liver fibrosis and cirrhosis caused by long-term cholestasis. 18β-GA treatment significantly reduces collagen deposition in the liver of BDL rats and the gene transcription of α-SMA and TGF-β1 in HSCs [65]. In addition, 18β-GA significantly inhibits hepatitis B surface antigen (HBsAg) and significantly improves HBV-induced liver dysfunction in humans and animals [66].
Cirrhosis
Cirrhosis is a common intrahepatic diffuse fibrosis caused by one or more of the following factors: hepatocyte necrosis, interstitial inflammatory reactions, or liver deformation and hardening. Cirrhosis eventually causes a variety of complications, including liver cancer. It has been reported that the long-term administration of 18β-GA can significantly inhibit liver fibrosis in SD rats by reducing interstitial inflammation and the occurrence of cirrhosis [67], although the detailed anticirrhotic mechanism of 18β-GA has not been further studied.
Hepatocellular carcinoma (HCC)
Hepatocellular carcinoma (HCC) is one of the most serious forms of liver diseases worldwide, and in the absence of proper care and treatment, HCC is one of the leading causes of death [22]. Although anticancer drugs and surgery are widely used for the treatment of liver cancer in the clinic, side effects and other adverse reactions influence the treatment outcomes of liver cancer. Recently, a traditional Chinese medicine product, which may reduce the side effects of standard chemotherapy, prolong survival and improve quality of life, has been used as an alternative therapy for patients with liver cancer [68]. A variety of Chinese medicines have been used alone to treat liver cancer with perceived benefits; however, their treatment effects are often difficult to evaluate due to various compounding factors and the complexity of the disease. On the other hand, the benefits of immunomodulatory and/or anti-inflammatory activities for cancer patients are widely accepted. One Chinese medicine commonly used is Bu-Zhong-Yi-Qi-Tang (BZYQT), which can strengthen immune functions, improve the defense system, and fight against liver cancer in HCC patients [69]. Danshen (Salvia miltiorrhiza) is a typical Chinese medicine that can exert anti-inflammatory, antifibrotic and anticancer effects by inhibiting p38 and NF-κB signaling [70]. Glycyrrhizic acid and glycyrrhetinic acid have been used as liver protective drugs for treating chronic hepatitis in China for over a decade. It was reported that 18β-GA can inhibit the proliferation of HepG2 cells by increasing reactive oxygen species, increasing the loss of mitochondrial membrane potential, and relieving the effects of hepatocellular carcinoma [71]. A recent study suggested that 18β-GA induces apoptosis in HCC cells by inducing cell cycle arrest, activating caspase-dependent pathways, and activating the PPARγ pathway [72]. 18β-GA reverses HSC-mediated immunosuppression in HCC and eventually abolishes HCC cell invasion and metastasis [4].
In addition, new formulations of 18β-GA using functionalized nanomaterials, including liposomes, micelles, and nanospheres, and other formulations have been used to treat hepatocellular carcinoma [73–76]. These modified formulations of 18β-GA using nanomaterials induce higher uptake of 18β-GA by HCC cells than unmodified formulations; thus, they are expected to have a stronger inhibitory effect on HCC. The potential mechanisms of the anticancer activity of 18β-GA involve antiproliferative, pro-apoptotic and anti-invasive/antimetastatic activities [77].
Autoimmune liver disease
Autoimmune liver disease (AILD) is attributed to autoimmune abnormalities characterized by the presence of autoantibodies in the circulation. AILD is mainly divided into the following three categories: autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC). Among those AILDs, AIH is mainly characterized by hepatitis, and PBC and PSC are mainly characterized by cholestasis [78]. Clinical or laboratory diagnosis of AILD, as a chronic liver injury, is usually based on partial symptoms. Interventions for AILD include but are not limited to the use of prednisolone to improve inflammation, the administration of ursodeoxycholic acid (UDCA) to improve cholestasis, and the use of rifampicin to improve pruritus. 18β-GA inhibits the development of autoimmune diseases in autoimmune lpr mice. 18β-GA also inhibits urinary protein excretion and serum IgG levels and improves autoimmune diseases in mice [79].
GL preparations have been used in combination with UDCA to treat AILD, and such combinations have been reported to have significant therapeutic effects. One such preparation, TianqingGanping combined with UDCA, was used to treat PSC patients and showed a significant effect in the early stage; initially, the effect was remarkable. However, after 1 year of treatment, the bile acid, total bilirubin, glutamyl transpeptidase (GGT) and alkaline phosphatase (ALP) levels of patients increased to varying degrees and fluctuated repeatedly, and the treatment effect decreased with the progression of the disease [80, 81]. It has been found that the combination of magnesium isoglycyrrhizinate and UDCA is more effective than the individual agents alone in patients with PBC [82, 83]. Diammonium glycyrrhizinate combined with UDCA can significantly improve the symptoms of autoimmune hepatitis patients and improve autoimmune function and liver function [84, 85]. 18β-GA has not been tested in patients with autoimmune liver disease in clinical trials. The exact reasons are unknown but might be related to the poor solubility of 18β-GA and insufficient experimental evidence.
Practical issues of 18β-GA as a hepatoprotective agent
The use of licorice as a detoxifying herb was reported in “Shennong Bencao Jing”, a classic Chinese herbology book published more than 2500 years ago. Later, many licorice-containing prescriptions were also recorded in traditional Chinese medicine masterpieces, such as Shanghan Lun (Han Dynasty), JinGui Yaolue (Han Dynasty) and Bencao Gangmu (Ming Dynasty). The successful extraction and purification of GL from licorice and semisynthetic preparations of glycyrrhizin by Japanese pharmaceutical companies were reported in the 1940s. Japanese scientists also combined glycyrrhizin (GL) with glycine and cysteine (methionine) to form a compound glycyrrhizin product, commonly known as SNMC, Meineng, and Qianglixin, which has been used for the treatment of chronic hepatitis since 1958 with apparent success. Two SNMC-like GL preparations, Ganlixin and TianqingGanmei, were developed in China as first- and second-generation liver protective medicines. The main component of Ganlixin is a mixture of α- and β-glycyrrhizic acid diammonium salts (also known as diammonium glycyrrhizinate). β-Glycyrrhizic acid diammonium can cause hypertension and edema in a small number of patients. TianqingGanmei, prepared from a pure α-GL derivative (magnesium isoglycyrrhizinate), is considered safer than Ganlixin and is thus widely regarded as a second-generation licorice-derived liver protective drug in China. 18β-GA is one of the major active metabolites of α-GL in vivo. Experiments conducted in our lab also demonstrated that the liver protective effect of 18β-GA against ANIT-induced liver damage is significantly stronger than that of diammonium glycyrrhizinate, a result consistent with findings reported in the literature (data not shown here). The strong hepatoprotective effects of 18β-GA in rodents lead us to speculate that 18β-GA may also be a liver protective metabolite of diammonium glycyrrhizinate and magnesium isoglycyrrhizinate.
The chemical structure of 18β-GA has similarities with steroid hormones, indicating that 18β-GA may play a hormone-like role in combination with steroid hormone receptors. The data indeed indicate that 18β-GA increases glucocorticoid activity and alleviates adverse reactions such as drug withdrawal and drug dependence. Data from the literature also indicate that 18β-GA can reduce the inactivation of glucocorticoids by inhibiting 11β-HSD2 to exert anti-inflammatory activity [86]. Further studies have confirmed that 18β-GA promotes the dissociation of the glucocorticoid receptor (GR)-HSP90 complex, which activates GR and negatively interacts with NF-κB and AP-1 to block inflammation [46, 87]. It has also been confirmed that 18β-GA can inhibit ROS production by activating the PI3K/AP-1/HO-1 signaling pathway and the GR/DUSP1 signaling pathway, thereby restoring glucocorticoid sensitivity and reversing glucocorticoid resistance [86].
One of the safety concerns of the long-term use of GL preparations, especially at high doses, is the development of pseudoaldosteronism [88–90]. The clinical manifestations of pseudoaldosteronism include elevated blood pressure, sodium retention, decreased urine potassium, and decreased plasma aldosterone concentrations. Studies have demonstrated that 18β-GA inhibits 11β-HSD2 in the kidney and leads to the corticosteroid-induced excessive accumulation of cortisol in renal tubular epithelial cells and pseudohyperaldosteronism [90–92].
The in vivo metabolic processes of GL and 18β-GA have not been extensively studied. After the oral administration of GL to humans and rats, the concentrations of 18β-GA in the urine are extremely low (below the detection limit (1 ng/100 g body weight)) [18, 93]. In contrast, the concentrations of 3-monoglucuronyl-glycyrrhetinic acid (3MGA, another important metabolite of GL) in the plasma and urine are reported to be much higher [93]. Studies in normal and Eisai hyperbilirubinemia rats (Mrp2-deficient) have confirmed that 3MGA (but not 18β-GA) accumulates in the renal tubule after the oral administration of 18β-GA. The accumulation of 3MGA in the kidney inhibits 11β-HSD2 and in turn leads to pseudohyperaldosteronism [94, 95].
Summary and prospect
In summary, 18β-GA has significant biological activities against liver injury. Recent studies have shown that 18β-GA has potent liver protective effects in animals (Table 1) and cells (Table 2), which may involve antioxidative (Fig. 3), anti-inflammatory (Fig. 4) and other functions (Fig. 5). However, due to the poor water solubility of 18β-GA, its liver protective effects are still limited to laboratory research. In recent years, many studies have found that 18β-GA is biomodified and targeted to the liver, with the data suggesting that it has significant therapeutic value in different liver disease models and is well tolerated [96, 97]. Preliminary studies have shown that there is a specific 18β-GA-binding protein on the surface of hepatocytes. The binding of GA to this protein is the highest in the liver followed by the kidney. A study of a fluorescent tag (FITC)-labeled 18β-GA analog (FITC-GA) found that there was competition between FITC-GA and the GA receptor (GA-R) and that 18β-GA and FITC-GA induced similar cytotoxicity in vitro. 18β-GA competitively binds to GA-R with an equilibrium dissociation constant (Kd) of 7.457 ± 2.122 pmol/L and a maximum binding count (Bmax) of 2.385 ± 0.175 pmol/(2.5 × 106) cells [98]. Studies have also reported that 18β-GA exerts pharmacological effects through intracellular endocytosis and specifically binds GA-R on the cell membrane. However, the specific protein structure of GA-R is currently unknown. We believe that the determination of GA-R, that is, the confirmation of that targets of 18β-GA, warrants further research. First, identifying these targets provides clues for the study of the hepatoprotective mechanism of 18β-GA and serves as a reference biomarker for studying the clinical effects of 18β-GA in the treatment of liver and other diseases. Second, based on these targets, novel liver protective agents could be developed and tested to potentially offer new options for the treatment of relevant human diseases.
Table 1.
Summary of in vivo studies on the effect of 18β-GA in liver injury
| Model | 18β-GA treated dose | Pharmacological activities | Mechanisms | References |
|---|---|---|---|---|
| CP-induced wistar rats liver injury | 18β-GA, ip 25, 50 mg/kg single dose | Antioxidant defenses and reducing inflammation | Inhibited activating Nrf2 and PPARc | [27] |
| HFD-induced SD rat NAFLD models | 18β-GA, ig 25, 50 mg/kg for 8 weeks | Reduced hepatic lipotoxicity and cell appotosis | Lysosomal and mitochondrial pathways | [53] |
| Carbon tetrachloride-induced mice liver fibrosis | 18β-GA, ip 15 mg/kg three times a week for 4 weeks | Inhibition of hepatic fibrosis | Inhibited nuclear accumulation of Smad3 in activated HSCs | [64] |
| LPS/D-GalN-induced Balb/c mice liver injury | 18β-GA, ip 10, 30 or 100 mg/kg single dose | Alleviation of mortality; anti-inflammation | Upregulating IRKM | [44] |
| Ischemia/reperfusion-induced C57BL/6 mice liver injury | 18β-GA, ip 100 mg/kg single dose | Anti-inflammation | Inhibiting TLR4 signaling cascade | [99] |
| Acetaminophen-induced Balb/c mice acute liver injury | 18β-GA, ip 100 mg/kg single dose | Alleviated hepatic damages with hepatocellular apoptosis | Inhibition of CYP2E1 expression and HMGB1-TLR4 pathway | [34] |
| CCl4-induced SD rat cirrhosis | 18β-GA, ig 72 mg/kg for 7 days) | Inhibition of hepatic fibrosis | Restoring the composition of Lactobacillus | [100] |
| ANIT-induced 129/Sv mice liver injury | 18β-GA, ip 50 mg/kg for 7 days | Inhibited bile acid cycle disruption | Increase transporter expression | [39] |
| ANIT-induced SD rat liver injury | 18β-GA, ip 60 mg/kg for 7 days | Increase transporter expression through Sirt1/FXR | [7] | |
| P. acnes-induced C57BL/6 mice liver injury | 18β-GA, ip 75 mg/kg for 1 day | Suppressed mortality and improving the liver dysfunction | Inhibition of macrophage inflammatory protein-1 | [43] |
| Methotrexate-induced wistar rats hepatotoxicity | 18β-GA, ig 50, 100 mg/kg 7 days | Attenuation of inflammation, oxidative stress and apoptosis | Inhibited activating Nrf2 and PPARγ | [28] |
| Realgar-induced ICR mice subchronic hepatotoxicity | 18β-GA, ig 16, 48 mg/kg for 8 weeks | Regulating changes in the lipid and energy metabolism | None | [101] |
| CCl4-induced Kunming mice chronic liver fibrosis | 18β-GA, ig 25, 50, 100 mg/kg for 30 days | Antioxidant defenses | Decreased nuclear Nrf2 expression | [61] |
| Retrorsine-induced SD rat hepatotoxicity | 18β-GA, ip 10 mg/kg for 30 min | Improving the liver dysfunction | None | [32] |
| BDL-induced SD rat chronic liver fibrosis | 18β-GA, iv 2 mg/kg, M6P26-HAS-GA, iv 10 mg/kg, three times a week for 4 weeks | Inhibition of hepatic fibrosis | Target hepatic stellate cell | [65] |
| CCl4-plus ethanol-induced SD rat liver cirrhosis | – | Inhibition of hepatic fibrosis | Target hepatic stellate cell | [63] |
| Dimethyl nitrosamin-induced SD rat liver injury | 18α-GL,18β-GL ip,150 mg/kg, three times a week for 4 weeks | Improving the liver dysfunction | None | [102] |
| Con-A-induced ICR mice immunological liver injury | 18α-GL, 18β-GL, ip 15, 30, 60 mg/kg for 5 days | Improving the liver dysfunction | None | [103] |
| CCl4-induced ICR mice chronic liver injury | 18β-GA, s.c. 10, 50, 100 mg/kg for 3 days | Attenuation of CCl4-induced hepatotoxicity | Inhibited cytochrome P450 2E1 expression | [10] |
| CCl4-induced wistar rat acute liver injury | 18β-GA, ip 50, 100 mg/kg for 30 min | Attenuation of acute liver injury | Inhibited β-glucuronidase | [104] |
| Acetylaminofluorene-induced Wistar rats hepatotoxicity | 18β-GA, ig 45, 75 mg/kg for 15 days | Attenuation of oxidative stress, inflammation and hyperproliferation | Restored the expressions of PCNA, COX- 2, iNOS and NF-κB | [38] |
CP cyclophosphamide, CCl4 carbon tetrachloride, Con-A concanavalin A, BDL bile duct ligation, LPS/d-GalN lipopolysaccharide, LPS/D-galactosamine, P. acnes Propionibacterium acnes, ANIT alpha-naphthylisothi, IRK-M interleukin-1 receptor-associated kinase-M, Nrf2 NF-E2-related factor 2, PPARg peroxisome proliferator-activated receptor-gamma, Sirt1 Sirtuin-1, FXR farnesoid X receptor, TLR4 Toll-like receptor 4, HFD high fat diet, NAFLD nonalcoholic fatty liver disease, HSC hepatic stellate cells, FAA fatty acid
Table 2.
Summary of in vitro studies on the effect of 18β-GA in liver injury
| Cell line | Dose and time | Pharmacological activities | Mechanisms | References |
|---|---|---|---|---|
| HL-7702 and HepG2 | 18β-GA and GA derivatives, 5, 10, 20 µM; 24 h | Induced cell cycle arrest at the G2/M phase, and inhibited cancer cells proliferation and migration | Triggered apoptosis through the mitochondrial pathway | [105] |
| HepaRG | 18β-GA and nanoparticle co-loading entecavir and glycyrrhetinic acid, 2.5, 5, 10, 20 µg/mL, 24 h | Glycyrrhetinic acid can enhance the accumulation of entecavir in HepaRG cell and liver for treating hepatitis B | Improved liver accumulation of entecavir and investigated its ability to deliver both drugs to liver | [106] |
| HOS and HT1080 cells | 20, 40 µM; 24, 48, or 72 h | 18β-GA inhibits sarcoma cell proliferation by inducing G0/G1-phase arrest | GA induced apoptosis through both extrinsic and intrinsic pathways and GA-induced autophagy, represents a promising therapeutic approach for the treatment of sarcoma | [107] |
| Hep3B | Serial of concentrations (0.78–25 µM); 24 h | Inhibited tumor growth | GA and poly(L-Histidine) mediated polymeric drug delivery system was targeted HCC, and released its encapsulated anticancer drug in the acidic microenvironment of HCC | [74] |
| HepG2 | GA modified curcuminsupramolecular pro-gelator (GA-Cur), serial of concentrations (for IC50); 48 h | Enhanced cellular uptake and better inhibition capacity | – | [108] |
| HepG2, HLE, LM3, and Hep3B | 18β-GA 0, 100, 150, 200 µM; 24 or 48 h | GA suppressed proliferation of various HCC cell lines | GA increased ATF4/CHOP-induced autophagy and IRE1α/XBP1s UPR pathways-induced apoptosis | [6] |
HCC hepatocellular carcinoma, ATF4 activating transcription factor 4, CHOP C/EBP homologous protein, IRE1α inositol-requiring enzyme 1α, XBP1 IRE1 activation-dependent X-box-binding protein-1, UPR unfolded protein response, HOS cell human osteosarcoma cell line, HT1080 cells human fibrosarcoma cell line, HLE HCC cell lines, LM3 human hepatocellular carcinoma cell lines
Fig. 3.
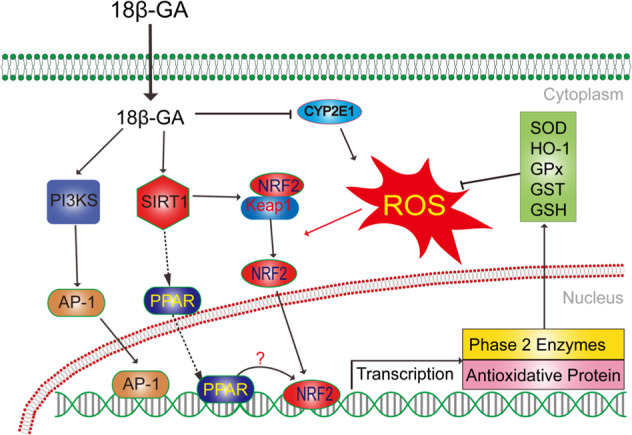
Networks of molecular signaling underlying antioxidative stress effects of 18β-glycyrrhetinic acid. PI3K: phosphoinositide 3-kinase; AP-1: activator protein-1; ROS: reactive oxygen species; GSH: glutathione; NRF2: NF-E2-related factor 2; PPAR: peroxisome proliferator-activated receptor-gamma; SIRT1: Sirtuin-1; Keap-1: Keleh-like ECH-associated protein-1; CYP2E1: cytochrome P450, family 2, subfamily E, polypeptide 1
Fig. 4.
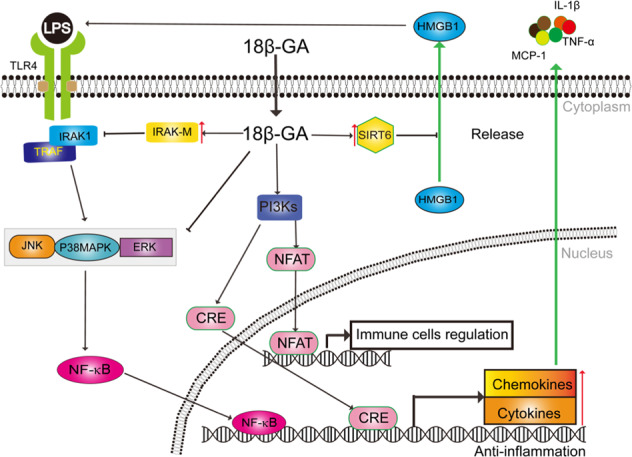
Networks of molecular signaling underlying anti-inflammation effects of 18β-glycyrrhetinic acid. NFAT: nuclear factor of activated T cells; NF-kB: Nuclear factor κB; CRE: cAMP Responsive Element; JNK: c-Jun N-terminal kinase; MAPK: mitogen-activated protein kinase; ERK: extracellular regulated protein kinases; IRAK: interleukin-1 receptor-associated kinase; TRAF: TNF receptor-associated factor; HMGB1: high mobility group boxe chromosomal protein; SIRT6: Sirtuin-6
Fig. 5.
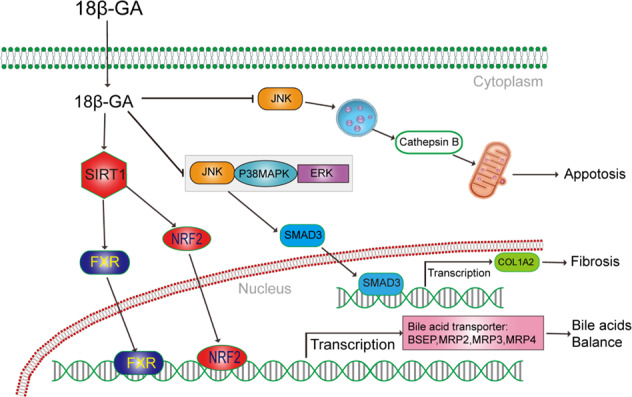
Networks of molecular signaling underlying other functions of 18β-glycyrrhetinic acid. SMAD3: sekelsky mothers against dpp3; BSEP: bile salt export pump; MRP: multidrug resistance associated protein; COL1A2: collagen, type I, alpha 2
Acknowledgements
This work was supported by the National New Drug Creation Program of China (No. 2018ZX09201017-004) and the “Strategic Priority Research Program” of the Chinese Academy of Sciences (No. XDA12050305).
Competing interests
The authors declare no competing interests.
References
- 1.Akao T, Akao T, Hattori M, Kanaoka M, Yamamoto K, Namba T, et al. Hydrolysis of glycyrrhizin to 18 beta-glycyrrhetyl monoglucuronide by lysosomal beta-D-glucuronidase of animal livers. Biochem Pharmacol. 1991;41:1025–9. doi: 10.1016/0006-2952(91)90210-v. [DOI] [PubMed] [Google Scholar]
- 2.Ishida T, Miki I, Tanahashi T, Yagi S, Kondo Y, Inoue J, et al. Effect of 18 beta-glycyrrhetinic acid and hydroxypropyl gamma cyclodextrin complex on indomethacin-induced small intestinal injury in mice. Eur J Pharmacol. 2013;714:125–31. doi: 10.1016/j.ejphar.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Kim YH, Kim DE, Lee SH. Effects of 18beta-glycyrrhetinic acid on fungal protease-induced airway inflammatory responses. Mediators Inflamm. 2018;2018:6461032. doi: 10.1155/2018/6461032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuang P, Zhao W, Su W, Zhang Z, Zhang L, Liu J, et al. 18beta-Glycyrrhetinic acid inhibits hepatocellular carcinoma development by reversing hepatic stellate cell-mediated immunosuppression in mice. Int J Cancer. 2013;132:1831–41. doi: 10.1002/ijc.27852. [DOI] [PubMed] [Google Scholar]
- 5.Cai H, Chen X, Zhang J, Wang J. 18beta-Glycyrrhetinic acid inhibits migration and invasion of human gastric cancer cells via the ROS/PKC-alpha/ERK pathway. J Nat Med. 2018;72:252–9. doi: 10.1007/s11418-017-1145-y. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Zhang ZQ, Song J, Liu QM, Wang C, Huang Z, et al. 18beta-Glycyrrhetinic-acid-mediated unfolded protein response induces autophagy and apoptosis in hepatocellular carcinoma. Sci Rep. 2018 doi: 10.1038/s41598-018-27142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu SY, Cui SC, Wang L, Zhang YT, Yan XX, Lu HL, et al. 18beta-Glycyrrhetinic acid protects against alpha-naphthylisothiocyanate-induced cholestasis through activation of the Sirt1/FXR signaling pathway. Acta Pharmacol Sin. 2018;39:1865–73. doi: 10.1038/s41401-018-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kowalska A, Kalinowska-Lis U. 18beta-Glycyrrhetinic acid: its core biological properties and dermatological applications. Int J Cosmet Sci. 2019;41:325–31.. doi: 10.1111/ics.12548. [DOI] [PubMed] [Google Scholar]
- 9.Li JY, Cao HY, Liu P, Cheng GH, Sun MY. Glycyrrhizic acid in the treatment of liver diseases: literature review. Biomed Res Int. 2014;2014:872139. doi: 10.1155/2014/872139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong HG, You HJ, Park SJ, Moon AR, Chung YC, Kang SK, et al. Hepatoprotective effects of 18beta-glycyrrhetinic acid on carbon tetrachloride-induced liver injury: inhibition of cytochrome P450 2E1 expression. Pharmacol Res. 2002;46:221–7. doi: 10.1016/s1043-6618(02)00121-4. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Jiang T, Li P, Mao Q. The protection of glycyrrhetinic acid (GA) towards acetaminophen (APAP)-induced toxicity partially through fatty acids metabolic pathway. Afr Health Sci. 2015;15:1023–7. doi: 10.4314/ahs.v15i3.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Sun R, Liu R. Natural products in licorice for the therapy of liver diseases: progress and future opportunities. Pharmacol Res. 2019;144:210–26. doi: 10.1016/j.phrs.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Chinese CMA. Society of Infectious Diseases & Expert Committee for Prevention and Management of Liver Inflammation. Consensus statement by the expert commlttee for prevention and management of liver inflammation in China. Chin J Hepatol. 2014;22:94–103. [Google Scholar]
- 14.Wukui C, Tianyan C, Yongping C, Ping C, Xuefei D, Xuesong G, et al. Consensus statement by the expert commlttee for clinical applications of glycyrrhizic acid in liver disease. Chin J Exp Clin Infect Dis. 2016;32:844–52. [Google Scholar]
- 15.Zeng C-X, Yang Q, Hu Q. A comparison of the distribution of two glycyrrhizic acid epimers in rat tissues. J Drug Metab. PH. 2006;31:253–8. doi: 10.1007/BF03190464. [DOI] [PubMed] [Google Scholar]
- 16.Yi F, Jian-Hua D, Su-Yi L, Xu-Ning Z, Qiang L, Jing Z, et al. Studies on distribution in mice tissues of α-glycyrrhizic acid and β-glycyrrhizic acid. Chin J Clin Pharm Ther. 2004;9:619–22. [Google Scholar]
- 17.Ablise M, Jie M, Casimov G, Hongzhi L, Shuyan M. Preparation of 18alpha-glycyrrhetinic acid and its methyl ester. Northwest Pharm J. 2009;24:58–9. [Google Scholar]
- 18.Ploeger B, Mensinga T, Sips A, Seinen W, Meulenbelt J, DeJongh J. The pharmacokinetics of glycyrrhizic acid evaluated by physiologically based pharmacokinetic modeling. Drug Metab Rev. 2001;33:125–47. doi: 10.1081/dmr-100104400. [DOI] [PubMed] [Google Scholar]
- 19.Takeda S, Ishthara K, Wakui Y, Amagaya S, Maruno M, Akao T, et al. Bioavailability study of glycyrrhetic acid after oral administration of glycyrrhizin in rats; relevance to the intestinal bacterial hydrolysis. J Pharm Pharmacol. 1996;48:902–5. doi: 10.1111/j.2042-7158.1996.tb05998.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhao K, Ding M, Cao H, Cao ZX. In-vitro metabolism of glycyrrhetinic acid by human and rat liver microsomes and its interactions with six CYP substrates. J Pharm Pharmacol. 2012;64:1445–51. doi: 10.1111/j.2042-7158.2012.01516.x. [DOI] [PubMed] [Google Scholar]
- 21.Robinson MW, Harmon C, O’Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13:267–76. doi: 10.1038/cmi.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatol. 2014;60:2099–108. doi: 10.1002/hep.27406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panackel C, Thomas R, Sebastian B, Mathai SK. Recent advances in management of acute liver failure. Indian J Crit Care Med. 2015;19:27–33. doi: 10.4103/0972-5229.148636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Jiang W, Wang J. Clinical analysis of 275 cases of acute drug-induced liver disease. Front Med China. 2007;1:58–61. doi: 10.1007/s11684-007-0012-8. [DOI] [PubMed] [Google Scholar]
- 25.Andrade RJ, Chalasani N, Bjornsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, et al. Drug-induced liver injury. Nat Rev Dis Prim. 2019;5:58. doi: 10.1038/s41572-019-0105-0. [DOI] [PubMed] [Google Scholar]
- 26.Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13:131–49. doi: 10.1038/nrgastro.2015.219. [DOI] [PubMed] [Google Scholar]
- 27.Mahmoud AM, Al Dera HS. 18beta-Glycyrrhetinic acid exerts protective effects against cyclophosphamide-induced hepatotoxicity: potential role of PPARgamma and Nrf2 upregulation. Genes Nutr. 2015;10:41. doi: 10.1007/s12263-015-0491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmoud AM, Hussein OE, Hozayen WG, Abd el-Twab SM. Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPAR gamma and Nrf2: protective effect of 18 beta-glycyrrhetinic acid. Chem-Biol Interact. 2017;270:59–72. doi: 10.1016/j.cbi.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Wu CH, Chen AZ, Yen GC. Protective effects of glycyrrhizic acid and 18beta-glycyrrhetinic acid against cisplatin-induced nephrotoxicity in BALB/c mice. J Agric Food Chem. 2015;63:1200–9. doi: 10.1021/jf505471a. [DOI] [PubMed] [Google Scholar]
- 30.Lefaki M, Papaevgeniou N, Tur JA, Vorgias CE, Sykiotis GP, Chondrogianni N. The dietary triterpenoid 18alpha-Glycyrrhetinic acid protects from MMC-induced genotoxicity through the ERK/Nrf2 pathway. Redox Biol. 2020;28:101317. doi: 10.1016/j.redox.2019.101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan K, Zhang H, Lin ZX. An overview on adverse drug reactions to traditional Chinese medicines. Br J Clin Pharmacol. 2015;80:834–43. doi: 10.1111/bcp.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin G, Nnane IP, Cheng TY. The effects of pretreatment with glycyrrhizin and glycyrrhetinic acid on the retrorsine-induced hepatotoxicity in rats. Toxicon. 1999;37:1259–70. doi: 10.1016/s0041-0101(98)00263-3. [DOI] [PubMed] [Google Scholar]
- 33.Chang YL, Chen CL, Kuo CL, Chen BC, You JS. Glycyrrhetinic acid inhibits ICAM-1 expression via blocking JNK and NF-kappaB pathways in TNF-alpha-activated endothelial cells. Acta Pharmacol Sin. 2010;31:546–53. doi: 10.1038/aps.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang G, Zhang L, Ma L, Jiang R, Kuang G, Li K, et al. Glycyrrhetinic acid prevents acetaminophen-induced acute liver injury via the inhibition of CYP2E1 expression and HMGB1-TLR4 signal activation in mice. Int Immunopharmacol. 2017;50:186–93.. doi: 10.1016/j.intimp.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 35.Chen D, Bellussi LM, Cocca S, Wang J, Passali GC, Hao X, et al. Glycyrrhetinic acid suppressed hmgb1 release by up-regulation of Sirt6 in nasal inflammation. J Biol Regul Homeost Agents. 2017;31:269–77. [PubMed] [Google Scholar]
- 36.Yan T, Wang H, Zhao M, Yagai T, Chai Y, Krausz KW, et al. Glycyrrhizin protects against acetaminophen-induced acute liver injury via alleviating tumor necrosis factor alpha-mediated apoptosis. Drug Metab Dispos. 2016;44:720–31. doi: 10.1124/dmd.116.069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen C, Zhang H, Zhang G, Meng Q. Isoniazid-induced hepatotoxicity in rat hepatocytes of gel entrapment culture. Toxicol Lett. 2006;167:66–74. doi: 10.1016/j.toxlet.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Hasan SK, Khan R, Ali N, Khan AQ, Rehman MU, Tahir M, et al. 18-beta Glycyrrhetinic acid alleviates 2-acetylaminofluorene-induced hepatotoxicity in Wistar rats: Role in hyperproliferation, inflammation and oxidative stress. Hum Exp Toxicol. 2015;34:628–41. doi: 10.1177/0960327114554045. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Fang ZZ, Meng R, Cao YF, Tanaka N, Krausz KW, et al. Glycyrrhizin and glycyrrhetinic acid inhibits alpha-naphthyl isothiocyanate-induced liver injury and bile acid cycle disruption. Toxicology. 2017;386:133–42. doi: 10.1016/j.tox.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhai D, Zhao Y, Chen X, Guo J, He H, Yu Q, et al. Protective effect of glycyrrhizin, glycyrrhetic acid and matrine on acute cholestasis induced by alpha-naphthyl isothiocyanate in rats. Planta Med. 2007;73:128–33. doi: 10.1055/s-2006-957067. [DOI] [PubMed] [Google Scholar]
- 41.Zheng X, Zhu S, Zhou Z, Liu W, Xu W. Glycyrrhetic acid derivative TY501 protects against lithocholic acid-induced cholestasis. Drug Res (Stuttg) 2018;68:370–7. doi: 10.1055/s-0043-122222. [DOI] [PubMed] [Google Scholar]
- 42.Ichai P, Samuel D. Epidemiology of liver failure. Clin Res Hepatol Gastroenterol. 2011;35:610–7. doi: 10.1016/j.clinre.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Xiao YC, Xu JW, Mao CM, Jin M, Wu Q, Zou J, et al. 18 beta-Glycyrrhetinic acid ameliorates acute propionibacterium acnes-induced liver injury through inhibition of macrophage inflammatory protein-1 alpha. J Biol Chem. 2010;285:1128–37. doi: 10.1074/jbc.M109.037705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin X, Gong X, Zhang L, Jiang R, Kuang G, Wang B, et al. Glycyrrhetinic acid attenuates lipopolysaccharide-induced fulminant hepatic failure in d-galactosamine-sensitized mice by up-regulating expression of interleukin-1 receptor-associated kinase-M. Toxicol Appl Pharmacol. 2017;320:8–16. doi: 10.1016/j.taap.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 45.Wang CY, Kao TC, Lo WH, Yen GC. Glycyrrhizic acid and 18beta-glycyrrhetinic acid modulate lipopolysaccharide-induced inflammatory response by suppression of NF-kappaB through PI3K p110delta and p110gamma inhibitions. J Agric Food Chem. 2011;59:7726–33. doi: 10.1021/jf2013265. [DOI] [PubMed] [Google Scholar]
- 46.Kao TC, Shyu MH, Yen GC. Glycyrrhizic acid and 18beta-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3beta signaling and glucocorticoid receptor activation. J Agric Food Chem. 2010;58:8623–9. doi: 10.1021/jf101841r. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Yang S, Zhang M, Wang Z, He X, Hou Y, et al. Glycyrrhetinic acid improves insulin-response pathway by regulating the balance between the Ras/MAPK and PI3K/Akt pathways. Nutrients. 2019;11:604. doi: 10.3390/nu11030604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim ME, Kim HK, Kim DH, Yoon JH, Lee JS. 18beta-Glycyrrhetinic acid from licorice root impairs dendritic cells maturation and Th1 immune responses. Immunopharmacol Immunotoxicol. 2013;35:329–35. doi: 10.3109/08923973.2013.768636. [DOI] [PubMed] [Google Scholar]
- 49.Disease GBD, Injury I. Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci. 2018;75:3313–27.. doi: 10.1007/s00018-018-2860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Younossi ZM, Golabi P, de Avila L, Minhui Paik J, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019 doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 52.Park M, Lee JH, Choi JK, Hong YD, Bae IH, Lim KM, et al. 18 beta-Glycyrrhetinic acid attenuates anandamide-induced adiposity and high-fat diet induced obesity. Mol Nutr Food Res. 2014;58:1436–46. doi: 10.1002/mnfr.201300763. [DOI] [PubMed] [Google Scholar]
- 53.Wu XD, Zhang LY, Gurley E, Studer E, Shang J, Wang T, et al. Prevention of free fatty acid-induced hepatic lipotoxicity by 18 beta-glycyrrhetinic acid through lysosomal and mitochondrial pathways. Hepatology. 2008;47:1905–15. doi: 10.1002/hep.22239. [DOI] [PubMed] [Google Scholar]
- 54.Yan TT, Wang H, Cao LJ, Wang Q, Takahashi S, Yagai T, et al. Glycyrrhizin alleviates nonalcoholic steatohepatitis via modulating bile acids and meta-inflammation. Drug Metab Disp. 2018;46:1310–9. doi: 10.1124/dmd.118.082008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung JC, Lee YH, Kim SH, Kim KJ, Kim KM, Oh S, et al. Hepatoprotective effect of licorice, the root of Glycyrrhiza uralensis Fischer, in alcohol-induced fatty liver disease. Bmc Complem Altern M. 2016 doi: 10.1186/s12906-016-0997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huo X, Yang S, Sun X, Meng X, Zhao Y. Protective effect of glycyrrhizic acid on alcoholic liver injury in rats by modulating lipid metabolism. Molecules. 2018;23:1623. [Google Scholar]
- 57.Huo X, Sun X, Cao Z, Qiao J, Yang S, Meng X, et al. Optimal ratio of 18alpha- and 18beta-glycyrrhizic acid for preventing alcoholic hepatitis in rats. Exp Ther Med. 2019;18:172–8. doi: 10.3892/etm.2019.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mormone E, George J, Nieto N. Molecular pathogenesis of hepatic fibrosis and current therapeutic approaches. Chem Biol Interact. 2011;193:225–31. doi: 10.1016/j.cbi.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boll M, Weber LW, Becker E, Stampfl A. Mechanism of carbon tetrachloride-induced hepatotoxicity. Hepatocellular damage by reactive carbon tetrachloride metabolites. Z Naturforsch C. 2001;56:649–59. doi: 10.1515/znc-2001-7-826. [DOI] [PubMed] [Google Scholar]
- 60.Zhang HY, Wang HL, Zhong GY, Zhu JX. Molecular mechanism and research progress on pharmacology of traditional Chinese medicine in liver injury. Pharm Biol. 2018;56:594–611. doi: 10.1080/13880209.2018.1517185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen S, Zou L, Li L, Wu T. The protective effect of glycyrrhetinic acid on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. PLoS One. 2013 doi: 10.1371/journal.pone.0053662.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo XL, Liang B, Wang XW, Fan FG, Jin J, Lan R, et al. Glycyrrhizic acid attenuates CCl4-induced hepatocyte apoptosis in rats via a p53-mediated pathway. World J Gastroenterol. 2013;19:3781–91. doi: 10.3748/wjg.v19.i24.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang JY, Zhang QS, Guo JS, Hu MY. Effects of glycyrrhetinic acid on collagen metabolism of hepatic stellate cells at different stages of liver fibrosis in rats. World J Gastroenterol. 2001;7:115–9. doi: 10.3748/wjg.v7.i1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moro T, Shimoyama Y, Kushida M, Hong YY, Nakao S, Higashiyama R, et al. Glycyrrhizin and its metabolite inhibit Smad3-mediated type I collagen gene transcription and suppress experimental murine liver fibrosis. Life Sci. 2008;83:531–9. doi: 10.1016/j.lfs.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 65.Luk JM, Zhang QS, Lee NP, Wo JY, Leung PP, Liu LX, et al. Hepatic stellate cell-targeted delivery of M6P-HSA-glycyrrhetinic acid attenuates hepatic fibrogenesis in a bile duct ligation rat model. Liver Int. 2007;27:548–57.. doi: 10.1111/j.1478-3231.2007.01452.x. [DOI] [PubMed] [Google Scholar]
- 66.Sato H, Goto W, Yamamura J, Kurokawa M, Kageyama S, Takahara T, et al. Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antivir Res. 1996;30:171–7. doi: 10.1016/0166-3542(96)00942-4. [DOI] [PubMed] [Google Scholar]
- 67.Zhao MQ, Han DW, Ma XH, Zhao YC, Yin L, Li CM. Preventive and therapeutic actions of glycyrrhizin, glycyrrhetic acid and crude saikosides on experimental liver cirrhosis in rats. Yao Xue Xue Bao. 1983;18:325–31. [PubMed] [Google Scholar]
- 68.Hong M, Li S, Tan HY, Wang N, Tsao SW, Feng Y. Current status of herbal medicines in chronic liver disease therapy: the biological effects, molecular targets and future prospects. Int J Mol Sci. 2015;16:28705–45. doi: 10.3390/ijms161226126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kao ST, Yang SL, Hsieh CC, Yang MD, Wang TF, Lin JG. Immunomodulation of Bu-Zhong-Yi-Qi-Tang on in vitro granulocyte colony-stimulating-factor and tumor necrosis factor-alpha production by peripheral blood mononuclear cells. Immunopharmacol Immunotoxicol. 2000;22:711–20. doi: 10.3109/08923970009016434. [DOI] [PubMed] [Google Scholar]
- 70.Parajuli DR, Park EJ, Che XH, Jiang WY, Kim YC, Sohn DH, et al. PF2401-SF, standardized fraction of Salvia miltiorrhiza, induces apoptosis of activated hepatic stellate cells in vitro and in vivo. Molecules. 2013;18:2122–34. doi: 10.3390/molecules18022122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hasan SK, Siddiqi A, Nafees S, Ali N, Rashid S, Ali R, et al. Chemopreventive effect of 18beta-glycyrrhetinic acid via modulation of inflammatory markers and induction of apoptosis in human hepatoma cell line (HepG2) Mol Cell Biochem. 2016;416:169–77. doi: 10.1007/s11010-016-2705-2. [DOI] [PubMed] [Google Scholar]
- 72.Tan J, Shen W, Shi W, Chen X, Sun D, Xu C, et al. ONTD induces growth arrest and apoptosis of human hepatoma Bel-7402 cells though a peroxisome proliferator-activated receptor gamma-dependent pathway. Toxicol Vitr. 2017;45:44–53. doi: 10.1016/j.tiv.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 73.Qu Y, Sun F, He F, Yu C, Lv J, Zhang Q, et al. Glycyrrhetinic acid-modified graphene oxide mediated siRNA delivery for enhanced liver-cancer targeting therapy. Eur J Pharm Sci. 2019 doi: 10.1016/j.ejps.2019.105036.. [DOI] [PubMed] [Google Scholar]
- 74.Zhang J, Zhang M, Ji J, Fang X, Pan X, Wang Y, et al. Glycyrrhetinic acid-mediated polymeric drug delivery targeting the acidic microenvironment of hepatocellular carcinoma. Pharmacol Res. 2015;32:3376–90. doi: 10.1007/s11095-015-1714-2. [DOI] [PubMed] [Google Scholar]
- 75.Chen J, Jiang H, Wu Y, Li Y, Gao Y. A novel glycyrrhetinic acid-modified oxaliplatin liposome for liver-targeting and in vitro/vivo evaluation. Drug Des Devel Ther. 2015;9:2265–75. doi: 10.2147/DDDT.S81722. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Li J, Chen T, Deng F, Wan J, Tang Y, Yuan P, et al. Synthesis, characterization, and in vitro evaluation of curcumin-loaded albumin nanoparticles surface-functionalized with glycyrrhetinic acid. Int J Nanomed. 2015;10:5475–87. doi: 10.2147/IJN.S88253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roohbakhsh A, Iranshahy M, Iranshahi M. Glycyrrhetinic acid and its derivatives: anti-cancer and cancer chemopreventive properties, mechanisms of action and structure-cytotoxic activity relationship. Curr Med Chem. 2016;23:498–517. doi: 10.2174/0929867323666160112122256. [DOI] [PubMed] [Google Scholar]
- 78.Sebode M, Weiler-Normann C, Liwinski T, Schramm C. Autoantibodies in autoimmune liver disease-clinical and diagnostic relevance. Front Immunol. 2018;9:609. doi: 10.3389/fimmu.2018.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Horigome H, Hirano T, Oka K. Therapeutic effect of glycyrrhetinic acid in MRL lpr/lpr mice: implications of alteration of corticosteroid metabolism. Life Sci. 2001;69:2429–38. doi: 10.1016/s0024-3205(01)01317-0. [DOI] [PubMed] [Google Scholar]
- 80.Guoli J. Clinical features of primary sclerosing cholangitis: analysis of 6 cases. Nei Mongol J Trid Chin Med. 2013;32:42–3. [Google Scholar]
- 81.Zhiqin W, Feng W, Lei W, Haiguang X, Hang X. Clinical features of primary sclerosing cholangitis: analysis of 16 cases. Chin Hepatol. 2010;15:349–50. [Google Scholar]
- 82.Xuejuan Z. The clinical observation and nursing care of UDCA combined with magnesium isoglycyrrhizinate in treatment of patients with primary biliary cirrhosis. Xinxueguanbing Fangzhi Zhishi J. 2014;22:115–7. [Google Scholar]
- 83.Xiaowei W. The clinical effect of UDCA combined with magnesium isoglycyrrhizinate in treatment of patients with primary biliary cirrhosis. Chin. J Gastroenterol. 2014;23:819–21. [Google Scholar]
- 84.Delong C, Haifang C, Xiaoxia W, Chengjun L. Clinical study on ursodeoxycholic acid capsules combined with diammonium glycyrrhizinate in treatment of autoimmune hepatitis. Drugs Clin. 2018;33:2615–9. [Google Scholar]
- 85.Wen-biao Y. Clinical analysis of glycyrrhizin in the treatment of patients with autoimmune hepatitis. J Prim Med. 2018;22:1581–2. [Google Scholar]
- 86.Kao TC, Wu CH, Yen GC. Glycyrrhizic acid and 18beta-glycyrrhetinic acid recover glucocorticoid resistance via PI3K-induced AP1, CRE and NFAT activation. Phytomed. 2013;20:295–302. doi: 10.1016/j.phymed.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 87.Bertorelli G, Bocchino V, Olivieri D. Heat shock protein interactions with the glucocorticoid receptor. Pulm Pharmacol Ther. 1998;11:7–12. doi: 10.1006/pupt.1998.0119. [DOI] [PubMed] [Google Scholar]
- 88.Flores-Robles BJ, Sandoval AR, Dardon JD, Blas CA. Lethal liquorice lollies (liquorice abuse causing pseudohyperaldosteronism) BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-201007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kusano E. How to diagnose and treat a licorice-induced syndrome with findings similar to that of primary hyperaldosteronism. Intern Med. 2004;43:5–6. doi: 10.2169/internalmedicine.43.5. [DOI] [PubMed] [Google Scholar]
- 90.Sabbadin C, Bordin L, Dona G, Manso J, Avruscio G, Armanini D. Licorice: from pseudohyperaldosteronism to therapeutic uses. Front Endocrinol. 2019 doi: 10.3389/fendo.2019.00484.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferrari P, Sansonnens A, Dick B, Frey FJ. In vivo 11beta-HSD-2 activity: variability, salt-sensitivity, and effect of licorice. Hypertension. 2001;38:1330–6. doi: 10.1161/hy1101.096112. [DOI] [PubMed] [Google Scholar]
- 92.Sontia B, Mooney J, Gaudet L, Touyz RM. Pseudohyperaldosteronism, liquorice, and hypertension. J Clin Hypertens (Greenwich) 2008;10:153–7. doi: 10.1111/j.1751-7176.2008.07470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Makino T, Ohtake N, Watanabe A, Tsuchiya N, Imamura S, Iizuka S, et al. Down-regulation of a hepatic transporter multidrug resistance-associated protein 2 is involved in alteration of pharmacokinetics of glycyrrhizin and its metabolites in a rat model of chronic liver injury. Drug Metab Dispos. 2008;36:1438–43. doi: 10.1124/dmd.108.021089. [DOI] [PubMed] [Google Scholar]
- 94.Makino T, Okajima K, Uebayashi R, Ohtake N, Inoue K, Mizukami H. 3-Monoglucuronyl-glycyrrhretinic acid is a substrate of organic anion transporters expressed in tubular epithelial cells and plays important roles in licorice-induced pseudoaldosteronism by inhibiting 11 beta-hydroxysteroid dehydrogenase 2. J Pharmacol Exp Ther. 2012;342:297–304. doi: 10.1124/jpet.111.190009. [DOI] [PubMed] [Google Scholar]
- 95.Kato H, Kanaoka M, Yano S, Kobayashi M. 3-Monoglucuronyl-glycyrrhetinic acid is a major metabolite that causes licorice-induced pseudoaldosteronism. J Clin Endocr Metab. 1995;80:1929–33. doi: 10.1210/jcem.80.6.7775643. [DOI] [PubMed] [Google Scholar]
- 96.Takahashi H, Onishi H, Machida Y. Glycyrrhetic acid-loaded microparticles: liver-specific delivery and therapeutic potential against carbon tetrachloride-induced hepatitis. J Pharm Pharmacol. 2004;56:437–44. doi: 10.1211/0022357023132. [DOI] [PubMed] [Google Scholar]
- 97.Han X, Wang Z, Wang M, Li J, Xu Y, He R, et al. Liver-targeting self-assembled hyaluronic acid-glycyrrhetinic acid micelles enhance hepato-protective effect of silybin after oral administration. Drug Deliv. 2016;23:1818–29. doi: 10.3109/10717544.2015.1108374. [DOI] [PubMed] [Google Scholar]
- 98.Sun YQ, Dai CM, Zheng Y, Shi SD, Hu HY, Chen DW. Binding effect of fluorescence labeled glycyrrhetinic acid with GA receptors in hepatocellular carcinoma cells. Life Sci. 2017;188:186–91.. doi: 10.1016/j.lfs.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 99.Jiang X, Kuang G, Gong X, Jiang R, Xie T, Tie H, et al. Glycyrrhetinic acid pretreatment attenuates liver ischemia/reperfusion injury via inhibiting TLR4 signaling cascade in mice. Int Immunopharmacol. 2019 doi: 10.1016/j.intimp.2019.105870.. [DOI] [PubMed] [Google Scholar]
- 100.Yuan T, Wang J, Chen L, Shan J, Di L. Glycyrrhizic acid improving the liver protective effect by restoring the composition of Lactobacillus. J Funct Foods. 2019;52:219–27.. [Google Scholar]
- 101.Huo T, Fang Y, Zhang Y, Wang Y, Feng C, Yuan M, et al. Plasma metabolomics study of the hepatoprotective effect of glycyrrhetinic acid on realgar-induced sub-chronic hepatotoxicity in mice via (1)H NMR analysis. J Ethnopharmacol. 2017;208:36–43. doi: 10.1016/j.jep.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 102.Jin T, Jian L. The comparing study of the anti-hepatofibrosis effect of 18alpha- and 18beta-Glycyrrhizic acid in rats. Chin J Mod Appl Pharmacol. 2006;23:102–4. [Google Scholar]
- 103.Hui Z, Zhidong P, Bo Z, Tingguo K. Study on protective effect of 18α- and 18β-glycyrrhizic acid on immunological liver injury in mice and their compatibility proportion. Trad Chin Drug Res Clin Pharmacol. 2015;26:476–81. [Google Scholar]
- 104.Shim SB, Kim NJ, Kim DH. Beta-glucuronidase inhibitory activity and hepatoprotective effect of 18 beta-glycyrrhetinic acid from the rhizomes of Glycyrrhiza uralensis. Planta Med. 2000;66:40–3. doi: 10.1055/s-2000-11109. [DOI] [PubMed] [Google Scholar]
- 105.Jin L, Dai LM, Ji M, Wang HS. Mitochondria-targeted triphenylphosphonium conjugated glycyrrhetinic acid derivatives as potent anticancer drugs. Bioorg Chem. 2019;85:179–90.. doi: 10.1016/j.bioorg.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 106.He S, Lin Q, Qu M, Wang L, Deng L, Xiao L, et al. Liver-targeted co-delivery of entecavir and glycyrrhetinic acid based on albumin nanoparticle to enhance the accumulation of entecavir. Mol Pharmacol. 2018;15:3953–61. doi: 10.1021/acs.molpharmaceut.8b00408. [DOI] [PubMed] [Google Scholar]
- 107.Shen S, Zhou M, Huang K, Wu Y, Ma Y, Wang J, et al. Blocking autophagy enhances the apoptotic effect of 18beta-glycyrrhetinic acid on human sarcoma cells via endoplasmic reticulum stress and JNK activation. Cell Death Dis. 2017 doi: 10.1038/cddis.2017.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen G, Li J, Cai Y, Zhan J, Gao J, Song M, et al. A glycyrrhetinic acid-modified curcumin supramolecular hydrogel for liver tumor targeting therapy. Sci Rep. 2017;7:44210. doi: 10.1038/srep44210. [DOI] [PMC free article] [PubMed] [Google Scholar]