Abstract
Background & aims
The aim of this study was to evaluate the nutritional support management in mechanically ventilated coronavirus disease 2019 (COVID-19) patients and explore the association between early caloric deficit and mortality, taking possible confounders (i.e. obesity) into consideration.
Methods
This was a prospective study carried out during the first pandemic wave in the intensive care units (ICUs) of two referral University Hospitals in Lombardy, Italy.
Two hundred twenty-two consecutive mechanically ventilated COVID-19 patients were evaluated during the ICU stay. In addition to major demographic and clinical data, we recorded information on the route and amount of nutritional support provided on a daily basis.
Results
Among patients still in the ICUs and alive on day 4 (N = 198), 129 (65.2%) and 72 (36.4%) reached a satisfactory caloric and protein intake, respectively, mainly by enteral route.
In multivariable analysis, a satisfactory caloric intake on day 4 was associated with lower mortality (HR = 0.46 [95%CI, 0.42–0.50], P < 0.001). Mild obesity (body mass index [BMI] ≥30 and < 35 kg/m2) was associated with higher mortality (HR = 1.99 [95%CI, 1.07–3.68], P = 0.029), while patients with moderate-severe obesity (BMI≥35 kg/m2) were less likely to be weaned from invasive mechanical ventilation (HR = 0.71 [95%CI, 0.62–0.82], P < 0.001).
Conclusions
This study confirmed the negative prognostic and clinical role of obesity in mechanically ventilated COVID-19 patients and suggested that early caloric deficit may independently contribute to worsen survival in this patients’ population. Therefore, any effort should be made to implement an adequate timely nutritional support in all COVID-19 patients during the ICU stay.
Keywords: Coronavirus disease 2019, Mechanical ventilation, Nutritional support, Early caloric deficit, Obesity, Mortality
1. Introduction
The novel coronavirus disease 2019 (COVID-19) pandemic has significantly affected the healthcare systems around the world and has caused a huge number of deaths [1].
Most patients have clinically mild symptoms and are likely to recover. However, many infected individuals develop severe clinical manifestations and a significant proportion becomes critically ill, requiring advanced organ support in the Intensive Care Unit (ICU), including medical nutritional therapy [2].
Approximately 90% of hospitalized patients has one or more underlying conditions, particularly hypertension, chronic lung disease, diabetes mellitus, cardiovascular disease, cancer, and obesity [1].
Previous research showed that in ICU underfeeding related to inability to deliver the required amount of nutrients is very common. Repetitive fasting periods, enteral tube complications and gastrointestinal intolerance are the most frequently reported problems [3].
In COVID-19 patients admitted to ICU, nutritional support can be particularly challenging, as new ICU wards are progressively being created in emergency circumstances, recruiting doctors and nurses from other medical specialties. Moreover, tolerance to enteral nutrition (EN) may be incomplete because of unstable hypoxemia, hypercapnia or acidosis, gastric distention and/or impaired emptying, erosive gastritis, diarrhea and other comorbidities, and of the frequent use of prone positioning during mechanical ventilation [4]. Hence, different nutritional support strategies have to be considered, like the use of supplemental parenteral nutrition (PN) or specific high protein-high caloric, highly digestible enteral formulas, which may not be easily available or manageable in every hospital [4].
The energy deficit accumulated by long-staying ICU patients during their first days of ICU stay may play an important role in ICU and hospital outcomes, including mortality and acquired infections [5]. However, the opportunity of reaching full caloric requirements by artificial nutrition remains a matter of debate, in particular among obese patients [6], as overzealous calories provision in the early phase of critical illness may be harmful, too [5]. Furthermore, obesity in COVID-19 patients seems associated with worse outcomes, including higher risk of hospitalization, admission to ICU, need of invasive mechanical ventilation (IMV) and mortality [7].
Aim of the present study was to evaluate the nutritional support management in mechanically ventilated COVID-19 patients and to explore the association between early caloric deficit and clinical outcomes, taking into consideration possible confounders, including obesity.
2. Methods
2.1. Study population
This was a sub-study of a prospective observational study on consecutive mechanically ventilated COVID-19 patients (positive result on real-time reverse-transcriptase–polymerase-chain-reaction assay of nasopharyngeal swab) admitted to the ICUs of two Italian referral hospitals in the outbreak region of Lombardy (March–May 2020) and in whom nutritional aspects were collected. The study and the sub-study were approved by the local Institutional Ethics Committees and aimed to: 1) describe the nutritional support of this patient population; 2) evaluate the impact of early caloric deficit on mortality and IMV-free survival. Only patients requiring extracorporeal membrane oxygenation were excluded.
2.2. Assessments
Weight was obtained by weighing beds when available, identity cards, history or information obtained from the families. Height was obtained by identity cards or information obtained from the families. In addition to major demographic and clinical data (main comorbidities, sequential organ failure assessment [SOFA] score, Simplified Acute Physiology Score [SAPS] II, protonation, time to admission to ICU, duration of ICU stay), we obtained information on the route (enteral and parenteral) and amount of nutritional support (protein and calories) provided on a daily basis from the electronic medical records. Specifically, nutritional support was progressively implemented to reach 80–100% of estimated requirements between day 4 and day 7. As indirect calorimetry was not available, energy and protein targets (25 kcal/kg/day and 1.3 g/kg/day, respectively) were calculated according to real or adjusted body weight (for obese patients) in agreement with the recent European guidelines [8]. Energy delivered intravenously via glucose solutions and propofol were taken into account. Early caloric and protein deficit was defined by the incapacity to reach 80% of estimated caloric and protein requirement on day 4.
2.3. Study outcomes
Mortality in ICU and IMV-free survival were the endpoints of interest.
2.4. Statistical analysis
We used the Stata software (release 16.1, StataCorp, College Station, TX, USA) for all computations. We considered a 2-sided p-value<0.05 as statistically significant. We described continuous data with the mean and standard deviation (SD) or the median and the inter-quartile range (IQR) when appropriate and categorical data as counts and percent. We fitted multivariable Cox models for death and weaning from IMV including caloric intake on day 4, BMI and a series of predefined confounders (Table 1 ). The satisfactory caloric intake on day 4 was modelled as time-dependent. We report hazard ratios (HR) and 95% confidence intervals (95%CI). We computed the Harrell's c statistic for discrimination. We plotted the model derived cumulative survival or cumulative hazard for the mortality and weaning from IMV, respectively. For all models, we computed Huber-White robust standard error to account for clustering within Center. We tested the interaction of BMI categories and caloric intake. No formal sample size was performed for the sub-study; we planned to enroll about 200 patients to observe around 100 deaths [1] to satisfy the predictors to events of 1:10 pragmatic rule for inclusion in a multivariable model.
Table 1.
Clinical, demographic characteristics of the population and predictors of outcome (multivariable Cox proportional hazard model).
| Feature | Overall population (N = 222) | Mortality Harrell'c 0.75, (95%CI 0.69-0.81) |
IMV weaning-free survival Harrell's c 0.70 (95%CI 0.70-0.71) |
||
|---|---|---|---|---|---|
| HR [95%CI] | P value | HR [95%CI] | P value | ||
| Female, N (%) | 49 (22.1) | 0.51 (0.45–0.54) | <0.001 | 1.37 (1.31–1.45) | <0.001 |
| Age (years), Mean (SD) | 58.6 (11.2) | ||||
| ≥60 years, N (%) | 109 (49.1) | 3.01 (1.26–7.17) | 0.013 | 0.75 (0.45–1.27) | 0.28 |
| Body mass index (kg/m2), Mean (SD) | 28.9 (5.5) | 0.21 | 0.93 | ||
| <30 kg/m2, N (%) | 154 (69.4) | 1.00 | 1.00 | ||
| 30–34.9 kg/m2, N (%) | 41 (18.5) | 1.99 (1.07–3.68) | 0.029 | 1.01 (0.88–1.16) | 0.88 |
| ≥35 kg/m2, N (%) | 27 (12.1) | 2.47 (0.59–10.28) | 0.21 | 0.71 (0.62–0.82) | <0.001 |
| Time to admission to ICU (days), Median (IQR) | 4 (1–7) | ||||
| ≥4 days, N (%) | 96 (43.2) | 1.29 (0.86–1.93) | 0.21 | 0.94 (0.85–1.03) | 0.19 |
| COPD, N (%) | 5 (2.3) | 3.08 (0.77–12.38) | 0.11 | 1.10 (0.33–3.65) | 0.88 |
| Diabetes, N (%) | 30 (13.5) | 1.87 (1.25–2.79) | 0.002 | 1.20 (0.44–3.29) | 0.72 |
| Hypertension, N (%) | 97 (43.7) | – | – | – | – |
| Ischemic heart disease, N (%) | 27 (12.2) | – | – | – | – |
| Cancer, N (%) | 18 (8.1) | – | – | – | – |
| Chronic kidney disease, N (%) | 4 (1.8) | – | – | – | – |
| SOFA score, Mean (SD) | 7.9 (2.4) | ||||
| ≥8, N (%) | 74 (33.3) | 1.58 (1.42–1.75) | <0.001 | 0.67 (0.62–0.72) | <0.001 |
| SAPS-II score, Mean (SD) | 40.1 (13.1) | – | – | – | – |
| ≥41, N (%) | 104 (46.9) | ||||
| PaO2/FiO2, Median (IQR) | 114 (83–156) | – | – | – | – |
| Pronation, N (%) | 65 (29.3) | 1.55 (0.88–2.75) | 0.13 | 0.53 (0.40–0.70) | <0.001 |
| Lymphocytes (x10ˆ3/ul), Median (IQR) | 0.60 (0.43–0.97) | – | – | – | – |
| Lactate dehydrogenase (U/L), Median (IQR) | 414 (307–587) | – | – | – | – |
| C-reactive protein (mg/dL), Mean (SD) | 15.5 (10.4) | – | – | – | – |
| Albumin (g/dL), Mean (SD) | 2.67 (053) | – | – | – | – |
| D-Dimer (ug/L), Median (IQR) | 1779 (955–7480) | – | – | – | – |
| hs-Troponine I (ng/L), Median (IQR) | 31 (10–77) | – | – | – | – |
| Calorie intake ≥ 80% on day 4, N (%)a | 129 (65.2) | 0.46 (0.42–0.50) | <0.001 | 0.97 (0.70–1.36) | 0.88 |
| Protein intake ≥ 80% on day 4, N (%)a | 72 (36.4) | – | – | – | – |
Abbreviations:IMV, invasive mechanical ventilation; ICU, intensive care unit, COPD, chronic obstructive pulmonary disease; SOFA, sequential organ failure assessment; SAPS-II, Simplified Acute Physiology Score-II; HR (95%CI), hazard ratio and 95% confidence interval; SD, standard deviation; IQR, interquartile range (25th-75th percentile).
Percentage calculated on those still alive in ICU on day 4 (N = 198).
3. Results
Two hundred twenty-two patients were included. EN was provided to 209 patients, while exclusive PN and supplemental PN were administered to 4 and 33 patients, respectively. In 9 patients it was not possible to provide any nutritional support. Among patients still in the ICU and alive on day 4 (N = 198), 129 (65.2%) and 72 (36.4%) reached a satisfactory caloric and protein intake, respectively. Among 174 patients alive on day 7, 134 (77.0%) and 81 (46.6%) were receiving an acceptable caloric and protein support, respectively. Only 7 (3.5%) patients underwent overfeeding with a caloric intake >35 kcal/kg on day 4. After a median ICU stay of 14 days [IQR, 8–25], 72 (32.4%) patients died (12 and 20 in the first 4 and 7 days, respectively). Median duration of IMV was 13 days [IQR, 7–24].
Sixty-five (29.3%) patients were proned: their caloric intake did not significantly differ as compared to that of non-proned patients.
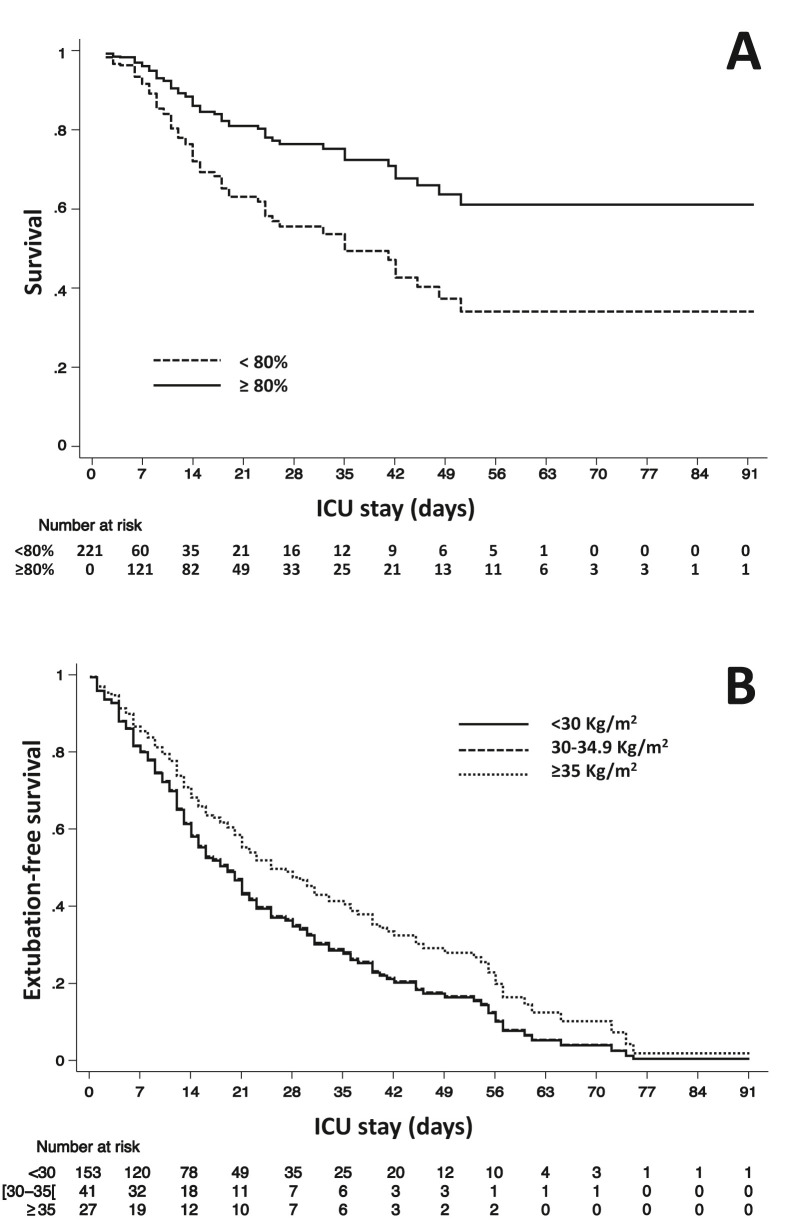
In multivariable analysis, reaching a satisfactory caloric intake on day 4 was associated with lower ICU mortality (HR 0.46 [95%CI 0.42–0.50], Table 1 and Fig. 1 A) but no association was found with earlier weaning from IMV (HR = 0.97 [95%CI, 0.70–1.36], P = 0.88). Mild obesity (body mass index [BMI] ≥30 and < 35 kg/m2 vs. <30) was associated with higher ICU mortality (Table 1), while patients with moderate-severe obesity (BMI≥35 kg/m2 vs. <30) were less likely to be weaned from invasive mechanical ventilation (HR = 0.71 [95%CI, 0.62–0.82], P < 0.001; Fig. 1B). Caloric and protein intake did not differ among different BMI categories and in diabetes patients.
Fig. 1.
Plot A, multivariable model adjusted cumulative survival according to the presence (dashed line) or absence (continuous line) of early caloric deficit; plot B, multivariable model adjusted cumulative extubation-free survival according to the absence (continuous line), the presence of mild (dashed line) or moderate-severe (dotted line) obesity.
There was no interaction between BMI categories and caloric intake, neither for survival (P = 0.38) nor for weaning from IMV (P = 0.93).
4. Discussion
To the best of our knowledge, this is the first prospective study describing the nutritional support management in mechanically ventilated COVID-19 patients and the association between early caloric deficit and clinical outcomes.
At multivariable analysis, in addition to SOFA score and gender, caloric deficit on day 4 emerged as an independent predictor of ICU mortality together with obesity and diabetes. This observation is relevant, as these two comorbidities could influence the physicians’ approach towards nutritional support, suggesting the need for a reduced provision of calories and/or glucose, which was not the case in our study. To note, the effect of low caloric intake on weaning from mechanical ventilation and ICU mortality did not differ among different BMI categories in our population. Although the observational nature of the study and the absence of a large sample size do not allow any conclusive statement and the absence of indirect calorimetry assessment may impair the data accuracy, we believe that our results could be useful in underlying the importance of adequate and timely nutritional support in all critically ill COVID-19 patients, even in case of obesity and/or diabetes.
Our findings are consistent with previous studies showing that the energy deficit accumulated by long-staying ICU patients during the first days since admission is associated with increased mortality [5]. Overall, nutritional support was administered according to guidelines and recent recommendations [2,5], as deducible from the wide and prevalent use of EN and the very low percentage of patients with overfeeding. Nevertheless, almost one third and two thirds of patients did not receive enough calories and proteins on day 4, respectively. In respect to this, as reported in other studies [8], we cannot exclude that inadequate protein intake also contributed to worsen prognosis, although a separate model of analysis including only protein intake, considered due to collinearity with caloric intake, did not show any effect. However, these data are not surprising, as both overfeeding and underfeeding in the ICUs are still a common and unresolved problem [3,8]. Of note, prone positioning did not appear to be a limiting factor for nutritional support.
Finally, the results regarding obesity were not unexpected, as worse prognosis has been already consistently shown by other studies with large samples [7]. Interestingly, although less than one third of the sample was obese, we also detected an association between severe obesity and IMV duration, which further underlines the detrimental consequences of this clinical condition. Providing adequate artificial nutritional support without harming obese and diabetic patients is still a challenging issue and our results further indicate its clinical relevance. Interestingly, in our two referral centers where the awareness of nutritional support is high, the 32% ICU mortality was lower than the 48% reported by previous literature [1]. Further comparative studies are certainly warranted.
As major limitation, we recognize the use of predictive equation in the estimation of energy needs. ESPEN guidelines [5] suggest that, if predictive equations are used, hypocaloric nutrition (<70% estimated needs) should be preferred over isocaloric nutrition. This may appear in contradiction with our results. However, the TICACOS study [9] has recently shown that, compared to indirect calorimetry, the use of predictive equations and a calorie target similar to our study (20–25 kcal/kg) was associated more negative energy balance in IMV patients. On the other hand, with the LEEP-COVID study [10], it has been highlighted that a progressive hypermetabolism throughout the ICU stay does exist in intubated COVID-19 patients. Overall, these findings further highlight the peculiarities of this patient population and the need of more accurate assessments of energy requirements in guiding nutrition delivery.
5. Conclusions
This observational study confirmed the negative prognostic and clinical role of obesity in mechanically ventilated COVID-19 patients and suggested that early caloric deficit may independently contribute to worsen survival in these patients, suggesting that any effort should be made to implement timely and adequate nutritional support during their ICU stay.
Author contributions
Caccialanza, Cereda, Guzzardella, Grasselli, Belliato and Mojoli had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of data analysis.
Caccialanza and Grasselli are chief investigators and act as guarantors for this work.
Concept and design: Caccialanza, Cereda, Guzzardella, Grasselli.
Acquisition, analysis, or interpretation of data: Caccialanza, Cereda, Belliato, Sciutti, Mojoli, Grasselli, Guzzardella, Savioli, Zanella, Mongodi, Crotti, Masi.
Drafting of the manuscript: Caccialanza, Cereda, Guzzardella, Grasselli, Klersy.
Critical revision of the manuscript for important intellectual content: Belliato, Mojoli, Grasselli.
Statistical analysis: Klersy.
Administrative, technical, or material support: Guzzardella, Sciutti, Savioli, Zanella, Mongodi, Masi, Crotti.
Conflict of interest disclosures
None of the authors has conflicts of interest to disclose.
Funding/support
This work was supported by the Fondazione IRCCS Policlinico San Matteo, the Fondazione IRCCS Ca’ Granda Ospedale Maggiore di Milano Policlinico and by an unconditioned grant from Nutricia Italia S.p.A.
Acknowledgments
We are grateful to all the employees of the Fondazione IRCCS Policlinico San Matteo and the Fondazione IRCCS Ca’ Granda Ospedale Maggiore di Milano Policlinico for their courageous efforts in struggling against the clinical and social COVID-19 emergency.
References
- 1.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., et al. COVID-19 Lombardy ICU Network Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thibault R., Seguin P., Tamion F., Pichard C., Singer P. Nutrition of the COVID-19 patient in the intensive care unit (ICU): a practical guidance. Crit Care. 2020;24(1):447. doi: 10.1186/s13054-020-03159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vallejo K.P., Martínez C.M., Matos Adames A.A., Fuchs-Tarlovsky V., Nogales G.C.C., Paz R.E.R., et al. Current clinical nutrition practices in critically ill patients in Latin America: a multinational observational study. Crit Care. 2017;21(1):227. doi: 10.1186/s13054-017-1805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minnelli N., Gibbs L., Larrivee J., Sahu K.K. Challenges of maintaining optimal nutrition status in COVID-19 patients in intensive care settings. J Parenter Enter Nutr. 2020;44(8):1439–1446. doi: 10.1002/jpen.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer P., Blaser A.R., Berger M.M., Alhazzani W., Calder P.C., Casaer M.P., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 6.Schetz M., De Jong A., Deane A.M., Druml W., Hemelaar P., Pelosi P., et al. Obesity in the critically ill: a narrative review. Intensive Care Med. 2019;45(6):757–769. doi: 10.1007/s00134-019-05594-1. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y., Lu Y., Huang Y.M., Wang M., Ling W., Sui Y., et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020;113:154378. doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zusman O., Theilla M., Cohen J., Kagan I., Bendavid I., Singer P. Resting energy expenditure, calorie and protein consumption in critically ill patients: a retrospective cohort study. Crit Care. 2016;20(1):367. doi: 10.1186/s13054-016-1538-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singer P., De Waele E., Sanchez C., Ruiz Santana S., Montejo J.C., Laterre P.F., et al. TICACOS international: a multi-center, randomized, prospective controlled study comparing tight calorie control versus liberal calorie administration study. Clin Nutr. 2021;40(2):380–387. doi: 10.1016/j.clnu.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Whittle J., Molinger J., MacLeod D., Haines K., Wischmeyer P.E., LEEP-COVID Study Group Persistent hypermetabolism and longitudinal energy expenditure in critically ill patients with COVID-19. Crit Care. 2020;24(1):581. doi: 10.1186/s13054-020-03286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]