Abstract
The purpose of this study was to evaluate the expression of the SARS-CoV-2 receptors ACE2 and TMPRSS2 in an immortalized human conjunctival epithelial cell line and in healthy human conjunctiva excised during ocular surgery, using Western blot, confocal microscopy and immunohistochemistry. The Western blot showed that ACE2 and TMPRSS2 proteins were expressed in human immortalized conjunctival cells, and this was confirmed by confocal microscopy images, that demonstrated a marked cellular expression of the viral receptors and their co-localization on the cell membranes. Healthy conjunctival samples from 11 adult patients were excised during retinal detachment surgery. We found the expression of ACE2 and TMPRSS2 in all the conjunctival surgical specimens analyzed and their co-localization in the superficial conjunctival epithelium. The ACE2 Western blot levels and immunofluorescence staining for ACE2 were variable among specimens.
These results suggest the susceptibility of the conjunctival epithelium to SARS-CoV-2 infection, even though with a possible interindividual variability.
Keywords: SARS-CoV-2, COVID-19, ACE2, TMPRSS2, Human conjunctiva, Conjunctival epithelial cells, Immunohistochemistry, Western blot
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is the novel coronavirus responsible for the Coronavirus Disease 2019 (COVID-19). Due to its rapid spread across the world, on March 11th, 2020, COVID-19 was recognized as a pandemic by the World Health Organization (WHO). Even though the main feature of COVID-19 is a severe interstitial pneumonia and the main transmission route of SARS-CoV-2 is airborne (by inhaling droplets or aerosols released by an infected individual), a possible transmission of the virus through the ocular surface is suspected (Lu et al., 2020; Wu et al., 2020). The ocular route is supported by case reports of COVID-19 in people who visited or worked in Wuhan hospitals wearing N95 masks and other personal protective equipment, but without any protective eye wear (Lu et al., 2020; Zhang et al., 2020). Moreover, a possible SARS-CoV-2 ocular involvement has been suggested by reports of conjunctivitis and ocular symptoms in patients affected by COVID-19 (Aggarwal et al., 2020; Wu et al., 2020): nevertheless, it is not clear whether conjunctiva is the site of viral entry or is secondarily involved by retrograde migration of the coronavirus from the upper respiratory tract through the lacrimal drainage ducts.
The evidence in favor of the susceptibility of ocular surface epithelia to SARS-CoV-2 infection is growing: SARS-CoV-2 RNA has been detected (even though with a small prevalence) in tears and conjunctival secretions of COVID-19 patients with active conjunctivitis (Aggarwal et al., 2020; Bernabei et al., 2020; Xia et al., 2020) or in postmortem ocular specimens of affected patients (Sawant et al., 2021); moreover, the nucleocapsid protein antigen of SARS-CoV-2 has been found in the ocular tissues of a patient previously infected with COVID-19 (Yan et al., 2020). In addition, the viral replication in ex-vivo cultures of human conjunctiva has been demonstrated (Hiu et al., 2020). Nevertheless, the ability of SARS-CoV-2 to infect the ocular surface tissues needs to be confirmed by further studies.
It has been demonstrated that the entry of SARS-CoV-2 into human cells is mainly mediated by the binding of the viral spike protein (S protein) to the host receptor angiotensin-converting enzyme-2 (ACE2); in addition, in order to facilitate the membrane fusion process, the S protein needs to be primed by the cellular transmembrane serine protease 2 (TMPRSS2) (Hoffmann et al., 2020).
The expression of these viral entry proteins in human cornea and conjunctiva, which if confirmed is a proof of the susceptibility of these tissues to SARS-CoV-2 infection, has been evaluated by recent studies with contradictory results, using different techniques: analysis of transcriptomic datasets (Hikmet et al., 2020; Sungnak et al., 2020), RNA sequencing (Collin et al., 2021; Ma et al., 2020; Lange et al., 2020; Leonardi et al., 2020), Western blot and/or immunohistochemistry (Lange et al., 2020; Li et al., 2021; Roehrich et al., 2020; Zhou et al., 2020). In this context, the purpose of this preliminary study was to evaluate the expression of the SARS-CoV-2 receptors ACE2 and TMPRSS2 in an immortalized human conjunctival epithelial cell line and in healthy human conjunctiva excised during ocular surgery, using Western blot and immunofluorescence staining.
The human conjunctival epithelial cells (HConEC) provided by Innoprot (Derio, Bizkaia, Spain) (Catalog Number: P10870, batch #111019) were isolated from human conjunctiva. They were kept in culture in Corneal Epithelium Cell Medium and maintained as previously described (Mencucci et al., 2020).
Healthy conjunctival samples (bulbar conjunctiva) from 11 adult patients were excised during retinal detachment surgery. All patients had preoperative nasopharyngeal swabs negative for SARS-CoV-2 RNA. Patients with history of previous HIV, HBV or HCV infection, previous ocular surgery and/or recent or active conjunctival inflammation were excluded. The study followed the tenets of the Declaration of Helsinki and was approved by the local Ethics Committee (Area Vasta Centro, Careggi Hospital, University of Florence, Italy). All study participants underwent informed consent and all tissue samples were analyzed anonymously.
Four surgical samples were immediately stored at −80 °C after removal, until processing for Western blot analysis; the remaining 7 were immediately fixed in 10% buffered formalin, then processed and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin for routine histological analysis.
Western blotting was performed as previously reported (Piazzini et al., 2019). Human conjunctival epithelial cells (3 wells/sample) were dissolved in 1% SDS. Conjunctival tissue was homogenized in 1% SDS with protease inhibitors (cOmplete Protease Inhibitor Cocktail, Roche). The human primary glioblastoma cell lineU87 MG (ATCC, Manassas, VA) served as negative control according to Human Protein Atlas (https://www.proteinatlas.org). Insoluble material was removed by centrifugation at 10,000 g for 15 min at 4 °C. BCA (bicinchoninic acid) Protein Assay was used to quantify the total protein levels. Lysates (20 μg/lane of protein) were resolved by electrophoresis on a 4–20% SDS-polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA, USA) and transferred onto nitrocellulose membranes. After blocking, the blots were incubated overnight at 4 °C with polyclonal-rabbit antibody against TPRMSS2 (catalog no. ab109131; Abcam, Cambridge, UK) and mouse monoclonal anti-ACE2 (catalog no. ab89111; Abcam) diluted 1:1000 in TBS-T containing 5% bovine serum albumin. β-actin (from Sigma, St Louis, MO, USA) was used as a loading control. Immunodetection was performed with HRP-conjugated secondary antibodies (1:2000 anti-mouse and anti-rabbit Amersham Biosciences, UK) in TBS-T containing 5% non-fat dry milk. After washing, the membranes and reactive bands were detected using chemiluminescence (ECLplus; Euroclone, Padova, Italy).
For immunofluorescence staining, the cells were seeded on glass coverslips and were washed with PBS, fixed in 2% (w/v) buffered paraformaldehyde for 10 min at room temperature (20 °C) (Becatti et al., 2018), and permeabilized with a 0.5% (v/v) Triton X-100 solution for 5 min. Then, cells were incubated for 120 min at room temperature (20 °C) with 1:200 rabbit polyclonal anti-TMPRSS2 or with 1:400 mouse monoclonal anti-ACE2 antibodies in Tris-buffered saline, pH 7.6, 0.1% Tween 20 (TBS-T) containing 3% bovine serum albumin. After washing, cells were incubated with Horse Anti-Mouse IgG Antibody (H+L), DyLight® 594 (DI-2594, 1:400, Vector Laboratories, Inc., USA) or with AlexaFluor 488 donkey anti-rabbit (1:400; A21206, Thermo Fisher Sci., USA). The cells were analyzed using a Leica TCS SP8 confocal scanning microscope (Leica Microsystems, Mannheim, Germany) equipped with an argon laser source and a Leica Plan Apo 63 × oil immersion objective.
Concerning immunofluorescence staining of healthy human conjunctival tissue, paraffin-embedded tissue sections (4 μm thick) were deparaffinized, rehydrated in a graded series of ethanol and boiled in sodium citrate buffer (10 mM, pH 6) for 10 min. Sections were washed in PBS and then incubated with a solution containing glycine (2 mg/ml) for 10 min to quench autofluorescence. After blocking with 1% bovine serum albumin (BSA) in PBS for 45 min, tissue sections were incubated with a mixture of mouse monoclonal anti-ACE2 (1:50) and rabbit monoclonal anti-TMPRSS2 (1:100) antibodies overnight at 4 °C. The slides were then washed 3 times for 5 min in PBS and incubated with a specific antibody mixture of Alexa Fluor 488-conjugated goat anti-mouse and Alexa Fluor 568-conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, OR, USA) diluted 1:200 in PBS with 1% BSA for 45 min at room temperature. Negative controls were performed using irrelevant isotype- and concentration-matched mouse and rabbit IgG (Sigma-Aldrich, Milan, Italy), while cross-reactivity of secondary antibodies was tested omitting primary antibodies. Human lung sections were used as positive control tissue, while human skin sections served as negative control tissue according to Human Protein Atlas (https://www.proteinatlas.org). Nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI; 1:1000; Chemicon International, Temecula, CA, USA). After washing in PBS, the immunolabeled sections were mounted with an antifade mounting medium (Biomeda Gel Mount; Electron Microscopy Sciences, Foster City, CA) and observed under a Leica DM4000 B microscope equipped with fully automated fluorescence axes (Leica Microsystems, Mannheim, Germany). Fluorescence images were collected using a Leica DFC310 FX 1.4-megapixel digital color camera equipped with the Leica software application suite LAS V3.8 (Leica Microsystems).
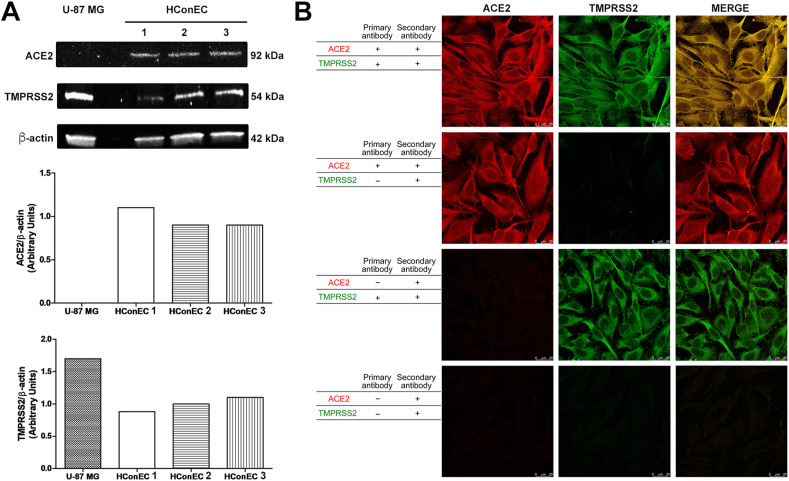
In this study, Western blot showed that ACE2 and TMPRSS2 proteins were expressed in human immortalized conjunctival cells (Fig. 1 A), and this was confirmed by confocal microscopy images (Fig. 1B), that demonstrated a marked cellular expression of the viral receptors and their co-localization on the cell membranes.
Fig. 1.
A: Qualitative and quantitative ACE2 and TMPRSS2 protein expression in immortalized human conjunctival epithelial cells (HConEC) (3 different samples) and U87 MG cells (negative control). Cell lysates were prepared from conjunctival epithelial and U87 MG cell samples and analyzed by Western blot, using antibodies against ACE2 or TMPRSS2. Beta-actin was used as the loading control. B: Confocal microscope analysis (63× magnification) of ACE2 and TMPRSS2 receptors expression in immortalized human conjunctival epithelial cells. To confirm staining and exclude experimental artefacts, slides were also stained solely with primary antibody (ACE2 or TMPRSS2) or secondary antibody.
Moreover, we analyzed the surgical specimens obtained from 11 patients who underwent retinal detachment surgery.
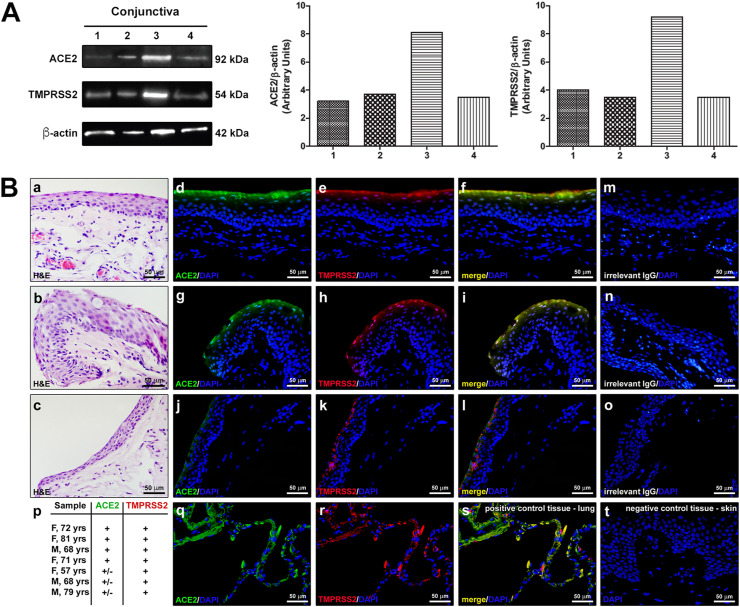
Using Western blot analysis, we found the expression of ACE2 and TMPRSS2 in all the conjunctival surgical specimens analyzed (4 patients). As shown in Fig. 2 A, both TMPRSS2 and ACE2 levels were variable between specimens.
Fig. 2.
Expression of ACE2 and TMPRSS2 in healthy human conjunctival epithelium. A: Representative qualitative and quantitative Western blot of human conjunctival surgical specimens of 4 patients (1: M, 68 yrs; 2: F, 33yrs; 3: F, 68 yrs; 4: M, 80 yrs). B: (a–c) Representative images of hematoxylin and eosin (H&E) staining of 3 different conjunctival samples. (d–l) Representative images of double immunofluorescence staining for ACE2 (green) and TMPRSS2 (red) in the same 3 conjunctival samples. Note the variability in the expression of ACE2 among samples: (d, g) strong expression, (j) weak expression. In the samples displaying strong ACE2 expression, ACE2 and TMPRSS2 are clearly co-localized (yellow staining in f, i) in the superficial conjunctival epithelium. (m–o) Images of negative controls obtained with irrelevant IgG. (p) Analysis of ACE2 and TMPRSS2 immunostaining in conjunctival samples from 7 patients; gender (F/M) and age in years (yrs) are indicated for each patient. In each sample, the immunostaining intensity was scored semiquantitatively (+and±for strong and weak staining, respectively) in 5 randomly chosen microscopic fields ( × 40 original magnification). Human lung (q–s) and skin (t) were used as positive and negative control tissue, respectively. Note the absence of immunostaining even in the superficial epidermis (t). In each immunofluorescence image, nuclei are counterstained in blue with DAPI. Scale bar: 50 μm (a-o, q-t). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The immunohistochemical analysis (Fig. 2B) performed on the conjunctival surgical specimens of the other 7 patients revealed the expression of ACE2 and TMPRSS2 and their co-localization in the superficial conjunctival epithelium in all samples. While the TMPRSS2 expression was similar in all samples, the immunofluorescence staining for ACE2 was more variable as assessed by semiquantitative analysis of immunostaining intensity. In the conjunctival stroma, no immunofluorescence staining for the two proteins was detected.
Several studies have demonstrated ACE2 expression in epithelial cells of tissues including lung, heart, ileum and kidneys (Hikmet et al., 2020; Hoffmann et al., 2020), whereas the results concerning its presence on the ocular surface, especially in corneal and conjunctival epithelia, are controversial. TMPRSS2 is known to be a key protein for the priming of the SARS-CoV-2 protein S to facilitate the membrane fusion and consequently the viral entry into the target cell. (Hoffmann et al., 2020; Sungnak et al., 2020). The TMPRSS2 expression profile in human tissues do not match that of ACE2 exactly, with very low expression in the adult heart (Sungnak et al., 2020; Liu et al., 2020), where other proteases such as cathepsin L and furin may act as substitutes of TMPRSS2 (Liu et al., 2020); however, the few recent studies about TMPRSS2 expression in the human conjunctival and/or corneal epithelium also led to contradictory results.
Our preliminary study found that the viral entry factors ACE2 and TMPRSS2 were co-expressed in an immortalized human conjunctival epithelial cell line and in surgical specimens of human conjunctiva, even though the analyzed human samples showed a variable ACE2 expression. These results suggest the susceptibility of the conjunctival epithelium to SARS-CoV-2 infection and the importance of eye protection for healthcare workers who are at high risk of contracting the virus.
Our results on the conjunctival tissues are in line with the immunohistochemistry and Western blot results published by Zhou et al. (2020), who found by immunohistochemical analysis the expression of ACE2 and TMPRSS2 in the conjunctiva, limbus, and cornea of postmortem human eyes, and in conjunctival surgical specimens. In all cases, the most prominent staining was found in the epithelia, with a weak or focal expression in the substantia propria in surgical conjunctival specimens (Zhou et al., 2020).
Similarly, Roehrich et al. (2020) in their immunohistochemical study on fresh donor corneas and primary explant cell cultures of corneal, limbal, and conjunctival epithelial cells from corneoscleral disks found that both corneal and conjunctival epithelia express ACE2, dendritic cell–specific intercellular adhesion molecule 3–grabbing nonintegrin (DC-SIGN), DC-SIGN–related proteins (DC-SIGNR or L-SIGN), and TMPRSS2, suggesting that the ocular surface is a potential route for the transmission of SARS-CoV-2. Analyzing transcriptomic datasets or using RNA sequencing, Sungnak et al. (2020), Collin et al. (2021) and Leonardi et al. (2020) confirmed the co-expression of ACE2 and TMPRSS2 in the ocular surface epithelia, even if with slightly different results: Sungnak et al. (2020) found both proteins only in the superficial conjunctival cells; Collin et al. (2021) found them in human adult limbal and corneal epithelium as well; Leonardi et al. (2020) reported that ACE2 was expressed in conjunctival samples at a low level and at a lower expression level in the cornea, while TMPRSS2 were expressed at intermediate levels in both the conjunctiva and cornea. Moreover, a letter by Li et al. (2021) suggested the overexpression of ACE2 in diseased conjunctival tissue.
In contrast, Ma et al. (2020), using RNA-sequencing in primary cell lines derived from human healthy conjunctival tissue and pterygia, found consistent expression of ACE2 in conjunctival and pterygium cell lines, whereas the expression of TMPRSS2 was present only in 1 pterygium cell line, but not in any conjunctival cell line. Moreover, Lange at al. (2020), using RNA sequencing were not able to identify the expression of ACE2 and TMPRSS2 in 38 healthy and diseased surgical specimens from previous conjunctival biopsies (excised between 1967 and 2007), and their immunohistochemical analysis performed with two monoclonal antibodies found an irrelevant ACE2 protein content in healthy conjunctival samples.
In our opinion, these contradictory results may be due mainly to an interindividual variability in the expression of SARS-CoV-2 entry factors, especially ACE2, in human ocular surface epithelia, as has been hypothesized for other human tissues (Bourgonje et al., 2020) and as has been suggested by reports of ACE2 overexpression in diseased conjunctiva (Li et al., 2021). This may cause a different interindividual susceptibility to SARS-CoV-2 infection. Western blot and immunohistochemical studies on a higher number of fresh conjunctival samples are needed to reach the statistical power to assess whether sex, age and other characteristics can influence the expression of SARS-CoV-2 entry factors. Moreover, infection studies with live virus are necessary to confirm the susceptibility of the ocular surface to SARS-CoV-2 infection.
To our knowledge, the present study is the first to evaluate, using Western blot and confocal microscopy, the expression of ACE2 and TMPRSS2 in an immortalized human conjunctival epithelial cell line: our results may pave the way for future in vitro infection studies on immortalized cell cultures, which are more easily available and less variable than primary cell cultures.
In conclusion, further immunohistochemical and viral infection studies are needed to better assess and characterize the ocular surface susceptibility to SARS-CoV-2 infection, and to evaluate its possible interindividual variability.
Funding declaration
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
References
- Aggarwal K., Agarwal A., Jaiswal N., Dahiya N., Ahuja A., Mahajan S., Tong L., Duggal M., Singh M., Agrawal R., Gupta V. Ocular surface manifestations of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. PloS One. 2020;15 doi: 10.1371/journal.pone.0241661. Https://doi.org/10.1371/journal.pone.0241661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becatti M., Barygina V., Mannucci A., Emmi G., Prisco D., Lotti T., Fiorillo C., Taddei N. Sirt1 protects against oxidative stress-induced apoptosis in fibroblasts from psoriatic patients: a new insight into the pathogenetic mechanisms of psoriasis. Int. J. Mol. Sci. 2018;19:1572. doi: 10.3390/ijms19061572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabei F., Versura P., Rossini G., Re M.C. There is a role in detection of SARS-CoV-2 in conjunctiva and tears: a comprehensive review. New Microbiol. 2020;43(4) [PubMed] [Google Scholar]
- Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J., Bolling M.C., Dijkstra G., Voors A.A., Osterhaus A.D., van der Voort P.H., Mulder D.J., van Goor H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J. Pathol. 2020;251:228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin J., Queen R., Zerti D., Dorgau B., Georgiou M., Djidrovski I., Hussain R., Coxhead J.M., Joseph A., Rooney P., Lisgo S., Figueiredo F., Armstrong L., Lako M. Co-expression of SARS-CoV-2 entry genes in the superficial adult human conjunctival, limbal and corneal epithelium suggests an additional route of entry via the ocular surface. Ocul. Surf. 2021;19:190–200. doi: 10.1016/j.jtos.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikmet F., Méar L., Edvinsson Å., Micke P., Uhlén M., Lindskog C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020;16 doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui K.P.Y., Cheung M.-C., Perera R.A.P.M., Ng K.-C., Bui C.H.T., Ho J.C.W., Ng M.M.T., Kuok D.I.T., Shih K.C., Tsao S.-W., Poon L.L.M., Peiris M., Nicholls J.M., Chan M.C.W. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir Med. 2020;8:687–695. doi: 10.1016/S2213-2600(20)30193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C., Wolf J., Auw-Haedrich C., Schlecht A., Boneva S., Lapp T., Horres R., Agostini H., Martin G., Reinhard T., Schlunck G. Expression of the COVID-19 receptor ACE2 in the human conjunctiva. J. Med. Virol. 2020;92:2081–2086. doi: 10.1002/jmv.25981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi A., Rosani U., Brun P. Ocular surface expression of SARS-CoV-2 receptors. Ocul. Immunol. Inflamm. 2020;28:735–738. doi: 10.1080/09273948.2020.1772314. [DOI] [PubMed] [Google Scholar]
- Li S., Li D., Fang J., Liu Q., Cao W., Sun X., Xu G. SARS-CoV-2 receptor ACE2 is expressed in human conjunctival tissue, especially in diseased conjunctival tissue. Ocul. Surf. 2021;19:249–251. doi: 10.1016/j.jtos.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Gai S., Wang X., Zeng J., Sun C., Zhao Y., Zheng Z. Single-cell analysis of SARS-CoV-2 receptor ACE2 and spike protein priming expression of proteases in the human heart. Cardiovasc. Res. 2020;116:1733–1741. doi: 10.1093/cvr/cvaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C., Liu X., Jia Z. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;22(10224):395. doi: 10.1016/S0140-6736(20)30313-5. e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D., Chen C.-B., Jhanji V., Xu C., Yuan X.-L., Liang J.-J., Huang Y., Cen L.-P., Ng T.K. Expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in human primary conjunctival and pterygium cell lines and in mouse cornea. Eye. 2020;34:1212–1219. doi: 10.1038/s41433-020-0939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencucci R., Favuzza E., Bottino P., Mazzantini C., Zanotto E., Pellegrini-Giampietro D.E., Landucci E. A new ophthalmic formulation containing antiseptics and dexpanthenol: in vitro antimicrobial activity and effects on corneal and conjunctival epithelial cells. Exp. Eye Res. 2020;201:108269. doi: 10.1016/j.exer.2020.108269. [DOI] [PubMed] [Google Scholar]
- Piazzini V., Landucci E., D'Ambrosio M., Tiozzo Fasiolo L., Cinci L., Colombo G., Pellegrini-Giampietro D.E., Bilia A.R., Luceri C., Bergonzi M.C. Chitosan coated human serum albumin nanoparticles: a promising strategy for nose-to-brain drug delivery. Int. J. Biol. Macromol. 2019;129:267–280. doi: 10.1016/j.ijbiomac.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Roehrich H., Yuan C., Hou J.H. Immunohistochemical study of SARS-CoV-2 viral entry factors in the cornea and ocular surface. Cornea. 2020;39:1556–1562. doi: 10.1097/ICO.0000000000002509. [DOI] [PubMed] [Google Scholar]
- Sawant O.B., Singh S., Wright R.E., 3rd, Jones K.M., Titus M.S., Dennis E., Hicks E., Majmudar P.A., Kumar A., Mian S.I. Prevalence of SARS-CoV-2 in human post-mortem ocular tissues. Ocul. Surf. 2021;19:322–329. doi: 10.1016/j.jtos.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., Talavera-López C., Maatz H., Reichart D., Sampaziotis F., Worlock K.B., Yoshida M., Barnes J.L., HCA Lung Biological Network SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Duan F., Luo C., Liu Q., Qu X., Liang L., Wu K. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in hubei province, China. JAMA Ophthalmol. 2020;138:575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J., Tong J., Liu M., Shen Y., Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020;92:589–594. doi: 10.1002/jmv.25725.ù. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Diao B., Liu Y., Zhang W., Wang G., Chen Z. Severe Acute respiratory Syndrome coronavirus 2 nucleocapsid protein in the ocular tissues of a patient previously infected with coronavirus disease 2019. JAMA Ophthalmol. 2020;138:1–4. doi: 10.1001/jamaophthalmol.2020.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Chen X., Chen L., Deng C. The evidence of SARS-CoV-2 infection on ocular surface. Ocul. Surf. 2020;18:360–362. doi: 10.1001/jamaophthalmol.2020.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Xu Z., Castiglione G.M., Soiberman U.S., Eberhart C.G., Duh E.J. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul. Surf. 2020;18:537–544. doi: 10.1016/j.jtos.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]